Highlights
-
•
CDV, CPV and CCoV nucleic acids were detected in samples from Portuguese wild carnivores.
-
•
Exposure to Canine parvovirus and Canine distemper virus was confirmed by serology.
-
•
Sample conservation status was significant for antibody detection.
-
•
Co-infections by multiple pathogens (two or three viruses) were recorded.
Keywords: Canine parvovirus, Canine distemper virus, Coronavirus, Real-time PCR, Iberian wild carnivores, Serology
Abstract
Pathogen surveillance in free-ranging carnivores presents challenges due to their low densitie and secretive nature. We combined molecular and serological assays to investigate infections by viral pathogens (Canine parvovirus (CPV), Canine distemper virus (CDV) and Canine coronavirus (CCoV)) in Portuguese carnivores (Canis lupus, Vulpes vulpes, Lutra lutra, Martes foina, M. martes, Meles meles, and Genetta genetta) over a period of 16 years. Additionally we explored spatio-temporal patterns of virus occurrence in Canis lupus. Our study identified CPV DNA in all carnivore species with an overall prevalence of 91.9 %. CPV was detected in all sampled years and seasons in Canis lupus, supporting its enzootic nature. CDV RNA was mainly detected in the Canidae family, with viral nucleic acid recorded between 2005 and 2008 with a peak prevalence of 67 % among the wolf population, followed by a sharp decline, suggesting an epizootic behaviour of the virus. Antibodies show that mustelids and viverrids were often exposed to CDV. CCoV was first recorded by molecular methods in wolf samples in 2002, remaining in the wolf populations with marked fluctuations over time. The dual serological and molecular approach provided important epidemiological data on pathogens of wild carnivores in Portugal. These programmes should also include monitoring of other potential reservoir hosts such as domestic cats and dogs.
1. Introduction
There is a great diversity of known pathogens among free ranging populations of carnivores, which can be transmitted to both domestic and stray animals. Thus, epidemiological surveillance and improvement of current methodologies for pathogen detection are crucial to understand the ecology, impact and dynamics of the diseases [1]. General knowledge about disease dynamics in free-ranging carnivores is sparse, mainly due lack of epidemiological information concerning pathogen distribution among wild populations [2].
Viruses are important pathogens of wild carnivores that can affect populations through increased mortality and/or decreased general health [3]. Canine parvovirus (CPV) and canine distemper virus (CDV) are known pathogens of domestic and wild carnivores worldwide [[4], [5], [6], [7], [8]]. Although CPV infection is a relatively new disease first reported in the late 1970s in domestic dogs [5], the virus has spread rapidly [7,9]. In Europe, the presence of CPV has been documented in a variety of free-ranging carnivore populations from canids to mustelids and viverrids, through serology or molecular methods [7,[10], [11], [12]]. Additionally, Duarte et al. 2013 [10] reported high exposure to parvoviruses in mesocarnivores from Portugal, but to date, no cases of mortality among wild carnivores have been reported in Iberian Peninsula [13,14].
Canine distemper virus (CDV) is a highly contagious viral pathogen mainly transmitted by aerosols through respiratory secretions, and responsible for a lethal systemic disease in dogs and other carnivores [15]. Domestic dogs have largely been responsible for introducing CDV to previously unexposed wildlife [16,17]. In Iberian Peninsula, the virus has already affected wild carnivores, being responsible for death or disease of common genet (Genetta genetta) [18], red fox (Vulpes vulpes) [8] and Iberian lynx (Lynx pardinus) [19]. Direct mortality due to canine distemper in wolves was documented in Portugal [20], and several cases have been reported in other parts of Europe (e.g. [21].
In contrast, knowledge on coronaviruses (CCoV) in free-ranging carnivores is very limited. Serological surveys have been providing evidence that some of these species may serve as hosts for this viral pathogen [11]. Nevertheless, CCoV seems to be enzootic worldwide in dogs [22,23], which likely transmit the virus to wild carnivores [11]. In Europe, wolf population have been found positive for this virus in France and Italy, although with prevalence <9 % [11]. Despite the sampling of several Iberian populations of wild carnivores, only a single coronavirus-positive has been reported from a mongoose (Herpestes ichneumon) [[24], [25], [26]].
Several carnivore species have suffered dramatic declines in Europe during the last two centuries, with human activities leading to fragmented populations and consequently genetic isolation [27,28]. Although infectious diseases are not usually the main factor affecting the survival of these species, virulent pathogens can act as a mortality source and cause epidemics. In fact, small fragmented populations, such as those of Iberian wolves, are thought to become more vulnerable to disease outbreaks [2,29]. Multi-host pathogens, such as CPV, CDV or CCoV, can be maintained in the system through domestic dogs or other reservoir hosts, potentially impairing the viability of smaller more vulnerable wolf populations [30].
Despite all three viruses having already been reported in the Portuguese territory in wild carnivores, either by detection of antibodies (Abs) or nucleic acids (see [8,10,31], an assessment of the potential threat posed is hampered by the difficulty of overlaying the different methods for analyses. The available diagnose tools used (serology or molecular) or the target material (scats or body tissues) impair, to some extent, the interpretation and comparison between datasets. Thus, with the present study we intend to investigate occurrence of infection and spatio-temporal patterns of selected viral pathogens (CDV, CCoV and CPV) circulating in free-ranging populations of carnivores from northern Portugal. The use of combined approaches that include serological (Abs) and molecular detection as means for an enhanced perception of the results and inter study comparisons is discussed. The resulting information has critical implications for understanding of disease dynamics in these species and broader conservation policies.
