Abstract
Objectives of the present study were to characterize the dose dependency of an intravenous Escherichia coli O111:H8 challenge in colostrum-fed Jersey calves and to identify any biochemical markers indicative of septicemia. Eighteen 3-week old colostrum-fed Jersey calves were completely randomized to 1 of 6 doses of E. coli O111:H8. The challenge doses included 0, 1.5 × 105, 1.5 × 106, 1.5 × 107, 1.5 × 108, and 1.5 × 109 colony-forming units (CFU) given intravenously as a bolus in 5 mL of sterile isotonic saline. Peripheral blood samples were collected at 0, 2, 4, 8, 12, 24, and 48 h relative to the challenge for biochemical, total leukocyte count, and differential analyses. Rectal temperatures were collected via indwelling rectal temperature probes at 5-min intervals, and hourly averages calculated from 2 d prior to the challenge till 2 d after the challenge. All calves survived the 48 h observation period following the challenge. Calves given 1.5 × 108 and 1.5 × 109 CFU displayed sickness behaviors (P < 0.01) beginning 0.5 h after the challenge and returned to that of the control calves by 6 and 32 h for calves challenged with 1.5 × 108 and 1.5 × 109 CFU, respectively. There were treatment × time interactions (P < 0.01) on total leukocyte counts and plasma glucose and zinc concentrations. Calves administered 1.5 × 108 and 1.5 × 109 CFU had leucopenia beginning 2 h after the challenge and returning to counts similar to the control calves within 24 h. Additionally, those calves were hypoglycemic from 4 to 12 h after the challenge with the degree of hypoglycemia inversely related to the dose of the E. coli. All calves challenged with E. coli had decreased plasma zinc concentrations, and the magnitude was inversely proportional to the challenge dose. There were treatment × time interactions (P < 0.001) on rectal temperatures following the challenge. All calves challenged with E. coli developed a febrile response, but the intensity and duration of the response were dependent on the challenge dose. These data indicate that calves intravenously challenged with 1.5 × 108 and 1.5 × 109 CFU of the E. coli O111:H8 showed immediate clinical and biochemical signs indicative of septicemia. However, calves administered 1.5 × 107 or less of the E. coli had febrile responses, but did not develop septicemia. Blood glucose and zinc concentrations may be dose responsive indicators that could potentially differentiate between a septicemic versus non-septicemic calf.
Keywords: Calf, Inflammatory response, Septicemia
1. Introduction
It is well documented that dairy calves are highly susceptible to disease and early mortality (Roy, 1990). Enteric disease is the primary cause of pre-weaning morbidity and mortality, and many enteric pathogens can cause bacteremia and septicemia among dairy calves. Thirty-one percent of scouring calves were bacteremic and survival rates were low, only 12% (Lofstedt et al., 1999). In agreement, Fecteau et al. (1997) in 2 experiments observed that 24 and 31% of severely ill calves were bacteremic and the mortality of blood culture positive calves was greater, 57.4%, versus 15.1% for blood culture negative calves. Fecteau et al. (2001) reported that of the 51 bacterial isolates, 51% were Escherichia coli, 25.5% from other gram negative enterics, 5.9% gram negative anaerobes, 11.8% gram positive cocci, and 5.9% gram positive rods. The high mortality in blood culture bacteria positive calves could be associated with septic shock due to an over-aggressive systemic acute phase response.
The systemic release of pro-inflammatory mediators such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in response to bacterial derived immunogens causes vasodilation, increases vascular permeability, and activates the clotting cascade all of which decrease perfusion of blood to many vital organs and can lead to irreversible organ damage and consequently death. Immediate administration of antibiotic and therapeutic treatments would need to be implemented for a septicemic calf; however, differentiating between a septicemic and non-septicemic calf with scours is difficult based on clinical observations alone (Fecteau et al., 1997). There is a need for an inexpensive and rapid on-farm test that can accurately and precisely identify septicemic calves. The objective of the present study was to characterize the pathogenesis of septicemia in colostrum-fed Jersey calves and identify any potential biochemical markers indicative of septicemia.
