Summary
Objectives
To determine the efficacy of methylprednisolone pulse therapy for children with Mycoplasma pneumoniae pneumonia (MP) that is refractory to antibiotic treatment.
Methods
Refractory patients were defined as cases showing clinical and radiological deterioration despite appropriate antibiotic therapy for 7 days or more. We identified 6 such children (male/female: 3/3) aged 3–9 years who were treated between 1998 and 2006. During the same period, 190 children with MP were admitted to our institution.
Results
Common laboratory findings of the patients included cytopenia, elevated serum lactate dehydrogenase and ferritin levels, and elevated urine β2-microglobulin levels, suggesting complication of hypercytokinemic condition. We initiated intravenous methylprednisolone at a dose of 30 mg/kg on 10.2 ± 2.8 clinical days and administered it once daily for 3 consecutive days. Fever subsided 4–14 h after initiation of steroid pulse therapy in all patients. This dramatic effect was accompanied by rapid improvement of radiological abnormalities including infiltrates and pleural effusion, followed by improvement of laboratory abnormalities. There were no adverse events of steroid therapy.
Conclusions
This is the first case-series study showing an effect of 3-day methylprednisolone pulse therapy on refractory MP in children. This therapy is apparently an efficacious and well-tolerated treatment for refractory MP.
Keywords: Methylprednisolone, Pulse, Refractory, Mycoplasma pneumoniae, Children
Introduction
Mycoplasma pneumoniae (M. pneumoniae) pneumonia (MP) is a community-acquired infection occurring primarily in previously healthy children and young adults. A recent prospective study in Japan showed that M. pneumoniae accounts for approximately 40% of childhood pneumonia with an increasing frequency according to age.1 MP is usually a benign self-limited disease. However, it may in rare situations develop into a severe life-threatening pneumonia, such as acute respiratory distress syndrome, necrotizing pneumonitis, and fulminant pneumonia.2, 3, 4, 5, 6, 7, 8
Macrolide is the first choice for MP. However, progression to severe pneumonia might occur despite appropriate antibiotic therapy. Although the details remain unclear, the pathogenesis of macrolide-nonresponsive and progressive condition is suggested to be related to cellular immunological responses.2, 4, 5, 7, 8 Based on the proposed mechanism, corticosteroid has been used for severe or refractory MP in adulthood with promising efficacy.2, 5, 7, 8 In contrast, little is known about the effect of corticosteroid therapy on such patients in a pediatric population. A recent study showed that oral prednisolone of 1 mg/kg/day for more than 1 week for severe MP in children may be helpful for reducing morbidity.9 However, appropriate methods of steroid use, in particular, those of dosage, administration route, or duration of treatment have not been fully clarified. In this report, we describe six pediatric patients with severe progressive MP, who dramatically responded to methylprednisolone pulse therapy for 3 days. We evaluated the efficacy of methylprednisolone pulse therapy in clinical and radiological conditions in refractory MP children.
Materials and methods
We investigated the medical records and chest radiographic findings of six previously healthy children with MP, who were treated with methylprednisolone pulse therapy at Nishi-Kobe Medical Center between January 1998 and December 2006. This is a pilot and retrospective study with a small number of subjects. During the study period, 190 children with MP were admitted to our institution. The mean age of the six children was 5.8 ± 2.3 years (range, 3–9 years); three were male and three were female. Serological M. pneumoniae testing consists of M. pneumoniae IgM enzyme immunoassay (Meridian ImmunoCard Mycoplasma Meridian Bioscience, Inc, Cincinnati, OH) and particle agglutination (PA) method (Serodia-Myco II, Fujirebio, Japan) using both acute and convalescent serum when available. MP was diagnosed in one patient based on positive results for IgM antibody in a single sample and in five patients by four-fold or greater rise in serum PA titers from the acute phase (<1:80[n = 4]–1:160[n = 1]) to convalescent phase (1:640–1:5120). All patients were treated initially with macrolides (erythromycin, clindamycin, or azithromycin), and in some cases, together with minocycline. Refractory pneumonia was defined as a case of prolonged fever and deterioration of radiological findings despite antibiotic treatment for 7 days or more. For the refractory cases, we intravenously administered methylprednisolone at a dose of 30 mg/kg once daily for 3 consecutive days. Microbiologic tests, to exclude other respiratory tract infections, included blood cultures, nasopharyngeal aspirate/swab for common respiratory tract virus antigens (respiratory scincytial virus, adenovirus, and influenza virus), and serology for Chlamydia pneumoniae. None of the tests detected any of the other pathogens examined.