2. Materials and methods
2.1. Sampling and study areas
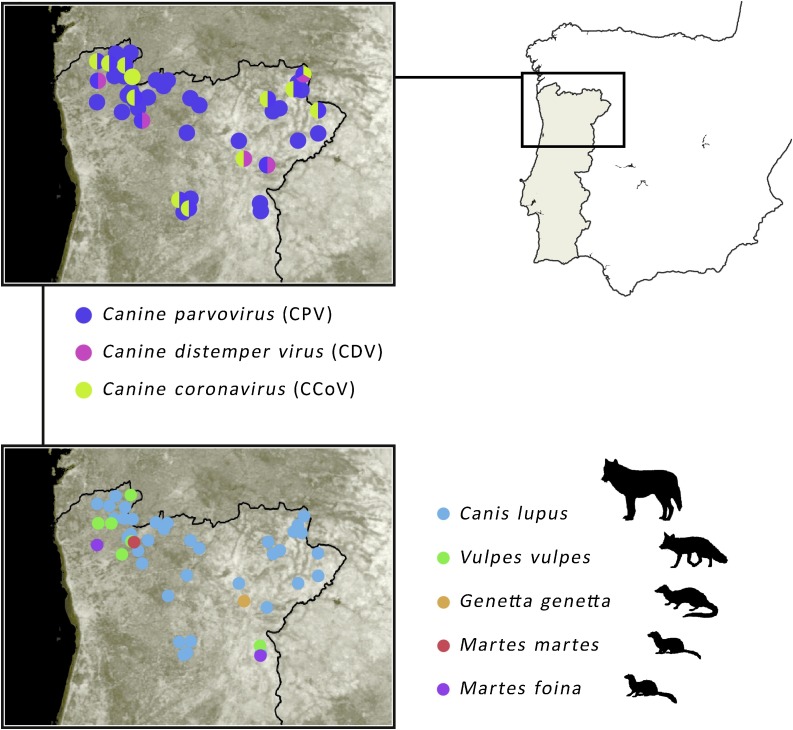
The study was conducted on 7 species of free-ranging carnivores, in a total of 62 individuals: 54 canids - Iberian wolf Canis lupus signatus (n = 42), and red fox Vulpes vulpes silacea (n = 12); 2 mustelids - Eurasian otter Lutra lutra (n = 1) and Eurasian badger Meles meles (n = 1); 3 viverrids - common genet Genetta genetta (n = 2), stone marten Martes foina (n = 3), and pine marten Martes martes (n = 1). Samples were collected from animals opportunistically found dead between 1995 and 2011 in northern Portugal, including four protected areas (Peneda-Gerês National Park, Montesinho Natural Park, Douro Internacional Natural Park and Alvão Natural Park) and in Beira Interior region comprising the Serra da Estrela Natural Park (Fig. 1 ). The main known cause of death was accidental road kills and shootings (Table 1 ).
Fig. 1.
Study sites in northern Portugal: bottom close up map shows the distribution of the samples per species; top close up map shows the overall detection of different viruses (CPV, CDV and CoV) through molecular analyses, highlighting the sites of double and triple co-infection.
Table 1.
Causes of dead of the free-ranging carnivore species included in this study. Samples were collected from individuals opportunistically found dead between 1995 and 2011 in northern Portugal. “Others” include trauma(s) and dead cause by wolves, dogs, and other animals.
| Cause of dead | n |
|---|---|
| Road killing | 13 |
| Hunting/ shooting | 5 |
| Snaring | 3 |
| Poisoning | 2 |
| Disease | 2 |
| Others | 3 |
| Unknown | 34 |
Samples were stored at -20 °C at the Institute for the Conservation of Nature and Forest (ICNF) tissue depository (BTVS/ICNB) and in the Monitoring System of Dead Wolves (SMLM/ICNF). The collection site, gender, age and the preservation status was recorded for each animal (Table S1). Sampled tissues from the Iberian wolf included liver, spleen and lymph node; liver, spleen, small intestine/rectum and lungs for the red fox and for the remaining animal species small intestine/rectum and lungs.
2.2. Molecular screening
Tissue homogenates were performed in phosphate buffered saline (PBS) solution and directly used for co-extraction of total DNA and RNA with the DNeasy tissue and blood kit (Qiagen, Germany), according to the manufactures instructions. Nucleic acids were extracted in a separate laboratory and after quantification in a Nanodrop 2000c (Thermoscientific) were kept at −80 °C until analysed. According to the organ availability, lung, liver or spleen tissue extracts were prepared as previously described [32], stored at −20 °C and later used for specific Ab detection.
We conducted Molecular screening with a TaqMan® quantitative PCR system already described [33,34]. CPV DNA was amplified by quantitative PCR (qPCR) in a 20 μl reaction with 25 ng of template, using the TaqMan® Gene Expression 2× Master Mix (Applied Biosystems), 0.3μM of primer forward, 0.3μM of primer reverse and 0.25μM of TaqMan probe. CDV and CCoV RNA were amplified by one step reverse transcription-qPCR (rt-qPCR) using the TaqMan® RNA-to-Ct (TM) 1 step kit, 0.3μM of primer forward, 0.3μM of primer reverse and 0.25μM of TaqMan probe in a 20 μl reaction with 25 ng of template.
We performed the amplification in the Applied 7300 instrument (Applied Biosystems) and the cycling conditions comprised an initial denaturation step at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 1 min at 60 °C. When the template was RNA the amplification cycle included an initial reverse transcription step at 48 °C for 15 min.
Reagents assemble and template addition was performed in separate areas to prevent sample contamination. As positive control for CPV, we used tenfold dilutions of CPV-2-780 916 Cornell strain (Tetradog®, Merial). Regarding CDV and CCoV each recombinant plasmid was used as positive control as already described [34]. Water was used as negative control representing 10 % of the total samples tested in each run.
2.3. Serological assays
We used lung, liver or spleen tissue extracts to detect CDV and CPV Abs by Indirect Enzyme-linked immunoassay commercial kits Ingezim Parvo Canino 1.5.CPV.K.1 and Ingezim Moquillo IgG 1.5.CDG.K.1 (Ingenaza, Spain), according to the manufacturer instructions. Tissue extracts were tenfold diluted for CDV and CPV testing. The anti-dog conjugate provided in the kits was used for detection of the primary Antigen (Ag)/Abs complex in the canid samples (wolves, red foxes); in the viverrid and mustelid samples it was replaced by the Protein A-Peroxidase from Staphylococcus aureus/horseradish (Sigma-Aldrich) [35].
2.4. Statistical analyses
For wolf and red fox we investigated the possible association between molecular findings and age (cub, juvenile, adult) and sex (male, female) of the animals with Fisher’s exact test. Taking advantage of the larger sample size of wolf, we used the same test to assess the effect of season (winter/ spring vs. summer/ autumn) on the infection status by all the three viruses. To do so, we only considered the interval of years in which each virus was detected. Both statistical analyses were computed using IBM SPSS Statistics 25.0 software. A 95 % confidence interval (CI) was calculated using the Wilson score interval method without correction for continuity (the Richard Lowry’s VassarStats online calculators: http://vassarstats.net/prop1.html).
