Abstract
It was previously reported that up-regulation of α-enolase protein was detected in 65% of patients with non-small cell lung cancers (NSCLC). Moreover, a high titer of anti-α-enolase antibodies was developed in a smaller proportion (7.4%) of these patients than in non-tumor-associated patients and healthy subjects. In the present study, we characterized polyclonal and single-chain variable fragment (scFv) anti-α-enolase antibodies from immunized chickens. The E. coli-derived recombinant α-enolase protein was purified to its high homogenicity as verified by SDS-PAGE. After the 4th immunization, a high titer of specific polyclonal anti-α-enolase antibodies was elicited in immunized chickens and specifically recognized the purified human α-enolase antigen as determined by Western blot and ELISA. The expressed heavy and light chain variable genes (VH and VL) were isolated from spleen B cells and amplified to construct phage antibody libraries containing scFv molecules. After four rounds of panning selection, the scFv antibodies of randomly chosen clones were expressed and their binding specificity to α-enolase protein was verified using competitive ELISA, flow cytometry and immunofluorescence staining. Nucleotide sequence analysis from 10 α-enolase binding clones showed that 3 (30%) clones used identical heavy and light genes for scFv antibody expression, as represented by EnL5. Notably, amino acid changes in complementarity-determining regions (CDRs) were more frequently observed than those in framework regions (FRs) in all clones, indicating a strong affinity selection through mutations. All together, it is believed that these polyclonal and scFv IgY antibodies may be helpful in the development of molecular diagnostic and therapeutic agents for lung cancers in the future.
Abbreviations: scFv, single-chain variable fragment; E. coli, Escherichia coli; VH, heavy chain variable region; VL, light chain variable region; CDRs, complementarity-determining regions; FRs, framework regions
Keywords: α-Enolase, scFv, Phage display libraries
1. Introduction
Enolase was originally characterized as an enzyme involved in glycolytic metabolism catalyzing the conversion of 2-phosphoglycerate into phosphoenolpyruvate. In mammals, there are three isoforms of enolase, called α-ENO1, β-ENO3 and γ-ENO2 (Redlitz et al., 1995). The α-enolase is a major form of enolase present in the early stages of embryonic development; it is expressed ubiquitously in various types of tissue, whereas γ-ENO2 and β-ENO3 are exclusively found in neuron and muscle cells (Antikainen et al., 2007, Chang et al., 2006). By using an alternative translation start codon, ENO1 can be translated into c-myc promoter binding protein 1 (MBP-1), which is preferentially localized in cell nuclei (Feo et al., 2000). Both ENO1 and MBP-1 proteins are known to be negative regulators for c-myc expression.
Recently, it was reported that α-enolase is a multifunctional protein which exhibits enzymatic, structural, and receptor functions (Chang et al., 2006, Lee et al., 2003). In addition to its glycolytic function, α-enolase has been found to play an important role in several biological and pathophysiological processes (Sirover, 1996, Sirover, 1999). In particular, several studies have found α-enolase to play important roles in tumorigenesis. For example, Redlitz et al. (1995) found α-enolase on the cell surface, functioning as one of the plasminogen receptors which may play a role in tumor invasion. Up-regulation of α-enolase has also been reported in several highly tumorigenic or metastatic cell lines (Chang et al., 2006, Ito et al., 2007, Peebles et al., 2003, Wu et al., 2002, Zhang et al., 2000). α-Enolase over-expression has been correlated with tumorigenicity in several types of cancer, which suggests its pathophysiologic role in cancer formation (Altenberg and Greulich, 2004). Furthermore, an autoantigen of α-enolase was identified in non-small cell lung cancer and its over-expression was highly correlated with poor survival outcomes (Chang et al., 2006). In addition to its roles in cancer, α-enolase has been implicated in numerous diseases, including autoimmune disorders, ischaemia and bacterial infection (Antikainen et al., 2007, Bogdanos et al., 2004, Gitlits et al., 2001, Jiang et al., 1997, Kinloch et al., 2005, Saulot et al., 2002).
Generation of monoclonal antibodies with high specificity to various target antigens through traditional hybridoma technology is an expensive and tedious process (Groves and Morris, 2000). In recent years, a phage display system which is more cost and time effective involving the in vitro cloning of antibody heavy and light genes, has been extensively used to identify and isolate monoclonal antibodies from recombinant antibody repertories (Barbas et al., 1991, Winter et al., 1994). Smaller recombinant engineered antibody fragments, e.g. antigen-binding fragment (Fab) or single-chain variable fragment (scFv), are now emerging as new applications in diagnosis and therapy for the biotechnology market. ScFv molecules in which the VH and VL domains are joined with a flexible polypeptide linker usually retain the specific, monovalent antigen-binding affinity of the parent IgG, while showing improved pharmacokinetics for tissue penetration (Holliger and Hudson, 2005). Among all the animals suitable for antigen immunization, domestic chicken is the most feasible and available host for scFv antibody library construction to screen efficient binders against various pathogen infections for diagnostic applications (Fehrsen et al., 2005, Finlay et al., 2006, Park et al., 2005). From the aforementioned studies, α-enolase might serve as a potential target for diagnostic or therapeutic application clinically. Accordingly, in the present study, we generated and characterized the anti-α-enolase polyclonal IgY antibodies in chicken and monoclonal scFv antibodies by a phage display system using ELISA, Western blotting, flow cytometry and immunofluorescence staining.
