Summary
Objectives
In places of mass gathering, rapid infection screening prior to definite diagnosis is vital during the epidemic season of a novel influenza. In order to assess the possibility of clinical application of a newly developed non-contact infection screening system, we conducted screening for influenza patients.
Materials and methods
The system is operated by a screening program via a linear discriminant analysis using non-contact derived variables, i.e., palmar pulse derived from a laser Doppler blood-flow meter, respiration rate determined by a 10-GHz microwave radar, and average facial temperature measured by thermography. The system was tested on 57 seasonal influenza (2008–2009) patients (35.7 °C ≤ body temperature ≤ 38.3 °C, 19–40 years) and 35 normal control subjects (35.5 °C ≤ body temperature ≤ 36.9 °C, 21–35 years) at the Japan Self-defense Forces Central Hospital.
Results
A significant linear discriminant function (p < 0.001) was determined to distinguish the influenza group from the control group (Mahalanobis D-square = 6.5, classification error rate > 10%). The system had a positive predictive value (PPV) of 93%, which is higher than the PPV value (PPV ≤ 65.4%) reported in the recent summary of studies using only thermography performed mainly in hospitals.
Conclusions
The proposed system appears promising for application in accurate screening for influenza patients at places of mass gathering.
Keywords: Non-contact, Screening, Infection, Pandemic influenza, Microwave radar, Thermography, Heart rate, Respiratory rate
Introduction
A novel influenza A virus, subtype H1N1 of swine-lineage (H1N1 swl) has circulated throughout the world rapidly.1 In mass gathering places, such as, hospital outpatient units, airport quarantine facilities, and public health centers, prior to definite diagnosis, rapid screening of infection is urgently needed during the epidemic season of a novel influenza.2 In order to conduct fast screening of people with infections, such as pandemic influenza, severe acute respiratory syndrome (SARS),3 or other emerging infectious diseases, we have developed a non-contact screening system to perform human medical inspections within several tens of seconds, from non-contact derived heart and respiratory rates, as well as facial temperature measured by a non-contact method. A number of countries have applied thermography at international airports in order to detect infectious passengers. A previous study revealed that the positive predictive value (PPV) varied from 3.5% to 65.4%.4 This low PPV indicates the limited effectiveness of thermography in detecting early-stage influenza symptoms.
As a result of infection, not only body temperature but also heart and respiratory rates increase. Therefore, in order to achieve more accurate screening, heart and respiratory rates are used as new parameters for screening. We conducted non-contact influenza screening within 30 s using the newly developed screening system. The system is operated by the screening program through a linear discriminant analysis using non-contact derived variables, i.e., palmar pulse determined using a laser Doppler blood-flow meter, respiration rate determined using a 10-GHz microwave radar, and average facial temperature measured by thermography. We have previously demonstrated the possibility of non-contact determination of exposure to toxic conditions via a 1215-MHz microwave radar,5, 6 which has been investigated for use in locating human subjects buried under earthquake rubble,7, 8, 9 in order to prevent secondary exposure of medical personnel to toxic materials under biochemical hazard conditions. Exposure to a toxin, namely, lipopolysaccharide,10 was determined by linear discriminant analysis using non-contact derived variables in an animal experiment.5 In a previous study, we assessed the performance of the newly developed non-contact screening system in a laboratory from an engineering viewpoint.11 In the present paper, for seasonal influenza (2008–2009) patients and normal control subjects, we investigate whether the newly developed screening system can distinguish influenza patients from normal control subjects at the Japan Self-defense Forces Central Hospital.