Lastly we tested if the samples preservation status would have an effect on Abs detection also using Fisher’s exact test.
3. Results
3.1. CPV: serology and viral nucleic acid detection
We detected CPV DNA in all the species with an overall prevalence of 91.9 % (Table 2 ). Particularly in C. lupus, CPV DNA was detected in all sampled years (Table 3 ; Fig. 2 ) with prevalence of infection close to 100 % across the four seasons. Thus, time of the year had no significant effect on the infection status in wolves (Fisher’s Exact Test, p = 1.000). Samples showed an average Ct of 34.6, ranging from 41.9 to 12.5 Cts (standard deviation 4.4). C. lupus liver samples showed a higher frequency of viral DNA positivity, but positive results were also obtained from the spleen and lymph node. Vulpes vulpes were also highly positive (83.3 %; Table 2) with viral DNA detected in the lungs, liver, spleen and small intestine. Both mustelids tested positive for CPV with viral DNA detected in the small intestine in L. lutra and lungs in M. meles (Table 2). Among the few screened viverrids, G. genetta yielded the lowest prevalence with 1 out of 2 animals positive (Table 2) in the lung. Both marten species generated positives in lungs and small intestine.
Table 2.
Frequency of viral nucleic acid across different carnivore species in northern Portugal. 95 % CI: Confidence Interval.
| Viral nucleic acid | Canidae |
Mustelidae |
Viverridae |
Overall prevalence % (CI) |
||||
|---|---|---|---|---|---|---|---|---|
| C. lupus | V. vulpes | L. lutra | M. meles | G. genetta | M. foina | M. martes | ||
| CPV | 41/42 (97.6) |
10/12 (83.3) |
1/1 (100) |
1/1 (100) |
1/2 (50.0) |
2/3 (66.7) |
1/1 (100) |
91.9 (81.5–97) |
| CDV | 3/42 (7.1) |
1/12 (8.3) |
0/1 (0.0) |
0/1 (0.0) |
1/2 (50.0) |
0/3 (0.0) |
0/1 (0.0) |
8.1 (3–18.5) |
| CCoV | 13/42 (31.0) |
4/12 (33.3) |
1/1 (100) |
0/1 (0.0) |
2/2 (100) |
0/3 (0.0) |
0/1 (0.0) |
32.3 (21.3–45.5) |
Table 3.
Distribution of viral nucleic acid and antibody detection, through the collection years.
|
C. lupus | ||||||
|---|---|---|---|---|---|---|
| Nucleic Acid detection |
Antibody detection |
|||||
| Collection year | Sample number | CPV | CDV | CCoV | Ac α CPV | Ac α CDV |
| 1995 | 1 | 1 | 0 | 0 | 1 | 0 |
| 1996 | 2 | 2 | 0 | 0 | 0 | 0 |
| 1997 | 1 | 1 | 0 | 0 | 1 | 1 |
| 1999 | 2 | 2 | 0 | 0 | 2 | 2 |
| 2000 | 3 | 3 | 0 | 0 | 3 | 2 |
| 2001 | 1 | 1 | 0 | 0 | 1 | 1 |
| 2002 | 3 | 3 | 0 | 2 | 3 | 2 |
| 2003 | 3 | 3 | 0 | 0 | 2 | 1 |
| 2004 | 8 | 7 | 0 | 5 | 4 | 6 |
| 2005 | 3 | 3 | 2 | 1 | 2 | 2 |
| 2006 | 1 | 1 | 0 | 1 | 1 | 1 |
| 2008 | 5 | 5 | 1 | 2 | 5 | 4 |
| 2009 | 6 | 6 | 0 | 1 | 3 | 1 |
| 2010 | 2 | 2 | 0 | 1 | 2 | 2 |
| 2011 | 1 | 1 | 0 | 0 | 1 | 1 |
| Total | 42 | 41 | 3 | 13 | 31 | 26 |
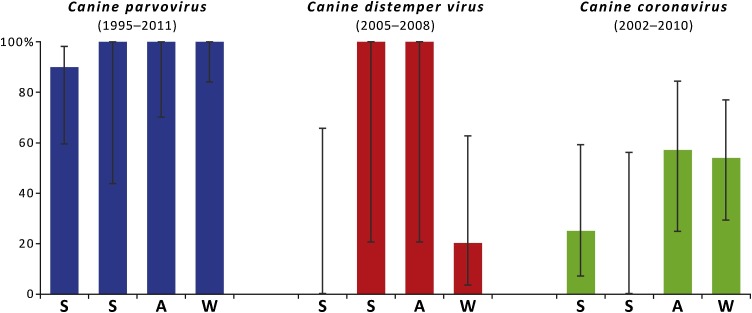
Fig. 2.
Prevalence of three viral pathogens in Canis lupus sampled from northern Portugal across the seasons of the year (respectively: S, spring; S, summer; A, autumn; W, winter) obtained through molecular detection. We considered the interval of years in which each virus was detected. Prevalence includes 95 % confidence intervals.
Antibodies against CPV were found in 60.0 % of the samples, including 33 canids where seroprevalence in wolves reached almost 74 % (Table 4 ). Nevertheless, evidence of a positive association was found between CPV seropositivity in wolves and the sample conservation status (Fisher’s Exact Test, p = 0.001), where better preserved samples had a higher likelihood of detecting Ab. Among mustelids, the two sampled individuals of M. foina had been exposed to CPV.
Table 4.
Prevalence of antibody detection across different carnivore species in northern Portugal. Confidence Interval: 95 % CI.
| Antibodies | Canidae |
Mustelidae |
Viverridae |
Overall prevalence % (CI) |
||||
|---|---|---|---|---|---|---|---|---|
| C. lupus | V. vulpes | L. lutra | M. meles | G. genetta | M. foina | M. martes | ||
| CPV | 31/42 (73.8) |
2/11 (18.2) |
1/1 (100) |
0/1 (0.0) |
0/1 (0.0) |
2/2 (100) |
0/2 (0.0) |
60.0 (46.6–72.2) |
| CDV | 26/42 (61.9) |
8/11 (72.7) |
1/1 (100) |
1/1 (100) |
0/1 (0.0) |
2/2 (100) |
1/2 (50.0) |
65.0 (51.5–76.6) |
3.2. CDV: serology and viral nucleic acid detection
CDV RNA was detected in two families (Canidae and Mustelidae) with an overall low prevalence (8.1 %) (Table 2). The organs with the higher yields were the liver and spleen in C. lupus, but the intestinal tract in V. vulpes and G. genetta. CDV RNA was only recorded in 2005 and 2008 (Table 3; Fig. 2). The wolf populations reached a frequency of 67 % (Table 3: 2/3) in 2005, followed by a decrease in 2008 (Table 3: 20 %; 1/5). We found a higher prevalence during the summer/ autumn months and progressively decreasing towards spring (Fig. 2). However, the small sample size did not allow for a statistically significant effect of seasonality (Fisher’s Exact Test, p = 0.083).