Results
Clinical characteristics and laboratory findings
All patients had symptoms and signs indicative of pneumonia on admission, including fever (>38 °C) and cough. In all cases, initial chest radiograph showed segmental or lobar infiltrates in a single or multiple lobes. None of the patients were transferred to the intensive care unit or received mechanical ventilation during their hospital stay. Table 1 shows clinical characteristics and laboratory findings of the six patients. White blood cell (WBC) count on admission was 5.4 ± 2.2 × 103/μl, and was decreased to 3.1 ± 1.8 × 103/μl at 9.0 ± 1.9 days after the onset of fever (clinical day). Lymphopenia was found on admission (1.7 ± 0.6 × 103/μl) and demonstrated increasingly profound depression during follow-up (1.1 ± 0.5 × 103/μl at 9.0 ± 1.9 clinical days). In parallel, hemoglobin and platelet counts mostly declined after admission. After steroid therapy, cytopenia was gradually recovered with clinical improvement. The maximum C-reactive protein (CRP) during their clinical course was 5.8 ± 6.2 mg/dl. Lactate dehydrogenase (LDH) was elevated on admission in all patients, and reached the maximum levels of 619 ± 152 IU/l. Serum ferritin and urine β2-microglobulin were elevated at the highest levels of 665 ± 545 ng/ml (n = 5) and 3212 ± 3706 μg/l (n = 4), respectively. Table 2 shows duration of fever and hospitalization of the six patients. The clinical day when we initiated steroid pulse therapy was 10.2 ± 2.8 days. The mean duration of fever prior to admission was 5.2 ± 1.3 days. All patients achieved defervescence 4–14 h after the initiation of steroid pulse therapy. In addition, it was accompanied by rapid resolution of clinical symptoms and radiological abnormalities as representatively described below. The duration of hospitalization after the start of steroid treatment was 6.2 ± 3.4 days. There were no adverse events in any patients during steroid treatment, and all patients were discharged without morbidity.
Table 1.
Clinical characteristics and laboratory findings of patient with refractory Mycoplasma pneumoniae pneumonia
| Patient | Age (year) | Sex | Total WBC, lymphocytes (×103/μl) |
CRP (mg/dl) |
LDH (IU/l) |
Ferritin (ng/ml) |
U-β2MG (μg/l) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| On admission | Follow-up |
On admission | Max. during follow-up | On admission | Max. during follow-up | Max. during follow-up | Max. during follow-up | ||||
| Before steroid therapy | After steroid therapy | ||||||||||
| 1 | 5 | F | 5.1, 1.8 | 1.5, 0.7 | 9.6, 1.4 | 2.3 | 2.3 | 346 | 366 | 950 | ND |
| 2 | 3 | F | 5.4, 1.9 | 3.2, 1.0 | 5.1, 1.1 | 5.1 | 17.7 | 395 | 675 | 210 | ND |
| 3 | 8 | M | 3.3, 1.0 | 2.0, 0.8 | 6.6, 2.2 | 0.9 | 1.9 | 304 | 745 | 227 | 8683 |
| 4 | 9 | F | 4.1, 1.0 | 2.9, 0.8 | 6.8, 1.9 | 4.5 | 5.0 | 698 | 778 | 1480 | 1998 |
| 5 | 6 | M | 4.7, 1.6 | 4.7, 1.6 | 7.6, 4.0 | 1.0 | 1.0 | 613 | 613 | ND | 1686 |
| 6 | 4 | M | 9.5, 2.7 | 4.2, 1.8 | 5.8, 2.3 | 6.7 | 6.7 | 468 | 534 | 457 | 479 |
U-β2MG: urine β2-microglobulin, max.: maximum level, ND: not determined.
