Highlights
-
•
Patients with exacerbation of airways disease had syndromic POCT for viruses.
-
•
Antibiotics were stopped early in patients testing positive for viruses.
-
•
There was no difference between influenza and non-influenza viruses.
-
•
Syndromic POCT for viruses should be favoured over testing for influenza alone.
Keywords: Point-of-care testing, Syndromic, Respiratory viruses, Airways disease, Antibiotic use, Hospital
Abstract
Background
The ResPOC study demonstrated that syndromic molecular point-of-care testing (POCT) for respiratory viruses was associated with early discontinuation of unnecessary antibiotics compared to routine clinical care. Subgroup analysis suggests these changes occur predominantly in patients with exacerbation of airways disease. Use of molecular POCT for respiratory viruses is becoming widespread but there is a lack of evidence to inform the choice between multiplex syndromic panels versus POCT for influenza only.
Materials/methods
We evaluated patients from the ResPOC study with exacerbation of asthma or COPD who were treated with antibiotics. The duration of antibiotics and proportion with early discontinuation were compared between patients testing positive and negative for viruses by POCT, and controls. Patients testing positive for viruses by POCT were compared according to virus types.
Results
118 patient with exacerbation of airways disease received antibiotics in the POCT group and 111 in the control group. In the POCT group 49/118 (42%) patients tested positive for viruses. Of those testing positive for viruses 17/49 (35%) had early discontinuation of antibiotics versus 9/69 (13%) testing negative and 7/111 (6%) of controls, p<0.0001. Of those positive for viruses by POCT 10/49 (20%) were positive for influenza, 21/49 (43%) for rhinovirus and 18/49 (37%) for other viruses. The proportion with early discontinuation of antibiotics was not different between the virus types (p = 0.34).
Conclusions
This data suggests that syndromic molecular POCT for respiratory viruses should be favoured over POCT for influenza alone in adults with exacerbation of airways disease.
Introduction
Antimicrobial resistance (AMR) is the arguably one of the greatest threats to global human health and is driven by the overuse of antibiotics.1 Antibiotics are prescribed to the vast majority of patients hospitalised with acute respiratory illness (ARI), including in clinical groups where viruses are strongly implicated in their aetiology. Most asthma exacerbations are associated with respiratory virus infection, especially rhinovirus2 and randomised controlled trials have failed to demonstrate any benefit from antibiotic treatment.3, 4 Additionally, a large retrospective observational study of nearly 20,000 patients hospitalised with an exacerbation suggests that antibiotic use does not improve outcome and is associated with higher costs.5 Professional societies strongly discourage the use of antibiotics in asthma exacerbation through national guidelines6, 7 but despite this antibiotic use in adults hospitalised with exacerbation of asthma remains very common.8, 9, 10 Patients hospitalised with exacerbation of COPD are also routinely treated with antibiotics despite viruses being frequently implicated in their aetiology12 and a lack of high quality evidence for benefit in patients with non-life threatening exacerbations.11 Several randomised trials of biomarker-directed therapy show that antibiotics use can be safely reduced in non-critically ill hospitalised patients with exacerbation of COPD and low levels of CRP or procalcitonin13, 14 supporting the concept that antibiotics are currently overused in this population. Diagnostic uncertainty regarding the aetiology of exacerbations of airways disease contributes to the ongoing overuse of antibiotics in these groups.
The UK department of health commissioned report into antimicrobial resistance specifically noted the importance of rapid diagnostic tests to help combat AMR through effective antibiotic stewardship and recommended that by 2020 all prescribing of antibiotics should be informed by a diagnostic test where one exists.1 Rapid molecular tests for respiratory viruses could potentially fulfil this need in patients hospitalised with exacerbation of airways disease.