CDV Abs were detected from 1997 onwards (Table 3) in 65.0 % of the samples where both canid species presented relatively high prevalences (C. lupus 61.9 %; V. vulpes 72.7 %) (Table 4). We found seropositive samples in all species but G. genetta (Table 4).
3.3. CCoV: viral nucleic acid detection
Through molecular analyses, we obtained an overall prevalence of CCoV of 32.3 %. CCoV RNA was detected in all three families. Thirteen C. lupus (31.0 %; Table 2) tested positive in the spleen and four V. vulpes (33.3 %; Table 2) in the spleen and small intestine. Positive results were also found in the two G. genetta and the single L. lutra, both in the rectum (Table 2).
The virus was first detected in a single fox in 1999. However, CCoV RNA was not recorded in our wolf samples until 2002. We did not detect the virus also in 2005 and after 2010(Table 3). Although the data shows higher prevalence in the cooler months with a trending decrease in detection from autumn to summer (with no positive animals found in the warmer months; Fig. 2), the differences are not statistically significant (spring/ summer vs. autumn/ winter: Fisher’s Exact Test, p=1.000).
3.4. Co-infection by multiple viruses
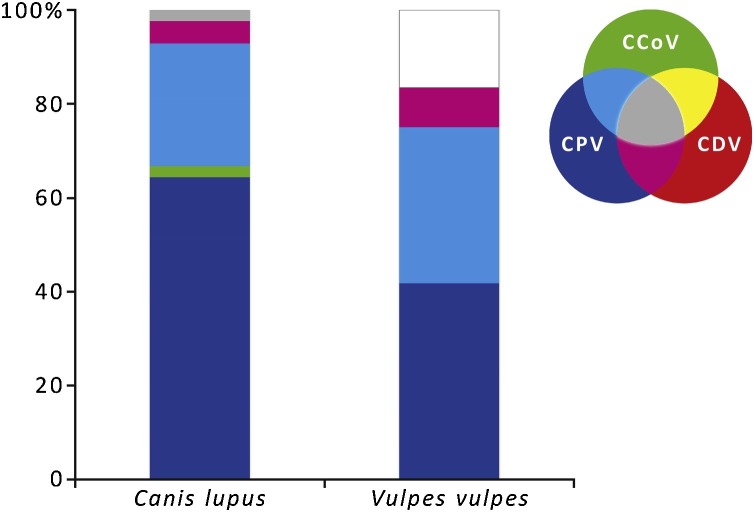
We recorded co-infections by multiple pathogens (two or three viruses) across the three families, representing 37.9 % (22/58) of the sampled species. CPV was the most frequent virus involved in mixed infections with nucleic acid from additional viruses detected in 95.5 % (21/22) of the co-infected individuals. Among these, CCoV mixed infections with CPV were most common in both wolves and red foxes (Fig. 3 ; Table 5 ). Unfortunately for most mixed infections, there was no information on the cause of death or any additional data that could help with the diagnosis.
Fig. 3.
Proportion of viral infections and co-infections in the two species of canids sampled from northern Portugal. Pathogen detection was assessed through nucleic acid detection. Non pathogen detection represented by white proportion.
Table 5.
Viral infections and co-infections in carnivores of northern Portugal evaluated through nucleic acid detection.
| Species | CPV | CCoV | CPV/CCoV | CPV/CDV | CDV/CCoV | CPV/CDV/CCoV | No pathogen | Total |
|---|---|---|---|---|---|---|---|---|
| C. lupus | 27 | 1 | 11 | 2 | 0 | 1 | 0 | 42 |
| V. vulpes | 5 | 0 | 4 | 1 | 0 | 0 | 2 | 12 |
| G. genetta | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| L. lutra | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| M. foina | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| M. martes | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| M. meles | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 37 | 0 | 17 | 4 | 1 | 1 | 2 | 62 |
Co-infection by the three viruses was detected in a single individual of C. lupus (Fig. 3; Table 5). Co-infection by CDV/CCoV was also infrequent and detected in one juvenile of G. genetta (Table 5).
No significant associations were detected between age (Fisher’s Exact Test, p 0.339), or sex (Fisher’s Exact Test, p 0.505), and viral nucleic acid positive samples in any of the two canid species.
4. Discussion
Diagnosis and detection of viruses and other pathogens in wildlife populations can be extremely difficult. Long-term collection of samples allowed us to show that all three taxonomic families (Canidae, Viverridae and Mustelidae) have been exposed to CPV, CDV and CCoV in northern Portugal. Viral detection in free-ranging animals was confirmed by molecular and serological data.
4.1. Canine parvovirus
Our study identified a high prevalence of CPV among all surveyed species. This seems to be particularly significant in the two species of canids, where wolves presented an infection rate close to 100 %, although with a high variability of viral loads (ranging from 41.9–12.5 Cts). CPV was detected in all sampled years and across all seasons in C. lupus, suggesting its enzootic nature in this study area. We believe that the social habits of Iberian wolves [36] may increase the frequency of intra-specific contacts and thus promoting the pathogen transmission. The findings mimic the results obtained by Duarte et al. [10], where some southern populations of mesocarnivores in Portugal also reached 100 % prevalence, using the same methodological approach. In foxes, for example, CPV DNA was detected in almost 79 % of the tissue samples, not far from 83.3 % obtained in our study and within the confidence interval. Contrastingly, other European studies described a much lower prevalence, ranging from 3.5–15.2% in wolf scats in Italy [11,12], or 12.1 % obtained in France [11,20], and even a total lack of viral DNA detection in tissue samples from several species (Germany [6].
CPV infection is typically established by oronasal route by contact with contaminated faeces and after initial replication in lymphoid organs CPV localizes predominantly in the intestinal tract [37]. Whenever matched tissue samples were available, mustelids and viverrids tested positive in the lung but not in the small intestine, contrasting with the canid samples, particularly from wolves, in accordance with this pattern of virus dissemination.
