Abstract
Background
In adults hospitalized with atypical community-acquired pneumonia (CAP), Legionnaires' disease is not uncommon. Legionnaire's disease can be differentiated from typical CAPs and from other atypical CAPs based on its characteristic pattern of extrapulmonary organ involvement. The first clinically useful diagnostic weighted point score system for the clinical diagnosis of Legionnaires' disease was developed by the Infectious Disease Division at Winthrop-University Hospital in the 1980s. It has proven to be diagnostically accurate and useful for more than two decades, but was time-consuming. Because Legionella spp. diagnostic tests are time-dependent and problematic, a need was perceived for a rapid, simple way to render a clinical, syndromic diagnosis of Legionnaires' disease pending Legionella test results. During the “herald wave” of the swine influenza (H1N1) pandemic in the New York area, our hospital, like others, was inundated with patients who presented to the Emergency Department with influenza-like illnesses (ILIs) for H1N1 testing/evaluation. Most patients with ILIs did not have swine influenza. Hospitalized patients with ILIs who tested positive with rapid influenza diagnostic tests (RIDTs) were placed on influenza precautions and treated with oseltamivir. Unfortunately, approximately 30% of adult patients admitted with an ILI had negative RIDTs. Because the definitive laboratory diagnosis of H1N1 pneumonia by reverse transcription-polymerase chain reaction(RT-PCR), testing was restricted by health departments, resulted in clinical and infection control dilemmas in determining which RIDT-negative patients did, in fact, have H1N1 pneumonia.
Objective
Accordingly, a diagnostic weighted point score system was developed for H1N1 pneumonia patients, based on RT-PCR positivity by the Infectious Disease Division at Winthrop-University Hospital. This diagnostic point score system for hospitalized adults with negative RIDTs was time-consuming. As the pandemic progressed, a simplified diagnostic swine influenza (H1N1) triad was developed for the rapid clinical diagnosis of probable H1N1 pneumonia, which also differentiated it from its mimics as well as from bacterial pneumonia, eg, Legionnaires' disease. During the “herald wave” of the H1N1 pandemic, we noticed an unexplained increase in Legionnaires' disease CAPs. Because clinical resources were stressed to the maximum during the pandemic, it was critically important to rapidly identify patients rapidly with Legionnaire's disease who did not require influenza precautions or oseltamivir, but who did require anti-Legionella antimicrobial therapy.
Methods
Based on the Winthrop-University Hospital Infectious Disease Division's diagnostic weighted point score system for Legionnaires' disease (modified), key indicators were identified and became the basis for the diagnostic Legionnaires' disease triad. The diagnostic Legionnaires' disease triad was used to make a clinical diagnosis of Legionnaires' disease until the results of Legionella diagnostic tests were reported. The diagnostic Legionnaires' disease triad diagnosed Legionnaires' disease in hospitalized adults with CAPs with extrapulmonary findings (atypical CAP) and relative bradycardia, accompanied by any three (ie, a triad) of the following: otherwise unexplained relative lymphopenia, early/mildly elevated serum transaminases (SGOT/SGPT), highly increased ferritin levels (≥2 × n), or hypophosphatemia. The diagnostic Legionnaires' disease triad provides clinicians with a rapid way to clinically diagnose Legionnaires' disease, pending Legionella test results.
Results
The accuracy of the diagnostic Legionnaires' disease triad was confirmed in our 9 cases of Legionnaires' disease by subsequent Legionella diagnostic testing.
Conclusions
The diagnostic Legionnaires' disease triad is particularly useful in situations where a rapid clinical syndromic diagnosis is needed, ie, during an H1N1 pandemic.