2. Materials and methods
2.1. α-Enolase protein expression and purification
The gene encoding α-enolase protein was cloned from PE089 cells by reverse transcription-PCR using gene-specific primers 5′-GGTGGAATTCTATCTATTCTCAAGATCCATGCC-3′ (forward) and 5′-ACTCCATGGTTACTTGGCCAAGGGGTTTCT-3′ (reverse). The PE089 cell line was originally obtained from effusion tumor cells of a 36-year-old patient with stage IV lung adenocarcinoma, which was kindly provided by Dr. Neng-Yao Shih from National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan. The resultant PCR fragment was cloned into the pGEX-KG vector at EcoRI and NcoI sites and transformed into the E. coli BL-21 (DE3) strain for expression. The gene was also subcloned into the pET21a vector using 5′-CCGCGTGAATTCGGGGATCCATGTCTATTCTCAAGATCC-3′ (forward) and 5′-CATGGAGTCGACCTCGAGCTTGGCCAAGGGGTTTCTG-3′ (reverse) primers and expressed as His-fused α-enolase. Individual clones were grown in 5 ml LB medium containing ampicillin (100 μg/ml) at 37°C overnight. The bacterial culture was diluted 10-fold in the same LB medium and further grown until the OD600 reached between 0.6 and 1.0. To induce GST-fused or His-fused α-enolase protein expression, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.5 mM in the culture. The cell pellet was re-suspended in 2 ml of 1× PBS containing 1% Triton x-100 and lysed by three cycles of freezing (−70 °C) and thawing (37 °C). After centrifugation, the resulting cellular lysate was incubated with Glutathione Sepharose 4B or a Ni2+-charged resin column to purify the α-enolase protein according to the manufacturer's instruction (General Electronics, Piscataway, NJ, USA).
2.2. Chicken immunization
Female white leghorn (Gallus domesticus) chickens were immunized with 100 μg purified α-enolase in an equal volume of Freund's complete adjuvant by an intramuscular injection. Three additional immunizations with incomplete adjuvant were performed at intervals of 7 days. After each immunization, IgY antibodies in sera and egg yolk were collected and titrated by an enzyme-linked immunosorbent assay (ELISA) to determine the presence of humoral anti-α-enolase immune response. Egg yolk was separated from the egg white for IgY purification using 10% Dextran sulphate as described previously (Akita and Nakai, 1993a, Akita and Nakai, 1993b). The purified total IgY antibodies from each egg were dissolved in 5 ml of TBS containing 0.05% sodium azide and stored at −20 °C.
2.3. Construction of scFv antibody library and biopanning
The antibody library was established based on the previous report (Andris-Widhopf et al., 2000). Briefly, chicken spleens were harvested 7 days after the final immunization. Two hundred milligrams of spleen were homogenized in liquid nitrogen and placed in 2 ml Trizol solution for RNA extraction using the manufacturer's protocol (Invitrogen, USA). Ten micrograms of total RNA was reversely transcribed into the first-strand cDNA using a SuperScript RT kit (Invitrogen, USA). After amplification using chicken-specific primers (Andris-Widhopf et al., 2000), PCR products of heavy and light chain variable (VH and VL) regions were subjected to a second round of PCR with a short- or long-linker (Andris-Widhopf et al., 2000) to form full-length scFv fragments, which were further digested with SfiI and cloned into the pComb3X vector. Recombinant DNAs were transformed into E. coli XL-1 blue strain by electroporation. Recombinant phage production was initiated by the addition of VCS-M13 helper phage, precipitated with 4% polyethylglycol 8000 and 3% NaCl (w/v), and finally re-suspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and stored at 4 °C. Then, 1011 plaque-forming units (pfu) of recombinant phages from the scFv antibody library were added to wells pre-coated with α-enolase protein (0.5 μg/well), and incubated at 37 °C for 2 h. After removing unbound phages, bound phages were eluted with 0.1 M HCl/glycine (pH 2.2)/0.1% BSA, neutralized with 2 M Tris base buffer and used to infect the XL-1 blue strain. The amplified phages were precipitated and recovered as described above for the next round of selection. The panning procedure was repeated three or four times. A panel of clones were randomly selected and grown from the final panning process. After 0.5 mM IPTG induction for 6 h, bacterial cells were collected and lysed by three cycles of freezing and thawing and/or sonication. The supernatants were analyzed for their scFv antibody expression and binding reactivity to α-enolase using Western blotting and ELISA. ScFv antibodies expressed in TOP 10F′ E. coli (Invitrogen, a nonsuppressor strain) and purified using Ni2+-charged sepharose as described by the manufacturer (Amersham Biosciences, UK) were also prepared in flow cytometric and immunofluoresence analyses.
2.4. Western blotting and ELISA
To detect the scFv antibody expression, the cellular lysates were subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). All the proteins were transferred onto nitrocellulose membranes (Amersham Biosciences, UK), which were then blocked with 5% skim milk in TBST for 1 h. Polyclonal goat anti-chicken IgY light chain antibodies (Bethyl Laboratories, Montgomery, TX, USA) were added at 1:3000 dilution and incubated for an additional hour. The membranes were washed with TBST three times for 5 min each. The bound antibodies were detected by adding horseradish peroxidase (HRP)-conjugated donkey anti-goat Ig antibodies (Sigma, St. Louis, MO, USA) at 1:3000 dilution. After three washings, the membranes were developed with diaminobenzidine (DAB) substrate until the desired intensity was reached. To examine their binding reactivity, the IgY purified from chicken 1 week after 4th immunization or the expressed scFv antibodies were incubated with the purified α-enolase immobilized on nitrocellulose membranes or ELISA plate wells. It was subsequently detected by adding goat anti-chicken IgY light chain and HRP-conjugated donkey anti-goat Ig antibodies as described (Lee et al., 2007a, Lee et al., 2007b). The ELISA tests were done in the duplicated wells for each sample.