Materials and methods
Non-contact screening system for medical inspection
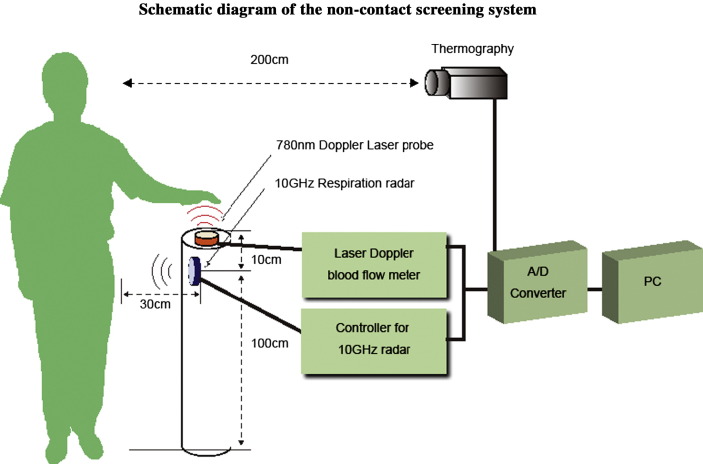
The technical details of the non-contact screening system have been reported in a previous paper.11 The non-contact screening system simultaneously measures heart rate, respiratory rate, and facial temperature (Figure 2, Figure 3, Figure 4 ). The non-contact monitoring system consists of a non-contact laser Doppler blood-flow meter (ALF21N, Advance, Tokyo), a prototype 10-GHz respiration radar (Tau-giken, Yokohama, Japan), an infrared thermograph (NEC SANEI, IS7800, Tokyo), and a personal computer with a data processing display program. The Doppler laser probe is used to measure the palmar pulse. The respiratory rate was measured using the 10-GHz respiration radar by monitoring the abdominal respiratory motion, and the facial surface temperature was measured via infrared thermography. The thermography was placed 200 cm from the face of the subject.
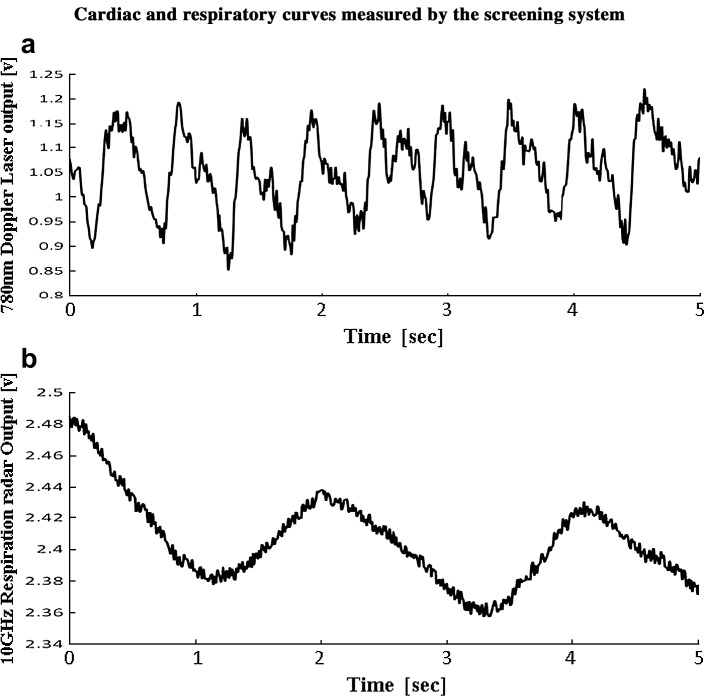
Figure 2.
(a) The laser Doppler blood-flow meter output includes cyclic oscillations that are attributed to the palmar pulse of the subject. (b) The 10-GHz microwave radar output includes cyclic oscillations induced by the respiratory motion of the abdomen of the subject.