The pathogen’s high stability in the environment and the ability to induce carrier states in susceptible hosts [38], support our findings. The high resistance of CPV to adverse environments [39], the higher sensitivity and specificity of qPCR, and the availability of target tissues for viral replication in vivo [10], may explain the higher detection of CPV DNA, with variability of the viral load. Nevertheless, there was a lower seroprevalence of CPV, as positive samples were approximately 30 % lower compared to results obtained through molecular assays. This discrepancy may be explained by several factors including: the use of lung, liver or spleen tissue extracts as the biological matrix for Ab detection; the use of the anti-dog conjugate in the ELISA assay for canids samples, and the lower sensitivity of ELISA assays for Abs detection versus qPCR for nucleic acid detection [37,40]. Additionally, we found a link between sample preservation status and seropositivity. Yet, the seropositivity rate obtained suggests a high rate of CPV exposure, but a low rate of CPV induced mortality [4], suggested by the perdominant low viral load detected in the samples.
4.2. Canine distemper virus
CDV was mainly detected in the Canidae family with an overall prevalence below 10 %. Viral RNA was only recorded between 2005 and 2008 with a prevalence peak in the first year among the wolf population (67 %) followed by a drop down, suggesting an epizootic behaviour of the virus. Similar infection pattern has been documented in several animal species worldwide [41], including in Europe [21,42,43], with CDV often implicated in disease outbreaks and mortality events. The highly infectious nature of CDV [44] may explain the sudden increase in prevalence.
CDV serological data shows a much higher proportion of positive samples (65 %) compared to nucleic acid detection. In addition to canids, we confirmed that mustelids and viverrids have also been exposed to CDV. The observed high seroprevalence suggests a high rate of circulation among the carnivore in northern Portugal and confirms these species as being highly susceptible to CDV infections. Considering CDV epidemiology, our finding may indicate CDV spill-over into wild populations, quite possibly from domestic and stray dogs acting as disease reservoirs [16,20,30,42].
4.3. Canine coronavirus
CCoV RNA was first recorded in our wolf samples in 2002, remaining with marked fluctuations over time. Both canid species present similar overall prevalence values. In comparison, the incidence was much lower in Italian and French wolf populations using faecal samples [11], which is not surprising given the low stability of viral particles in scats [45]. CCoV RNA has been detected in dog tissues across Europe [46], but despite previous efforts, only a single positive mongoose has been recorded in Portugal [10]. Thus, our results represent the first record of viral infections in wild populations of C. lupus and V. vulpes in Iberia, as well as for L. lutra and G. genetta.
Although our prevalence data are poorly informative about the impact of infection and disease in the populations, previous studies have shown that CCoV infection seems not to be frequently associated with fatal disease; instead, it often causes mild, self-limiting enteritis followed by rapid recovery [47]. Nevertheless, mortality may occur as a consequence of mixed infections with any of the two studied virus, CPV and/ or CDV [47,48].
4.4. Viral co-infection
A proportion close to 40 % of the carnivores was involved in mix infections where CPV dominated most of the combinations in all affected species. The latter is not surprising giving the high prevalence of CPV among the carnivore populations, as previously discussed. Conversely, when compared to previous studies [10,11], we found the overall proportion of co-infections we obtained to be relatively high.
Infections by CCoV seem to be aggravated when occur simultaneously with CPV [49,50] and/or CDV [51], which could explain seven of the unknown deaths in wolves where we detected any of the two combinations and even one individual infected by the three viruses. Yet, Mech & Goyal [52] showed that the negative impact of CPV seems to be attenuated once the virus becomes enzootic in a population, which could explain the apparent lack of outbreaks in our system, even with such a high prevalence of co-infections. However, this suggestion should be interpreted with caution due to the absence of demographic data and survival of pups for the populations here considered.
As our data does suggest that CDV is not enzootic in the northern Portugal populations of wolf, individuals may be more vulnerable to CDV infection but as well as to co-infection with CDV and CCoV. Nevertheless, only a single individual was detected with this mixed infection but cause of death was unknown.
Lastly, dual infection by CDV/CPV seems to be overall less frequent, at least in wild carnivores in European as we only detected in three canids in our study. The lack of case reports makes it hard to predict impacts and consequences of infections. However, research on other species highlighted a case of fatal canine distemper infection in African wild dogs with concurrent infection by CPV in 2/6 individuals, leading the authors to believe that co-infections may have contributed to the fatal outcome in some of the dogs [53].
4.5. Broad implications and final considerations
Free-ranging carnivores are of vital importance to the stability and integrity of most ecosystems, but some viral infectious diseases have shown the potential to negatively impact wild populations and cause declines [54,55]. Almost half of the known infectious diseases of free-ranging carnivores are of viral origin [3]. Although the three viruses here studied have been associated with mortality in wild and domestic canids worldwide, and some viruses could, theoretically, threaten the viability of small isolated populations (e.g. CPV [29]), their impact in wild carnivore populations is still largely unknown, particularly among the northern Portuguese populations. Despite high prevalence levels of CPV and CDV our populations, none of these pathogens seem to have been associated with declines in Iberia, or listed among the major threats to the wild carnivores [56]. This can be explained by a greater impact of fragmentation (road-kills) and illegal hunting, and/ or simple lack of robust data and regular disease surveillance. Nevertheless, our results suggest a high and common exposure to CPV and CDV, with a higher seroprevalence of Abs when compared to similar studies conducted in the Iberian Peninsula [8,31]. Furthermore, we detected different antibody titers towards CPV and CDV, suggesting different contact timings between the animals and the viral pathogens. Immune response towards CPV and CDV infection is considered lifelong, thus detection of seropositive animals implies exposure but also protection against the virus [57].
Considering the availability of recent data regarding CDV and CPV in Portugal [10, 31; this study], it is suggested an active viral circulation among the animal population studied. Unvaccinated dogs, as well as wolf prey species, or scattered infectious faeces/urine, make the transmission of viral pathogens between populations a likely scenario [58]. The control and widespread vaccination of domestic dogs and cats could reduce the potential spill-over of pathogens. Additionally, constant evolution of virus through mutations in the genome [59,60] present a challenge for pathogen detection and consequently for adequate wild carnivore populations’ management. This means that the results obtained in this and other studies may underestimate the infectious pressure, particularly when a single diagnostic test is used. Thus, continuous surveillance of viral pathogens and longitudinal studies in stray and wild animal populations are crucial to detect new viral variants potentially escaping both the host immune system and detection methods.
