Abstract
Background
Community-acquired pneumonia (CAP) in an immunocompetent host may be severe because of a variety or combination of host and microbial factors. In patients with severe cardiopulmonary dysfunction, even relatively avirulent pathogens, that is, Mycoplasma pneumoniae, Moraxella catarrhalis, may compromise borderline cardiac/heart function and present clinically as severe CAP. Alternately, patients with Streptococcus pneumoniae and impaired humoral immunity/splenic dysfunction may present as severe CAP. With the exception of Legionnaire's disease, influenza, and adenovirus, pathogen virulence is not a key determinant of CAP severity.
Methods
Diagnostically, patients with severe CAP may be approached based on the pattern of infiltrates on chest x-ray together with the severity of hypoxemia (ie, increased A-a gradient: >35).
Results
We present the case of an immunocompetent adult who presented with severe CAP during peak influenza season. Direct fluorescent antibody testing of his respiratory secretions was negative for influenza, adenovirus, and other respiratory viruses. Diagnostic bronchoscopy was negative for bacterial and fungal pathogens. The only clues to the cause of his severe CAP was the presence of relative lymphopenia, atypical lymphocytosis and elevated serum transaminases. After influenza and adenovirus were ruled out, cytomegalovirus (CMV) CAP was considered. The diagnosis of CMV CAP was made serologically by demonstrating highly elevated IgM CMV titers. Because the diagnosis was made during the patient's recovery late in hospitalization, he did not receive CMV antiviral therapy.
Conclusion
This case should remind clinicians that influenza and adenovirus are diagnostic considerations in patients presenting with severe CAP with diffuse bilateral interstitial infiltrates accompanied by severe hypoxemia in normal hosts. If influenza and adenovirus are ruled out, then CMV CAP, although rare, should be considered, particularly when viral CAP is accompanied by relative lymphopenia, atypical lymphocytosis and increased serum transaminases.
Cytomegalovirus (CMV) is a DNA virus in the family Herpesviridae. CMV transmission from person to person requires body fluid contact. CMV initially penetrates host epithelial cells and produces cell enlargement, thus the term “cytomegalovirus.” After initial infection by the immune system suppresses the virus and CMV remains latent unless reactivated by impaired cell-mediated immunity (CMI).1, 2, 3
There are many clinical manifestations of CMV infection that may be divided into acute primary infection and reactivation. Acute infection occurs in neonates via vertical transmission from the mother. In immunocompetent adults, acute infection is manifested by an infectious mononucleosis-like syndrome that is benign and self-limited. In immunocompromised hosts (ie, organ transplant recipients), patients with impaired CMI, and patients with human immunodeficiency virus (HIV), severe disease is often seen.4, 5 Acute CMV or reactivation of latent infection can affect almost any organ. However, the most common manifestations are retinitis, pneumonia, encephalitis, hepatitis, and colitis. Severe infection is rare in immunocompetent hosts.1, 2, 3, 6, 7 We present a case of severe CMV community-acquired pneumonia (CAP) in an immunocompetent host.
Case Report
A 64-year-old man was admitted with shortness of breath. He had a 2-day history of a “flu-like illness” characterized by fever, myalgias, and progressive breathlessness. The patient had seen his primary physician, who prescribed oseltamivir and moxifloxacin, but the shortness of breath progressed and he went to the hospital for further evaluation and treatment. On admission, the patient's temperature was 103°F with a simultaneous pulse of 107 beats/min. He appeared to be in mild to moderate respiratory distress. He had a negative medical history, was a normal host, and had no risk factors for HIV. His physical examination results were unremarkable except for bilateral diffuse rales.
