Abstract
The renin angiotensin system (RAS) is intricately involved in normal cardiovascular homeostasis. Excessive stimulation by the octapeptide angiotensin II contributes to a range of cardiovascular pathologies and diseases via angiotensin type 1 receptor (AT1R) activation. On the other hand, tElsevier Inc.he angiotensin type 2 receptor (AT2R) is thought to counter-regulate AT1R function. In this review, we describe the enhanced expression and function of AT2R in various cardiovascular disease settings. In addition, we illustrate that the RAS consists of a family of angiotensin peptides that exert cardiovascular effects that are often distinct from those of Ang II. During cardiovascular disease, there is likely to be an increased functional importance of AT2R, stimulated by Ang II, or even shorter angiotensin peptide fragments, to limit AT1R-mediated overactivity and cardiovascular pathologies.
Keywords: Angiotensin II, AT2 receptor, AT1 receptor, Cardiovascular disease
Abbreviations: ACE, angiotensin converting enzyme; ACE2, angiotensin converting enzyme 2; Ang II, angiotensin II; Ang III, angiotensin III; Ang IV, angiotensin IV; Ang (1–7), angiotensin (1–7); ATBP50, AT2R-binding protein of 50 kDa; ATIP-1, AT2 receptor interacting protein-1; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor; AT4R, angiotensin II type 4 receptor; BK, bradykinin; BP, blood pressure; cGMP, cyclic guanine 3′,5′-monophosphate; ECM, extracellular matrix; eNOS, endothelial nitric oxide synthase; ERK-1/2, extracellular-regulated kinases-1,2; IRAP, insulin-regulated aminopeptidase; l-NAME, NG-nitro-l arginine methyl ester; LVH, left ventricular hypertrophy; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; MI, myocardial infarction; MMP, matrix metalloproteinase; mRNA, messenger ribonucleic acid; NF-κβ, nuclear transcription factor-κβ; NO, nitric oxide; O2−, superoxide; PC12W, rat pheochromocytoma cell line; RAS, renin angiotensin system; ROS, reactive oxygen species; SHR, spontaneously hypertensive rat; TIMP-1, tissue inhibitor of metalloproteinase-1; TNFα, tumour-necrosis factor α; VSMC, vascular smooth muscle cell; WKY, Wistar-Kyoto rat
1. Introduction
1.1. Conventional aspects of renin angiotensin system
The major effector peptide of the renin angiotensin system (RAS) is the octapeptide angiotensin II (Ang II), which was originally considered a circulating endocrine hormone (Unger et al., 1996, Csikos et al., 1997), with major effects involving vascular/cardiac contractility and fluid and electrolyte homeostasis. However, identification of components of the RAS in various organs has extended our understanding of the RAS (Zimmerman and Dunham, 1997, Akasu et al., 1998), and it is now well accepted that in addition to systemic effects, local synthesis of Ang II exerts both autocrine and paracrine functions, influencing cellular growth and regional haemodynamics in a tissue-specific manner (Johnston, 1992, Zimmerman and Dunham, 1997, de Gasparo et al., 2000, Paul et al., 2006).
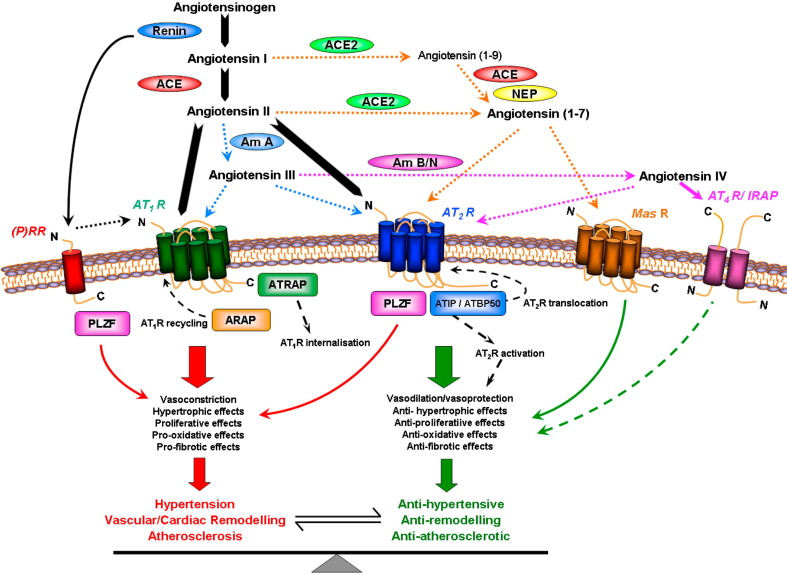
As has been well documented, the ‘classical’- or ‘renal’-RAS involves the conversion of the hepatic-derived precursor peptide angiotensinogen to angiotensin I (Ang I), which is catalysed by circulating renin released from the kidney. The conversion of Ang I to Ang II is then primarily facilitated by angiotensin converting enzyme (ACE) which is found in endothelial cells, and due to the large surface area, this conversion is prominent in the lungs. Alternatively (but to a much lesser degree), Ang I may be directly converted to Ang (1–7) by tissue endopeptidases (Ferrario et al., 1997). Ang II is susceptible to degradation by aminopeptidases, resulting in fragments such as Ang III and Ang IV, which may in themselves possess biological activity (Ferrario and Iyer, 1998, de Gasparo et al., 2000). (see Fig. 1 and Section 2.4).
Fig. 1.
Summary of the RAS incorporating the Ang peptide family and physiological effects mediated via ATR subtypes. Under the classical RAS schema, Ang II is produced, via renin and ACE, to act with equal affinity on two ATR subtypes, AT1R and AT2R (large arrows). However, it is now appreciated that a number of breakdown products of Ang II, namely Ang (1–7), Ang III and Ang IV, exert their own unique effects that are distinct (and often opposite) to those of Ang II. Such effects are often mediated via newly recognized receptors such as MasR for Ang (1–7) and AT4R (also known as IRAP) for Ang IV, or additionally via AT2R stimulation. ACE2 is also a new pathway for the formation of Ang (1–7). Newly identified Ang receptor binding proteins associated with different ATR subtypes may also modify ATR activation. Thus, over-stimulation of AT1R (and (P)RR) by Ang II, which can contribute to a plethora of cardiovascular disease processes, may be counter-regulated by a number of non-AT1R mechanisms. Most notably, AT2R stimulation usually causes opposing effects to AT1R, as indicated. It is also likely that the MasR exerts a similar counter-regulatory role, whereas the evidence is more preliminary and speculative for AT4R/IRAP. In terms of mediators, Ang II itself stimulates AT2R whereas the shorter Ang peptides stimulate their cognate receptors and possibly also AT2R.
1.2. Angiotensin receptors and peptides
Based on the differing affinities of several natural and synthetic ligands, two major subtypes of Ang receptors have been identified and cloned, the Ang subtype 1 receptor (AT1R) and subtype 2 receptor (AT2R) (de Gasparo et al., 2000). Both AT1 and AT2R possess similar affinity for Ang II (Timmermans et al., 1991, Griendling et al., 1996), however, affinity for entities such as the biphenyl tetrazoles, losartan and candesartan, and compounds such as PD123319 and CGP42112, vary significantly. For example, the AT1R antagonist, losartan, displays ~10 000-fold greater affinity for the AT1 than the AT2R (Timmermans et al., 1991), and candesartan cilexetil, which in vivo is metabolised to candesartan, has 80 times higher affinity for the AT1R than losartan (Shibouta et al., 1993, Morsing, 1999). On the other hand, the tetrahydroimidazopyridine compounds, PD123177 and PD123319, have been shown to be 3500-fold more selective for the AT2R than the AT1R (Timmermans et al., 1991, Timmermans et al., 1993), while CGP42112 exhibits over 1000-fold selectivity for AT2R (Whitebread et al., 1989).
All of the classical actions of Ang II, including vasoconstriction, effects on fluid and electrolyte homeostasis, and influences on cellular growth and differentiation, have been shown to be due to stimulation of AT1R located on the plasma membrane of cells. In addition, there has been much recent interest in the cardiovascular effects of other peptides of the RAS, such as Ang 1–7, Ang III and Ang IV, which are formed by various amino- and endopeptidases (see Fig. 1), and are believed to influence cardiovascular function both due to modulation of classical Ang II-mediated actions, and by exerting their own specific biological actions via individual cognate receptors.
For example, the G-protein-coupled receptor encoded by the Mas protooncogene was identified as a functional receptor for Ang (1–7) (Santos et al., 2003). A number of cardiovascular effects have been attributed to Ang (1–7) (Santos et al., 2000) acting via MasR (Ferrario et al., 2005b, Santos and Ferreira, 2007) although there is evidence that Ang (1–7) can act independently of Ang (1–7)/MasR (Walters et al., 2005). As will be discussed later, the resurgence of interest in the effects of Ang (1–7) has been buoyed by the discovery of angiotensin converting enzyme 2 (ACE2). There is also evidence for a binding site which recognizes Ang IV, but which has no affinity for either losartan or PD123319 (Wright et al., 1993), and has been designated the angiotensin II type 4 receptor (AT4R) (Swanson et al., 1992, Wright et al., 1995). However, this site has recently been purified from bovine adrenal membranes, and identified as the enzyme, insulin-regulated aminopeptidase (IRAP) (Albiston et al., 2001). IRAP has been localised in tissues such as heart, lung, kidney, and brain, and ligand binding results in effects such as enhancement of cognitive function (Wright et al., 1993, Wright et al., 1999) modulation of blood flow (Swanson et al., 1992, Coleman et al., 1998), increased natriuresis (Ardaillou & Chansel, 1997) and inhibition of cardiomyocyte hypertrophy (Baker and Aceto, 1990, Baker et al., 1992).
Thus, at present, there are 4 ATR subtypes (see Fig. 1). However, for the purpose of this review, the following discussion will predominantly focus on the AT2R and its functional relevance in cardiovascular disease.
1.3. Angiotensin AT2R
Like the well characterised AT1R, the AT2R is a member of the 7 transmembrane spanning receptor family. The AT2R shares 34% sequence homology to the AT1R (Guthrie, 1995, Unger et al., 1996), and is expressed abundantly in foetal tissue, but levels decline rapidly after birth. Consequently, it was originally believed that the AT2R was primarily involved in cellular growth and differentiation (Capponi, 1996, Akishita et al., 1999, Yamada et al., 1999). In adults, the AT2R has been localised to the heart, kidney, adrenal gland, brain, uterus, pancreas, retina, skin, and both endothelial and vascular smooth muscle cells (VSMCs) of the vasculature (Viswanathan and Saavedra, 1992, Leung et al., 1997, Nora et al., 1998, Allen et al., 1999, Wang et al., 1999, Wheeler-Schilling et al., 1999, Roulston et al., 2003), and importantly, expression is also increased in numerous cardiovascular pathologies (see Section 3 and Table 2).
Table 2.