2.5. Sequence analysis
The nucleotide sequence determination of heavy and light variable regions from chosen clones was carried out by an auto-sequencer machine (ABI PRISM 377; PerkinElmer, National Health Research Institute) using ompseq (5′-AAGACAGCTATCGCGATTGCAGTG-3′) and HRML-F (5′-GGTGGTTCCTCTAGATCTTCC-3′) primers. The results were analyzed using alignment program BLAST and Vector NTI (http://www.ncbi.nlm.nih.gov/BLAST). The overall mutation rate was defined as: (number of amino acids that differ between cloned and germline genes/total number of amino acids in germline gene) × 100%. The nucleotide sequences of heavy and light chain variable regions of EnL1, EnL2, EnL3, EnL4 and EnL5 scFv clones were deposited in GenBank (accession numbers are 1330277, 1330291, 1330296, 1330299 and 1330301, respectively).
2.6. Flow cytometry analysis
The PE089 cells have been cultured in RPMI 1640 supplemented with 5% fetal bovine serum, 2 mmol/L glutamine, and antibiotics for at least 40 passages in vitro. A total of 2 × 106 cells were harvested and fixed with 2% paraformaldehyde as described (Abcam, MA, USA). The α-enolase expressed in the PE089 cells was detected with purified scFv EnL2 and EnL5 antibodies, visualized with mouse anti-HA (1:200) and Cy-2-conjugated goat anti-mouse antibodies (1:200) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The results were analyzed using the FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Negative controls were performed as described above, omitting the primary scFv EnL2 and EnL5 antibodies, while positive controls were performed using rabbit polyclonal anti-human enolase antibodies (1:200) (Kelowna Inc., Taipei, Taiwan) instead of scFv EnL2 and EnL5 antibodies.
2.7. Immunofluorescence staining
PE089 cells (2 × 105 cells/ml) were seeded on cover glass and fixed by incubating with 4% paraformaldehyde (freshly prepared by mixing equal volume of 8% paraformaldehyde in 1× PBS solution) (Biotrin International Ltd., Dublin, Ireland) on ice for 15 min. After fixing, the cells were dehydrated in a sequential treatment of 70%, 95% and 99% methanol and rehydrated with 95% and 70% methanol. The slides were then overlaid with blocking buffer (1%BSA in 1× PBS) at RT for 1 hr. Following washing with 1× PBS, scFv antibodies were incubated with cells at RT for one additional h. Finally, their binding to α-enolase protein was detected by mouse anti-HA antibodies, followed by goat anti-mouse antibodies conjugated with Cy-2. Nuclei were also counterstained with PI solution as suggested (Invitrogen, USA). We examined the slides with a Confocal Spectral Microscope Imaging System (TCS SP5, Leica).
3. Results
3.1. Characterization of purified recombinant α-enolase and polyclonal anti-α-enolase IgY antibodies
To produce antigen for chicken immunization, the α-enolase gene was cloned, expressed as both His-fused and GST-fused recombinant proteins and purified as described in Section 2. After electrophoresis and Coomassie blue staining, purified His-fused and GST-fused α-enolase were visualized as a single band of 48 and 75 kD (lanes 2 and 3 in Fig. 1A, respectively). The identity of GST-fused α-enolase was verified using anti-GST antibodies as shown in lane 3, Fig. 1B. Similarly, polyclonal IgY antibodies produced in chickens immunized with purified His-fused α-enolase were able to clearly recognize both His-fused and GST-fused α-enolase immobilized on Western blots (lanes 2 and 3 in Fig. 1C).
Fig. 1.
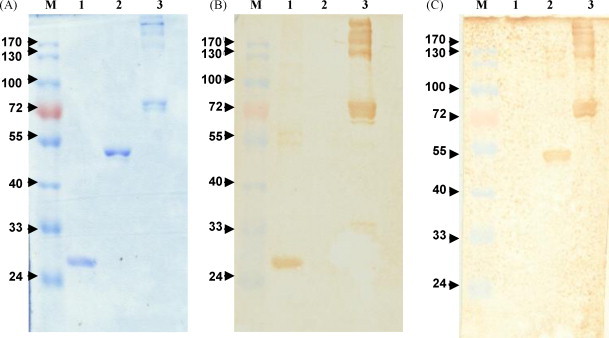
Characterization of recombinant α-enolase and polyclonal anti-α-enolase IgY antibodies. Samples in each panel are protein markers (lane M), purified GST (lane 1), purified α-enolase (lane 2), and purified GST-α-enolase fusion protein (lane 3). Purified proteins visualized by Coomassie blue staining (panel A) were blotted onto nitrocellulose paper and probed with anti-GST antibodies (panel B) or sera from 4th-immunized chicken (panel C). The molecular weight of recombinant α-enolase protein is about 48 kD.