Legionnaire's disease is the term given to pneumonias caused by any Legionella spp. Legionella pneumophila is the most common Legionella sp. causing pneumonia in humans. Six Legionella species account for the majority of pneumonias in humans. Legionnaires' disease is a systemic infection involving the lungs, and is accompanied by a variety of extrapulmonary manifestations.1, 2
Legionnaires' disease is the cause of community-acquired pneumonia (CAP) as well as nursing home-acquired pneumonia or hospital-acquired pneumonia, ie, nosocomial pneumonias.3, 4, 5 The Legionella sp. CAP occurs sporadically in communities where Legionella is in the aqueous environment.3 Legionnaire's disease as a cause of nursing home-acquired or nosocomial pneumonias occur in outbreaks.4 Outbreaks in nursing homes or hospitals are invariably related to the aerosolization of Legionella-contaminated water, eg, cooling towers, air-conditioning units, ice cubes, showers, or water in general.6, 7, 8, 9, 10
Legionella are small, aerobic, Gram-negative bacilli that do not stain well using a Gram stain. In tissue, they appear as coccobacillary forms, and in a solid-culture medium are filamentous. Legionella spp. require L-cysteine for growth, and iron salts enhance growth. Legionella cannot be cultured on standard solid media, and are best cultured on buffered yeast charcoal extract agar.3
A diagnosis of Legionnaires' disease may be rendered through culturing respiratory secretions or lung specimens. Legionella may also be demonstrated in respiratory secretions by direct fluorescent antibody (DFA) techniques, using polyclonal or monoclonal antibodies. The positivity of DFAs decreases rapidly after treatment, but the DFAs of respiratory secretions, when positive, allow for the most rapid diagnostic test available early in Legionnaires' disease. More commonly, Legionnaires' disease is diagnosed serologically by demonstrating a single high titer or rising titers between acute and convalescent specimens. Legionella seroconversion commonly takes 4 to 6 weeks. For this reason, many patients with Legionnaires' disease often have negative initial titers, making early diagnosis difficult. Very few patients have repeat titers at 4 to 6 weeks after discharge. Legionnaires' disease attributable to Legionella pneumophila (serotypes 01-06) may also be diagnosed using the Legionella antigen test. Legionella antigenuria takes 2 to 3 weeks to become detectable in urine, but persists for months after an infection. The main value of the Legionella urinary antigen test involves its persistent positivity for weeks or months, permitting a retrospective diagnosis, particularly in cases where Legionella titers were initially negative. However, the Legionella antigen test is positive only when Legionnaires' disease is caused by Legionella pneumophila (serotypes 01-06).3, 5
Since the first description of Legionnaires' disease after the Philadelphia outbreak of 1976, physicians have sought to determine whether any clinical or nonclinical laboratory features of Legionnaires' disease would permit differentiation of Legionnaires' disease from typical bacterial CAPs.1 Typical bacterial CAPs are most commonly attributable to S. pneumoniae, H. influenzae, or M. catarrhalis, and are infections confined to the lungs, without extrapulmonary findings. Legionnaire's disease, like other atypical CAP pathogens, is a systemic infection with pneumonia.11, 12, 13, 14, 15
The most common atypical CAPs are associated with extrapulmonary findings, ie, zoonotic and nonzoonotic atypical CAPs. The most common atypical zoonotic CAPs are attributable to Q fever, psittacosis, or tularemia. The most common nonzoonotic atypical pneumonias are M. pneumoniae, C. pneumoniae, and Legionnaires' disease (Table I, Table II ).
Table I.
Diagnostic features of nonzoonotic atypical pneumonias
| Key characteristics | Mycoplasma pneumoniae | Legionnaire's disease | Chlamydophilia pneumoniae |
|---|---|---|---|
| Symptoms | |||
| Mental confusion | − | + | − |
| Prominent headache | − | ± | − |
| Meningismus | − | − | − |
| Myalgias | ± | ± | ± |
| Ear Pain | + | − | ± |
| Pleuritic pain | − | ± | − |
| Abdominal pain | − | + | − |
| Diarrhea | + | + | − |
| Signs | |||
| Rash | ±∗ | − | − |
| Nonexudative pharyngitis | + | − | + |
| Hemoptysis | − | ± | − |
| Wheezing | − | − | + |
| Lobar consolidation | − | ± | − |
| Cardiac involvement | ±† | −‡ | − |
| Splenomegaly | − | − | − |
| Relative bradycardia | − | + | − |
| Laboratory abnormalities | |||
| WBC count | ↑/N | ↑ | N |
| Acute thrombocytosis | ± | − | − |
| Hyponatremia | − | + | − |
| Hypophosphatemia | − | + | − |
| ↑ AST/ALT (SGOT/SGPT) | − | + | − |
| ↑ CPK | − | + | − |
| ↑ CRP (>30) | − | + | − |
| ↑ Ferritin (>2 × n) | − | + | − |
| ↑ Cold agglutinins (≥1:64) | + | − | − |
| Microscopic hematuria | − | + | − |
| Chest x-ray | |||
| Infiltrates | Patchy | Patchy or consolidation | “Circumscribed” lesions |
| Bilateral hilar adenopathy | − | − | − |
| Pleural effusion | ± (small) | ± | − |
| Diagnostic tests | |||
| Direct isolation (culture) | ± | + | ± |
| Serology (specific) | CF | IFA | CF |
| Legionella IFA titers | − | ↑↑↑ | − |
| Legionella DFA | − | + | − |
| Legionella urinary antigen | − | +¶ | − |
ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CF, Complement fixation; CYE, Charcoal yeast agar; DFA/IFA, Direct/indirect fluorescent antibody test; N, Normal; WBC, White blood cells; +, Usually present; ±, Sometimes present; −, Usually absent; ↑, Increased; ↓, Decreased; ↑↑↑, Markedly increased.