Status of AT2R expression and function in different cardiovascular pathologies
| Disease/setting | AT2R expression (direction of change; localization) | Function | References |
|---|---|---|---|
| Hypertension (vessels) | ↑ aorta SHR, 2K1C, banding (↑ young, ↓ adult- mesenteric SHR) | Anti-hypertrophic (vasoconstriction) | 14, 24, 28, 34, 39, 41, 42 |
| Normotension (vessels) | Present VSMC, EC | Vasodilatation | |
| LVH | ↑'s and ↓'s reported | Hypertrophic/anti-hypertrophic | 18, 26, 33, 35 |
| Heart failure | Mainly ↑ infarcted heart (fibrotic regions) | Anti-growth | 2, 7, 12, 16, 22, 35, 38 |
| Cardiac fibrosis | Mainly ↑ | Anti-fibrotic | 6, 35, 38 |
| Stroke | ↑ infarcted brain | Neuroprotective | 19, 43 |
| Renal disease | Mainly ↑ | Renoprotective | 4, 8 |
| Pronatriuretic | |||
| Diabetes: Type 1 | ↑'s & ↓'s kidney, ↑ heart, ↑ vasculature | Renoprotective | 1, 5, 10, 11, 17, 20, 29, 30, 37 |
| Type 2 | ↑ kidney (tubular) | Pronatriuretic | |
| Atherosclerosis | ↑ plaque and vessel wall | Vasoprotective/anti-growth | 15, 36, 44 |
| Neointimal formation | ↑ neointima | Vasoprotective/anti-growth | 21, 23, 32, 40 |
| Females | Mainly ↑ vasculature, kidney | Vasoprotective | 3, 9, 23, 27, 31 |
| Aging | ↑ heart, aortic and mesenteric arteries | (Vasoconstriction-mesentery) | 13, 25, 39 |
References: 1. Arun, K.H., et al. (2004). J Hypertens, 22, 2143–52. 2. Asano, K., et al. (1997). Circulation, 95, 1193–200. 3. Baiardi, G., et al. (2005). Regul Pept, 124, 7–17. 4. Bautista, R., et al. (2001). Hypertension, 38, 669–73. 5. Bonnet, F., et al. (2002). J Hypertens, 20, 1615–24. 6. Brink, M., et al. (1996). J. Mol. Cell. Cardiol., 28, 1789–99. 7. Busche, S., et al. (2000). Am. J. Pathol., 157, 605–11. 8. Cao, Z., et al. (2002). J Am Soc Nephrol, 13, 1773–87. 9. de P Rodrigues, S.F., et al. (2006). Life Sci, 78, 2280–5. 10. Hakam, A.C. & Hussain, T. (2005). Hypertension, 45, 270–5. 11. Hakam, A.C., et al. (2006). Am J Physiol Renal Physiol, 290, F503–8. 12. Haywood, G.A., et al. (1997). Circulation, 95, 1201–6. 13. Heymes, C., et al. (1998). Endocrinology., 139, 2579–87. 14. Hiyoshi, H., et al. (2004). Hypertension, 43, 1258–63. 15. Johansson, M.E., et al. (2005). J Hypertens, 23, 1541–9. 16. Lee, S., et al. (2001). Cardiovasc Res, 51, 131–9. 17. Li, C., et al. (2005). Cardiovasc Drugs Ther, 19, 105–12. 18. Lopez, J.J., et al. (1994). Am J Physiol, 267, H844–52. 19. Lu, Q., et al. (2005). Neuroreport, 16, 1963–7. 20. Mezzano, S., et al. (2003). Kidney Int Suppl, S64–70. 21. Nakajima, M., et al. (1995). Proc. Natl. Acad. Sci. U. S. A., 92, 10663–7. 22. Nio, Y., et al. (1995). J Clin Invest, 95, 46–54. 23. Okumura, M., et al. (2005). Hypertension, 46, 577–83. 24. Otsuka, S., et al. (1998). Hypertension, 32, 467–72. 25. Pinaud, F., et al. (2007). Hypertension, 50, 96–102. 26. Regitz-Zagrosek, V., et al. (1995). Circulation, 91, 1461–71. 27. Sampson, A.K., et al. (2008). Hypertension, 52, 666–71. 28. Savoia, C., et al. (2006). J Hypertens, 24, 2417–22. 29. Savoia, C., et al. (2007). Hypertension, 49, 341–6. 30. Sechi, L.A., et al. (1994). Diabetes, 43, 1180–4. 31. Silva-Antonialli, M.M., et al. (2004). Cardiovasc Res, 62, 587–93. 32. Suzuki, J., et al. (2002). Circulation, 106, 847–53. 33. Suzuki, J., et al. (1993). Circ Res, 73, 439–47. 34. Touyz, R.M., et al. (1999). Hypertension, 33, 366–72. 35. Tsutsumi, Y., et al. (1998). Circ Res, 83, 1035–46. 36. Vinh, A., et al. (2008). Cardiovasc Res, 77, 178–87. 37. Wehbi, G.J., et al. (2001). Am J Physiol Renal Physiol, 280, F254–65. 38. Wharton, J., et al. (1998). J Pharmacol Exp Ther, 284, 323–36. 39. Widdop, R.E., et al. (2008). Clin Exp Pharmacol Physiol, 35, 386–90. 40. Wu, L., et al. (2001). Circulation, 104, 2716–21. 41. Yayama, K., et al. (2004). J Pharmacol Exp Ther, 308, 736–43. 42. You, D., et al. (2005). Circulation, 111, 1006–11. 43. Zhu, Y.Z., et al. (2000). Neuroreport, 11, 1191–4. 44. Zulli, A., et al. (2006). J Histochem Cytochem, 54, 147–50.
Signaling mechanisms differ markedly to those associated with AT1R (Touyz & Berry, 2002). The topic of AT2R signalling has recently been extensively reviewed (Hannan and Widdop, 2004, Kaschina and Unger, 2003, Steckelings et al., 2005), and so will only be mentioned briefly. One of the most frequently reported signaling pathways for AT2R stimulation in vasculature has been increased production of cyclic guanine 3′,5′-monophosphate (cGMP), nitric oxide (NO) and bradykinin (BK), as was first described by Siragy and Carey, 1996, Siragy and Carey, 1997: see Widdop et al. (2003) for review. In cultured endothelial (Chaki and Inagami, 1993, Caputo et al., 1995, Saito et al., 1996, Pueyo et al., 1998), PC12W (Zhao et al., 2003), and N1E-115 neuroblastoma cells, and in isolated blood vessels from animal (Boulanger et al., 1995, Seyedi et al., 1995, Thorup et al., 1998, Thorup et al., 1999, Hannan et al., 2003), and human (Batenburg et al., 2004) studies, Ang II has been shown to increase NO and/or cGMP levels via AT2R and/or AT1R stimulation. Cross talk between AT2R and AT1R is also implicated by the recent finding that NO/cGMP activates a cGMP-dependent protein kinase causing decreased RhoA activity and consequently decreased AT1R-mediated vasoconstriction (Savoia et al., 2006a, Savoia et al., 2005). Involvement of BK and NO/cGMP signalling pathways has also been demonstrated following AT2R stimulation in vivo, in the vasculature of rats (Siragy and Carey, 1996, Siragy and Carey, 1997, Siragy and Carey, 1999, Siragy et al., 2000, Siragy et al., 2001, Walters et al., 2005), and mice (Tsutsumi et al., 1999), and in cardiac growth responses (Liu et al., 1997, Bartunek et al., 1999).
Several signaling pathways involve activation of protein phosphatases, whose function is to dephosphorylate and thus inactivate MAP kinases such as ERK-1 and ERK-2, resulting in inhibition of both AT1R- and other ‘classical’ growth factor-mediated pathways involved in cellular growth and differentiation (Stoll et al., 1995, Tsuzuki et al., 1996). Increased PTPase activity following AT2R stimulation has been demonstrated in PC12W cells (Bottari et al., 1992, Brechler et al., 1994), N1E-115 neuroblastoma cells (Nahmias et al., 1995) and R3T3 fibroblasts (Tsuzuki et al., 1996). In PC12W cells and adult rat ventricular myocytes, induction of the PTPase MKP-1 is G-protein-dependent (Horiuchi et al., 1997, Fischer et al., 1998). However, studies performed in N1E-115 cells demonstrated that AT2R-mediated activation of PTPase did not involve a G-protein, but rather induced the soluble PTPase, SHP-1 (Bedecs et al., 1997). Thus Ang II stimulation of AT2R may result in activation of PTPases via both G-protein-dependent and -independent mechanisms. In addition, stimulation of serine/threonine phosphatases, and subsequent inhibition of ERK-1 and -2, appears to be particularly important in neuronal tissue, in which activation of the serine/threonine phosphatase PP2A has been demonstrated to cause opening of a delayed rectifier K+ channel (Kang et al., 1994), upregulation of AT2R mRNA, and increased apoptosis (Shenoy et al., 1999).
AT2R stimulation may also activate lipid-signalling pathways. Ang II stimulation of neonatal rat cardiomyocytes (Lokuta et al., 1994), rabbit proximal tubule epithelia (Jacobs & Douglas, 1996), and cultured neurons (Zhu et al., 1998), increased PLA2 activity and arachadonic acid (AA) release. Long-term stimulation of AT2R by Ang II has also been shown to increase synthesis of ceramides, which may then activate stress kinases and caspases involved in the induction of apoptosis (Gallinat et al., 1999, Lehtonen et al., 1999).
2. Emerging aspects of renin angiotensin system
2.1. Renin/prorenin
It is now recognized that renin exists in two forms, mature renin which can actively cleave angiotensinogen, and the proenzyme, prorenin. Prorenin lacks enzymatic activity, but is transformed into mature renin following cleavage of the 43-amino acid N-terminal propeptide which covers the enzymatic cleft and prevents angiotensinogen access and subsequent cleavage. Interestingly, although synthesized in a limited number of tissues, it has been suggested that prorenin represents up to 90% of total plasma renin in normal subjects, and in certain physiological and pathological conditions, such as pregnancy and diabetes, can circulate at 100-fold higher concentrations than mature renin (Danser et al., 1998). This excess circulating prorenin cannot be activated in the circulation, which has sparked research into the existence of a renin/prorenin receptor. Indeed it was demonstrated that a major source of renin/prorenin in cardiac tissue is due to sequestration and uptake from the circulation (Danser et al., 1994, Muller et al., 1998) suggesting a functional role for prorenin.
A specific renin/prorenin receptor (P)RR was first identified in cultured human mesangial cells (Nguyen et al., 2002), and has since been found to be expressed at relatively high levels in rat and human heart, brain, placenta and adipocytes, and at lower levels in kidney and liver (Danser and Deinum, 2005, Nguyen, 2006, Achard et al., 2007). The (P)RR consists of 350 amino acids, possesses a single transmembrane domain, and exclusively binds renin/prorenin. Binding of renin/prorenin to (P)RR has been shown to have 2 major consequences: increased catalytic activity of renin/prorenin, and activation of (P)RR-mediated signal transduction cascades. Binding of renin to its receptor increases angiotensinogen conversion to Ang I by five-fold (Nguyen et al., 2002), and prorenin, which is virtually inactive in solution, also displays enzymatic activity following receptor binding. This activation of prorenin is not due to proteolysis of the pro-segment which covers the catalytic site, but rather it has been hypothesized that prorenin undergoes a conformational change when bound to the (P)RR, which unmasks the catalytic site and thus activates the proenzyme without removal of the propeptide (Nguyen et al., 2002). Importantly, increased renin/prorenin activity at the cell surface may result in greater Ang I and Ang II levels in the immediate vicinity of Ang receptors and thus increase efficiency of Ang II binding. In addition, receptor-bound renin/prorenin appears to induce intracellular signaling via activation of the MAP kinases, ERK1/2, which is distinct from Ang II-mediated effects (Nguyen et al., 2002). Thus activation and potentiation of renin/prorenin enzymatic activity, together with specific (P)RR-mediated signaling, could conceivably have striking effects on cardiovascular regulation.
In light of these data, recent studies have investigated the role of renin/prorenin and its receptor in physiological and pathophysiological conditions. In particular, a role for the (P)RR in animal models of diabetes and hypertension has recently been identified. Ichihara et al., used a decoy peptide which corresponds with the handle region in the prorenin pro-segment (handle region peptide = HRP), to competitively prevent non-proteolytic activation of prorenin. This group reported that HRP treatment prevented the development of diabetic nephropathy in streptozotocin (STZ)-induced diabetic rats (Ichihara et al., 2004), and also decreased perivascular fibrosis and left ventricular hypertrophy (LVH) in spontaneously hypertensive stroke prone rats (Ichihara et al., 2006). However, several groups have since demonstrated that HRP is unable to prevent renin/prorenin binding and subsequent Ang I generation in mouse VSMCs (Batenburg et al., 2008), or to inhibit renin/prorenin-induced ERK1/2 phosphorylation in cultured VSMCs (Feldt et al., 2008b), or monocytes (Feldt et al., 2008a), casting doubt on the validity of HRP as a peptide to prevent prorenin activation. Similarly, recent studies have been unable to confirm the beneficial effects of HRP in vivo, and thus saw no improvement in end-organ damage following HRP administration in double-transgenic rats overexpressing renin and angiotensinogen genes (Feldt et al., 2008b), or in renovascular hypertensive 2-kidney, 1-clip (2K1C) rats (Muller et al., 2008).
Nevertheless, over-expression of (P)RR in rats increased systolic blood pressure and heart rate which was shown to gradually worsen with increased aged (Burckle et al., 2006), lending weight to the notion of (P)RR-mediated cardiovascular regulation. Although the exact mechanisms by which these effects occur require further investigation, it appears that they are not simply due to increased synthesis of Ang II and subsequent potentiation of Ang II-mediated effects. Huang et al. (2006), demonstrated that treatment of cultured mesangial cells with human and rat recombinant renin increased levels of the pro-fibrotic cytokine transforming growth factor, TGF-β1, and that this effect was not influenced by an inhibitor of the enzymatic activity of renin (RO 42-5892), an AT1R antagonist (losartan) or an ACE inhibitor (enalapril), but was significantly inhibited by renin siRNA (Huang et al., 2006). Furthermore, evidence of a direct interaction between (P)RR and the transcription factor, promyelocytic zinc finger protein (PLZF), has recently identified a novel signal transduction cascade involving renin/(P)RR/PLZF, activation of which results in cellular proliferation via upregulation of PI3K-p85α (Schefe et al., 2006). Interestingly, PLZF has also been found to associate with AT2R in the heart (Senbonmatsu et al., 2003), and such an interaction has been suggested as explanation for the AT2R-mediated cardiac growth-promoting effects deduced from several AT2R knockout studies (Senbonmatsu et al., 2000, Ichihara et al., 2001, Ichihara et al., 2002) (see Section 3.2).
