Abstract
Lipids were long believed to have a structural role in biomembranes and a role in energy storage utilizing cellular lipid droplets and plasma lipoproteins. Research over the last decades has identified an additional role of lipids in cellular signaling, membrane microdomain organization and dynamics, and membrane trafficking. These properties make lipids an attractive target for pathogens to modulate host cell processes in order to allow their survival and replication. In this review we will summarize the often ingenious strategies of pathogens to modify the lipid homeostasis of host cells, allowing them to divert cellular processes. To this end pathogens take full advantage of the complexity of the lipidome. The examples are categorized in generalized and emerging principles describing the involvement of lipids in host–pathogen interactions. Several pathogens are described that simultaneously induce multiple changes in the host cell signaling and trafficking mechanisms. Elucidation of these pathogen-induced changes may have important implications for drug development. The emergence of high-throughput lipidomic techniques will allow the description of changes of the host cell lipidome at the level of individual molecular lipid species and the identification of lipid biomarkers.
Keywords: Host-pathogen, Pathogen vacuole, Lipids, Phospholipids, Lipidomics, Lipid droplets, Lipid-enriched microdomains, Lipid rafts, Membrane trafficking, Mycobacterium, Toxoplasma, Clamydia
1. Introduction
Lipids are loosely defined as biological molecules with hydrophobic or amphipathic properties that render them soluble in organic solvents [1]. Despite the fact that this definition includes thousands of different chemical structures, a more defined classification seemed unnecessary for a long period of time. Lipids were believed to have two general functions: a structural role in biomembranes and a role in energy storage in cells (lipid droplets) and body fluids (lipoproteins). During the last two decades, however, we have come to realize that lipids have a multitude of different and essential functions in the cell. First indications for the involvement of specific lipids in biological processes came from the identification of platelet-activating factor as a lipid molecule [2]. The groundbreaking work of Irvine and Berridge in the 1980s showed the involvement of lipids in intracellular cellular signaling by the generation of second messengers from phosphoinositides [3]. Almost concomitantly the discovery was made that diacylglycerol and phosphatidic acid are biologically active lipids, as well as lipidic breakdown products of cellular sphingolipids [4], [5], [6], [7]. Since the identification of the phosphatidylinositol transfer protein as an essential factor for protein trafficking from the trans-Golgi network (TGN) in yeast in the early 1990s [8], an overwhelming body of evidence showed the involvement of lipids in the regulation of membrane traffic [9]. Lipids have also been implicated in the generation of lateral heterogeneity in biological membranes, creating membrane domains that are often referred to as lipid rafts [10]. By specific recruitment of proteins and lipids to these domains while excluding others, many biological processes such as cell migration, the immune system, and the cell cycle are affected or regulated.
Thus, lipids have a multitude of functions in many biological processes and we are beginning to understand the long-time mysterious reason why nature synthesizes thousands of different lipids. This multitude of functions makes lipids also an attractive target for pathogens. In the innate immune system, microbes can be internalized by a phagocytosis-like process in order to be degraded by the acidic environment in subsequent phagolysosomal compartments. Some pathogens, however, escape the immune system by interacting with the cellular machinery at any one of these steps, allowing their survival and multiplication. Today there are many indications for an important role of lipids in various stages of host–pathogen interactions. One of the first examples for such involvement comes from the bacterium Vibrio cholerae that secretes the enterotoxin cholera toxin. The receptor of the toxin is a lipid termed GM1 [11]. This protein-lipid interaction results in the formation of a membrane pore, ultimately causing severe diarrhea.
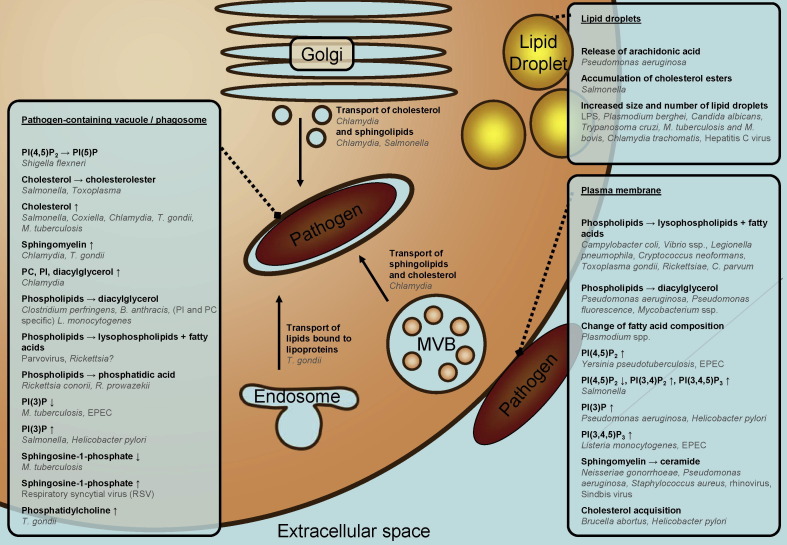
In this review we will summarize the often ingenious strategies that pathogens utilize to divert cellular processes by modifying cellular lipid homeostasis. To this end pathogens take full advantage of the complexity of the lipidome (Fig. 1 ). The examples are categorized in generalized and emerging principles describing the involvement of lipids in host–pathogen interactions.
Fig. 1.
Overview of modifications of the host cell lipidome by various pathogens. MVB: multivesicular body; EPEC: Enteropathogenic E. coli; PC: phosphatidylcholine; PI: phosphatidylinositol; PI(3)P: phosphatidylinositol-3-phosphate; PI(5)P: phosphatidylinositol-5-phosphate; PI(3,4)P2: phosphatidylinositol-3,4-bisphosphate; PI(4,5)P2: phosphatidylinositol-4,5-bisphosphate; PI(3,4,5)P3: phosphatidylinositol-3,4,5-trisphosphate.
Many lipid-derived (signaling) molecules that classify as lipids themselves, and that might have a role in host–pathogen interaction, like prostaglandins, leukotrienes, quinones, vitamins, and tocopherols will not be discussed here. This still leaves us with thousands of different lipid species in the (phospho)lipid and sterol classes.
Until recently, only limited possibilities for lipid analysis were available, often only capable of lipid identification into different classes. Now the field of lipidology enters a new era, with mass spectrometry-based techniques providing novel tools to analyze samples at the single lipid species level and at the same time at the whole lipidome level [12], [13], [14]. We already see preliminary examples of and the urgent need for lipidomic approaches in various fields, including that of host–pathogen interaction, to unravel the involvement of lipids in infectious diseases [15]. This review aims to give an overview of the current status of our knowledge on the involvement of lipids in host–pathogen interactions, indicating that lipids from both host and pathogen play key roles in infection processes.
2. Modulation of the fatty acid moiety of host cell lipids
2.1. Fatty acid modulation of host cell lipids upon infection
During development in the erythrocyte, the malaria causing parasites, Plasmodium spp. modify the protein and lipid composition and various properties of the plasma membrane of their host cell [16], [17], [18]. In the membrane of erythrocytes parasitized by Plasmodium falciparum the composition of individual phospholipid classes, as well as that of their fatty acid constituents is altered [17], [18], [19]. Fatty acid alterations are not observed in all lipid classes. Alterations in the fatty acid composition of phosphatidylcholine and phosphatidylethanolamine of erythrocyte membranes were observed for Plasmodium knowlezi [20]. Intraerythrocytic development of Plasmodium chabaudi is also associated with alterations in the overall fatty acid composition of the plasma membrane of the host cell [21], but not in that of the host cell’s plasma membrane phosphatidylcholine [22]. At this moment it is not clear whether the observed alterations in fatty acid composition are due to enzymatic modifications in situ of the phospholipids in the plasma membrane of the erythrocyte, or to selective uptake of phospholipid molecular species from the erythrocyte plasma membrane by the parasite. Alterations in fatty acid composition of host cell lipids are not limited to parasitic infections. Changes in phospholipid fatty acid composition were also observed in mouse tissues after infection with Bacillus Calmette-Guérin, an attenuated mutant of Mycobacterium bovis [23], while human immunodeficiency virus-1 (HIV-1) infection of cultured lymphocytes was found to result in alterations of the fatty acid composition of the membrane lipids of the lymphocytes [24].
Pathogens can modify the fatty acid composition of host cell lipids in several ways. One way is the modulation of host cell fatty acid uptake from the environment. Enteropathogenic Escherichia coli (EPEC) inhibits the uptake of short-chain fatty acids, as illustrated by inhibition of butyrate uptake into Caco-2 cells during infection [25]. Genome-wide RNAi screens in Drosophila cells infected by Listeria monocytogenes or Mycobacterium fortuitum indicated the involvement of a member of the CD36 family of scavenger receptors, which are presumed fatty acid translocases [26], [27]. A second way to modify the host cell fatty acid composition is the modulation of the activity or expression of enzymes involved in fatty acid synthesis. RNAi screening in Drosophila SL2 cells indicated that infection by M. fortuitum or L. monocytogenes requires sufficient expression of acetyl-CoA carboxylase, fatty acid synthase and stearoyl-CoA 9-desaturase [26]. Likewise, inhibition of acetyl-CoA carboxylase or fatty acid synthase in the host cells inhibits Hepatitis C virus (HCV) RNA replication [28], [29]. Moreover, during infection HCV induces an upregulation of the expression of mRNAs encoding enzymes of fatty acid synthesis [28], [29]. It is not clear whether alterations in expression levels of enzymes involved in fatty acid biosynthesis also result in altered fatty acid profiles of membrane lipids. In the next sub-sections we will focus on a third way for pathogens to modulate the fatty acid composition of host cell lipids which involves phospholipase activities, lipase activities, and/or cholesterol acyltransferase activities to efficiently modify of the fatty acid composition of host cell lipids by fatty acid exchange.
2.2. Pathogen and host cell phospholipases
Phospholipases, enzymes that cleave phospholipids, are involved in modification of membrane composition, in cell signaling pathways and in inflammatory cascades. Based on the position within the phospholipid at which the cleavage takes place, they are classified into four major groups: phospholipase A, B, C and D (PLA, PLB, PLC and PLD, respectively). Phospholipases from bacteria, viruses and parasites act as virulence factors by modifying host cell lipids to their advantage [30], [31]. They exert their action by being involved in a variety of aspects of host–pathogen interaction, such as causing membrane damage (hemolysis in the case of erythrocytes), entry into the host, and exit from vacuoles (Fig. 2 ). Phospholipases of pathogens may also be involved in acquisition of fatty acids for nutritional purposes (see Section 8), and activation of host signaling pathways to regulate the immune response (see Section 3). Lastly, pathogens can also trigger the activity of host cell phospholipases. Distinct isoforms of PLA2 were found to mediate the ability of Salmonella typhimurium and Shigella flexneri to induce the transepithelial migration of neutrophils [32].
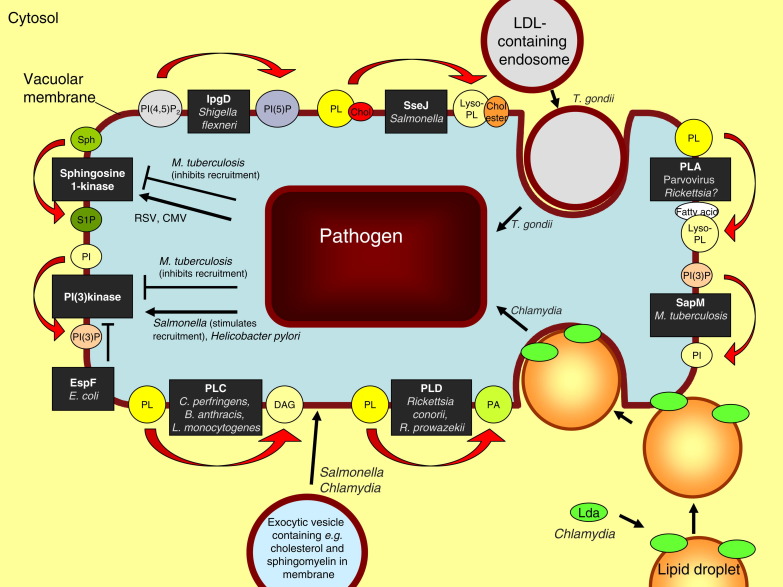
Fig. 2.
Lipid metabolism at the pathogen-containing vacuole. The various effectors and enzymes are drawn in the vacuolar membrane. However, their exact location relative to the membrane is, in most cases, unknown. PLA: phospholipase A1 or A2; PLC: phospholipase C; PLD: phospholipase D; PL: phospholipid; PA: phosphatidic acid; DAG: diacylglycerol; Sph: sphingosine; S1P: sphingosine-1-phosphate; RSV: respiratory syncytial virus; CMV: cytomegalovirus; Chol: cholesterol; LDL: low-density lipoprotein; EspF: Escherichia coli effector molecule F; SapM: M. tuberculosis effector molecule; IpgD: Shigella flexneri effector molecule; SseJ: Salmonella SPI-2 effector molecule; Lda: Lipid droplet-associated Chlamydial effector proteins. For other abbreviations: see legend to Fig. 1.
Pseudomonas aeruginosa injects an effector protein ExoU with PLA2 activity directly into host cells by means of a type 3 secretion system [33], [34], [35]. This PLA2 requires Cu2+, Zn2+-superoxide dismutase (SOD1) from the host cell as an activating factor [36]. Recently, ExoU was also found to have lysophospholipase A activity [37]. ExoU injected into the host cell is cytotoxic. By lysing cell membranes it may contribute to the ability of Pseudomonas to disseminate rapidly from lung tissue into the bloodstream. However, the cellular effects of ExoU are not limited to localised cell death and tissue destruction. This protein triggers an arachidonic acid-dependent inflammatory cascade in vivo [38]. Recent data indicate that ExoU liberates the arachidonic acid from intracellular lipid droplets [39]. In addition, it activates several transcription factors that control proliferative responses and proinflammatory cytokine production (for review, see [30]. S. typhimurium injects an effector protein SseJ into the host cell cytoplasm from within a vacuole in which the bacterium resides after its uptake into the host cell. SseJ exhibits both PLA and glycerophospholipid:cholesterol acyltransferase activity [40], [41]. The exact role of this enzyme, which is activated by as yet unidentified proteinaceous host cell factor(s), is not yet clear [40]. SseJ is not cytotoxic [42].
2.3. Phospholipases and host cell plasma membrane damage
Many pathogenic bacteria secrete or contain in their outer membrane a phospholipase activity, which can destabilize the erythrocyte plasma membrane. This hemolytic activity has been characterized for several pathogens: In the case of Campylobacter coli [43], Vibrio ssp. [44], [45], [46] and Legionella pneumophila [47] it is a PLA, while in the case of P. aeruginosa [48], [49] and Pseudomonas fluorescence [50] it is a PLC. The observation of a positive correlation between the presence of PLC activity and the hemolytic activity in a range of Mycobacterium isolates indicates that also in Mycobacterium species a PLC is involved in hemolysis [51]. In some cases the bacteria may acquire iron from the lysed erythrocytes. PLA secreted by L. pneumophila may act as an important agent in causing lung disease by destruction of phospholipids of pulmonary surfactant and damage to cell membranes by the generated lysophosphatidylcholine [52].
In addition to causing lysis of cells, membrane degradation by hydrolysis of phospholipids and (in the case of PLA activity) formation of lytic phospholipids may also facilitate entry of pathogens into host cells, while in the gastrointestinal tract phospholipases may also degrade the mucus layer overlying these cells. A PLA2 of Helicobacter pylori plays a role in colonization of the gastric mucosa [53], [54]. A similar role may be played by a PLA secreted by Campylobacter pylori, which was shown to degrade phospholipids of gastric mucus [55]. The Plasmodium berghei phospholipase Pb PL is involved in migration of sporozoites through cells to gain entry into the bloodstream [56]. Pb PL is most similar in sequence to LCAT, a member of the PLA2 family of serine lipases. Like LCAT it contains the GxSxG motif found in many serine lipases, and the catalytic triad of serine, histidine and aspartate. The enzyme was shown to have PLA activity [56]. The penetration of host cells by Rickettsia rickettsii, R. prowazekii and R. conorii also appears to be mediated by a PLA2 of rickettsial origin [57], [58]. A PLA2 may also be involved in host cell invasion by the protozoan parasites Cryptosporidium parvum [59] and Toxoplasma gondii [60], [61]. PLB secreted by Cryptococcus neoformans is important in the binding of this fungus to human epithelial cells prior to its internalization, possibly due to the release of fatty acids from host cell plasma membranes or pulmonary surfactant [62].
2.4. Phospholipases and vacuolar membrane damage
After invasion into host cells or phagocytosis by macrophages, bacteria reside in a vacuole or phagosome. Some intracellular pathogens escape from this vacuole into the cytoplasm to successfully survive and replicate [63], [64]. This vacuole escape involves the action of pore-forming proteins or phospholipases or both [64]. Clostridium perfringens escapes from its macrophage phagosome by use of its α-toxin (a PLC) in cooperation with its perfringolysin O, a cholesterol-dependent cytolysin [65]. The combined action of phospholipase and a pore-forming protein in escape from the vacuole is also seen for the well-studied L. monocytogenes, an intracellular pathogen that replicates in the cytosol of host cells [63], [64]. After invasion into cells, L. monocytogenes escapes from its vacuole by the combined action of the pore-forming protein listeriolysin O (LLO) and a phosphatidylinositol-specific PLC (PI-PLC), both secreted by the bacterium [63], [64], [66], [67]. It has been suggested that LLO forms holes into the vacuole membrane, which allow L. monocytogenes phospholipase to access exposed membrane leaflet [63]. However, in a liposome lysis assay membrane permeabilization by L. monocytogenes PI-PLC in cooperation with LLO was found to be independent of phospholipid hydrolysis [68]. In addition to directly invading a cell, L. monocytogenes can also spread from one cell to another without leaving the intracellular environment. After such cell-to-cell spread the bacterium resides in a secondary vacuole surrounded by a double membrane. The escape from this double-membrane vacuole requires the coordinated action of LLO, PI-PLC and PC-PLC (a PC-preferring PLC also secreted by L. monocytogenes) [67], [69], [70]. Recent data indicate that the PI-PLC and PC-PLC act specifically in the initial degradation of the inner membrane of the double-membrane vacuole, after which the outer membrane is disrupted by LLO [71]. PLCs may likewise be involved in phagosomal escape of Bacillus anthracis. Cooperation between the cholesterol-dependent cytolysin anthrolysin O and three PLCs in B. anthracis mediated macrophage-associated growth and survival of this pathogen, while ectopic expression of anthrolysin O from B. anthracis in Bacillus subtilis conferred limited phagosomal escape upon the recombinant bacterium [72]. However, at present it is unclear whether the mediation by the combined action of anthrolysin O and the PLCs of growth and survival of B. anthracis in macrophages is actually related to an effect on phagosomal escape. Also various Rickettsia species lyse their phagosomal compartment after entry into a host cell. The lysis of the vacuolar membrane has been attributed to the activity of rickettsia PLA2 [57], [58], [73]. The failure to identify a rickettsia PLA2 from the rickettsia genome, together with the identification of a PLD in R. prowazekii and R. conorii has led to the suggestion that a PLD is involved in the escape from the vacuole [74]. This is supported by the observation that expression of the R. prowazekii PLD gene in S. typhimurium, which normally does not escape from its vacuole, conferred on the Salmonella the ability of vacuole escape [75]. There are also viruses that use a phospholipase to escape from a host cell vesicle: parvovirus virions deploy a capsid-tethered PLA2 to breach the endosomal membrane after entry into a cell [76].
2.5. Lipases
Lipases are enzymes hydrolyzing tri-, di- and monoacylglycerols and are in some cases involved in pathogenesis. Burkholderia cepacia secretes a lipase that increases its invasion into epithelial cells without affecting plasma membrane or tight junction integrity [77]. P. aeruginosa uses a secreted lipase together with a secreted PLC to induce inflammatory mediator release from human platelets and leukocytes. Among these inflammatory mediators are the lipidic 12-hydroxyeicosatetraenoic acid (12-HETE) and leukotriene B4 [78], [79]. A lipase secreted by Staphylococcus aureus decreases phagocytosis and intracellular killing by human granulocytes of staphylococci, but not the phagocytic killing of pneumococci or streptococci. The effect of the lipase was reported to be partly retained after heat inactivation, indicating that the effect of the lipase is not exerted by enzyme action alone [80]. Also Candida parapsilosis secretes a lipase which inhibits phagocytosis and intracellular killing by macrophages [81]. In addition to these functions, lipases secreted by pathogens may also be involved in generation of fatty acids from host cell lipids for the pathogens’ energy production or complex lipid synthesis (see Section 8).
2.6. Cholesterol acyltransferases
Several bacteria secrete enzymes with glycerophospholipid:cholesterol acyltransferase (GCAT) activity. These GCATs transfer an acyl chain from a phospholipid onto cholesterol, resulting in the formation of cholesterol esters and lysophospholipids. In addition, they possess phospholipase A activity. They are members of the GDSL lipase family, which is characterized by the presence of a conserved GDSL motif and a catalytic triad (S-D-H) [82], [83]. Cholesterol is an important component of eukaryotic membranes, but is absent from the membranes of most prokaryotes. Acylation by a pathogen of host cell cholesterol might therefore be a good tool for the pathogen to specifically target host cells using an enzyme that in principle does not discriminate between host cell and pathogen lipids. Lowering the cholesterol level in the membranes of host cells may lead to increased fluidity of these membranes. In addition, GCAT/PLAs may influence signaling pathways in the host cell by generating lysophospholipids and fatty acids, or by affecting lipid raft composition through their effects on cholesterol. Here we will describe 3 examples of host–pathogen interactions involving GCAT activity that have been described in some detail.
In a survey of the distribution of a GCAT originally found in the culture supernatant of Aeromonas hydrophila [84] this enzyme was found to be present in the culture media of all Aeromonas species tested, in that of Vibrio anguillarum and V. parahaemolyticus, and in that of S. aureus [85]. The enzyme has been purified from Aeromonas salmonicida and has a preference for phospholipids carrying short-chain or unsaturated fatty acids [86]. In addition to GCAT activity, the enzyme also possesses phospholipase A2 and lysophospholipase A activity [87]. The enzyme is a major lethal toxin to fish, and lytic toward fish erythrocytes, especially when bound to lipopolysaccharide [88]. The AspA serine protease, another toxin secreted by A. salmonicida, is responsible for the proteolytic activation of pro-GCAT to GCAT after secretion of the former from the bacterium [89], [90]. Despite their toxicity, however, neither the GCAT, nor the AspA appear to be essential for the virulence of A. salmonicida [91].
The second example comes from the intracellular pathogen L. pneumophila that secretes a GCAT activity termed PlaC. PlaC is probably secreted via a type 2 secretion system. It requires activation which is either directly or indirectly dependent on the type 2 secreted zinc metalloprotease ProA [92]. Beside GCAT activity, PlaC also possesses PLA and lysophospholipase A activity. As cholesterol is not present in the membrane of Legionella, PlaC may play a role in modification of host cell membranes. However, PlaC is not essential for infection of and replication within Acanthamoeba castellanii (L. pneumophila’s natural host) and U937 macrophages [92].
A third member of the GDSL lipase family is the S. typhimurium effector protein SseJ. This protein was recently found to have GCAT activity [40], [41], PLA activity [40] and deacylase activity towards the artificial substrate para-nitrophenyl butyrate [93], but no lysophospholipase activity [40]. SseJ is translocated by a type 3 secretion system into the cytosol of a host cell from within the Salmonella-containing vacuole (SCV) in which the Salmonella resides after its entry into the host cell [94]. The GCAT and PLA activities of SseJ are both potentiated by proteinaceous factors from the host cell [40]. SseJ destabilizes the SCV membrane in the absence of another Salmonella effector SifA [42], and antagonizes the stimulatory effect of SifA on the formation of Salmonella-induced filaments (Sifs), which are tubular extensions of the SCVs [42], [95]. SifA prevents the microtubule motor kinesin from being recruited to the SCV [96]. The GCAT activity of SseJ may lower the cholesterol level of the SCV membrane and lead to storage of the resulting cholesterol esters in lipid droplets [41]. The decreased cholesterol level in the SCV membrane may lead to increased fluidity of this membrane, thus facilitating kinesin-mediated rupture of the SCV around Salmonella lacking SifA [40]. Furthermore, it was suggested that by lowering the amount of cholesterol in the SCV membrane SseJ could lower the amount of SifA on the SCV, thereby decreasing the level of Sif formation [40]. Alternatively, the formation of lysophospholipid by SseJ may aid in giving the Sif membrane the right amount of curvature.
Another type of acyltransferase is produced by S. aureus. The elimination of S. aureus from staphylococcal abscesses is mediated by the generation of bactericidal fatty acids, possibly by the activity of leukocytes [97]. However, several species of Staphylococcus produce an enzyme, termed fatty acid modifying enzyme (FAME), which can inhibit the bactericidal activity of these fatty acids by esterifying them with an alcohol [98]. Cholesterol, which the bacterium may obtain from the host cell membranes, is a particularly good alcohol substrate for this enzyme. FAME has not yet been purified and characterized in detail. The enzyme does not require ATP for its activity and is probably not a cholesterol esterase acting in reverse [98].