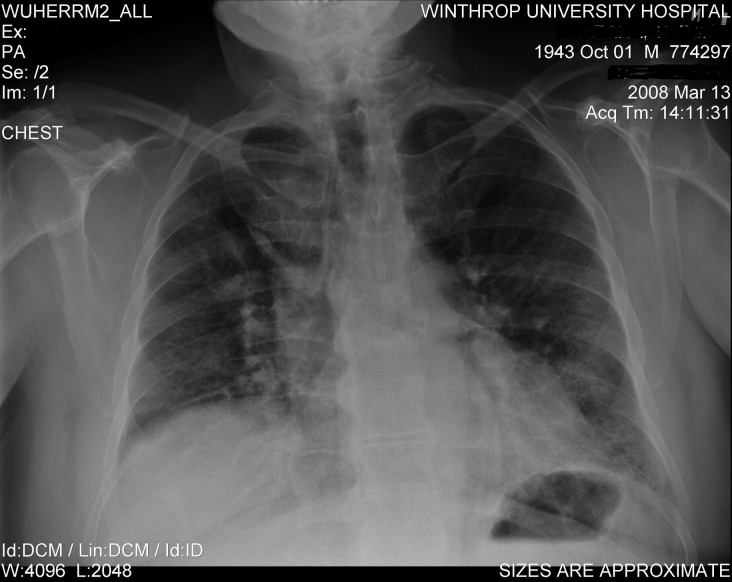
Admission laboratory tests revealed a white blood cell count of 35 K/mm3 (polymorphonuclear neutrophils = 83%, lymphocytes = 14%, atypical lymphocytes = 5%, and monocytes = 3%), a platelet count of 581 K/mm3 (n = 160-392 K/mm3), an erythrocyte sedimentation rate of 82 mm/h (n = 1-20 mm/h), a C-reactive protein level of 11.3 mg/L (n = <3 mg/L), a blood urea nitrogen level of 24 mg/dL (n = 8-21 mg/dL), and a creatinine level of 0.9 mg/dL (n = 0.6-1.2 mg/dL). Serum transaminases were mildly elevated: serum glutamate oxaloacetate transaminase = 171 IU/L (n = 13-39 IU/L), serum glutamate pyruvate transaminase = 135 IU/L (n = 4-36 IU/L), and alkaline phosphatase = 181 IU/L (n = 25-100 IU/L). Respiratory fluorescent antibody panel for influenza A and B virus, respiratory syncytial virus, adenovirus, parainfluenza virus, and metapneumovirus was negative. His initial chest x-ray showed bilateral interstitial markings that rapidly progressed over 24 hours (Fig 1, Fig 2). Diagnostic bronchoscopy was unrevealing, and bronchoalveolar lavage (BAL) was negative for Pneumocystis (carinii) jiroveci pneumonia. Bacterial and fungal cultures were negative. BAL cytology was negative for viral inclusions.
Fig 1.
Admission chest x-ray of severe CMV CAP in an immunocompetent adult.
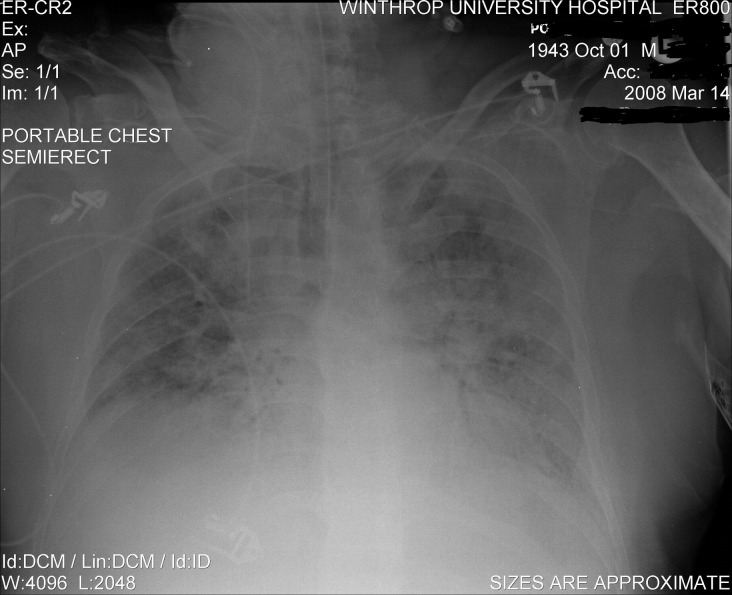
Fig 2.
Repeat chest x-ray of severe CMV CAP on hospital day 2.