3.2. Chicken immunization
Sera and eggs were collected from chickens before and after each immunization. We purified total IgY antibodies in egg yolk and detected the presence of their heavy and light chain fragments using horse radish peroxidase-conjugated anti-chicken IgY antibodies (data not shown). The purified IgY antibodies were used to test for their binding activity to α-enolase immobilized on nitrocellulose membrane (Fig. 1C) or ELISA plate wells. As shown in Fig. 2 , the IgY antibodies prepared from the egg yolk after the 4th immunization specifically bind to α-enolase but not bovine serum albumin when titered at 1:16,000 dilution, suggesting a strong humoral antibody response was elicited in the chicken host. In contrast, the IgY antibodies from pre-immunized chicken eggs showed very little binding signal to either antigen.
Fig. 2.
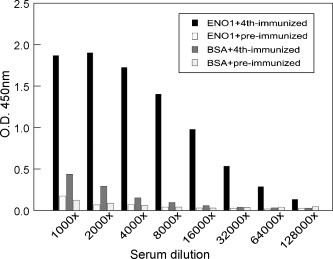
Humoral IgY responses in chicken after the 4th immunization analyzed by ELISA. Purified α-enolase protein and bovine serum antigen (BSA) were coated on plate wells. A series of diluted IgY antibodies were examined for their specific binding activity to either α-enolase or BSA. Solid and open bars represent the binding of IgY from 4th- or pre-immunized chickens to α-enolase, respectively. In addition, their binding to BSA presented by deep and light gray bars were shown in parallel as negative controls.
3.3. Construction of scFv antibody library and biopanning
The chickens were sacrificed 8 weeks after the last immunization and total RNA was extracted from the enlarged spleens for antibody library construction. The amplification of full-length scFv gene fragments was carried out using 2 consecutive PCR steps. In the primary PCR, VH gene products were amplified as 400 bp in size using primers containing short (GGSSRSS) and long (GGSSRSSSSGGGGSGGGG) linkers as presented as EnVH.S (Fig. 3A, lane 2) and EnVH.L (Fig. 3A, lane 3), respectively. Accordingly, the VL gene was amplified as a band of 350 bp and loaded in lane 4 in Fig. 3A (EnVL). Subsequently, the amplified VH and VL were joined to form full-length scFv gene fragments of approximately 750 bp presented as EnscFv.S (lane 2) and EnscFv.L (lane 3) in Fig. 3B. Several phage displaying antibody libraries were constructed and two of which were used to screen the specific anti-α-enolase scFv antibodies (Table 1 ).
Fig. 3.
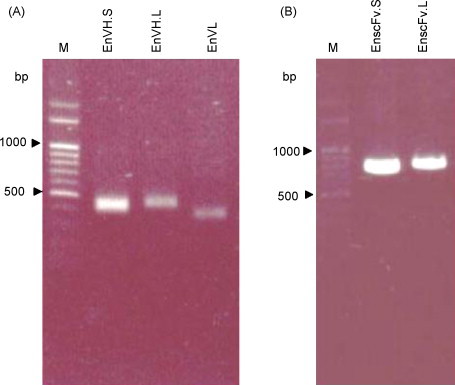
PCR amplification of the variable regions in chicken immunoglobulin genes. Variable regions of the light chain (EnVL) and heavy chain with short-linker (EnVH.S) or with long-linker (EnVH.L) were amplified successfully (panel A). The second round of PCR generated the full-length scFv gene fragments with short (EnscFv.S) or long (EnscFv.L) linkers (panel B).
Table 1.
The size of anti-α-enolase scFv gene libraries and eluted phage titer of each panning.
| Library | Size | Panning cycle | Eluted phage numbers |
|---|---|---|---|
| EnscFv.S | 2.4 × 104 | 1st | 2.2 × 104 |
| 2nd | 1.2 × 104 | ||
| 3rd | 1.3 × 104 | ||
| 4th | 1.8 × 104 | ||
| EnscFv.L | 3.5 × 105 | 1st | 1.6 × 104 |
| 2nd | 1.4 × 104 | ||
| 3rd | 5.8 × 104 | ||
| 4th | 7.2 × 105 | ||
3.4. Characterization of anti-α-enolase scFv clones
Four rounds of panning cycles were carried out as described above. After each panning, fifteen clones were randomly selected and analyzed for 750 bp fragment inserts in the pCom3X cloning vector and for scFv antibody protein expression. Our data (not shown) indicated that 67% (10/15), 87% (13/15), 100% (15/15) and 100% (15/15) of clones from each round of panning had the full-length inserts. Moreover, ten clones with 750 bp inserts from the last round of panning were induced to express their scFv antibodies. These results indicated that the expression level of immunoglobulin genes with highly conserved sequences could be dramatically different even under identical experimental conditions.
3.5. Gene sequencing and ELISA analysis
The nucleotide sequences of the variable regions of heavy and light chain genes of 10 clones were determined and aligned to the germline gene sequences of chicken immunoglobulin. The results revealed that 30% of the sequenced clones (EnL5, EnL6 and EnL7) shared identical heavy and light genes leading to the similar profiles of their scFv antibody expression as seen in lanes 5–7 in Fig. 4 . The overall mutation rates as compared to the germline gene sequences range from 18.6% to 27.4% and from 13.5% to 23.1% in heavy and light chain variable regions, respectively (Fig. 5 ). The binding activities of the expressed EnL1 to EnL7 scFv antibodies against α-enolase were analyzed using ELISA. We found that those scFv antibody fragments exhibit significant binding activity to α-enolase as compared to 2 other scFv antibodies which were previously characterized and known to specifically recognize SARS-CoV spike protein. In particular, EnL2, EnL4, EnL5 and EnL6 scFv antibodies showed stronger positive reactivity than polyclonal IgY purified from chicken immunized with the human α-enolase molecule (Fig. 6 ).