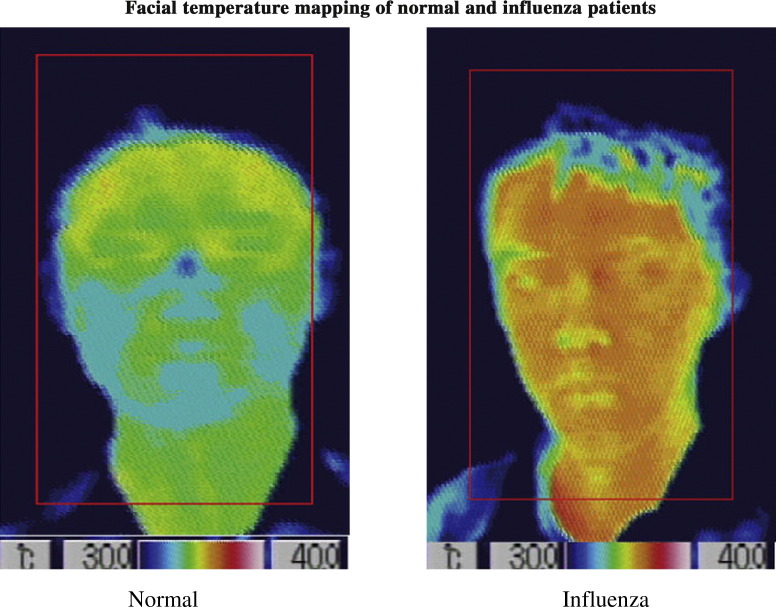
Figure 3.
The screening system automatically captures the facial area and calculates the average facial temperature of the area within the red rectangle, excluding the facial background area.
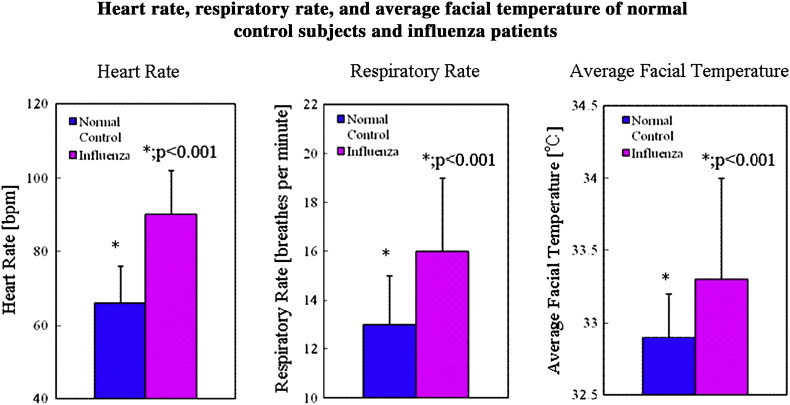
Figure 4.
The heart rates of influenza patients are significantly larger than that of the normal control subjects (p < 0.001) (left). The respiratory rates of influenza patients are higher than those of the normal control subjects (p < 0.001) (middle). The average facial temperatures of influenza patients are also significantly larger than those of the normal control subjects (p < 0.001) (right).
We designed a cylindrical post for monitoring vital signs (CPV) (diameter: 22 cm, height: 110 cm) with a disk-shaped plastic cover on top (Fig. 1). The CPV incorporates a Doppler laser probe for palmar pulse measurement and a 10-GHz respiration radar (Fig. 1). The heart and respiratory rates and the facial temperature are measured simultaneously while the subject holds his/her left palm over the CPV for 30 s.
Figure 1.
The system is operated by a screening program via a linear discriminant analysis using non-contact derived variables, i.e., palmar pulse determined using a laser Doppler blood-flow meter, respiration rate determined using a 10-GHz microwave radar, and average facial temperature measured by thermography.
The outputs of the laser Doppler blood-flow meter, the respiration radar, and the infrared thermography were transferred to a personal computer and then analyzed and displayed in real time, using the screening program. The screening program incorporates band pass filters for monitoring respiratory and heart rates in order to eliminate background noise. The heart rate is obtained by fast Fourier transform (FFT), and the respiratory rate is determined by the respiration curve measured by the respiration radar. The pulse wave, the respiratory curve, and the thermograph are displayed in real time on a graphic terminal. The screening program calculates average facial temperature and displays the screening result (“potential infection” or “pass”) obtained by a linear discriminant function from the non-contact derived heart rate, the respiratory rate, and the average facial temperature.