Normal range: LDH 120-230 IU/L, Ferritin: 4.0–64.2 ng/ml, urine β2-microglobulin: 3–100 μg/l.
Table 2.
Duration of fever and hospitalization of patients with refractory M. pneumoniae pneumonia
| Patient | Clinically day at start of steroid therapy | Duration of fever |
Duration of hospitalization |
||
|---|---|---|---|---|---|
| Before admission (day) | After steroid therapy (h) | Total (day) | After steroid therapy (day) | ||
| 1 | 10 | 5 | 6 | 17 | 12 |
| 2 | 11 | 5 | 4 | 12 | 6 |
| 3 | 15 | 6 | 4 | 13 | 4 |
| 4 | 8 | 5 | 5 | 11 | 8 |
| 5 | 10 | 7 | 14 | 7 | 4 |
| 6 | 7 | 3 | 4 | 6 | 3 |
The first clinical day is defined as the first day of fever.
We describe the clinical courses of two representative cases
Patient 1
A 5-year-old girl had been in excellent health until she developed fever and productive cough 5 days before admission to our hospital. At presentation, body temperature was 39.7 °C, pulse rate was 160/min. Auscultation of the lungs demonstrated coarse crackle over the left lung. Chest X-ray film showed left lower lobar consolidation. Laboratory findings on admission included WBC 5.1 × 103/μl with neutrophils 3.0 × 103/μl and lymphocytes 1.8 × 103/μl, hemoglobin (Hb) 12.3 g/dl, platelet count (Plt) 188 × 103/μl, LDH 346 IU/l and CRP 2.3 mg/dl. We administered parental cefotiam (90 mg/kg/day) and oral erythromycin (40 mg/kg/day). Fever and productive cough continued for the next 4 days, and the respiratory condition deteriorated day by day. She became severely dyspneic and breath sounds were hardly ausculated over the left lung. Since hypoxemia progressed to an oxygen saturation of 91%, we started oxygen supplement. On the 10th clinical day, WBC was decreased to 1.5 × 103/μl with neutrophils of 0.6 × 103/μl and lymphocytes of 0.7 × 103/μl. The serum ferritin level was increased to 950 ng/ml. Chest radiograph showed marked aggravation of left lower lobar infiltration complicated by pleural effusion (Fig. 1A). Antibiotics were switched to panipenem-betamipron (45 mg/kg/day) and minocycline (4 mg/kg/day). Computed tomography (CT) of the chest demonstrated extensive consolidation of the left lower lobe complicated by air bronchogram and pleural effusion (Fig. 1C). On that day, we initiated methylprednisolone pulse therapy once daily for 3 days. Six hours after the initiation of steroid therapy, she became afebrile. On the next day, dyspnea was resolved. Chest radiograph on that day showed dramatic improvement (Fig. 1B). Five days after the initiation of steroid therapy, laboratory findings were normalized. She was discharged on the 17th day of admission without sequelae.
Figure 1.