2. Materials and methods
2.1. Experimental design and calves
All animal care was conducted according to the Guide for the Care and Use of Agriculture Animals in Agricultural Research and Teaching and approved by the Institutional Animal Care and Use Committee of the USDA-ARS. Twenty Jersey bull calves (1 ± 1 d of age) were acquired from a local commercial dairy in January 2010 and transported 130 km to the Livestock Issue Research Unit calf facility in Liberty, TX. The calves were given 4 quarts of pooled colostrum within the first 12 h of life. Cows in the herd are routinely vaccinated against rotavirus, coronavirus, and the J-5 lipopolysaccharide (LPS) core and K99+ antigens of E. coli. Peripheral blood samples were collected immediately upon arrival and serum collected and quantified for total serum protein using a temperature controlled refractometer (Atago Inc., Bellevue, WA). Antibodies to the J-5 LPS core antigen could have attenuated the response in the current study if compared with calves that received colostrum from cows that were not vaccinated against the J-5 LPS core antigen or did not receive colostrum (Deluyker et al., 2004). Calves were individually housed in stainless steel pens (1 m × 2 m) with a slotted rubber mat floor in a thermo-controlled room (ranged from 20.3 to 21.9 °C). The calf nursery was illuminated with fluorescent lighting 24 h a day. All calves were fed 454 g/d as-fed of a commercial, non-medicated, 20% fat and 20% all milk protein, milk replacer (Lawley's Inc., Stockton, CA) for a 21-d adaptation period. During this period, calves with scours were treated therapeutically with acidified oral electrolytes (Bluelite C, Tech Mix, Stewart, MN) and bismuth subsalicylate added directly to the milk replacer. Calves were fed twice daily at 0800 and 1630 h.
On d 18 all calves were fitted with indwelling rectal temperature probes (Reuter et al., 2010). The rectal temperature probes consisted of a DST micro-T small thermo logger (Star Oddi, Reykjavik, Iceland) enclosed in a 1 mm outside diameter × 4.5 mm long stainless steel fabricated capsule that was attached to a 0.75 mm outside diameter and 11 mm long PVC tube. The PVC tube was attached to half a fabricated piece of powder-coated aluminum pipe, 11 mm long × 5 mm internal diameter and secured to the tail using Co-flex flexible bandage (Andover Healthcare, Andover, MA). Rectal temperatures were collected via the DST micro-T small thermo logger at 5-min intervals, and hourly averages calculated from −48 h prior to the challenge till 48 h after the challenge.
Prior to d 21, one calf died. On d 21, eighteen of the remaining 19 calves were completely randomized to 1 of 6 intravenous treatment doses of an E. coli O111:H8. The E. coli expressed the intimin and shiga-like toxin 1 genes. The challenge doses included 0, 1.5 × 105, 1.5 × 106, 1.5 × 107, 1.5 × 108, and 1.5 × 109 colony-forming units (CFU) given as a bolus by venapuncture in 5 mL of sterile isotonic saline immediately following the AM feeding. The challenge doses were prepared from a 12 h, log-growth culture in tryptic soy broth. Briefly, 40 mL of tryptic soy broth was inoculated with a pure culture of the E. coli O111:H8 from a Microbank bead (Pro-lab Diagnostics, Austin, TX). The culture was incubated for 12 h at 37 °C and 200 rpm. The quantity of free LPS was determined in duplicate cultures, grown under the same conditions used in the challenge culture, by a commercial laboratory (Nelson Laboratories, Salt Lake City, UT) using the limulus amebocyte lysate method. Confirmation of challenge doses was performed approximately 30 min before the challenge was administered by spread plating serial dilutions. The range of the E. coli challenge doses was based on data from Smith and Halls (1968), and 21 d old calves were used in the current study to ensure that all calves were free of any clinical signs of enteric or systemic disease. Additionally, a 48 h observation period was evaluated based on the clinical course of the disease observed by Smith and Halls (1968) after various challenge strains of E. coli were administered intravenously. The E. coli O111:H8 strain was chosen in the present study because our lab has used it in our phagocytic and oxidative burst assays and it was shown to be rapidly phagocytized and produce a robust oxidative burst (Hulbert et al., 2011).
2.2. Observations and sampling
The displaying of sickness behaviors was assessed before and at 30 min intervals through 8 h, then hourly till 12 h, and finally at 24, 32, and 48 h relative to the challenge by a single blinded observer. Behavior was classified as 1 = normal, alert, response to stimuli quick; 2 = depressed, response to stimuli decreased; 3 = lethargic, response to stimuli greatly reduced; 4 = morbid, little or no response to stimuli. All attitude data were collected by 1 observer. Ten mL of peripheral blood were collected by venapuncture at −24, 0, 2, 4, 8, 12, 24, and 48 h relative to the challenge into heparinized vacutainers for leukocyte and differential count analyses. Following leukocyte and differential analyses, whole blood was centrifuged at 1200 × g for 15 min and plasma removed and stored at −40 °C until biochemical analyses.