Recently the non-peptide renin inhibitor, aliskiren, was approved for the treatment of hypertension. Animal and clinical studies have revealed striking depressor effects of aliskiren (Jensen et al., 2008). More recently, aliskiren has proven to be beneficial in several pathophysiological settings including hypertension and diabetic nephropathy (Feldman et al., 2008), cardiac remodelling and fibrosis (Pilz et al., 2005, Whaley-Connell et al., 2008) as well as atherosclerosis (Lu et al., 2008). Moreover, Nussberger et al. (2008) reported that in comparison with anti-hypertensive treatments including AT1R blockade, a β-blocker and calcium channel antagonist, aliskiren was shown to have equally potent blood pressure lowering effects as well as anti-atherosclerotic effects. The history and efficacy of aliskiren in the treatment of hypertension has recently been reviewed by Jensen et al. 2008).
Therefore, these recent studies support a role for the (P)RR in pathological states and future studies using (P)RR knockout mice may provide more insight into the therapeutic potential of aliskiren and the development of (P)RR inhibitors.
2.2. ACE2
ACE2 is a recently discovered homologue of ACE, with 56% similar homology with the N-terminal domain of ACE (Donoghue et al., 2000, Tipnis et al., 2000, Vickers et al., 2002). In contrast to the wide distribution of ACE, ACE2 expression was initially thought to be restricted to endothelial and VSMCs of the heart and kidney (Donoghue et al., 2000, Tipnis et al., 2000, Crackower et al., 2002) although a more widespread distribution is emerging (see Hamming et al., 2007). Unlike the dipeptidyl carboxypeptidase ACE, ACE2 cleaves a single amino acid from the C-termimal of the peptide substrate. Thus, ACE2 cleaves Ang I to the inactive Ang (1–9), which may then be converted to the vasodilator peptide, Ang (1–7), by ACE. More importantly, ACE2 may also directly metabolise Ang II to form Ang (1–7), and this reaction occurs at a faster rate than the formation of Ang (1–9) from Ang I (Carey and Siragy, 2003, Rice et al., 2004). Thus, ACE2 may counter-regulate ACE activity by simultaneously decreasing Ang II levels and increasing Ang (1–7) formation (Hamming et al., 2007). While ACE2 shares significant sequence homology with ACE, it is not sensitive to ACE inhibitors.
Early investigations regarding ACE2 suggested that ACE2 mRNA was increased in both human and animal models of heart failure (Goulter et al., 2004, Burrell et al., 2005) and decreased in genetically hypertensive rats (Crackower et al., 2002), sparking interest that ACE2 may play an important modulatory role on the RAS in certain cardiovascular pathologies. Furthermore, Ishiyama et al. (2004) have reported that ACE2 mRNA expression was increased by AT1R inhibition following myocardial infarction (MI), and spironolactone treatment also increased ACE2 in heart failure patients (Keidar et al., 2005). Both AT1R blockade and ACE inhibition increased cardiac ACE2 expression in Lewis rats, whereas activity of the enzyme was increased by AT1R inhibition only (Ferrario et al., 2005a). However, in direct contrast, Batlle et al. (2006) found no evidence of increased ACE2 activity in biopsies of human heart failure patients. Such inconsistency in results regarding ACE2 expression and activity may be at least partially due to the methods employed, as only poor correlation between ACE2 mRNA and both protein expression and activity has recently been reported in diabetic mice (Wysocki et al., 2006).
Initial studies regarding ACE2 function performed in ACE2 knockout mice (Crackower et al., 2002), resulted in severe impairment of cardiac contractility, which was normalized in double ACE/ACE2 knockout mice, suggesting a counter-regulatory function of ACE2 on the RAS (Crackower et al., 2002). However, several alternative lines of ACE2 knockout mice have since been generated, which exhibit modestly elevated basal blood pressure and normal cardiac phenotype, despite significantly elevated circulating Ang II levels (Gurley et al., 2006). Furthermore, normal cardiac function has been shown in ACE2 knockout mice (Yamamoto et al., 2006), although ACE2 deletion did result in a greater hypertrophic response to pressure overload compared to wild type mice. Similarly, in normotensive rats, ACE2 over-expression induced by lentiviral administration of ACE2 mRNA, did not affect basal cardiac function, however, transgenic animals displayed significantly blunted cardiac hypertrophic and fibrotic responses to Ang II infusion compared to control animals (Huentelman et al., 2005). In an analogous study performed by the same group, ACE2 over-expression in SHR and WKY rats, reduced blood pressure (BP), decreased left ventricular wall thickness, increased left ventricular end diastolic pressure, and attenuated cardiac perivascular fibrosis in hypertensive animals only (Diez-Freire et al., 2006). These data suggest that cardiac phenotype is not solely dependent on ACE2 expression, but support the notion of a cardioprotective role for ACE2 in situations of cardiac stress.
Interestingly, all strains of ACE2 knockout mice reported to date have increased plasma and tissue levels of Ang II, due to both decreased metabolism of plasma Ang II, and increased tissue synthesis as a result of elevated Ang I, suggesting an important function of ACE2 to regulate Ang II levels (Crackower et al., 2002, Gurley et al., 2006, Yamamoto et al., 2006). In addition, ACE2 is known to undergo proteolytic shedding of its extracellular ectodomain to release a soluble form of ACE2 in plasma that maintains catalytic activity (Lambert et al., 2005, Warner et al., 2005). Moreover, ACE2 acts as a receptor for the severe-acute respiratory syndrome (SARS) coronavirus (Li et al., 2003) where it may serve a protective role (Hamming et al., 2007).
2.3. Ligand-independent effects of AT2R
Although the AT2R is a member of the G-protein-coupled receptor (GPCR) superfamily, it is well recognized that AT2R signal transduction does not always occur via classic G-protein-dependent pathways. Recent studies of GPCR modulation and function, with particular focus on areas such as constitutive activity, formation of homo- and hetero-oligimers, and interaction with receptor-associated proteins have afforded fresh insights into GPCR signalling, and provide information that may assist in resolving previous controversies regarding AT2R-mediated function.
2.3.1. Constitutive activity
AT2R may possess constitutive activity, as several investigators have reported that AT2R expression exerts cellular effects without ligand binding. Over-expression of AT2R in cultured fibroblasts, CHO cells and VSMCs, caused apoptosis via p38 MAPK and caspase-3 signalling pathways (Miura & Karnik, 2000). Moreover, the degree of apoptosis was not sensitive to either Ang II or PD123319, but showed significant correlation with the level of AT2R protein expression (Miura & Karnik, 2000). In addition, over-expression of AT2R in cultured VSMC has also been shown to downregulate AT1aR in a ligand-independent manner (Jin et al., 2002). This effect on AT1aR expression was suggested to be due to potentiated BK/NO signaling, as not only was BK and iNOS protein increased by AT2R over-expression, but both the B2R antagonist, HOE 140, and the NO synthase inhibitor, l-NAME, ameliorated the decrease in AT1aR expression (Jin et al., 2002). The same group also reported a similar downregulation of AT1a and TGF-β receptor expression in VSMC from WKY, which was associated with reduced basal and Ang II-induced DNA synthesis (Su et al., 2002). Interestingly, AT1a and TGF-β receptor expression, and both basal and Ang II-stimulated markers of cellular growth, were not altered by AT2R over-expression in VSMCs from SHR, suggesting a disturbance of gene regulation in this model of genetic hypertension, which was suggested to contribute to the exaggerated growth of VSMCs from SHR (Su et al., 2002).
A recent study by D'Amore et al. (2005) also showed constitutive activity of AT2R in the context of cardiac growth. In this study, transfection of increasing titres of AT2R in cultured neonatal cardiomyocytes resulted in cellular hypertrophy, which was not influenced by AT2R ligand binding, and also did not affect AT1R-mediated hypertrophic signaling, providing evidence for parallel stimulatory roles of AT1R and AT2R in cardiac hypertrophy. Interestingly, Falcon et al. utilised microarray expression analysis to identify genes whose expression was regulated by AT2R expression. This group identified ~5224 genes which were regulated independently of AT2R-ligand binding, with proposed functions on cell migration, protein processing, intracellular signaling and DNA repair (Falcon et al., 2005). Moreover, it was found that AT2R over-expression inhibited human coronary arterial endothelial cells migration in a ligand-independent manner, albeit in an experimental system in which AT2R expression was increased to levels much greater than those which occur in either physiological or pathophysiological settings (Falcon et al., 2005).
2.3.2. Receptor dimerisation
It is well established that GPCRs are susceptible to receptor dimerisation, and that such an interaction between receptors may affect both receptor activation and signaling, as has been well described for AT1R (Oro et al., 2007). Like AT1R which are reported to form heterodimers (AbdAlla et al., 2000, AbdAlla et al., 2001b), both hetero- and homo-oligomers of AT2Rs have been reported. AT2R were first shown to directly bind to AT1R in PC12W cells and fetal fibroblasts, and subsequent stimulation of cells with Ang II resulted in decreased expression of the of AT1R-associated G-proteins, Gαi/o and Gαq/11, suggesting that such an interaction between receptor subtypes may contribute to AT2R-mediated antagonism of AT1R (AbdAlla et al., 2001a). Furthermore, this effect on AT1R-mediated signaling was independent of AT2R stimulation, as PD123319 had no influence on Gαi/o and Gαq/11 activation (AbdAlla et al., 2001a). In the same study, AT2–AT1R heterodimers were decreased in myometrial biopsies from pregnant compared to non-pregnant women, and expression levels paralleled levels of AT1R-mediated signaling, demonstrating a functional relevance of heterodimerisation. However, a study in which cultured cardiomyocytes were transfected with AT1R and AT2R failed to detect any influence of AT2R over-expression on AT1R signaling pathways (D'Amore et al., 2005), suggesting that further confirmation of AT1/AT2R heterodimerisation is required.
AT2Rs have also recently been shown to form heterodimers with BK receptors (B2R) (Abadir et al., 2006). Similarly to that seen by AT1–AT2R dimerisation in PC12W cells, Abadir et al. found that the rate of formation of AT2–B2R heterodimers was influenced by the level of expression of both receptors, and was not dependent on ligand binding. Furthermore, conditions which maximized AT2–B2R dimer expression also resulted in maximal NO and cGMP production (Abadir et al., 2006), demonstrating that heterodimers are indeed functional.
In addition, homooligomerisation due to disulfide bonding between AT2R was shown to occur in transfected CHO cells, and was localised to the plasma membrane (Miura et al., 2005). In this cell line, such an interaction between AT2R resulted in apoptosis, as was indicated by increased caspase3-like activity. Interestingly, apoptosis was unaffected by treatment with either Ang II or PD123319, but was prevented by inhibition of disulfide bonding by dithiothreitol, suggesting that not only was AT2R-mediated apoptosis ligand-independent, but that homodimerisation of the AT2R was essential to the observed effect (Miura et al., 2005).
2.3.3. Angiotensin receptor binding proteins
GPCRs are now thought to interact with a range of other accessory proteins (see Bockaert et al., 2003). Recent studies have identified and sequenced 2 distinct AT1R-associated binding proteins, ARAP1 and ATRAP, which either promote recycling of the AT1R to the plasma membrane (ARAP1), or induce receptor internalization (ATRAP) (Daviet et al., 1999, Guo et al., 2003, Lopez-Ilasaca et al., 2003). Renal-specific over-expression of ARAP1 in mice resulted in hypertension, decreased urine output, and renal hypertrophy (Guo et al., 2006), and as these effects were abrogated by AT1R inhibition, indicate a functional role for ARAP1 in potentiation of AT1R signaling. Conversely, ATRAP has been found to be co-localised with AT1R in renal tubules (Tsurumi et al., 2006), and in vitro studies have demonstrated that over-expression of this protein decreases AT1R-mediated signaling and cellular proliferation, thus suggesting that ATRAP may act as a negative regulator of AT1R signaling (Cui et al., 2000, Lopez-Ilasaca et al., 2003). This field has recently been reviewed (Mogi et al., 2007).