Fig. 4.
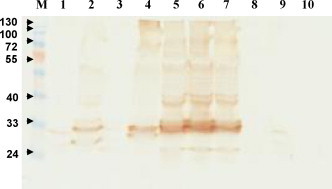
Expression of scFv antibodies analyzed by Western blotting. Identical amounts of total cellular lysates from 30 clones were loaded onto SDS-PAGE and transferred to nitrocellulose papers. The presence of scFv antibodies was detected by goat anti-chicken light chain antibodies at 1:3000 dilution, followed by HRP-conjugated donkey anti-goat IgG. The predicted molecular weight of scFv fragment is approximately 35 kDa. The blot is a representative result of scFv expression in 10 selected clones.
Fig. 5.

Sequence analysis of VL and VH genes of scFv antibodies. The nucleotide sequences of VH and VL of 10 clones were determined and translated into amino acid sequences to be aligned with those of the chicken germline gene. FR: framework region; CDR: complementarity-determining region. Sequence gaps were introduced to maximize the alignment; these are indicated by blank spaces. Dots indicate the consensus sequences. Framework region (FR) and complementarity-determining region (CDR) boundaries are indicated above germline sequences.
Fig. 6.
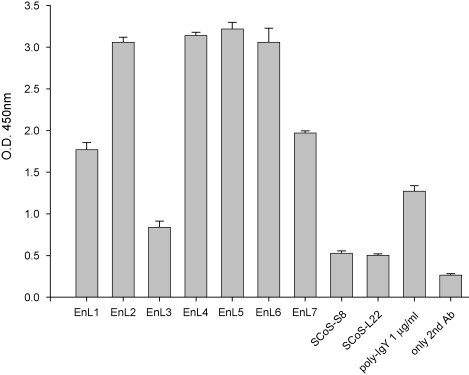
Binding activity of scFv antibodies to purified α-enolase analyzed by ELISA. Cellular lysates containing scFv antibodies from randomly selected clones from the 4th panning cycle were examined for their binding to purified α-enolase coated onto the plate wells. Binding activity was detected using the goat anti-chicken light chain antibodies at 1:3000 dilution, followed by HRP-conjugated donkey anti-goat IgG and measured at 450 nm. Two anti-SARS-CoV scFv antibodies (SCoS-S8 and SCoS-L22) were used as negative controls. One additional control experiment was carried out as described without adding primary recombinant scFv antibodies. Polyclonal IgY antibodies from chickens immunized with purified α-enolase were used as a positive control. The ELISA data were represented as means of the duplicated experiments.
3.6. FACS analysis
To test the binding reactivity of these cloned scFv antibodies, human α-enolase intrinsically expressed in PE089 tumor cells was analyzed for its expression on the membrane surface which was subsequently analyzed by flow cytometry. EnL2 and EnL5 scFv antibodies purified as a single band on SDS-PAGE (data not shown) were able to detect α-enolase protein in PE089 cells, and the binding signal is comparable to that of commercially available rabbit polyclonal antibodies specific for α-enolase as demonstrated in Fig. 7 .
Fig. 7.
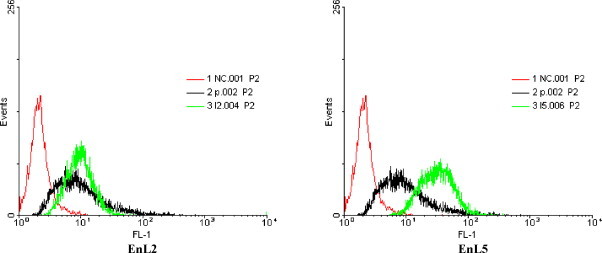
Binding activity of scFv antibodies to purified α-enolase analyzed by flow cytometry. Surface associated α-enolase on PE089 cells was detected using purified EnL2 and EnL5 scFv antibodies, mouse anti-HA (1:200) and Cy-2-conjugated goat anti-mouse antibodies (1:200). The grey thin lines indicate negative control, cells treated with DMSO alone and stained with fluorescence-labeled Abs against surface markers; the gray solid lines indicate cells stained with fluorescence-labeled Ig isotype controls; and the black solid lines indicate cells stained with scFv antibodies EnL2 and EnL5 fluorescence-labeled Abs against surface α-enolase. The results of one representative experiment of three separate experiments are shown.
3.7. Immunofluorescence and laser scanning microscopy
We also applied immunocytochemical staining to assess the binding ability of purified EnL2 and EnL5 scFv antibodies against α-enolase molecules expressed in PE089 cells. It has been demonstrated that the α-enolase molecule is mainly expressed and translocated on the nuclear membrane of PE089 cells (personal communication with Dr. N-Y Shih). Accordingly, our recombinant EnL2 and EnL5 scFv antibodies exhibited significant binding signal around the cell nuclear membrane as shown in Fig. 8 . In contrast, two negative controls using Cy-2-conjugated goat anti-mouse antibodies only or 4L8 clone expressing a scFv antibody specific for SARS-CoV spike protein instead showed no reactivity at all. The cell morphology and distribution under light microscopy are included in the most left panel for comparison. Taken together, the results provided further evidence to show that phage display technology might be a better alternative over hybridoma protocol for the cloning and generation of scFv antibodies against specific antigens.