2.3. Blood leukocyte and plasma metabolite analyses
Within 30 min of blood collection, whole blood was analysed for total leukocyte counts and differential analyses of neutrophils and mononuclear cells using a Cell Dyn 3700 (Abbot Laboratories, Abbott, IL). The setting on the Cell Dyne 3700 for bovine blood did not further differentiate the mononuclear cells into lymphocytes and monocytes, therefore lymphocyte counts encompass both lymphocytes and monocytes. Plasma glucose and urea nitrogen were analysed using commercially available enzymatic kits (Stanbio Laboratory, Boerne, TX). All procedures were followed per the manufacturer's instructions for the manual method except reduced sample and reagent volumes were used in a 96-well microplate method. Absorbance was measured on a SpectraMax 340PC (Molecular Devices, Sunnyvale, CA). Control serum (Randox Laboratories, Jefferson City, WV) was used to calculate the inter-assay coefficients of variations of 4.5 and 5.8% for plasma glucose and urea nitrogen, respectively. Intra-assay coefficients of variations were 3.7 and 4.2% for plasma glucose and urea nitrogen, respectively. Plasma cytokines, tumor necrosis factor-α, interleukin-6, and interferon-γ, were measured using a multi-plexed sandwich-based chemiluminescence ELISA (Searchlight-Aushon Biosystems, Inc., Billerica, MA). The inter- and intra-assay coefficients of variations were 9.2 and 12.1%, respectively. Plasma haptoglobin was quantified as described by Makimura and Suzuki (1982). The inter- and intra-assay coefficients of variation for the haptoglobin assay were 1.8 and 1.9%, respectively. Plasma zinc concentrations were analysed by atomic absorption spectrometry (Smimadzu AA-6300, Columbia, MD) after plasma protein was precipitated by mixing equal volumes of plasma and 20% trichloroacetic acid and incubating at 100 °C for 20 min. Intra- and inter-assay coefficients of variation were 2.1 and 2.6%, respectively.
2.4. Statistical analyses
All repeated, continuous data were analysed by restricted maximum likelihood ANOVA for a completely randomized design using the Mixed procedure of SAS (version 9.2, SAS Institute, Cary, NC). A linear, mixed model with the fixed effects of treatment, time, and the interaction of treatment × time was fitted. Mean biochemical measurements at −24 and 0 h and mean rectal temperature from −48 to 0 h relative to the E. coli challenge were used as covariates in the model. The mixed model was run with all available covariance structures for the within-subject measurement and the appropriate covariance structure for unequal spacing was chosen based on the Schwarz's Bayesian information criterion. The sickness score data were analysed by generalized estimating equations with a poisson distribution using the GenMod procedure of SAS. Calf was the repeated unit and the exchangeable covariance structure was used to fit the data. Means separation was performed at each time for significant treatment × time interactions by using a sliced-effect multiple comparison approach with a Tukey–Kramer adjustment (version 9.2, SAS Institute, Cary, NC). Least squares means (±SEM) are reported throughout. A treatment difference of P ≤ 0.05 was considered statistically significant, and 0.05 < P ≤ 0.10 was considered a tendency.
3. Results
3.1. Sickness scores
All but 1 calf had total serum protein concentrations greater than 5.0 g/100 mL. Serum protein concentrations averaged 5.58 ± 0.256 g/100 mL. The quantity of free endotoxin was 10 μg (±coefficient of variation of 22%) in the calves challenged with 1.5 × 109 CFU. When expressed per kg of BW those calves received approximately 0.32 μg free LPS/kg BW. Calves challenged with either 1.5 × 108 or 1.5 × 109 CFU as a bolus IV developed sickness behaviors within 30 min post challenge (P < 0.01; Fig. 1 ). The acute response, over the first 12 h, was dose dependent; whereas 1.5 × 109 CFU challenged calves had a more severe response and their behavior continued to be altered until 32 h post-challenge. Calves challenged with 1.5 × 108 CFU sickness behaviors had returned to normal within 6 h post-challenge; whereas the calves challenged with 1.5 × 107 CFU showed mild signs of sickness behaviors only at 12 h post-challenge. Finally, calves challenged with either 1.5 × 105 or 1.5 × 106 CFU did not show any signs of sickness during the 48 h post-challenge observation period.
Fig. 1.
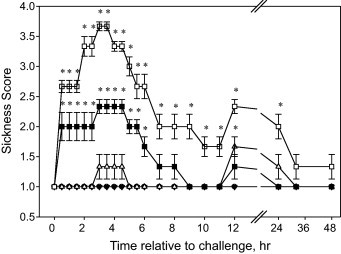
Effects of intravenous E. coli challenge dose on sickness scores (LSmeans ± SEM). ● = 0; ○ = 1.5 × 105; ▾ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. Treatment × time P < 0.001; sliced time effects reported as *P < 0.05.
3.2. Cytokine responses
Calves challenged with either 1.5 × 108 or 1.5 × 109 CFU had elevated peripheral pro-inflammatory cytokine concentrations (Fig. 2a and b). The elevated concentrations of TNF-α were not statistically significant, which is likely due to the sampling frequency in the current study. There were dose dependent responses (P < 0.001) in the concentration of IL-6; whereas calves challenged with 1.5 × 109 CFU had a greater magnitude and persistent response than calves challenged with 1.5 × 108 CFU (Fig. 2b). Calves challenged with 1.5 × 107 CFU or less did not have concentrations of either of the pro-inflammatory cytokines, TNF-α or IL-6, above baseline at any time following the challenge. Peripheral concentrations of interferon-γ (IFN-γ) were elevated in all calves except those not challenged or challenged with 1.5 × 105 CFU (Fig. 2c). Concentrations of IFN-γ peaked (P < 0.01) in calves challenged with 1.5 × 109 four h post-challenge and returned to baseline by 24 h. Concentrations of IFN-γ began increasing in all other doses at 12 h post-challenge and had returned back to baseline by 48 h. Furthermore, calves challenged with the intermediate dose of E. coli, 1.5 × 107 CFU, had the greatest response at 12 h post-challenge.