Testing of the non-contact screening system at a hospital to distinguish influenza patients from normal control subjects
The subjects of the present study were 57 patients admitted with headache, sore throat, and other symptoms from January to March, 2009. The patients were diagnosed to have seasonal influenza based on results obtained using QuickVue Rapid SP influ kits (Quidel Corp., USA). These patients were mainly men (86.0%) and were all Self-Defense Forces members. The average age of the patients was 25 years (19–40 years). All influenza patients were treated with an Oseltamivir or a Zanamivir hydrate, and their axillary temperatures averaged 37.0 ± 0.6 °C (35.7 °C ≤ body temperature ≤ 38.3 °C) after hospitalization.
35 Normal control subjects (30 male and five female) were students at the Institute of Medical Radiology Technologists at the Japan Self-Defense Force Central Hospital. These subjects had no symptoms of fever, headache, or sore throat. The average age of the subjects was 26 years (20–35 years). Their axillary temperatures averaged 36.6 ± 0.3 °C (35.5 °C ≤ body temperature ≤ 36.9 °C).
Measurements using the screening system were performed at 10:00–11:00 a.m. for the above hospitalized influenza patients and normal control subjects. The heart rate, respiratory rate, and average facial temperature of each subject were determined using the screening system. The axillary temperatures were measured using a clinical thermometer (Omron MC-107BW) for both influenza patients and normal control subjects. All subjects who normally wore eyeglasses removed them for the facial temperature measurements.
In order to assess the possibility of distinguishing between influenza-infected subjects and normal control subjects, a linear discriminant function was determined using non-contact derived data. Linear discriminant analysis is generally used for data classification. In our previous paper, the exposure to a toxin was determined by linear discriminant analysis using non-contact derived variables in a laboratory experiment, an F-test was used to evaluate the significance of the linear discriminant function, which can predict group membership for p < 0.05.5
The linear equation was defined as follows:
where Z(X 1,X 2, X 3) is a linear discriminant function, A is a constant, B 1, B 2, and B 3 are regression coefficients corresponding to the heart rate, the respiratory rate, and the average facial temperature, respectively, and X 1, X 2, and X 3 are variables representing heart rate, respiratory rate, and average facial temperature, respectively.
The optimum combinations of regression coefficients and the constant (A) were determined by StatMate III (ATMS, Tokyo) statistical software using the variables derived by the non-contact screening system. We evaluated whether the calculated Z(X 1,X 2,X 3) function can discriminant influenza patients from normal control subjects. Mahalanobis D2 and the classification error rate were calculated from the non-contact derived variables, where D2 is an index of the extent to which the discriminant function discriminants between criterion groups.
Quantitative data are expressed as mean values ± SDs. Statistical analysis was performed using StatMate III (ATMS, Tokyo) software.
Ethical approval
The present study was approved by the Ethics Committee of Japan Self-defense Forces Central Hospital.
Results
Measurements were taken while each subject stood holding the left palm over the top of the CPV. The Doppler laser output exhibits a cyclic oscillation induced by cardiac oscillation with a period of 0.53 s (Fig. 2(a)). A respiratory oscillation having a period of 3.8 s was observed using a 10-GHz radar (Fig. 2(b)).
The screening system automatically captures the facial area and calculates the average facial temperature of the area within the red rectangle (Fig. 3) which includes part of neck, excluding the facial background area (dark blue part f the collar which corresponds to the area temperature < 30 °C), was calculated.
The average heart rate was 66 ± 10 bpm for normal control subjects and 90 ± 12 bpm for influenza patients, which is significantly larger than that of normal control subjects (p < 0.001) (Fig. 4, left panel). The average respiratory rate was 13 ± 2 bpm for normal control subjects and 16 ± 3 bpm for influenza patients, which is significantly larger than that of the normal control subjects (p < 0.001) (Fig. 4, center panel). The average facial temperature was 32.9 ± 0.3 °C for normal control subjects and 33.3 ± 0.7 °C for influenza patients, which is significantly larger than that of the normal control subjects (p < 0.001) (Fig. 4, right panel).
