Highlights
-
•
Rapid detection methods used as first diagnostic test for CARVs may delayed the start of antiviral therapy in a significant number of influenza and RSV cases.
-
•
Syndromic multiplex RT-PCR-based prospective clinical CARV survey in allo-HCT recipients translates into a lower mortality rate as compared to standard clinical practice based on RSV and influenza virus rapid detection test.
-
•
We found that donor/recipient HLA mismatch, CARV LRTD and high-risk ISI were also associated with higher mortality.
Keywords: Prospective respiratory virus surveillance program, Community-acquired respiratory virus, Allogeneic hematopoietic stem cell transplantation, Respiratory virus infection, Immunodeficiency score index, Influenza, Respiratory syncytial virus, Parainfluenza virus
Abstract
Background
There is a lack of studies comparing clinical outcomes among retrospective versus prospective cohorts of allogeneic stem cell transplant (allo-HCT) recipients with community acquired respiratory virus (CARV) infections.
Methods
We compare outcomes in two consecutive cohorts of allo-HCT recipients with CARV infections. The retrospective cohort included 63 allo-HCT recipients with 108 CARV infections from January 2013 to April 2016 who were screened and managed following standard clinical practice based on influenza and respiratory syncytial virus rapid antigen detection methods. The prospective cohort was comprised of 144 consecutive recipients with 297 CARV episodes included in a prospective interventional clinical surveillance program (ProClinCarvSur-P) based on syndromic multiplex PCR as first-line test from May 2016 to December 2018 at a single transplant center.
Results
CARV infections in the retrospective cohort showed more severe clinical features at the time of diagnosis compared to the prospective cohort (fever 83% vs. 57%, hospital admission 69% vs. 28% and lower respiratory tract 58% vs. 31%, respectively, p ≤ 0.002 for all comparisons). Antiviral therapy was more commonly prescribed in the prospective cohort (69 vs. 43 treated CARV episodes), particularly at the upper respiratory tract disease stage (34 vs. 12 treated CARV episodes). Three-month all-cause mortality was significantly higher in the retrospective cohort (n = 23, 37% vs. n = 10, 7%, p < 0.0001). Multivariate logistic regression analysis showed that recipients included in ProClinCarvSur-P had lower mortality rate [odds ratio 0.31, 95% confidence interval 0.12–0.7, p = 0.01].
Conclusion
This study report on outcome differences when reporting retrospective vs. prospective CARV infections after allo-HCT. Recipients included in a ProClinCarvSur-P had lower mortality.
Introduction
Respiratory tract infections are the second leading cause of mortality and morbidity worldwide1 and community acquired respiratory viruses (CARV) are the most common cause.2 This statistic and the recent 2009 H1N1 influenza pandemic have raised awareness in national and transnational health authorities who support developing CARV epidemiological survey systems.3, 4, 5, 6 These monitoring activities definitely contribute to health improvement and are extremely useful for national policy makers in identifying groups at high risk and for selecting influenza strains for yearly vaccine production. However, these systems get positive CARV results derived from standard clinical practice (StCP) through sentinel centers. Currently, StCP focuses on testing influenza and respiratory syncytial virus (RSV) using either rapid antigen detection or RT-PCR test in patients with respiratory symptoms requiring hospital admission.7 , 8 Consequently, the current survey systems have substantial limitations in the knowledge regarding epidemiology and relevance of other CARV types.
The seasonality of CARV infections in allogeneic hematopoietic stem cell transplantation (allo-HCT) recipients mirrors the incidence of infection in the community.9 However, CARV infections in immunocompromised patients, in particular recipients of allo-HCT, are more severe than in the general population, showing long viral shedding, higher rates of progression from the upper (URTD) to the lower respiratory tract disease (LRTD) and higher mortality rates, irrespective of the CARV type.10, 11, 12 In these patients, StCP focused on influenza and RSV seems suboptimal since this strategy led to significant mortality rates after any CARV LRTD ranging from 6% to 83%.13, 14, 15, 16, 17 This fact emphasize that actions are required to improve outcomes in this scenario.
The implementation of prospective interventional clinical CARV surveillance programs (ProClinCarvSur-P) in transplant centers, expanding virus detection through syndromic multiplex PCR panels as a first-line test13 , 18 could be of value to this aim. Such a program should ideally include the following elements: information/education on CARV infections/transmission, facilities for immediate risk-assessment, diagnosis and monitoring, the availability of highly sensitive syndromic multiplex PCR platforms, a real-time alert system for practitioners and an updated CARV management protocol including transmission control measures. The expected clinical benefits would be identifying CARV at an earlier stage of the disease (at the beginning of respiratory symptoms and before hospital admission), limiting outbreak situations and timely therapy with antiviral drugs or supportive care measures. The final end point would be a reduction in direct (CARV transmission, progression to LRTD, hospital and/or ICU admission and mortality)19 and indirect CARV effects (air flow obstruction, bacterial co-infection, COPD exacerbations and/or cardiovascular events and all-cause mortality).20 , 21 To date there is a lack of studies demonstrating the clinical benefit of conducting such a program compared to StCP.22
The current study analyzes the effects of instituting a ProClinCarvSur-P by comparing clinical outcomes in allo-HCT recipients with documented CARV infections before and after its implementation in a single transplant center in Spain.
Patients and Methods
Study population and design
This study included two consecutive cohorts of adult (>18 years) allo-HCT recipients with microbiologically-documented CARV infection diagnosed at the hematology division of the Hospital Universitari i Politècnic La Fe in Valencia, Spain from January 2013 to December 2018. The institutional review boards approved the study in accordance with the Declaration of Helsinki and informed consent was obtained from patients included in the prospective cohort.
The first cohort (retrospective cohort) included all allo-HCT recipients with documented CARV infection episodes diagnosed between January 2013 and April 2016 (40-month period). The second cohort (prospective cohort) was comprised of consecutive allo-HCT recipients with molecularly-documented CARV infections who were diagnosed during the ProClinCarvSur-P, between May 2016 and December 2018 (31-month period).
From January 2013 to April 2016, our allo-HCT CARV StCP focused on evaluating recipients with respiratory symptoms in the general emergency unit, where influenza A/B and RSV were tested by rapid antigen and/or nucleic acid amplification detection methods.7 In cases of negative results and respiratory symptoms worsening or hospital admission, syndromic multiplex PCR test was used in specimens of either the upper or lower respiratory tract provided that radiological signs suggested a viral etiology (ground glass opacities, interstitial infiltrates or bilateral micronodules). Influenza was treated with oseltamivir while RSV LRTD was treated with aerolized ribavirin.