Fig. 8.
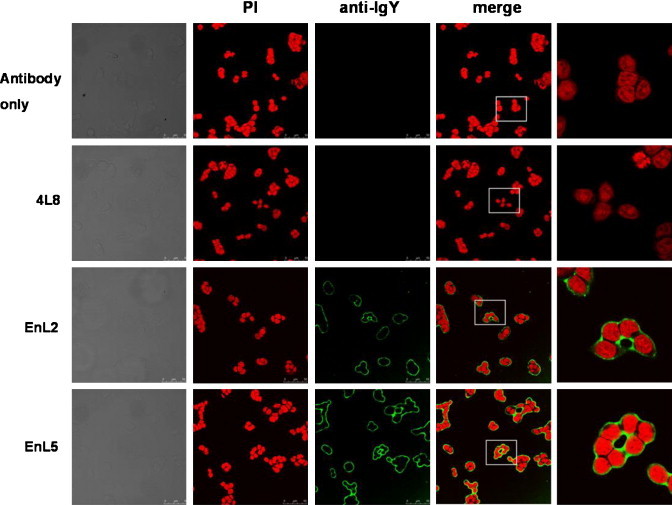
Immunofluorescent staining of α-enolase protein in PE089 cells. Cells were fixed and their α-enolase expression was detected using purified EnL2 and EnL5 scFv antibodies, followed by mouse anti-HA and Cy-2-conjugated goat anti-mouse antibodies. The nucleus (red) was visualized by PI staining. Both EnL2 and EnL5 scFv antibodies clearly stained nuclear membrane (green) in PE089 cells. An anti-SARS-CoV scFv antibody, 4L8, did not show any reactivity with nuclear membrane. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4. Discussion
The objective of this study was to produce and characterize a panel of recombinant antibodies against human α-enolase for their potential diagnostic application in the future. The recombinant α-enolase proteins were successfully expressed in E. coli and purified to a great extent of homogeneity as shown in Fig. 1. A specific humoral response in immunized chickens was steadily observed after a 2nd booster injection. The polyclonal IgY antibodies from the 4th immunization showed a strong reactivity to α-enolase antigen using a 1:16,000 dilution. Moreover, the high titer of anti-α-enolase IgY antibodies was continuously detected 4 months after final immunization (data not shown). Taken together, our data indicated that α-enolase protein is highly immunogenic in experimental chicken, and this system has been applied to elicit polyclonal IgY antibodies with high specificity against numerous genetically distinctive antigens (Chalghoumi et al., 2008, Mine and Kovacs-Nolan, 2002, Shin et al., 2002).
The expression of scFv antibody in E. coli was examined on randomly chosen clones after the 4th panning step. As is often encountered in protein expression studies, the expressed levels and stability in our characterized scFv clones containing evolutionarily close immunoglobulin genes were also unpredictable and various (Graves et al., 2008, Jana and Deb, 2005). As shown in Fig. 4, EnL1, EnL3 and EnL9 scFv antibodies were barely detected as determined on Western blots as compared to EnL5, EnL6 and EnL7 clones. Indeed, the amount of expressed scFv antibodies of EnL5, EnL6 and EnL7 was clearly visualized on Coomassie blue-stained SDS-PAGE (data not shown). These 3 clones exhibited identical protein expression patterns, suggesting they might be derived from one parental clone. This speculation was further supported by subsequent sequence analysis on the heavy and light chain variable genes of these clones. We did not detect any scFv antibodies expressed in clone EnL8 or EnL10 under identical conditions. Combined with the fact that all the immunoglobulin genes are highly conserved as mentioned earlier, our data indicate that the levels of their expression as scFv molecules in E. coli cells vary tremendously from clone to clone.
Numerous antibodies composed of single-chain variable fragment (scFv) have been cloned and generated against broad tumor-associated antigens using a phage display system (Robinson et al., 2008, Sun et al., 2009). We have constructed several immune antibody libraries from the spleen of chicken immunized with α-enolase by this novel technology. Of these antibody libraries, two, one constructed with a short-linker (6 × 104 clones) and the other with a long-linker (3.5 × 105 clones), were panned against recombinant human α-enolase. Several specific scFv antibodies with high binding activity, as represented by clones EnL2 and EnL5, were obtained from the long-linker containing library. On the contrast, the scFv antibodies characterized in the short-linker containing library are of low affinity and specificity. Our pilot studies demonstrated that the specific anti-α-enolase scFv antibody clones could not be readily detected in the original library constructed with a short-linker in a plaque lift assay (data not shown), suggesting the heavy and light variable genes specific for α-enolase might not be amplified, cloned, expressed or displayed on the surface of M13 viral particles. However, the reasons for such results are not exactly known.
The power of the phage display technology is its effectiveness to enrich specific antibodies with high affinity in an in vitro panning procedure (Barbas et al., 1991). Sequence analysis data indicated all the clones contained heavy and light chain variable genes derived from the chicken germline in which 3 clones (EnL5, EnL6 and EnL7) use the same heavy and light genes for their scFv antibody expression Together with the finding that the ratio of recombinant clones with full-length of scFv gene inserts increased from 67% to 100% during the panning cycles, the data supported very strongly that mono-specific anti-α-enolase with high affinity could be steadily generated using phage display antibody technology. Notably, high mutation rates ranging from 14.3% to 100% were found in CDR regions of both VL and VH genes; in particular the entire CDR3 region of VH genes of chicken germline was substituted by amino acids with various length and sequences in all the characterized clones (Fig. 5). It was previously reported that somatic hypermutations to increase antibody affinity occurred more frequently in the CDR than FR regions of the rearranged V genes (Gearhart and Bogenhagen, 1983). Thus, all EnL clones in the study accumulated more mutations in their CDR regions as a result of affinity selection of B cells.