The ResPOC study was a large pragmatic randomised controlled trial that evaluated the clinical impact of syndromic (i.e. comprehensive multiplex PCR) point-of-care testing (POCT) for respiratory viruses in adults presenting to hospital with acute respiratory illness.10 It demonstrated that syndromic POCT for respiratory viruses using the FimArray Respiratory Panel was associated with several clinical benefits compared to routine clinical care with laboratory testing, including the earlier discontinuation of antibiotics, without any increase in adverse events. Subgroup analysis from the trial suggests that these antibiotic changes occurred predominantly in patients with exacerbation of airways disease testing positive for viruses, with little difference seen in antibiotic use in other clinical groups such as patients with community acquired pneumonia or in patients testing negative for viruses. Several observational studies support the findings of ResPOC, demonstrating a reduction in unnecessary antibiotics with rapid molecular testing for respiratory viruses in a variety of clinical setting.15, 16 In addition a post hoc analysis from the trial itself suggests that the very rapid turnaround times for results seen with POCT compared to laboratory testing are critical in modifying antibiotic use in hospitalised patients.17
Several rapid molecular test platforms for respiratory viruses are now available with the potential for deployment as POCTs in clinical areas and their use in hospitals is becoming more widespread.18 However, there is a lack of evidence to inform clinicians and hospitals in the choice between multiplex syndromic panels such as the FilmArray RP, which test for a comprehensive range of respiratory viruses, and platforms that test for influenza only, or for influenza and RSV. Rapid, accurate influenza detection is clearly a priority for hospitals during periods of intense influenza transmission, to correctly and rapidly administer influenza antivirals and to efficiently utilise infection control facilities. In addition multiplex syndromic panels are more expensive than uniplex tests for influenza and it is not currently clear what additional clinical value is gained by testing for the other virus types in hospitalised patients.
In this further analysis we examine in detail the changes in antibiotic use associated with syndromic molecular POCT among patients with exacerbation of airways diseases, and determine the importance of changes in antibiotic management occurring due to the detection of non-influenza viruses.
Methods
The study design, inclusion and exclusion criteria, outcome measures and baseline population characteristics of the ResPOC study have been previously described in the original report of this trial.10 In brief, adults presenting to the emergency department or acute medical unit with ARI were recruited at presentation and randomised 1:1 to receive POCT for respiratory viruses using the FilmArray Respiratory Panel or routine clinical care. The study was approved by the North West – Preston Regional Ethics Committee (NW/14/1467). The protocol is publically available.19
In this further analysis we evaluated patients with exacerbation of asthma or COPD who were treated with antibiotics. The duration of antibiotics and proportion treated with a single dose or short course (defined as less than 24 h) were compared between patients testing positive for viruses by POCT, those testing negative by POCT and control patients. Patients testing positive for viruses by POCT were then compared according to the virus types detected. Kaplan-Meier curves were generated for duration of antibiotic use.
Clinical data
Early discontinuation of antibiotics was defined as receipt of less than 24 h of antibiotics before stopping (including those that only received a single dose). Where rhino/enterovirus was co-detected with influenza or RSV, they (influenza and RSV) were considered to be dominant and the patient was classified accordingly.
Statistical analysis
Statistical analyses were done using Prism version 7.0 (Graphpad software; La Jolla, CA, USA) and Stata version 13.1 (StataCorp; College Station, TX, USA). We compared duration of antibiotic use between groups using median differences and the Kruskal–Wallis test and differences in proportions using Chi Squared test or Fisher's exact test, as appropriate. Kaplan–Maier curves for the duration of antibiotics were generated and compared using the log rank test.
Results
There were 143 patients with exacerbation of airways disease in the POCT group (62 with asthma and 81 with COPD) and 118 (83%) of these received antibiotics. There were 140 patients with exacerbation of airways disease in the control group (57 with asthma and 83 with COPD) and 111 (79%) of these received antibiotics, difference in antibiotic use between POCT and control group of 4% (95%CI – 6 to 13%), p = 0.55. In the POCT group 62/143 (43%) patients tested positive for respiratory viruses and 49/62 (79%) of these received antibiotics, 81/143 (57%) patients tested negative for viruses and 69/81 (85%) of these received antibiotics, difference in antibiotic use between POCT positive and negative patients of 6% (95%CI – 7 to 20%), p = 0.38. Baseline characteristics of patients testing positive and negative by POCT and control patients were well matched including physiological observations and laboratory results. Only 48/140 (34%) patients in the control group were tested for respiratory viruses using laboratory PCR and the median turnaround time for results was 31.1 h compared with 1.6 h for those tested with POCT, p<0.0001, Table 1 .
Table 1.