§Mental confusion only if meningoencephalitis.
Erythema multiforme.
Myocarditis, heart block, or pericarditis.
Unless endocarditis.
Often not positive early, but antigenuria persists for weeks. Useful only for diagnosing L. pneumophila (serogroups 01-06), but not other species or serogroups.
Adapted from Cunha.5
Table II.
Diagnostic features of zoonotic atypical pneumonias
| Key characteristics | Psittacosis | Q fever | Tularemia |
|---|---|---|---|
| Symptoms | |||
| Mental confusion | − | ± | − |
| Prominent headache | + | + | + |
| Meningismus | − | − | − |
| Myalgias | + | + | + |
| Ear pain | − | − | − |
| Pleuritic pain | ± | ± | ± |
| Abdominal pain | − | − | − |
| Diarrhea | − | − | − |
| Signs | |||
| Rash | ± ∗ | − | − |
| Nonexudative pharyngitis | − | − | ± |
| Hemoptysis | − | − | ± |
| Lobar consolidation | + | + | + |
| Cardiac involvement | ± † | ± ‡ | − |
| Splenomegaly | + | + | − |
| Relative bradycardia | + | + | − |
| Chest x-ray | |||
| Infiltrates | Patchy consolidation | “Ovoid or round infiltrates” | Patchy consolidation |
| Bilateral hilar adenopathy | − | − | ± |
| Pleural effusion | − | − | Bloody |
| Laboratory abnormalities | |||
| WBC count | ↓ | ↑/N | ↑/N |
| Acute thrombocytosis | − | + | − |
| Hyponatremia | ± | ± | ± |
| Hypophosphatemia | − | − | − |
| ↑ AST/ALT (SGOT/SGPT) | + | + | − |
| ↑ Cold agglutinins | − | ± | − |
| Anti-smooth muscle antibodies | − | ± | − |
| Microscopic hematuria | − | − | + |
| Diagnostic tests | |||
| Direct isolation (culture) | − | − | − |
| Serology (specific) | CF | CF | TA |
ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CF, Complement fixation; IFA, Indirect fluorescent antibody test; N, Normal; TA, Tube agglutinins; WBC, White blood cells; +, Usually present; ±, Sometimes present; −, Usually absent; ↑, Increased; ↓, Decreased; ↑↑↑, Markedly increased.
Horder's spots (facial spots resembling abdominal rash of typical fever, ie, rose spots).
Myocarditis.
Endocarditis.
Adapted from Cunha.5
Each of the atypical CAP pathogens, ie, zoonotic and nonzoonotic, has its own characteristic pattern of extrapulmonary involvement. The presumptive clinical diagnosis of CAPs takes into account this critically important clinical concept. The clinical features related to organ involvement in each atypical pneumonia permit a presumptive clinical diagnosis. Because the pattern of extrapulmonary organ involvement is different and characteristic of each atypical CAP pathogen, clinicians can easily differentiate not only typical from atypical CAPs, but also zoonotic from nonzoonotic atypical CAP pathogens.5, 11, 12, 13, 14, 15
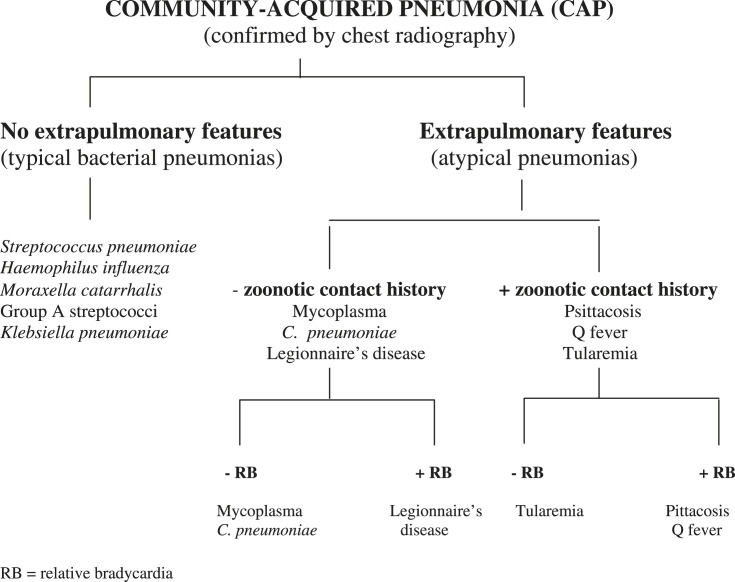
In the literature, studies sought to identify individual markers to differentiate Legionnaires' disease from typical CAPs. Invariably, this approach has been unsuccessful, because studies used individual parameters with no diagnostic specificity, eg, fever or hyponatremia, which may be present in both typical and atypical CAPs.3, 16, 17, 18 A presumptive clinical diagnosis of Legionnaire's disease is optimally based on a clinical syndromic diagnosis. Key extrapulmonary findings are associated with Legionnaires' disease, and by virtue of this fact, are diagnostically more useful in clinical syndromic diagnoses.5, 15, 19, 20, 21 To take into account the differing diagnostic significance of various clinical signs and symptoms as well as nonspecific laboratory findings, a diagnostic weighted point score system was developed at Winthrop-University Hospital in 1989.8, 20 Critical in an efficient approach to atypical CAPs is the zoonotic contact history, and the presence or absence of relative bradycardia (Fig 1 and Table III ).