CRediT authorship contribution statement
Gonçalo M. Rosa: Conceptualization, Methodology, Writing - original draft. Nuno Santos: Resources, Writing - review & editing. Ricardo Grøndahl-Rosado: Methodology, Investigation. Francisco Petrucci Fonseca: Resources, Writing - review & editing. Luis Tavares: Project administration, Funding acquisition, Writing - review & editing. Isabel Neto: Resources, Data curation. Clara Cartaxeiro: Methodology. Ana Duarte: Methodology, Writing - original draft, Writing - review & editing, Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
We acknowledge the financial support by Portuguese National Funds, through FCT – Fundação para a Ciência e Tecnologia, within the project UID/BIA/00329/2013, UID/CVT/00276/2013CIISA – CIISA, Centre for Interdisciplinary Research in Animal Health, Faculty of Veterinary Medicine, University of Lisbon and PEst-OE/AGR/UI0276/2014, which enabled this work. N. Santos was supported by the post-doctoral fellowship (SFRH/BPD/
116596/2016) from Fundação para a Ciência e Tecnologia. For the biological samples we acknowledge BTVS (Banco de Tecidos de Vida Selvagem) and SMLM (Sistema de Monitorização de Lobos Mortos) of ICNF (Instituto de Conservação da Natureza e das Florestas, Portugal). We would also like to acknowledge all the colleagues and collaborators who collected the samples in the field.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cimid.2020.101432.
Contributor Information
Gonçalo M. Rosa, Email: goncalo.m.rosa@gmail.com.
Nuno Santos, Email: nuno.santos@cibio.up.pt.
Ricardo Grøndahl-Rosado, Email: rcrosado@gmail.com.
Francisco Petrucci Fonseca, Email: fpfonseca@fc.ul.pt.
Luis Tavares, Email: ltavares@fmv.ulisboa.pt.
Isabel Neto, Email: isaneto@fmv.ulisboa.pt.
Clara Cartaxeiro, Email: cartaxeiro@fmv.ulisboa.pt.
Ana Duarte, Email: anaduarte@fmv.ulisboa.pt.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Aguirre A. Wild canids as sentinels of ecological health: a conservation medicine perspective. Parasit. Vectors. 2009;2(Suppl. 1) doi: 10.1186/1756-3305-2-S1-S7. S7–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith K.F., Acevedo-Whitehouse K., Pedersen A.B. The role of infectious diseases in biological conservation. Anim. Conserv. 2009;12(1):1–12. [Google Scholar]
- 3.Murray D.L., Kapke C.A., Evermann J.F., Fuller T.K. Infectious disease and the conservation of free-ranging large carnivores. Anim. Conserv. 1999;2(4):241–254. doi: 10.1111/j.1469-1795.1999.tb00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almberg E.S., Mech L.D., Smith D.W., Sheldon J.W., Crabtree R.L. A serological survey of infectious disease in Yellowstone National Park’s canid community. PLoS One. 2009;4(9) doi: 10.1371/journal.pone.0007042. e7042–e7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eugster A.K., Nairn C. Diarrhea in puppies: parvovirus-like particles demonstrated in their feces. Southwest Vet. 1977;30 59–59. [Google Scholar]
- 6.Frölich K., Streich W.J., Fickel J., Jung S., Truyen U., Hentschke J., Dedek J., Prager D., Latz N. Epizootiologic investigations of parvovirus infections in free-ranging carnivores from Germany. J. Wildl. Dis. 2005;41(1):231–235. doi: 10.7589/0090-3558-41.1.231. [DOI] [PubMed] [Google Scholar]
- 7.Miranda C., Thompson G. Canine parvovirus: the worldwide occurrence of antigenic variants. J. Gen. Virol. 2016;97(9):2043–2057. doi: 10.1099/jgv.0.000540. [DOI] [PubMed] [Google Scholar]
- 8.Sobrino R., Arnal M.C., Luco D.F., Gortazar C. Prevalence of antibodies against canine distemper virus and canine parvovirus among foxes and wolves from Spain. Vet. Microbiol. 2008;126(1–3):251–256. doi: 10.1016/j.vetmic.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Steinel A., Parrish C.R., Bloom M.E., Truyen U. Parvovirus infections in wild carnivores. J. Wildl. Dis. 2001;37(3):594–607. doi: 10.7589/0090-3558-37.3.594. [DOI] [PubMed] [Google Scholar]
- 10.Duarte M.D., Henriques A.M., Barros S.C., Fagulha T., Mendonça P., Carvalho P., Monteiro M., Fevereiro M., Basto M.P., Rosalino L.M., Barros T., Bandeira V., Fonseca C., Cunha M.V. Snapshot of viral infections in wild carnivores reveals ubiquity of parvovirus and susceptibility of egyptian mongoose to feline Panleukopenia virus. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059399. e59399–e59399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar B., Duchamp C., Möstl K., Diehl P.-A., Betschart B. Comparative survey of canine parvovirus, canine distemper virus and canine enteric coronavirus infection in free-ranging wolves of central Italy and south-eastern France. Eur. J. Wildl. Res. 2014;60(4):613–624. doi: 10.1007/s10344-014-0825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinello F., Galuppo F., Ostanello F., Guberti V., Prosperi S. Detection of canine parvovirus in wolves from Italy. J. Wildl. Dis. 1997;33(3):628–631. doi: 10.7589/0090-3558-33.3.628. [DOI] [PubMed] [Google Scholar]
- 13.Decaro N., Desario C., Addie D.D., Martella V., Vieira M.J., Elia G., Zicola A., Davis C., Thompson G., Thiry E., Truyen U., Buonavoglia C. The study molecular epidemiology of canine parvovirus, Europe. Emerging Infect. Dis. 2007;13(8):1222–1224. doi: 10.3201/eid1308.070505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filipov C., Desario C., Patouchas O., Eftimov P., Gruichev G., Manov V., Filipov G., Buonavoglia C., Decaro N. A ten-year molecular survey on parvoviruses infecting carnivores in Bulgaria. Transbound. Emerg. Dis. 2014:1–5. doi: 10.1111/tbed.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deem S.L., Spelman L.H., Yates R.A., Montali R.J. Canine distemper in terrestrial carnivores: a review. J. Zoo Wildl. Med. 2000;31(4):441–451. doi: 10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Frolich K., Czupalla O., Haas L., Hentschke J., Dedek J., Fickel J. Epizootiological investigations of canine distemper virus in free-ranging carnivores from Germany. Vet. Microbiol. 2000;74(4):283–292. doi: 10.1016/s0378-1135(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy A.J., Shaw M.-A., Goodman S.J. Pathogen evolution and disease emergence in carnivores. Proc. R. Soc. B: Biol. Sci. 2007;274(1629):3165–3174. doi: 10.1098/rspb.2007.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Peña M., Vázquez S., Alemañ N., López-Beceiro A., Muñoz F., Pereira J.L., Nieto J.M. Canine distemper in a genet (Gennetta gennetta), associated with endogenous lipid pneumonia. J. Comp. Pathol. 2001;124(2):207–211. doi: 10.1053/jcpa.2000.0432. [DOI] [PubMed] [Google Scholar]