From May 2016, we carried out the ProClinCarvSur-P in the transplant unit which has previously been described, in part, elsewhere.9 , 23 The main changes introduced in this period were yearly educational/informative clinical sessions for healthcare workers (physicians and nurses) and for recipients during the pre-transplant workup. Allo-HCT recipients with respiratory symptoms underwent CARV screening by syndromic multiplex RT-PCR panels as a first-line test. We attended recipients with respiratory symptoms in the outpatient transplant clinic during working hours. Transmission control measures were reinforced in the outpatient clinic following guidelines.24 We assessed the immunodeficiency scoring index (ISI)25 at the time of CARV screening and established an electronic real-time alert system for positive CARV results. We treated RSV and HPiV with oral ribavirin at early stage of the disease in the outpatient clinic according to our prior interventional protocol.26 Influenza infections were treated with oseltamivir at dose of 75 mg/12 h or 150 mg/12 h in severe cases or cases with long lasting symptoms (>3 weeks). In RSV and HPiV, oral ribavirin was given at a loading dose (maximum daily dose of 30 mg/kg) in the outpatient clinic whereas aerolized ribavirin at 2 g/8 h each course lasting 2 h was used in cases with LRTD. We also boosted annual flu vaccination awareness information. Finally, we prospectively collected clinical and laboratory variables at the time of CARV screening. Further details of the retrospective and ProClinCarvSur-P cohorts and management are provided in the Patients and Methods Supplementary file.
CARV episodes analysis criteria and definitions
The recipients/CARV episodes included in the current study had no evidence of disease relapse before CARV detection. In recipients who died after developing more than one CARV episode, we counted the most recent CARV episode for mortality, while the previous one was censored as alive at the time of the following CARV episode. We did not routinely check for CARV-negativity.
URTD was defined as a combination of upper respiratory symptoms (rhinorrhea, sinusitis, otitis, or pharyngitis) as well as positive CARV diagnosis by PCR test in the upper respiratory samples, and absence of LRTD symptoms and/or any indication of pulmonary infiltrates in chest X-ray or CT scan radiology results. We classified LRTD as possible, probable or confirmed, as previously described.27 There were no probable episodes because bronchoscopies were not performed in patients without radiological proof of pulmonary involvement.
Technical and diagnostic considerations
Patients with URTD and/or LRTD symptoms underwent nasopharyngeal aspiration, nasopharyngeal swabs or an induced sputum test, while BAL was performed in patients with radiological signs of LRTD whenever possible. From January 2013 to April 2016, first-line CARV screening was based on influeza and RSV rapid antigen detection and/or nucleic acid amplification test (NAAT) through an in vitro rapid immunochromatographic assay that detected RSV and Influenza A & B (Alere BinaxNOWⓇ) and/or the automated Simplexa™ Flu A/B & VRS Direct assay (SPX, Focus Diagnostics, Cypress, CA), repectvely. At both periods, a RT-PCR multiplex platform consisted of the CLART® PneumoVir DNA array assay (Genomica, Coslada, Spain). This RT-PCR can detect adenoviruses (ADVs); human bocavirus (HBoV); human coronavirus (HCoV) types 229E; influenza A virus A/H1N1, A/H3N2; influenza B and C; human metapneumovirus (hMPV); HPiV 1, 2, 3, and 4; RSV A-B; and enterovirus/rhinovirus (EvRh). From July 2018 onwards CARV screening was performed by BioFire FilmArray® Respiratory Panel (BioFire Diagnostics (a bioMerieux company), Salt Lake City, UT). This RT-PCR is able to detect 15 respiratory viruses: influenza virus types A, B and C (with influenza A subtyping), ADV, HCoV HKU1, NL63, 229E and OC43, hMPV, EvRh, HPiV types 1–4 and RSV, and also detects three bacteria: Mycoplasma pneumoniae, Chlamydia pneumoniae and Bordetella pertussis.
Endpoints and statistical analysis
The primary objective was to evaluate the clinical benefit of ProClinCarvSur-P by comparing three-month all-cause mortality from the time of CARV diagnosis in recipients receiving StCP care versus those diagnosed during ProClinCarvSur-P care. Secondary endpoints included identification of other risk factors (RFs) associated with all-cause mortality and overall survival (OS).
Frequencies were compared using the χ2 test for categorical variables. Differences between medians were compared using the Mann–Whitney U test. Univariate and multivariate analyses of how ProClinCarvSur-P, clinical and biological variables associated with three-month all-cause mortality were calculated using logistic regression models. For multivariate analysis, only variables with parameter estimates showing a P value ≤ 0.10 in the univariate analysis were finally included. Two-sided exact P values were reported and p values ≤ 0.05 were considered statistically significant. OS probabilities of CARV episodes according to different clinical and biological variables was estimated from time of CARV detection using Kaplan-Meier curves28 and univariate comparisons were made with the log-rank test.29 The data was analyzed with the SPSS (version 20.0) statistical package.
Results
Patient characteristics
Overall, 207 allo-HCT recipients (63 in the retrospective cohort and 144 in the prospective cohort) developed 404 episodes of CARV URTD and/or LRTD (108 CARV episodes in the retrospective cohort and 297 in the prospective cohort). Clinical and transplant characteristics of both cohorts are detailed in Table 1 . The study population comprised a high-risk cohort, since 63% of the recipients were allografted from alternative donors [adult unrelated donor (URD), single cord blood units (CBU) or haplo-identical family donors]. Donor/recipient human leukocyte antigen (HLA)-mismatch (considering high resolution typing of HLA A, B, C, DRB1 and DQB1) represented 45% of the cohort. The most notable differences among the cohorts included more CBU allo-HCT and ATG use in the retrospective cohort and more URD, prior HCT and reduced intensity conditioning regimen in the prospective cohort (p < 0.05 for all comparisons).
Table 1.
Patient characteristics in the prospective and retrospective cohort.