Fig 1.
Clinical approach to community-acquired pneumonia. RB, relative bradycardia.
Adapted from Cunha.5
Table III.
Relative bradycardia (temperature-pulse relationships)
| Temperature | Appropriate pulse Response (beats/min) | Relative bradycardia (pulse deficit) Pulse (beats/min) |
|---|---|---|
| 106°F (41.1°C) | 150 | <140 |
| 105°F (41.1°C) | 140 | <130 |
| 104°F (41.1°C) | 130 | <120 |
| 103°F (41.1°C) | 120 | <110 |
| 102°F (41.1°C) | 110 | <100 |
| Criteria for relative bradycardia |
|---|
| Inclusive |
| • Patient must be an adult. |
| • Temperature of ≥102°F. |
| • Pulse must be taken simultaneously with the temperature elevation. |
| Exclusive |
| • Patient without arrhythmia, second/third-degree heart block, or pacemaker-induced rhythm. |
| • Patient must not be on β-blockers, verapamil, or diltiazem. |
| Causes of relative bradycardia | |
|---|---|
| Infectious | Noninfectious |
| • Legionnaires' disease | • β-blockers |
| • Psittacosis | • Verapamil |
| • Q fever | • Diltiazem |
| • Typhoid fever | • Central nervous system disorders |
| • Typhus | • Lymphomas |
| • Babesiosis | • Factitious fever |
| • Malaria | • Drug fever |
| • Leptospirosis | |
| • Yellow fever | |
| • Dengue fever | |
| • Viral hemorrhagic fever | |
| • Rocky Mountain spotted fever | |
Adapted from Cunha.5
The Winthrop-University Hospital diagnostic weighted point score system was found to be fairly sensitive and specific, and it has been modified and improved. The Winthrop-University Hospital Infectious Disease Division's diagnostic weighted point score system (modified) for Legionnaires' disease provides physicians with an accurate presumptive clinical diagnosis22 (Table IV ). Because most recommendations regarding the empiric treatment of CAP include coverage against Legionella, the Winthrop-University Hospital Infectious Disease Division's diagnostic weighted point score system (modified) was not intended to be the basis of empiric therapy. Rather, it was to be used to render a clinical syndromic diagnosis of Legionnaires' disease, and to prompt clinicians who would otherwise not suspect Legionnaires' disease to order appropriate Legionella diagnostic tests.22
Table IV.