Fig. 2.
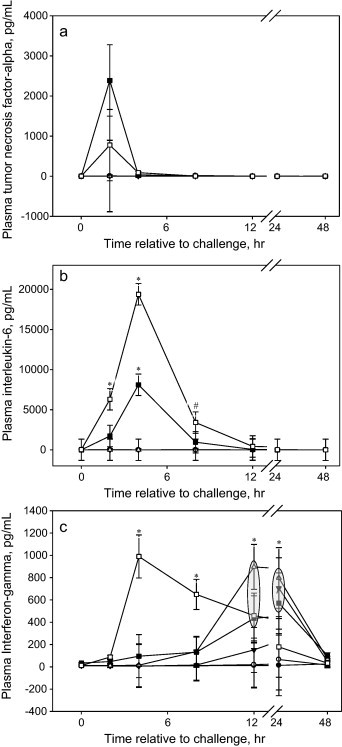
(a) Effects of intravenous E. coli challenge dose on plasma concentrations of tumor necrosis factor-α (LSmeans ± SEM). ● = 0; ○ = 1.5 × 105; ▾ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. No effects of treatment or treatment × time were evident. (b) Effects of intravenous E. coli challenge dose on plasma concentrations of interleukin-6 (LSmeans ± SEM). ● = 0; ○ = 1.5 × 105; ▾ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. Treatment × time P < 0.001; sliced time effects reported as *P < 0.01 and #P < 0.10. (c) Effects of intravenous E. coli challenge dose on plasma concentrations of interferon-γ (LSmeans ± SEM). ● = 0; ○ = 1.5 × 105; ▾ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. Treatment × time P < 0.001; sliced time effects reported as *P < 0.05;  denotes multiple treatments at a particular significance.
denotes multiple treatments at a particular significance.
3.3. Total leukocytes and differentials
Acute leucopenia, within 2 h post-challenge, were observed (P < 0.01) in calves administered either 1.5 × 108 or 1.5 × 109 CFU and persisted through 12 h post-challenge (Fig. 3a). The calves challenged with 1.5 × 107 CFU developed a mild leucopenia at 12 and 48 h post-challenge. There were treatment × time interactions for the percentage of circulating neutrophils (P < 0.01); however, individual treatment peripheral neutrophil percentages were highly variable (Fig. 3b). Calves challenged with 1.5 × 105 CFU had reduced percentages of peripheral neutrophils at 4 h post-challenge; whereas calves challenged with 1.5 × 106 CFU had increased percentages of neutrophils in circulation at 12 h post-challenge. Additionally, calves challenged with 1.5 × 109 CFU had increased percentages at 12 h post-challenge.
Fig. 3.
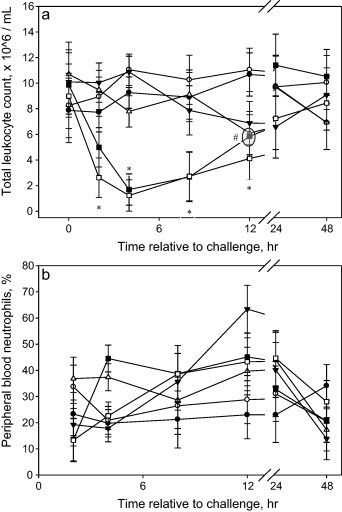
(a) Effects of intravenous E. coli challenge dose on peripheral blood total leukocytes (LSmeans ± SEM). ● = 0; ○ = 1.5 × 105; ▾ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. Treatment × time P < 0.001; sliced time effects reported as *P < 0.05 and #P < 0.10;  denotes multiple treatments at a particular significance. (b) Effects of intravenous E. coli challenge dose on the percentage of peripheral blood neutrophils (LSmeans ± SEM). ● = 0; ○ = 1.5 × 105; ▾ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. Treatment × time P < 0.01; sliced time effects reported as *P < 0.05.
denotes multiple treatments at a particular significance. (b) Effects of intravenous E. coli challenge dose on the percentage of peripheral blood neutrophils (LSmeans ± SEM). ● = 0; ○ = 1.5 × 105; ▾ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. Treatment × time P < 0.01; sliced time effects reported as *P < 0.05.