Baseline characteristics and outcome of patients with exacerbation of airways disease, in patients testing positive and negative by POCT and in control patients.
| POCT group n = 143 |
Control group n = 140 | p value | ||
|---|---|---|---|---|
| POCT positive n = 62 | POCT negative n = 81 | |||
| Age, years [range] | 61 [19–93] | 65 [18–93] | 60 [19–92] | 0.11 |
| Male sex | 21 (34) | 47 (58) | 69 (49) | 0.75 |
| White ethnicity | 59 (95) | 79 (98) | 136 (97) | 0.69 |
| Current smoker | 20 (30) | 28 (35) | 40 (29) | 0.65 |
| Influenza vaccine* | 38 (61) | 55 (68) | 94 (67) | 0.66 |
| Duration of symptoms, days⁎⁎ | 4 [3–6] | 4 [2–6] | 4 [2.3–5] | 0.52 |
| Pulse rate, bpm | 103 [90–110] | 95 [83–110] | 100 [82–112] | 0.1 |
| Respiratory rate, bpm | 25 [20–29] | 24 [20–28] | 24 [20–27] | 0.13 |
| Supplemental O2 | 16 (26) | 19 (24) | 21 (15) | 0.13 |
| Temperature, °C | 36.7 [36.2–37.2] | 36.6 [36.1–37] | 36.6 [36.3–37.2] | 0.45 |
| CRP, mg/L | 23 [9–65] | 13 [4–53] | 17 [6–50] | 0.23 |
| WCC, X109/L | 10.8 [8.1–13.3] | 10.6 [8.9–14.3] | 10.4 [7.9–13.4] | 0.31 |
| CXR performed | 62 (100) | 80 (99) | 137 (97) | 0.34 |
| Respiratory viral PCR | 62 (100) | 81 (100) | 48 (34) | <0.0001 |
| Turnaround time for result, hours | 1.6 [1.3–3.0] | 1.6 [1.3–3.0] | 31.1 [25.1–49.2] | <0.0001 |
| Length of stay, days | 2.7 [0.9–5.3] | 3.0 [0.9–5.2] | 3.1 [1.3–6.5] | 0.57 |
| 30 day mortality | 0 (0) | 2 (2.5) | 2 (1.5) | 0.46 |
| Re-admission¶ | 3 (5) | 21 (26) | 25 (18) | 0.0042 |
POCT, point-of-care test. CRP, C reactive protein. WCC, white cell count. CXR, chest X-ray. PCRP, polymerase chain reaction.
Influenza vaccine receipt for the current influenza season when recruited.
Duration of illness prior to presentation. Data are presented a median [inter-quartile range] and number (%) except where stated otherwise.
Within 30 days of discharge.
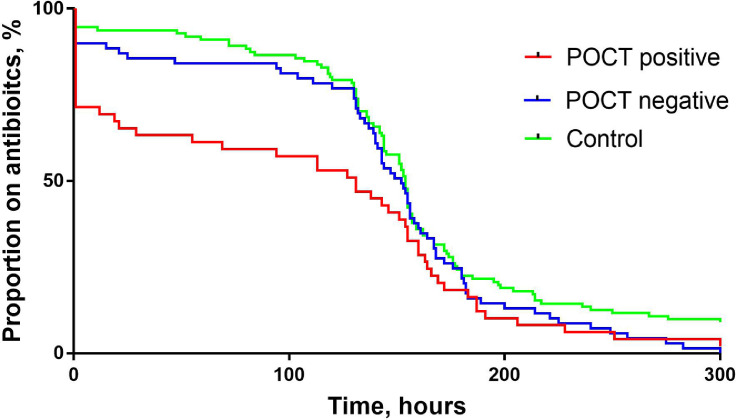
Of those testing positive for viruses by POCT and who received antibiotics, 17/49 (35%) had early discontinuation of antibiotics versus 9/69 (13%) in those testing negative and 7/111 (6%) in control patients, p<0.0001. The median duration of antibiotic was 5.5 days in those testing positive by POCT versus 6.3 days in those testing negative by POCT and 6.4 days in the control group, p = 0.012. The median duration of IV antibiotics was 1 h (i.e., a single dose) in those testing positive by POCT versus 6.5 in those testing negative and 26 h on the control group, p = 0.09. Antibiotic use by POCT result and in control patients is shown in Table 2 . Kaplan–Meier curve analysis for duration of antibiotics is shown in Fig. 1 . Although the curves for the three groups (POCT positive, POCT negative and control) becomes similar after approximately 6 days from starting antibiotics, the curves show that there is a sharp reduction in antibiotic use early on for those testing positive for viruses by POCT, p = 0.034.
Table 2.