Similar proteins that modulate AT2R expression at the cell membrane have also been identified. ATIP1 (AT2-interacting protein 1) is the product of the human Mitochondrial Tumour Suppressor gene, MTUSI, and, in contrast to AT2R, is widely expressed throughout the body suggesting both AT2R-dependent and -independent functions (Nouet et al., 2004). Alternatively, this mismatch in ATIP/AT2R expression could represent an important mechanism by which ATIP modulates AT2R function in situations of pathological re-expression of AT2R. Binding of endogenous ATIP1 to the C-terminal tail of the AT2R inhibited mitogenic pathway signalling, an effect which was potentiated by ligand activation, but was also present in the absence of such stimuli (Nouet et al., 2004). An identical protein, designated ATBP50 (AT2R-binding protein of 50 kDa), has also been identified in mice. Binding of the Golgi membrane-associated ATBP50 to AT2R was shown to promote AT2R cell-surface expression, and this effect was prevented by downregulation of ATBP50 by use of siRNA (Wruck et al., 2005). In mouse neuroblastoma N1E-115 cells, stimulation of AT2R inhibited EGF-induced ERK1/2 activation and cellular proliferation, and interestingly, not only was this effect blocked by PD123319, but it was also significantly inhibited by ATBP50 siRNA, indicating a functional modulatory interaction between ATBP50 and AT2R-mediated anti-mitogenic signalling (Wruck et al., 2005).
By contrast, AT2R also exert growth-promoting effects when coexpressed with the transcription factor PLZF (Senbonmatsu et al., 2003). PLZF is highly expressed in cardiac tissue, and was found to colocalise with AT2R in the cell membrane upon Ang II stimulation. Within 30 minutes of Ang II administration, AT2R and PLZF were shown to translocate to the internal compartment, and nuclear PLZF induced p70S6 kinase activation (essential for protein synthesis), via increased expression of the phosphatidylinositol-3 kinase p85α subunit (Senbonmatsu et al., 2003).
Taken collectively, these recent data on interacting proteins demonstrate that AT2R-mediated growth effects may vary dramatically depending on the presence and type of AT2R-binding proteins, and highlights the importance of future determination of AT2R-modulatory factors, in addition to AT2R expression, in the elucidation of AT2R function in a given tissue (e.g., see Section 3.2 for differential cardiac modulation).
2.4. Angiotensin peptide fragments
Another emerging concept of the RAS is the unique roles of shorter Ang II peptide fragments, such as Ang (1–7), Ang III and Ang IV. These peptides were initially thought to be inactive breakdown products of Ang II, however, they are now recognized as active components of the RAS, often with their own unique biological profile.
2.4.1. Ang (1–7)
There has been a resurgence of interest in the actions of the N-terminal heptapeptide Ang (1–7) since the discovery of ACE2 and the realization that this peptide can be efficiently produced by this additional pathway. As already mentioned, Ang (1–7) can be formed directly from Ang I via neutral endopeptidase. Alternatively, ACE2 is a carboxypeptidase that cleaves the C-terminal amino acid from either Ang I or Ang II to form Ang (1–9) or Ang (1–7), respectively. Ang (1–7) evokes a range of acute central and peripheral effects, the most prominent being vasodilatation, inhibition of VSMC proliferation, vasopressin release and fluid and electrolyte homeostasis (Santos et al., 2000). Interestingly, the mechanism of action of Ang (1–7) is not always straightforward since it can mediate multiple effects via a variety of ATRs including AT1R, AT2R or an Ang (1–7)-sensitive site that is recognized by the analogue A-779 (for review, see Santos et al., 2000). More recently, it was postulated that Ang (1–7) is the endogenous ligand for the MasR, mainly on the basis of cardiovascular actions of Ang (1–7) being abolished in MasR-deficient mice (Santos et al., 2003, Pinheiro et al., 2004). Other studies have suggested that the MasR can heterodimerise with AT1R to inhibit the effects of Ang II (Kostenis et al., 2005). In addition, extracellular matrix (ECM) remodeling in the heart leading to collagen accumulation and impaired heart function was seen in MasR-deficient mice (Santos et al., 2006). In the last few years, a number of important chronic effects of Ang (1–7) have been identified as a result of exogenous infusion of the peptide. In particular, Ang (1–7) exerted anti-growth and anti-fibrotic effects in rat models of MI, neointimal formation and fibrosis (Strawn et al., 1999, Loot et al., 2002, Benter et al., 2006, Grobe et al., 2006, Grobe et al., 2007). The ATR subtype responsible for these cardiovascular protective effects was examined in only one of the afore-mentioned studies, in which it was only partially mediated by the MasR (Grobe et al., 2007), and AT2R involvement was not examined. By contrast, we found that Ang (1–7) caused vasodepressor effects, during AT1R blockade, utilizing BK and NO pathways, that were abolished by AT2R blockade but not Ang (1–7) receptor blockade (Walters et al., 2005). Analogous findings of specific AT2R- but not MasR-sensitivity to Ang (1–7) have recently been reported (Lara Lda et al., 2006). The likely physiological relevance of these findings is underscored by the fact that the cleavage of Ang (1–7) by ACE2 from Ang II provides a double effect, i.e. the shunting towards Ang (1–7) promotes the formation of a counter-regulatory peptide while reducing the levels, and thus action, of pro-excitatory Ang II (Hamming et al., 2007). Updated biochemical and functional aspects of Ang (1–7) have recently been reviewed (Ferrario et al., 2005b, Ferrario, 2006, Reudelhuber, 2006, Santos and Ferreira, 2007).
2.4.2. Ang III
The Ang (2–8) fragment, Ang III, is readily cleaved from Ang II via aminopeptidase A. Ang III is usually considered a less potent analogue of Ang II that exerts similar AT1R-mediated effects to the parent octapeptide (de Gasparo et al., 2000). This lack of potency of Ang III is not usually the case in the central nervous system where it is proposed that Ang III is the main mediator of centrally-mediated pressor effects of Ang II, following conversion from the latter (Reaux et al., 2001, Wright et al., 2003). However, this view has recently been challenged (Kokje et al., 2007). Interestingly, large bolus doses of Ang III were reported to exert a biphasic effect on BP in anaesthetized rats consisting of an initial pressor followed by depressor effect; the latter component being AT2R-mediated (Scheuer & Perrone, 1993). More recently, an AT2R-mediated vasodepressor effect of Ang III was unmasked during AT1R blockade in conscious SHR and involved NO and BK signaling pathways (Walters et al., 2003), in an identical manner to Ang (1–7) (Walters et al., 2005). Similarly, Padia et al.showed that Ang III evoked natriuretic effects in conscious rats via AT2R stimulation, and that inhibition of aminopeptidase N (and thus prevention of the conversion of Ang III to Ang IV), potentiated sodium excretion (Padia et al., 2006 , 2007). Furthermore, Ang III-mediated natriuresis was shown to involve the NO/BK cascade which this group has championed as being a hallmark of AT2R activation (Siragy and Carey, 1996, Siragy and Carey, 1997, Siragy and Carey, 1999, Padia et al., 2006).
2.4.3. Ang IV
Ang IV is formed by the cleavage of Ang III by aminopeptidase B or N. The role of Ang IV in cardiovascular pathophysiology, like other Ang peptide fragments, is emerging as a possible mediator in cardiovascular disease. Following identification of the AT4R as an aminopeptidase (Albiston et al., 2001), it has been proposed that AT4R ligands act by inhibiting IRAP catalytic activity, thereby reducing IRAP cleavage of substrates such as lys-bradykinin and vasopressin and prolonging their biological activity (Lew et al., 2003). The precise mechanisms of IRAP modulation by Ang IV is yet to be elucidated, however, the recent identification of a site of Ang IV interaction distinct from the active site of IRAP, suggests that Ang IV may utilise an allosteric mechanism to modulate IRAP activity (Caron et al., 2003). Currently, available data suggests that Ang IV exerts central effects on learning and memory (Chai et al., 2004). AT4R/IRAP is also upregulated in a rabbit balloon vascular injury model suggesting a possible role in vascular repair or remodeling (Moeller et al., 1999). On the other hand, Esteban et al. (2005) recently demonstrated that Ang IV activated NFκB and subsequently increased pro-inflammatory mediator expression in cultured VSMCs, via AT4R and independently of AT1R or AT2R. The latter two studies suggest Ang IV may play a role in diseases such as atherosclerosis or neointimal hyperplasia. However, Ang IV also induced vasodilatation, (Kramar et al., 1997, Patel et al., 1998, Chen et al., 2000) and has been shown to increase eNOS activation and subsequent NO release (Patel et al., 1998, Hill-Kapturczak et al., 1999) which represents a protective effect in the vasculature. In this context, we have recently shown that chronic treatment with Ang IV improved endothelial dysfunction in ApoE-deficient mice, and this vasoprotective effect most likely resulted from increased NO bioavailability (Vinh et al., 2008a, Vinh et al., 2008b). Clearly further research is required to elucidate the role of Ang IV in cardiovascular pathology.
2.5. Endogenous AT2R ligands?
Given the contextual actions of the various Ang peptide fragments, it is likely that there will be a greater scrutiny of the relative levels of various peptides in the future. For example, it was recently reported that hypercholesterolemia, evoked by a fat-enriched diet in LDL-deficient mice, stimulated production of many angiotensin peptide fragments, with the greatest increases seen in Ang II and Ang IV plasma concentrations (Ang (1–7) was not analysed) (Daugherty et al., 2004). Whether or not all of these peptide fragments have modulatory roles in the progression of atherosclerosis or other cardiovascular diseases remains to be seen. It is well known that plasma Ang II levels are elevated following AT1R blockade. However, in many instances, AT1R antagonists as well as ACE inhibitors both elevate plasma Ang (1–7) (Campbell et al., 1991, Campbell et al., 1994), and a similar finding has been made for plasma Ang IV levels in hypertensive patients (Shibasaki et al., 1999). Of course, these changes do not necessarily reflect changes in tissue levels which are notoriously difficult to measure. Nevertheless, it is well established that there is differential regulation of Ang II and related peptides in plasma versus tissue (Campbell et al., 1991, Campbell et al., 1994, Campbell et al., 1995, Campbell, 1996). Clearly, the plasma and tissue profiles of various Ang peptides are likely to be an important consideration for the full understanding of cardiovascular pathophysiology. New mas spectroscopy techniques (Jankowski et al., 2005) for detection and quantification of plasma and tissue Ang peptides simultaneously should help address this issue.
As is clearly evident from the preceding discussion, there are an increasing number of reports that Ang peptides other than Ang II can cause a range of cardiovascular effects via non-AT1R (see Table 1 ). However, there are often mismatches when comparing between functional and binding studies. For example, Ang (1–7) has relatively low binding affinity for AT2R relative to Ang II and Ang III (Rowe et al., 1995, Bouley et al., 1998), and a low affinity for AT1R (Rowe et al., 1995, Santos et al., 2003) and yet many of its reported physiological effects occur via interaction with these sites (see Santos et al., 2000). Indeed, we have reported that Ang (1–7) can lower BP via functional stimulation of AT2R (Walters et al., 2005). Moreover there are other functional and molecular data to support this claim that Ang (1–7) can stimulate AT2R (Jaiswal et al., 1993, Muthalif et al., 1998, Hansen et al., 2000, Heitsch et al., 2001, De Souza et al., 2004, Castro et al., 2005, Lara Lda et al., 2006) often despite low potency (Hansen et al., 2000). Much less data are available pertaining to Ang IV in this respect. This hexapeptide has a much lower binding affinity than Ang II for AT2R (Bouley et al., 1998, Hansen et al., 2000), unlike AT4R; nevertheless there are functional data indicating that Ang IV exerts vascular effects via AT2R (Loufrani et al., 1999, Faure et al., 2006). Indeed, our own recent data has demonstrated that chronic Ang IV treatment resulted in vasoprotective effects on endothelial function via both AT4R and AT2R stimulation (Vinh et al., 2008a, Vinh et al., 2008b), consistent with findings in a stroke model (Faure et al., 2006).
Table 1.
Endogenous Ang peptides and synthetic ligands and their relative affinity for various ATR subtypes
| Peptide structure | AT1R | AT2R | MasR | AT4R | |
|---|---|---|---|---|---|
| Endogenous ligands | |||||
| Ang II (1–8) | Asp-Arg-Val-Tyr-Ile-His-Pro-Phe | +++ | +++ | + | |
| Ang II (1–7) | Asp-Arg-Val-Tyr-Ile-His-Pro | + | ++ | +++ | |
| Ang III (2–8) | Arg-Val-Tyr-Ile-His-Pro-Phe | ++ | +++ | ||
| Ang IV (3–8) | Val-Tyr-Ile-His-Pro-Phe | + | + | +++ | |
| Synthetic ligands | |||||
| Sartan compounds | – | (+++) | |||
| PD123319 | – | (+++) | |||
| CGP42112 | Nicotinoyl-Tyr-Lys(Z-Arg)-His-Pro-Ile | +++ | |||
| Compound 21 | – | +++ | |||
| AVE 0991 | – | + | +++ | ||
| A-779 | Asp-Arg-Val-Tyr-Ile-His-D-Ala | (+++) | |||
| Divalinal-Ang IV | Valψ(CH2-NH2)-Tyr-Valψ(CH2-NH2)-His-Pro-Phe | (+++) | |||
+ indicates relative affinity for receptor based on binding and functional data (+++) indicates compounds that are antagonists.