During the patient's hospitalization, he became progressively hypoxemic and required intubation and mechanical ventilation. His A-a gradient on room air was 194. Mycoplasma pneumoniae, C. pneumoniae, Herpes simplex virus (HSV) 1 and 2, and Q fever titers were negative. Legionella titers and urinary legionella antigen testing were also negative. Atypical lymphocytosis persisted and peaked at 13%. He slowly improved over 14 days, and antibiotics were discontinued after 20 days. His oxygen requirements gradually decreased, and he was eventually extubated.
Influenza A titers were 1:8 (n = <1:8), and influenza B titers were 1:16 (n = 1:8). Cold agglutinins were repeatedly negative. CMV polymerase chain reaction (PCR) was negative. His initial immunoglobulin IgM CMV titer was 1.87 (n < 1.1 ISR). Ten days later, his IgM CMV titer was 2.4 (n < 1.1). His initial IgG CMV titer was 3.26 (n < 1.1), and 10 days later the IgG CMV titer was 4.28 (n < 1.1), confirming the diagnosis of CMV pneumonia. The patient was eventually discharged and remains in good health after hospitalization.
Discussion
CAP may be caused by a variety of typical and atypical bacterial pathogens, as well as some viruses. The severity of CAP depends on host factors (ie, cardiopulmonary function), immune system status (ie, splenic function), and, in some cases, the virulence of the pathogen. Patients with preexisting severe cardiac or pulmonary disease, if pneumonia develops, may present with severe CAP. Superimposed CAP stresses already compromised cardiopulmonary function and may manifest clinically as severe CAP. Even relatively avirulent pathogens (eg, M. pneumoniae, M. catarrhalis) may manifest as severe CAP in patients with impaired cardiopulmonary function. With CAP caused by encapsulated bacteria (eg, Streptococcus pneumoniae, Haemophilus influenzae), splenic function is the primary determinant of CAP severity. Any of the above factors alone or in combination in immunocompetent hosts may present clinically as severe CAP. Nonetheless, some pathogens are inherently highly virulent and often present as severe CAP. All other things being equal, CAP is likely to be severe in patients with Legionnaire's disease, influenza, systemic acute respiratory distress syndrome (SARS), hanta pulmonary virus syndrome (HPS), or adenovirus.8, 9, 10
CMV pneumonia occurs almost exclusively in patients with impaired CMI/impaired T-lymphocyte function (eg, organ transplant recipients and those with HIV) or who are receiving immunomodulating/immunosuppressive agents.1, 2, 3, 4, 5, 8 CMV CAP results from the reactivation of a previous/latent dormant CMV infection. Although clinically similar, HSV-I pneumonia, an often overlooked but important cause of nosocomial pneumonia, does not present as CAP.8, 11, 12, 13, 14
In the compromised host with impaired CMI, CMV CAP pneumonia presents early (<48 hours) with fever and profound/prolonged hypoxemia with minimal or no pulmonary infiltrates. Pulmonary infiltrates may appear later (>48 hours) with CMV CAP. Nonspecific laboratory abnormalities often associated with CMV CAP include leukopenia, relative lymphopenia, and thrombocytopenia.8, 15, 16 Atypical lymphocytes may or may not be present in the peripheral smear. CMV infection regularly involves the liver, manifested in CMV CAP by mild elevations of serum transaminases.1, 2, 3 In patients with HIV, CMV is an “innocent bystander” copathogen in approximately 75% of cases with Pneumocystis (carinii) jiroveci pneumonia (PCP). In patients with PCP, the underlying CMV does not need to be treated. In patients with HIV with PCP, in contrast, PCP resolves without specifically treating the CMV component. However, in organ transplant recipients, CMV CAP is treated with specific anti-CMV antiviral therapy.8, 17 Because CMV CAP is rare in immunocompetent adults, the natural history of CMV CAP is not well understood.