| Characteristics | Retrospective cohort (n = 63) | Prospective cohort (n = 144) | p value |
|---|---|---|---|
| Age (years), median (range) | 46 (18–65) | 44 (16–70) | 0.6 |
| Male, n (%) | 37 (58) | 81 (56) | 0.7 |
| Baseline disease, n (%) | |||
| • AL and other myeloid disorders | 49 | 108 | 0.4 |
| • Lymphoid disorders | 13 | 31 | |
| • Others | 1 | 5 | |
| Disease status at transplant, n (%) | |||
| • CR | 54 (86) | 99 (69) | 0.1 |
| • PR | 2 (3) | 12 (8) | |
| • Refractory/active disease | 7 (11) | 33 (23) | |
| Prior Auto-HCT, n (%) | 7 (11) | 38 (26) | 0.03 |
| Period of transplant, n (%) | |||
| • 2016–2018 | 2 | 115 | |
| • 2013–2015 | 43 | 15 | |
| • 2010–2012 | 10 | 6 | |
| • Before 2010 | 8 | 8 | |
| Conditioning regimen, n (%) | |||
| • RIC | 15 (24) | 60 (42) | 0.04 |
| Type of donor, n (%) | |||
| • HLA-identical sibling donor | 22 (35) | 55 (38) | 0.001 |
| • Unrelated donor | 3 (5) | 34 (24) | |
| • Umbilical cord blood | 30 (48) | 18 (13) | |
| • Haploidentical family donor | 7 (11) | 37 (26) | |
| PB stem cell source, n (% | 33 (53) | 121 (84) | 0.01 |
| HLA fully-matched, n (%) | 25 (40) | 89 (62) | 0.02 |
| ATG as a part of conditioning regimen, n (%) | 29 (46) | 24 (17) | 0.001 |
| GvHD prophylaxis, n (%) | |||
| • Sir-Tac | 1 (2) | 1 (1) | 0.001 |
| • CsA + MTX | 23 (37) | 30 (21) | |
| • Post-Cy | 7 (11) | 89 (62) | |
| • CsA + PDN and Others | 32 (51) | 24 (17) | |
| Length of observation period, months | 40 | 31 | |
| Number CARV episodes | 108 | 297 | |
| Median time from allo-HCT to CARV, days (range) | 252 (−7-6177) | 193 (−7-4894) | 0.03 |
| Median F/U after CARV, days (range) | 613 (6-2147) | 217 (1-799) | |
Abbreviations: AL, acute leukemia; MDS, myelodysplastic syndrome; MPD, myeloproliferative disease; CR, complete remission; PR, partial remission; Auto-HCT, autologous stem cell transplantation; RIC, reduced intensity conditioning; ATG, anti-thymocyte globulin; Sir, sirolimus; Tac, tacrolimus; CsA, cyclosporine A; MTX, methotrexate; Post-Cy, post-transplant cyclophosphamide; PDN, prednisone; SC, stem cell; allo-HCT, allogeneic hematopoietic stem cell transplantation; CARV, community-acquired respiratory virus; F/U, follow-up.
CARV types
Table 2 summarizes the most common CARV types isolated, and the corresponding rates of URTD and LRTD in each group. Although the length of both periods was similar, the retrospective cohort included only 108 CARV episodes in 63 recipients whereas the prospective cohort included 297 CARV episodes in 144 recipients. As expected, CARV epidemiology differed between groups. RSV followed by influenza virus was dominant in the retrospective group whereas EvRh followed by RSV was the most common CARV in the prospective cohort. Recipients with LRTD were significantly higher in the retrospective cohort (58%) than in the prospective cohort (31%), (p ≤ 0.0001).
Table 2.
Type of CARV and mortality by CARV type according to prospective vs. retrospective nature of cohort and CARV upper or lower respiratory tract disease.
| EvRh | RSV | Influ | HPiV | hMPV | AdV | HCoV | HBoV | |
|---|---|---|---|---|---|---|---|---|
| Prospective CARV episodes, n | 126 | 60 | 58 | 48 | 27 | 4 | 15 | 15 |
| 90-day overall mortality, n (%)a | 5 (4) | 3 (5) | 1 (2) | 1 (2) | 0 | 0 | 0 | 0 |
| CARV URTD, n (%)a | 91 (72) | 42 (70) | 40 (69) | 30 (65) | 16 (59) | 3 (75) | 12 (80) | 12 (80) |
| 90-day overall mortality, n (%)a | 3 (3) | 1 (2) | 0 | 1 (3) | 0 | 0 | 0 | 0 |
| CARV LRTD, n (%)a | 35 (28) | 18 (30) | 18 (31) | 17 (35) | 11 (41) | 1 (25) | 3 (20) | 3 (20) |
| 90-day overall mortality, na | 2 (6) | 2 (11) | 1 (6) | 0 | 0 | 0 | 0 | |
| Retrospective CARV episodes, n | 24 | 43 | 31 | 15 | 11 | 3 | 0 | 1 |
| 90-day overall mortality, n (%)a | 6 (25) | 11 (26) | 4 (13) | 4 (27) | 4 (36) | 1 (25) | - | 0 |
| CARV URTD, n (%)a | 11 (46) | 14 (32) | 14 (45) | 7 (46) | 4 (36) | 0 | - | 0 |
| 90-day overall mortality, n (%)a | 0 | 0 | 0 | 0 | 0 | - | - | - |
| CARV LRTD, n (%)a | 13 (54) | 29 (68) | 17 (55) | 8 (54) | 7 (64) | 2 (75) | - | 1 (100) |
| 90-day overall mortality, n (%)a | 6 (46) | 11 (38) | 4 (23) | 4 (50) | 4 (57) | 1 (50) | - | 0 |
Abbreviations: CARV, community-acquired respiratory virus; EvRh, Enterovirus/rhinovirus; ADV, adenovirus; RSV, respiratory syncytial virus; HPiV, human parainfluenza virus; hMPV, human metapneumovirus; HCoV, human coronavirus; Influ, human influenza virus; AdV, adenovirus; IFD, invasive pulmonary fungal disease; URTD, upper respiratory tract disease; LRTD, lower respiratory tract disease.
The sum total of the episodes does not match the overall number of episodes (n = 404) since multiple CARVs were detected in the same respiratory sample in 66 (16%) CARV episodes.
Clinical and biological characteristics of CARV infection episodes
Patients’ clinical and biological characteristics at the time of CARV infection in both groups are summarized in Table 3 . CARV episodes were diagnosed earlier after stem cell infusion in the prospective cohort (median of 193 days vs. 253 days, p = 0.03). Use of immunosuppressant drugs and higher ISI score were significantly over-represented in the retrospective cohort (p ≤ 0.05 for all comparisons). CARV infections in this group were characterized by higher rates of fever, hospital admission and antiviral therapy (p ≤ 0.002 for all comparisons).
Table 3.
Clinical and biological characteristics of respiratory virus infection episodes in allo-HSCT recipients according to prospective clinical CARV survey vs. retrospective standard clinical practice.