On the other hand, there are substantial binding data suggesting that Ang III has 5–10 times higher affinity for AT2R over AT1R (Dudley et al., 1990, Timmermans et al., 1991, Rosenstrom et al., 2004), and indeed possesses higher affinity than Ang II itself at AT2R (Mukoyama et al., 1995, Bouley et al., 1998, Hansen et al., 2000). These binding data fit with the relatively few functional studies that have examined ATR subtype effects of Ang III, since both in vivo (Walters et al., 2003, Padia et al., 2006, Padia et al., 2007, Padia et al., 2008) and cellular signaling (Lorenzo et al., 2002) studies have implicated an AT2R-selective effect of this peptide.
Intriguingly, Ang (1–7) and Ang III both exerted AT2R-mediated effects (Walters et al., 2003, Walters et al., 2005, Padia et al., 2006) under conditions in which Ang II itself was ineffective (Gohlke et al., 1998), which raises the distinct possibility that these smaller Ang peptide fragments are endogenous AT2R ligands. Likewise, the effects of chronic Ang (1–7) or Ang IV infusions are strikingly different to those of Ang II itself (Grobe et al., 2006, Grobe et al., 2007, Vinh et al., 2008a, Vinh et al., 2008b). Therefore, at the very least, we should consider that Ang peptides other than Ang II have a major role in the cardiovascular system as endogenous non-AT1R ligands stimulating multiple ATR subtypes (see Table 1).
Of particular note, AT2R does not desensitize since concentration–response curves to AT2R-mediated vasorelaxation are highly reproducible, unlike AT1R-mediated contractile effects (Widdop et al., 2002). The reproducible nature of AT2R function is consistent with a lack of AT2R internalization previously reported (Mukoyama et al., 1995, Hein et al., 1997) and hence lack of desensitization. Cellular trafficking of both AT1 and AT2R expressed in human embryonic kidney 293 cells indicated that AT2R cell-surface binding was not altered after prolonged exposure to Ang II (Mukoyama et al., 1995), and fluorescently labeled AT2R were also not internalized after agonist exposure (Hein et al., 1997). By contrast, AT1R were rapidly internalized (Hein et al., 1997, Thomas, 1999), which is consistent with functional data (Widdop et al., 2002). Thus it is possible that, in the event of raised circulating or tissue levels of Ang peptides, such fragments may maintain efficacy at least at the AT2R. Indeed, the ability of failing human hearts to produce Ang (1–7) via ACE2 was directly correlated with AT2R, but not AT1R, density (Zisman et al., 2003), further supporting the concept of endogenous AT2R ligands modulating the effects of Ang II.
2.6. Novel ATR ligands
The Ang II derived peptide CGP42112 has long been the gold standard for determining functional and selective AT2R activity (Whitebread et al., 1989, de Gasparo et al., 2000). Recently, non-peptide selective AT2R agonists have been developed. Initially, a non-peptide agonist for both AT1R and AT2R was identified (Wan et al., 2004a) and this was soon followed by the first selective non-peptide AT2R agonist, Compound 21, which was active in 2 in vivo bioassays where it enhanced alkaline secretion from rat intestine and lowered BP in anaesthetized SHR (Wan et al., 2004b). In addition, a number of other peptide-based AT2R mimetics have been made by the same research group following extensive structure–activity relationships using Ang II or analogues (Johannesson et al., 2004, Georgsson et al., 2005, Rosenstrom et al., 2005, Georgsson et al., 2006). More recently, new peptide-based ligands have been designed that may become lead compounds for future drug development (Georgsson et al., 2007).
AVE0991 is a non-peptide compound that was first described as a Ang (1–7) mimetic, as it competed for Ang (1–7) binding in bovine aortic endothelial cells, and increased NO release in a similar manner to Ang (1–7) (Wiemer et al., 2002). The functional antidiuretic and vasodilator effects of AVE0991 are absent in MasR-deficient mice (Pinheiro et al., 2004, Lemos et al., 2005), although in some instances either MasR blockade (with A-779) or AT2R blockade (with PD123319) can markedly attenuate the effects of AVE0991 (Wiemer et al., 2002, Pinheiro et al., 2004). More recently, chronic AVE0991 administration was reported to attenuate heart failure induced by MI (Ferreira et al., 2007) although the ATR subtype mediating this effect was not investigated.
Ang IV mimetics also exist. Modifications to the valine in position 1 of Ang IV lead to the formation of an analogue, norleucine-Ang IV (Nle1-Ang IV), which exhibits similar agonistic properties but 100 fold higher affinity for IRAP compared with Ang IV (Sardinia et al., 1994). In addition, Leu-Val-Val-hemorphin-7 (LVV-hemorphin-7), which was first identified as a ligand based on its ability to displace 125I-Ang IV (Lee et al., 2003) also mimics the biological effects of Ang IV such as enhanced memory and learning retention. In contrast, divalinal-Ang IV, is used as an antagonist to inhibit Ang IV mediated effects (Wright et al., 1995).
3. Role of AT2R in cardiovascular pathological states
Having discussed the physiological and pharmacological effects mediated by AT2R, it is appropriate to discuss the specific role of AT2R in cardiovascular pathologies, given that this ATR subtype is usually upregulated in a range of settings (refer to Table 2 ).
3.1. Hypertension
Essential or primary hypertension refers to the condition of elevated arterial BP without known cause, and although usually asymptomatic in its earlier stages, has been shown to be closely correlated with the occurrence of future cardiovascular disorders such as left ventricular hypertrophy (LVH), cardiac failure, arteriosclerosis, and stroke (Unger et al., 1996, Weber, 1997, Simon et al., 1998). Considering the well documented anti-hypertensive effect of AT1R antagonists, and that AT2R oppose AT1R-mediated actions in many situations, it is tempting to speculate that AT2R stimulation may contribute to BP regulation (Widdop et al., 2003).
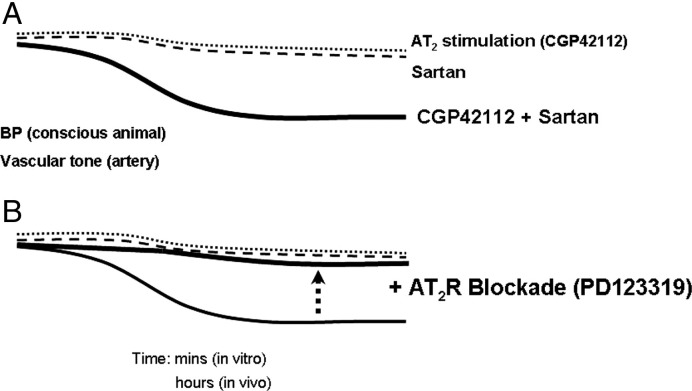
Initially, in vivo studies deduced a vasodilator function of the AT2R since Ang II evoked either an augmented vasodepressor effect in the presence of AT1R blockade or an enhanced vasoconstriction during AT2R blockade (Scheuer and Perrone, 1993, Munzenmaier and Greene, 1996). However, similar studies performed in SHR were unable to demonstrate corresponding Ang II-mediated vasodilatation during AT1R blockade (Gohlke et al., 1998). Such divergence in responses in these experiments may be reconciled by the subtle hypotensive effect of AT2R stimulation, which may have been masked by the concomitant dominant AT1R-mediated pressor action of Ang II infusion. In order to avoid such confounding influences of AT1R stimulation on potential AT2R vasodilator function, investigators have assessed the effect of selective AT2R agonists and antagonists during AT1R blockade (see Fig. 2 ). Using this approach, selective stimulation of AT2R by CGP42112 lowered BP, provided that there was a background of AT1R blockade in conscious rats (Barber et al., 1999, Carey et al., 2001). Furthermore, this BP-lowering response to AT2R stimulation was shown to be associated with increased blood flow in renal, mesenteric and hindquarter circulations indicating widespread vasodilatation (Li & Widdop, 2004), which highlights the fact that modest AT2R-mediated vasorelaxation observed in isolated blood vessels can translate into significant in vivo haemodynamic effects. A similar acute BP-lowering effect of AT2R stimulation was also deduced in a recent study in which the anti-hypertensive action of losartan was potentiated in rats following transient peripheral over-expression of AT2R. This hypotensive action was blocked by PD123319, and persisted over the same time frame as enhanced AT2R expression (reduced towards basal levels 7 days after viral transduction), lending weight to the importance of relative AT1/AT2R expression to AT2R-mediated functional effects (Li et al., 2006).
Fig. 2.
The in vitro and in vivo vasodilator effects of AT2R stimulation are often difficult to detect because of the overriding effects of AT1R-mediated vasoconstriction. This state can be dramatically changed by performing AT2R stimulation against a background of low-dose AT1R antagonist (sartan), even using sartan doses that are sub-threshold for BP-lowering (A). Under these circumstances, AT2R-mediated vasodilatation can be unmasked and subsequently abolished by concomitant AT2R blockade using PD123319 (B).
These acute in vivo findings are consistent with numerous reports of AT2R-mediated vasorelaxation in a wide variety of locations, including mesenteric, renal, coronary, cerebral and uterine vascular beds, which has been shown to be via BK/NO/cGMP signaling pathways. AT2R-mediated vasorelaxation has also been indirectly implicated in conduit vessels such as the aorta since aortic banding markedly increased aortic AT2R expression as well as activating the eNOS/cGMP axis (Hiyoshi et al., 2004, Yayama et al., 2004, Yayama et al., 2006). Consequently, Ang II-mediated contraction via AT1R stimulation was reduced in this vessel Yayama, 2004 #1686; Hiyoshi, 2004 #1685}. Recent evidence further suggests that such recruitment of NO/cGMP mechanisms activates a cGMP-dependent protein kinase (cGKI) resulting in downregulation of RhoA activity, which is known to involved in AT1R-mediated vasoconstriction (Savoia et al., 2006a). Interestingly, AT1R blockade was shown to increase AT2R expression (2–3-fold) and NO production, and to suppress NAD(P)H-driven superoxide generation, in arteries of hypertensive SHRSP, but not normotensive WKY rats (Savoia et al., 2006a). This potentiated NO signaling decreased RhoA/Rho kinase activation, reduced MLC phosphorylation, and subsequently unmasked AT2R-mediated vasorelaxation to Ang II, providing evidence for mechanisms of AT1/AT2R crosstalk at a signaling level (Savoia et al., 2005). For further detailed analysis of AT2R-mediated relaxation/vasodilatation, see (Hannan and Widdop, 2004, Henrion et al., 2001 Widdop et al., 2008).
The vast majority of in vitro vascular studies on AT2R have used arteries obtained from normotensive animals; interestingly, there is little evidence of AT2R-mediated vasorelaxation in arteries taken from untreated hypertensive animals (Matrougui et al., 1999, Matrougui et al., 2000, Cosentino et al., 2005) which contrasts the afore-mentioned in vivo data (Barber et al., 1999, Li and Widdop, 2004, Walters et al., 2005). Moreover, in some cases, Ang II in fact evoked contraction of mesenteric arteries obtained from SHR that involved both AT1R and AT2R (Touyz et al., 1999).
Pressure overload induced by aortic banding in rats (Yayama et al., 2004) and mice (Hiyoshi et al., 2004), or 2K1C hypertension (Hiyoshi et al., 2005), has been shown to upregulate vascular AT2R expression and thus attenuate the AT1R-mediated contractile response to Ang II. Increased AT2R expression in the context of pressure overload was suggested to be due to stimulation of AT1R by increased circulating Ang II, as AT1R inhibition prevented the upregulation of AT2R expression due to aortic banding (Yayama et al., 2004). On the other hand, vascular AT2R was reduced in mesenteric arteries, but increased in aortae, in spontaneously hypertensive rats compared to normotensive controls (Touyz et al., 1999, Widdop et al., 2008). Thus, site-specific changes in vascular AT2R expression associated with hypertension, and the need to block tonic AT1R activity, may account for some of the discrepancies between in vitro and in vivo hypertensive studies.