As compared to the traditional hybridoma method, our present study indicated that it is feasible to generate poly-specific IgY antibodies in chicken and subsequently mono-specific scFv antibodies using phage display technology. These scFv antibodies could be applied to delineate the potential biological functions of α-enolase protein played in the lung carcinogenesis and to develop valuable reagents for clinical applications.
Acknowledgments
This study was supported by grants NSC 91-2314-B-038-037, NSC-92-2314-B-038-063, NSC 96-2320-B-038-005 and NSC 97-2320-B-038-012-MY2 (Yi-Yuan Yang) from the National Science Council (NSC) and DOH97-TD-G-111-022 (Neng-Yao Shih) from National Health Research Institutes (NHRI) in Taiwan.
References
- Akita E.M., Nakai S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J. Immunol. Methods. 1993;160:207–214. doi: 10.1016/0022-1759(93)90179-b. [DOI] [PubMed] [Google Scholar]
- Akita E.M., Nakai S. Production and purification of Fab’ fragments from chicken egg yolk immunoglobulin Y (IgY) J. Immunol. Methods. 1993;162:155–164. doi: 10.1016/0022-1759(93)90380-p. [DOI] [PubMed] [Google Scholar]
- Altenberg B., Greulich K.O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Andris-Widhopf J., Rader C., Steinberger P., Fuller R., Barbas C.F., III Methods for the generation of chicken monoclonal antibody fragments by phage display. J. Immunol. Methods. 2000;242:159–181. doi: 10.1016/s0022-1759(00)00221-0. [DOI] [PubMed] [Google Scholar]
- Antikainen J., Kuparinen V., Lahteenmaki K., Korhonen T.K. Enolases from Gram-positive bacterial pathogens and commensal lactobacilli share functional similarity in virulence-associated traits. FEMS Immunol. Med. Microbiol. 2007;51:526–534. doi: 10.1111/j.1574-695X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Barbas C.F., III, Kang A.S., Lerner R.A., Benkovic S.J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanos D.P., Gilbert D., Bianchi I., Leoni S., Mitry R.R., Ma Y., Mieli-Vergani G., Vergani D. Antibodies to soluble liver antigen and alpha-enolase in patients with autoimmune hepatitis. J. Autoimmune Dis. 2004;1:4. doi: 10.1186/1740-2557-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalghoumi R., Thewis A., Portetelle D., Beckers Y. Production of hen egg yolk immunoglobulins simultaneously directed against Salmonella enteritidis and Salmonella typhimurium in the same egg yolk. Poult. Sci. 2008;87:32–40. doi: 10.3382/ps.2007-00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G.C., Liu K.J., Hsieh C.L., Hu T.S., Charoenfuprasert S., Liu H.K., Luh K.T., Hsu L.H., Wu C.W., Ting C.C., Chen C.Y., Chen K.C., Yang T.Y., Chou T.Y., Wang W.H., Whang-Peng J., Shih N.Y. Identification of alpha-enolase as an autoantigen in lung cancer: its overexpression is associated with clinical outcomes. Clin. Cancer Res. 2006;12:5746–5754. doi: 10.1158/1078-0432.CCR-06-0324. [DOI] [PubMed] [Google Scholar]
- Fehrsen J., van Wyngaardt W., Mashau C., Potgieter A.C., Chaudhary V.K., Gupta A., Jordaan F.A., du Plessis D.H. Serogroup-reactive and type-specific detection of bluetongue virus antibodies using chicken scFvs in inhibition ELISAs. J. Virol. Methods. 2005;129:31–39. doi: 10.1016/j.jviromet.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Feo S., Arcuri D., Piddini E., Passantino R., Giallongo A. ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter-binding protein 1 (MBP-1) FEBS Lett. 2000;473:47–52. doi: 10.1016/s0014-5793(00)01494-0. [DOI] [PubMed] [Google Scholar]
- Finlay W.J., Shaw I., Reilly J.P., Kane M. Generation of high-affinity chicken single-chain Fv antibody fragments for measurement of the Pseudonitzschia pungens toxin domoic acid. Appl. Environ. Microbiol. 2006;72:3343–3349. doi: 10.1128/AEM.72.5.3343-3349.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P.J., Bogenhagen D.F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc. Natl. Acad. Sci. U.S.A. 1983;80:3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlits V.M., Toh B.H., Sentry J.W. Disease association, origin, and clinical relevance of autoantibodies to the glycolytic enzyme enolase. J. Investig. Med. 2001;49:138–145. doi: 10.2310/6650.2001.34040. [DOI] [PubMed] [Google Scholar]
- Graves P.E., Henderson D.P., Horstman M.J., Solomon B.J., Olson J.S. Enhancing stability and expression of recombinant human hemoglobin in E. coli: progress in the development of a recombinant HBOC source. Biochim. Biophys. Acta. 2008;1784:1471–1479. doi: 10.1016/j.bbapap.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Groves D.J., Morris B.A. Veterinary sources of nonrodent monoclonal antibodies: interspecific and intraspecific hybridomas. Hybridoma. 2000;19:201–214. doi: 10.1089/02724570050109602. [DOI] [PubMed] [Google Scholar]