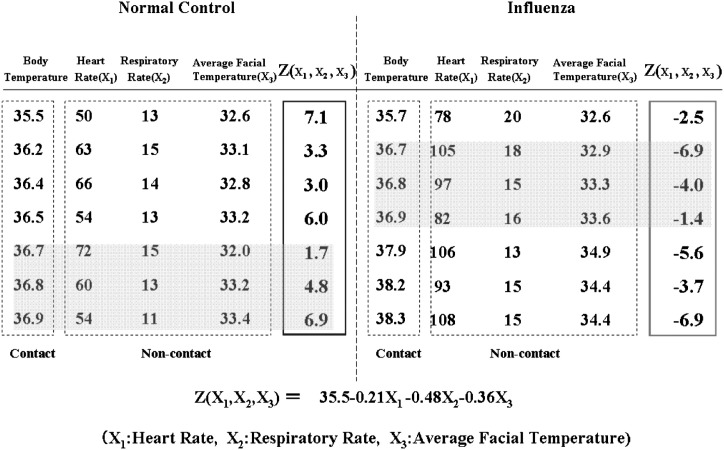
In order to distinguish the influenza patients group from the normal control subjects group, the Z(X 1,X 2,X 3) function was calculated by statistical software (StatMate III) using non-contact derived data, which is shown in part in Table 1 . The derived Z(X 1,X 2,X 3) is a statistically significant function (p < 0.001, as determined by an F-test):
where X 1 is the heart rate, X 2 is the respiratory rate, and X 3 is the average facial temperature.
Table 1.
Z values of the normal group and the influenza group as calculated from data, which is shown in part in this table (heart rate, respiratory rate, and average facial temperature) using linear discriminant analysis. All Z values are positive in the normal group and are negative in the influenza group. The linear discriminant function Z(X1,X2,X3) calculated from the non-contact derived variables distinguishes the influenza group from the normal control group. The Z value remains effective in distinguishing influenza patients regardless of their temperatures (Cf, gray areas), influenza patients without fever indicate relatively high heart and respiratory rates compared with normal controls within the same body temperature range.
The Z values calculated by this formula are shown in part in Table 1. In this table, Z values were positive in the normal control subjects group (left) and negative in the influenza group (right), although some influenza patients have lower average facial temperatures compared to some of the normal subjects, because their relatively high heart and respiratory rates compensate for these average facial temperature inversions. The sign of the Z value can be used to distinguish the influenza group from the normal control group. The Mahalanobis D2 calculated from the non-contact derived variables was 6.5. The classification error rate corresponding to the Mahalanobis D2 was more than 10%.
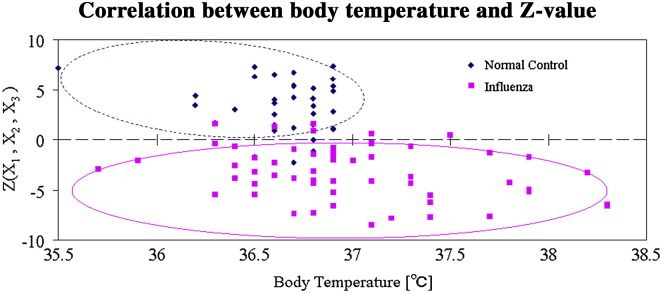
A total of 51 influenza patients, bounded by the solid line ellipse in Fig. 5 , indicated a negative Z value, six influenza patients showed a positive Z value, and the corresponding positive predictive value (PPV) and sensitivity are 93% and 88%, respectively. The Z value remains effective in distinguishing influenza patients regardless of their temperatures as indicated in Fig. 5. A total of 30 normal control subjects, bounded by the dotted line ellipse in Fig. 5 indicated a positive Z value, and five normal control subjects showed negative Z values. The corresponding negative predictive value (NPV) and specificity are 82% and 89%, respectively.
Figure 5.