Indeed, vascular AT2R expression is generally increased by chronic AT1R blockade (Savoia et al., 2005, You et al., 2005, Savoia et al., 2006a, Savoia et al., 2006b), and such treatment can unmask AT2R-mediated vasorelaxation in previously unresponsive aortic vessels from SHR (Cosentino et al., 2005). In this context, we have recently reported that the AT2R vascular phenotype is dependent on basal arterial BP and level of AT2R expression, at least in mesenteric arteries (You et al., 2005). Ang II (in the presence of AT1R blockade) evoked AT2R-mediated vasorelaxation in mesenteric arteries from WKY rats whereas analogous experiments using mesenteric arteries from SHR resulted in Ang II causing AT2R-mediated contraction. At the same time, AT2R expression, determined by Western blot analysis, was reduced in mesenteric arteries from SHR compared with WKY rats. Strikingly, when basal BP of SHR was normalized to WKY-like levels with either an ACE inhibitor or an AT1R antagonist, the AT2R-mediated response was converted from contraction to relaxation. Mesenteric AT2R expression was increased with chronic anti-hypertensive treatment, in line with AT2R-mediated vasorelaxation (You et al., 2005). Moreover, this upregulation of AT2R expression appeared to be related to the level of basal BP since treatment of SHR with the non-specific anti-hypertensive, hydralazine, also increased AT2R expression and converted AT2R-mediated vasoconstriction to Ang II in mesenteric vessels into vasorelaxation. Vascular AT2R localization was also performed using fluorescently-labelled Ang II in the presence of AT1R blockade. These studies confirmed the upregulation of AT2R following anti-hypertensive treatments and identified that AT2R were re-expressed at the level of the endothelium (You et al., 2005). Conversely, it was recently reported that C-reactive protein caused systolic hypertension that was directly related to downregulation of vascular AT2R (Vongpatanasin et al., 2007).
AT2R function has also been indirectly examined by determining the effect on BP of AT2R blockade during AT1R inhibition. In relatively short-term studies performed in rats with either renovascular hypertension or which had been sodium-depleted, the hypotensive effect of AT1R blockade was reversed by simultaneous administration of PD123319, suggesting a vasodilator role of the AT2R (Gigante et al., 1998, Siragy and Carey, 1999, Siragy et al., 2000). However, in analogous experiments, Duke et al. (2005a) reported that AT2R contributed to the BP-lowering and mesenteric vasodilator effect of candesartan in normotensive, but not hypertensive (SHR, 2K1C) rats.
There are a limited number of clinical studies investigating vascular AT2R function in vivo, and these studies have not always reported AT2R-mediated vasodilator effects, which most likely reflect the different patient populations studied. In healthy male volunteers, intravascular administration of PD123319 had no effect on systemic vascular resistance or arterial stiffness, providing no evidence for acute AT2R-mediated haemodynamic effects (Phoon and Howes, 2001, Brillante et al., 2005). However, in other studies by the same investigators, treatment of adult male subjects (Gilles et al., 2004) or elderly women (Phoon & Howes, 2002) with an AT1R antagonist, uncovered acute haemodynamic effects due to AT2R, deduced from short-term infusions of PD123319, thus supporting a role for AT2R during AT1R blockade, albeit with some variation that likely reflects the different patient populations (Phoon and Howes, 2002, Gilles et al., 2004).
On the other hand, investigations into chronic AT2-mediated regulation of BP have yielded less conclusive results. In AT2R knockout mice (Hein et al., 1995, Ichiki et al., 1995, Gross et al., 2000a), and rats administered AT2R antisense to ‘knockdown’ receptor expression (Wang et al., 2004), both an increase in basal BP and/ or potentiation of the pressor response to exogenous Ang II have been attributed to the absence of AT2R modulation of vascular tone. In mice with targeted over-expression of vascular (Tsutsumi et al., 1999) or cardiac (Masaki et al., 1998) AT2R, there was no change in basal BP, although acute Ang II infusion decreased BP. Stimulation of AT2R by chronic Ang II infusion during AT1R blockade failed to decrease BP (Cao et al., 1999, Diep et al., 1999), and similarly, chronic AT2R inhibition either had no (Liu et al., 1997, Tea et al., 2000) or minimal (Varagic et al., 2001) influence on the anti-hypertensive effect of chronic AT1R blockade. Thus in terms of chronic haemodynamic control, and in contrast to the well documented acute vasodilator/relaxation effects of AT2R stimulation, AT2R activation appears to exert only subtle influences on long-term BP regulation. Further studies using chronic AT2R agonist treatment are clearly warranted to address this issue.
Compared to the extensively investigated role of AT2R in vascular reactivity, longer term effects on vascular remodeling in hypertension have been less well studied. Vascular remodelling associated with hypertension occurs at all levels of the vascular tree, and increased local expression of RAS components and subsequent enhanced local Ang II production (Shiota et al., 1992, Jandeleit-Dahm et al., 1997, Wang et al., 2003), implies an important role of the RAS in this process. Furthermore, other factors involved in cellular growth and ECM production which are known to be stimulated by Ang II, such as growth factors (Parker et al., 1998, Su et al., 2002), factors involved in the inflammatory response (Chou et al., 1998) and reactive oxygen species (ROS) (Virdis et al., 2002, Keidar et al., 2004, Touyz et al., 2003), are also upregulated in the hypertensive vasculature. The present overview is limited to discussion of vascular remodeling directly related to hypertension, as reports of AT2R-mediated vascular effects in the specific contexts of atherosclerosis and diabetes (Savoia et al., 2006b) will be discussed in following sections.
Early direct evidence for a component of the vascular anti-hypertrophic effect of sartan-treatment being due to AT2R stimulation was provided by Tea et al. (2000), who showed that simultaneous AT2R inhibition returned aortic mass, smooth muscle cell number and DNA synthesis back to control levels in SHR. We have similarly shown that chronic PD123319 treatment completely reversed sartan-mediated aortic remodeling in both adult and senescent SHR (unpublished data), similarly to aged normotensive rats (Jones et al., 2004). Importantly, this AT2R-mediated vascular anti-hypertrophic effect was not simply a consequence of sartan-induced BP-lowering, as simultaneous PD123319 administration had no further influence on BP in these studies (Tea et al., 2000, Jones et al., 2004). Similarly, in Ang II-induced hypertension, simultaneous blockade of AT1 and AT2R by Sar-Ile resulted in greater media:lumen ratio than that due to AT1R blockade alone in both aortic and mesenteric vessels, suggesting an AT2R-mediated anti-hypertrophic effect (Brassard et al., 2005). Regulation of collagen synthesis also appears to be altered in hypertension, since AT1R-mediated Ang II stimulation of collagen production was potentiated in SHR and occurred via both p38 and ERK1/2 signalling pathways, whereas collagen production was reduced, and dependent on only ERK1/2 activation in WKY (Touyz et al., 2001). In addition, Su et al. (2002) showed that over-expression of AT2R in VSMCs from WKY rats decreased AT1R and TGF-β receptor expression, but that this response was absent in VSMCs of SHR, suggesting a deficiency in inhibitory mechanisms in this model of genetic hypertension.
Furthermore, both basal media:lumen ratio, and vascular hypertrophy due to pressure overload was significantly elevated in aortic, femoral (Brede et al., 2001), and coronary (Akishita et al., 2000b, Wu et al., 2002) vessels of AT2R knockout mice compared to wild type controls, indicating an AT2R-mediated protective effect on vascular hypertrophy. Moreover, consistent with results from pharmacological AT1/AT2R inhibition in rats, the anti-hypertrophic action of AT1R inhibition was reduced in coronary vessels from AT2R knockout mice, supporting a role for AT2R stimulation in the beneficial remodeling effects of AT1R antagonists (Wu et al., 2002).
Taken collectively, these experimental results suggest that, although an acute vasodilator role of AT2R is well documented, chronic BP regulation seems to be only minimally influenced by AT2R stimulation. However, AT2R have consistently been shown to be important in the regulation of vascular structure, which may indirectly influence BP maintenance in the longer term. Indeed, restoration of AT2R-mediated vasorelaxation in mesenteric arteries from SHR was correlated with anti-hypertensive efficacy (You et al., 2005).
3.2. LVH
LVH is considered a major predictor of cardiovascular morbidity and mortality (Levy et al., 1990), and results in changes in the structural organization of the heart, which necessarily influences cardiac function. When the relative proportions of cell types within the heart remain unchanged, hypertrophy is termed adaptive or physiological, however, by far the most prevalent form of cardiac hypertrophy involves disproportionate changes within the cardiac tissue, which ultimately decrease cardiac function, and results in LVH and subsequent progression to heart failure (Swynghedauw, 1999, Zhu et al., 2003). Since the cloning of the AT2R over a decade ago, and despite considerable investigation into the role of AT2R in cardiac hypertrophy, the exact function of AT2R in cardiac tissue still remains somewhat controversial (Booz, 2004, Reudelhuber, 2005).
Importantly, the relatively low expression of the AT2R compared with that of the AT1R, is upregulated in certain conditions, such that increased AT2R expression has been reported in patients with heart failure, ischemic heart disease, and dilated cardiomyopathy (Regitz-Zagrosek et al., 1995, Asano et al., 1997, Haywood et al., 1997, Tsutsumi et al., 1998, Wharton et al., 1998). Moreover, a correlation between AT2R density and severity of heart failure has been reported (Rogg et al., 1996), raising the obvious question of whether or not such increased AT2R expression represents a causative or a reactive consequence of LVH.
In animal models, AT2R expression is also increased by cardiac hypertrophy and heart failure, either in terms of absolute numbers, or relative to AT1R expression (Suzuki et al., 1993, Lopez et al., 1994, Nio et al., 1995, Ohkubo et al., 1997, Bartunek et al., 1999, Busche et al., 2000). In vitro studies have demonstrated increased AT2R expression due to stabilization of mRNA following cardiomyocyte stretch, providing a potential mechanism for observed enhancement of AT2R density during situations of cardiac overload (Kijima et al., 1996).
In accordance with the notion that the AT2R opposes AT1R mediated effects, Liu et al. (1997) demonstrated in a rat model of heart failure that the anti-hypertrophic effects of AT1R blockade on LV volume and cardiomyocyte size were reversed by concomitant AT2R inhibition. Consistent with a protective function of AT2R, AT2R-deficient mice showed a greater hypertrophic response to MI than that of wild type controls (Brede et al., 2003, Oishi et al., 2003), and also attenuated response to AT1R blockade (Xu et al., 2002). In more recent studies in which AT2R were over-expressed via a lentiviral vector gene delivery system in cardiomyocytes of 5 day old SHR (Metcalfe et al., 2004), or Sprague-Dawley rats (Falcon et al., 2004), AT2R over-expression reduced cardiac hypertrophy at 21 weeks of age compared to control SHR, and attenuated Ang II-induced cardiac hypertrophy and fibrosis, respectively. Similarly, in isolated perfused adult hypertrophied hearts (Bartunek et al., 1999), Ang II-induced production of early response genes were enhanced by AT2R inhibition, providing evidence for AT2R-mediated regulation of cellular growth at the level of gene expression.
On the other hand, a lack of effect of AT2R on indices of cellular growth has also been shown in pharmacological studies. We and others have deduced no influence of AT2Rs on cardiac hypertrophy from ‘PD-reversal’ studies, in which simultaneous PD123319 administration did not modify the cardiac anti-hypertrophic effect of AT1R blockade in either aged WKY (Jones et al., 2004) or SHR (Varagic et al., 2001) rats. Similarly, the cardiac growth-inhibitory effect of valsartan, was of similar magnitude in aortic-banded AT2R knockout mice to that of animals expressing AT2R (Wu et al., 2002). Ang II infusion also resulted in a comparable increase in cardiac hypertrophy in mice with cardiac specific over-expression of AT2R, to that of wild type mice (Sugino et al., 2001, Kurisu et al., 2003).
In direct contrast, several in vivo studies have suggested a growth-stimulatory role of AT2R. Mice in which AT2R were over-expressed using the ventricular-specific myosin light-chain promoter, exhibited dilated cardiomyopathy which was associated with increases in both myocyte cross sectional area and fibrosis (Yan et al., 2003). Similarly, in AT2R knockout mice, the cardiac hypertrophic response to aortic banding, Ang II infusion or MI was absent in AT2R-deficient animals (Senbonmatsu et al., 2000, Ichihara et al., 2001, Ichihara et al., 2002), indicating a requisite presence of AT2R for cardiomyocyte hypertrophy. Interestingly, D'Amore et al. (2005) recently showed that AT2R evoked constitutive growth in cultured neonatal cardiomyocytes. The research groups who reported a growth-promoting function of AT2R also found evidence for increased signalling via p70S6 kinase, which is a well documented hypertrophic mechanism (Senbonmatsu et al., 2000, Yan et al., 2003). In a subsequent study (Senbonmatsu et al., 2003), AT2R-mediated cellular hypertrophy involving p70S6 kinase was dependent on PLZF activation as previously discussed (Section 2.3.3). Nevertheless, the cardiac hypertrophic effects of AT2R are controversial, with either no effect or hypertrophy being attributed to cardiac AT2R in different AT2R knockout strains subjected to various cardiac loads (Akishita et al., 2000b, Senbonmatsu et al., 2000, Widdop et al., 2003). Thus, it is possible that these discrepancies may relate to differential PLZF expression and/ or activity in the 2 AT2R knockout strains. In this context, as previously mentioned, a novel signal transduction pathway involving renin/(P)RR/PLZF has been described, that displayed both excitatory and inhibitory actions of PLZF (Schefe et al., 2006).