- Holliger P., Hudson P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- Ito S., Honma T., Ishida K., Wada N., Sasaoka S., Hosoda M., Nohno T. Differential expression of the human alpha-enolase gene in oral epithelium and squamous cell carcinoma. Cancer Sci. 2007;98:499–505. doi: 10.1111/j.1349-7006.2007.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S., Deb J.K. Strategies for efficient production of heterologous proteins in Escherichia coli. Appl. Microbiol. Biotechnol. 2005;67:289–298. doi: 10.1007/s00253-004-1814-0. [DOI] [PubMed] [Google Scholar]
- Jiang B.H., Agani F., Passaniti A., Semenza G.L. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 1997;57:5328–5335. [PubMed] [Google Scholar]
- Kinloch A., Tatzer V., Wait R., Peston D., Lundberg K., Donatien P., Moyes D., Taylor P.C., Venables P.J. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R1421–1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Chung H.S., Kim H.S., Oh S.H., Ha M.K., Baik J.H., Lee S., Bang D. Human alpha-enolase from endothelial cells as a target antigen of anti-endothelial cell antibody in Behcet's disease. Arthritis Rheum. 2003;48:2025–2035. doi: 10.1002/art.11074. [DOI] [PubMed] [Google Scholar]
- Lee Y.C., Leu S.J., Hu C.J., Shih N.Y., Huang I.J., Wu H.H., Hsieh W.S., Chiang B.L., Chiu W.T., Yang Y.Y. Chicken single-chain variable fragments against the SARS-CoV spike protein. J. Virol. Methods. 2007;146:104–111. doi: 10.1016/j.jviromet.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.C., Leu S.J., Hung H.C., Wu H.H., Huang I.J., Hsieh W.S., Chiu W.T., Hsieh M.S., Cheng T.F., Yang Y.Y. A dominant antigenic epitope on SARS-CoV spike protein identified by an avian single-chain variable fragment (scFv)-expressing phage. Vet. Immunol. Immunopathol. 2007;117:75–85. doi: 10.1016/j.vetimm.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine Y., Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. J. Med. Food. 2002;5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- Park K.J., Park D.W., Kim C.H., Han B.K., Park T.S., Han J.Y., Lillehoj H.S., Kim J.K. Development and characterization of a recombinant chicken single-chain Fv antibody detecting Eimeria acervulina sporozoite antigen. Biotechnol. Lett. 2005;27:289–295. doi: 10.1007/s10529-005-0682-8. [DOI] [PubMed] [Google Scholar]
- Peebles K.A., Duncan M.W., Ruch R.J., Malkinson A.M. Proteomic analysis of a neoplastic mouse lung epithelial cell line whose tumorigenicity has been abrogated by transfection with the gap junction structural gene for connexin 43, Gja1. Carcinogenesis. 2003;24:651–657. doi: 10.1093/carcin/bgg008. [DOI] [PubMed] [Google Scholar]
- Redlitz A., Fowler B.J., Plow E.F., Miles L.A. The role of an enolase-related molecule in plasminogen binding to cells. Eur. J. Biochem. 1995;227:407–415. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- Robinson M.K., Hodge K.M., Horak E., Sundberg A.L., Russeva M., Shaller C.C., von Mehren M., Shchaveleva I., Simmons H.H., Marks J.D., Adams G.P. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br. J. Cancer. 2008;99:1415–1425. doi: 10.1038/sj.bjc.6604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulot V., Vittecoq O., Charlionet R., Fardellone P., Lange C., Marvin L., Machour N., Le Loet X., Gilbert D., Tron F. Presence of autoantibodies to the glycolytic enzyme alpha-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheum. 2002;46:1196–1201. doi: 10.1002/art.10252. [DOI] [PubMed] [Google Scholar]
- Shin J.H., Yang M., Nam S.W., Kim J.T., Myung N.H., Bang W.G., Roe I.H. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin. Diagn. Lab. Immunol. 2002;9:1061–1066. doi: 10.1128/CDLI.9.5.1061-1066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M.A. Minireview. Emerging new functions of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in mammalian cells. Life Sci. 1996;58:2271–2277. doi: 10.1016/0024-3205(96)00123-3. [DOI] [PubMed] [Google Scholar]
- Sirover M.A. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta. 1999;1432:159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- Sun Y., Shukla G.S., Weaver D., Pero S.C., Krag D.N. Phage-display selection on tumor histological specimens with laser capture microdissection. J. Immunol. Methods. 2009;347:46–53. doi: 10.1016/j.jim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Griffiths A.D., Hawkins R.E., Hoogenboom H.R. Making antibodies by phage display technology. Annu. Rev. Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- Wu W., Tang X., Hu W., Lotan R., Hong W.K., Mao L. Identification and validation of metastasis-associated proteins in head and neck cancer cell lines by two-dimensional electrophoresis and mass spectrometry. Clin. Exp. Metastasis. 2002;19:319–326. doi: 10.1023/a:1015515119300. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cilley R.E., Chinoy M.R. Suppression subtractive hybridization to identify gene expressions in variant and classic small cell lung cancer cell lines. J. Surg. Res. 2000;93:108–119. doi: 10.1006/jsre.2000.5957. [DOI] [PubMed] [Google Scholar]