| Prospective CARV | Retrospective CARV | p value | |
|---|---|---|---|
| (n = 297) | (n = 108) | ||
| Immunodeficiency Scoring Index, n (%)a | |||
| ANC < 0.5 × 109/L (3pts) | 21 (7) | 18 (17) | 0.007 |
| ALC< 0.2 × 109/L (3pts) | 41 (14) | 35 (32) | <0.0001 |
| Age ≥ 40 y (2pts) | 181 (62) | 67 (62) | 0.9 |
| Myeloablative conditioning regimen (1pt) | 182 (61) | 85 (78) | 0.001 |
| GvHD (acute or chronic; 1pt) | 126 (42) | 79 (73) | <0.0001 |
| Corticosteroids (1pt) | 92 (31) | 91 (86) | <0.0001 |
| Recent or pre-engraftment allo-HCT (1pt) | 27 (9) | 21 (19) | 0.008 |
| ISI, n (%) | <0.0001 | ||
| • Low risk (0–2) | 114 (38) | 10 (9) | |
| • Moderate risk (3–6) | 160 (54) | 68 (63) | |
| • High risk (7–12) | 23 (8) | 30 (28) | |
| Other characteristicsa | |||
| On IS, n (%) | 213 (71) | 101 (94) | <0.0001 |
| ALC< 0.1 × 109/L, n (%) | 30 (10) | 19 (18) | 0.06 |
| ALC < 0.5 × 109/L, n (%) | 73 (25) | 49 (45) | 0.001 |
| RVI characteristics and clinical consequences | |||
| CARV LRTD, n (%) | 93 (31) | 63 (58) | <0.0001 |
| • Possible | 41 (49) | 32 (51) | |
| • Proven | 52 (51) | 31 (49) | |
| Hospital admission, n (%) | 82 (28) | 75 (69) | <0.0001 |
| Antiviral therapy, n (%) | 69 (23) | 43 (40) | 0.002 |
| • URTD | 34 | 12 | |
| • LRTD | 35 | 31 | |
| • HPiVb | 9 | 1 | |
| ∘ URTD/LRTD | 1 / 8 | 0 / 1 | |
| • RSVb | 22 | 21 | |
| ∘ URTD/LRTD | 11 / 11 | 3 / 18 | |
| • Influenzab | 46 | 25 | |
| ∘ URTD/LRTD | 29 / 17 | 10 / 15 | |
| • hMPVb | 5 | 4 | |
| ∘ URTD/LRTD | 2 / 3 | 0 / 4 | |
| CARV during the first year of allo-HCT, n (%) | 108 (36) | 39 (33) | 1 |
| Fever during CARV, n (%) | 169 (57) | 90 (83) | <0.0001 |
| Co-virus infection | 45 (15) | 21 (19) | 0.3 |
| Bronchoalveolar lavage | 59 (20) | 39 (36) | 0.001 |
| Co-bacterial pneumonia | 15 (5) | 10 (9) | 0.1 |
| Seasonal influenza vaccination | 79 (27) | 3 (3) | <0.0001 |
| Day + 30 overall mortality rate, n (%) | 2 (0.6) | 11 (10) | <0.0001 |
| Day + 60 overall mortality rate, n (%) | 4 (1.2) | 18 (17) | <0.0001 |
| Day + 90 overall mortality rate, n (%) | 10 (3) | 23 (21) | <0.0001 |
Abbreviations: CARV, community-acquired respiratory virus; IFD, invasive pulmonary infectious fungal disease; ATG, anti-thymocyte globulin; GvHD, graft-versus-host disease; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; IS, immunosuppressants; LRTD, lower respiratory tract disease; URTD, upper respiratory tract disease; HPiV, human parainfluenza virus; RSV, respiratory syncytial virus; hMPV, human metapneumovirus; Allo-HCT, allogeneic hematopoietic stem cell transplantation;.
All variables were captured at the time of CARV diagnosis.
The sum total antiviral therapies do not match the overall number of antiviral therapies since multiple CARVs were detected in the same respiratory sample.
With regards to the time of antiviral therapy (ribavirin and oseltamivir) in RSV and influenza cases, we compared the median time in starting antiviral therapy in the StCP vs. the ProClinCarvSur-P cohort. Although the ProClinCarvSur-P cohort start antivirals early [median of 3 days (range 0–21 days) vs. 5 days (range 0–83)], the differences did not reach statistical significance (p = 0.5). We also compared the timing of antiviral therapy in the StCP cohort in recipients with RSV and/or influenza viruses who were diagnosed by rapid detection technics as compared to those who were diagnosed by syndromic multiplex RT-PCR as a second diagnostic step. Among the 69 episodes of RSV and/or influenza (38 RSV, 26 influenza and 5 with both VRS and influenza) 52 episodes (75%) were diagnosed by rapid detection tests whereas 17 episodes (25%) were diagnosed by syndromic multiplex PCR in a second step. Eight out of 17 cases diagnosed by PCR (47%) received antiviral as compared to 32 out of 52 diagnosed by rapid test (61%) (p = 0.2). The median time in days of starting antiviral therapy from the time of diagnosis was 2 days (range 0–30) in the rapid detection cohort vs. 7 days (range 1–83) in the multiplex PCR cohort (p = 0.02). Day 90 mortality was 9 out of 52 (17%) in the rapid test cohort vs. 6 out of 17 (35%) in the multiplex PCR group (p = 0.17).
Mortality rates and cause of death
Death by day 90 after CARV diagnosis occurred in 23 (21%) of 108 CARV episodes versus 10 (3%) of 297 CARV episodes in the retrospective and prospective cohorts, respectively (p ≤ 0.0001). Median time to death was 189 days (range, 35–1032) and 175 days (range, 90–1084) after stem cell infusion, respectively. The overall three-month all-cause mortality was 37% (23 out of 63 recipients) in the retrospective cohort versus 7% (10 out of 144 recipients) in the prospective cohort (p < 0.0001). Differences in mortality were even higher in recipients with CARV LRTD in the retrospective cohort (37% vs. 5%, p < 0.0001). We compared causes of death at different time points among cohorts and we did not find significant differences. In the retrospective cohort, 11 recipients died by day 30 after CARV documentation due to mixed bacterial/CARV co-infection (n = 4), CARV-related respiratory failure (n = 3), fungal/CARV co-infection (n = 2), GvHD (n = 1) and disease relapse (n = 1). By day 60, 7 additional recipients died due to mixed bacterial/CARV co-infection (n = 2), CARV (n = 1), fungal/CARV co-infection (n = 1), bacterial infection (n = 1)], GVHD (n = 1) and relapse (n = 1). Finally, 5 more recipients died by day 90 due to CARV-related respiratory failure (n = 1), fungal/CARV co-infection (n = 1), GvHD (n = 2) and relapse (n = 1). In the prospective cohort 2 recipients died by day 30 due to CARV-related respiratory failure (n = 1) and pulmonary secondary malignancy (n = 1). Two other recipients died by day 60, one due to CARV respiratory failure and another caused by severe GvHD and hemorrhage. Six additional recipients died by day 90 due to bacterial infection (n = 2), CARV-related respiratory failure (n = 2) and disease relapse (n = 2).
Analysis of risk factors for three-month all-cause mortality and overall survival
Logistic regression univariate and multivariate analyses of RFs for three-month all-cause mortality after overall CARV infection episodes and LRTD are shown in Table 4 .
Table 4.