Antibiotic use in patients with exacerbation of airways disease testing positive and negative by POCT and in control patients, n = 143.
| POCT positive | POCT negative | Control | p value | |
|---|---|---|---|---|
| n = 62 | n = 81 | n = 140 | ||
| Received any antibiotic | 49/62 (79) | 69/81 (85) | 111/140 (79) | 0.51 |
| Received IV antibiotics | 20/62 (32) | 28/81 (35) | 43/140 (31) | 0.84 |
| Duration of any antibiotic, days | 5.5 [0–6.9] | 6.3 [5.4–7.4] | 6.4 [5.5–7.4] | 0.012 |
| Duration of IV antibiotic, hours | 1 [1 –48] | 6.5 [1–62] | 26 [1–72] | 0.09 |
| Received a single dose of antibiotics only | 14/49 (29) | 7/69 (10) | 6/111 (5) | 0.0001 |
| Received <24 h antibiotics | 17/49 (35) | 9/69 (13) | 7/111 (6) | <0.0001 |
POCT, point-of-care test. IV, intravenous. Data are presented a median [inter quartile range] and number (%) except where stated otherwise.
Fig. 1.
Kaplan Meier curve showing antibiotic use over time in patients testing positive and negative by POCT and for control patients. Log rank test, p = 0.034.
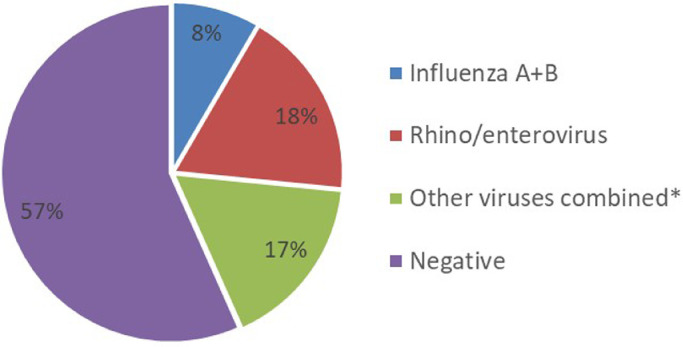
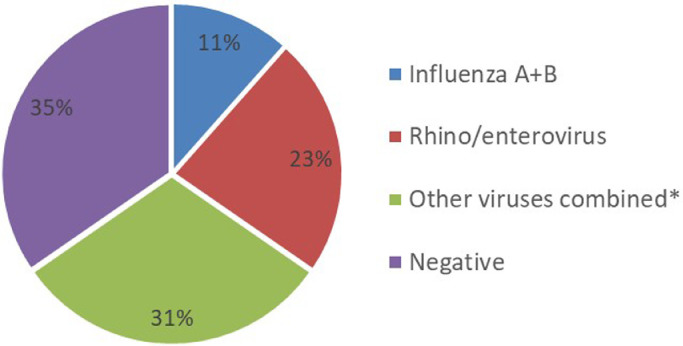
Of those tested for viruses by POCT 12/143 (8%) were positive for influenza A or B, 26/143, (18%) positive for rhino/enterovirus and 24/143 (17%) positive for other viruses combined (RSV, parainfluenza 1–4, human metapneumovirus and human coronavirus), Fig. 2 . There were 4 co-detections; RSV with rhino/enterovirus×2, Influenza A with Human coronavirus, and RSV with hMPV and human coronavirus. In patients who received the POC test and had early discontinuation of antibiotics, 3/26 (11%) had influenza detected, 6/26 (23%) had rhino/enterovirus detected 8/26, (31%) had other viruses detected and 9/26 (35%) had no virus detected, Fig. 3 .
Fig. 2.
Proportion of patients with viruses detected in POCT group, n = 143
*RSV, parainfluenza virus 1–4, human metapneumovirus, and human coronavirus.
Fig. 3.
Viruses detected in patients with early discontinuation of antibiotics in POCT group, n = 26. *RSV, parainfluenza virus 1–4, human metapneumovirus, and human coronavirus.