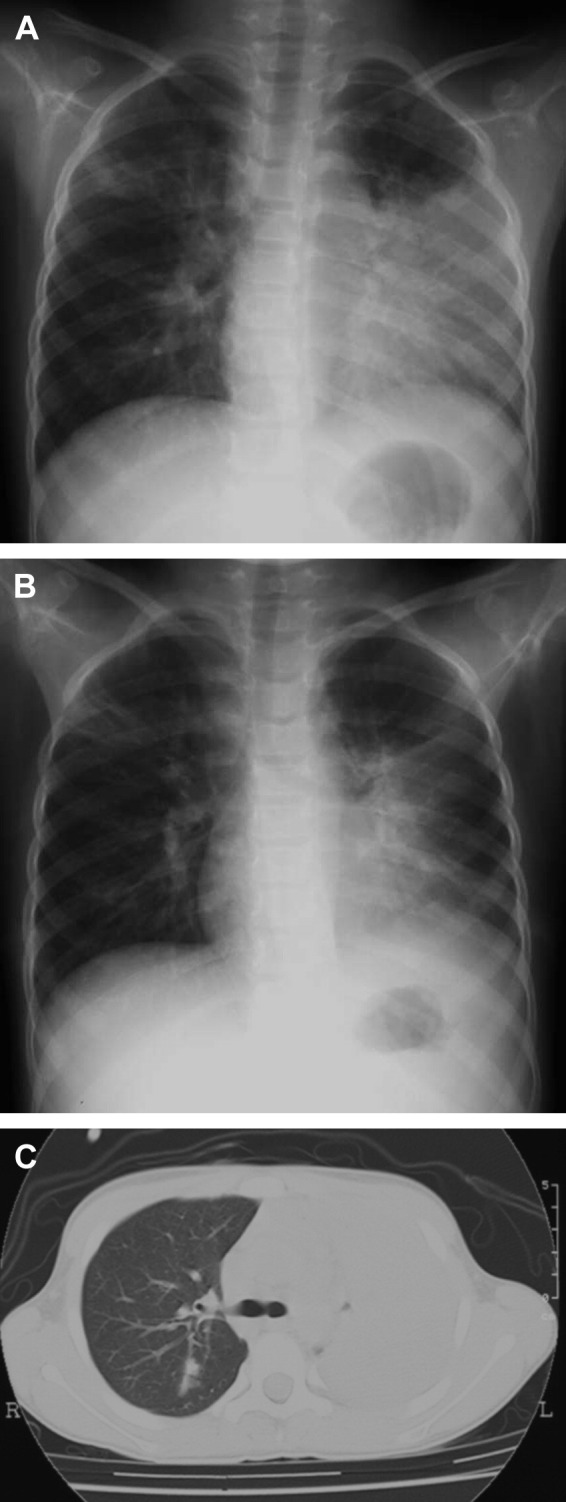
Chest radiograph before (A) (10th day of admission) and after (B) (11th day of admission) methylprednisolone pulse therapy, and computed tomography of the chest before steroid therapy (C) (10th day of admission) in patient 1.
Patient 4
A 9-year-old girl with unremarkable medical history was hospitalized because of 5-day history of fever, cough and shortness of breath. On admission, body temperature was 39.4 °C, pulse rate was 128/min and respiration rate was 40/min. Coarse crackles were heard with decreased breath sounds over the left upper lung. Peripheral blood cell counts were as follows: WBC 4.1 × 103/μl with neutrophils 3.0 × 103/μl and lymphocytes 1.0 × 103/μl, Hb 12.4 g/dl and Plt 117 × 103/μl. Blood chemistry results included LDH of 698 IU/l and CRP of 4.5 mg/dl. Chest radiograph demonstrated a left upper lobe infiltrate. Although a three-drug antibiotic regimen that included flomoxef (80 mg/kg/day), clarithromycin (12 mg/kg/day), and minocycline (4 mg/kg/day) was started, the patient remained febrile and respiratory conditions deteriorated to severe dyspnea over the next two days. Auscultation of the lungs demonstrated severely diminished breath sounds over the left lung. Leucopenia (3.8 × 103/μl), thrombocytopenia (Plt 122 × 103/μl), and liver dysfunction (aspirate aminotransferase/alanine aminotransferase: 97/52 IU/l) were also noted. The serum ferritin level and urine β2-microglobulin increased to 1480 ng/ml and 1998 μg/l, respectively. Two days after admission, chest radiograph and CT demonstrated aggravation of consolidation of the left upper lobe complicated by pleural effusion (Fig. 2A and C). On that day, high-dose corticosteroid therapy was started and antibiotics were substituted to panipenem-betamipron (60 mg/kg/day) and azithromycin (10 mg/kg/day). The patient became afebrile 5 h after the first steroid infusion. On the next day, she had only mild dyspnea and chest radiograph showed dramatic improvement of infiltration and decrease of pleural effusion (Fig. 2B). Five days after admission, laboratory findings were as follows: WBC 6.8 × 103/μl, LDH 436 IU/l, ferritin 376 ng/ml, and urine β2-microglobulin 901 μg/l. She fully recovered and was discharged 11 days after admission.
Figure 2.