CAP CMV is clinically indistinguishable from other viral pneumonias. As with CMV, other viral pneumonias may be accompanied by leukopenia, relative lymphopenia, or thrombocytopenia. Viral pneumonias typically present with minimal or few infiltrates on chest x-ray and are accompanied by severe hypoxemia with a high A-a gradient (ie, >35).8, 18
The diagnosis of CMV CAP may be made serologically or pathologically. The serologic diagnosis of CMV depends on demonstrating an elevated CMV IgM titer. CMV PCR is often negative, but if positive it is also diagnostic. Pathologically, CMV inclusion bodies may be seen in transbronchial/open lung biopsy specimens pathognomonic for CMV, that is, cytoplasmic inclusions, cellular gigantism, and “kidney bean-shaped” intranuclear inclusions. BAL is usually negative and therefore unhelpful in diagnosing CMV CAP. In contrast, in HSV-1 nosocomial pneumonia, cytopathic examination of bronchial epithelial cells is diagnostic for HSV-1. Therefore, in the absence of viral inclusion bodies in transbronchial, percutaneous, or open lung biopsy specimens, the clinical diagnosis of CMV CAP is based on the clinical presentation of viral pneumonia plus increased IgM CMV titers or a positive CMV PCR.3, 8, 19
In the case presented, an immunocompetent adult presented with severe CAP requiring intensive care and ventilatory support.20, 21, 22, 23, 24 His hypoxemia was out of proportion to auscultation and chest x-ray findings. Minimal bilateral, symmetrical, interstitial chest x-ray infiltrates on the chest x-ray with an increased A-a gradient (>35) and minimal infiltrates suggested a process causing an oxygen diffusion defect (ie, viral pneumonia).8, 22, 24 Because he was admitted during influenza season, the initial working diagnosis was viral influenza. However, rapid influenza testing and respiratory viral fluorescent antibody panel were negative for influenza and other respiratory viruses. Viral influenza and adenoviral titers were also negative. Because he was an immunocompetent host, CMV was not an initial diagnostic consideration.5, 6 The possibility of CMV was later considered in view of some of his otherwise unexplained laboratory findings, for example, atypical lymphocytosis (5%-13%) and increased serum transaminases (serum glutamate oxaloacetate transaminase = 103-331 IU/L, n = 13-39 IU/L; serum glutamate oxaloacetate transaminase = 164-441 IU/L, n = 4-36 IU/L). Diagnostic bronchoscopy was performed, and cytology was negative for HSV-1 and CMV. Viral cultures of bronchial washings were negative for CMV. Because the diagnosis was confirmed after the patient's recovery, which was slow and prolonged, he was not treated for CMV CAP.
The clinical approach to a patient with severe CAP depends on the assessment of the cardiopulmonary and splenic function, distribution of the pulmonary infiltrates on chest x-ray or chest computed tomography scan, and degree of hypoxemia/magnitude of the A-a gradient.8, 22, 23, 24
Patients with severe CAP may be approached clinically as having either focal or nonfocal infiltrates. Those with focal chest x-ray infiltrates without a large A-a gradient (<35) have severe CAP usually as the result of impaired cardiopulmonary/splenic function. The pathogens causing severe CAP with focal chest x-ray infiltrates without a large diffusion defect (ie, A-a gradient < 35) are the typical bacterial CAP pathogens: S. pneumoniae, H. influenzae, M. catarrhalis, M. pneumoniae, and C. pneumoniae. In contrast, in patients presenting with severe CAP with focal chest x-ray infiltrates and a large A-a gradient (>35), Legionnaire's disease or, less commonly, adenoviral CAP is the primary diagnostic consideration. If a patient presents with severe CAP and no or minimal infiltrates, when accompanied by a large A-a gradient (>35), then an interstitial infectious process should be considered. Interstitial pathogens causing pneumonia with an oxygen diffusion defect are respiratory viruses: influenza, respiratory syncytial virus, systemic acute respiratory distress syndrome and hanta pulmonary virus syndrome.8 PCP is found virtually only in individuals with HIV and immunosuppressed patients (ie, transplants), steroids (Table I). 8, 22 In an “apparently normal” host, the diagnosis of otherwise unexplained PCP should prompt HIV testing.8 Influenza A is the prototypical cause of severe viral CAP. Patients with severe influenza A have a severe oxygen diffusion defect and die of profound hypoxemia without bacterial suprainfections. A minority of patients with influenza have superimposed S. aureus CAP. Influenza with superimposed bacterial pneumonia is easily recognized in patients because there are focal infiltrates on chest x-ray. Later in viral influenza (ie, >48 hours), there may be bilateral patchy chest x-ray infiltrates which are not nonsegmental or lobar infiltrates, and indicate a superimposed bacterial pneumonia.8, 18
Table I.