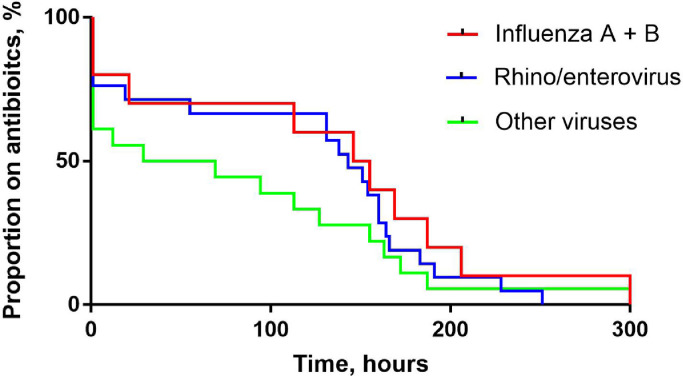
Among those positive for viruses receiving antibiotics, the proportion with early discontinuation of antibiotics was not different between the virus types, 3/10 (30%) for influenza, 6/21 (29%) for rhinovirus and 8/18 (44%) for other viruses combined, p = 0.54. Antibiotic use by virus type is shown in Table 3 . A higher proportion of patients with influenza received IV antibiotics compared to those with rhino/enterovirus or other virus types (53% vs. 23% and 21%, p = 0.044) but the median duration of IV antibiotic or any antibiotic was not different between the virus types, although the small numbers of individual virus types make definitive statement difficult. Survival analysis did not show any differences in early antibiotic discontinuation between virus types, p = 0.53, Fig. 4 .
Table 3.
Antibiotic use in patients with exacerbation of airways disease testing positive by POCT, according to virus type, n = 62.
| Influenza A or B n = 12 | Rhino/enterovirus n = 26 | Other viruses Combined*n = 24 | p value | |
|---|---|---|---|---|
| Received any antibiotic | 10/12 (83) | 21/26 (81) | 18/24 (75) | 0.42 |
| Received IV antibiotic | 7/12 (58) | 6/26 (23) | 5/24 (21) | 0.044 |
| Duration of antibiotics, days | 6.3 [0.7–8.0] | 6.0 [0.4–6.9] | 2.0 [0–6.5] | 0.25 |
| Duration of IV antibiotic, hours | 1 [1–68] | 30 [1–91] | 1 [1–28] | 0.26 |
| Received a single dose of antibiotics only | 2/10 (20) | 5/21 (24) | 7/18 (39) | 0.46 |
| Received <24 h antibiotics | 3/10 (30) | 6 /21(29) | 8/18 (44) | 0.55 |
IV, intravenous. Data are presented a median [inter quartile range] and number (%) except where stated otherwise.
Respiratory syncytial virus (RSV), parainfluenza viruses 1–4, human metapnumovirus and human coronaviruses.
Fig. 4.
Kaplan Meier curve showing antibiotic use over time for patients testing positive by POCT for influenza, rhino/enterovirus and other viruses combined (RSV, parainfluenza virus 1–4, human metapneumovirus, and human coronavirus). Log rank test, p = 0.53.
Discussion
This study shows that syndromic molecular POCT for respiratory viruses in adults presenting to hospital with exacerbation of airways disease, leads to a reduction in antibiotic use due to early discontinuation in those testing positive for viruses. Most viruses detected in these patients were non-influenza viruses with rhino/enterovirus being the most commonly detected single virus type, and there was no difference in the rate of antibiotic discontinuation between different virus types. As noted in the ResPOC study, POCT did not lead to a reduction in the proportion treated with antibiotics compared to routine clinical care however this is unsurprising as patients are started on antibiotics very early in their patient journey when admitted to hospital and this was often before the results of POCT can be made available. The reason that antibiotics are stopped early in patients with exacerbation of airways disease testing positive for viruses is unknown but is likely to be due to clinicians feeling the detection of a virus adequately explains their acute illness and giving them confidence that a bacterial infection is unlikely and no longer needs to be ‘covered’ with antibiotics, in combination with other data such as CRP level and radiology results.
As expected rhino/enterovirus was the most frequently detected virus in this group but the type of virus detected did not seem to influence the decision to stop antibiotics early, although as the numbers in each groups were small we cannot definitively exclude a difference. This suggests that for patients with exacerbation of airways disease respiratory virus testing should be performed at the point-of-care using a comprehensive syndromic multiplex panel rather than a molecular POCT for influenza alone, which would not detect the majority of viruses associated with early antibiotic discontinuation. It could be argued that for many of the patients in the study treated with antibiotics that they should not have been treated in any case, on clinical grounds alone, given that antibiotic use in patients with asthma exacerbation is already strongly discouraged by national society guidelines. However, despite these guidelines inappropriate antibiotic use in hospitalised patients with asthma remains very common8, 9, 10 and diagnostic uncertainty regarding the possibility of bacterial infection, especially in the early part of hospital admission before all diagnostic information is available, is likely to be a key driver of this practice. The early provision of accurate results regarding exacerbation aetiology seems to assist in rational antibiotic decision making and is a potential alternative or complementary strategy to biomarker-directed therapy.