A total of 51 influenza patients, bounded by the solid line ellipse, had negative Z values, and six influenza patients had positive Z values. The positive predictive value (PPV) and sensitivity are 93% and 88%, respectively. A total 30 normal control subjects, bounded by the dotted line ellipse, had positive Z values, and five normal control subjects had negative Z values. The negative predictive value (NPV) and specificity are 82% and 89%, respectively.
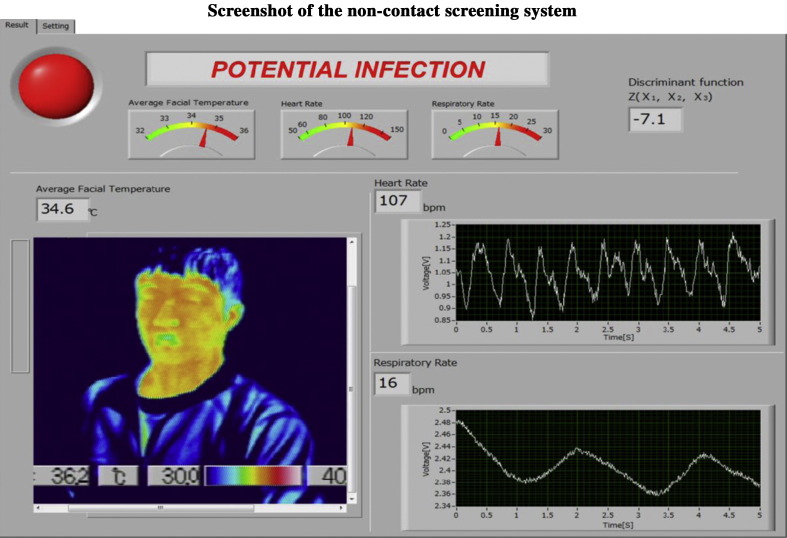
The execution screen of the non-contact screening system displays the screening result, i.e., “potential infection” or “pass”, which is judged by the sign of the non-contact derived Z value (Fig. 6 ).
Figure 6.
Screenshot of an individual being analyzed using the non-contact screening system. The heart and respiratory curves, the facial temperature mapping, and the screening result, i.e., “potential infection” or “pass”, are displayed.
Discussions
Some previous studies pointed out that there are many variables that can affect the accuracy of mass screening systems using only thermography. Ambient temperature, heavy makeup, alcohol and perspiration are some examples of such varaiables.12, 13, 14 However, this non-contact screening system monitors not only temperature but also heart and respiratory rates. In this sense, it may offer an increase in accuracy in detecting infected individuals.
The influenza patients who were the subjects of the present study were treated with an Oseltamivir or a Zanamivir hydrate, and some of them had normal temperatures after medication. The newly developed non-contact screening system using discriminant function is effective in distinguishing influenza patients regardless of their temperatures. This can be attributed to the fact that even in the cases of influenza patients with normal temperatures, their heart and respiratory rates are relatively high compared with normal subjects.
The non-contact screening system showed the PPV of 93%, which is higher than the reported PPV in the recent summary of the existing studies on fever screening using only non-contact infrared thermometers performed mainly in hospitals (the PPV varied from 3.5% to 65.4%).11 It must be noted, however, in this “case-control” study, the percentage of febrile subject is 26% which is much higher than actual febrile patients percentages. The prevalence of a disease influences PPV, therefore, a larger scale study needs to be conducted. Also, in order to conduct more accurate screening, it is necessary to accumulate data for people of a variety of ages, races, body morphologies, and anamnesis.
In summary, the discriminant function of this proposed system is effective in distinguishing influenza patients regardless of their temperatures, which enables higher PPV rate of 93% compared to the conventional method using only thermography. There are some limitations in detecting infected individuals before symptoms appear. However, the system saves time and effort for screening at airport quarantines and other mass gathering places and contributes to reduce the risk of secondary exposure and transmission of infection.