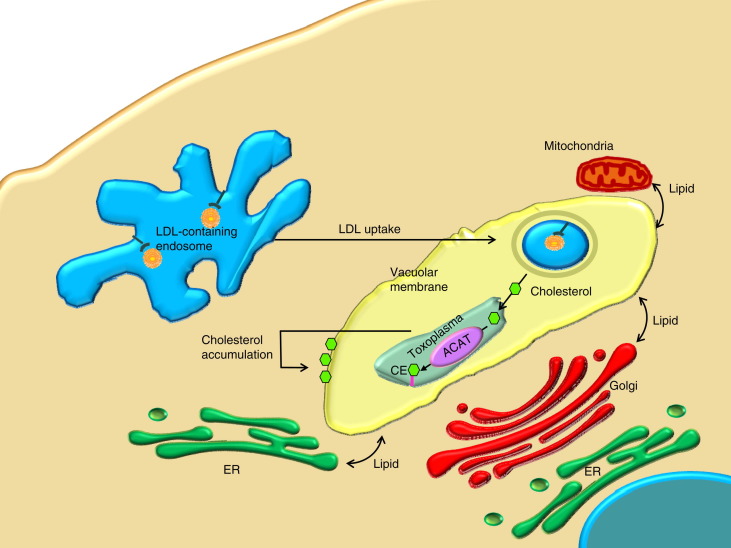
As a final example of the involvement of cholesterol acyltransferase in host–pathogen interactions we mention the apicomplexan parasitic protozoa T. gondii that resides in a parasitophorous vacuole. In the vacuole the parasite depends on host cell cholesterol derived from endocytosed low-density lipoproteins (Fig. 3 ) (see Section 8 for further details). Replication of T. gondii in its vacuole is dependent on the conversion of cholesterol to cholesterol ester. The parasite expresses two isoforms of acyl-CoA:cholesterol acyltransferase (ACAT), which differ from mammalian ACAT in their substrate affinity and specificity, and in their mechanism of regulation [99]. The endogenous ACAT activities of T. gondii are thought to be involved in the parasite’s cholesterol ester synthesis and lipid droplet biogenesis [99], [100] (Fig. 3).
Fig. 3.
Effects of Toxoplasma gondii on the host cell lipidome. ACAT: acyl-CoA:cholesterol acyltransferase; ER: endoplasmic reticulum; LDL: low-density lipoprotein; CE: cholesterolester.
Utilization of host cholesterol is also critical for the intraerythrocytic proliferation of a related apicomplexan parasite, P. falciparum [101]. The genome of this parasite was screened for genes that may be involved in acyl coenzyme A:DAG acyltransferase (DGAT) [102]. In addition to this gene, a sequence was identified which, based on its homology to the sequences for human and mouse lecithin:cholesterol acyltransferase (LCAT), was suggested to encode a plasmodial LCAT [102]. LCAT transfers an acyl chain from phosphatidylcholine (lecithin) to cholesterol, similarly to the GCATs described above. Despite the presence of the sequence for the putative LCAT in the Plasmodium, this parasite was found not to produce cholesterol esters, at least not while residing in erythrocytes [102].
3. Pathogen interference with lipid signaling in host cells
3.1. Phosphoinositide signaling during phagocytosis
Lipids play a major role in cellular signaling [103], [104]. In general, signaling lipids have a rapid turnover and are present in minute amounts. This does not exclude the possibility that signaling lipids transiently appear at high concentrations in subdomains of the membrane. Phosphoinositides are an important class of signaling lipids and they are involved in numerous cellular signaling cascades. Phosphatidylinositol can be phosphorylated at its 3, 4 and 5 position in all possible combinations, leading to 7 different phosphoinositide species. Phosphorylation at other positions is also possible but less common. Phosphoinositides are involved in cellular signaling via two different mechanisms: (1) their hydrolysis yields second messengers that transmit downstream signals; and (2) they serve as a docking site for proteins with domains that recognize specific phosphoinositides [105]. In the latter case, phosphoinositides must be present in stoichiometric amounts and must be generated at (or targeted to) specific organelles or membrane domains. Therefore, phosphoinositides help define the identity of an organelle or of a domain by recruitment of specific proteins [106].
Phosphoinositide metabolism plays a central role in the regulation of receptor-mediated endocytosis and phagocytosis [107], [108]. It contributes to the dynamics of the entire phagocytic maturation process, starting with the formation of a phagocytic cup at the plasma membrane and ending with the maturation process transforming a phagosome into a phagolysosome. During these processes, lipid kinases and phosphatases act in concert to regulate the extensive interconversion of various phosphoinositides. In brief: at the phagocytic cup, rapid interconversions between PI(4)P, PI(3,4)P2, PI(4,5)P2, and PI(3,4,5)P3 take place [109], [110]. Some of the phosphoinositides are involved in the recruitment of cytoskeletal elements, necessary for the formation of a phagocytic cup [111]. During the subsequent maturation process, formation of PI(3)P is essential, as well as the action of PI(3) kinases. Multiple waves of PI(3)P attract effector proteins such as EEA1 and Hrs to the phagosome, which is important for downstream signaling events [112]. In later stages of phagosome maturation, PI(3,5)P2 is generated through phosphorylation of PI(3)P by PIKfyve [113]. As a result, PI(3)P effector proteins are released from the membrane and PI(3,5)P2 effector proteins are recruited to the phagosomes.
3.2. Modulation of phosphoinositide signaling by pathogens
Pathogens have evolved ingenious strategies to subvert phosphoinositide metabolism by interference with many of the interconversion steps (for excellent reviews on this topic, see [114], [115]). Here we will discuss some general principles, updated with recent examples. By interference with phosphoinositide metabolism, pathogens can affect either the uptake process (A) or the phagosomal maturation process (B).
(A) L. monocytogenes is a Gram-positive pathogen that secretes an effector named InlB via its type 3 secretion system. The Listeria surface protein InlB promotes bacterial internalization into host cells by activation of type I phosphatidylinositol 3-kinase (PI3KI) [116], resulting in the generation of PI(3,4,5)P3. The effector protein In1B also results in the co-recruitment of type II phosphatidylinositol 4-kinases (PI4KII) to phagosomes [117]. The mechanism of action PI4KII remains to be established, as knockdown of PI4KII did not affect the levels of phosphoinositides but did affect the uptake process.
Yersinia pseudotuberculosis activates PIP(5)KIα to form PI(4,5)P2 and causes activation of the small GTPase Rac1, involved in membrane ruffling during the uptake in epithelial cells [118].
An ingenious example of modulation of host cell phosphoinositide signaling is provided by P. aeruginosa that can only enter cells via basolateral membranes. To enable its uptake at the apical membrane, P. aeruginosa stimulates a PI(3) kinase at the apical membrane to increase local phosphoinositide levels that are phosphorylated at the 3 position, including PI(3,4,5)P3 [119]. Formation of PI(3,4,5)P3 leads to the generation of domains in the apical membrane resembling a basolateral membrane [120]. This transformation makes the apical membrane accessible for P. aeruginosa entry [121].
S. typhimurium secretes SigD (also known as SopB) via a type 3 secretion system. SigD is a phosphatase that, at least in vitro, is capable of dephosphorylating inositol phosphates and phosphoinositides at various positions [122]. This effector influences phosphoinositide metabolism in several ways, affecting both the uptake process and the maturation process (see below). By elimination of host cell PI(4,5)P2, SigD promotes membrane fission at the plasma membrane during Salmonella invasion [123], [124]. At the same time, SigD phosphatase activity leads to an accumulation of PI(3,4)P2 and PI(3,4,5)P3 on invasion ruffles, suggesting the concurrent recruitment to or activation at the plasma membrane of PI(3)-kinase [125].
Enteropathogenic E. coli (EPEC) also induces a transient PI(4,5)P2 accumulation at bacterial infection sites, resulting in local actin accumulation and induction of PI(3,4,5)P3 clustering [126].
(B) After uptake, pathogens disrupt phosphoinositide signaling by secretion of lipid modifying enzymes, allowing them to interfere with the maturation process. As these enzymes lead to propagation of the infection, they are often regarded as virulence factors [127]. As mentioned before, Salmonella secretes SigD which affects phosphoinositide metabolism at the plasma membrane of the host cell. The same enzyme activity of SigD is, however, also active at the Salmonella-containing vacuole (SCV). After entry of the Salmonella, SigD is delivered from the plasma membrane to the SCV via a process controlled by SigD ubiquitination [128]. At the SCV, SigD promotes formation of PI(3)P [125]. In this process SigD does not dephosphorylate phosphoinositides to produce PI(3)P, as suggested earlier [129], but promotes recruitment of Rab5 and its effector the PI(3) kinase Vps34, which in turn results in the formation of PI(3)P [125]. The mechanism described for the effector protein SigD is reminiscent of the effector IpgD that is secreted by S. flexneri. IpgD is a phosphatase that dephosphorylates PI(4,5)P2 to PI(5)P. PI(4,5)P2 is a key regulator of the actin cytoskeleton and in this way the cytoskeletal organization is affected [130], [131]. Furthermore, IpgD is required for the formation of PI(3)-kinase products during S. flexneri invasion, possibly by activating a class I PI(3)-kinase [132].
Other pathogens that manipulate the phosphorylation at the 3 position of phosphoinositides to disrupt phagosome maturation include (i) Enteropathogenic E. coli that secretes EspF to block host PI(3) kinase activity [133], [134]; (ii) Helicobacter pylori that strongly activates PI(3) kinases to disrupt actin cytoskeleton regulation and to cause a delayed phagocytosis [135]; and (iii) Mycobacterium tuberculosis that targets phosphoinositide metabolism is several ways. Via an unknown mechanism, M. tuberculosis secretes SapM, a lipid phosphatase that hydrolyzes PI(3)P (Fig. 4 ) and inhibits phagosome-late endosome fusion in vitro, thereby arresting phagosomal maturation [136]. In addition, M. tuberculosis interferes with PI-3 kinase hVPS34 by using phosphatidylinositol homologues [137] (Fig. 4). This will be further discussed in Section 4.
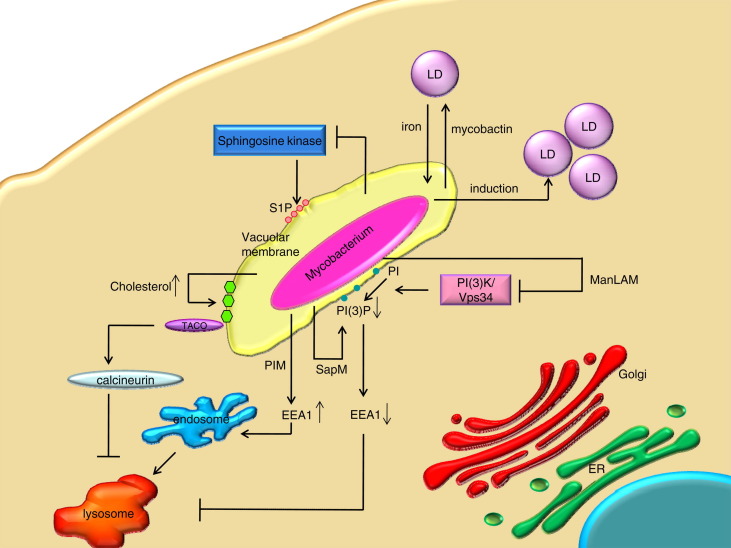
Fig. 4.
Effects of mycobacteria on the host cell lipidome. LD: lipid droplet; S1P: sphingosine-1-phosphate; SapM: M. tuberculosis effector molecule; PI(3)P: phosphatidylinositol-3-phosphate; EEA1: Early endosomal antigen 1; PI(3)K/Vps34: phosphoinositide 3- kinase; PIM: phosphatidylinositol mannoside; ManLam: mannose-capped form of Lam (lipoarabinomannan); ER: endoplasmic reticulum; TACO: tryptophan aspartate containing coat protein.
3.3. Sphingolipid signaling
Sphingolipids are a major class of membrane lipids, virtually absent from mitochondria and the ER, but constituting 20–35 mol% of plasma membrane lipids [138]. As a bulk lipid, sphingomyelin is involved in the stability of the lipid scaffold of lipid-enriched microdomains or lipid rafts. These microdomains are important targets for pathogens and will be dealt with separately (see Section 6). During the past two decades it became clear that sphingosine and related lipids containing a sphingoid base have an important role in cellular signaling, in cell function including the cell cycle, and in apoptosis [104]. The sphingolipid metabolites ceramide (Cer) and sphingosine (Sph) are associated with growth arrest and apoptosis. Many stress stimuli increase the levels of Cer and Sph, whereas suppression of apoptosis is associated with increased intracellular levels of sphingosine-1-phosphate (S1P). The balance between the apoptotic signals of Cer and Sph and the pro-survival signal of S1P is referred to as the “sphingolipid rheostat” [139].
3.4. Pathogen targeting of sphingosine-1-phosphate
S1P directly stimulates Ca2+ release from the endoplasmic reticulum (ER) [140], [141]. The sphingosine kinase 1-dependent increase in cytosolic [Ca2+] is necessary for the fusion and fission events during phagosomal maturation [142]. S1P also activates phagosome actin assembly required for killing of pathogenic mycobacteria [108]. Hence, inhibition of S1P may benefit pathogen survival in host cells and indeed, lysates from macrophages that were treated with M. tuberculosis inhibited sphingosine kinase activity, indicating direct inhibition of the enzyme by mycobacterial components [142] (Fig. 4). In addition, M. tuberculosis interferes with sphingosine kinase 1 translocation to the Mycobacterial phagosome, thereby inhibiting S1P accumulation and phagosomal maturation [143].
In contrast, some viruses that do depend on the phagocytic process, such as respiratory syncytial virus (RSV), stimulate sphingosine kinase activity resulting in increased levels of S1P. The pro-survival signal of S1P delays host cell death and results in increased viral replication [144]. Cytomegalovirus also activates sphingosine kinase, but in this case an elevated level of dihydrosphingosine-1-phosphate (dhS1P, also known as sphinganine-1-phosphate) was observed [145]. Sphingosine kinase can use both sphingosine and dihydrosphingosine (dhSph) as a substrate, resulting in the synthesis of S1P or dhS1P, respectively. As dhSph is a temporary intermediate in early steps of the synthesis of sphingolipids in the ER, levels of dhSph and its metabolites such as dhS1P are usually very low. Hence little is known about their potential biological (signaling) activity. dhS1P has been shown to be an S1P receptor agonist [146]. Opposite effects of dhS1P and S1P have been observed in transforming growth factor-beta/Smad signaling [147]. Thus, potential overlap and divergence in their biological activities have yet to be defined. It is interesting to note that the lipid composition of HIV-1, an enveloped retrovirus, shows an enrichment of the unusual sphingolipid dihydrosphingomyelin [148].
3.5. Pathogens aid pharmaceutical targeting of sphingolipid metabolism
Many naturally occurring and synthetic sphingoid base-like compounds have been identified that interfere with various steps of the complex interconversion of sphingolipid metabolism [149]. These compounds bear promise for therapeutic interventions for cancer cells and pathogenic microorganisms. For example, specific inhibition of serine palmitoyltransferase (SPT), the first step in the synthesis of sphingolipids, suppresses virus replication [150], [151], [152]. In several of these cases, it is not clear whether the inhibition is the result of inhibition of cellular signaling, or is due to an altered interaction of pathogens with lipid rafts, in which sphingolipids play an important role (see Section 6).
Several of the naturally occurring sphingoid base-like compounds that interfere with sphingolipid metabolism are produced by pathogenic fungi (for an excellent review, see [149]). Fusarium moniliforme (=F. verticillioides) for example produces fumonisins which mimic metabolites of sphingolipid metabolism. Fumonisins inhibit ceramide synthase and are major toxins involved in inducing apoptosis [153], [154], [155], [156]. The effects of the compounds produced by fungi are very diverse, ranging from causing disease to immunosuppression [157]. Some of these compounds act as (irreversible) enzyme inhibitors, whereas others act as molecular mimics, closely resembling host lipids (see Section 4).
3.6. Other signaling lipids
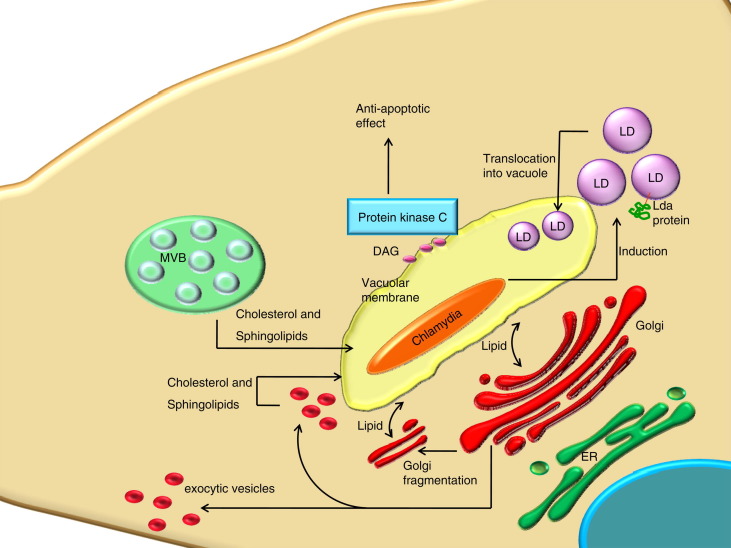
Upon host cell infection with Chlamydia, diacylglycerol (DAG) accumulates at the inclusion vacuole (Fig. 5 ), as detected by the expression of a fluorescently tagged C1 domain that specifically interacts with DAG [158]. The accumulation of DAG at the vacuole results in the recruitment of protein kinase Cδ, resulting in an anti apoptotic effect of bacterial infection.
Fig. 5.
Effects of Chlamydia trachomatis on the host cell lipidome. LD: lipid droplet; DAG: diacylglycerol; ER: endoplasmic reticulum; MVB: multivesicular body.
Leishmania major induces cholesterol depletion in host cells [159]. Recent evidence suggests that this affects the DC40 signaling complex (signalosome) composition and effector function of this parasite [160]. This is the first time that a pathogen is shown to be capable of modulating lipid levels to such an extent, that the biophysical properties of biological membranes are altered. These biophysical properties are likely to be mediated via lipid rafts which are discussed below (see Section 6).
4. Molecular mimicry of lipids
4.1. Sphingolipids
One of the most complex forms of molecular mimicry occurs in sphingolipid metabolism. Sphingolipids play an important role in the regulation of the delicate balance between the pathogen and the host. Pathogens that cannot produce sphingolipids themselves are often capable of utilizing host sphingolipids to promote their virulence [161], [162]. This is illustrated by Chlamydia trachomatis. The bacterial membrane contains up to 4% of (modified) sphingolipids although the bacterium is not capable of sphingolipid synthesis de novo [163]. The observations implying that pathogens can take up and modify host cell sphingolipids will be further discussed below (see Section 8). Pathogens capable of producing sphingolipids often produce types of sphingolipid species that are not present in mammalian hosts and that are specific to plants and fungi, including phytoceramide, inositolphosphorylceramide (IPC), and products thereof [156], [161], [162]. The functions of these pathogen-specific sphingolipids are not fully understood. Some are essential for pathogen function itself, as is seen for example in Leishmania. In this case, inhibition of sphingolipid synthesis results in an accumulation of small intracellular vesicles in internal vacuoles and the flagellar pocket [164], [165]. The small vesicles in internal vacuoles resemble those occurring within the multivesicular bodies or multivesicular tubules of the endocytic pathway. These observations are consistent with the enrichment of sphingolipids in the endocytic pathway and multivesicular bodies [138]. Another potential function for these pathogen-specific sphingolipids is that they contribute to virulence. In this respect it is interesting to note that pathogen-specific sphingolipids are not a substrate for host cell enzymes involved in sphingolipid metabolism and thus are not converted to other sphingolipid metabolites in host cells [162]. This does not exclude the possibility, however, that several of these pathogen-specific sphingolipids can bind to host cell enzymes involved in sphingolipid metabolism. In fact, by molecular mimicry several of these compounds are highly efficient inhibitors of host cell enzymes involved in sphingolipid metabolism [149]. Given the crucial role of sphingolipids in host cell function, including proliferation, differentiation and apoptosis, these compounds can effectively affect the host organisms, and cause disease or death. In fundamental and applied research, these inhibitors are used to study the involvement of sphingolipid metabolism in biological processes. For pharmaceutical research these compounds offer great potential for the development of anti-infectious disease drugs.
4.2. Phosphoinositides
Phosphatidylinositol with a mannose attached to the inositol headgroup at the 2 position (PIM) is one of the most common phospholipids in the mycobacterial envelope [166]. Additional mannose groups can be attached at the 6 position of the inositol ring, giving rise to PIM2-PIM6. PIM stimulates Rab5 and EEA1 to enhance fusion of phagosomes with early endosomes (Fig. 4) instead of fusion with lytic organelles [136]. PIM is the precursor of lipoarabinomannan (LAM), which is a heavily glycosylated form of PI, and of ManLAM (mannose-capped form of LAM). ManLAM interferes with a PI(3) kinase that synthesizes PI(3)P and thereby with subsequent recruitment of EEA1, affecting late endosomal and lysosomal trafficking events [137], [167], [168] (Fig. 4). The molecular mechanism(s) of action of PIMs and LAMs are not known. M. tuberculosis may disrupt phosphoinositide signaling by molecular mimicry of phosphoinositides. It has been suggested that especially the structure of PIM1 and PIM2 may resemble that of phosphoinositides, with the mannose residues replacing the phosphate groups [169]. The effect of PIM is independent of PI(3)K as it stimulates early endosomal fusion in the presence of wortmannin [170]. However, it is difficult to envision the binding of PIMs to phosphoinositide-specific protein domains in which electrostatic interactions of the phosphate groups play an important role [171]. Deretic and colleagues [170] suggest that PIMs may act either by insertion in membranes and subsequent recruitment of effector proteins [172], or by modifying the biophysical properties of the membrane bilayer.
4.3. Cholesterol
As will be discussed below (see Section 6), cholesterol plays an important role in host–pathogen interactions. In the case of Mycobacteria, cholesterol is involved in mycobacterial entry and survival [173], [174]. Mycolic acids are major components of the cell wall of Mycobacteria [166]. These α-alkyl β-hydroxy long-chain fatty acids may resemble the structure of cholesterol according to several criteria, including binding to cholesterol-specific antibodies, binding to Amphotericin B, and binding to each other [175]. Thus, free mycolic acids from microbacterial origin seem to be capable of mimicking cholesterol. It is not clear how a small subset of mycolic acids via their cholesterol-like hydrophobic properties contribute to mycobacterial pathogenesis.
4.4. Lipid membrane mimicry
Many highly pathogenic viruses including influenza virus, vaccinia virus, and HIV are enveloped, i.e. they have a membrane around the nucleocapsid containing the viral genome. Whereas the proteins on the viral envelope are almost exclusively virally encoded, all lipids originate from the host and are recruited from host membranes. Thus, the lipid composition of the virus resembles the composition of the host cell membrane [176], [177]. This form of mimicry may allow viruses to avoid immune detection. An example illustrating this principle is the recent observation that two rare antibodies against HIV-1 with potential clinical application were in fact reactive with the phospholipid cardiolipin [178]. Because of autoantigen mimicry, current HIV-1 vaccines may not induce these types of antibodies.
Vaccinia uses membrane mimicry to enter host cells [179]. The uptake of the virus by the host cell is critically dependent on the presence of phosphatidylserine in the viral membrane. By having phosphatidylserine at the outside of the membrane, Vaccinia mimics apoptotic bodies. This mimicking of apoptotic bodies causes efficient uptake of Vaccinia due to macropinocytosis by phagocytosing cells.
Schistosoma mansoni binds and ingests LDL particles, and breaks them down to serve as a source for lipids [180], [181]. Next to lipid acquisition S. mansoni also uses LDL for immune evasion, by covering its surface with LDL to mask its own antigens [180].
4.5. Glycosylphosphatidylinositol (GPI)
GPI is a complex structure comprising a phosphoethanolamine linker, a glycan core, and a phosphatidylinositol tail. It is well known as a C-terminal post-translational modification of proteins [182]. Much less is known about the function of free glycosylphosphatidylinositol molecules that abundantly cover the cell surface of several parasites, including Leishmania, Trypanosoma, T. gondii, and malaria parasites (P. falciparum). The free GPIs of these protozoan pathogens closely resemble the mammalian GPI anchor, but differ from each other with respect to the composition of the glycan head group and/or the fatty acid-moieties of the lipid anchor [183], [184]. Differences between free GPI structures are responsible for sometimes opposite biological effects. Depending on the isolation of GPIs from T. Brucei, P. falciparum, or Leishmania, the GPI isolates activated or inhibited macrophage function [185], [186], [187]. Very little is known about the mechanism of action of free GPI on host cells. If free GPI from pathogens would act by molecular mimicry on host cells, this would suggest a signaling role of GPI in mammalian cells. So far, however, the only function ascribed to GPI in mammalian cells is to serve as anchoring device for peripheral proteins. Alternatively, the signal of pathogen-derived GPI might be mediated via Toll-like receptors (TLR) and be involved in induction of secretion of the cytokine tumor necrosis factor (TNF). Indeed, parasite GPIs have been shown to activate TLR1, TLR2, TLR4, and TLR6 [188], [189], [190] A glycosylphosphatidylinositol-based treatment alleviates Trypanosomiasis-associated immunopathology [191].