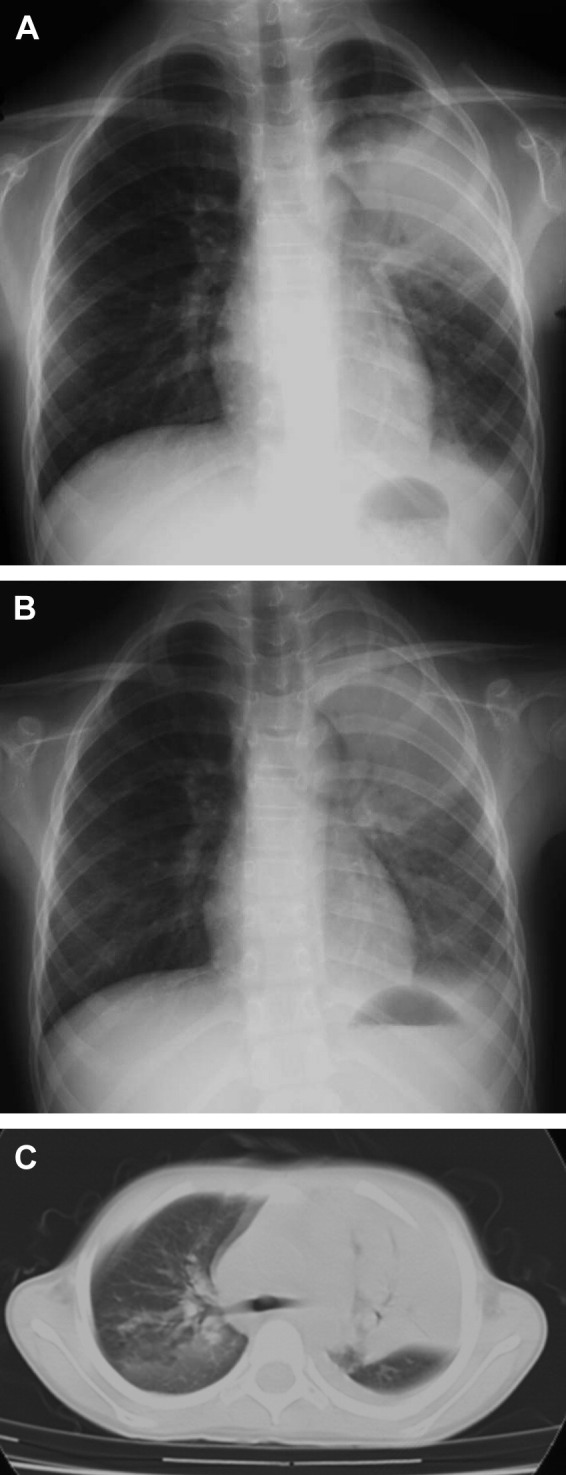
Chest radiograph before (A) (8th day of admission) and after (B) (9th day of admission) methylprednisolone pulse therapy, and computed tomography of the chest before steroid therapy (C) (8th day of admission) in patient 4.
Discussion
Cell-mediated immunity of the host plays an important role in the development of the pulmonary lesions in MP. The role of T-cells in the pathogenesis of Mycoplasma infection can be defined by an examination in immunocompromized hosts10 and by apparent correlation of the development of a delayed hypersensitivity skin reaction to M. pneumoniae with the severity of disease.11 Furthermore, Tanaka et al. demonstrated that the levels of serum interleukin-18, which promotes Th1 cytokine responses, in patients with severe MP were higher than those in mild cases and correlated with the number of affected lobes.12
We report rapid clinical and radiological improvement of refractory MP in children after methylprednisolone pulse therapy. These cases would show the beneficial effects of corticosteroids on severe and refractory MP. The mechanisms underlying the effects of steroids remain to be elucidated. Based on the rapid improvements of clinical symptoms and pulmonary lesions, it has been proposed that hypercytokinemia and resultant immunological dysregulation plays a crucial role in the action.13, 14 Recent evidence indicated that a similar pathogenesis was responsible for the development of acute respiratory distress syndrome and multiorgan failure in severe cases of corona virus (severe acute respiratory syndrome) and avian H5N1 influenza virus.15, 16 Accumulated evidence has shown that high-dose corticosteroid is effective for such patients.15, 16 These severe viral diseases share common laboratory findings, such as cytopenia, high serum levels of ferritin, transaminase, and LDH, similar to those we observed in refractory MP patients. Lymphopenia was observed in all of our patients and it recovered after steroid therapy, and this finding might be associated with dysregulation of cellular immunity. High-dose methylprednisolone has an ability to downregulate the cell-mediated immune response, and therefore may have a profound effect by reducing the immune-mediated pulmonary injury seen in severe mycoplasma infection.