- 19.Meli M.L., Simmler P., Cattori V., Martínez F., Vargas A., Palomares F., López-Bao J.V., Simón M.A., López G., León-Vizcaino L., Hofmann-Lehmann R., Lutz H. Importance of canine distemper virus (CDV) infection in free-ranging Iberian lynxes (Lynx pardinus) Vet. Microbiol. 2010;146(1–2):132–137. doi: 10.1016/j.vetmic.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Müller A., Silva E., Santos N., Thompson G. Domestic dog origin of canine distemper virus in free-ranging wolves in Portugal as revealed by hemagglutinin gene characterization. J. Wildl. Dis. 2011;47(3):725–729. doi: 10.7589/0090-3558-47.3.725. [DOI] [PubMed] [Google Scholar]
- 21.Di Sabatino D., Lorusso A., Di Francesco C.E., Gentile L., Di Pirro V., Bellacicco A.L., Giovannini A., Di Francesco G., Marruchella G., Marsilio F., Savini G. Arctic lineage-canine distemper virus as a cause of death in apennine wolves (Canis lupus) in Italy. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0082356. e82356–e82356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., Castagnaro M., Tempesta M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006;12(3):492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratelli A. Genetic evolution of canine coronavirus and recent advances in prophylaxis. Vet. Res. 2006;37(2):191–200. doi: 10.1051/vetres:2005053. [DOI] [PubMed] [Google Scholar]
- 24.Millán J., Rodríguez A. A serological survey of common feline pathogens in free-living European wildcats (Felis silvestris) in central Spain. Eur. J. Wildl. Res. 2009;55(3):285–291. doi: 10.1007/s10344-008-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pena L., Garcia P., Jimenez M.A., Benito A., Alenza M.D.P., Sanchez B. Histopathological and immunohistochemical findings in lymphoid tissues of the endangered Iberian lynx (Lynx pardinus) Comp. Immunol. Microbiol. Infect. Dis. 2006;29(2–3):114–126. doi: 10.1016/j.cimid.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roelke M.E., Johnson W.E., Millán J., Palomares F., Revilla E., Rodríguez A., Calzada J., Ferreras P., León-Vizcaíno L., Delibes M., O’Brien S.J. Exposure to disease agents in the endangered Iberian lynx (Lynx pardinus) Eur. J. Wildl. Res. 2008;54(2):171–178. doi: 10.1007/s10344-007-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temple H.J., Terry A. Office for Official Publications of the European Communities; Luxembourg: 2007. The Status and Distribution of European Mammals. Viii + 48pp-Viii + 48pp. [Google Scholar]
- 28.Lucchini V., Galov A., Randi E. Evidence of genetic distinction and long-term population decline in wolves (Canis lupus) in the Italian Apennines. Mol. Ecol. 2004;13(3):523–536. doi: 10.1046/j.1365-294x.2004.02077.x. [DOI] [PubMed] [Google Scholar]
- 29.Mech L.D., Goyal S.M. Canine parvovirus effect on wolf population change and pup survival. J. Wildl. Dis. 1993;29(2):330–333. doi: 10.7589/0090-3558-29.2.330. [DOI] [PubMed] [Google Scholar]
- 30.Almberg E.S., Cross P.C., Smith D.W. Persistence of canine distemper virus in the Greater Yellowstone Ecosystem’s carnivore community. Ecol. Appl. 2010;20(7):2058–2074. doi: 10.1890/09-1225.1. [DOI] [PubMed] [Google Scholar]
- 31.Santos N., Almendra C., Tavares L. Serologic survey for canine distemper virus and canine parvovirus in free-ranging wild carnivores from Portugal. J. Wildl. Dis. 2009;45(1):221–226. doi: 10.7589/0090-3558-45.1.221. [DOI] [PubMed] [Google Scholar]
- 32.Ferroglio E., Rossi L., Gennero S. Lung-tissue extract as an alternative to serum for surveillance for brucellosis in chamois. Prev. Vet. Med. 2000;43(2):117–122. doi: 10.1016/s0167-5877(99)00090-2. [DOI] [PubMed] [Google Scholar]
- 33.Castanheira P., Duarte A., Gil S., Cartaxeiro C., Malta M., Vieira S., Tavares L. Molecular and serological surveillance of canine enteric viruses in stray dogs from Vila do Maio, Cape Verde. BMC Vet. Res. 2014;10(1) doi: 10.1186/1746-6148-10-91. 91–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gil S., Leal R.O., Duarte A., McGahie D., Sepulveda N., Siborro I., Cravo J., Cartaxeiro C., Tavares L.M. Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Res. Vet. Sci. 2013;94(3):753–763. doi: 10.1016/j.rvsc.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindmark R., Thorén-Tolling K., Sjöquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J. Immunol. Methods. 1983;62(1):1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- 36.Packard J.M. Wolves: Behavior, Ecology, and Conservation. 2003. Wolf behaviour: reproductive, social and intelligent; pp. 35–65. [Google Scholar]
- 37.Greene Decaro N.C.E. In: Canine Viral Enteritis. Greene C.E., editor. Saunders Elsevier; St Louis, Missouri: 2012. pp. 67–80. [Google Scholar]
- 38.Almberg E.S., Cross P.C., Dobson A.P., Smith D.W., Hudson P.J. Parasite invasion following host reintroduction: a case study of Yellowstone’s wolves. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012;367(1604):2840–2851. doi: 10.1098/rstb.2011.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon J.C., Angrick E.J. Canine parvovirus: environmental effects on infectivity. Am. J. Vet. Res. 1986;47(7):1464–1467. [PubMed] [Google Scholar]