3.4. Blood metabolites and haptoglobin
Acute hypoglycemia was observed (P < 0.001) in calves administered either 1.5 × 108 or 1.5 × 109 CFU, but had returned to baseline concentrations by 12 h post-challenge (Fig. 4a). There was no effect of intravenous E. coli dose on the concentrations of plasma urea nitrogen (data not shown). Twelve h post-challenge plasma haptoglobin concentrations were increased (P < 0.05) in calves challenged with 1.5 × 109 CFU, which tended (P < 0.10) to also be elevated at 24 h post-challenge (Fig. 4b). At 24 and 48 h post-challenge calves administered the intermediate dose of E. coli, 1.5 × 107 CFU, had the greatest increase (P < 0.01) in plasma haptoglobin concentrations. At 24 h post-challenge, calves administered 1.5 × 106 had elevated (P < 0.01) haptoglobin concentrations. Plasma zinc concentrations decreased (P < 0.001) in all calves that were challenged with the E. coli (Fig. 4c). The magnitude of the decrease in plasma zinc was inversely proportional to the challenge dose.
Fig. 4.
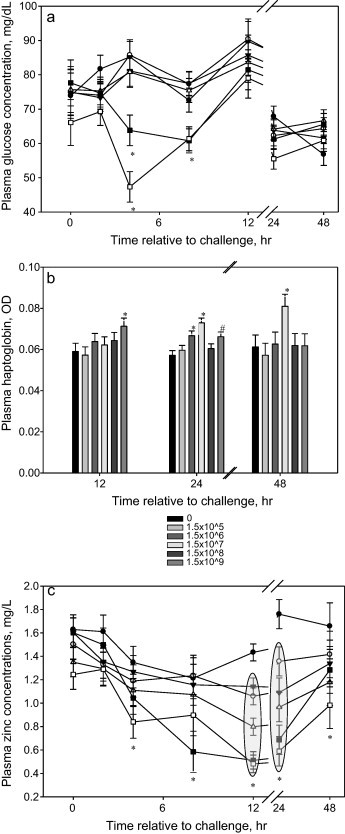
(a) Effects of intravenous E. coli challenge dose on the concentration of plasma glucose (LSmeans ± SEM). ● = 0; ○ = 1.5 × 105; ▾ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. Treatment × time P < 0.001; sliced time effects reported as *P < 0.05. (b) Effects of intravenous E. coli challenge dose on the concentration of plasma haptoglobin (LSmeans ± SEM). Treatment × time P = 0.57; treatment P = 0.62; sliced time effects reported as *P < 0.05 and #P < 0.10.
3.5. Rectal temperatures
All calves challenged with intravenous E. coli developed a febrile response (P < 0.001; Fig. 5 ). Calves challenged with lower doses of E. coli, 1.5 × 105 and 1.5 × 106 CFU had febrile responses that persisted from 20 to 35 h and 10 to 35 h post-challenge, respectively. The calves challenged with the intermediate dose, 1.5 × 107 CFU, had the greatest peak febrile response, peaking between 11 and 14 h post-challenge. Further, all calves challenged with greater or equal to 1.5 × 107 CFU had febrile responses that persisted through 48 h post-challenge.
Fig. 5.
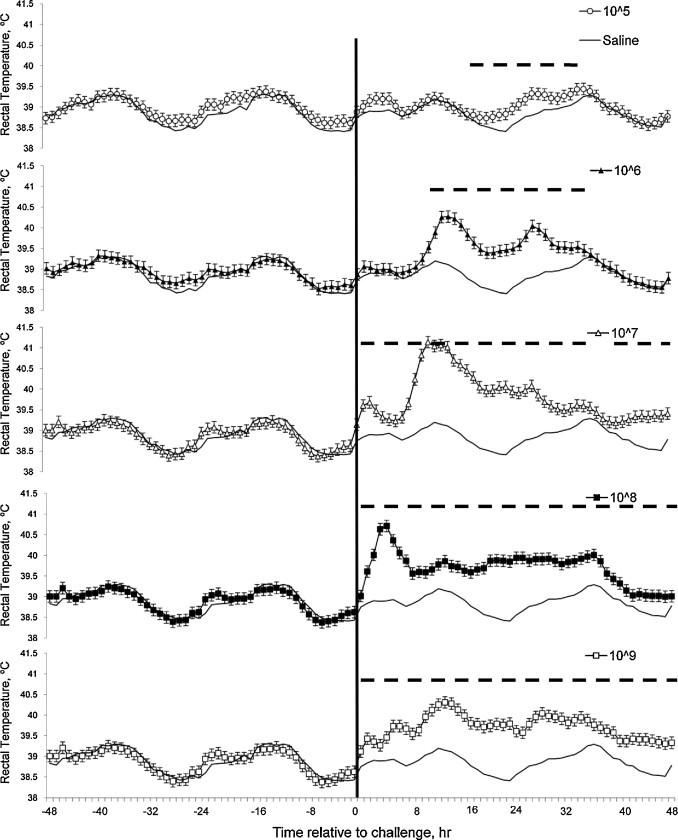
Effects of intravenous E. coli challenge dose on rectal temperatures (LSmeans ± SEM). Black line = 0; ○ = 1.5 × 105; ▴ = 1.5 × 106; ▵ = 1.5 × 107; ■ = 1.5 × 108; □ = 1.5 × 109 colony-forming units. Treatment × time P < 0.001; sliced time effects (P < 0.01) intervals reported as ---.