The appropriate courses of corticosteroid therapy in children are not well established. The reported corticosteroid treatment regimens used for severe pediatric MP varied widely. Lee et al. administered oral prednisolone of 1 mg/kg/day for 3–7 days, then weaned over 1 week in 15 patients but showed improvement of clinical and radiological findings over several days.9 We employed methylprednisolone pulse therapy at a dose of 30 mg/kg over 3 consecutive days. This course was selected based on the successful experiences that patients with severe acute lung injury2 or refractory pneumonia17 caused by M. pneumoniae well responded to 1 g methylprednisolone pulse for 1–2 days. Our treatment resulted in rapid deferverscence in all six patients within 14 h after initiation of steroid therapy as well as prompt clinical improvement. This would allow for shorter hospital stay and treatment period than those under the standard dosage of prednisolone therapy. Of additional note is that there were no adverse events during pulse therapy. However, dosage and duration of steroids should be modified according to disease severity and chronicity. The dosage that we used was high, and has generally been applied in cases of severe lung disease with life-threatening events.2, 17 Therefore, the efficacy and clinical outcome of different steroid regimens should be carefully determined in a well-designed comparative study.
Macrolide-resistant M. pneumoniae (MR M. pneumoniae) has been increasingly isolated in Japan, accounting for 15%–20% of clinical isolates or polymerase chain reaction-positive specimens isolated between 2000 and 2004.18, 19 Given that we did not perform an antimicrobial susceptibility test, whether nonresponsiveness in our patients to antibiotics is related to the macrolide-resistance was not defined. However, Suzuki et al. reported that although MR MP patients showed more febrile days than macrolide-sensitive MP patients, there was no apparent treatment failure or serious illness in MR MP patients.20 Thus, the refractory condition does not appear to be a mere reflection of MR M. pneumoniae infection.
In conclusion, this is the first case-series report indicating management of methylprednisolone pulse therapy associated with a good outcome in children with severe refractory MP. This therapy showed rapid clinical improvement without major adverse events in this series. However, a larger prospective study is needed to define the benefits of high-dose corticosteroid therapy in this condition.
References
- 1.Bamba M., Jozaki K., Sugaya N., Tamai S., Ishihara J., Kori T. Prospective surveillance for atypical pathogens in children with community-acquired pneumonia in Japan. J Infect Chemother. 2006;12:36–41. doi: 10.1007/s10156-005-0422-y. [DOI] [PubMed] [Google Scholar]
- 2.Radisic M., Torn A., Gutierrez P., Defranchi H.A., Pardo P. Severe acute lung injury caused by Mycoplasma pneumoniae: potential role for steroid pulses in treatment. Clin Infect Dis. 2000;31:1507–1511. doi: 10.1086/317498. [DOI] [PubMed] [Google Scholar]
- 3.Wang R.S., Wang S.Y., Hsieh K.S., Chiou Y.H., Huang I.F., Cheng M.F. Necrotizing pneumonitis caused by Mycoplasma pneumoniae in pediatric patients. Pediatr Infect Dis J. 2004;23:564–567. doi: 10.1097/01.inf.0000130074.56368.4b. [DOI] [PubMed] [Google Scholar]
- 4.Takiguchi Y., Shikama N., Aotsuka N., Koseki H., Terano T., Hirai A. Fulminant Mycoplasma pneumoniae pneumonia. Intern Med. 2001;40:345–348. doi: 10.2169/internalmedicine.40.345. [DOI] [PubMed] [Google Scholar]