Winthrop-University Hospital Infectious Disease Division's diagnostic weighted point system for diagnosing Legionnaires' disease in adults (modified)
| Presentation | Qualifying conditions1 | Point score |
|---|---|---|
| Clinical features | ||
| Temperature >102°F∗ | With relative bradycardia | +5 |
| Headache | Active onset | +2 |
| Mental confusion/lethargy∗ | Not drug-induced | +4 |
| Ear pain | Acute onset | −3 |
| Nonexudative pharyngitis | Acute onset | −3 |
| Hoarseness | Acute not chronic | −3 |
| Sputum (purulent) | Excluding AECB | −3 |
| Hemoptysis∗ | Mild/moderate | −3 |
| Chest pain (pleuritic) | −3 | |
| Loose stools/watery diarrhea∗ | Not drug-induced | +3 |
| Abdominal pain∗ | With/without diarrhea | +2 |
| Renal failure∗ | Acute (not chronic) | +3 |
| Shock/hypotension∗ | Excluding cardiac/pulmonary causes | −5 |
| Splenomegaly | Excluding non-CAP causes | −5 |
| Lack of response to B-lactam antibiotics | After 72 h (excluding viral pneumonias) | +5 |
| Laboratory tests | ||
| Chest x-ray | Rapidly progressive asymmetric infiltrates∗ (excluding severe influenza[human, avian, swine], HPS, SARS) | +3 |
| Severe hypoxemia with ↑ A-a gradient (>35)∗ | Acute onset | −2 |
| Hyponatremia∗ | Acute onset | +1 |
| Hypophosphatemia | Acute onset | +5 |
| ↑ SGOT/SGPT (early/mild/transient)∗ | Acute onset | +2 |
| ↑ Total bilirubin | Acute onset | +1 |
| ↑ LDH (>400)∗ | Acute onset | −5 |
| ↑ CPK∗ | Acute onset | +4 |
| ↑ CRP∗ | Acute onset | +5 |
| ↑Cold agglutinin titers (≥1:64) | Acute onset | −5 |
| Relative lymphopenia (<21%)∗ | Acute onset | +5 |
| ↑ Ferritin (>2 × n)∗ | +5 | |
| Microscopic hematuria∗ | Excluding trauma, BPH, Foley catheter, bladder/renal neoplasms | +2 |
| Total point score | Legionnaires' Disease very likely | >15 |
| Legionnaires' Disease likely | 5–15 | |
| Legionnaires' Diseaseunlikely | <5 |
AECB, Acute exacerbation of chronic bronchitis; BPH, Benign prostatic hyperplasia; SARS, Severe acute respiratory syndrome; HPS, Hantavirus pulmonary syndrome; SGOT/SGPT, Serum glutamate oxaloacetate transaminase/serum glutamate pyruvate transaminase; LDH, Lactate dehydrogenase; CPK, Creatine phosphokinase; CRP, C-reactive protein; ↑, increased.
†In adults, otherwise unexplained, acute, and associated with the pneumonia.
Otherwise unexplained (acute and associated with the pneumonia).
Although the Winthrop-University Hospital Infectious Disease Division's diagnostic weighted point score system for Legionnaires' disease (modified) has been accurate and useful, its use requires a familiarity with the diagnostic point score system, and it is time-consuming.
The need to develop a rapid, presumptive clinical diagnosis of Legionnaires' disease arose during the “herald wave” of the swine influenza (H1N1) pandemic in New York. Winthrop-University Hospital was at the epicenter of the H1N1 pandemic in New York. The Emergency Department (ED) of the hospital was inundated with hundreds of patients with influenza-like illnesses (ILIs) who presented for H1N1 testing and evaluation. The Quick Vue (A/B) test (Quidel, San Diego) for influenza A was the primary screening test for H1N1 pneumonia used in the ED. Because H1N1 is a variant of influenza A, it was hoped the rapid influenza A screening test would be a useful screening test for H1N1. Patients who were rapid influenza A test-positive were also reverse transcription polymerase chain reaction (RT-PCR)-positive for H1N1. It was soon realized that 30% of patients who clinically had H1N1 pneumonia had negative rapid influenza A tests in the ED. Other tests, eg, respiratory fluorescent antibody (FA) viral panel tests, were ordered to increase the diagnostic yield. Unfortunately, these rapid influenza diagnostic tests (RIDTs) did not correlate well with RT-PCR testing for H1N1 pneumonia. Accordingly, there was an urgent need to develop clinical criteria for the probable diagnosis of H1N1 pneumonia in patients with negative RIDTs.23
The Infectious Disease Division of Winthrop-University Hospital developed a diagnostic weighted point score system for the probable diagnosis of H1N1 pneumonia in a hospitalized adult with negative RIDTs, based on the previous Legionnaires' disease clinical diagnostic weighted point score system. At the end of July 2009, the Centers for Disease Control (Atlanta, GA) acknowledged that the designation of probable H1N1 was equivalent to definite H1N1 via RT-PCR testing. This was announced because health departments restricted RT-PCR testing, leaving physicians with no definitive way to rule in or rule out H1N1 pneumonias in those with negative RIDTs whose specimens for RT-PCR testing were restricted.