Univariate and multivariate analysis of risk factors for overall mortality after CARV infection and LRTD.
| Variables | Log. Regr. Overall Mortality by day 90 (n = 405) |
Log. Regr. Overall Mortality LRTD CARV by day 90 (n = 156) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||
| OR (95% C.I.).% (95%C.I.) | P value | OR (95% C.I.) | P value | OR (95% C.I.).% (95%C.I.) | P value | OR (95% C.I.) (95%C.I.) | P value | |
| Type of donor, n (%) | ||||||||
| HLA-identical sibling donor | 1 | 1 | ||||||
| Alternative donor | 6.4 (1.9–21.5) | 0.002 | NS | 5.9 (1.7–20.5) | 0.005 | NS | ||
| HLA mismatch | 7 (2.6–18.6) | <0.0001 | 5.98 (2.1–16.9) | 0.001 | 7.7 (2.5–23.5) | <0.0001 | 6.5 (1.9–21.8) | 0.002 |
| ATG as a part of conditioning | 5.2 (2.5–11) | <0.0001 | NS | 6.6 (2.7–16) | <0.0001 | NS | ||
| GVHD prophylaxis | NS | NS | ||||||
| Sir-Tac | 1 | 1 | ||||||
| CsA + MTX | 15.1 (1.1–217) | 0.04 | 18.5 (0.8–417) | 0.06 | ||||
| Post-Cy | 8.9 (0.8–96) | 0.07 | 14.7 (0.7–292) | 0.07 | ||||
| CsA + PDN and Others | 1.3 (0.13–13.2) | 0.8 | 1.47 (0.08–24.9) | 0.78 | ||||
| ProClinCarvSur-P | 0.13 (0.06–0.3) | <0.0001 | 0.31 (0.12–0.7) | 0.01 | 0.09 (0.03–0.28) | <0.0001 | 0.19 (0.06–0.6) | 0.006 |
| CARV LRTD | 10.7 (4–28) | <0.0001 | 6.6 (2.3–18.7) | <0.0001 | NT | |||
| • No | 1 | |||||||
| • Proven | 13.8 (4.9–38) | <0.0001 | ||||||
| • Possible | 1.8 (0.8–4.1) | 0.18 | ||||||
| On IS | 2.2 (0.75–6.4) | 0.15 | 2.1 (0.5–9.7) | 0.3 | ||||
| ALC < 0.5 × 109/L, n (%) | 5.3 (2.5–11.4) | <0.0001 | NT | 3.3 (1.3–7.9) | 0.009 | NT | ||
| ALC < 0.2 × 109/La | 6.4 (3–13.5) | <0.0001 | NT | 3.8 (1.6–8.8) | 0.002 | NT | ||
| ALC < 0.1 × 109/L | 3.08 (1.3–7) | 0.008 | NT | 1.7 (0.7–4.3) | 0.26 | |||
| ANC < 0.5 × 109/La | 2.86 (1.15–7.1) | 0.024 | NT | 2.1 (0.8–5.9) | 0.13 | |||
| Age ≥ 40 yearsa | 1.3 (0.6–2.7) | 0.5 | 1.2 (0.5–3) | 0.7 | ||||
| Active GvHD at time of RVIa | 3.3 (1.5–7.6) | 0.004 | NT | 2.5 (0.95–6.6) | 0.06 | NT | ||
| Periengraftmenta | 0.7 (0.2–2.4) | 0.6 | 0.5 (0.13–1.8) | 0.3 | ||||
| Allo-HCT ≤ 6 months | 1.7 (0.8–3.4) | 0.14 | 1.3 (0.6–2.9) | 0.56 | ||||
| Myeloablativea | 2 (0.85–4.7) | 0.11 | 2 (0.77–5.4) | 0.14 | ||||
| Corticosteroidsa | 6.2 (2.5–15.4) | <0.0001 | NT | 10.1 (2.3–44.4) | 0.002 | NT | ||
| Antiviral therapy | 0.56 (0.27–1.1) | 0.12 | 0.8 (0.3–1.85) | 0.6 | ||||
| Type of RVI | ||||||||
| Mono infection | 1 | 1 | ||||||
| Co-infection | 1.4 (0.6–3.4) | 0.4 | 1.8 (0.6–4.8) | 0.24 | ||||
| ISI | ||||||||
| Low risk (0–2) | 1 | 1 | 1 | 1 | ||||
| Moderate risk (3–6) | 11.7 (3.7–37.5) | <0.0001 | 3.1 (0.8–12.6) | 0.1 | 15.6 (1.9–127) | 0.01 | 8.7 (0.89–85) | 0.06 |
| High risk (7–12) | 6 (2.7–13.5) | <0.0001 | 3.1 (1.2–7.9) | 0.01 | 3.8 (1.6–9.3) | 0.003 | 3.4 (1.2–9.5) | 0.02 |
Abbreviations: C.I., confidence interval; Log. Regr., Logistic regression model; OR, Odds Ratio; IFD, invasive pulmonary fungal disease; ATG, anti-thymocyte globulin; Sir, sirolimus; Tac, tacrolimus; CsA, cyclosporine A; MTX, methotrexate; Post-Cy, post-transplant cyclophosphamide; PDN, prednisone; ProClinCarvSur-P, prospective clinical CARV surveillance program; CARV LRTD, community-acquired respiratory virus lower respiratory tract disease; GvHD, graft-versus-host disease; Allo-HCT, allogeneic hematopoietic stem cell transplantation; ISI, immunodeficiency score index; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NS, not significant; NT, not tested.
These variables were included in the ISI score and then not tested in the multivariate model.
We studied the 404 evaluable recipient/episode pairs to identify transplant and CARV conditions associated with mortality. Multivariate analysis identified four RFs: donor/recipient HLA mismatch [odds ratio (OR) 5.98, 95% confidence interval (C.I.) 2.1–16.9, p = 0.001], CARV lower respiratory tract disease (OR 6.6, 95% C.I. 2.3–18.7, p ≤ 0.0001), high-risk ISI score (OR 3.1, 95% C.I. 1.2–7.9, p = 0.01) and ProClinCarvSur-P (OR 0.31, 95% C.I. 0.12–0.7, p = 0.01).
We analyzed episodes of CARV involving LRTD to determine RFs for mortality in these cases (n = 156). Multivariate analysis identified three independent RFs associated with mortality: again, donor/recipient HLA mismatch, high-risk ISI score and ProClinCarvSur-P (OR 6.5, 95% C.I. 1.9–21.8, p = 0.002; OR 3.4, 95% C.I. 1.2–9.5; OR 0.19, 95% C.I. 0.06–0.6, p ≤ 0.02, respectively).