4. Discussion
The dose response impacts of an intravenous E. coli O111:H8 challenge and the pathogenesis of the disease states in colostrum fed Jersey calves were investigated. The 2 largest doses of E. coli O111:H8 used in this study induced acute signs of septicemia including sickness behavior, febrile responses, increased peripheral pro-inflammatory cytokines, hypoglycemia, and leucopenia. Many of the responses were dose dependent. Calves challenged with 1.5 × 108 CFU displayed severely depressed behaviors while calves challenged with 1.5 × 109 CFU became recumbent and 2 of the 3 calves had no reaction to stimuli during their peak response. The greatest scores for sickness behaviors among calves treated with the two highest doses occurred between 3 and 4.5 h post-challenge; after which these calves began to recover. Calves challenged with 1.5 × 108 CFU had recovered within 7 h, but the latency until recovery for calves challenged with 1.5 × 109 CFU was longer, occurring within 32 h. Smith and Halls (1968) also observed immediate sickness behaviors in both colostrum deprived and adequate Jersey calves when challenged intravenously with 109.7 CFU of E. coli 078:K80, which persisted from 1–5 h post-challenge. In the current study, the hypoglycemia was more severe in calves challenged with 1.5 × 109 CFU than those challenged with 1.5 × 108 CFU. Dose dependent clinical and biochemical responses to endotoxin were observed in cattle, but it was dependent on the response variable (Jacobsen et al., 2004). This is evident in the current study where the magnitude and temporal response of the leucopenia were similar for calves challenged with either of the two high doses of E. coli. The acute responses observed in these animals was similar to those observed when Jersey calves are challenged with purified LPS; however, the febrile response was sustained through 48 h post-challenge using the live pathogen; whereas rectal temperatures in Jersey calves returned to baseline within 24 h after the LPS challenge in a previous study (Ballou et al., 2008). It is probable that the acute signs of septicemia in the calves challenged with either 1.5 × 108 or 1.5 × 109 CFU were at least partially attributable to the concentrations of free LPS in the challenge preparations.
Twelve h post-challenge 2 of the 3 calves challenged with 1.5 × 107 CFU displayed depressed sickness behaviors, which corresponded with their peak rectal temperature, mild leucopenia, and the nadir in their plasma zinc concentrations. Calves challenged with 1.5 × 107 CFU had the greatest peaks in rectal temperatures, which occurred 11–14 h post-challenge. The greater magnitude in febrile response observed in these calves may have occurred in response to an attenuated counter anti-inflammatory response. A robust counter anti-inflammatory response is often observed during endotoxemia and septicemia (Ejima et al., 2003). Although calves challenged with either 1.5 × 105 or 1.5 × 106 CFU did not display any sickness behaviors or changes in leucocytes or biochemical responses during the 48 h post-challenge observation period, they did develop dose-dependent febrile and decreased plasma zinc concentration responses. Calves challenged with 1.5 × 105 CFU displayed a slight febrile response from 20 to 35 h post-challenge; whereas calves challenged with 1.5 × 106 CFU had febrile responses of greater magnitude and length, lasting from 10 to 35 h post-challenge. These data indicate calves challenged with less than or equal to 1.5 × 107 CFU developed acute phase responses, but did not become septicemic. This could be a reflection of the good colostrum status of these calves or the E. coli O111:H8 used in the current study. These data also indicate that rectal temperatures and plasma zinc concentrations are very sensitive indicators of infection, but not necessarily specific for septicemia.
Pro-inflammatory cytokines, TNF-α and IL-6 were only acutely elevated in calves challenged with the 2 largest doses, 1.5 × 108 and 1.5 × 109 CFU, of E. coli O111:H8. Peripheral inflammation during septicemia and endotoxemia can directly activate the central nervous system and alter the thermoregulatory center of the brain (Givalois et al., 1994); however, in localized inflammation, febrile responses are still observed, even in the absence of peripheral pro-inflammatory cytokines (Zhang et al., 2008). The current data indicate that the febrile and decreased plasma zinc concentration responses during infection without concurrent septicemia were independent of peripheral concentrations of the pro-inflammatory cytokines, TNF-α and IL-6. Additionally, the leucopenia and increased plasma haptoglobin concentrations in calves challenged with 1.5 × 107 CFU were not influenced by peripheral cytokine concentrations. In contrast, it is well documented in rats and humans that acute phase protein synthesis is responsive to the pro-inflammatory cytokines, TNF-α and IL-1 and IL-6 (Koj, 1985, Gabay et al., 1996). Furthermore, IL-6 is considered a major inducer of acute phase protein synthesis (Heinrich et al., 1990). Despite elevations in haptoglobin observed at 12 and 24 h post-challenge in the 1.5 × 109 CFU challenged calves, the current data indicate that the intermediate dose of E. coli O111:H8, 1.5 × 107 CFU, had the greatest and most sustained increase in plasma haptoglobin. Localized inflammation or other peripheral factors not measured in the current study may have influenced the response. The greater response observed in calves challenged with 1.5 × 107 CFU could be due to less of an acute counter anti-inflammatory response that was likely observed in calves challenged with either 1.5 × 108 or 109 CFU.