The Winthrop-University Hospital Infectious Disease Division's weighted diagnostic point score system (modified) for H1N1 pneumonia worked well throughout the “herald wave” of the pandemic.24, 25 The H1N1 diagnostic weighted point score system and its rapid, simplified version permitted the efficient triage of adult patients with ILIs and negative RIDTs and an accurate implementation of influenza precautions and oseltamivir therapy.24, 25
During the “herald wave” of swine influenza pneumonia, other patients with ILIs did not manifest H1N1 pneumonia. Still others presented with mimics of H1N1 pneumonia, eg, exacerbations of chronic bronchitis, asthma, or congestive heart failure, or with CAPs, eg, Legionnaires' disease.4, 5, 24, 25 Although the H1N1 pneumonia diagnostic point score system clearly differentiated H1N1 from its mimics, there was an urgent need for the rapid identification of patients with Legionnaires' disease who required no influenza precautions and different therapy.24, 25
Accordingly, the Winthrop-University Hospital Infectious Disease Division developed a rapid, presumptive diagnostic approach to identify Legionnaires' disease during the “herald wave” of the H1N1 pandemic. In reviewing the Winthrop-University Hospital Infectious Disease Division diagnostic weight point score system (modified) for Legionnaires' disease, a few key diagnostic markers were identified, eg, relative bradycardia, relative lymphopenia (Table V ), hypophosphatemia (Table VI ), and elevated serum ferritin levels (Table VII ). These key markers for Legionnaires' disease formed the basis of the diagnostic Legionnaires' disease triad. The Winthrop-University Hospital Infectious Disease Division's diagnostic Legionnaires' disease triad permitted a rapid presumptive clinical diagnosis for Legionella CAP. The diagnostic Legionnaires' disease triad was consistently predictive of a diagnosis of Legionnaires' disease, as confirmed by serologic methods or Legionella antigen tests (Table VIII ).
Table V.
Differential diagnosis significance of relative lymphopenia∗
| Infectious causes | Noninfectious causes |
|---|---|
|
|
(≤21%; normal range, 21% to 52%).
Adapted from Cunha.5
Table VI.
Differential diagnosis of hypophosphatemia
| Infectious causes | Noninfectious causes |
|---|---|
|
|
Table VII.
Differential diagnosis of highly elevated serum ferritin levels∗
| Infectious causes | Noninfectious causes |
|---|---|
| Acute | Malignancies |
| • Legionnaires' disease | • Preleukemias |
| • WNE | • Lymphomas |
| • Multiple myeloma | |
| • Hepatomas | |
| • Breast cancer | |
| • Colon cancer | |
| • Prostate cancer | |
| • Lung cancer | |
| • Liver/CNS metastases | |
| Chronic | Myeloproliferative disorders |
| • HIV | |
| • CMV | |
| • TB | |
| Rheumatic/inflammatory disorders | |
| • Rheumatoid arthritis | |
| • Adult Still's disease | |
| • SLE | |
| • TA | |
| Renal disease | |
| • Acute renal failure | |
| • Chronic renal failure | |
| Liver disease | |
| • Hemochromatosis | |
| • Cirrhosis | |
| • α-1 antitrypsin deficiency | |
| • CAH | |
| • Cholestatic jaundice | |
| Miscellaneous | |
| • Sickle-cell anemia | |
| • Multiple blood transfusions |
WNE, West Nile encephalitis; HIV, Human immunodeficiency; virus; CNS, Central nervous system; CMV, Cytomegalovirus; SLE, Systemic lupus erythematosus; TB, Tuberculosis; TA, Temporal arteritis; CAH, Chronic acute hepatitis.
> 2 × normal.
Adapted from Cunha.5
Table VIII.
Winthrop-University Hospital Infectious Disease Division's rapid clinical diagnosis of legionnaires' disease: the Legionnaires' disease diagnostic triad
| Entry criteria | Key clinical features | Any 3 key laboratory features (diagnostic triad) |
|---|---|---|
| • Signs and symptoms of atypical CAP (CAP + extrapulmonary features) plus | • Fever >102° F with relative bradycardia∗ (See Table III) | • Relative lymphopenia∗ (See Table V) |
| • Negative recent/close zoonotic vector contact history plus |
• Mildly/transiently elevated serum transaminases∗ | |
| • New single/multiple focal infiltrates on chest x-ray∗ | • Hypophosphatemia∗ (See Table VI) | |
| • Highly elevated serum ferritin levels (>2 × n)∗ (See Table VII) |
Otherwise unexplained.
We present a case of Legionnaire's disease that occurred during the “herald wave” of the H1N1 pandemic at Winthrop-University Hospital. A rapid, presumptive diagnosis of Legionnaires' disease was rendered in this patient, using the Winthrop-University Hospital Infectious Disease Division's diagnostic Legionnaires' disease triad. The Winthrop-University Hospital Infectious Division's diagnostic Legionnaires' disease triad permitted a rapid presumptive clinical diagnosis and clearly differentiated this and other cases of Legionnaires' disease from other CAPs, as well as from H1N1 pneumonia.