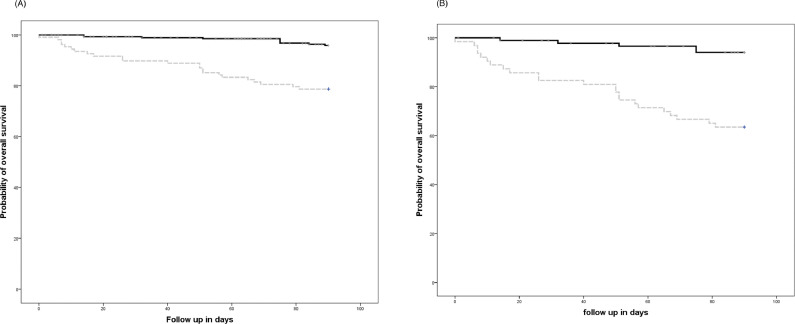
CARV infection in the retrospective cohort had lower three-month OS after CARV diagnosis (78% vs. 97%, p ≤ 0.0001). Likewise, OS was significantly lower in allo-HCT recipients with CARV LRTD in the retrospective cohort (63% vs. 94%, respectively, p ≤ 0.0001) (Fig. 1 A and B). OS according to variables previously identified as RFs for mortality were also significantly lower in the retrospective cohort (Table 5 ).
Fig. 1.
(A) Three-month overall survival according to the community acquired respiratory virus (CARV) infection period (96% in the prospective cohort vs. 78% in the standard clinical practice cohort, p < 0.0001); (B) Three-month overall survival in recipients with CARV LRTD according to the CARV infection period (94% in the prospective cohort vs. 63% in the standard clinical practice cohort, p < 0.0001).
Table 5.
Day 90 overall survival per CARV episodes according to patient's characteristics among cohorts.
| Characteristics | Retrospective cohort | Prospective cohort | P value |
|---|---|---|---|
| (n = 108 episodes) | (n = 297 episodes) | Log.Rank | |
| Prior ASCT, n (%) | |||
| • Yes | 9 out of 11 (82) | 71 out of 73 (97) | 0.04 |
| • No | 76 out of 97 (78) | 216 out of 224 (96) | <0.0001 |
| Conditioning regimen, n (%) | |||
| • MAC | 67 out of 85 (79) | 174 out of 182 (96) | <0.0001 |
| • RIC | 18 out of 23 (78) | 113 out of 115 (98) | <0.0001 |
| Type of donor, n (%) | |||
| • HLA-identical sibling donor | 35 out of 37 (95) | 109 out of 110 (99) | 0.1 |
| • Unrelated donor | 1 out of 4 (75) | 55 out of 55 (100) | 0.004 |
| • Umbilical cord blood | 37 out of 55 (67) | 43 out of 47 (92) | <0.0001 |
| • Haploidentical family donor | 8 out of 10 (80) | 76 out of 81 (94) | 0.1 |
| HLA, n (%) | |||
| • Fully-matched | 39 out of 42 (93) | 168 out of 170 (99) | 0.04 |
| • HLA mismatch | 46 out of 66 (70) | 119 out of 127 (94) | <0.0001 |
| ATG as a part of conditioning regimen, n (%) | |||
| • Yes | 35 out of 52 (67) | 49 out of 52 (94) | 0.001 |
| • No | 50 out of 56 (89) | 238 out of 245 (97) | 0.026 |
| With active GvHD n (%) | |||
| • Yes | 60 out of 69 (76) | 120 out 126 (95) | <0.0001 |
| • No | 25 out of 29 (86) | 167 out of 171 (98) | 0.006 |
| On immunosuppressants | |||
| • Yes | 79 out of 101 (78) | 206 out of 213 (97) | <0.0001 |
| • No | 6 out of 7 (86) | 81 out of 84 (96) | 0.2 |
| Absolute lymphocyte count | |||
| • ALC < 0.5 × 109/L | 32 out of 49 (65) | 68 out of 73 (93) | <0.0001 |
| • ALC > 0.5 × 109/L | 51 out of 57 (89) | 217 out of 222 (98) | 0.011 |
| • ALC < 0.2 × 109/L | 20 out of 35 (57) | 38 out of 41 (93) | 0.0001 |
| Corticosteroids | |||
| • Yes | 70 out of 91 (77) | 86 out of 92 (94) | 0.002 |
| • No | 15 out of 17 (88) | 200 out of 204 (98) | 0.03 |
| ISI score group | |||
| • Low risk (0–2) | 10 out of 10 (100) | 109 out of 114 (97) | 0.5 |
| • Moderate risk (3–6) | 58 out of 68 (85) | 156 out of 160 (98) | 0.001 |
| • High risk (7–12) | 17 out of 30 (57) | 21 out of 23 (91) | 0.009 |
Abbreviations: ASCT, autologous stem cell transplantation; MAC, myeloablative conditioning; RIC, reduced intensity conditioning; HLA, human leucocyte antigen; ATG, anti-thymocyte globulin; GvHD, graft-versus-host disease; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; ISI, immunodeficiency score index.
Discussion
This study shows that application of ProClinCarvSur-P in allo-HCT recipients was independently associated with reduced three-month all-cause mortality after CARV compared to influenza and RSV-based StCP. The significant decrease in mortality rate was observed not only in relative numbers [21% vs. 3% of CARV episodes in the retrospective and ProClinCarvSur-P cohort, respectively], but significantly in absolute numbers [23 out of 63 recipients (37%) vs. 10 out of 144 recipients (7%) in the retrospective and ProClinCarvSur-P cohort, respectively]. Additionally, we found that donor/recipient HLA mismatch, CARV LRTD and high-risk ISI were also associated with higher mortality.
The differences in mortality reported herein prompt several questions. Was the mortality rate in the retrospective cohort abnormally high? The first observation was the unusually high proportion of moderate to high-risk ISI (91%)9 , 25 , 30 suggesting that the StCP mostly focused on recipients at higher risk. However, the mortality rates of different CARV types with LRTD in this cohort (46% in EvRh, 38% in RSV, 23% in influenza, 50% HPiV and 57% in hMPV) were inside the intervals of that previously reported, ranging from 21% to 41% in allo-HCT recipients with EvRh LRTD,31 , 32 from 21% to 83% for RSV LRTD,17 , 20 , 22 , 25 , 31 , 33, 34, 35 6% to 45% for influenza LRTD,30 , 31 , 36 27% to 55% for HPiV LRTD27 , 31 , 37 , 38 and finally 6% to 43% for hMPV LRTD.39 , 40 , 41 Second, was the retrospective cohort subject to selection bias from retrospective studies reporting severe cases? We captured all documented CARV infection by retrieving data from the microbiology electronic system and crossing data with our transplant database. Our data rather point to the inaccuracy of our StCP CARV screening selecting recipients with severe features (58% of them at the LRTD stage, 83% with fever and 69% requiring hospital admission). Additionally, we observed that the likely lower sensitivity of rapid detection technics might have delayed the diagnosis, and importantly the start of antiviral drugs (2 days vs. 7 days, p = 0.02), in a significant proportion of recipients with RSV and influenza (25%) who initially tested negative through rapid detection technics. Although not statistically significant these recipients showed higher mortality rates in the StCP cohort (35% vs. 17%, p = 0.17). Our findings support the idea that the StCP resulted in a delay in starting antiviral therapy in a significant number of recipients which may also help to explain in part the differences in mortality among periods. Third, are the cohorts comparable, given the high proportion of risk conditions associated with mortality and the lower number of CARV episodes in the retrospective cohort? The fact that the cohorts had different risk conditions was a direct consequence of different CARV screening and management strategies between periods. However, when outcome comparisons were matched according to high-risk conditions of CARV mortality, we also observed significantly worse outcomes in the retrospective cohort (see Table 5). Therefore, our data suggest that the StCP likely led to CARV screening in high-risk recipients with severe features, delaying the start of antiviral therapy and consequently poorer outcomes, whereas the ProClinCarvSur-P led to screening and treating CARV earlier, in a larger number of recipients and with consequently improved outcomes.