5. Cholesterol and pathogens
Cholesterol is a ubiquitous component of all mammalian membranes. It influences the biophysical properties of biological membranes and is central to the organization, dynamics, function, and sorting of lipid bilayers in vivo [192]. Cholesterol is enriched in the plasma membrane of eukaryotic cells, where it is non-uniformly distributed. Within the plasma membrane, it is enriched in microdomains that play an important role in cellular signaling [193], [194]. Because of the crucial roles of cholesterol, its (sub)cellular levels are carefully maintained [192], [195], [196], [197]. The crucial roles played by cholesterol also make this lipid an attractive target for many pathogens via which they can influence host cell dynamics. In addition, the absence of cholesterol from prokaryotes makes it an almost ideal biomarker that pathogens can use to recognize and infect mammalian host cells. Hence, pathogens have developed various ingenious ways to make use of the presence of cholesterol for the recognition of and interaction with host cell membranes. The interaction of pathogens with lipid rafts will be discussed in the next section. Here we will discuss indications for raft-independent interactions of pathogens with cholesterol.
5.1. Effector binding to cholesterol
Several type 3 secretion systems (T3SSs) have now been identified in Gram-negative bacteria to deliver virulence effector proteins into eukaryotic target cells via a needle-like structure. They have been classified into three distinct phylogenetic groups in which e.g. Salmonella and Shigella entry-associated T3SSs are closely related and the T3SSs of EPEC and Pseudomonas are more divergent [198]. At the tip of the needle, bacterial proteins insert into the host cell membrane to form a translocon that perturbs the bilayer [199], [200]. The translocon components SipB and IpaB of Salmonella and Shigella, respectively, are cholesterol-binding proteins. Cholesterol is required for the binding of SipB to the plasma membrane, and secretion of effectors by the T3SSs of Salmonella, Shigella and EPEC depends on cholesterol [201]. Of the Pseudomonas translocon components PopB and PopD, PopB directly binds cholesterol, while in addition both PopB and PopD can bind phosphatidylserine [202]. PopB together with PopD causes a cholesterol-dependent aggregation of liposomes [203]. This aggregation occurs in liposomes containing 15% cholesterol and is independent of the presence of sphingomyelin, suggesting that this effect is lipid raft-independent. The biological significance of the aggregation observed in this assay is not clear. Under different experimental conditions, PopB and PopD synergistically permeabilize vesicles without causing aggregation in a cholesterol-independent manner [202].
5.2. Cholesterol accumulation on pathogen-containing vacuoles
Host cell cholesterol accumulates at parasitophorous vacuoles of several intracellular pathogens (see Section 8). At later stages of infection the Salmonella-containing vacuole (SCV) contains up to 30% of the cellular cholesterol pool in both epithelial cells and macrophages [204]. The function of cholesterol recruitment to the pathogen-containing vacuoles is not clear.
First, it may be used as a nutrient, as was recently shown for mycobacteria [205]. See Section 8 for further details.
Second, cholesterol may influence the interaction of pathogen-containing vacuoles with other cellular organelles, which depends on membrane-trafficking pathways [206]. Various intracellular trafficking pathways of eukaryotic cells are sensitive to cholesterol, including ER to Golgi transport [207], intra-Golgi transport [208], endosomal transport [209], [210], [211], and phagosomal maturation [212], [213]. Cholesterol levels may affect intracellular trafficking in various ways, including the recruitment of proteins and lipids to the pathogen-containing vacuole. For example, cholesterol was found to mediate the phagosomal association of TACO/coronin-1, affecting the degradation of mycobacteria in lysosomes [174] (Fig. 4). Coronin-1 is required for activation of the Ca2+-dependent phosphatase calcineurin, thereby blocking lysosomal delivery of mycobacteria [214]. This mechanism acts in concert with the effect of mycobacterium on sphingosine kinase (discussed in Section 3.4 and elsewhere [215]).
Third, the Salmonella effector SseJ has been described to use cholesterol as an acceptor of acyl chains and might therefore use cholesterol at SCVs as a substrate [40], [41]. In addition, this function may also imply cholesterol-specific recruitment of SseJ to the SCVs. Vice versa, SseJ may be involved in the recruitment of cholesterol to the SCV, as overexpression of SseJ results in formation of cholesterol-rich membrane structures [42].
5.3. Pathogen uptake and modification of cholesterol
Cholesterol in the membranes of Helicobacter spp. [216], [217], [218], S. aureus [217], Anaplasma phagocytophilum [219] and Chlamydia EB and RB membranes [163] is of host origin. A sterol-binding protein in Toxoplasma was recently identified that may optimize pathogen handling of host cell derived cholesterol [220]. Helicobacter pylori extracts cholesterol from epithelial cells and converts it into cholesteryl α-glucosides [221]. Cholesterol and cholesteryl glucosides represent 1.6% and 25%, respectively, of the total lipids of H. pylori [222]. The abundant presence of glucosylated cholesterol inhibits phagocytosis of and T-cell activation by H. Pylori, providing an immune escape mechanism for this pathogen.
6. Lipid rafts
The organization of biological membranes is based on interactions between membrane proteins and lipids. Recent evidence suggests that in these membranes specialized microdomains (so called lipid rafts) exist that are characterized by an enrichment of cholesterol, sphingolipids, and a specific subset of proteins. By selective inclusion and exclusion of proteins, rafts are involved in many cellular processes such as endocytosis, signaling, protein sorting and intracellular membrane trafficking. It is beyond the scope of this review to describe details and to discuss controversies surrounding the biological function and properties of lipid rafts. These topics are covered by many review articles [10], [223], [224], [225], [226], [227], [228], [229], [230].
The biophysical properties and the high concentration of signaling molecules make lipid rafts a natural target for pathogens through which they communicate with host cells and hijack membrane-trafficking pathways. This topic has also been the focus of several reviews to which the reader is referred [231], [232], [233], [234], [235]. An updated list now includes over 100 pathogens that have been suggested to interact with lipid rafts (Table 1, Table 2, Table 3, Table 4 ). Not in all cases the involvement of rafts, rather than e.g. cholesterol, in the host–pathogen interaction has been unambiguously established. The early methodology to show an involvement of lipid rafts in biological processes is rather diffuse, and uses detergents and cholesterol-extracting agents. Only a combination of several independent methods, which also include morphological and functional studies, can establish the involvement of lipids rafts in host–pathogen interactions. Hence, especially data from early publications, when these additional methods were not yet available, should be interpreted with some caution.
Table 1.
Bacteria interacting with lipid rafts.
| Bacterium | Involved in | Pathogen molecule involved | Raft molecule involved | Refs. |
|---|---|---|---|---|
| Anaplasma phagocytophilum | Entry | GPI-anchored proteins | [236] | |
| Afipia felis | Entry via macropinocytosis | [237] | ||
| Brucella abortus | Entry | 1,2 cyclic glycan | Class A scavenger receptor | [238], [239], [240] |
| Phagosomal maturation | ||||
| Brucella suis | Entry/ intracellular survival | [238] | ||
| Campylobacter jejuni | Entry | [241] | ||
| Chlamydia pneumonia | Entry | [242] | ||
| Chlamydia psittaci | Entry | [242] | ||
| Chlamydia trachomatis (E and F) | Entry | [242], [243], [244] | ||
| Ehrlichia chaffeensis | Entry | GPI-anchored protein | [236] | |
| Escherichia coli FimH expressing | Entry/survival | FimH | CD48/CD55 uroplakin-1a | [245], [246], [247], [248], [249] |
| Escherichia coli Enteropathogenic | Induces lipid rafts during pedestal formation | [250], [251] | ||
| Escherichia coli Uropathogenic | Transcellular translocation | [252] | ||
| Escherichia coli diffusely adhering (DAEC) | Entry | AfaE | CD55 | [253], but see also [254] |
| Francisella tularensis | Entry /survival | [255] | ||
| Helicobacter pylori | Adhesion | HpaA | LacCer | [256] |
| Legionella pneumophila | Intracellular survival | [257] | ||
| Listeria monocytogenes | Signaling | Internalin | E-cadherin | [258] |
| Mycobacterium avium | Entry | Polar lipid fraction | [259] | |
| Mycobacterium kansasii | Entry | CR3 in association with GPI-anchored protein(s) | [260] | |
| Mycobaterium tuberculosis | Entry/survival | Receptor-Ck | [174], [261] | |
| Mycobaterium bovis | Entry | [174] | ||
| Mycoplasma fermentans | [262] | |||
| Porphyromonas gingivalis | Entry/intracellular survival | [263], [264] | ||
| Pseudomonas aeruginosa | Entry/signaling | [265], [266], [267] | ||
| Salmonella typhimurium | Entry/intracellular survival | SipB (T3SS) | Cholesterol | [201], [204], [268], [269] |
| Effector proteins | PipB and PipB2 | |||
| Shigella flexneri | Entry | Components of the T3SS: IpaB | CD44 | [270], [271] |
| Yersinia enterolitica | Intracellular replication | [272] | ||
| Neisseria gonorrhoeae | Entry | CEACAM | [273], [274] | |
| Staphylococcus aureus | Entry | asialoGM1/TLR2 | [275] | |
| Gram-negative bacteria | Binding/Entry | Lipopolysaccheride (LPS) | CD14 | [276] |
Table 2.
Bacterial toxins interacting with lipid rafts.
| Bacterial toxina | ||||
|---|---|---|---|---|
| Actinobacillus actinomycetemcomitus | Binding | Cytolethal distending toxin | [277], [278] | |
| Aeromonas hydrophila | Binding–oligomerization | Aerolysin | CD14 (GPI-anchored) | [279], [280] |
| Bacillus anthracis | Oligomerization – Clathrin-coated pits | anthrax toxin (protective antigen) | Anthrax toxin receptor (ATR) | [281], [282] |
| Anthrolysin O (a CDC) | ||||
| Bacillus thuringiensis | Binding/oligomerization | cry1A toxin | Aminopeptidase N | [283], [284], [285] |
| Clostridium botulinum | Binding | Neurotoxin | [286], [287] | |
| Clostridium difficilus | Binding | Toxin TcdA and TcdB | [288] | |
| Clostridium perfringens | Binding/oligomerization | epsilon-Toxin | [289] | |
| Clostridium perfringens | Binding/internalization | iota-Toxin | [290] | |
| Clostridium perfringens | Binding | theta-Toxin (CDC)b (=perfringolysin) | Cholesterol | [291], [292] |
| Clostridium septicum | Binding | α-Toxin | GPI-anchored proteins | [293] |
| Clostridium tetani | Binding/internalization | Tetanus toxin | [294] | |
| Escherichia coli | Binding/oligomerization | Heat-labile enterotoxin LTII | GD1 | [295] |
| Escherichia coli | Binding/oligomerization | Heat-labile enterotoxin LTI | GM1 | [296] |
| Helicobacter pylori | Binding/oligomerization | VacA | SMc | [297], [298] |
| Listeria monocytogenes | Binding/oligomerization | Listeriolysin (a CDC)b | Cholesterol | [258], [299], [300] |
| Vacuole lysis (via T3SS-raft interaction) | ||||
| Shigella dysenteriae | Binding/oligomerization | Shiga toxin | Gb3 | [301], [302] |
| Streptococcus pyogenes | Oligomerization | streptolysin (CDC)b | [303] | |
| Vibrio cholera | Binding/Caveolae | Vibrio cholera cytolysin (CDC)b | Cholesterol | [303], [304] |
| Cholera toxin | GM1 | |||
For a complete overview of CDC, secreted by 26 different pathogens, the reader is referred to a recent review by Rosado et al. [305].
CDC: Cholesterol-dependent cytolysin.
For a list additional toxins that interact with sphingomyelin (SM) but are secreted by other types of pathogens, the reader is referred to a recent review by Shogomori and Kobayashi [306].
Table 3.
Viruses interacting with lipid rafts.
| Virus | ||||
|---|---|---|---|---|
| Avian sarcoma and leukosis virus | Entry | GPI-anchored receptor TVA800 | [307] | |
| Bluetongue virus | Protein localisation | VP5 | [308] | |
| Coxsackievirus | Entry/Golgi targeting | [309] | ||
| Dengue virus | Entry | Entry: HSP70/90 | [310], [311] | |
| Association | Association: non-structural glycoprotein NS1 | |||
| Ebola virus | Entry | Entry: fusion glycoprotein GP2 | [312], [313], [314] | |
| Budding/assembly | Budding/assembly: VP40 | Budding/assembly: Recruitment of TSG101 | ||
| Echovirus 1 | Entry/non-caveolar endocytosis/caveosomes/trafficking | alpha2beta1 integrin | [315], [316], [317] | |
| Echovirus 6 | Entry | DAF GPI-anchored) | [318] | |
| Echovirus 11 | Entry raft dependent | DAF for raft-dependent entry | [319] | |
| Ecotropic murine leukemia virus | Entry/budding | [320] | ||
| Epstein-barr virus | Signaling | [321], [322] | ||
| Hepatitis C virus | Entry | Entry: CD81 and class B scavenger receptor | [323], [324], [325], [326] | |
| Replication | Replication: NS proteins | Replication: hVAP-33 | ||
| Herpes simplex virus | Entry/binding | Entry: glycoprotein B | [327], [328] | |
| Budding | Budding: UL11 | |||
| Herpesvirus saimiri | Down regulation TCR | Tip | [329] | |
| HIV | Entry | Binding: gp120 | Binding: CD4/CXCR4 | [148], [330], [331], [332], [333], [334], [335], [336], [337], [338], [339], [340] |
| Transcytosis | Transcytosis: Gp41 | Transcytosis: GalCer | ||
| Assembly/budding/release | Assembly: Gag | Assembly: Annexin2 | ||
| Human herpes virus-6 | Entry | Glycoprotein Q1 | CD46 | [341] |
| Human herpesvirus 8 = Kaposi’s sarcoma-associated herpes virus | Manipulation of signaling | [342] | ||
| Human T-cell leukemia virus | Entry/budding/ | [343], [344], [345] | ||
| Signaling in T-cell proliferation | Signaling: Tax1 | Signaling: IκB | ||
| Influenza virus | Entry/budding/ assembly | hemagglutinin and neuraminidase | [346], [347], [348] | |
| Marburg virus | Entry/budding | [313] | ||
| Measles virus | Budding/assembly | [349], [350], [351] | ||
| Newcastle disease virus | Assembly/release | [352], [353] | ||
| Pseudorabies virus | Entry/proteins localisation | Us9 | [354], [355], [356] | |
| Respiratory syncitial virus | Entry in caveolae | F protein | [357], [358], [359] | |
| Assembly | ||||
| Rhinovirus | Entry | [360] | ||
| rotaviruses | Entry/replication | VP4 | [361], [362], [363] | |
| SARS CoV | Entry | S-protein | ACE-2 | [364], [365] |
| Sendai Virus | Budding/assembly | [366], [367] | ||
| Semliki forest virus | Entry and exit | Entry: Fusion peptide | [368], [369] | |
| Simian virus 40 | Entry/Caveolae/trafficking/ | MHCI | [370], [371] | |
| Vaccinia virus | Entry | [372], [373] | ||
| Varicella zoster virus | Viral envelope integrity | [374] | ||
| West Nile virus | Entry | [375] | ||
Table 4.
Protozoa, fungi and other pathogens interacting with lipid rafts.
| Protozoon | ||||
|---|---|---|---|---|
| Cryptosporidium parvum | Entry/attachment | [376] | ||
| Entamoeba histolytica | Attachment | [377] | ||
| Leishmania | Intracellular survival | Lipophosphoglycan | [378] | |
| Plasmodium falciparum | Entry | [379], [380], [381], [101] | ||
| Theileria parva | Signaling | [382] | ||
| Toxoplasma gondii | Invasion/Intracellular survival | [383] | ||
| Fungus | ||||
| Paracoccidiodis brasiliensis | Adhesion/signaling | [384] | ||
| Other | ||||
| Prion protein PrPC | Localisation to host cell membrane | PrPsc | [385], [386] | |
The basic property of lipid rafts that is exploited by pathogens is their intrinsic ability to transiently oligomerize. This property is abused by pathogens, resulting in stabilized, altered or disrupted oligomeric structures which (i) provide a mechanism for pathogen uptake, (ii) affect host cell signaling; (iii) alter intracellular trafficking including pathogen vacuole maturation, and (iv) offer strategies for exit from the host cell. Here we will focus on the role of lipids in the raft-mediated interactions between host cells and pathogens.
6.1. Raft lipids as receptors for pathogens
Several pathogens directly target sphingolipids in raft structures (Table 1). Recently, VacA was identified as a bacterial virulence factor, secreted by the gastric pathogen Helicobacter pylori that exploits a plasma membrane sphingolipid, sphingomyelin (SM), as a cellular receptor [298]. Helicobacter pylori may exploit the capacity of SM to partition into lipid rafts in order to access the raft-associated cellular machinery. Other pathogens that exploit lipid-binding toxins to localise to lipid rafts include toxigenic E. coli, which secrete the heat-labile enterotoxin LTI targeting GM1, or which secrete the heat-labile enterotoxin LTII targeting GD1, Shigella dysenteriae that secrete Shiga toxin targeting Gb3, and Vibrio cholera that secrete cholera toxin targeting GM1. The multimeric toxins have the potential to cluster rafts, thus allowing high affinity toxin-binding through relatively weak individual binding forces of carbohydrate binding sites [387], [388]. In addition, the raft-dependent interaction also determines their mechanism of uptake. It is becoming increasingly clear that there are clathrin-dependent and clathrin-independent endocytic pathways which differ in their requirement of lipid rafts [389], [390], [391], [392].
The family of cholesterol-dependent cytolysins (CDCs) binds cholesterol in membranes, resulting in oligomeric ring structures in membranes that create pores with diameters ranging from 1 to 50 nm [305]. CDCs localise to lipid rafts, but how this property relates to their mode of action is not clear, as the biophysical properties of lipid rafts hamper the interaction of cholesterol with CDCs [393].
6.2. Lipid-dependent reorganization of rafts
Several lipids are targeted by pathogens in order to change the oligomeric state of lipid rafts, resulting in (i) stabilization, (ii) alteration, or (iii) disruption of lipid rafts. Examples of the first possibility, that of stabilization of lipid rafts, are provided by Neisseriae gonorrhoeae, P. aeruginosa, S. aureus, rhinovirus or Sindbis virus, which activate the enzyme acid sphingomyelinase to release ceramide in the outer leaflet of the cell membrane [235]. Ceramide molecules have the tendency to induce the formation of ceramide-enriched microdomains, to function as a fusogen that triggers the spontaneous fusion of ceramide-enriched membrane microdomains, and to stabilize large ceramide-enriched membrane macrodomains [394], [395], [396]. The second possibility of lipid targeting by pathogens is that lipids cause a reorganization/alteration of lipid rafts. This possibility has been suggested for lipoarabinomannan (LAM), an integral component of the M. tuberculosis that markedly alters the morphology of lipid domains in membranes [397]. As a result phagosome maturation is inhibited [398]. The third possibility is that pathogens target lipids to cause a disruption of lipid raft structures. Brucella abortus secretes 1,2 cyclic glycan, a component that resembles cyclodextrins and extracts cholesterol from membranes, resulting in disruption of lipid rafts [399].
Together these results confirm that lipids are essential for the stability of microdomains in biological membranes. In model membranes that consist of only a specific subset of lipids, lipid rafts can easily be observed (see e.g. [400]). In biological membranes, the lipid composition is much more complex and heterogeneous, resulting in the suggestion that protein interactions are necessary to stabilize lipid rafts (see e.g. [229] for a discussion on this topic). The fact that pathogens can alter the oligomeric state of rafts in biological membranes by targeting lipids suggests that lipids provide a major driving force determining the biophysical state and hence physiological role of rafts.
6.3. Lipid rafts in budding of viruses
It appears that several enveloped viruses select raft-like domains to exit from cells. HIV viral membranes contain raft markers GM1, Thy-1 and CD59 [334]. HIV viral membranes are Triton X-100 resistant and contain typical raft lipids [148]. Other viruses known to assemble on or bud from lipid rafts in host cell membranes include influenza virus [176], measles virus [401] and Newcastle disease virus [352] (see Table 1 for a comprehensive list). As HIV-1 particles excluded a bona fide raft marker (flotillin-1), this indicates that the virus buds from a subset of cellular microdomains. The function of lipid rafts in virus assembly can be manifold, including recruitment of a subset of host lipids and proteins into viral membranes and determination of the oligomeric state of viral proteins. Another surprising possibility was suggested by the finding that assembly of the non-enveloped Rotavirus is dependent on cholesterol, suggesting that rafts may control the proper incorporation of viral proteins into virions [402].
7. Lipid droplets
Lipid droplets (LDs), also termed lipid bodies or adiposomes, are lipid storage organelles found in animals, plants and microorganisms [403]. Lipid droplets are thought to be formed by accumulation of neutral lipids between the membrane leaflets of the ER and bud from there [403], [404], [405], although some variations of this model have been presented [406], [407]. Lipid droplets consist of a neutral core composed of triacylglycerols, cholesterol esters, retinol esters and diacylglycerols surrounded by a monolayer of phospholipids. In addition, lipid droplets contain a specific and dynamic subset of structural and functional proteins that are involved in the biogenesis, turnover, and biological functions of these organelles. Recent proteomic approaches have identified a large variety of proteins associated with lipid droplets, which indicates that they are much more than lipid storage depots. A wide range of cellular functions have been suggested, including an involvement of lipid droplets in lipid transport and metabolism, membrane trafficking, intracellular signaling, production of inflammatory mediators, and in protein folding and aggregation [408], [409].
These properties make them attractive targets for pathogens and indeed, recent work shows that several pathogens interact with lipid droplets.
7.1. Pathogen induction of lipid droplet formation
A variety of pathogens induce the formation of lipid droplets in host cells. For example, bacterial lipopolysaccharide (LPS), present in all Gram-negative bacteria, induces lipid droplet accumulation in host cells in a Toll-like receptor 4 (TLR4)-dependent way, which, in case of macrophages, results in formation of foam cells [410], [411]. Other examples include the induction of lipid droplet formation by Plasmodium berghei in mouse kidney and liver cells [412], [413] and the induction of lipid droplet formation by Candida albicans in macrophages and hepatocytes by use of an extracellular lipase [414]. The induction of lipid droplet formation by hepatitis C virus (HCV), Trypanosoma cruzi, M. tuberculosis, Chlamydia trachomatis, and Mycobacterium bovis (M. bovis) will be discussed in some detail below.
7.2. Lipid droplets and pathogen assembly
Hepatitis C virus induces the formation of more and larger lipid droplets in hepatic cells. In addition, structural proteins of HCV have been found to localise to lipid droplets [415]. The HCV capsid protein (core protein) localises to the LDs and recruits non-structural proteins and replication complexes, which appears critical for the production of virus particles [416], [417]. The core protein most likely replaces adipose differentiation-related protein (ADRP), a major protein associated with the surface of lipid droplets, by progressively coating the entire surface. The absence of ADRP then results in lipid droplet aggregation around the nucleus [418]. The presence of the replication machinery and the observation of virions in close proximity to the LDs might indicate virus assembly at or in close proximity to lipid droplets. Recent work from Roingeard et al. [419] indicates that the overexpression of HCV core protein induces virion budding from ER membranes in close proximity to the LD rather than at membranes directly apposed to the lipid droplets.
7.3. Pathogen-vacuole interactions with lipid droplets
Trypanosoma cruzi induces both lipid droplet formation and eicosanoid production in macrophages [420]. This suggests that lipid droplets may have a role in eicosanoid production. Indeed, subsequent work showed that lipid droplets in cells infected with T. cruzi are found in close proximity to, attached to, and even internalized into the parasite-containing vacuole. Thus, a close interaction of lipid droplets with the pathogen vacuole allows a possible discharge of lipid droplet content [421]. A direct interaction between lipid droplets and pathogen vacuoles is, however, not always necessary for the induction eicosanoid production: the membrane compound LAM of Mycobacterium induces a TLR2-dependent, but phagocytosis-independent induction of lipid droplets and eicosanoids in macrophages [422].
Cells infected with M. tuberculosis also show a close association of the mycobacterial phagosome with lipid droplets. In this case the close association of lipid droplets with the pathogen-containing vacuole is hijacked for the acquisition of iron (Fig. 4). M. tuberculosis secretes the metal-free siderophore mycobactin and after binding to host cell cytosolic iron, mycobactin is targeted to lipid droplets. The close interaction with lipid droplets subsequently allows the pathogen to acquire host cell iron [423].