Interestingly, a study performed by Lako-Futo et al. may go some way towards explaining such equivocal AT2R-mediated effects on cardiac hypertrophy. In acute studies performed in rats, blockade of the AT2R with PD123319 during 62 h Ang II infusion, resulted in enhanced expression of genes associated with the promotion of cardiac hypertrophy (c-fos, endothelin-1, and IGF-1), but also increased expression of anti-hypertrophic factors (ANF and BNP), and decreased growth factors (VEGF and FGF-1) (Lako-Futo et al., 2003). These results suggest that in addition to opposing AT1R-mediated effects, AT2R stimulation may produce growth-stimulatory actions which partially offset AT2R-mediated growth-inhibitory effects. Thus, it is conceivable that even slight alterations in conditions may shift the balance of AT2R-mediated actions from a situation in which pro- and anti-hypertrophic effects remain in equilibrium, to that in which either growth-promoting or growth inhibiting- pathways are favoured.
In this context, it is interesting to note that the most consistent reports of a cardioprotective effect of AT2R in vivo, have been determined from experiments performed in models of MI-induced heart failure. The vast majority of investigators have deduced AT2R-mediated improvement of LV function and remodelling from multiple studies performed in mouse models of either AT2R-deletion (Brede et al., 2003, Oishi et al., 2003) or over-expression (Yang et al., 2002, Bove et al., 2005). In fact, only 1 study has deduced a negative role for AT2R stimulation in this context (Ichihara et al., 2002), and these discordant results may be due to the alternative strain of knockout mouse used by Ichihara et al., as has been previously discussed (see Widdop et al., 2003). AT2R-mediated cardioprotection has been shown to involve NO signalling, as NO synthase inhibition prevented the beneficial effects of AT2R over-expression on LV function (Bove et al., 2004). BK has also been implicated in cardiac AT2R-mediated effects, as pharmacological inhibition of B2R in rats (Liu et al., 1997), or B2R deletion in mice (Isbell et al., 2007), was shown to prevent the anti-fibrotic influence of AT2R stimulation. In addition, AT2R stimulation has been shown to be involved in the beneficial effects of sartans post-MI, as AT1R inhibition was found to be less effective during AT2R blockade (Liu et al., 1997) or AT2R-deletion (Xu et al., 2002), and potentiated by AT2R-over-expression (Voros et al., 2006).
Interestingly, genetic influences in humans have also been reported which add another degree of complexity. The AT2R gene polymorphism -1332G/A (also known as 1675G/A) has also been linked with LVH. In hypertensive subjects, LVH has been associated with either the A allele (Schmieder et al., 2001) or the G allele (Alfakih et al., 2004) in 2 separate studies, with the method of LVH determination (echocardiography versus magnetic resonance imaging) suggested as explanation for these discrepancies (Alfakih et al., 2004). Premature coronary artery disease has also been associated with the G allele (Alfakih et al., 2005). However, the interpretation of these findings is somewhat unclear as the same group has reported that the G allele represents either decreased AT2R function (Nishimura et al., 1999, Alfakih et al., 2004, Alfakih et al., 2005) or increased AT2R function (Warnecke et al., 2005, Strauss and Hall, 2006).
Clearly, neither animal nor human studies to date have been able to delineate a consistent influence of AT2R stimulation on LVH. Current equivocal results may be partly explained by the range of animal models, indices measured, drugs, doses and treatment times employed by investigators, but most likely reflect the complex and context-specific nature of AT2R-mediated cardiac growth effects, probably also involving ligand-independent effects. Given the correlation between LVH and the poor long-term prognosis of patients exhibiting such modification of cardiac structure (Levy et al., 1990, Rihal et al., 1994, Senni et al., 1998), this area of AT2R function undoubtedly requires further research.
3.3. Cardiac fibrosis
In contrast to the range of equivocal results regarding the influence of AT2R on LVH, the majority of experimental data relating to cardiac fibrosis report anti-fibrotic effects of AT2R stimulation. Indeed, increased AT2R expression in the heart has been specifically localised to cardiac fibroblasts, suggesting a link between upregulation of AT2R expression and fibrosis present in hypertrophied and failing hearts (Brink et al., 1996, Tsutsumi et al., 1998, Wharton et al., 1998). Such an association between AT2R expression and cardiac disease states, and previous suggestions of AT2R-mediated anti-growth effects, has fuelled interest in the AT2R as a potential target for cardiac anti-fibrotic therapies.
It is well accepted that Ang II is an important mediator of ECM homeostasis, with numerous reports of either AT1R blockade or ACE inhibitor treatment resulting in decreased cardiac fibrosis in both humans and animal models. Importantly, in both physiological (e.g. heart valve leaflets) and pathophysiological (e.g. MI) sites of repair, AT2R are expressed at high levels, along with other RAS components such as angiotensinogen, ACE, and AT1R (Weber et al., 1997). Moreover, important factors involved in Ang II-mediated connective tissue formation, including TGF-β1 and its receptors, and type I and III collagens, are also expressed in AT2R-rich fibroblasts and myofibroblasts (Katwa et al., 1997, Lijnen et al., 2003).
Although 2 separate groups have reported a stimulatory effect of AT2R on cardiac collagen accumulation (Senbonmatsu et al., 2000, Ichihara et al., 2001, Ichihara et al., 2002, Yan et al., 2003), the bulk of data from numerous in vivo studies clearly support an inhibitory effect of AT2R on cardiac fibrosis. In ‘PD123319 reversal’ experiments, AT2R inhibition reversed the anti-fibrotic effects of AT1R blockade both in SHRs (Varagic et al., 2001), and in aged normotensive WKY (Jones et al., 2004) and aged SHR (unpublished data). Similarly, the anti-fibrotic effect of AT1R inhibition was found to be attenuated in AT2R-deficient mice, since the sartan-mediated reduction in cardiac fibrosis following aortic banding or Ang II infusion, was of smaller magnitude in AT2R knockout mice compared to wild type littermates (Wu et al., 2002, Xu et al., 2002). Other investigators have also deduced an inhibitory effect of the AT2R on cardiac fibrosis, as the promotion of interstitial fibrosis by either aortic banding or l-NAME was augmented in AT2R knockout mice (Akishita et al., 2000b, Wu et al., 2002, Gross et al., 2004) and cardiac specific over-expression of AT2R diminished the amount of both perivascular and interstitial fibrosis induced by Ang II infusion (Kurisu et al., 2003).
However, the exact mechanisms by which AT2R may modulate collagen deposition are not completely understood. The accumulation of collagen within the heart is a dynamic process, with total collagen content representing the net effect of both collagen synthesis and degradation. In terms of collagen production, a stimulatory role for AT1R in terms of collagen synthesis is well established, however, there are contrasting reports of the contribution of AT2R to this process. In rat cardiac fibroblasts, collagen production was reduced by inhibition of either AT1 or AT2R (Brilla et al., 1994, Brilla et al., 1995), and chronic blockade of both AT1R and AT2R with Sar1Ile8Ang II in rats also decreased collagen deposition, suggesting a stimulatory role of AT2R in collagen synthesis (Brassard et al., 2005). In addition, Mifune et al. (2000) also reported that AT2R stimulation by CGP42112 induced collagen synthesis in VSMCs and mesangial cells, but that CGP42112 treatment had the opposite effect in cultured fibroblasts, indicating heterogeneous AT2R-mediated responses in different cell types. In contrast, several other investigators have reported a lack of effect of AT2R on collagen secretion, since Ang II stimulation of collagen production in cultured rat and porcine cardiac fibroblasts, and rat mesenteric VSMCs was inhibited by AT1R blockade, but unaffected by AT2R inhibition (Lijnen et al., 2000, Touyz et al., 2001, Warnecke et al., 2001). On the other hand, the influence of AT2R on collagen degradation has not been extensively investigated. In one of the earliest studies to investigate AT2R-specific effects on collagen metabolism, the decrease in collagenase activity due to Ang II stimulation of cultured cardiac fibroblasts was abolished by AT2R blockade but not influenced by AT1R inhibition (Brilla et al., 1994). In contrast, Min et al. (2004) demonstrated a diminution of collagenase activity by AT2R blockade which was associated with increased tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) expression, and selective AT2R stimulation with CGP42112 increased matrix metalloproteinase-1 (MMP-1) production in human monocytes (Kim et al., 2005).
3.4. Stroke and neuroprotection
On the basis of several large-scale clinical trials, it is now accepted that chronic inhibition of the RAS can provide neuroprotection, with reduced occurrence of stroke in high-risk populations (Dahlof et al., 2002, Arnold et al., 2003; Chapman et al., 2004, Schrader et al., 2005). Neuroprotection has also been demonstrated by candesartan cilexetil in an acute setting (Schrader et al., 2003). Moreover, drugs that target the renin–angiotensin system offer benefits that extend beyond the control of BP (Arnold et al., 2003, Chapman et al., 2004).
In accordance with human clinical trials, animal studies have also concluded that inhibition of the RAS protects against neuronal injury following stroke, often in a BP-independent manner (Ito et al., 2002, Groth et al., 2003). Similarly, AT1R knock out mice, exhibited smaller infarct size following temporary middle cerebral artery occlusion (Walther et al., 2002). Underlying mechanisms may involve the inhibition of AT1R-mediated cerebrovascular pathological growth and inflammation, as well as improved cerebrovascular compliance and autoregulation (Nishimura et al., 2000, Saavedra et al., 2006). In addition, Sugawara et al. (2005) recently demonstrated that a non-hypotensive dose of an AT1 receptor antagonist reduced superoxide production and improved neurological outcome following global ischemia (Sugawara et al., 2005).
Not surprisingly considering the contribution of AT2R to the effects of AT1R inhibition in other settings, attention has recently turned to the potential role of the AT2R in neuroprotection, particularly since AT2R is reported to be upregulated in stroke (Zhu et al., 2000, Li et al., 2005b, Lu et al., 2005), specifically in the ischemic area of the brain (Mogi et al., 2006). Moreover, AT2R-deficient mice display a larger ischemic area and a more severe neurological deficit following stroke when compared to wild type (Iwai et al., 2004), implicating a neuroprotective role for the AT2R. In this context, the neuroprotective effect of valsartan was reduced in AT2R knockout animals (Iwai et al., 2004, Mogi et al., 2006), and either peripheral (Lu et al., 2005) or central (Li et al., 2005b) administration of an AT2R antagonist reversed the neuroprotection evoked by AT1R blockade, suggesting opposing roles for AT1R and AT2R during stroke.
The potential mechanisms of AT2R-mediated neuroprotection in reducing infarct volume following stroke are currently under investigation. AT2R-mediated apoptosis (Zhu et al., 2000, Grammatopoulos et al., 2002) is one possibility. Indeed, AT2R is capable of inducing apoptosis in PC12W cells through the induction of ceramides and the activation of MKP-1 signalling (Dai et al., 1999). Furthermore, AT2R knockout mice have higher neuronal numbers compared to controls, suggesting that AT2R deficit antagonises angiotensin II-induced apoptosis (Von Bohlen und Halbach et al., 2001). Another possible AT2R-mediated neuroprotective influence involves neuronal regeneration. Earlier studies (Laflamme et al., 1996) demonstrated that in vitro stimulation of AT2R was associated with intense neurite outgrowth; an effect that was negated by PD123319. Reinecke et al. (2003) demonstrated that angiotensin II can act similarly to NGF, promoting remyelination, neurite outgrowth and functional recovery following sciatic nerve crush in adult rats via AT2R (Reinecke et al., 2003). In this model of nerve injury, co-administration of losartan and Ang II accelerated regeneration whereas PD123319 alone or in combination with Ang II, prevented recovery. Similar findings were reported using an optic nerve crush model, whereby stimulation of AT2R on retinal ganglion cells significantly increased neurite outgrowth (Lucius et al., 1998). Stroth et al. (1998) identified that the AT2R is involved in the regulation of cytoskeleton proteins essential for neurite extension (Stroth et al., 1998). They examined the expression of several microtubule components in PC12W cells and found Ang II and NGF differentially regulate microtubule proteins and, in the case of Ang II, the upregulation of dendritic proteins was mediated via AT2R. Likewise, it was found that selective activation of AT2R increased the expression of beta III-tubulin, and the microtubule-associated proteins, tau and MAP2, resulting in increased elongation of neuritis (Cote et al., 1999). In a recent series of elegant studies from Unger's laboratories, it was found that central administration of PD 123319 abolished the neuroprotective effects of central AT1 receptor blockade in conscious rats, both in terms of infarct size and neurological outcome following a transient unilateral medial cerebral artery occlusion (Li et al., 2005b). These authors indicated that AT2R was upregulated during stroke and supported neuronal survival and neurite outgrowth in vulnerable peri-ischemic brain areas, as has recently been reviewed (Thone-Reineke et al., 2006).