Differential diagnosis of severe community-acquired pneumonia in immunocompetent hosts⁎
| Chest x-ray infiltrates | Potential pathogens⁎ |
|---|---|
| Focal infiltrates | |
| (A-a gradient < 35) | S. pneumoniae |
| H. influenzae† | |
| M. catarrhalis† | |
| M. pneumoniae† | |
| C. pneumoniae† | |
| Focal infiltrates | |
| (A-a gradient > 35) | Legionnaire's disease |
| Adenovirus | |
| Early: <48 h | |
| No/minimal diffuse infiltrates | |
| (A-a gradient > 35) | Influenza |
| Adenovirus | |
| RSV | |
| Later: >48 h | |
| Bilateral diffuse patchy infiltrates | Influenza |
| (A-a gradient > 35) | CMV |
| SARS | |
| HPS | |
| PCP‡ |
RSV, Respiratory syncytial virus; CMV, cytomegalovirus; SARS, severe acute respiratory syndrome; HPS, hantavirus pulmonary syndrome; PCP, pneumocystis carinii pneumonia.
Excluding zoonotic atypical pneumonias (eg, tularemia).
Only with severely impaired cardiopulmonary function.
Only in HIV and immunocompromised hosts with impaired T-lymphocyte function.
The clinical presentation of respiratory viral pathogens presenting as severe CAP is difficult to differentiate clinically. Many clinical and laboratory features overlap. Common differential diagnostic problems include differentiating viral influenza from adenoviral CAP.8, 18, 22, 23, 24 Ordinarily, CMV CAP in immunocompetent hosts is not a diagnostic consideration. In the case presented, a viral influenza was suspected because the case occurred during the peak of the influenza season. Adenoviral CAP also occurs during the late winter and early spring months and was also a likely diagnostic possibility. Because the patient did not have leukopenia, relative lymphopenia, or thrombocytopenia, CMV was considered as a possible pathogen only after influenza and adenoviral CAP were ruled out. Although it is true that influenza and adenovirus pneumonia may be associated with mildly increased serum transaminases, tests for these pathogens were negative, prompting testing for an unusual diagnosis (ie, CMV). The patient's IgM CMV titer was highly elevated (5.65 ISR; n < 1.1 ISR), which confirmed the diagnosis in this case (Table II). 8, 18 We believe the mildly elevated influenza B titers were the result of CMV cross-reactivity.8, 25, 26
Table II.
Differential diagnosis of severe viral community-acquired pneumonia
| Influenza | Adenovirus | CMV | |
|---|---|---|---|
| Symptoms | |||
| Onset | Acute | Acute | Acute |
| Presentation | Acute/severe | Subacute/moderate | Subacute/moderate |
| Myalgias | + | ± | ± |
| Neck/back pain | + | − | − |
| Signs | |||
| Fever | + | + | + |
| Dry cough | + | ± | ± |
| Conjunctival suffusion | + | ± | − |
| Blood tinged sputum | + | − | − |
| Laboratory tests | |||
| CBC: | |||
| Leukocytosis | − | − | ± |
| Leukopenia | + | + | ± |
| Relative lymphopenia | + | + | ± |
| Atypical lymphocytosis | − | + | + |
| Thrombocytopenia | + | + | ± |
| Cold agglutinin titers (<1:64) | ± | ± | ± |
| Chest x-ray | |||
| No/minimal infiltrate (early <48 h) | + | + | + |
| Bilateral diffuse infiltrates (later >48 h) | + | ± | − |
| Focal infiltrates | −⁎ | + | − |
| Severe hypoxemia (A-a gradient >35) | + | ± | ± |
| Fluorescent respiratory viral panel | + | + | − |
| ↑ Influenza IgM titers | + | − | − |
| ↑ Adenoviral IgM titers | − | + | − |
| ↑ CMV IgM titers | − | − | + |
| + CMV PCR | − | − | ± |
| Diagnostic bronchoscopy | |||
| BAL cytology | − | − | − |
| TBB (viral inclusions) | + | + | + |
Ig, Immunoglobulin; PCR, polymerase chain reaction; CBC, complete blood count; CMV, cytomegalovirus; BAL, bronchoalveolar lavage; TBB, transbronchial biopsy.