Lipid droplets are also crucial in Chlamydia trachomatis intracellular survival and replication. In epithelial cells three Chlamydia proteins (Lda) were found to localise to host cell lipid droplets (Fig. 5). Similar to what had been observed for HCV, Lda association with lipid droplets occurred with a concomitant decrease of ADRP, suggesting a replacement of lipid droplet coat protein with a pathogen protein, allowing functional control of this organelle. For example, lipid droplet-associated Lda may be involved in the observed accumulation of lipid droplets around the Chlamydia-containing vacuole (inclusion) [424]. Alternatively, the chlamydial Lda proteins may function in the recently described translocation of lipid droplets into the Chlamydia inclusion, probably for the acquisition of nutrients [425]. See Section 8 for further details.
7.4. Pathogen-localised lipid droplets in detoxification
As a consequence of a hemoglobin diet for blood-feeding organisms such as P. falciparum, they produce large amounts of heme, a toxic molecule that can disrupt membranes, inhibit enzymatic processes and initiate oxidative damage. P. falciparum uses lipid droplets in close proximity to its digestive vacuole to detoxify heme by formation of haemozoin crystals within the hydrophobic environment of lipid droplets [426], [427]. Lipid droplet-mediated detoxification of host derived heme has also been suggested for Schistosoma mansoni and Rhodnius prolixus, allowing the formation of multicrystalline assemblies in the guts of these organisms [428].
In summary LDs are involved in a wide variety of host–pathogen interactions, such as inflammatory responses, pathogen assembly, pathogen nutrient acquisition and detoxification processes. As we are only beginning to understand the biological functions of lipid droplets, studies on the involvement of lipid droplets in host–pathogen interactions will also contribute to this area of research.
8. Lipid acquisition
The host provides an appealing habitat for numerous microorganisms and excluding them from critical nutritive resources has its role in host defense [429]. This nutrient restriction also provides an interesting pharmaceutical target, as many pathogen enzymes that utilize host cell lipids bear resemblance to host cell enzymes, but distinct differences allow the design of specific drugs to selectively inhibit pathogen enzymes. A recent publication by Brinster et al. [430], however, casts some doubts on this strategy, as inhibition of bacterial fatty acid synthesis is fully bypassed by the uptake of exogenous fatty acids. There is an enormous amount of literature available that indicates that pathogens require host cell lipids and it is beyond the scope of this review to give an extensive overview. For certain pathogens, it may seem obvious why they acquire host cell lipids as they are auxotroph for fatty acids (e.g. [431]), or cholesterol [205]. In many other cases, however, the reason for lipid acquisition from the host cell is not clear (e.g. [432]). Here, we will focus on the general principles, illustrated with prominent and recent examples.
8.1. Metabolic fate of acquired lipids
One function of acquired lipids is to modulate host–pathogen interactions and virulence, as has been discussed in other sections of this review (see also e.g. [433]). A second function of acquired lipids is to serve as an energy source. For example, Corynebacterium jeikeium cannot synthesize fatty acids, but upon their acquisition they are utilized for subsequent β-oxidation, as the bacterium contains all the enzymes required for this process [431]. Even cholesterol can be used as a substrate for energy production, for example by Rhodococcus species, soil bacteria related to M. tuberculosis [434]. To ensure expedited energy supply, Gram-negative bacteria can induce the release of fatty acids from host cells by stimulating adipose lipolysis via the lipid A moiety of LPS [435]. A third function of acquired lipids is to provide building blocks for pathogen assembly. In general, pathogens can synthesize the typical prokaryotic lipids phosphatidylserine, phosphatidylethanolamine, and phosphatidylglycerol and must acquire lipids generally associated with eukaryotes such as sphingomyelin, cholesterol, phosphatidylcholine and phosphatidylinositol [163]. Hence, several pathogens are auxotrophic for these lipids (e.g. sphingolipids [162] or cholesterol [99], [219]). Host cell lipids can also be used as a carbon source, as has been described for e.g. M. tuberculosis [205], [429], Salmonella [436], Candida albicans [437], and Cryptococcus neoformans [438]. The acquisition of a viral envelope from host cell membranes could also be considered as an effective mechanism to acquire building blocks for pathogen assembly. This has been discussed elsewhere in this review (Section 4).
Thus, there are various ways by which pathogens utilize host cell lipids. In the next section we will discuss which lipids are known to be acquired by pathogens via interactions with a host cell and describe the mechanisms involved.
8.2. Lipid acquisition by extracellular pathogens
Extracellular pathogens often reside in the host’s nutrient-rich body fluids. Trypanosoma brucei acquires lysophosphatidylcholine from plasma pools. Lysophospholipids are more soluble in aqueous solution when compared to di-acylated phospholipids and are more readily imported by the parasite. After their uptake, two lysophosphatidylcholine molecules are then used for the generation of one molecule of phosphatidylcholine by 3 consecutive enzymatic steps involving a lyso-phospholipase A1, an acyl-CoA ligase and a lysophosphatidylcholine:acyl-CoA acyltransferase [439], [440].
T. brucei also incorporates host cholesterol from plasma into its membranes. Cholesterol is not water soluble, but in this case T. brucei selective absorbs LDL particles from the plasma by receptor-mediated endocytosis [441], [442] and acquires cholesterol from these particles. Trichomonas vaginalis and Schistosoma mansoni also internalize LDL particles for the acquisition of essential host lipids [180], [181], [443]. The endocytic uptake by T. vaginalis is mediated by a receptor for apolipoprotein CIII.
Extracellular pathogens acquire lipids from other body fluids as well. For example, P. aeruginosa acquires lipids from lung surfactant [444] and Salmonella ssp. might utilize phospholipids from intestinal mucus [445], although this has not been confirmed in vivo. Finally, extracellular pathogens can obtain lipids from the extracellular leaflet of the plasma membrane, as has been shown for Helicobacter pylori, which acquires cholesterol from lipid rafts in the plasma membrane [221].
8.3. Lipid acquisition inside a host cell via the pathogen vacuole
Many pathogens reside within membrane-enclosed vacuoles and determine the properties of these structures to avoid host cell-mediated degradation. The vacuolar membrane is the barrier between host and pathogen, and can at the same time function as source of lipids to the pathogen. Indeed, several host cell-derived lipids accumulate in the membrane of pathogen vacuoles. Cholesterol accumulates in the vacuoles of e.g. Coxiella [446], Salmonella [204], Chlamydia [447], T. gondii [448], [449], [450], and M. tuberculosis [212]. Sphingomyelin accumulates in Chlamydia inclusions [451] and T. gondii vacuoles [452]. Diacylglycerol and the phospholipids PI and PC accumulate in Chlamydia inclusions [158], [163]. T. gondii also preferentially recruits PC over other phospholipids to its parasitophorous vacuole [453]. How are these lipids delivered to the vacuole? In the case of Chlamydia, the observation that host transmembrane proteins are not delivered to the vacuole has intriguing implications for the mechanisms of transport [454]. Only for a few pathogens, including Chlamydia and T. gondii, the molecular mechanisms of lipid transport to the pathogen vacuole have been described in some detail. These pathogens will be discussed in some more detail, with evidence from other pathogens added as additional examples.
8.4. Lipid acquisition via vesicular trafficking to the pathogen vacuole
Chlamydia intercepts exocytic vesicles from the secretory pathway to acquire host cell lipid nutrients. In an elegant series of experiments, the group of Hackstadt provided evidence for the diversion of Golgi-derived vesicles, en route to the plasma membrane, to the pathogen vacuole (Fig. 5) (reviewed in [455]). The transport of both sphingomyelin [451] and cholesterol [447] was shown to be Brefeldin A-sensitive, suggesting a Golgi origin of these vesicles. In contrast, Golgi-derived glycoproteins en route to the plasma membrane were not intercepted by the pathogen [456]. These results indicate that only a subfraction of vesicles is targeted for delivery to the pathogen vacuole. Indeed, recent evidence suggests that Chlamydia preferentially intercepts basolaterally-directed sphingomyelin-containing exocytic vesicles [457]. Whether the intercepted vesicles represent bona fide exocytic vesicles derived from the Golgi complex remain to be established, as it cannot be excluded that Chlamydia induces an alternative or modified exocytic pathway from the Golgi to the plasma membrane. Recently, it was shown that Chlamydia trachomatis causes fragmentation of the Golgi complex and the generation of Golgi ministacks surrounding the bacterial inclusion [458]. This process is mediated by bacteria-induced proteolytic cleavage of the Golgi matrix protein Golgin-84. This Golgi fragmentation is involved in lipid acquisition, as inhibition of the cleavage of Golgin-84 also inhibited the transport of lipids to the pathogen vacuole. These observations open the possibility that lipid transport is not only mediated by exocytic vesicles, but also by Golgi fragments (Fig. 5). T. gondii also induces Golgi fragmentation in the host cell, but in this case, the Golgi fragments seem dispensable for parasite invasion and replication [459]. It will interesting to determine whether the lipids from these Golgi fragments are transported by fusion of the Golgi fragments with the vacuole or by the generation of contact sites between the Golgi fragments and the bacterial vacuole (see also next Section 8.5). The Golgi fragments generated by knockdown of Golgin-84 are different from the Golgi fragments caused by Brefeldin A. First, in the presence of Brefeldin A, de novo synthesized sphingomyelin and cholesterol from the exocytic pathway are no longer delivered to the Chlamydia vacuole [447], [451]. Second, whereas Golgin-84-induced Golgi fragmentation enhances bacterial replication [458], Brefeldin A does not affect bacterial multiplication [447], [460]. The ineffectiveness of Brefeldin A on bacterial multiplication indicates that alternative pathways must be available to provide Chlamydia with lipids. Indeed, recent evidence suggests that Chlamydia can also recruit lipids from multivesicular bodies that are rich in cholesterol and sphingomyelin [461], [462] (Fig. 5).
S. typhimurium is also known to redirect exocytic vesicles from the Golgi complex toward the Salmonella-containing vacuole [463]. This process is dependent on effectors encoded by the Salmonella pathogenicity island 2 (SPI-2). Similar to Chlamydia, the Salmonella vacuole is also in close proximity to the Golgi complex. In contrast to Chlamydia, however, Salmonella replication is affected by Brefeldin A [464]. Legionella pneumophilla also intercepts vesicular traffic, in this case from the endoplasmic reticulum exit sites, and the pathogen-containing vacuole becomes surrounded by these vesicles [465]. These vesicles attach and fuse with the Legionella-containing vacuole, allowing the acquisition of ER-derived proteins. Similar to Legionella, Brucella abortus interacts with the ER exit sites and its replication is inhibited by blockage of Sar1 activity, which disrupts the ER exit sites [466]. In the cases of Salmonella, Legionella and Brucella, it remains to be established whether, as a result of these specific interceptions of exocytic vesicles, the pathogens acquire lipids.
8.5. Lipid acquisition via novel translocation mechanisms across vacuolar membranes
Pathogen-containing vacuoles are often found in close proximity to other organelles (see also Fig. 3, Fig. 5) such as the Golgi complex (Chlamydia [458], Salmonella [464]), the ER (Brucella [466], Legionella [465], T. gondii [467], and Plasmodium [468]), mitochondria (T. gondii [467]), lipid droplets (Chlamydia [424], P. falciparum [427], Trypanosoma cruzi [421]), multivesicular bodies (Chlamydia [462]) and possibly recycling endosomes (Mycobacterium, [205]). When the vacuole comes in close contact with another organelle, the two membranes may form local contact sites as an efficient means to transfer lipids from one membrane to the other [469], [470].
Alternative models for lipid delivery to the pathogen have been suggested for cholesterol acquisition in the vacuole of T. gondii, as studied in an elegant series of experiments by Coppens and coworkers. The intracellular parasite T. gondii acquires cholesterol from the host. T. gondii scavenges cholesterol from LDL that is taken up by the host cell (Fig. 3) and does not utilize cholesterol synthesized de novo by the host cell. During Toxoplasma infection, cholesterol from LDL travels from the endosome to the parasitophorous vacuole and is taken up by the parasite in a microtubule dependent way [448], [449]. The characteristics of cholesterol acquisition, such as temperature, energy, and microtubule dependency, supported a mechanism involving vesicular transport [449]. Recent evidence, however, allowed Coppens et al. [471] to propose an unconventional model that involves the inward budding of endosomal/lysosomal vesicles into the parasitophorous vacuole and subsequent fission of the vacuolar membrane, generating double membrane-layered vesicles in the vacuole.
A similar mechanism may apply to the appearance of lipid droplets in the Chlamydia vacuole [425]. Lipid droplets were found docked at the surface of the vacuole and can translocate from the host cell cytoplasm into the vacuolar lumen [425]. How entire organelles such as lipid droplets or vesicles derived from multivesicular bodies are translocated across the vacuolar membrane remains to be determined. Under physiological conditions, inward budding (i.e. into the lumen of an organelle) is so far only observed for multivesicular bodies. The ESCRT machinery provides the driving force for this process [472]. Pathogens such as HIV hijack this machinery to bud from the plasma membrane. It remains to be established whether the inward budding into the parasitophorous vacuole also requires elements of the ESCRT machinery, or whether specific virulence effectors, secreted by the pathogens, are sufficient for this process. Clearly, the uptake of lipid droplets and vesicles from multivesicular bodies is dependent on pathogen-secreted factors [425], [471]).
9. Conclusions and future perspectives
Lipids from host cells as well as from pathogens play important roles in the ability of pathogens to escape from the immune system. Pathogens make extensive use of the complexity of the host cell lipidome and have evolved very sophisticated mechanisms to use the diversity and complexity of the lipidome of their host to their advance. This is best illustrated by Mycobacterium tuberculosis, that uses a large portion of its coding capacity to the production of enzymes involved in lipogenesis and lipolysis [473]. Pathogens not only use lipids as building blocks or as a nutrient source, but also to influence the host cell physiology, enabling their survival and replication.
This review was written at the onset of the emergence of high-throughput lipidomic techniques. Lipidomic analysis of host–pathogen interactions by mass spectrometry will provide a great opportunity for future research and can be expected to reveal the specific roles of individual lipid species. This will greatly aid in the generation of more specific drugs against pathogens. An example of drug development that interferes with lipid metabolism is the statins. Statins have been reported to inhibit the replication of various pathogens in host cells, including viruses [29], [474], [475], [476], [477], [478], [479], [480], [481], [482], [483], bacteria [484], [485], [486], [487], [488], [489] and parasites [490], [491], [492], [493]. Clinical studies have been carried out concerning the use of statins for the management of septic patients [494]. Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase and reduce the biosynthesis of cholesterol, but also that of farnesylpyrophosphate and geranylgeranylpyrophosphate. The latter two compounds serve as lipid attachments for a post-translational modification, termed prenylation, of a great number of regulatory proteins in eukaryotes, among which small GTP-binding proteins. These GTP-binding proteins have crucial roles in intracellular inflammatory signaling [495]. The inhibition by statins of the prenylation of regulatory proteins in the eukaryotic cells is in most cases thought to cause the suppressing effect of statins on the replication of the microorganisms. In the case of the suppression of Salmonella replication in host cells by statins [485], the inhibition of host cell prenylation of the bacterial effector protein SifA [496] may also play a role.
Another opportunity of lipidomic analysis is lipid profiling of host–pathogen interactions. Lipid profiling may become an important indicator for the metabolic condition of a cell or organism. The specific depletion, presence or modulation of certain lipid species may generate lipid fingerprints unique to infection by specific pathogens, allowing the identification of biomarkers.
The study on host–pathogen interactions is a two-edged sword: On the one hand, these studies advance our molecular understanding of normal cellular processes, as they reveal novel intracellular signaling and trafficking pathways/mechanisms. One such example is the surprising capacity of lipid droplet organelles to pass biological membranes. Many novel and unanticipated findings are expected to emerge from these studies, in combination with novel methods of lipidomic analysis. On the other hand, these studies generate knowledge about pathogen-mediated changes in host cell metabolism. In this respect it is revealing that most pathogens studied so far do not play a single trick with the host cell but simultaneously induce several changes in the host cell signaling and trafficking mechanisms, both at the level of the proteome and of the lipidome. In this review, we have described several pathogens, such as T. gondii (Fig. 3), Mycobacteria (Fig. 4), Salmonella and Chlamydia (Fig. 5), which appeared in multiple sections, illustrating that they take full advantage of the complexity of the host cell lipidome.
This may have important implications for drug development. The goal to develop one drug for one pathogen in order to control host cell infection may be too simplistic. For most pathogens, a multidrug approach that aims at several pathogen-derived effector molecules or their host cell targets may be more effective. Moreover, the pathogens described in this review change the host cell lipidome in a unique way, characteristic for that particular pathogen. Thus lipidomic techniques will soon provide us with a detailed view on changes of the host cell lipidome in response to pathogen infection. Although lipidomic methods are technically more challenging than proteomic techniques, they may soon catch up and bypass proteomic analyses. Most lipids exist across species, allowing the application of lipidomic analyses to all species, independently of the knowledge of the genomic organization. In the field of host–pathogen interactions, with many pathogen genomes still unknown, this is a great advantage. Thus, lipidomic analysis of host–pathogens can be expected to contribute significantly to the fight against infectious diseases.
Acknowledgements
The authors are supported by Grants from The Netherlands Organisation for Scientific Research (NWO-ALW 817.02.023), The Dutch Technology Foundation (STW UDG.6488), and the European Union (Framework Program 7, Grant agreement no. 202272). They would like to thank Dr. Dora Kaloyanova for critical reading of the manuscript.
References
- 1.Fahy E., Subramaniam S., Brown H.A., Glass C.K., Merrill A.H., Murphy R.C. A comprehensive classification system for lipids. J Lipid Res. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Benveniste J. Platelet-activating factor, a new mediator of anaphylaxis and immune complex deposition from rabbit and human basophils. Nature. 1974;249:581–582. doi: 10.1038/249581a0. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M.J., Irvine R.F. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 4.Hannun Y.A., Bell R.M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- 5.Moolenaar W.H., Kruijer W., Tilly B.C., Verlaan I., Bierman A.J., de Laat S.W. Growth factor-like action of phosphatidic acid. Nature. 1986;323:171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- 6.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 7.Bell R.M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986;45:631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- 8.Bankaitis V.A., Aitken J.R., Cleves A.E., Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- 9.Haucke V., Di Paolo G. Lipids and lipid modifications in the regulation of membrane traffic. Curr Opin Cell Biol. 2007;19:426–435. doi: 10.1016/j.ceb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons K., Vaz W.L. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 11.King C.A., Van Heyningen W.E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973;127:639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- 12.Van Meer G. Cellular lipidomics. EMBO J. 2005;24:3159–3165. doi: 10.1038/sj.emboj.7600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenk M.R. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 14.Dennis E.A. Lipidomics joins the omics evolution. Proc Natl Acad Sci USA. 2009;106:2089–2090. doi: 10.1073/pnas.0812636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms J.B. Host–pathogen interactions: lipids grease the way. Eur J Lipid Sci Technol. 2006;108:895–897. [Google Scholar]
- 16.Cabantchik Z.I. Properties of permeation pathways induced in the human red cell membrane by malaria parasites. Blood Cells. 1990;16:421–432. [PubMed] [Google Scholar]
- 17.Simões A.P., Roelofsen B., Op den Kamp J.A.F. Lipid compartmentalization in erythrocytes parasitized by Plasmodium spp. Parasitol Today. 1992;8:18–21. doi: 10.1016/0169-4758(92)90305-l. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao L.L., Howard R.J., Aikawa M., Taraschi T.F. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite Plasmodium falciparum. Biochem J. 1991;274:121–132. doi: 10.1042/bj2740121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vial H.J., Ancelin M.L., Philippot J.R., Thuet M.J. Biosynthesis and dynamics of lipids in Plasmodium-infected mature mammalian erythrocytes. Blood Cells. 1990;16:531–555. [PubMed] [Google Scholar]
- 20.Simões A.P., Moll G.N., Beaumelle B., Vial H.J., Roelofsen B., Op den Kamp J.A.F. Plasmodium knowlesi induces alterations in phosphatidylcholine and phosphatidylethanolamine molecular species composition of parasitized monkey erythrocytes. Biochim Biophys Acta. 1990;1022:135–145. doi: 10.1016/0005-2736(90)90107-y. [DOI] [PubMed] [Google Scholar]
- 21.Wunderlich F., Fiebig S., Vial H., Kleinig H. Distinct lipid compositions of parasite and host cell plasma membranes from Plasmodium chabaudi-infected erythrocytes. Mol Biochem Parasitol. 1991;44:271–277. doi: 10.1016/0166-6851(91)90013-v. [DOI] [PubMed] [Google Scholar]
- 22.Simões A.P., Fiebig S., Wunderlich F., Vial H., Roelofsen B., Op den Kamp J.A.F. Plasmodium chabaudi-parasitized erythrocytes: phosphatidylcholine species of parasites and host cell membranes. Mol Biochem Parasitol. 1993;57:345–348. doi: 10.1016/0166-6851(93)90211-f. [DOI] [PubMed] [Google Scholar]
- 23.Jackson S.K., Stark J.M., Taylor S., Harwood J.L. Changes in phospholipid fatty acid composition and triacylglycerol content in mouse tissues after infection with bacille Calmette-Guérin. Br J Exp Pathol. 1989;70:435–441. [PMC free article] [PubMed] [Google Scholar]
- 24.Klein A., Mercure L., Gordon P., Bruser B., Ramcharitar S., Malkin A. The effect of HIV-1 infection on the lipid fatty acid content in the membrane of cultured lymphocytes. AIDS. 1990;4:865–867. doi: 10.1097/00002030-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Borthakur A., Gill R.K., Hodges K., Ramaswamy K., Hecht G., Dudeja P.K. Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol. 2006;290:G30–G35. doi: 10.1152/ajpgi.00302.2005. [DOI] [PubMed] [Google Scholar]
- 26.Agaisse H., Burrack L.S., Philips J.A., Rubin E.J., Perrimon N., Higgins D.E. Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science. 2005;309:1248–1251. doi: 10.1126/science.1116008. [DOI] [PubMed] [Google Scholar]