Discussion
It was long thought that no good way existed to differentiate typical CAPs clinically from atypical CAPs. This notion was based on studies that examined individual clinical and laboratory parameters that individually did not differentiate typical from atypical CAPs.3, 16, 17, 18 However, certain key extrapulmonary findings are more diagnostically important than others.11, 14, 15 When key extrapulmonary findings are combined in a diagnostic weighted point score system, physicians can clearly differentiate Legionnaires' disease from a typical CAP. The Winthrop-University Hospital Infectious Disease Division's diagnostic weighted point score system (modified) has been used to diagnose Legionnaires' disease over the years.22 However, the use of a diagnostic weighted point system for Legionnaires' disease requires physicians to be familiar with the weighted point scores of each entry. Although the Winthrop-University Hospital Infectious Disease Division's diagnostic weighted point score system to diagnose Legionnaires' disease (modified) is accurate, it is also time-consuming.22 Therefore, there was a need to render a presumptive diagnosis of Legionnaires' disease rapidly, based on a simplified system.
During the “herald wave” of the H1N1 pandemic in the New York area, such a need was realized. In addition to the H1N1 pneumonia of adult patients admitted to our institution via the ED, there was a higher than usual number of cases with Legionnaires' disease. Because of the high clinical volume imposed by H1N1, we needed a rapid, simplified method to render a presumptive clinical diagnosis of Legionnaires' disease. The diagnostic Legionnaires' disease triad was developed to meet this need.
The diagnostic Legionnaires' disease triad was used in a clinical context under pandemic conditions to effect a rapid, presumptive diagnosis of sporadic cases of Legionnaires' disease among cases of H1N1 pneumonia. All cases of Legionnaires' disease during the late spring and summer in admitted patients at Winthrop-University Hospital were effectively identified using the diagnostic Legionnaires' disease triad.
Our case report demonstrates the value of using the diagnostic Legionnaires' disease triad to render a rapid, presumptive clinical diagnosis of Legionnaires' disease, pending Legionella diagnostic test results. An early presumptive diagnosis of Legionnaires' disease permitted early initiation of appropriate anti-Legionella antimicrobial therapy.
The take-home lesson for clinicians is that by using the Winthrop-University Hospital Infectious Disease Division's diagnostic Legionnaires' disease triad, a rapid, simple, and accurate presumptive clinical diagnosis can be rendered. Clinicians should realize that a clinical syndromic diagnosis of Legionnaires' disease is possible using either the diagnostic weighed point score system (modified) or (more rapidly and simply) the diagnostic Legionnaires' disease triad. The diagnostic Legionnaires' disease triad is based on the modified diagnostic weighted point score system. The key clinical indicators of the diagnostic Legionnaires' disease triad can only occur together at the same time in patients with the Legionnaires' disease CAP. The diagnostic Legionnaires' disease triad's effectiveness was demonstrated under unusual circumstances, ie, during the “herald wave” of the H1N1 pandemic, which attested to its clinical usefulness in a difficult clinical setting.26, 27, 28
References
- 1.Lattimer G.L., Ormsbee R.A. Marcel Dekker, Inc; New York: 1981. Legionnaires' disease. [Google Scholar]
- 2.Lattimer G.L., Rhodes L.V. Legionnaire's Disease: Clinical findings and one-year follow-up. JAMA. 1978;240:1169–1171. doi: 10.1001/jama.240.11.1169. [DOI] [PubMed] [Google Scholar]
- 3.Edelstein P.H. Manual of clinical microbiology. 9th ed. ASM Press; Washington, DC: 2007. Legionella; pp. 835–849. [Google Scholar]
- 4.Cunha B.A. Legionnaire's Disease: clinical differentiation from typical and other atypical lpneumonias. Infect Dis Clin North Am. 2010;24:73–105. doi: 10.1016/j.idc.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunha B.A., editor. Pneumonia essentials. 3rd ed. Jones & Bartlett; Sudbury, MA: 2010. [Google Scholar]
- 6.Coletta F.S., Fein A.M. Radiological manifestation of Legionella/Legionella-like organisms. Semin Respir Infect. 1998;13:109–115. [PubMed] [Google Scholar]
- 7.Nguyen T.M., Ilef D., Jarraud S., Rouil L., Campese C., Che D. A community-wide outbreak of Legionnaires' disease linked to industrial cooling towers—how far can contaminated aerosols spread? J Infect Dis. 2006:102. doi: 10.1086/498575. 1993. [DOI] [PubMed] [Google Scholar]