Adapted from Cunha BA. Pneumonia essentials. 3rd edition. Jones & Bartlett, Sudbury, MA, 2009.
Only with coexistent S. aureus CAP.
Severe CMV infections are rare in immunocompetent hosts. In the largest review published to date, 34 cases of CMV infection ranging from isolated encephalitis to disseminated disease with multiple organ involvement were described.7 In this case series, CMV CAP was the sole manifestation of CMV infections in only 1 case (1/34). CMV CAP was present in 9 of 34 cases. In 5 of 9 CMV CAP cases, lung infection was accompanied by hepatic involvement, an important clue to the diagnosis. The diagnosis of CMV infection was confirmed serologically in 23 of 34 patients. The single patient with isolated CMV CAP without liver involvement survived without anti-CMV therapy, as was the case in the patient presented.7
Conclusions
In severe CAP with normal/near-normal chest x-rays and profound hypoxemia accompanied by leukopenia, relative lymphopenia, thrombocytopenia, or elevated serum transaminases, the primary diagnostic considerations should be viral influenza and adenovirus. The diagnosis of influenza should be doubted without leukopenia and relative lymphopenia, which are key features of influenza with both diagnostic and prognostic significance. The diagnosis of severe influenza or adenovirus CAP should be questioned in the absence of leukopenia with relative lymphopenia, and alternate diagnoses should be considered. However, if viral influenza and adenovirus are ruled out in patients with otherwise unexplained atypical lymphocytosis and increased serum transaminases, clinicians should consider the possibility of CMV in immunocompetent adults presenting with severe CAP.
References
- 1.Ho M. 2nd ed. Plenum Publishing Corp; New York: 1991. Cytomegalovirus biology and infection. [Google Scholar]
- 2.Britt W.J., Alford C.A. CMV. In: Fields N.B., Knipe D.M., Hawley P.M., editors. Fields virology. 3rd ed. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 2493–2523. [Google Scholar]
- 3.Pass R.F. Cytomegalovirus. In: Knipe D.M., Howley P.M., editors. Fields virology volume 2. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2675–2705. [Google Scholar]
- 4.Meyers J.D., Spencer H.C., Watts J.C., Gregg M.B., Stewart J.A., Troupin R.H., Thomas E.D. CMV pneumonia after human marrow transplantation. Ann Intern Med. 1975;82:181–188. doi: 10.7326/0003-4819-82-2-181. [DOI] [PubMed] [Google Scholar]
- 5.Mera J.R., Whimbey E., Elting L. Cytomegalovirus pneumonia in adult nontransplantation patients with cancer: review of 20 cases occurring from 1964 through 1990. Clin Infect Dis. 1996;22:1046–1050. doi: 10.1093/clinids/22.6.1046. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J.L., Corey G.R. Cytomegalovirus infection in the normal host. Medicine. 1985;64:100–114. doi: 10.1097/00005792-198503000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Eddleston M., Peacock S., Juniper M., Warrell D.A. Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 1997;24:52–56. doi: 10.1093/clinids/24.1.52. [DOI] [PubMed] [Google Scholar]
- 8.Cunha B.A. Community acquired pneumonia. In: Cunha B.A., editor. Pneumonia essentials. 2nd ed. Physicians Press; Royal Oak, MI: 2008. pp. 116–134. [Google Scholar]