- 27.Philips J.A., Rubin E.J., Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 28.Su A.I., Pezacki J.P., Wodicka L., Brideau A.D., Supekova L., Thimme R. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci USA. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapadia S.B., Chisari F.V. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitkiewicz I., Stockbauer K.E., Musser J.M. Secreted bacterial phospholipase A2 enzymes: better living through phospholipolysis. Trends Microbiol. 2007;15:63–69. doi: 10.1016/j.tim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Heffernan B.J., Thomason B., Herring-Palmer A., Shaughnessy L., McDonald R., Fisher N. Bacillus anthracis phospholipases C facilitate macrophage-associated growth and contribute to virulence in a murine model of inhalation anthrax. Infect Immun. 2006;74:3756–3764. doi: 10.1128/IAI.00307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mumy K.L., Bien J.D., Pazos M.A., Gronert K., Hurley B.P., McCormick B.A. Distinct isoforms of phospholipase A2 mediate the ability of Salmonella enterica serotype typhimurium and Shigella flexneri to induce the transepithelial migration of neutrophils. Infect Immun. 2008;76:3614–3627. doi: 10.1128/IAI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato H., Frank D.W., Hillard C.J., Feix J.B., Pankhaniya R.R., Moriyama K. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips R.M., Six D.A., Dennis E.A., Ghosh P. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J Biol Chem. 2003;278:41326–41332. doi: 10.1074/jbc.M302472200. [DOI] [PubMed] [Google Scholar]
- 35.Sato H., Frank D.W. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 36.Sato H., Feix J.B., Frank D.W. Identification of superoxide dismutase as a cofactor for the Pseudomonas type III toxin, ExoU. Biochemistry. 2006;45:10368–10375. doi: 10.1021/bi060788j. [DOI] [PubMed] [Google Scholar]
- 37.Tamura M., Ajayi T., Allmond L.R., Moriyama K., Wiener-Kronish J.P., Sawa T. Lysophospholipase A activity of Pseudomonas aeruginosa type III secretory toxin ExoU. Biochem Biophys Res Commun. 2004;316:323–331. doi: 10.1016/j.bbrc.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 38.Saliba A.M., Nascimento D.O., Silva M.C.A., Assis M.C., Gayer C.R.M., Raymond B. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell Microbiol. 2005;7:1811–1822. doi: 10.1111/j.1462-5822.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 39.Plotkowski M.C., Brandão B.A., de Assis M.C., Feliciano L.F.P., Raymond B., Freitas C. Lipid body mobilization in the ExoU-induced release of inflammatory mediators by airway epithelial cells. Microb Pathog. 2008;45:30–37. doi: 10.1016/j.micpath.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Lossi N.S., Rolhion N., Magee A.I., Boyle C., Holden D.W. The Salmonella SPI-2 effector SseJ exhibits eukaryotic activator-dependent phospholipase A and glycerophospholipid: cholesterol acyltransferase activity. Microbiology. 2008;154:2680–2688. doi: 10.1099/mic.0.2008/019075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nawabi P., Catron D.M., Haldar K. Esterification of cholesterol by a type III secretion effector during intracellular Salmonella infection. Mol Microbiol. 2008;68:173–185. doi: 10.1111/j.1365-2958.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Albert J., Yu X.J., Beuzón C.R., Blakey A.N., Galyov E.E., Holden D.W. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol. 2002;44:645–661. doi: 10.1046/j.1365-2958.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 43.Grant K.A., Belandia I.U., Dekker N., Richardson P.T., Park S.F. Molecular characterization of pldA, the structural gene for a phospholipase A from Campylobacter coli, and its contribution to cell-associated hemolysis. Infect Immun. 1997;65:1172–1180. doi: 10.1128/iai.65.4.1172-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinoda S., Matsuoka H., Tsuchie T., Miyoshi S.I., Yamamoto S., Taniguchi H. Purification and characterization of a lecithin-dependent haemolysin from Escherichia coli transformed by a Vibrio parahaemolyticus gene. J Gen Microbiol. 1991;137:2705–2711. doi: 10.1099/00221287-137-12-2705. [DOI] [PubMed] [Google Scholar]
- 45.Lee J.H., Ahn S.H., Kim S.H., Choi Y.H., Park K.J., Kong I.S. Characterization of Vibrio mimicus phospholipase A (PhlA) and cytotoxicity on fish cell. Biochem Biophys Res Commun. 2002;298:269–276. doi: 10.1016/s0006-291x(02)02434-8. [DOI] [PubMed] [Google Scholar]
- 46.Koo B.S., Lee J.H., Kim S.C., Yoon H.Y., Kim K.A., Kwon K.B. Phospholipase A as a potent virulence factor of Vibrio vulnificus. Int J Mol Med. 2007;20:913–918. [PubMed] [Google Scholar]
- 47.Flieger A., Rydzewski K., Banerji S., Broich M., Heuner K. Cloning and characterization of the gene encoding the major cell-associated phospholipase A of Legionella pneumophila, plaB, exhibiting hemolytic activity. Infect Immun. 2004;72:2648–2658. doi: 10.1128/IAI.72.5.2648-2658.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasil M.L., Berka R.M., Gray G.L., Nakai H. Cloning of a phosphate-regulated hemolysin gene (phospholipase C) from Pseudomonas aeruginosa. J Bacteriol. 1982;152:431–440. doi: 10.1128/jb.152.1.431-440.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostroff R.M., Vasil A.I., Vasil M.L. Molecular comparison of a nonhemolytic and a hemolytic phospholipase C from Pseudomonas aeruginosa. J Bacteriol. 1990;172:5915–5923. doi: 10.1128/jb.172.10.5915-5923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossignol G., Merieau A., Guerillon J., Veron W., Lesouhaitier O., Feuilloley M.G.J. Involvement of a phospholipase C in the hemolytic activity of a clinical strain of Pseudomonas fluorescens. BMC Microbiol. 2008;8:189. doi: 10.1186/1471-2180-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez A., Mve-Obiang A., Vray B., Rudnicka W., Shamputa I.C., Portaels F. Detection of phospholipase C in nontuberculous mycobacteria and its possible role in hemolytic activity. J Clin Microbiol. 2001;39:1396–1401. doi: 10.1128/JCM.39.4.1396-1401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flieger A., Gong S., Faigle M., Mayer H.A., Kehrer U., Mußotter J. Phospholipase A secreted by Legionella pneumophila destroys alveolar surfactant phospholipids. FEMS Microbiol Lett. 2000;188:129–133. doi: 10.1111/j.1574-6968.2000.tb09183.x. [DOI] [PubMed] [Google Scholar]
- 53.Berstad A.E., Berstad K., Berstad A. PH-activated phospholipase A2: an important mucosal barrier breaker in peptic ulcer disease. Scand J Gastroenterol. 2002;37:738–742. doi: 10.1080/00365520212493. [DOI] [PubMed] [Google Scholar]
- 54.Dorrell N., Martino M.C., Stabler R.A., Ward S.J., Zhang Z.W., McColm A.A. Characterization of Helicobacter pylori PldA, a phospholipase with a role in colonization of the gastric mucosa. Gastroenterology. 1999;117:1098–1104. doi: 10.1016/s0016-5085(99)70394-x. [DOI] [PubMed] [Google Scholar]
- 55.Slomiany B.L., Kasinathan C., Slomiany A. Lipolytic activity of Campylobacter pylori: effect of colloidal bismuth subcitrate (De-Nol) Am J Gastroenterol. 1989;84:1273–1277. [PubMed] [Google Scholar]
- 56.Bhanot P., Schauer K., Coppens I., Nussenzweig V. A surface phospholipase is involved in the migration of Plasmodium sporozoites through cells. J Biol Chem. 2005;280:6752–6760. doi: 10.1074/jbc.M411465200. [DOI] [PubMed] [Google Scholar]
- 57.Silverman D.J., Santucci L.A., Meyers N., Sekeyova Z. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect Immun. 1992;60:2733–2740. doi: 10.1128/iai.60.7.2733-2740.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker D.H., Feng H.M., Popov V.L. Rickettsial phospholipase A2 as a pathogenic mechanism in a model of cell injury by typhus and spotted fever group rickettsiae. Am J Trop Med Hyg. 2001;65:936–942. doi: 10.4269/ajtmh.2001.65.936. [DOI] [PubMed] [Google Scholar]
- 59.Pollok R.C.G., McDonald V., Kelly P., Farthing M.J.G. The role of Cryptosporidium parvum-derived phospholipase in intestinal epithelial cell invasion. Parasitol Res. 2003;90:181–186. doi: 10.1007/s00436-003-0831-8. [DOI] [PubMed] [Google Scholar]
- 60.Saffer L.D., Long Krug S.A., Schwartzman J.D. The role of phospholipase in host cell penetration by Toxoplasma gondii. Am J Trop Med Hyg. 1989;40:145–149. doi: 10.4269/ajtmh.1989.40.145. [DOI] [PubMed] [Google Scholar]
- 61.Cassaing S., Fauvel J., Bessières M.H., Guy S., Séguéla J.P., Chap H. Toxoplasma gondii secretes a calcium-independent phospholipase A2. Int J Parasitol. 2000;30:1137–1142. doi: 10.1016/s0020-7519(00)00101-6. [DOI] [PubMed] [Google Scholar]
- 62.Ganendren R., Carter E., Sorrell T., Widmer F., Wright L. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 2006;8:1006–1015. doi: 10.1016/j.micinf.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 63.Goebel W., Kuhn M. Bacterial replication in the host cell cytosol. Curr Opin Microbiol. 2000;3:49–53. doi: 10.1016/s1369-5274(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 64.Hybiske K., Stephens R.S. Exit strategies of intracellular pathogens. Nat Rev Microbiol. 2008;6:99–110. doi: 10.1038/nrmicro1821. [DOI] [PubMed] [Google Scholar]
- 65.O’Brien D.K., Melville S.B. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. Perfringens in host tissues. Infect Immun. 2004;72:5204–5215. doi: 10.1128/IAI.72.9.5204-5215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Camilli A., Tilney L.G., Portnoy D.A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith G.A., Marquis H., Jones S., Johnston N.C., Portnoy D.A., Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldfine H., Knob C., Alford D., Bentz J. Membrane permeabilization by Listeria monocytogenes phosphatidylinositol-specific phospholipase C is independent of phospholipid hydrolysis and cooperative with listeriolysin O. Proc Natl Acad Sci USA. 1995;92:2979–2983. doi: 10.1073/pnas.92.7.2979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Vazquez-Boland J.A., Kocks C., Dramsi S., Ohayon H., Geoffroy C., Mengaud J. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gedde M.M., Higgins D.E., Tilney L.G., Portnoy D.A. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alberti-Segui C., Goeden K.R., Higgins D.E. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell Microbiol. 2007;9:179–195. doi: 10.1111/j.1462-5822.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 72.Heffernan B.J., Thomason B., Herring-Palmer A., Hanna P. Bacillus anthracis anthrolysin O and three phospholipases C are functionally redundant in a murine model of inhalation anthrax. FEMS Microbiol Lett. 2007;271:98–105. doi: 10.1111/j.1574-6968.2007.00713.x. [DOI] [PubMed] [Google Scholar]
- 73.Winkler H.H., Miller E.T. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells) Infect Immun. 1982;38:109–113. doi: 10.1128/iai.38.1.109-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Renesto P., Dehoux P., Gouin E., Touqui L., Cossart P., Raoult D. Identification and characterization of a phospholipase D-superfamily gene in rickettsiae. J Infect Dis. 2003;188:1276–1283. doi: 10.1086/379080. [DOI] [PubMed] [Google Scholar]
- 75.Whitworth T., Popov V.L., Yu X.J., Walker D.H., Bouyer D.H. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar Typhimurium mediates phagosomal escape. Infect Immun. 2005;73:6668–6673. doi: 10.1128/IAI.73.10.6668-6673.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farr G.A., Zhang L.G., Tattersall P. Parvoviral virions deploy a capsid-tethered lipolytic enzyme to breach the endosomal membrane during cell entry. Proc Natl Acad Sci USA. 2005;102:17148–17153. doi: 10.1073/pnas.0508477102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mullen T., Markey K., Murphy P., McClean S., Callaghan M. Role of lipase in Burkholderia cepacia complex (Bcc) invasion of lung epithelial cells. Eur J Clin Microbiol Infect Dis. 2007;26:869–877. doi: 10.1007/s10096-007-0385-2. [DOI] [PubMed] [Google Scholar]
- 78.König B., Jaeger K.E., König W. Induction of inflammatory mediator release (12-hydroxyeicosatetraenoic acid) from human platelets by Pseudomonas aeruginosa. Int Arch Allergy Immunol. 1994;104:33–41. doi: 10.1159/000236706. [DOI] [PubMed] [Google Scholar]
- 79.König B., Jaeger K.E., Sage A.E., Vasil M.L., König W. Role of Pseudomonas aeruginosa lipase in inflammatory mediator release from human inflammatory effector cells (platelets, granulocytes, and monocytes. Infect Immun. 1996;64:3252–3258. doi: 10.1128/iai.64.8.3252-3258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rollof J., Braconier J.H., Söderström C., Nilsson-Ehle P. Interference of Staphylococcus aureus lipase with human granulocyte function. Eur J Clin Microbiol Infect Dis. 1988;7:505–510. doi: 10.1007/BF01962601. [DOI] [PubMed] [Google Scholar]
- 81.Gácser A., Trofa D., Schäfer W., Nosanchuk J.D. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest. 2007;117:3049–3058. doi: 10.1172/JCI32294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akoh C.C., Lee G.C., Liaw Y.C., Huang T.H., Shaw J.F. GDSL family of serine esterases/lipases. Prog Lipid Res. 2004;43:534–552. doi: 10.1016/j.plipres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Upton C., Buckley J.T. A new family of lipolytic enzymes? Trends Biochem Sci. 1995;20:178–179. doi: 10.1016/s0968-0004(00)89002-7. [DOI] [PubMed] [Google Scholar]
- 84.MacIntyre S., Buckley J.T. Presence of glycerophospholipid: cholesterol acyltransferase and phospholipase in culture supernatant of Aeromonas hydrophila. J Bacteriol. 1978;135:402–407. doi: 10.1128/jb.135.2.402-407.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacIntyre S., Trust T.J., Buckley J.T. Distribution of lycerophospholipid-cholesterol acyltransferase in selected bacterial species. J Bacteriol. 1979;139:132–136. doi: 10.1128/jb.139.1.132-136.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buckley J.T. Substrate specificity of bacterial glycerophospholipid: cholesterol acyltransferase. Biochemistry. 1982;21:6699–6703. doi: 10.1021/bi00269a013. [DOI] [PubMed] [Google Scholar]
- 87.Buckley J.T., Halasa L.N., MacIntyre S. Purification and partial characterization of a bacterial phospholipid: cholesterol acyltransferase. J Biol Chem. 1982;257:3320–3325. [PubMed] [Google Scholar]
- 88.Lee K.K., Ellis A.E. Glycerophospholipid:cholesterol acyltransferase complexed with lipopolysaccharide (LPS) is a major lethal exotoxin and cytolysin of Aeromonas salmonicida: LPS stabilizes and enhances toxicity of the enzyme. J Bacteriol. 1990;172:5382–5393. doi: 10.1128/jb.172.9.5382-5393.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eggset G., Bjørnsdottir R., McQueen Leifson R., Arnesen J.A., Coucheron D.H., Jørgensen T.Ø. Extracellular glycerophospholipid:cholesterol acyltransferase from Aeromonas salmonicida: activation by serine protease. J Fish Dis. 1994;17:17–29. [Google Scholar]
- 90.Whitby P.W., Landon M., Coleman G. The cloning and nucleotide sequence of the serine protease gene (aspA) of Aeromonas salmonicida ssp. Salmonicida. FEMS Microbiol Lett. 1992;99:65–71. doi: 10.1016/0378-1097(92)90289-z. [DOI] [PubMed] [Google Scholar]
- 91.Vipond R., Bricknell I.R., Durant E., Bowden T.J., Ellis A.E., Smith M. Defined deletion mutants demonstrate that the major secreted toxins are not essential for the virulence of Aeromonas salmonicida. Infect Immun. 1998;66:1990–1998. doi: 10.1128/iai.66.5.1990-1998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banerji S., Bewersdorff M., Hermes B., Cianciotto N.P., Flieger A. Characterization of the major secreted zinc metalloprotease-dependent glycerophospholipid:cholesterol acyltransferase, PlaC, of Legionella pneumophila. Infect Immun. 2005;73:2899–2909. doi: 10.1128/IAI.73.5.2899-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ohlson M.B., Fluhr K., Birmingham C.L., Brumell J.H., Miller S.I. SseJ deacylase activity by Salmonella enterica serovar Typhimurium promotes virulence in mice. Infect Immun. 2005;73:6249–6259. doi: 10.1128/IAI.73.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miao E.A., Miller S.I. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc Natl Acad Sci USA. 2000;97:7539–7544. doi: 10.1073/pnas.97.13.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Birmingham C.L., Jiang X., Ohlson M.B., Miller S.I., Brumell J.H. Salmonella-induced filament formation is a dynamic phenotype induced by rapidly replicating Salmonella enterica serovar typhimurium in epithelial cells. Infect Immun. 2005;73:1204–1208. doi: 10.1128/IAI.73.2.1204-1208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boucrot E., Henry T., Borg J.P., Gorvel J.P., Méresse S. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 2005;308:1174–1178. doi: 10.1126/science.1110225. [DOI] [PubMed] [Google Scholar]
- 97.Dye E.S., Kapral F.A. Characterization of a bactericidal lipid developing within staphylococcal abscesses. Infect Immun. 1981;32:98–104. doi: 10.1128/iai.32.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mortensen J.E., Shryock T.R., Kapral F.A. Modification of bactericidal fatty acids by an enzyme of Staphylococcus aureus. J Med Microbiol. 1992;36:293–298. doi: 10.1099/00222615-36-4-293. [DOI] [PubMed] [Google Scholar]
- 99.Nishikawa Y., Quittnat F., Stedman T.T., Voelker D.R., Choi J.Y., Zahn M. Host cell lipids control cholesteryl ester synthesis and storage in intracellular Toxoplasma. Cell Microbiol. 2005;7:849–867. doi: 10.1111/j.1462-5822.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 100.Sonda S., Ting L.M., Novak S., Kim K., Maher J.J., Farese R.V., Jr. Cholesterol esterification by host and parasite is essential for optimal proliferation of Toxoplasma gondii. J Biol Chem. 2001;276:34434–34440. doi: 10.1074/jbc.M105025200. [DOI] [PubMed] [Google Scholar]
- 101.Lauer S., VanWye J., Harrison T., McManus H., Samuel B.U., Hiller N.L. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 2000;19:3556–3564. doi: 10.1093/emboj/19.14.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nawabi P., Lykidis A., Ji D., Haldar K. Neutral-lipid analysis reveals elevation of acylglycerols and lack of cholesterol esters in Plasmodium falciparum-infected erythrocytes. Eukaryot Cell. 2003;2:1128–1131. doi: 10.1128/EC.2.5.1128-1131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 104.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 105.Lemmon M.A. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 106.De Matteis M.A., Godi A. PI-loting membrane traffic. Nat Cell Biol. 2004;6:487–492. doi: 10.1038/ncb0604-487. [DOI] [PubMed] [Google Scholar]
- 107.Yeung T., Ozdamar B., Paroutis P., Grinstein S. Lipid metabolism and dynamics during phagocytosis. Curr Opin Cell Biol. 2006;18:429–437. doi: 10.1016/j.ceb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Anes E., Kühnel M.P., Bos E., Moniz-Pereira J., Habermann A., Griffiths G. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol. 2003;5:793–802. doi: 10.1038/ncb1036. [DOI] [PubMed] [Google Scholar]
- 109.Botelho R.J., Teruel M., Dierckman R., Anderson R., Wells A., York J.D. Localized biphasic changes in phosphatidylinositol-4, 5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151:1353–1368. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dewitt S., Tian W., Hallett M.B. Localised PtdIns(3, 4, 5)P3 or PtdIns(3, 4)P2 at the phagocytic cup is required for both phagosome closure and Ca2+ signalling in HL60 neutrophils. J Cell Sci. 2006;119:443–451. doi: 10.1242/jcs.02756. [DOI] [PubMed] [Google Scholar]
- 111.Scott C.C., Dobson W., Botelho R.J., Coady-Osberg N., Chavrier P., Knecht D.A. Phosphatidylinositol-4, 5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol. 2005;169:139–149. doi: 10.1083/jcb.200412162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chua J., Deretic V. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J Biol Chem. 2004;279:36982–36992. doi: 10.1074/jbc.M405082200. [DOI] [PubMed] [Google Scholar]
- 113.Ikonomov O.C., Sbrissa D., Foti M., Carpentier J.L., Shisheva A. PIKfyve controls fluid phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell. 2003;14:4581–4591. doi: 10.1091/mbc.E03-04-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pizarro-Cerdá J., Cossart P. Subversion of phosphoinositide metabolism by intracellular bacterial pathogens. Nat Cell Biol. 2004;6:1026–1033. doi: 10.1038/ncb1104-1026. [DOI] [PubMed] [Google Scholar]
- 115.Steinberg B.E., Grinstein S. Pathogen destruction versus intracellular survival: the role of lipids as phagosomal fate determinants. J Clin Invest. 2008;118:2002–2011. doi: 10.1172/JCI35433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ireton K., Payrastre B., Cossart P. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J Biol Chem. 1999;274:17025–17032. doi: 10.1074/jbc.274.24.17025. [DOI] [PubMed] [Google Scholar]
- 117.Pizarro-Cerdá J., Payrastre B., Wang Y.J., Veiga E., Yin H.L., Cossart P. Type II phosphatidylinositol 4-kinases promote Listeria monocytogenes entry into target cells. Cell Microbiol. 2007;9:2381–2390. doi: 10.1111/j.1462-5822.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 118.Wong K.W., Isberg R.R. Arf6 and phosphoinositol-4-phosphate-5-kinase activities permit bypass of the Rac1 requirement for β1 integrin-mediated bacterial uptake. J Exp Med. 2003;198:603–614. doi: 10.1084/jem.20021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kierbel A., Gassama-Diagne A., Mostov K., Engel J.N. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol Biol Cell. 2005;16:2577–2585. doi: 10.1091/mbc.E04-08-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gassama-Diagne A., Yu W., ter Beest M., Martin-Belmonte F., Kierbel A., Engel J. Phosphatidylinositol-3, 4, 5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol. 2006;8:963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- 121.Kierbel A., Gassama-Diagne A., Rocha C., Radoshevich L., Olson J., Mostov K. Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane. J Cell Biol. 2007;177:21–27. doi: 10.1083/jcb.200605142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou D., Chen L.M., Hernandez L., Shears S.B., Galán J.E. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol. 2001;39:248–259. doi: 10.1046/j.1365-2958.2001.02230.x. [DOI] [PubMed] [Google Scholar]
- 123.Terebiznik M.R., Vieira O.V., Marcus S.L., Slade A., Yip C.M., Trimble W.S. Elimination of host cell PtdIns(4, 5)P2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4:766–773. doi: 10.1038/ncb854. [DOI] [PubMed] [Google Scholar]
- 124.Mason D., Mallo G.V., Terebiznik M.R., Payrastre B., Finlay B.B., Brumell J.H. Alteration of epithelial structure and function associated with PtdIns(4, 5)P2 degradation by a bacterial phosphatase. J Gen Physiol. 2007;129:267–283. doi: 10.1085/jgp.200609656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mallo G.V., Espina M., Smith A.C., Terebiznik M.R., Alemán A., Finlay B.B. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol. 2008;182:741–752. doi: 10.1083/jcb.200804131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sason H., Milgrom M., Weiss A.M., Melamed-Book N., Balla T., Grinstein S. Enteropathogenic Escherichia coli subverts phosphatidylinositol 4, 5-bisphosphate and phosphatidylinositol 3, 4, 5-trisphosphate upon epithelial cell infection. Mol Biol Cell. 2009;20:544–555. doi: 10.1091/mbc.E08-05-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hilbi H. Modulation of phosphoinositide metabolism by pathogenic bacteria. Cell Microbiol. 2006;8:1697–1706. doi: 10.1111/j.1462-5822.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 128.Patel J.C., Hueffer K., Lam T.T., Galán J.E. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell. 2009;137:283–294. doi: 10.1016/j.cell.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hernandez L.D., Hueffer K., Wenk M.R., Galán J.E. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304:1805–1807. doi: 10.1126/science.1098188. [DOI] [PubMed] [Google Scholar]
- 130.Qualmann B., Kessels M.M. Endocytosis and the cytoskeleton. Int Rev Cytol. 2002;220:93–144. doi: 10.1016/s0074-7696(02)20004-2. [DOI] [PubMed] [Google Scholar]
- 131.Niebuhr K., Giuriato S., Pedron T., Philpott D.J., Gaits F., Sable J. Conversion of PtdIns(4, 5)P2 into PtdIns(5)P by the S. flexneri effector IpgD reorganizes host cell morphology. EMBO J. 2002;21:5069–5078. doi: 10.1093/emboj/cdf522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pendaries C., Tronchère H., Arbibe L., Mounier J., Gozani O., Cantley L. PtdIns5(P) activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J. 2006;25:1024–1034. doi: 10.1038/sj.emboj.7601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Celli J., Olivier M., Finlay B.B. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 2001;20:1245–1258. doi: 10.1093/emboj/20.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quitard S., Dean P., Maresca M., Kenny B. The enteropathogenic Escherichia coli EspF effector molecule inhibits PI-3 kinase-mediated uptake independently of mitochondrial targeting. Cell Microbiol. 2006;8:972–981. doi: 10.1111/j.1462-5822.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 135.Allen L.A.H., Allgood J.A., Han X., Wittine L.M. Phosphoinositide3-kinase regulates actin polymerization during delayed phagocytosis of Helicobacter pylori. J Leukoc Biol. 2005;78:220–230. doi: 10.1189/jlb.0205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vergne I., Chua J., Lee H.H., Lucas M., Belisle J., Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fratti R.A., Backer J.M., Gruenberg J., Corvera S., Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol. 2001;154:631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Meer G., Lisman Q. Sphingolipid transport: rafts and translocators. J Biol Chem. 2002;277:25855–25858. doi: 10.1074/jbc.R200010200. [DOI] [PubMed] [Google Scholar]
- 139.Hait N.C., Oskeritzian C.A., Paugh S.W., Milstien S., Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 140.Birbes H., El Bawab S., Obeid L.M., Hannun Y.A. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 141.Spiegel S., Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 142.Malik Z.A., Thompson C.R., Hashimi S., Porter B., Iyer S.S., Kusner D.J. Cutting edge: Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J Immunol. 2003;170:2811–2815. doi: 10.4049/jimmunol.170.6.2811. [DOI] [PubMed] [Google Scholar]