The three-month all-cause mortality rate observed in the ProClinCarvSur-P cohort (7%) compares favorably to those previously reported, especially in recipients with LRTD.17 , 20 , 22 , 25 , 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 The low mortality rate found in the current study supports the value of ProClinCarvSur-P in reducing mortality. This assumption is supported by prior experience in another institution running the same ProClinCarvSur-P in allo-HCT recipients achieving low mortality rates (<5%) in recipients with RSV and/or HPiV LRTD.26 Although we suppose that several elements of the ProClinCarvSur-P have significantly contributed to these encouraging results, in our opinion the improvement has three major key points. First, the decision to using syndromic multiplex RT-PCR panels as the primary CARV test, a factor that undoubtedly increased sensitivity and specificity compared to rapid detection technics42 increasing CARV type detections. Consequently, a large number of CARV episodes (108 vs. 297 CARV episodes) and recipients (63 vs. 144 recipients) have been identified in the ProClinCarvSur-P cohort. Second, the CARV real-time electronic alert system was extremely useful for contacting recipients who tested positive in order to start antiviral therapy at an earlier stage of the disease. Third, the identification of CARV at earlier stage allowed us to early intervention with antiviral drugs in many cases (69 vs. 43 CARV episodes treated), particularly those with CARV URTD (34 vs. 12 CARV URTD episodes treated), RSV URTD (11 vs. 3 treated), HPiV (9 vs. 1 treated) and influenza (46 vs. 25 treated). Additionally, ProClinCarvSur-P may have promoted closer follow-up of recipients with CARV, which in turn could have contributed to identifying and treating indirect CARV effects. We can hypothesize that the observed improvement in CARV that have no effective antivirals drugs could be related to several factors. First, the identification of these viruses in recipients with airflow obstruction may have contributed to limit the misinterpretation of these pulmonary patterns as GvHD-related obliterant bronchiolitis, avoiding corticosteroids exposure that surely have reduced the CARV LRTD severity and/or the risk of all-cause mortality. Second, the close clinical monitoring in recipients with documented CARV infection may have led to early introduction of antibiotics in cases of bacterial co-infection suspicion reducing the rate of hospital admission in the ProClinCarvSur-P cohort (28% vs. 69%) and consequently limiting the severity of this indirect CARV effect. The sum of all these clinical circumstances may have contributed to the overall improvement observed in these CARVs. Finally, one of the most rewarding effects of annual clinical CARV sessions was the increased awareness of CARV infections and care taken by healthcare workers (including microbiologists, fellows, transplant physicians and nurses). Altogether, ProClinCarvSur-P may have contributed towards refining CARV screening, collection techniques and processing, greater acuity in identifying recipients with mild respiratory symptoms, timeliness of feedback of positive results and awareness of CARV transmission routes. Of note, our current policy also translated into an increased influenza vaccination rate (from 3% to 27%) which has also surely played a role in reducing influenza prevalence, influenza LRTD progression and hospital admission.9 Of note, this study confirms the value of ISI score in predicting mortality in several CARV type.43 The ISI was developed by investigators from the MD Anderson Cancer Center to predict LRTD progression and mortality in allo-HCT with RSV.25 The high-risk ISI category consistently predicts the severity of respiratory virus infections irrespective of the CARV type in our series.
Although the implementation of ProClinCarvSur-P is time and resource consuming, we are enthusiastic of its potential value to reduce all-cause mortality after CARV infection in a relatively short time. It is likely that its application could be expanded to other medical areas with probably similar benefits. National and transnational health authorities should promote such programs in tertiary health care centers worldwide by giving them priority and funding, such as in the World health organization BRaVe initiative against respiratory virus.44 Additionally, these programs could bring epidemiological data more realistic to public health authorities.
We acknowledge certain limitations of this study, such as the inclusion of several CARV types, use of two different PCR methods differing (minimally) in analytical performance and the non-prospective randomized study design. Nonetheless, our study has strengths that merit consideration, notably the inclusion of contemporary cohorts in the molecular era and the prospective data collection in the most recent cohort.
In conclusion, we provide evidence that establishing a ProClinCarvSur-P based on multiplex PCR testing is associated with major clinical benefit by reducing three-month all-cause mortality in allo-HCT recipients with CARV infection. Further prospective studies are warranted to confirm this finding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2019.12.022.
Appendix. Supplementary materials
References
- 1.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark T.W., Medina M.J., Batham S., Curran M.D., Parmar S., Nicholson K.G. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69:507–515. doi: 10.1016/j.jinf.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention. 2018. Flu activity & surveillance. https://www.cdc.gov/flu/weekly/fluactivitysurv.htm.
- 4.Red nacional de vigilancia epidemiológica. Instituto de salud Carlos III. Boletín Epidemiológico Semanal eISSN: 2173-9277. https://revistas.isciii.es
- 5.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Flu News Europe, Joint ECDC–WHO. https://flunewseurope.org.
- 6.World Health organization. https://www.who.int/influenza/rsv/rsv_lab/en/.