- 9.Cunha B.A. Severe Legionella pneumonia: rapid diagnosis with Winthrop-University Hospital's weighted point score system (modified) Heart Lung. 2008;37:312–321. doi: 10.1016/j.hrtlng.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Acute respiratory disease associated with adenovirus serotype 14—four states, 2006-2007. MMWR Morb Mortal Wkly Rep. 2007;56:1181–1184. [PubMed] [Google Scholar]
- 11.Cunha B.A. Herpes simplex-1 (HSV-1) pneumonia. Infect Dis Pract. 2005;29:375–378. [Google Scholar]
- 12.Eisenstein L., Cunha B.A. Herpes simplex virus type 1 (HSV-1) pneumonia presenting as failure to wean. Heart Lung. 2003;32:65–66. doi: 10.1067/mhl.2003.4. [DOI] [PubMed] [Google Scholar]
- 13.Cunha B.A., Eisenstein L.E., Dillard T., Krol V. Herpes simplex virus (HSV) pneumonia in a heart transplant: diagnosis and therapy. Heart Lung. 2007;36:72–78. doi: 10.1016/j.hrtlng.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Mohan S.S., Hamid N., Cunha B.A. A cluster of nosocomial HSV-1 pneumonia in an intensive care unit. Infect Control Hosp Epidemiol. 2006;26:1255–1257. doi: 10.1086/508843. [DOI] [PubMed] [Google Scholar]
- 15.Salomon N., Perlman D.C. Cytomegalovirus pneumonia. Semin Respir Infect. 1999;14:353–358. [PubMed] [Google Scholar]
- 16.Forward K. Community-acquired pneumonia due to cytomegalovirus, herpes simplex 1 virus, and human herpesvirus-6. In: Marrie T.J., editor. Community-acquired pneumonia. Plenum Publishing Corp; New York: 2001. pp. 665–678. [Google Scholar]
- 17.Millar A.B., Patou G., Miller R.F., Grundy J.E., Katz D.R., Weller I.V., Semple S.J. CMV in the lungs of patients with AIDS: respiratory pathogen or passenger? Am Rev Respir Dis. 1990;141:1474–1477. doi: 10.1164/ajrccm/141.6.1474. [DOI] [PubMed] [Google Scholar]
- 18.Cunha B.A. The clinical diagnosis of severe viral influenza A. Infection. 2008;36:92–93. doi: 10.1007/s15010-007-7255-9. [DOI] [PubMed] [Google Scholar]
- 19.Buffone G.J., Frost A., Samo T., Demmler G.J., Cagle P.T., Lawrence E.C. The diagnosis of CMV pneumonitis in lung and heart/lung transplant patients by PCR compared with traditional laboratory criteria. Transplantation. 1993;56:342–347. doi: 10.1097/00007890-199308000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Cunha B.A. Severe community-acquired pneumonia. J Crit Illn. 1997;12:711–721. [Google Scholar]
- 21.Cunha B.A. Severe community-acquired pneumonia. Crit Care Clin. 1998;14:105–118. doi: 10.1016/s0749-0704(05)70384-5. [DOI] [PubMed] [Google Scholar]
- 22.Cunha B.A. Pulmonary infections in the compromised host. Infect Dis Clin. 2001;16:591–596. doi: 10.1016/s0891-5520(05)70161-6. [DOI] [PubMed] [Google Scholar]
- 23.Cunha B.A. Severe community acquired pneumonia: determinants of severity and approach to therapy. Infect Med. 2005;22:53–58. [Google Scholar]
- 24.Cunha B.A. Severe community acquired pneumonia in the critical care unit. In: Cunha B.A., editor. Infectious disease in critical care medicine. 2nd ed. Informa; NY: 2007. pp. 157–167. [Google Scholar]
- 25.Eisenstein L., Cunha B.A. Diagnostic significance of elevated cold agglutinin titers in infectious disease. Infect Dis Pract. 2007;31:577–578. [Google Scholar]
- 26.Cunha B.A. Mycoplasma pneumoniae pneumonia: diagnostic significance of highly elevated cold agglutinin titers. Eur J Clin Microbiol Infect Dis. 2008;27:1017–1019. doi: 10.1007/s10096-008-0526-2. [DOI] [PubMed] [Google Scholar]