- 5.Chan E.D., Welsh C.H. Fulminant Mycoplasma pneumoniae pneumonia. West J Med. 1995;162:133–142. [PMC free article] [PubMed] [Google Scholar]
- 6.Koletsky R.J., Weinstein A.J. Fulminant Mycoplasma pneumoniae infection. Report of a fatal case and a review of the literature. Am Rev Respir Dis. 1980;122:491–496. doi: 10.1164/arrd.1980.122.3.491. [DOI] [PubMed] [Google Scholar]
- 7.Fujishiro M., Takezawa S., Hasejima N., Kobayashi H., Yamato K. A case of fulminant Mycoplasma pneumoniae pneumonia successfully treated by steroid therapy. Nihon Kokyuki Gakkai Zasshi. 1999;37:476–480. [in Japanese with English abstract] [PubMed] [Google Scholar]
- 8.Miyashita N., Obase Y., Ouchi K., Kawasaki K., Kawai Y., Kobashi Y. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol. 2007;56:1625–1629. doi: 10.1099/jmm.0.47119-0. [DOI] [PubMed] [Google Scholar]
- 9.Lee K.Y., Lee H.S., Hong J.H., Lee M.H., Lee J.S., Burgner D. Role of prednisolone treatment in severe Mycoplasma pneumonia pneumonia in children. Pediatr Pulmonol. 2006;41:263–268. doi: 10.1002/ppul.20374. [DOI] [PubMed] [Google Scholar]
- 10.Foy H.M., Ochs H., Davis S.D., Kenny G.E., Luce R.R. Mycoplasma pneumoniae infections in patients with immunodeficiency syndromes: report of four cases. J Infect Dis. 1973;127:388–393. doi: 10.1093/infdis/127.4.388. [DOI] [PubMed] [Google Scholar]
- 11.Mizutani H., Kitayama T., Hayakawa A., Nagayama E. Delayed hypersensitivity in Mycoplasma pneumoniae infection. Lancet. 1971;1(7691):186–187. doi: 10.1016/s0140-6736(71)91956-8. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H., Narita M., Teramoto S., Saikai T., Oashi K., Igarashi T. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121:1493–1497. doi: 10.1378/chest.121.5.1493. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh C.C., Tang R.B., Tsai C.H., Chen W. Serum interleukin-6 and tumor necrosis factor-α concentrations in children with mycoplasma pneumonia. J Microbiol Immunol Infect. 2001;34:109–112. [PubMed] [Google Scholar]
- 14.Ishida Y., Hiroi K., Tauchi H., Oto Y., Tokuda K., Kida K. Hemaphagocytic lymphohistiocytosis secondary to mycoplasma pneumoniae infection. Pediatr Int. 2004;46:174–177. doi: 10.1046/j.1442-200x.2004.01878.x. [DOI] [PubMed] [Google Scholar]
- 15.Ho J.C., Ooi G.C., Mok T.Y., Chan J.W., Hung I., Lam B. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med. 2003;168:1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- 16.Carter M.J. A rationale for using steroids in the treatment of severe cases of H5N1 avian influenza. J Med Microbiol. 2007;56:875–883. doi: 10.1099/jmm.0.47124-0. [DOI] [PubMed] [Google Scholar]
- 17.Cimolai N. Corticosteroids and complicated Mycoplasma pneumoniae infection. Pediatr Pulmonol. 2006;41:1008–1009. doi: 10.1002/ppul.20424. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka M., Narita M., Okazaki N., Ohya H., Yamazaki T., Ouchi K. Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob Agents Chemother. 2004;48:4624–4630. doi: 10.1128/AAC.48.12.4624-4630.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morozumi M., Hasegawa K., Kobayashi R., Inoue N., Iwata S., Kuroki H. Emergence of macrolide-resistant Mycoplasma pneumoniae with a 23S rRNA gene mutation. Antimicrob Agents Chemother. 2005;49:2302–2306. doi: 10.1128/AAC.49.6.2302-2306.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S., Yamazaki T., Narita M., Okazaki N., Suzuki I., Andoh T. Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob Agents Chemother. 2006;50:709–712. doi: 10.1128/AAC.50.2.709-712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