There are additional reasons beyond the antibiotic stewardship benefits for choosing to test using a comprehensive syndromic POCT for a range of respiratory viruses rather than with a POCT for influenza alone. Although some molecular POCT platforms for influenza have diagnostic accuracy comparable to laboratory PCR, others have demonstrated inferior sensitivity in non-industry sponsored, real world studies20 potentially reducing their usefulness in the hospital setting where missed diagnoses have serious consequences including nosocomial transmission of influenza. Although influenza may represent a priority for hospital infection control teams during peak influenza season, the detection of other respiratory viruses is also important for infection control purposes as other viruses also pose a serious risk of nosocomial transmission and outbreaks, which are especially dangerous to immunocompromised patients in certain high risk areas such as haematology wards and renal units. In some hospitals a two-step testing process is adopted where a patient with a negative influenza POCT is then tested using a multiplex laboratory PCR, increasing costs and prolonging the time before result are available compared with a comprehensive syndromic POCT. Regulatory consideration are also important and rapid diagnostic platforms may not be able to be used at the POCT in certain countries due to regulatory requirements, for example the current FilmArray system is not fully CLIA waived in the US. A further significant consideration is obviously the higher cost associated with syndromic panels versus molecular POCT for influenza and there is clearly a need for health economic studies to provide evidence for the cost effectives of such an approach considering the incremental benefits of syndromic testing.
A lower rate of re-admission was seen in those testing positive for viruses versus those testing negative or control patients. The reason for this is not clear but it may be that non-viral exacerbations have a higher rate of subsequent exacerbations or even that clarity around the aetiology of exacerbation may reduce readmission.
Although the ResPOC study is the only published randomised controlled trial of syndromic respiratory virus testing at the point-of-care, other non-randomised, pre and post intervention studies have suggested similar benefits from rapid syndromic respiratory virus testing in terms of reducing unnecessary antibiotic use, in both paediatric and adults patients presenting to hospital.15, 16
The strengths of this study include the randomised nature of the parent study, the large overall cohort of patients studied and the pragmatic nature with broad generalisability to other UK and international centres. Its limitations include being a single-centre trial and the relatively small numbers of patients with asthma and COPD with each respiratory virus detected. Although it is likely to be generalisable to other centres we cannot rule out that the changes seen are dependent on the processes of care in UK hospitals. It is also currently uncertain as to how molecular POCT for respiratory viruses should be best implemented within the NHS and other health systems. Potential models include training clinical staff to perform the testing or the development of dedicated point-of-care testing laboratories staffed by technicians and located within, or close to, acute areas.21
In conclusion syndromic molecular POCT for respiratory viruses in adults with exacerbation of airways disease is associated with early discontinuation of unnecessary antibiotics in those testing positive for viruses. Most viruses detected were non-influenza viruses and there was no difference in antibiotic discontinuation between different virus types. These results suggest that syndromic molecular POCT for respiratory viruses should be favoured over POCT for influenza alone in adults with exacerbation of airways disease.
Funding
University of Southampton. The manufactures of the molecular test platform (Biofire Diagnostics LLC, Salt Lake City, Utah, US) had no role in the study conception, design, data analysis or manuscript preparation. The corresponding author had full access to all of the data and the final responsibility to submit for publication. This report is independent research supported by the National Institute for Health Research (NIHR Post Doctoral Fellowship, Dr Tristan Clark, PDF 2016-09-061). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Competing interests
All authors declare that they have no conflicts of interests.
Acknowledgements
We thank all of the patients and clinical staff at the Southampton General Hospital including; clinicians, nurses, and laboratory technicians. We thank the directors, research nurses, data managers, clinical trials assistants and laboratory staff at the NIHR Southampton Clinical Research Facility and the NIHR Southampton Biomedical Research Centre. We thank the staff at the R&D department, University Hospital Southampton NHS Foundation Trust and the NIHR Clinical Research Network, Wessex for their support throughout the trial.