- 40.Decaro N., Buonavoglia C. Canine parvovirus—A review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012;155(1):1–12. doi: 10.1016/j.vetmic.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapil S., Yeary T.J. Canine distemper spillover in domestic dogs from urban wildlife, the veterinary clinics of North America. Small Anim. Pract. 2011;41(6):1069–1086. doi: 10.1016/j.cvsm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martella V., Bianchi A., Bertoletti I., Pedrotti L., Gugiatti A., Catella A., Cordioli P., Lucente M.S., Elia G., Buonavoglia C. Canine distemper epizootic among red foxes, Italy, 2009. Emerging Infect. Dis. 2010;16(12):2007–2009. doi: 10.3201/eid1612.100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekulin K., Hafner-Marx A., Kolodziejek J., Janik D., Schmidt P., Nowotny N. Emergence of canine distemper in Bavarian wildlife associated with a specific amino acid exchange in the haemagglutinin protein. Vet. J. 2011;187(3):399–401. doi: 10.1016/j.tvjl.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 44.Greene C.E., Vandevelde M. Canine distemper. In: S.L. Elsevier/Saunders, Mo, editor. Infectious Diseases of the Dog and Cat. 4th ed. 2020. pp. 25–42. [Google Scholar]
- 45.Pratelli A., Buonavoglia D., Martella V., Tempesta M., Lavazza A., Buonavoglia C. Diagnosis of canine coronavirus infection using nested-PCR. J. Virol. Methods. 2000;84(1):91–94. doi: 10.1016/S0166-0934(99)00134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Decaro N., Cordonnier N., Demeter Z., Egberink H., Elia G., Grellet A., Le Poder S., Mari V., Martella V., Ntafis V., von Reitzenstein M., Rottier P.J., Rusvai M., Shields S., Xylouri E., Xu Z., Buonavoglia C. European surveillance for pantropic canine coronavirus. J. Clin. Microbiol. 2013;51(1):83–88. doi: 10.1128/JCM.02466-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Decaro N., Buonavoglia C. Canine coronavirus: not only an enteric pathogen, the veterinary clinics of North America. Small Anim. Pract. 2011;41(6):1121–1132. doi: 10.1016/j.cvsm.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Decaro N., Buonavoglia C. An update on canine coronaviruses: viral evolution and pathobiology. Vet. Microbiol. 2008;132(3–4):221–234. doi: 10.1016/j.vetmic.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pratelli A., Tempesta M., Greco G., Martella V., Buonavoglia C. Development of a nested PCR assay for the detection of canine coronavirus. J. Virol. Methods. 1999;80(1):11–15. doi: 10.1016/S0166-0934(99)00017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decaro N., Martella V., Desario C., Bellacicco A.L., Camero M., Manna L., d’Aloja D., Buonavoglia C. First detection of canine parvovirus type 2c in pups with haemorrhagic enteritis in Spain. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2006;53(10):468–472. doi: 10.1111/j.1439-0450.2006.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decaro N., Camero M., Greco G., Zizzo N., Tinelli A., Campolo M., Pratelli A., Buonavoglia C. Canine distemper and related diseases: report of a severe outbreak in a kennel. New Microbiol. 2004;27:177–182. [PubMed] [Google Scholar]
- 52.Mech L.D., Goyal S.M. Parsing demographic effects of canine parvovirus on a Minnesota wolf population. J. Vet. Med. Anim. Health. 2011;3(2):27–30. [Google Scholar]
- 53.Goller K.V., Fyumagwa R.D., Nikolin V., East M.L., Kilewo M., Speck S., M??ller T., Matzke M., Wibbelt G. Fatal canine distemper infection in a pack of African wild dogs in the Serengeti ecosystem, Tanzania. Vet. Microbiol. 2010;146(3–4):245–252. doi: 10.1016/j.vetmic.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Randall D.A., Williams S.D., Kuzmin I.V., Rupprecht C.E., Tallents L.A., Tefera Z., Argaw K., Shiferaw F., Knobel D.L., Sillero-Zubiri C., Laurenson M.K. Rabies in endangered Ethiopian wolves. Emerging Infect. Dis. 2004;10(12):2214–2217. doi: 10.3201/eid1012.040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roelke-Parker M.E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., O’Brien S.J., Pospischil A., Hofmann-Lehmann R., Lutz H., Mwamengele G.L.M., Mgasa M.N., Machange G.A., Summers B.A., Appel M.J.G. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379(6564):441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Queiroz A.I., Pc A., Barroso I., Beja P., Fernandes M., Freitas L., Mathias M.L., Mira A., Palmeirim J.M., Preito R., Rainho A., Rodrigues L., Santos-Reis M., Sequeira M. Felis silvestris. In: Cabral M.J., Almeida J., Almeida P.R., Dellinger T., Ferrand de Almeida N., Oliveira M.E., Palmeirim J.M., Queiroz A.I., L& R., Santos-Reis M., editors. Livro Vermelho Dos Vertebrados de Portugal. Instituto da Conservação da Natureza; Lisboa Portugal: 2005. [Google Scholar]
- 57.Schultz R.D., Thiel B., Mukhtar E., Sharp P., Larson L.J. Age and long-term protective immunity in dogs and cats. J. Comp. Pathol. 2010;142(Suppl (0)):S102–S108. doi: 10.1016/j.jcpa.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Bronson E., Emmons L.H., Murray S., Dubovi E.J., Deem S.L. Serosurvey of pathogens in domestic dogs on the border of Noel Kempff Mercado National Park, Bolivia. J. Zoo Wildl. Med. 2008;39(1):28–36. doi: 10.1638/2006-0046.1. [DOI] [PubMed] [Google Scholar]
- 59.Battilani M., Bassani M., Forti D., Morganti L. Analysis of the evolution of feline parvovirus (FPV) Vet. Res. Commun. 2006;30(1):223–226. [Google Scholar]
- 60.Shackelton L.A., Parrish C.R., Truyen U., Holmes E.C. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc. Nat. Acad. Sci. U. S. A. 2005;102(2):379–384. doi: 10.1073/pnas.0406765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.