Peripheral concentrations of IFN-γ were elevated in calves challenged with greater or equal to 1.5 × 106 CFU; however, the responses were dose dependent. Calves challenged with 1.5 × 109 CFU had acute responses with greatest elevation of IFN-γ at 4 h. Previous research from our laboratories has shown that IFN-γ concentrations in plasma increase following an intravenous LPS challenge among beef calves, peaking at 4–5 h and returning to baseline concentrations within 12 h (Carroll et al., 2009). The concentrations of IFN-γ in plasma of these Jersey calves challenged with 1.5 × 109 CFU were approximately 10 fold higher than observed by Carroll et al. (2009) (990 versus 120 pg/mL). The current data taken together with the data from Carroll et al. (2009) suggest that some immunogens from the live culture are stimulating IFN-γ secretion in addition to what is observed from LPS alone. The source of the IFN-γ measured in plasma is unknown, but could either come from direct stimulation of natural killer cells or activated T-lymphocytes. In addition, calves challenged with 1.5 × 108 CFU, having displayed clinical, hematological, and biochemical responses indicative of septicemia had a very different temporal IFN-γ response, peaking at 12 h and returning to baseline within 48 h post challenge, than that observed in calves challenged with either 1.5 × 109 CFU or purified LPS (Carroll et al., 2009). The temporal response observed in calves challenged with 1.5 × 108 CFU was similar to that for calves challenged with either 1.5 × 106 or 1.5 × 107 CFU. The more delayed cytokine response observed among these calves is likely a reflection of the link between the innate and adaptive immune responses, but secretion from natural killer cells cannot be ruled out. Similar temporal results have been observed in rats challenged with Streptococcus pneumonia and furthermore using interferon-γ knock-outs they were able to show a protective role for interferon-γ (Rubins and Pomeroy, 1997).
Finally, identifying sensitive and specific biochemical markers that can be used on farm to identify a severely ill calf as either septicemic or non-septicemic would be helpful in determining appropriate therapeutic treatment. Hypoglycemia, leucopenia, and decreased plasma mineral concentrations in calves following LPS challenges have been consistently reported (Kinsbergen et al., 1994, Deluyker et al., 2004, Ballou et al., 2008). The current data suggest that plasma glucose and zinc maybe dose–responsive indicators of acute E. coli septicemia/endotoxemia. The diagnostic value of using either plasma glucose or zinc concentrations to identify a calf that is septicemic versus non-septicemic calf with scours is unknown; future research should determine the sensitivity and specificity of each metabolite to screen severely ill calves for septicemia. It is plausible that non-septicemic calves with scours could also have decreased plasma glucose and/or zinc concentrations due to decreased absorption of nutrients.
In conclusion, calves challenged with 1.5 × 108 and 1.5 × 109 CFU of E. coli O111:H8 rapidly developed signs of septicemia/endotoxemia. The degree of hypoglycemia and the decrease in plasma zinc observed was directly related to the challenge dose. Calves challenged with the intermediate dose of 1.5 × 107 CFU developed a robust acute phase response with a stronger febrile response and more sustained acute phase protein synthesis. However, the acute phase response of the calves was independent of increases in peripheral concentrations of either the pro-inflammatory cytokines, TNF-α or IL-6. All calves developed a febrile response, indicating that rectal temperatures are a sensitive measure of infection, but non-specific for degree of infection. All Jersey calves fed colostrum survived 48 h following the E. coli O111:H8 challenge.
Acknowledgements
Mention of trade names or commercial products in this manuscript is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. The authors thank J.W. Dailey, of Livestock Issues Research Unit, USDA-ARS Lubbock, TX for his technical support.