Acknowledgements
The authors would like to thank Mr. Kakeru Ujikawa, for his efforts and assistance. The present study was supported by Grants-in-Aid for Scientific Research (21510181) founded by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Carr M.J., Gunson R., Maclean A., Coughlan S., Fitzgerald M., Scully M. Development of a real-time RT-PCR for the detection of swine-lineage influenza A (H1N1) virus infections. J Clin Virol. 2009;45:196–199. doi: 10.1016/j.jcv.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottfredsson M., Halldórsson B.V., Jónsson S. Lessons from the past: familial aggregation analysis of fatal pandemic influenza (Spanish flu) in Iceland in 1918. Proc Natl Acad Sci U S A. 2008;29:1303–1308. doi: 10.1073/pnas.0707659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipsitch M., Cohen T., Cooper B., Robins J.M., Ma S., James L. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitar D., Goubar A., Desenclos J.C. International travels and fever screening during epidemics: a literature review on the effectiveness and potential use of non-contact infrared thermometers. Eurosurveillance. 2009;12:19115. [PubMed] [Google Scholar]
- 5.Matsui T., Hagisawa K., Ishizuka T. A novel method to prevent secondary exposure of medical and rescue personnel to toxic materials under biochemical hazard conditions using microwave radar and infrared thermography. IEEE Trans Biomed Eng. 2004;51:2184–2188. doi: 10.1109/TBME.2004.834250. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi M., Ishihara M., Matsui T. Biomedical engineering's contribution to defending the homeland, technological and biomedical initiatives being taken in Japan to counter bioterrorism. IEEE Eng Med Biol. 2004;23:175–186. doi: 10.1109/memb.2004.1297190. [DOI] [PubMed] [Google Scholar]
- 7.Chen K.M., Huang Y., Zhang J., Norman A. Microwave life-detection systems for searching human subjects under earthquake rubble or behind barrier. IEEE Trans Biomed Eng. 2000;27:105–113. doi: 10.1109/10.817625. [DOI] [PubMed] [Google Scholar]
- 8.Chen D., Misra D., Wang H., Chuang H.R. An X-band microwave life-detection system. IEEE Trans Biomed Eng. 1986;33:697–702. doi: 10.1109/TBME.1986.325760. [DOI] [PubMed] [Google Scholar]
- 9.Chuang H.R., Chen Y.F., Chen K.M. Automatic clutter-canceller for microwave life-detection system. IEEE Trans Instrum Meas. 1991;40:747–750. [Google Scholar]
- 10.Leclerc J., Corseaux D., Haddad E. A single endotoxin injection in the rabbit causes prolonged blood vessel dysfunction and a procoagulant state. Crit Care Med. 2000;28:3672–3678. doi: 10.1097/00003246-200011000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Matsui T., Suzuki S., Ujikawa K., Usui T., Gotoh S., Sugamata M. The development of a non-contact screening system for rapid medical inspection at a quarantine depot using a laser Doppler blood-flow meter, microwave radar and infrared thermography. J Med Eng Technol. 2009;33(6):481–487. doi: 10.1080/03091900902952675. [DOI] [PubMed] [Google Scholar]
- 12.Ng E.Y.K., Acharya R.U. A review of remote-sensing infrared thermography for indoor mass blind fever screening in containing an epidemic. IEEE Eng Med Biol. 2009;28:76–83. doi: 10.1109/MEMB.2008.931018. [DOI] [PubMed] [Google Scholar]
- 13.Chan L.S., Cheung G.T., Lauder I.J., Kumana C.R., Lauder I.J. Screening for fever by remote-sensing infrared thermographic camera. J Travel Med. 2004;11:273–279. doi: 10.2310/7060.2004.19102. [DOI] [PubMed] [Google Scholar]
- 14.Ng E.Y.K., Muljo W., Wong B.S. Study of facial skin and aural temperature. IEEE Eng Med Biol Mag. 2006;25:68–74. doi: 10.1109/memb.2006.1636353. [DOI] [PubMed] [Google Scholar]