- 143.Thompson C.R., Iyer S.S., Melrose N., VanOosten R., Johnson K., Pitson S.M. Sphingosine kinase 1 (SK1) is recruited to nascent phagosomes in human macrophages: inhibition of SK1 translocation by Mycobacterium tuberculosis. J Immunol. 2005;174:3551–3561. doi: 10.4049/jimmunol.174.6.3551. [DOI] [PubMed] [Google Scholar]
- 144.Monick M.M., Cameron K., Powers L.S., Butler N.S., McCoy D., Mallampalli R.K. Sphingosine kinase mediates activation of extracellular signal-related kinase and Akt by respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;30:844–852. doi: 10.1165/rcmb.2003-0424OC. [DOI] [PubMed] [Google Scholar]
- 145.Machesky N.J., Zhang G., Raghavan B., Zimmerman P., Kelly S.L., Merrill A.H. Human cytomegalovirus regulates bioactive sphingolipids. J Biol Chem. 2008;283:26148–26160. doi: 10.1074/jbc.M710181200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tamama K., Kon J., Sato K., Tomura H., Kuwabara A., Kimura T. Extracellular mechanism through the Edg family of receptors might be responsible for sphingosine-1-phosphate-induced regulation of DNA synthesis and migration of rat aortic smooth-muscle cells. Biochem J. 2001;353:139–146. [PMC free article] [PubMed] [Google Scholar]
- 147.Bu S., Kapanadze B., Hsu T., Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-β/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem. 2008;283:19593–19602. doi: 10.1074/jbc.M802417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brügger B., Glass B., Haberkant P., Leibrecht I., Wieland F.T., Kräusslich H.G. The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pruett S.T., Bushnev A., Hagedorn K., Adiga M., Haynes C.A., Sullards M.C. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J Lipid Res. 2008;49:1621–1639. doi: 10.1194/jlr.R800012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Amemiya F., Maekawa S., Itakura Y., Kanayama A., Matsui A., Takano S. Targeting lipid metabolism in the treatment of hepatitis C virus infection. J Infect Dis. 2008;197:361–370. doi: 10.1086/525287. [DOI] [PubMed] [Google Scholar]
- 151.Umehara T., Sudoh M., Yasui F., Matsuda C., Hayashi Y., Chayama K. Serine palmitoyltransferase inhibitor suppresses HCV replication in a mouse model. Biochem Biophys Res Commun. 2006;346:67–73. doi: 10.1016/j.bbrc.2006.05.085. [DOI] [PubMed] [Google Scholar]
- 152.Sakamoto H., Okamoto K., Aoki M., Kato H., Katsume A., Ohta A. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat Chem Biol. 2005;1:333–337. doi: 10.1038/nchembio742. [DOI] [PubMed] [Google Scholar]
- 153.Merrill A.H., van Echten G., Wang E., Sandhoff K. Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J Biol Chem. 1993;268:27299–27306. [PubMed] [Google Scholar]
- 154.Wang E., Norred W.P., Bacon C.W., Riley R.T., Merrill A.H., Jr Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- 155.Wang H., Jones C., Ciacci-Zanella J., Holt T., Gilchrist D.G., Dickman M.B. Fumonisins and Alternaria alternata lycopersici toxins: sphinganine analog mycotoxins induce apoptosis in monkey kidney cells. Proc Natl Acad Sci USA. 1996;93:3461–3465. doi: 10.1073/pnas.93.8.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Merrill A.H. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 157.Miyake Y., Kozutsumi Y., Nakamura S., Fujita T., Kawasaki T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem Biophys Res Commun. 1995;211:396–403. doi: 10.1006/bbrc.1995.1827. [DOI] [PubMed] [Google Scholar]
- 158.Tse S.M.L., Mason D., Botelho R.J., Chiu B., Reyland M., Hanada K. Accumulation of diacylglycerol in the Chlamydia inclusion vacuole: possible role in the inhibition of host cell apoptosis. J Biol Chem. 2005;280:25210–25215. doi: 10.1074/jbc.M501980200. [DOI] [PubMed] [Google Scholar]
- 159.Chakraborty D., Banerjee S., Sen A., Banerjee K.K., Das P., Roy S. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J Immunol. 2005;175:3214–3224. doi: 10.4049/jimmunol.175.5.3214. [DOI] [PubMed] [Google Scholar]
- 160.Rub A., Dey R., Jadhav M., Kamat R., Chakkaramakkil S., Majumdar S. Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nat Immunol. 2009;10:273–280. doi: 10.1038/ni.1705. [DOI] [PubMed] [Google Scholar]
- 161.Hanada K. Sphingolipids in infectious diseases. Jpn J Infect Dis. 2005;58:131–148. [PubMed] [Google Scholar]
- 162.Heung L.J., Luberto C., Del Poeta M. Role of sphingolipids in microbial pathogenesis. Infect Immun. 2006;74:28–39. doi: 10.1128/IAI.74.1.28-39.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wylie J.L., Hatch G.M., McClarty G. Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J Bacteriol. 1997;179:7233–7242. doi: 10.1128/jb.179.23.7233-7242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Denny P.W., Goulding D., Ferguson M.A.J., Smith D.F. Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol Microbiol. 2004;52:313–327. doi: 10.1111/j.1365-2958.2003.03975.x. [DOI] [PubMed] [Google Scholar]
- 165.Zhang K., Showalter M., Revollo J., Hsu F.F., Turk J., Beverley S.M. Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 2003;22:6016–6026. doi: 10.1093/emboj/cdg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brennan P.J., Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 167.Rosenberger C.M., Finlay B.B. Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat Rev Mol Cell Biol. 2003;4:385–396. doi: 10.1038/nrm1104. [DOI] [PubMed] [Google Scholar]
- 168.Fratti R.A., Chua J., Vergne I., Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci USA. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wenk M.R. Lipidomics of host–pathogen interactions. FEBS Lett. 2006;580:5541–5551. doi: 10.1016/j.febslet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 170.Vergne I., Fratti R.A., Hill P.J., Chua J., Belisle J., Deretic V. Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell. 2004;15:751–760. doi: 10.1091/mbc.E03-05-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lemmon M.A. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 172.Beatty W.L., Rhoades E.R., Ullrich H.J., Chatterjee D., Heuser J.E., Russell D.G. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 173.de Chastellier C., Thilo L. Pathogenic Mycobacterium avium remodels the phagosome membrane in macrophages within days after infection. Eur J Cell Biol. 2002;81:17–25. doi: 10.1078/0171-9335-00220. [DOI] [PubMed] [Google Scholar]
- 174.Gatfield J., Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288:1647–1650. doi: 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 175.Benadie Y., Deysel M., Siko D.G.R., Roberts V.V., Van Wyngaardt S., Thanyani S.T. Cholesteroid nature of free mycolic acids from M. Tuberculosis. Chem Phys Lipids. 2008;152:95–103. doi: 10.1016/j.chemphyslip.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 176.Nayak D.P., Hui E.K. The role of lipid microdomains in virus biology. Subcell Biochem. 2004;37:443–491. doi: 10.1007/978-1-4757-5806-1_14. [DOI] [PubMed] [Google Scholar]
- 177.Chazal N., Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev. 2003;67:226–237. doi: 10.1128/MMBR.67.2.226-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haynes B.F., Fleming J. St. Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 179.Mercer J., Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 180.Bennett M.W., Caulfield J.P. Specific binding of human low-density lipoprotein to the surface of schistosomula of Schistosoma mansoni and ingestion by the parasite. Am J Pathol. 1991;138:1173–1182. [PMC free article] [PubMed] [Google Scholar]
- 181.Rumjanek F.D., Campos E.G., Afonso L.C.C. Evidence for the occurrence of LDL receptors in extracts of schistosomula of Schistosoma mansoni. Mol Biochem Parasitol. 1988;28:145–152. doi: 10.1016/0166-6851(88)90062-x. [DOI] [PubMed] [Google Scholar]
- 182.Mayor S., Riezman H. Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol. 2004;5:110–120. doi: 10.1038/nrm1309. [DOI] [PubMed] [Google Scholar]
- 183.McConville M.J., Ferguson M.A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McConville M.J., Menon A.K. Recent developments in the cell biology and biochemistry of glycosylphosphatidylinositol lipids (review) Mol Membr Biol. 2000;17:1–16. doi: 10.1080/096876800294443. [DOI] [PubMed] [Google Scholar]
- 185.Tachado S.D., Gerold P., Schwarz R., Novakovic S., McConville M., Schofield L. Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma, and Leishmania: activation of protein tyrosine kinases and protein kinase C by inositolglycan and diacylglycerol moieties. Proc Natl Acad Sci USA. 1997;94:4022–4027. doi: 10.1073/pnas.94.8.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Almeida I.C., Camargo M.M., Procópio D.O., Silva L.S., Mehlert A., Travassos L.R. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 2000;19:1476–1485. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moore K.J., Labrecque S., Matlashewski G. Alteration of Leishmania donovani infection levels by selective impairment of macrophage signal transduction. J Immunol. 1993;150:4457–4465. [PubMed] [Google Scholar]
- 188.Krishnegowda G., Hajjar A.M., Zhu J., Douglass E.J., Uematsu S., Akira S. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gowda D.C. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 2007;23:596–604. doi: 10.1016/j.pt.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 190.Debierre-Grockiego F., Campos M.A., Azzouz N., Schmidt J., Bieker U., Resende M.G. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 191.Stijlemans B., Baral T.N., Guilliams M., Brys L., Korf J., Drennan M. A glycosylphosphatidylinositol-based treatment alleviates trypanosomiasis-associated immunopathology. J Immunol. 2007;179:4003–4014. doi: 10.4049/jimmunol.179.6.4003. [DOI] [PubMed] [Google Scholar]
- 192.Simons K., Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 193.Simons K., Toomre D. Lipid rafts and signal transduction. Nature Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 194.Anderson R.G.W., Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 195.Ikonen E., Jansen M. Cellular sterol trafficking and metabolism: spotlight on structure. Curr Opin Cell Biol. 2008;20:371–377. doi: 10.1016/j.ceb.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 196.Maxfield F.R., Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 197.Soccio R.E., Breslow J.L. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol. 2004;24:1150–1160. doi: 10.1161/01.ATV.0000131264.66417.d5. [DOI] [PubMed] [Google Scholar]
- 198.Cornelis G.R. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat Rev Mol Cell Biol. 2002;3:742–752. doi: 10.1038/nrm932. [DOI] [PubMed] [Google Scholar]
- 199.Fivaz M., van der Goot F.G. The tip of a molecular syringe. Trends Microbiol. 1999;7:341–343. doi: 10.1016/s0966-842x(99)01574-7. [DOI] [PubMed] [Google Scholar]
- 200.Büttner D., Bonas U. Port of entry–the type III secretion translocon. Trends Microbiol. 2002;10:186–192. doi: 10.1016/s0966-842x(02)02331-4. [DOI] [PubMed] [Google Scholar]
- 201.Hayward R.D., Cain R.J., McGhie E.J., Phillips N., Garner M.J., Koronakis V. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol Microbiol. 2005;56:590–603. doi: 10.1111/j.1365-2958.2005.04568.x. [DOI] [PubMed] [Google Scholar]
- 202.Faudry E., Vernier G., Neumann E., Forge V., Attree I. Synergistic pore formation by type III toxin translocators of Pseudomonas aeruginosa. Biochemistry. 2006;45:8117–8123. doi: 10.1021/bi060452+. [DOI] [PubMed] [Google Scholar]
- 203.Schoehn G., Di Guilmi A.M., Lemaire D., Attree I., Weissenhorn W., Dessen A. Oligomerization of type III secretion proteins PopB and PopD precedes pore formation in Pseudomonas. EMBO J. 2003;22:4957–4967. doi: 10.1093/emboj/cdg499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Catron D.M., Sylvester M.D., Lange Y., Kadekoppala M., Jones B.D., Monack D.M. The Salmonella-containing vacuole is a major site of intracellular cholesterol accumulation and recruits the GPI-anchored protein CD55. Cell Microbiol. 2002;4:315–328. doi: 10.1046/j.1462-5822.2002.00198.x. [DOI] [PubMed] [Google Scholar]
- 205.Pandey A.K., Sassetti C.M. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci USA. 2008;105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Haas A. The phagosome: compartment with a license to kill. Traffic. 2007;8:311–330. doi: 10.1111/j.1600-0854.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 207.Ridsdale A., Denis M., Gougeon P.Y., Ngsee J.K., Presley J.F., Zha X. Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins. Mol Biol Cell. 2006;17:1593–1605. doi: 10.1091/mbc.E05-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Stüven E., Porat A., Shimron F., Fass E., Kaloyanova D., Brügger B. Intra-Golgi protein transport depends on a cholesterol balance in the lipid membrane. J Biol Chem. 2003;278:53112–53122. doi: 10.1074/jbc.M300402200. [DOI] [PubMed] [Google Scholar]
- 209.Simons K., Gruenberg J. Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol. 2000;10:459–462. doi: 10.1016/s0962-8924(00)01847-x. [DOI] [PubMed] [Google Scholar]
- 210.Gruenberg J. Lipids in endocytic membrane transport and sorting. Curr Opin Cell Biol. 2003;15:382–388. doi: 10.1016/s0955-0674(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 211.Mukherjee S., Maxfield F.R. Lipid and cholesterol trafficking in NPC. Biochim Biophys Acta. 2004;1685:28–37. doi: 10.1016/j.bbalip.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 212.de Chastellier C., Thilo L. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cell Microbiol. 2006;8:242–256. doi: 10.1111/j.1462-5822.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 213.Huynh K.K., Gershenzon E., Grinstein S. Cholesterol accumulation by macrophages impairs phagosome maturation. J Biol Chem. 2008;283:35745–35755. doi: 10.1074/jbc.M806232200. [DOI] [PubMed] [Google Scholar]
- 214.Jayachandran R., Sundaramurthy V., Combaluzier B., Mueller P., Korf H., Huygen K. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell. 2007;130:37–50. doi: 10.1016/j.cell.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 215.Trimble W.S., Grinstein S. TB or not TB: calcium regulation in mycobacterial survival. Cell. 2007;130:12–14. doi: 10.1016/j.cell.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 216.Hirai Y., Haque M., Yoshida T., Yokota K., Yasuda T., Oguma K. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol. 1995;177:5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Haque M., Hirai Y., Yokota K., Oguma K. Steryl glycosides: a characteristic feature of the Helicobacter spp.? J Bacteriol. 1995;177:5334–5337. doi: 10.1128/jb.177.18.5334-5337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Testerman T.L., McGee D.J., Mobley H.L.T. Helicobacter pylori growth and urease detection in the chemically defined medium Ham’s F-12 nutrient mixture. J Clin Microbiol. 2001;39:3842–3850. doi: 10.1128/JCM.39.11.3842-3850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lin M., Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lige B., Jayabalasingham B., Zhang H., Pypaert M., Coppens I. Role of an ancestral D-bifunctional protein containing two sterol-carrier protein-2 domains in lipid uptake and trafficking in toxoplasma. Mol Biol Cell. 2009;20:658–672. doi: 10.1091/mbc.E08-05-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wunder C., Churin Y., Winau F., Warnecke D., Vieth M., Lindner B. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:10301–10308. doi: 10.1038/nm1480. [DOI] [PubMed] [Google Scholar]
- 222.Haque M., Hirai Y., Yokota K., Oguma K. Lipid profiles of Helicobacter pylori and Helicobacter mustelae grown in serum-supplemented and serum-free media. Acta Med Okayama. 1995;49:205–211. doi: 10.18926/AMO/30380. [DOI] [PubMed] [Google Scholar]
- 223.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 224.Jacobson K., Mouritsen O.G., Anderson R.G.W. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 225.Shaw A.S. Lipid rafts: now you see them, now you don’t. Nat Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- 226.Devaux P.F., Morris R. Transmembrane asymmetry and lateral domains in biological membranes. Traffic. 2004;5:241–246. doi: 10.1111/j.1600-0854.2004.0170.x. [DOI] [PubMed] [Google Scholar]
- 227.Kusumi A., Koyama-Honda I., Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5:213–230. doi: 10.1111/j.1600-0854.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 228.Munro S. Lipid rafts. Elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 229.Helms J.B., Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5:247–254. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- 230.Mukherjee S., Maxfield F.R. Membrane Domains. Annu Rev Cell Dev Biol. 2004;20:839–866. doi: 10.1146/annurev.cellbio.20.010403.095451. [DOI] [PubMed] [Google Scholar]
- 231.Lafont F., van der Goot F.G. Bacterial invasion via lipid rafts. Cell Microbiol. 2005;7:613–620. doi: 10.1111/j.1462-5822.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- 232.Rosenberger C.M., Brumell J.H., Finlay B.B. Microbial pathogenesis: lipid rafts as pathogen portals. Curr Biol. 2000;10:R823–R825. doi: 10.1016/s0960-9822(00)00788-0. [DOI] [PubMed] [Google Scholar]
- 233.Mañes S., del Real G. Martínez-AC. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 234.Pelkmans L., Helenius A. Insider information: what viruses tell us about endocytosis. Curr Opin Cell Biol. 2003;15:414–422. doi: 10.1016/s0955-0674(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 235.Riethmüller J., Riehle A., Grassmé H., Gulbins E. Membrane rafts in host–pathogen interactions. Biochim Biophys Acta. 2006;1758:2139–2147. doi: 10.1016/j.bbamem.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 236.Lin M., Rikihisa Y. Obligatory intracellular parasitism by Ehrlichia chaffeensis and Anaplasma phagocytophilum involves caveolae and glycosylphosphatidylinositol-anchored proteins. Cell Microbiol. 2003;5:809–820. doi: 10.1046/j.1462-5822.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- 237.Schneider B., Schueller C., Utermoehlen O., Haas A. Lipid microdomain-dependent macropinocytosis determines compartmentation of Afipia felis. Traffic. 2007;8:226–240. doi: 10.1111/j.1600-0854.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 238.Naroeni A., Porte F. Role of cholesterol and the ganglioside GM1 in entry and short-term survival of Brucella suis in murine macrophages. Infect Immun. 2002;70:1640–1644. doi: 10.1128/IAI.70.3.1640-1644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Watarai M., Makino S., Fujii Y., Okamoto K., Shirahata T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell Microbiol. 2002;4:341–355. doi: 10.1046/j.1462-5822.2002.00195.x. [DOI] [PubMed] [Google Scholar]
- 240.Kim S., Watarai M., Suzuki H., Makino S., Kodama T., Shirahata T. Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb Pathog. 2004;37:11–19. doi: 10.1016/j.micpath.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 241.Wooldridge K.G., Williams P.H., Ketley J.M. Host signal transduction and endocytosis of Campylobacter jejuni. Microb Pathog. 1996;21:299–305. doi: 10.1006/mpat.1996.0063. [DOI] [PubMed] [Google Scholar]
- 242.Stuart E.S., Webley W.C., Norkin L.C. Lipid rafts, caveolae, caveolin-1, and entry by Chlamydiae into host cells. Exp Cell Res. 2003;287:67–78. doi: 10.1016/s0014-4827(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 243.Jutras I., Abrami L., Dautry-Varsat A. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect Immun. 2003;71:260–266. doi: 10.1128/IAI.71.1.260-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Norkin L.C., Wolfrom S.A., Stuart E.S. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp Cell Res. 2001;266:229–238. doi: 10.1006/excr.2001.5202. [DOI] [PubMed] [Google Scholar]
- 245.Malaviya R., Gao Z., Thankavel K., van der Merwe P.A., Abraham S.N. The mast cell tumor necrosis factor α response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc Natl Acad Sci USA. 1999;96:8110–8115. doi: 10.1073/pnas.96.14.8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shin J.S., Gao Z., Abraham S.N. Bacteria-host cell interaction mediated by cellular cholesterol/glycolipid-enriched microdomains. Biosci Rep. 1999;19:421–432. doi: 10.1023/a:1020216323271. [DOI] [PubMed] [Google Scholar]
- 247.Baorto D.M., Gao Z., Malaviya R., Dustin M.L., van der Merwe A., Lublin D.M. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389:636–639. doi: 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- 248.Selvarangan R., Goluszko P., Popov V., Singhal J., Pham T., Lublin D.M. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect Immun. 2000;68:1391–1399. doi: 10.1128/iai.68.3.1391-1399.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Duncan M.J., Li G., Shin J.S., Carson J.L., Abraham S.N. Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem. 2004;279:18944–18951. doi: 10.1074/jbc.M400769200. [DOI] [PubMed] [Google Scholar]
- 250.Zobiack N., Rescher U., Laarmann S., Michgehl S., Schmidt M.A., Gerke V. Cell-surface attachment of pedestal-forming enteropathogenic E. coli induces a clustering of raft components and a recruitment of annexin 2. J Cell Sci. 2002;115:91–98. doi: 10.1242/jcs.115.1.91. [DOI] [PubMed] [Google Scholar]
- 251.Allen-Vercoe E., Waddell B., Livingstone S., Deans J., DeVinney R. Enteropathogenic Escherichia coli Tir translocation and pedestal formation requires membrane cholesterol in the absence of bundle-forming pili. Cell Microbiol. 2006;8:613–624. doi: 10.1111/j.1462-5822.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 252.Chassin C., Vimont S., Cluzeaud F., Bens M., Goujon J.M., Fernandez B. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J Am Soc Nephrol. 2008;19:2364–2374. doi: 10.1681/ASN.2007121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Peiffer I., Servin A.L., Bernet-Camard M.F. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect Immun. 1998;66:4036–4042. doi: 10.1128/iai.66.9.4036-4042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kansau I., Berger C., Hospital M., Amsellem R., Nicolas V., Servin A.L. Zipper-like internalization of Dr-positive Escherichia coli by epithelial cells is preceded by an adhesin-induced mobilization of raft-associated molecules in the initial step of adhesion. Infect Immun. 2004;72:3733–3742. doi: 10.1128/IAI.72.7.3733-3742.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tamilselvam B., Daefler S. Francisella targets cholesterol-rich host cell membrane domains for entry into macrophages. J Immunol. 2008;180:8262–8271. doi: 10.4049/jimmunol.180.12.8262. [DOI] [PubMed] [Google Scholar]
- 256.Fantini J., Garmy N., Yahi N. Prediction of glycolipid-binding domains from the amino acid sequence of lipid raft-associated proteins: application to HpaA, a protein involved in the adhesion of Helicobacter pylori to gastrointestinal cells. Biochemistry. 2006;45:10957–10962. doi: 10.1021/bi060762s. [DOI] [PubMed] [Google Scholar]
- 257.Watarai M., Derre I., Kirby J., Growney J.D., Dietrich W.F., Isberg R.R. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J Exp Med. 2001;194:1081–1096. doi: 10.1084/jem.194.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Seveau S., Bierne H., Giroux S., Prévost M.C., Cossart P. Role of lipid rafts in E-cadherin- and HGF-R/Met-mediated entry of Listeria monocytogenes into host cells. J Cell Biol. 2004;166:743–753. doi: 10.1083/jcb.200406078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Maldonado-García G., Chico-Ortiz M., Lopez-Marin L.M., Sánchez-García F.J. High-polarity Mycobacterium avium-derived lipids interact with murine macrophage lipid rafts. Scand J Immunol. 2004;60:463–470. doi: 10.1111/j.0300-9475.2004.01511.x. [DOI] [PubMed] [Google Scholar]
- 260.Peyron P., Bordier C., N’Diaye E.N., Maridonneau-Parini I. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J Immunol. 2000;165:5186–5191. doi: 10.4049/jimmunol.165.9.5186. [DOI] [PubMed] [Google Scholar]
- 261.Kaul D., Anand P.K., Verma I. Cholesterol-sensor initiates M. tuberculosis entry into human macrophages. Mol Cell Biochem. 2004;258:219–222. doi: 10.1023/b:mcbi.0000012851.42642.be. [DOI] [PubMed] [Google Scholar]
- 262.Yavlovich A., Katzenell A., Tarshis M., Higazi A.A.R., Rottem S. Mycoplasma fermentans binds to and invades HeLa cells: involvement of plasminogen and urokinase. Infect Immun. 2004;72:5004–5011. doi: 10.1128/IAI.72.9.5004-5011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang M., Hajishengallis G. Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell Microbiol. 2008;10:2029–2042. doi: 10.1111/j.1462-5822.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tsuda K., Furuta N., Inaba H., Kawai S., Hanada K., Yoshimori T. Functional analysis of α5β1 integrin and lipid rafts in invasion of epithelial cells by Porphyromonas gingivalis using fluorescent beads coated with bacterial membrane vesicles. Cell Struct Funct. 2008;33:123–132. doi: 10.1247/csf.08012. [DOI] [PubMed] [Google Scholar]
- 265.Kannan S., Audet A., Huang H., Chen L.J., Wu M. Cholesterol-rich membrane rafts and Lyn are involved in phagocytosis during Pseudomonas aeruginosa infection. J Immunol. 2008;180:2396–2408. doi: 10.4049/jimmunol.180.4.2396. [DOI] [PubMed] [Google Scholar]
- 266.Yamamoto N., Yamamoto N., Petroll M.W., Cavanagh H.D., Jester J.V. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:1348–1355. doi: 10.1167/iovs.04-0542. [DOI] [PubMed] [Google Scholar]
- 267.Grassmé H., Jendrossek V., Riehle A., von Kürthy G., Berger J., Schwarz H. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 268.Garner M.J., Hayward R.D., Koronakis V. The Salmonella pathogenicity island 1 secretion system directs cellular cholesterol redistribution during mammalian cell entry and intracellular trafficking. Cell Microbiol. 2002;4:153–165. doi: 10.1046/j.1462-5822.2002.00181.x. [DOI] [PubMed] [Google Scholar]