References
- Ballou M.A., Cruz G.D., Pittroff W., Keisler D.H., DePeters E.J. Modifying the acute phase response of Jersey calves by supplementing milk replacer with omega-3 fatty acids from fish oil. J. Dairy Sci. 2008;91:3478–3487. doi: 10.3168/jds.2008-1016. [DOI] [PubMed] [Google Scholar]
- Carroll J.A., Reuter R.R., Chase C.C., Jr., Coleman S.W., Riley D.G., Spiers D.E., Arthington J.D., Galyean M.L. Profile of the bovine acute-phase response following an intravenous bolus-dose lipopolysaccharide challenge. Innate Immunity. 2009;15:81–89. doi: 10.1177/1753425908099170. [DOI] [PubMed] [Google Scholar]
- Deluyker H.A., Rossitto P., Van Oye S.N., Cullor J.S. Efficacy of an Escherichia coli J-5 mutant strain bacterin in the protection of calves from endotoxin disease caused by subcutaneous challenge with endtoxins from Escherichia coli. Vaccine. 2004;23:709–717. doi: 10.1016/j.vaccine.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Ejima K., Layne M.D., Carvajal I.M., Kritek P.A., Baron P.M., Chen Y., Vom Saal J., Levy B.D., Yet S., Perrella M.A. Cyclooxygenase-2 deficient mice are resistant to endotoxin-induced inflammation and death. FASEB J. 2003;17:1325–1327. doi: 10.1096/fj.02-1078fje. [DOI] [PubMed] [Google Scholar]
- Fecteau G., Parẻ J., Van Metre D.C., Smith B.P., Holmberg C.A., Guterbock W., Jang S. Use of a clinical sepsis score for predicting bacteremia in neonatal dairy calves on a calf rearing farm. Can. Vet. J. 1997;38:101–104. [PMC free article] [PubMed] [Google Scholar]
- Fecteau G., Fairbrother J.M., Higgins R., Van Metre D.C., Parẻ J., Smith B.P., Holmberg C.A., Jang S. Virulence factors in Escherichia coli isolated from the blood of bacteremic neonatal calves. Vet. Microbiol. 2001;78:241–249. doi: 10.1016/s0378-1135(00)00299-6. [DOI] [PubMed] [Google Scholar]
- Gabay C., Singwe M., Genin B., Meyer O., Mentha G., Le Coultre C., Vischer R., Guerne P.A. Circulating levels of IL-11 and leukaemia inhibitory factor (LIF) do not significantly participate in the production of acute phase proteins by the liver. Clin. Exp. Immunol. 1996;105:260–265. doi: 10.1046/j.1365-2249.1996.d01-757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givalois L., Dornand J., Mekaouche M., Solier M.D., Bristow A.F., Ixart G., Siaud P., Assenmacher I., Barbenel G. Temporal cascade of plasma level surges in ACTH corticosterone, and cytokines in endotoxin-challenged rats. Am. J. Physiol. 1994;267:R164–R170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem. J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert, L.E., Cobb, C.J., Carroll, J.A., Ballou, M.A., 2011. Effects of changing feeding milk replacer from twice to once daily on Holstein calf innate immune responses before and after weaning. J. Dairy Sci., in press. [DOI] [PubMed]
- Jacobsen S., Andersen P.H., Toelboell T., Heegaard P.M.H. Dose dependency and individual variability of the lipopolysaccharide-induced bovine acute phase protein response. J. Dairy. Sci. 2004;87:3330–3339. doi: 10.3168/jds.S0022-0302(04)73469-4. [DOI] [PubMed] [Google Scholar]
- Kinsbergen M., Bruckmaier R.M., Blum J.W. Metabolic, endocrine and haematological responses to intravenous E. coli endotoxin administration in 1-week-old calves. J. Vet. Med. A. 1994;41:530–547. doi: 10.1111/j.1439-0442.1994.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Koj A. The Acute-Phase Response to Injury and Infection. Elsevier; Amersterdam, Netherlands: 1985. Definition and classification of acute-phase proteins. pp. 189–197. [Google Scholar]
- Lofstedt J., Dohoo I.R., Duizer G. Model to predict septicemia in diarrheic calves. J. Vet. Intern. Med. 1999;2:81–88. doi: 10.1111/j.1939-1676.1999.tb01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimura S., Suzuki N. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Jpn. J. Vet. Sci. 1982;44:15–21. doi: 10.1292/jvms1939.44.15. [DOI] [PubMed] [Google Scholar]
- Reuter R.R., Carroll J.A., Hulbert L.E., Dailey J.W., Galyean M.L. Technical note: development of a self-contained, indwelling rectal temperature probe for cattle research. J. Anim. Sci. 2010;88:3291–3295. doi: 10.2527/jas.2010-3093. [DOI] [PubMed] [Google Scholar]
- Roy J.H.B. 5th ed. vol. 1. Butterworth Publishers Inc.; Boston, MA: 1990. The Calf. (Management of Health). [Google Scholar]
- Rubins J.B., Pomeroy C. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 1997;65:2975–2977. doi: 10.1128/iai.65.7.2975-2977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.W., Halls S. The experimental infection of calves with bacteriaemia-producing strains of Escherichia coli: the influence of colostrum. J. Med. Microbiol. 1968;1:61–78. doi: 10.1099/00222615-1-1-61. [DOI] [PubMed] [Google Scholar]
- Zhang H., Ching S., Chen Q., Li Q., An Y., Quan N. Localized inflammation in peripheral tissue signals the CNS for sickness response in the absence of interleukin-1 and COX-2 in blood and brain. Neuroscience. 2008;157:895–907. doi: 10.1016/j.neuroscience.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]