References
- 1.O'Neill J. Tackling drug‐resistant infections globally: final report and recommendations. Rev Antimicrob Resist. 2016 https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf Accessed 28th March 2019. [Google Scholar]
- 2.Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A. Viruses and bacteria in acute asthma exacerbations e a GA2LEN-DARE* systematic review. Allergy. 2011;66:458e68. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston SL, Szigeti M, Cross M, Brightling C, Chaudhuri R, Harrison T. AZALEA trial Team. Azithromycin for acute exacerbations of Asthma: the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176(11):1630–1637. doi: 10.1001/jamainternmed.2016.5664. [DOI] [PubMed] [Google Scholar]
- 4.Graham VA, Milton AF, Knowles GK, Davies RJ. Routine antibiotics in hospital management of acute asthma. Lancet. 1982;1(8269):418–420. doi: 10.1016/s0140-6736(82)91619-1. [DOI] [PubMed] [Google Scholar]
- 5.Stefan MS, Shieh MS, Spitzer KA, Pekow PS, Krishnan JA, Au DH. Association of antibiotic treatment with outcomes in patients hospitalized for an asthma exacerbation treated with systemic corticosteroids. JAMA Intern Med. 2019 doi: 10.1001/jamainternmed.2018.5394. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma. Global strategy for asthma management and prevention. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-tracked_v1.3.pdf. Published 2015. Accessed 29th March 2019.
- 7.BTS/SIGN British Guideline on the Management of Asthma. 2016. https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma. Accessed 29th March 2019.
- 8.Lindenauer PK, Stefan MS, Feemster LC, Shieh MS, Carson SS, Au DH. Use of antibiotics among patients hospitalized for exacerbations of asthma. JAMA Intern Med. 2016;176(9):1397–1400. doi: 10.1001/jamainternmed.2016.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark TW, Medina MJ, Batham S, Curran MD, Parmar S, Nicholson KG. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69:507–515. doi: 10.1016/j.jinf.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brendish N, Malachira A, Armstrong L, Houghton R, Aitken S, Nyimbili E. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10 doi: 10.1002/14651858.CD010257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohan A, Chandra S, Agarwal D, Guleria R, Broor S, Gaur B. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15(3):536–542. doi: 10.1111/j.1440-1843.2010.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stolz D, Christ-Crain M, Bingisser R, Leuppi J, Miedinger D, Müller C. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 14.Prins HJ, Duijkers R, van der Valk P, Schoorl M, Daniels JMA, van der Werf TS. CRP-guided antibiotic treatment in acute exacerbations of COPD admitted to hospital. Eur Respir J. 2019 doi: 10.1183/13993003.02014-2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Rappo U, Schuetz AN, Jenkins SG, Calfee DP, Walsh TJ, Wells MT. Impact of early detection of respiratory viruses by multiplex PCR assay on clinical outcomes in adult patients. J Clin Microbiol. 2016;54(8):2096–2103. doi: 10.1128/JCM.00549-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers BB, Shankar P, Jerris RC, Kotzbauer D, Anderson EJ, Watson JR. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139(5):636e41. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 17.Brendish NJ, Malachira AK, Beard KR, Ewings S, Clark TW. Impact of turnaround time on outcome with point-of-care testing for respiratory viruses: a post hoc analysis from a randomised controlled trial. Eur Respir J. 2018;52(2) doi: 10.1183/13993003.00555-2018. [DOI] [PubMed] [Google Scholar]
- 18.Brendish NJ, Schiff HF, Clark TW. Point-of-care testing for respiratory viruses in adults: the current landscape and future potential. J Infect. 2015;71(5):501–510. doi: 10.1016/j.jinf.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brendish NJ, Malachira AK, Clark TW. Molecular point-of-care testing for respiratory viruses versus routine clinical care in adults with acute respiratory illness presenting to secondary care: a pragmatic randomised controlled trial protocol (ResPOC) BMC Infect Dis. 2017;17(1):128. doi: 10.1186/s12879-017-2219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merckx J, Wali R, Schiller I, Caya C, Gore GC, Chartrand C. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med. 2017;167(6):394–409. doi: 10.7326/M17-0848. [DOI] [PubMed] [Google Scholar]
- 21.Point of Care Tests for Influenza and other Respiratory Viruses . Support for hospital trusts considering the introduction of point of care testing (POCT) for influenza and other respiratory viruses during winter 2018 to 2019. Public Heath Engl. 2018 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/762344/point_of_care_tests_for_influenza_and_other_respiratory_viruses.pdf Accessed 29th March 2019. [Google Scholar]