- 7.Hirsch H.H., Martino R., Ward K.N., Boeckh M., Einsele H., Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uyeki T.M., Bernstein H.H., Bradley J.S., Englund J.A., File T.M., Fry A.M. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1–e47. doi: 10.1093/cid/ciy866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piñana J.L., Pérez A., Montoro J., Giménez E., Dolores Gómez M., Lorenzo I. Clinical effectiveness of influenza vaccination after allogeneic hematopoietic stem cell transplantation: a cross-sectional prospective observational study. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemaly R.F., Shah D.P., Boeckh M.J. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;59(Suppl (5)):S344–S351. doi: 10.1093/cid/ciu623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lima C.R., Mirandolli T.B., Carneiro L.C., Tusset C., Romer C.M., Andreolla H.F. Prolonged respiratory viral shedding in transplant patients. Transpl Infect Dis. 2014;16(1):165–169. doi: 10.1111/tid.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y.J., Guthrie K.A., Waghmare A., Walsh E.E., Falsey A.R., Kuypers J. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis. 2014;209:1195–1204. doi: 10.1093/infdis/jit832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana L., Strasfeld L. Respiratory virus infections of the stem cell transplant recipient and the hematologic malignancy patient. Infect Dis Clin North Am. Jun 2019;33(2):523–544. doi: 10.1016/j.idc.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Versluys A.B., Boelens J.J. Morbidity and mortality associated with respiratory virus infections in allogeneic hematopoietic cell transplant: too little defense or harmful immunity? Front. Microbiol. 2018;9:2795. doi: 10.3389/fmicb.2018.02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erard V., Chien J.W., Kim H.W., Nichols W.G., Flowers M.E., Martin P.J. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J. Infect. Dis. 2006;193:1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin D.E., Weatherby L.B., Huang W.Y., Rosenberg D.M., Cook S.F., Walker A.M. Impact of patient characteristics on the risk of influenza/ILI-related complications. BMC Health Serv Res. 2001;1:8. doi: 10.1186/1472-6963-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah D.P., Ghantoji S.S., Shah J.N., El Taoum K.K., Jiang Y., Popat U. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68(August (8)):1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers B.B., Shankar P., Jerris R.C., Kotzbauer D., Anderson E.J., Watson J.R. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139:636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 19.Chemaly R.F., Ghosh S., Bodey G.P., Rohatgi N., Safdar A., Keating M.J. Raad II. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85(September (5)):278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 20.Khawaja F., Chemaly RF. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica. 2019;104(July (7)):1322–1331. doi: 10.3324/haematol.2018.215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doan Q., Enarson P., Kissoon N., Klassen T.P., Johnson D.W. Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department. Cochrane Database Syst Rev. 2014;(9) doi: 10.1002/14651858.CD006452.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah J.N., Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117(10):2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 23.Piñana J.L., Gómez M.D., Montoro J., Lorenzo I., Pérez A., Giménez E. Incidence, risk factors and outcome of pulmonary invasive fungal disease after respiratory virus infection in allogeneic hematopoietic stem cell transplantation recipients. Transpl Infect Dis. 2019:e13158. doi: 10.1111/tid.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomblyn M., Chiller T., Einsele H., Gress R., Sepkowitz K., Storek J. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(October (10)):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah D.P., Ghantoji S.S., Ariza-Heredia E.J., Shah J.N., El Taoum K.K., Shah P.K. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123(21):3263–3268. doi: 10.1182/blood-2013-12-541359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piñana J.L., Hernández-Boluda J.C., Calabuig M., Ballester I., Marín M., Madrid S. A risk-adapted approach to treating respiratory syncytial virus and human parainfluenza virus in allogeneic stem cell transplantation recipients with oral ribavirin therapy: a pilot study. Transpl Infect Dis. 2017;19(August (4)):e12729. doi: 10.1111/tid.12729. [DOI] [PubMed] [Google Scholar]
- 27.Seo S., Xie H., Campbell A.P., Kuypers J.M., Leisenring W.M., Englund J.A. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis. 2014;58:1357–1368. doi: 10.1093/cid/ciu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein J.P., Rizzo J.D., Zhang M.J., Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28:909–915. doi: 10.1038/sj.bmt.1703260. [DOI] [PubMed] [Google Scholar]
- 29.Klein J.P., Rizzo J.D., Zhang M.J., Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant. 2001;28:1001–1011. doi: 10.1038/sj.bmt.1703271. [DOI] [PubMed] [Google Scholar]
- 30.Kmeid J., Vanichanan J., Shah D.P., El Chaer F., Azzi J., Ariza-Heredia E.J. Outcomes of influenza infections in hematopoietic cell transplant recipients: application of an immunodeficiency scoring index. Biol Blood Marrow Transplant. 2016;22(March (3)):542–548. doi: 10.1016/j.bbmt.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo S., Waghmare A., Scott E.M., Xie Hu, Kuypers J.M., Hackman RC. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica. 2017;102(June):1120–1130. doi: 10.3324/haematol.2016.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SE1 J., R S., Shore T.B., Satlin M.J., Schuetz A.N., Magro C. Human rhinovirus infections of the lower respiratory tract in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2013;15(October (5)):474–486. doi: 10.1111/tid.12111. Epub 2013 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo S., Campbell A.P., Xie H., Chien J.W., Leisenring W.M., Englund J.A. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant. 2013;19(4):589–596. doi: 10.1016/j.bbmt.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarthy A.J., Kingman H.M., Kelly C. The outcome of 26 patients with respiratory syncytial virus infection following allogeneic stem cell transplantation. Bone Marrow Transplant. 1999;24(12):1315–1322. doi: 10.1038/sj.bmt.1702078. [DOI] [PubMed] [Google Scholar]
- 35.Waghmare A., Campbell A.P., Xie H., Seo S., Kuypers J., Leisenring W. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis. 2013;57(12):1731–1741. doi: 10.1093/cid/cit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ljungman P., de la Camara R., Perez-Bercoff L., Abecasis M., Bartolo J., Campuzano N. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica. 2011;96(August (8)):1231–1235. doi: 10.3324/haematol.2011.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo S., Xie H., Leisenring W.M., Kuypers J.M., Sahoo F.T., Goyal S. Risk factors for parainfluenza virus lower respiratory tract disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(January (1)):163–171. doi: 10.1016/j.bbmt.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah D.P., Shah P.K., Azzi J.M., Chemaly R.F. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett. 2016;370(January (2)):358–364. doi: 10.1016/j.canlet.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Chaer F., Shah D.P., Kmeid J., Ariza-Heredia E.J., Hosing C.M., Mulanovich V.E., Chemaly R.F. Burden of human metapneumovirus infections in patients with cancer: risk factors and outcomes. Cancer. 2017 Jun 15;123(12):2329–2337. doi: 10.1002/cncr.30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renaud C., Xie H., Seo S., Kuypers J., Cent A., Corey L. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant. Aug 2013;19(8):1220–1226. doi: 10.1016/j.bbmt.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spahr Y., Tschudin-Sutter S., Baettig V., Compagno F., Tamm M., Halter J. Community-acquired respiratory paramyxovirus infection after allogeneic hematopoietic cell transplantation: a single-center experience. Open Forum Infect Dis. 2018;5(April (5)):ofy077. doi: 10.1093/ofid/ofy077. eCollection 2018 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chartrand C., Leeflang M.M., Minion J., Brewer T., Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 2012;156:500–511. doi: 10.7326/0003-4819-156-7-201204030-00403. [DOI] [PubMed] [Google Scholar]
- 43.Piñana J.L., Pérez A., Montoro J.HR, Lorenzo I., Giménez E., Gómez M.D. The effect of timing on community acquired respiratory virus infection mortality during the first year after allogeneic hematopoietic stem cell transplantation: a prospective epidemiological survey. Bone Marrow Transplant. 2019 doi: 10.1038/s41409-019-0698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health organization. Battle against Respiratory Viruses (BRaVe) initiative. https://www.who.int/influenza/patient_care/clinical/research_agenda/en/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.