- 269.Knodler L.A., Vallance B.A., Hensel M., Jäckel D., Finlay B.B., Steele-Mortimer O. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes. Mol Microbiol. 2003;49:685–704. doi: 10.1046/j.1365-2958.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- 270.Lafont F., Tran Van Nhieu G., Hanada K., Sansonetti P., van der Goot F.G. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 2002;21:4449–4457. doi: 10.1093/emboj/cdf457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Oliferenko S., Paiha K., Harder T., Gerke V., Schwärzler C., Schwarz H. Analysis of CD44-containing lipid rafts: Recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol. 1999;146:843–854. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sato Y., Kaneko K., Sasahara T., Inoue M. Novel pathogenetic mechanism in a clinical isolate of Yersinia enterocolitica KU14. J Microbiol. 2006;44:98–105. [PubMed] [Google Scholar]
- 273.Grassmé H., Gulbins E., Brenner B., Ferlinz K., Sandhoff K., Harzer K. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605–615. doi: 10.1016/s0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 274.Hauck C.R., Grassmé H., Bock J., Jendrossek V., Ferlinz K., Meyer T.F. Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of Neisseria gonorrhoeae. FEBS Lett. 2000;478:260–266. doi: 10.1016/s0014-5793(00)01851-2. [DOI] [PubMed] [Google Scholar]
- 275.Soong G., Reddy B., Sokol S., Adamo R., Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–1489. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ulevitch R.J., Tobias P.S. Recognition of endotoxin by cells leading to transmembrane signaling. Curr Opin Immunol. 1994;6:125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 277.Fong K.P., Pacheco C.M.F., Otis L.L., Baranwal S., Kieba I.R., Harrison G. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell Microbiol. 2006;8:1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Boesze-Battaglia K., Besack D., McKay T., Zekavat A., Otis L., Jordan-Sciutto K. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal-distending toxin. Cell Microbiol. 2006;8:823–836. doi: 10.1111/j.1462-5822.2005.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Abrami L., van der Goot F.G. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J Cell Biol. 1999;147:175–184. doi: 10.1083/jcb.147.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wang P.Y., Kitchens R.L., Munford R.S. Bacterial lipopolysaccharide binds to CD14 in low-density domains of the monocyte-macrophage plasma membrane. J Inflamm. 1995;47:126–137. [PubMed] [Google Scholar]
- 281.Abrami L., Liu S., Cosson P., Leppla S.H., van der Goot F.G. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160:321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bourdeau R.W., Malito E., Chenal A., Bishop B.L., Musch M.W., Villereal M.L. A Cholesterol-dependent Cytolysin Secreted by Bacillus anthracis. J Biol Chem. 2009;284:14645–14656. doi: 10.1074/jbc.M807631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Knight P.J.K., Knowles B.H., Ellar D.J. Molecular cloning of an insect aminopeptidase N that serves as a receptor for Bacillus thuringiensis CryIA(c) toxin. J Biol Chem. 1995;270:17765–17770. doi: 10.1074/jbc.270.30.17765. [DOI] [PubMed] [Google Scholar]
- 284.Zhuang M., Oltean D.I., Gómez I., Pullikuth A.K., Soberón M., Bravo A. Heliothis virescens and Manduca sexta lipid rafts are involved in Cry1A toxin binding to the midgut epithelium and subsequent pore formation. J Biol Chem. 2002;277:13863–13872. doi: 10.1074/jbc.M110057200. [DOI] [PubMed] [Google Scholar]
- 285.Bravo A., Gómez I., Conde J., Muñoz-Garay C., Sánchez J., Miranda R. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim Biophys Acta. 2004;1667:38–46. doi: 10.1016/j.bbamem.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 286.Nishiki T., Tokuyama Y., Kamata Y., Nemoto Y., Yoshida A., Sekiguchi M. Binding of botulinum type B neurotoxin to Chinese hamster ovary cells transfected with rat synaptotagmin II cDNA. Neurosci Lett. 1996;208:105–108. doi: 10.1016/0304-3940(96)12557-x. [DOI] [PubMed] [Google Scholar]
- 287.Petro K.A., Dyer M.A., Yowler B.C., Schengrund C.L. Disruption of lipid rafts enhances activity of botulinum neurotoxin serotype A. Toxicon. 2006;48:1035–1045. doi: 10.1016/j.toxicon.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 288.Nusrat A., von Eichel-Streiber C., Turner J.R., Verkade P., Madara J.L., Parkos C.A. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Miyata S., Minami J., Tamai E., Matsushita O., Shimamoto S., Okabe A. Clostridium perfringens ε-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J Biol Chem. 2002;277:39463–39468. doi: 10.1074/jbc.M206731200. [DOI] [PubMed] [Google Scholar]
- 290.Nagahama M., Yamaguchi A., Hagiyama T., Ohkubo N., Kobayashi K., Sakurai J. Binding and internalization of Clostridium perfringens iota-toxin in lipid rafts. Infect Immun. 2004;72:3267–3275. doi: 10.1128/IAI.72.6.3267-3275.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shimada Y., Maruya M., Iwashita S., Ohno-Iwashita Y. The C-terminal domain of perfringolysin O is an essential cholesterol-binding unit targeting to cholesterol-rich microdomains. Eur J Biochem. 2002;269:6195–6203. doi: 10.1046/j.1432-1033.2002.03338.x. [DOI] [PubMed] [Google Scholar]
- 292.Waheed A.A., Shimada Y., Heijnen H.F.G., Nakamura M., Inomata M., Hayashi M. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts) Proc Natl Acad Sci USA. 2001;98:4926–4931. doi: 10.1073/pnas.091090798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gordon V.M., Nelson K.L., Buckley J.T., Stevens V.L., Tweten R.K., Elwood P.C. Clostridium septicum alpha toxin uses glycosylphosphatidylinositol-anchored protein receptors. J Biol Chem. 1999;274:27274–27280. doi: 10.1074/jbc.274.38.27274. [DOI] [PubMed] [Google Scholar]
- 294.Herreros J., Ng T., Schiavo G. Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. Mol Biol Cell. 2001;12:2947–2960. doi: 10.1091/mbc.12.10.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wolf A.A., Jobling M.G., Wimer-Mackin S., Ferguson-Maltzman M., Madara J.L., Holmes R.K. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J Cell Biol. 1998;141:917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lencer W.I., Hirst T.R., Holmes R.K. Membrane traffic and the cellular uptake of cholera toxin. Biochim Biophys Acta. 1999;1450:177–190. doi: 10.1016/s0167-4889(99)00070-1. [DOI] [PubMed] [Google Scholar]
- 297.Ricci V., Galmiche A., Doye A., Necchi V., Solcia E., Boquet P. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol Biol Cell. 2000;11:3897–3909. doi: 10.1091/mbc.11.11.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gupta V.R., Patel H.K., Kostolansky S.S., Ballivian R.A., Eichberg J., Blanke S.R. Sphingomyelin functions as a novel receptor for Helicobacter pylori VacA. PLoS Pathog. 2008;4:e1000073. doi: 10.1371/journal.ppat.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Coconnier M.H., Lorrot M., Barbat A., Laboisse C., Servin A.L. Listeriolysin O-induced stimulation of mucin exocytosis in polarized intestinal mucin-secreting cells: evidence for toxin recognition of membrane-associated lipids and subsequent toxin internalization through caveolae. Cell Microbiol. 2000;2:487–504. doi: 10.1046/j.1462-5822.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- 300.Gekara N.O., Weiss S. Lipid rafts clustering and signalling by listeriolysin O. Biochem Soc Trans. 2004;32:712–714. doi: 10.1042/BST0320712. [DOI] [PubMed] [Google Scholar]
- 301.Lindberg A.A., Brown J.E., Strömberg N., Westling-Ryd M., Schultz J.E., Karlsson K.A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987;262:1779–1785. [PubMed] [Google Scholar]
- 302.Sandvig K., van Deurs B. Membrane traffic exploited by protein toxins. Annu Rev Cell Dev Biol. 2002;18:1–24. doi: 10.1146/annurev.cellbio.18.011502.142107. [DOI] [PubMed] [Google Scholar]
- 303.Zitzer A., Bittman R., Verbicky C.A., Erukulla R.K., Bhakdi S., Weis S. Coupling of cholesterol and cone-shaped lipids in bilayers augments membrane permeabilization by the cholesterol-specific toxins streptolysin O and Vibrio cholerae cytolysin. J Biol Chem. 2001;276:14628–14633. doi: 10.1074/jbc.M100241200. [DOI] [PubMed] [Google Scholar]
- 304.Shogomori H., Futerman A.H. Cholera toxin is found in detergent-insoluble rafts/domains at the cell surface of hippocampal neurons but is internalized via a raft- independent mechanism. J Biol Chem. 2001;276:9182–9188. doi: 10.1074/jbc.M009414200. [DOI] [PubMed] [Google Scholar]
- 305.Rosado C.J., Kondos S., Bull T.E., Kuiper M.J., Law R.H.P., Buckle A.M. The MACPF/CDC family of pore-forming toxins. Cell Microbiol. 2008;10:1765–1774. doi: 10.1111/j.1462-5822.2008.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shogomori H., Kobayashi T. Lysenin: a sphingomyelin specific pore-forming toxin. Biochim Biophys Acta. 2008;1780:612–618. doi: 10.1016/j.bbagen.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 307.Narayan S., Barnard R.J.O., Young J.A.T. Two retroviral entry pathways distinguished by lipid raft association of the viral receptor and differences in viral infectivity. J Virol. 2003;77:1977–1983. doi: 10.1128/JVI.77.3.1977-1983.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Bhattacharya B., Roy P. Bluetongue virus outer capsid protein VP5 interacts with membrane lipid rafts via a SNARE domain. J Virol. 2008;82:10600–10612. doi: 10.1128/JVI.01274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Triantafilou K., Triantafilou M. Lipid-raft-dependent Coxsackievirus B4 internalization and rapid targeting to the Golgi. Virology. 2004;326:6–19. doi: 10.1016/j.virol.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 310.Reyes-del Valle J., Chávez-Salinas S., Medina F., del Angel R.M. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J Virol. 2005;79:4557–4567. doi: 10.1128/JVI.79.8.4557-4567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Noisakran S., Dechtawewat T., Avirutnan P., Kinoshita T., Siripanyaphinyo U., Puttikhunt C. Association of dengue virus NS1 protein with lipid rafts. J Gen Virol. 2008;89:2492–2500. doi: 10.1099/vir.0.83620-0. [DOI] [PubMed] [Google Scholar]
- 312.Panchal R.G., Ruthel G., Kenny T.A., Kallstrom G.H., Lane D., Badie S.S. In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc Natl Acad Sci USA. 2003;100:15936–15941. doi: 10.1073/pnas.2533915100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bavari S., Bosio C.M., Wiegand E., Ruthel G., Will A.B., Geisbert T.W. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Freitas M.S., Gaspar L.P., Lorenzoni M., Almeida F.C.L., Tinoco L.W., Almeida M.S. Structure of the Ebola fusion peptide in a membrane-mimetic environment and the interaction with lipid rafts. J Biol Chem. 2007;282:27306–27314. doi: 10.1074/jbc.M611864200. [DOI] [PubMed] [Google Scholar]
- 315.Marjomäki V., Pietiäinen V., Matilainen H., Upla P., Ivaska J., Nissinen L. Internalization of echovirus 1 in caveolae. J Virol. 2002;76:1856–1865. doi: 10.1128/JVI.76.4.1856-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Xing L., Huhtala M., Pietiäinen V., Käpylä J., Vuorinen K., Marjomäki V. Structural and functional analysis of integrin α2I domain interaction with echovirus 1. J Biol Chem. 2004;279:11632–11638. doi: 10.1074/jbc.M312441200. [DOI] [PubMed] [Google Scholar]
- 317.Karjalainen M., Kakkonen E., Upla P., Paloranta H., Kankaanpää P., Liberali P. A Raft-derived, Pak1-regulated entry participates in α2β1 integrin-dependent sorting to caveosomes. Mol Biol Cell. 2008;19:2857–2869. doi: 10.1091/mbc.E07-10-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lévêque N., Norder H., Zreik Y., Cartet G., Falcon D., Rivat N. Echovirus 6 strains derived from a clinical isolate show differences in haemagglutination ability and cell entry pathway. Virus Res. 2007;130:1–9. doi: 10.1016/j.virusres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 319.Stuart A.D., McKee T.A., Williams P.A., Harley C., Shen S., Stuart D.I. Determination of the structure of a decay accelerating factor-binding clinical isolate of echovirus 11 allows mapping of mutants with altered receptor requirements for infection. J Virol. 2002;76:7694–7704. doi: 10.1128/JVI.76.15.7694-7704.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lu X., Xiong Y., Silver J. Asymmetric requirement for cholesterol in receptor-bearing but not envelope-bearing membranes for fusion mediated by ecotropic murine leukemia virus. J Virol. 2002;76:6701–6709. doi: 10.1128/JVI.76.13.6701-6709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ikeda M., Longnecker R. Cholesterol is critical for Epstein-Barr virus latent membrane protein 2A trafficking and protein stability. Virology. 2007;360:461–468. doi: 10.1016/j.virol.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Dykstra M., Cherukuri A., Pierce S.K. Rafts and synapses in the spatial organization of immune cell signaling receptors. J Leukoc Biol. 2001;70:699–707. [PubMed] [Google Scholar]
- 323.Aizaki H., Lee K.J., Sung V.M.H., Ishiko H., Lai M.M.C. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology. 2004;324:450–461. doi: 10.1016/j.virol.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 324.Shi S.T., Lee K.J., Aizaki H., Hwang S.B., Lai M.M.C. Hepatitis C virus RNA replication occurs on a detergent-resistant membrane that cofractionates with caveolin-2. J Virol. 2003;77:4160–4168. doi: 10.1128/JVI.77.7.4160-4168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kapadia S.B., Barth H., Baumert T., McKeating J.A., Chisari F.V. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81:374–383. doi: 10.1128/JVI.01134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Gao L., Aizaki H., He J.W., Lai M.M.C. Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft. J Virol. 2004;78:3480–3488. doi: 10.1128/JVI.78.7.3480-3488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Bender F.C., Whitbeck J.C., Ponce de Leon M., Lou H., Eisenberg R.J., Cohen G.H. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J Virol. 2003;77:9542–9552. doi: 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Koshizuka T., Kawaguchi Y., Nozawa N., Mori I., Nishiyama Y. Herpes simplex virus protein UL11 but not UL51 is associated with lipid rafts. Virus Genes. 2007;35:571–575. doi: 10.1007/s11262-007-0156-2. [DOI] [PubMed] [Google Scholar]
- 329.Cho N.H., Kingston D., Chang H., Kwon E.K., Kim J.M., Lee J.H. Association of herpesvirus saimiri tip with lipid raft is essential for downregulation of T-cell receptor and CD4 coreceptor. J Virol. 2006;80:108–118. doi: 10.1128/JVI.80.1.108-118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ono A., Freed E.O. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci USA. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Guyader M., Kiyokawa E., Abrami L., Turelli P., Trono D. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J Virol. 2002;76:10356–10364. doi: 10.1128/JVI.76.20.10356-10364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Rawat S.S., Viard M., Gallo S.A., Blumenthal R., Puri A. Sphingolipids, cholesterol, and HIV-1: a paradigm in viral fusion. Glycoconj J. 2006;23:189–197. doi: 10.1007/s10719-006-7924-4. [DOI] [PubMed] [Google Scholar]
- 333.Ablan S., Rawat S.S., Viard M., Wang J.M., Puri A., Blumenthal R. The role of cholesterol and sphingolipids in chemokine receptor function and HIV-1 envelope glycoprotein-mediated fusion. Virol J. 2006;3:104. doi: 10.1186/1743-422X-3-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nguyen D.H., Hildreth J.E.K. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Alfsen A., Iniguez P., Bouguyon E., Bomsel M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J Immunol. 2001;166:6257–6265. doi: 10.4049/jimmunol.166.10.6257. [DOI] [PubMed] [Google Scholar]
- 336.Mañes S., del Real G., Lacalle R.A., Lucas P., Gómez-Moutón C., Sánchez-Palomino S. Martínez-AC. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Carter G.C., Bernstone L., Sangani D., Bee J.W., Harder T., James W. HIV entry in macrophages is dependent on intact lipid rafts. Virology. 2009;386:192–202. doi: 10.1016/j.virol.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Viard M., Parolini I., Rawat S.S., Fecchi K., Sargiacomo M., Puri A. The role of glycosphingolipids in HIV signaling, entry and pathogenesis. Glycoconj J. 2004;20:213–222. doi: 10.1023/B:GLYC.0000024253.48791.d9. [DOI] [PubMed] [Google Scholar]
- 339.Harrist A.V., Ryzhova E.V., Harvey T., González-Scarano F. Anx2 interacts with HIV-1 Gag at phosphatidylinositol (4, 5) bisphosphate-containing lipid rafts and increases viral production in 293T cells. PLoS ONE. 2009;4:e5020. doi: 10.1371/journal.pone.0005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kamiyama H., Yoshii H., Tanaka Y., Sato H., Yamamoto N., Kubo Y. Raft localization of CXCR4 is primarily required for X4-tropic human immunodeficiency virus type 1 infection. Virology. 2009;386:23–31. doi: 10.1016/j.virol.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 341.Tang H., Kawabata A., Takemoto M., Yamanishi K., Mori Y. Human herpesvirus-6 infection induces the reorganization of membrane microdomains in target cells, which are required for virus entry. Virology. 2008;378:265–271. doi: 10.1016/j.virol.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 342.Raghu H., Sharma-Walia N., Veettil M.V., Sadagopan S., Caballero A., Sivakumar R. Lipid rafts of primary endothelial cells are essential for Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J Virol. 2007;81:7941–7959. doi: 10.1128/JVI.02848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wielgosz M.M., Rauch D.A., Jones K.S., Ruscetti F.W., Ratner L. Cholesterol dependence of HTLV-I infection. AIDS Res Hum Retroviruses. 2005;21:43–50. doi: 10.1089/aid.2005.21.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Niyogi K., Hildreth J.E.K. Characterization of new syncytium-inhibiting monoclonal antibodies implicates lipid rafts in human T-cell leukemia virus type 1 syncytium formation. J Virol. 2001;75:7351–7361. doi: 10.1128/JVI.75.16.7351-7361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Huang J., Ren T., Guan H., Jiang Y., Cheng H. HTLV-1 Tax is a critical lipid raft modulator that hijacks IκB kinases to the microdomains for persistent activation of NF-κB. J Biol Chem. 2009;284:6208–6217. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- 346.Leser G.P., Lamb R.A. Influenza virus assembly and budding in raft-derived microdomains: a quantitative analysis of the surface distribution of HA, NA and M2 proteins. Virology. 2005;342:215–227. doi: 10.1016/j.virol.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 347.Takeda M., Leser G.P., Russell C.J., Lamb R.A. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc Natl Acad Sci USA. 2003;100:14610–14617. doi: 10.1073/pnas.2235620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Barman S., Nayak D.P. Lipid raft disruption by cholesterol depletion enhances influenza A virus budding from MDCK cells. J Virol. 2007;81:12169–12178. doi: 10.1128/JVI.00835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Manié S.N., Debreyne S., Vincent S., Gerlier D. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J Virol. 2000;74:305–311. doi: 10.1128/jvi.74.1.305-311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Scheiffele P., Rietveld A., Wilk T., Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 351.Avota E., Müller N., Klett M., Schneider-Schaulies S. Measles virus interacts with and alters signal transduction in T-cell lipid rafts. J Virol. 2004;78:9552–9559. doi: 10.1128/JVI.78.17.9552-9559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Laliberte J.P., McGinnes L.W., Peeples M.E., Morrison T.G. Integrity of membrane lipid rafts is necessary for the ordered assembly and release of infectious Newcastle disease virus particles. J Virol. 2006;80:10652–10662. doi: 10.1128/JVI.01183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Laliberte J.P., McGinnes L.W., Morrison T.G. Incorporation of functional HN-F glycoprotein-containing complexes into Newcastle disease virus is dependent on cholesterol and membrane lipid raft integrity. J Virol. 2007;81:10636–10648. doi: 10.1128/JVI.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Desplanques A.S., Nauwynck H.J., Tilleman K., Deforce D., Favoreel H.W. Tyrosine phosphorylation and lipid raft association of pseudorabies virus glycoprotein E during antibody-mediated capping. Virology. 2007;362:60–66. doi: 10.1016/j.virol.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 355.Desplanques A.S., Nauwynck H.J., Vercauteren D., Geens T., Favoreel H.W. Plasma membrane cholesterol is required for efficient pseudorabies virus entry. Virology. 2008;376:339–345. doi: 10.1016/j.virol.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lyman M.G., Curanovic D., Enquist L.W. Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts. PLoS Pathog. 2008;4:e1000065. doi: 10.1371/journal.ppat.1000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Brown G., Rixon H.W.M., Sugrue R.J. Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin-1. J Gen Virol. 2002;83:1841–1850. doi: 10.1099/0022-1317-83-8-1841. [DOI] [PubMed] [Google Scholar]
- 358.Werling D., Hope J.C., Chaplin P., Collins R.A., Taylor G., Howard C.J. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50–58. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]
- 359.Fleming E.H., Kolokoltsov A.A., Davey R.A., Nichols J.E., Roberts N.J. Respiratory syncytial virus F envelope protein associates with lipid rafts without a requirement for other virus proteins. J Virol. 2006;80:12160–12170. doi: 10.1128/JVI.00643-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Grassmé H., Riehle A., Wilker B., Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- 361.Cuadras M.A., Greenberg H.B. Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo. Virology. 2003;313:308–321. doi: 10.1016/s0042-6822(03)00326-x. [DOI] [PubMed] [Google Scholar]
- 362.Iša P., Realpe M., Romero P., López S., Arias C.F. Rotavirus RRV associates with lipid membrane microdomains during cell entry. Virology. 2004;322:370–381. doi: 10.1016/j.virol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 363.Cuadras M.A., Bordier B.B., Zambrano J.L., Ludert J.E., Greenberg H.B. Dissecting rotavirus particle-raft interaction with small interfering RNAs: insights into rotavirus transit through the secretory pathway. J Virol. 2006;80:3935–3946. doi: 10.1128/JVI.80.8.3935-3946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Lu Y., Liu D.X., Tam J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem Biophys Res Commun. 2008;369:344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Ali A., Avalos R.T., Ponimaskin E., Nayak D.P. Influenza virus assembly: effect of influenza virus glycoproteins on the membrane association of M1 protein. J Virol. 2000;74:8709–8719. doi: 10.1128/jvi.74.18.8709-8719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Ali A., Nayak D.P. Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology. 2000;276:289–303. doi: 10.1006/viro.2000.0556. [DOI] [PubMed] [Google Scholar]
- 368.Ahn A., Gibbons D.L., Kielian M. The fusion peptide of Semliki Forest virus associates with sterol-rich membrane domains. J Virol. 2002;76:3267–3275. doi: 10.1128/JVI.76.7.3267-3275.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Marquardt M.T., Phalen T., Kielian M. Cholesterol is required in the exit pathway of Semliki Forest virus. J Cell Biol. 1993;123:57–65. doi: 10.1083/jcb.123.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Norkin L.C. Simian virus 40 infection via MHC class I molecules and caveolae. Immunol Rev. 1999;168:13–22. doi: 10.1111/j.1600-065x.1999.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 371.Parton R.G., Lindsay M. Exploitation of major histocompatibility complex class I molecules and caveolae by simian virus 40. Immunol Rev. 1999;168:23–31. doi: 10.1111/j.1600-065x.1999.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 372.Chung C.S., Huang C.Y., Chang W. Vaccinia virus penetration requires cholesterol and results in specific viral envelope proteins associated with lipid rafts. J Virol. 2005;79:1623–1634. doi: 10.1128/JVI.79.3.1623-1634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Orynbayeva Z., Kolusheva S., Groysman N., Gavrielov N., Lobel L., Jelinek R. Vaccinia virus interactions with the cell membrane studied by new chromatic vesicle and cell sensor assays. J Virol. 2007;81:1140–1147. doi: 10.1128/JVI.01345-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Hambleton S., Steinberg S.P., Gershon M.D., Gershon A.A. Cholesterol dependence of varicella-zoster virion entry into target cells. J Virol. 2007;81:7548–7558. doi: 10.1128/JVI.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Medigeshi G.R., Hirsch A.J., Streblow D.N., Nikolich-Zugich J., Nelson J.A. West Nile virus entry requires cholesterol-rich membrane microdomains and is independent of αvβ3 integrin. J Virol. 2008;82:5212–5219. doi: 10.1128/JVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Nelson J.B., O’Hara S.P., Small A.J., Tietz P.S., Choudhury A.K., Pagano R.E. Cryptosporidium parvum infects human cholangiocytes via sphingolipid-enriched membrane microdomains. Cell Microbiol. 2006;8:1932–1945. doi: 10.1111/j.1462-5822.2006.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Mittal K., Welter B.H., Temesvari L.A. Entamoeba histolytica: lipid rafts are involved in adhesion of trophozoites to host extracellular matrix components. Exp Parasitol. 2008;120:127–134. doi: 10.1016/j.exppara.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Dermine J.F., Duclos S., Garin J., St-Louis F., Rea S., Parton R.G. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J Biol Chem. 2001;276:18507–18512. doi: 10.1074/jbc.M101113200. [DOI] [PubMed] [Google Scholar]
- 379.Samuel B.U., Mohandas N., Harrison T., McManus H., Rosse W., Reid M. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J Biol Chem. 2001;276:29319–29329. doi: 10.1074/jbc.M101268200. [DOI] [PubMed] [Google Scholar]