- 8.Phares C.R., Russell E., Thigpen M.C., Service W., Crist M.B., Salyers M. Legionnaires' disease among residents of a long-term care facility: the sentinel event in a community outbreak. Am J Infect Control. 2007;5:319–323. doi: 10.1016/j.ajic.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Carbonara S., Monno L., Longo B. Legionella is often overlooked as a cause of NHAP outbreaks. Curr Opin Pulm Med. 2009;15:261–273. doi: 10.1097/MCP.0b013e3283287c3f. [DOI] [PubMed] [Google Scholar]
- 10.Roig J., Aguilar X., Ruiz J., Domingo C., Mesalles E., Manterola J. Comparative study of Legionella pneumophila and other nosocomial-acquired pneumonias. Chest. 1991;99:344–350. doi: 10.1378/chest.99.2.344. [DOI] [PubMed] [Google Scholar]
- 11.Levy P.Y., Teysseire N., Etienne J., Raoult D. A nosocomial outbreak of Legionella pneumophila caused by contaminated transesophageal echocardiography probes. Concise Commun. 2004;24:619–622. doi: 10.1086/502263. [DOI] [PubMed] [Google Scholar]
- 12.Cunha B.A., Quintiliani R. The atypical pneumonias: a diagnostic and therapeutic approach. Postgrad Med. 1979;66:95–102. doi: 10.1080/00325481.1979.11715248. [DOI] [PubMed] [Google Scholar]
- 13.Cunha B.A., Ortega A.M. Atypical pneumonia. Extrapulmonary clues guide the way to diagnosis. Postgrad Med. 1996;99:123–128. [PubMed] [Google Scholar]
- 14.Cunha B.A. The extrapulmonary manifestations of community-acquired pneumonias. Chest. 1998;112:945. doi: 10.1378/chest.114.3.945. [DOI] [PubMed] [Google Scholar]
- 15.Cunha B.A. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. 2006;12:12–24. doi: 10.1111/j.1469-0691.2006.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunha B.A. Atypical pneumonias: current clinical concepts focusing on Legionnaires' disease. Curr Opin Pulmon Med. 2008;14:183–194. doi: 10.1097/MCP.0b013e3282f79678. [DOI] [PubMed] [Google Scholar]
- 17.Miyashita N., Fukano H., Yoshida K., Niki Y., Matsushima T. Is it possible to distinguish between atypical pneumonia and bacterial pneumonia? Evaluation of the guidelines for community acquired pneumonia in Japan. Respir Med. 2004;98:952–960. doi: 10.1016/j.rmed.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Masiá M., Gutiérrez F., Padilla S., Soldán B., Mirete C., Shum C. Clinical characterisation of pneumonia caused by atypical pathogens combining classic and novel predictors. Clin Microbiol Infect. 2007;13:153–161. doi: 10.1111/j.1469-0691.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 19.Fiumefreddo R., Zaborsky R., Haeuptle J., Christ-Crain M., Trampuz A., Steffen I. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulm Med. 2009;9:4–7. doi: 10.1186/1471-2466-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strampfer M.J., Cunha B.A. Legionnaires' disease. Semin Respir Infect. 1987;2:228–234. [PubMed] [Google Scholar]
- 21.Cunha B.A. Clinical diagnosis of Legionnaires' disease. Semin Respir Infect. 1998;13:116–127. [PubMed] [Google Scholar]
- 22.Cunha B.A. The clinical diagnosis of Legionnaires' disease: diagnostic value of combining non-specific laboratory tests. Infection. 2008;6:395–397. doi: 10.1016/j.jinf.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Cunha B.A. Severe Legionella pneumonia: rapid diagnosis with Winthrop-University Hospital's weighted point score system (modified) Heart Lung. 2008;37:312–321. doi: 10.1016/j.hrtlng.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunha B.A., Pherez F.M., Strollo S. Swine Influenza (H1N1): diagnostic dilemma early in the pandemic. Scand J Infect Dis. 2009;41:900–902. doi: 10.3109/00365540903222465. [DOI] [PubMed] [Google Scholar]
- 25.Cunha B.A., Syed U., Strollo S. The Winthrop-University Hospital Infectious Disease Division's swine influenza (H1N1) pneumonia diagnostic weighted point score system in hospitalized adults with negative rapid influenza diagnostic tests. Heart Lung. 2009;38:534–538. doi: 10.1016/j.hrtlng.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunha B.A., Syed U., Mickail N., Strollo S. Rapid clinical diagnosis of fatal swine influenza (H1N1) pneumonias: diagnostic swine influenza triad. Heart Lung. 2010;39:78–86. doi: 10.1016/j.hrtlng.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunha B.A. Swine influenza (H1N1) pneumonia: clinical considerations. Infect Dis Clin North Am. 2010;24:203–228. doi: 10.1016/j.idc.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunha B.A., Mickail N., Thekkel V. Unexplained increased incidence of Legionnaire's Disease during the “Herald Wave” of the H1N1 influenza pandemic. Infect Control Hosp Epidemiol. 2010;31:562–563. doi: 10.1086/652453. [DOI] [PubMed] [Google Scholar]