- 380.Murphy S.C., Hiller N.L., Harrison T., Lomasney J.W., Mohandas N., Haldar K. Lipid rafts and malaria parasite infection of erythrocytes. Mol Membr Biol. 2006;23:81–88. doi: 10.1080/09687860500473440. [DOI] [PubMed] [Google Scholar]
- 381.Murphy S.C., Fernandez-Pol S., Chung P.H., Prasanna Murthy S.N., Milne S.B., Salomao M. Cytoplasmic remodeling of erythrocyte raft lipids during infection by the human malaria parasite Plasmodium falciparum. Blood. 2007;110:2132–2139. doi: 10.1182/blood-2007-04-083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Baumgartner M., Angelisová P., Setterblad N., Mooney N., Werling D., Horejsí V. Constitutive exclusion of Csk from Hck-positive membrane microdomains permits Src kinase-dependent proliferation of Theileria-transformed B lymphocytes. Blood. 2003;101:1874–1881. doi: 10.1182/blood-2002-02-0456. [DOI] [PubMed] [Google Scholar]
- 383.Mordue D.G., Desai N., Dustin M., Sibley L.D. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med. 1999;190:1783–1792. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Maza P.K., Straus A.H., Toledo M.S., Takahashi H.K., Suzuki E. Interaction of epithelial cell membrane rafts with Paracoccidioides brasiliensis leads to fungal adhesion and Src-family kinase activation. Microbes Infect. 2008;10:540–547. doi: 10.1016/j.micinf.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 385.Gilch S., Kehler C., Schätzl H.M. The prion protein requires cholesterol for cell surface localization. Mol Cell Neurosci. 2006;31:346–353. doi: 10.1016/j.mcn.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 386.Sarnataro D., Campana V., Paladino S., Stornaiuolo M., Nitsch L., Zurzolo C. PrPC association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol Biol Cell. 2004;15:4031–4042. doi: 10.1091/mbc.E03-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Lafont F., Abrami L., van der Goot F.G. Bacterial subversion of lipid rafts. Curr Opin Microbiol. 2004;7:4–10. doi: 10.1016/j.mib.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 388.Schengrund C.L. “Multivalent” saccharides: development of new approaches for inhibiting the effects of glycosphingolipid-binding pathogens. Biochem Pharmacol. 2003;65:699–707. doi: 10.1016/s0006-2952(02)01553-8. [DOI] [PubMed] [Google Scholar]
- 389.Conner S.D., Schmid S.L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 390.Sandvig K., Lyngaas Torgersen M., Andersen Raa H., van Deurs B. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem Cell Biol. 2008;129:267–276. doi: 10.1007/s00418-007-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Marsh M., Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Mayor S., Pagano R.E. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Nelson L.D., Johnson A.E., London E. How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction. J Biol Chem. 2008;283:4632–4642. doi: 10.1074/jbc.M709483200. [DOI] [PubMed] [Google Scholar]
- 394.Grassmé H., Jendrossek V., Bock J., Riehle A., Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- 395.Holopainen J.M., Subramanian M., Kinnunen P.K.J. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37:17562–17570. doi: 10.1021/bi980915e. [DOI] [PubMed] [Google Scholar]
- 396.Kolesnick R.N., Goñi F.M., Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 2000;184:285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 397.Hayakawa E., Tokumasu F., Nardone G.A., Jin A.J., Hackley V.A., Dvorak J.A. A Mycobacterium tuberculosis-derived lipid inhibits membrane fusion by modulating lipid membrane domains. Biophys J. 2007;93:4018–4030. doi: 10.1529/biophysj.107.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Welin A., Winberg M.E., Abdalla H., Särndahl E., Rasmusson B., Stendahl O. Incorporation of Mycobacterium tuberculosis lipoarabinomannan into macrophage membrane rafts is a prerequisite for the phagosomal maturation block. Infect Immun. 2008;76:2882–2887. doi: 10.1128/IAI.01549-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Arellano-Reynoso B., Lapaque N., Salcedo S., Briones G., Ciocchini A.E., Ugalde R. Cyclic β-1, 2-glucan is a Brucella virulence factor required for intracellular survival. Nat Immunol. 2005;6:618–625. doi: 10.1038/ni1202. [DOI] [PubMed] [Google Scholar]
- 400.Dietrich C., Bagatolli L.A., Volovyk Z.N., Thompson N.L., Levi M., Jacobson K. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Vincent S., Gerlier D., Manié S.N. Measles virus assembly within membrane rafts. Journal of Virology. 2000;74:9911–9915. doi: 10.1128/jvi.74.21.9911-9915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Mohan K.V., Muller J., Atreya C.D. Defective rotavirus particle assembly in lovastatin-treated MA104 cells. Arch Virol. 2008;153:2283–2290. doi: 10.1007/s00705-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Murphy D.J. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res. 2001;40:325–438. doi: 10.1016/s0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 404.Martin S., Parton R.G. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 405.Wolins N.E., Brasaemle D.L., Bickel P.E. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 406.Robenek H., Hofnagel O., Buers I., Robenek M.J., Troyer D., Severs N.J. Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci. 2006;119:4215–4224. doi: 10.1242/jcs.03191. [DOI] [PubMed] [Google Scholar]
- 407.Ploegh H.L. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007;448:435–438. doi: 10.1038/nature06004. [DOI] [PubMed] [Google Scholar]
- 408.Welte M.A. Proteins under new management: lipid droplets deliver. Trends Cell Biol. 2007;17:363–369. doi: 10.1016/j.tcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 409.Goodman J.M. The gregarious lipid droplet. J Biol Chem. 2008;283:28005–28009. doi: 10.1074/jbc.R800042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Pacheco P., Bozza F.A., Gomes R.N., Bozza M., Weller P.F., Castro-Faria-Neto H.C. Lipopolysaccharide-induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular loci involved in eicosanoid metabolism. J Immunol. 2002;169:6498–6506. doi: 10.4049/jimmunol.169.11.6498. [DOI] [PubMed] [Google Scholar]
- 411.Cao F., Castrillo A., Tontonoz P., Re F., Byrne G.I. Chlamydia pneumoniae-induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect Immun. 2007;75:753–759. doi: 10.1128/IAI.01386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Rodriguez-Acosta A., Finol H.J., Pulido-Méndez M., Marquez A., Andrade G., González N. Liver ultrastructural pathology in mice infected with Plasmodium berghei. J Submicrosc Cytol Pathol. 1998;30:299–307. [PubMed] [Google Scholar]
- 413.Pulido-Méndez M., Finol H.J., Girón M.E., Aguilar I. Ultrastructural pathological changes in mice kidney caused by Plasmodium berghei infection. J Submicrosc Cytol Pathol. 2006;38:143–148. [PubMed] [Google Scholar]
- 414.Paraje M.G., Correa S.G., Renna M.S., Theumer M., Sotomayor C.E. Candida albicans-secreted lipase induces injury and steatosis in immune and parenchymal cells. Can J Microbiol. 2008;54:647–659. doi: 10.1139/w08-048. [DOI] [PubMed] [Google Scholar]
- 415.Barba G., Harper F., Harada T., Kohara M., Goulinet S., Matsuura Y. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Miyanari Y., Atsuzawa K., Usuda N., Watashi K., Hishiki T., Zayas M. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:961–969. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 417.Shavinskaya A., Boulant S., Penin F., McLauchlan J., Bartenschlager R. The lipid droplet binding domain of hepatitis C virus core protein is a major determinant for efficient virus assembly. J Biol Chem. 2007;282:37158–37169. doi: 10.1074/jbc.M707329200. [DOI] [PubMed] [Google Scholar]
- 418.Boulant S., Douglas M.W., Moody L., Budkowska A., Targett-Adams P., McLauchlan J. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic. 2008;9:1268–1282. doi: 10.1111/j.1600-0854.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 419.Roingeard P., Hourioux C., Blanchard E., Prensier G. Hepatitis C virus budding at lipid droplet-associated ER membrane visualized by 3D electron microscopy. Histochem Cell Biol. 2008;130:561–566. doi: 10.1007/s00418-008-0447-2. [DOI] [PubMed] [Google Scholar]
- 420.Melo R.C.N., D’Avila H., Fabrino D.L., Almeida P.E., Bozza P.T. Macrophage lipid body induction by Chagas disease in vivo: putative intracellular domains for eicosanoid formation during infection. Tissue Cell. 2003;35:59–67. doi: 10.1016/s0040-8166(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 421.Melo R.C.N., Fabrino D.L., Dias F.F., Parreira G.G. Lipid bodies: structural markers of inflammatory macrophages in innate immunity. Inflamm Res. 2006;55:342–348. doi: 10.1007/s00011-006-5205-0. [DOI] [PubMed] [Google Scholar]
- 422.D’Avila H., Melo R.C.N., Parreira G.G., Werneck-Barroso E., Castro-Faria-Neto H.C., Bozza P.T. Mycobacterium bovis bacillus Calmette-Guérin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 2006;176:3087–3097. doi: 10.4049/jimmunol.176.5.3087. [DOI] [PubMed] [Google Scholar]
- 423.Luo M., Fadeev E.A., Groves J.T. Mycobactin-mediated iron acquisition within macrophages. Nat Chem Biol. 2005;1:149–153. doi: 10.1038/nchembio717. [DOI] [PubMed] [Google Scholar]
- 424.Kumar Y., Cocchiaro J., Valdivia R.H. The obligate intracellular pathogen Chlamydia trachomatis targets host lipid droplets. Curr Biol. 2006;16:1646–1651. doi: 10.1016/j.cub.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 425.Cocchiaro J.L., Kumar Y., Fischer E.R., Hackstadt T., Valdivia R.H. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci USA. 2008;105:9379–9384. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Pisciotta J.M., Coppens I., Tripathi A.K., Scholl P.F., Shuman J., Bajad S. The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochem J. 2007;402:197–204. doi: 10.1042/BJ20060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Jackson K.E., Klonis N., Ferguson D.J.P., Adisa A., Dogovski C., Tilley L. Food vacuole-associated lipid bodies and heterogeneous lipid environments in the malaria parasite, Plasmodium falciparum. Mol Microbiol. 2004;54:109–122. doi: 10.1111/j.1365-2958.2004.04284.x. [DOI] [PubMed] [Google Scholar]
- 428.Oliveira M.F., Kycia S.W., Gomez A., Kosar A.J., Bohle D.S., Hempelmann E. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett. 2005;579:6010–6016. doi: 10.1016/j.febslet.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 429.Schaible U.E., Kaufmann S.H.E. A nutritive view on the host–pathogen interplay. Trends Microbiol. 2005;13:373–380. doi: 10.1016/j.tim.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 430.Brinster S., Lamberet G., Staels B., Trieu-Cuot P., Gruss A., Poyart C. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature. 2009;458:83–86. doi: 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- 431.Hansmeier N., Chao T.C., Daschkey S., Müsken M., Kalinowski J., Pühler A. A comprehensive proteome map of the lipid-requiring nosocomial pathogen Corynebacterium jeikeium K411. Proteomics. 2007;7:1076–1096. doi: 10.1002/pmic.200600833. [DOI] [PubMed] [Google Scholar]
- 432.Stephens R.S., Kalman S., Lammel C., Fan J., Marathe R., Aravind L. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 433.Jain M., Petzold C.J., Schelle M.W., Leavell M.D., Mougous J.D., Bertozzi C.R. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc Natl Acad Sci USA. 2007;104:5133–5138. doi: 10.1073/pnas.0610634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Van der Geize R., Yam K., Heuser T., Wilbrink M.H., Hara H., Anderton M.C. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci USA. 2007;104:1947–1952. doi: 10.1073/pnas.0605728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zu L., He J., Jiang H., Xu C., Pu S., Xu G. Bacterial endotoxin stimulates adipose lipolysis via Toll-like receptor 4 and extracellular signal-regulated kinase pathway. J Biol Chem. 2009;284:5915–5926. doi: 10.1074/jbc.M807852200. [DOI] [PubMed] [Google Scholar]
- 436.Fang F.C., Libby S.J., Castor M.E., Fung A.M. Isocitrate lyase (AceA) is required for Salmonella persistence but not for acute lethal infection in mice. Infect Immun. 2005;73:2547–2549. doi: 10.1128/IAI.73.4.2547-2549.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Prigneau O., Porta A., Poudrier J.A., Colonna-Romano S., Noël T., Maresca B. Genes involved in β-oxidation, energy metabolism and glyoxylate cycle are induced by Candida albicans during macrophage infection. Yeast. 2003;20:723–730. doi: 10.1002/yea.998. [DOI] [PubMed] [Google Scholar]
- 438.Rude T.H., Toffaletti D.L., Cox G.M., Perfect J.R. Relationship of the glyoxylate pathway to the pathogenesis of Cryptococcus neoformans. Infect Immun. 2002;70:5684–5694. doi: 10.1128/IAI.70.10.5684-5694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Bowes A.E., Samad A.H., Jiang P., Weaver B., Mellors A. The acquisition of lysophosphatidylcholine by African trypanosomes. J Biol Chem. 1993;268:13885–13892. [PubMed] [Google Scholar]
- 440.Mellors A., Samad A. The acquisition of lipids by African trypanosomes. Parasitol Today. 1989;5:239–244. doi: 10.1016/0169-4758(89)90255-x. [DOI] [PubMed] [Google Scholar]
- 441.Coppens I., Baudhuin P., Opperdoes F.R., Courtoy P.J. Receptors for the host low density lipoproteins on the hemoflagellate Trypanosoma brucei: purification and involvement in the growth of the parasite. Proc Natl Acad Sci USA. 1988;85:6753–6757. doi: 10.1073/pnas.85.18.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Coppens I., Opperdoes F.R., Courtoy P.J., Baudhuin P. Receptor-mediated endocytosis in the bloodstream form of Trypanosoma brucei. J Protozool. 1987;34:465–473. doi: 10.1111/j.1550-7408.1987.tb03216.x. [DOI] [PubMed] [Google Scholar]
- 443.Peterson K.M., Alderete J.F. Trichomonas vaginalis is dependent on uptake and degradation of human low density lipoproteins. J Exp Med. 1984;160:1261–1272. doi: 10.1084/jem.160.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Son M.S., Matthews W.J., Kang Y., Nguyen D.T., Hoang T.T. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun. 2007;75:5313–5324. doi: 10.1128/IAI.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Krivan H.C., Franklin D.P., Wang W., Laux D.C., Cohen P.S. Phosphatidylserine found in intestinal mucus serves as a sole source of carbon and nitrogen for salmonellae and Escherichia coli. Infect Immun. 1992;60:3943–3946. doi: 10.1128/iai.60.9.3943-3946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Howe D., Heinzen R.A. Coxiella burnetii inhabits a cholesterol-rich vacuole and influences cellular cholesterol metabolism. Cell Microbiol. 2006;8:496–507. doi: 10.1111/j.1462-5822.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- 447.Carabeo R.A., Mead D.J., Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci USA. 2003;100:6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Coppens I., Sinai A.P., Joiner K.A. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J Cell Biol. 2000;149:167–180. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Sehgal A., Bettiol S., Pypaert M., Wenk M.R., Kaasch A., Blader I.J. Peculiarities of host cholesterol transport to the unique intracellular vacuole containing Toxoplasma. Traffic. 2005;6:1125–1141. doi: 10.1111/j.1600-0854.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 450.Besteiro S., Bertrand-Michel J., Lebrun M., Vial H., Dubremetz J.F. Lipidomic analysis of Toxoplasma gondii tachyzoites rhoptries: further insights into the role of cholesterol. Biochem J. 2008;415:87–96. doi: 10.1042/BJ20080795. [DOI] [PubMed] [Google Scholar]
- 451.Hackstadt T., Scidmore M.A., Rockey D.D. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci USA. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.de Melo E.J.T., de Souza W. Pathway of C6-NBD-ceramide on the host cell infected with Toxoplasma gondii. Cell Struct Funct. 1996;21:47–52. doi: 10.1247/csf.21.47. [DOI] [PubMed] [Google Scholar]
- 453.Charron A.J., Sibley L.D. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J Cell Sci. 2002;115:3049–3059. doi: 10.1242/jcs.115.15.3049. [DOI] [PubMed] [Google Scholar]
- 454.Dautry-Varsat A., Balañá M.E., Wyplosz B. Chlamydia- host cell interactions: recent advances on bacterial entry and intracellular development. Traffic. 2004;5:561–570. doi: 10.1111/j.1398-9219.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 455.Fields K.A., Hackstadt T. The chlamydial inclusion: escape from the endocytic pathway. Annu Rev Cell Dev Biol. 2002;18:221–245. doi: 10.1146/annurev.cellbio.18.012502.105845. [DOI] [PubMed] [Google Scholar]
- 456.Scidmore M.A., Fischer E.R., Hackstadt T. Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J Cell Biol. 1996;134:363–374. doi: 10.1083/jcb.134.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Moore E.R., Fischer E.R., Mead D.J., Hackstadt T. The chlamydial inclusion preferentially intercepts basolaterally directed sphingomyelin-containing exocytic vacuoles. Traffic. 2008;9:2130–2140. doi: 10.1111/j.1600-0854.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Heuer D., Lipinski A.R., Machuy N., Karlas A., Wehrens A., Siedler F. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- 459.Romano J.D., Bano N., Coppens I. New host nuclear functions are not required for the modifications of the parasitophorous vacuole of Toxoplasma. Cell Microbiol. 2008;10:465–476. doi: 10.1111/j.1462-5822.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 460.Hackstadt T., Rockey D.D., Heinzen R.A., Scidmore M.A. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 1996;15:964–977. [PMC free article] [PubMed] [Google Scholar]
- 461.Beatty W.L. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J Cell Sci. 2006;119:350–359. doi: 10.1242/jcs.02733. [DOI] [PubMed] [Google Scholar]
- 462.Beatty W.L. Late endocytic multivesicular bodies intersect the chlamydial inclusion in the absence of CD63. Infect Immun. 2008;76:2872–2881. doi: 10.1128/IAI.00129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Kuhle V., Abrahams G.L., Hensel M. Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic. 2006;7:716–730. doi: 10.1111/j.1600-0854.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 464.Salcedo S.P., Holden D.W. SseG, a virulence protein that targets Salmonella to the Golgi network. EMBO J. 2003;22:5003–5014. doi: 10.1093/emboj/cdg517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Kagan J.C., Roy C.R. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 466.Celli J., Salcedo S.P., Gorvel J.P. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc Natl Acad Sci USA. 2005;102:1673–1678. doi: 10.1073/pnas.0406873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Sinai A.P., Webster P., Joiner K.A. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110:2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 468.Bano N., Romano J.D., Jayabalasingham B., Coppens I. Cellular interactions of Plasmodium liver stage with its host mammalian cell. Int J Parasitol. 2007;37:1329–1341. doi: 10.1016/j.ijpara.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 469.Holthuis J.C.M., Levine T.P. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 470.Levine T., Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol. 2006;18:371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 471.Coppens I., Dunn J.D., Romano J.D., Pypaert M., Zhang H., Boothroyd J.C. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–274. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 472.Hurley J.H., Emr S.D. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Cole S.T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 474.Gower T.L., Graham B.S. Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro. Antimicrob Agents Chemother. 2001;45:1231–1237. doi: 10.1128/AAC.45.4.1231-1237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Ye J., Wang C., Sumpter R., Brown M.S., Goldstein J.L., Gale M. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci USA. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.del Real G., Jimenéz-Baranda S., Mira E., Lacalle R.A., Lucas P., Gómez-Moutón C. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med. 2004;200:541–547. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Potena L., Frascaroli G., Grigioni F., Lazzarotto T., Magnani G., Tomasi L. Hydroxymethyl-glutaryl coenzyme A reductase inhibition limits cytomegalovirus infection in human endothelial cells. Circulation. 2004;109:532–536. doi: 10.1161/01.CIR.0000109485.79183.81. [DOI] [PubMed] [Google Scholar]
- 478.Ikeda M., Kato N. Life style-related diseases of the digestive system: cell culture system for the screening of anti-hepatitis C virus (HCV) reagents: suppression of HCV replication by statins and synergistic action with interferon. J Pharmacol Sci. 2007;105:145–150. doi: 10.1254/jphs.fm0070050. [DOI] [PubMed] [Google Scholar]
- 479.Kim S.S., Peng L.F., Lin W., Choe W.H., Sakamoto N., Schreiber S.L. A cell-based, high-throughput screen for small molecule regulators of hepatitis C virus replication. Gastroenterology. 2007;132:311–320. doi: 10.1053/j.gastro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 480.Nabatov A.A., Pollakis G., Linnemann T., Paxton W.A., de Baar M.P. Statins disrupt CCR5 and RANTES expression levels in CD4+ T lymphocytes in vitro and preferentially decrease infection of R5 versus X4 HIV-1. PLoS One. 2007;2:e470. doi: 10.1371/journal.pone.0000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Amet T., Nonaka M., Dewan M.Z., Saitoh Y., Qi X., Ichinose S. Statin-induced inhibition of HIV-1 release from latently infected U1 cells reveals a critical role for protein prenylation in HIV-1 replication. Microbes Infect. 2008;10:471–480. doi: 10.1016/j.micinf.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 482.Bader T., Fazili J., Madhoun M., Aston C., Hughes D., Rizvi S. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- 483.Moriyama T., Sorokin A. Repression of BK virus infection of human renal proximal tubular epithelial cells by pravastatin. Transplantation. 2008;85:1311–1317. doi: 10.1097/TP.0b013e31816c4ec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Dechend R., Gieffers J., Dietz R., Joerres A., Rupp J., Luft F.C. Hydroxymethylglutaryl coenzyme A reductase inhibition reduces Chlamydia pneumoniae-induced cell interaction and activation. Circulation. 2003;108:261–265. doi: 10.1161/01.CIR.0000083367.93022.78. [DOI] [PubMed] [Google Scholar]
- 485.Catron D.M., Lange Y., Borensztajn J., Sylvester M.D., Jones B.D., Haldar K. Salmonella enterica serovar Typhimurium requires nonsterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Infect Immun. 2004;72:1036–1042. doi: 10.1128/IAI.72.2.1036-1042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Erkkilä L., Jauhiainen M., Laitinen K., Haasio K., Tiirola T., Saikku P. Effect of simvastatin, an established lipid-lowering drug, on pulmonary Chlamydia pneumoniae infection in mice. Antimicrob Agents Chemother. 2005;49:3959–3962. doi: 10.1128/AAC.49.9.3959-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Botelho-Nevers E., Espinosa L., Raoult D., Rolain J.M. Lovastatin, but not pravastatin, limits in vitro infection due to Coxiella burnetii. J Antimicrob Chemother. 2008;62:845–847. doi: 10.1093/jac/dkn282. [DOI] [PubMed] [Google Scholar]
- 488.Botelho-Nevers E., Rolain J.M., Espinosa L., Raoult D. Statins limit Rickettsia conorii infection in cells. Int J Antimicrob Agents. 2008;32:344–348. doi: 10.1016/j.ijantimicag.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 489.Schmeck B., Beermann W., N’Guessan P.D., Hocke A.C., Opitz B., Eitel J. Simvastatin reduces Chlamydophila pneumoniae-mediated histone modifications and gene expression in cultured human endothelial cells. Circ Res. 2008;102:888–895. doi: 10.1161/CIRCRESAHA.107.161307. [DOI] [PubMed] [Google Scholar]
- 490.Chen G.Z., Foster L., Bennett J.L. Antischistosomal action of mevinolin: evidence that 3-hydroxy-methylglutaryl-coenzyme a reductase activity in Schistosoma mansoni is vital for parasite survival. Naunyn Schmiedeberg’s Arch Pharmacol. 1990;342:477–482. doi: 10.1007/BF00169467. [DOI] [PubMed] [Google Scholar]
- 491.Haughan P.A., Chance M.L., Goad L.J. Synergism in vitro of lovastatin and miconazole as anti-leishmanial agents. Biochem Pharmacol. 1992;44:2199–2206. doi: 10.1016/0006-2952(92)90347-l. [DOI] [PubMed] [Google Scholar]
- 492.Grellier P., Valentin A., Millerioux V., Schrevel J., Rigomier D. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors lovastatin and simvastatin inhibit in vitro development of Plasmodium falciparum and Babesia divergens in human erythrocytes. Antimicrob Agents Chemother. 1994;38:1144–1148. doi: 10.1128/aac.38.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Soliman M.F.M., Ibrahim M.M. Antischistosomal action of atorvastatin alone and concurrently with medroxyprogesterone acetate on Schistosoma haematobium harboured in hamster: surface ultrastructure and parasitological study. Acta Trop. 2005;93:1–9. doi: 10.1016/j.actatropica.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 494.Kopterides P., Falagas M.E. Statins for sepsis: a critical and updated review. Clin Microbiol Infect. 2009;15:325–334. doi: 10.1111/j.1469-0691.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 495.Terblanche M., Almog Y., Rosenson R.S., Smith T.S., Hackam D.G. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7:358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 496.Reinicke A.T., Hutchinson J.L., Magee A.I., Mastroeni P., Trowsdale J., Kelly A.P. A Salmonella typhimurium effector protein SifA is modified by host cell prenylation and S-acylation machinery. J Biol Chem. 2005;280:14620–14627. doi: 10.1074/jbc.M500076200. [DOI] [PubMed] [Google Scholar]