At first glance, it seems contradictory that AT2R would be involved in both apoptosis and neuronal regeneration, however, during early stages, neuronal injury will initiate a series of events that are identical for apoptosis and regeneration (Lucius et al., 1998). AT2R-mediated activation of the MKP-1 pathway brings the cell to a point where it may enter the repair process, or programmed cell death, depending on the energy state of the cell. Thus AT2R contribute to the cellular mechanisms allowing adaptation to ischemic insult, by stimulating both tissue repair and programmed cell death (Lucius et al., 1999). The apoptotic process can be neuroprotective as it serves to conserve energy by reducing the occurrence of inflammatory reactions, allowing more energy to be directed to the recovery of viable cells. Thus, AT2R may play a role in determining the fate of damaged neurons. Indeed, unmasking the apoptotic and regenerative effects of the AT2R by AT1R blockade could enhance tissue repair following injury and account for the reduction in infarct volume observed in animals treated with AT1R antagonists (Lucius et al., 1998). In very recent studies, Mogi et al. (2006) have proposed that AT2R stimulation increases the expression of a neuroprotective factor, methyl methanesulfonate sensitive 2, which is involved in neuronal differentiation (Mogi et al., 2006).
While most research has focused on the neuronal effects of the AT2R in stroke, the vasodilator role of AT2R cannot be ignored as a possible contributor to any AT2R neuroprotective effect, by maintaining perfusion of penumbral regions following stroke. In accordance with many studies describing AT2R-mediated relaxation of peripheral arteries (see Section 3.1), Ang II caused AT1R-mediated vasoconstriction in cerebral arteries both in vitro and in vivo; an effect of Ang II that was converted to AT2R-mediated vasodilatation in the presence of an AT1R antagonist (Vincent et al., 2005). The fact that a sartan itself leads to an upregulation of AT2R in cerebral vessels (Zhou et al., 2006) is consistent with a vascular component to the neuroprotective effect of AT2R stimulation. Thus, the neuroprotective role of the AT2R appears to be complex, and is likely to involve a delicate interplay with AT1R on apoptotic, neuronal regenerative and vascular components in the central nervous system (Saavedra et al., 2006). Curiously, the direct effect of AT2R stimulation in the setting of stroke has not been reported.
3.5. Renal disease
AT2R are also developmentally regulated in the kidney, with AT2R expression higher than that of AT1R expression in the foetal kidney (Ciuffo et al., 1993, Shanmugam et al., 1995, Ozono et al., 1997). However, the relative proportion of these receptors reverses within days of birth, such that AT1R expression is higher than AT2R in the adult. AT2R are detected at relatively low levels in adult kidney, although reports of the extent and location of AT2R varies considerably depending on techniques used. Generally, AT2R mRNA and protein have been found to be distributed throughout tubular and vascular segments of the renal cortex and medulla, however, the demonstration of glomerular AT2R is more variable (Ozono et al., 1997, Miyata et al., 1999, Cao et al., 2000, Ruiz-Ortega et al., 2003). Discrepancies between studies may involve species differences since renal AT2R are more easily detected in mice than rats (Armando et al., 2002, Baiardi et al., 2005). AT2R are detected in human kidneys, primarily in associated vasculature (Zhuo et al., 1996, Matsubara et al., 1998), but also in tubular and glomerular tissue of patients with glomerular lesions (Mifune et al., 2001).
In terms of models of heightened RAS activation, there have been variable effects reported for renal AT2R expression. Sodium depletion upregulated renal AT2R (Ozono et al., 1997); AT2R was downregulated only in the ischaemic kidney from 2K1C rats (Wang et al., 1999) whereas Ang II infusion per se did not alter renal AT2R expression (Wang et al., 1999). AT1R was also downregulated in the former 2 models, while adult Ren-2 gene transgenic rats, TGR(mRen-2)27 exhibited increased AT1R but not AT2R (Zhuo et al., 1999). However, the functional consequences of such changes in ATR expression were not examined.
On the other hand, in models of overt renal damage, such as induced by renal ablation, subtotal nephrectomy, or kidney damage caused by protein overload, AT2R were invariably upregulated (Bautista et al., 2001, Cao et al., 2002, Ruiz-Ortega et al., 2003, Hashimoto et al., 2004, Tejera et al., 2004, Vazquez et al., 2005). Ex vivo analysis indicated that increased AT2R was associated with enhanced renal vasodilatation in perfused kidneys (Bautista et al., 2001) and increased tubular apoptosis following persistent proteinuria (Tejera et al., 2004). However, Cao et al. (2002) reported that blockade of AT2R with PD123319 actually conferred renoprotection, as evidenced by a reduction in albuminuria, as did AT1R blockade. These results suggested that both AT1R and AT2R were renoprotective in the subtotal nephrectomy model (Cao et al., 2002), although recent data using the same model disputes those findings. Vazquez et al. (2005) found that there was a time-dependent increase in AT2R expression over 30 days after renal ablation. Moreover, PD123319 exacerbated renal ischaemia and damage as well as causing a marked increase in telemetered BP (Vazquez et al., 2005). Thus, on balance, AT2R appear to be renoprotective, in keeping with other studies in which mice treated with PD123319, or in mice with AT2R deleted, exhibit marked increases in renal fibrosis (Ma et al., 1998, Morrissey and Klahr, 1999). Similarly, vascular over-expression of AT2R ameliorated glomerular injury in the mouse remnant kidney model since there were reductions in albuminuria and glomerular expression of platelet-derived growth factor and transforming growth factor (Hashimoto et al., 2004).
In addition to the well described AT2R renal vasodilator function, AT2R are thought to exert pronatriuretic effects. Seminal studies by Siragy and Carey, 1996, Siragy and Carey, 1997, Siragy and Carey, 1999 (Jin et al., 2001, Jin et al., 2004) demonstrated that increased renal interstitial levels of cGMP, NO and BK were dependent on AT2R activation, and led to natriuresis. Moreover, Carey and colleagues have recently postulated that Ang III may be the endogenous AT2R ligand in the kidney since, during AT1R blockade, renal interstitial infusion of Ang III, but not Ang II, caused natriuresis. This effect of Ang III was blocked by PD123319, indicative of an AT2R mechanism (Padia et al., 2006), which is consistent with Ang III-mediated depressor effects being AT2R-mediated (Walters et al., 2003). Moreover, stimulation of dopamine D1-like receptors with fenoldopam caused natriuresis due to recruitment and activation of AT2R in renal proximal tubules (Salomone et al., 2007). The pressure–natriuresis relationship was also impaired in AT2R knockout mice which had elevated basal renal resistance and altered arachidonic acid metabolism (Gross et al., 2000b). More recent pharmacological studies have directly addressed the role of AT2R in natriuresis. In obese Zucker rats, CGP42112 increased urinary and sodium excretion, in line with increased expression of tubular AT2R, whereas this effect was absent in lean rats. Moreover, PD123319 reversed the natriuretic and diuretic effects of candesartan, suggesting an involvement of AT2R in the effect of the latter compound (Hakam & Hussain, 2005), consistent with earlier studies (Siragy and Carey, 1999, Siragy et al., 1999). Thus, AT2R mediates natriuresis directly, or indirectly after AT1R blockade (Hakam and Hussain, 2005, Padia et al., 2006), thereby opposing the well known antinatriuretic effects of AT1R activation. Mechanisms underlying the natriuretic effects of AT2R may involve altered proximal tubule bicarbonate reabsorption involving phosholipase A and arachidonic acid (Haithcock et al., 1999), or by direct inhibition by AT2R of the proximal tubule sodium pump via NO/cGMP signalling as has recently been postulated (Hakam and Hussain, 2006a, Hakam and Hussain, 2006b). Furthermore, renal AT2R have recently been shown to inhibit renin biosynthesis and subsequent local Ang II production, thus providing an additional mechanism for modulation of AT1R-mediated effects (Siragy et al., 2007).
The regional haemodynamic effect of Ang II within the rabbit kidney itself has also been investigated by measuring cortical and medullary blood flows. As expected, we found that AT2R counteracted AT1R-mediated vasoconstriction in the kidney cortex. By contrast, Ang II evoked AT1R-mediated medullary vasodilatation that was opposed by activation of AT2R (Duke et al., 2003). Moreover, in 2K1C hypertensive rats, the AT2R antagonist increased basal medullary flow, suggestive of AT2R-mediated medullary vasoconstriction (Duke et al., 2005b). Thus, the medullary circulation behaves somewhat differently to other vascular beds in response to Ang II. Likewise, endogenous Ang II acting via both AT1R and AT2R would appear to enhance renal neurovascular function in rabbit kidneys (Rajapakse et al., 2005, Rajapakse et al., 2006).
3.6. Diabetes
Diabetes is one of the major causes of renal failure and blindness and is an important risk factor for the development of cardiovascular disease, where its presence is also associated with a worse prognosis (Gilbert et al., 2003). Although hyperglycaemia is a major determinant in the development of long-term complications of diabetes there are also glucose-independent mechanisms that contribute to the pathogenesis of organ injury in this disease. Activation of local RAS seems to be involved in the progression of kidney damage, with results of large clinical trials confirming the beneficial effects of ACE inhibitors and AT1R antagonists (Brenner et al., 2001, Lewis et al., 2001, Parving et al., 2001), however as yet the role of the AT2R in the pathogenesis of diabetic nephropathy remains to be elucidated.
There are relatively few studies that have examined ATR distribution in this disease. Of these, the majority of studies have focussed on the kidney as diabetic nephropathy is one of the major complications associated with both type 1 and type 2 diabetes. Using the STZ-model of type 1 diabetes, both reductions (Kalinyak et al., 1993, Cheng et al., 1994, Brown et al., 1997, Bonnet et al., 2002) and increases (Brown et al., 1997, Wehbi et al., 2001) in AT1R have been reported. On the other hand, AT2R expression has been found to be markedly increased in brush-border and basolateral membrane of the kidney (Hakam et al., 2006), and either not detected in the glomeruli of either diabetic or control rats (Hakam et al., 2006) or found to be significantly decreased (Wehbi et al., 2001). In a long-term study of type 1 diabetes, both AT1R and AT2R gene and protein levels were reduced in kidneys of diabetic SHR (both glomeruli and tubulo-interstitial cells) compared with both non-diabetic SHR and a normotensive model in which only the AT2R showed a tendency towards a reduced expression (Bonnet et al., 2002). Less information is available regarding type 2 diabetes. Using the obese hyperinsulinaemic Zucker rat as a model of type 2 diabetes, AT2R density was also found to be increased in the brush-border and basolateral membrane of the kidney (Hakam & Hussain, 2005) which is consistent with findings from kidney biopsies of patients with type 2 diabetes in which the AT2R levels were also found to be increased in tubular cells with a concomitant reduction of AT1R expression (Mezzano et al., 2003).
Thus it is difficult to reach consensus on overall receptor changes in the 2 types of diabetes due to differences in methodologies and duration and severity of diabetes. Unlike experimental renal failure, which is fast-onset and exhibits a clear upregulation of AT2R, diabetes-induced changes in renal ATR expression reflect the continuum of the diabetic progression. Indeed, it is highly likely that the relative expression of AT1 and AT2R in heterogeneous tissue compartments in the kidney is important in determining the effects of Ang II in progressive diabetic nephropathy.
In this context, in STZ-induced diabetic rats, there are 50- and 80-fold increases in AT2R in brush-border and basolateral membranes. Diabetes elevated urinary flow and urinary sodium excretion which was blocked by the selective AT2R antagonist, PD123319 in diabetic but not control rats (Hakam et al., 2006). A similar effect of the AT2R was observed in the type 2 diabetic model, the obese Zucker rat, with the AT2R agonist, CGP42112, promoting an increase in sodium and urine excretion in obese but not lean Zucker rats (Hakam & Hussain, 2005). On the basis of the effects of PD123319, there was a basal effect of AT2R to promote natriuresis in type 1 diabetes (Hakam et al., 2006) but not in type 2 diabetes (Hakam & Hussain, 2005) which most likely reflects the profoundly greater upregulation of tubular AT2R in the STZ-model compared with the type 2 model. The increase in natriuresis and diuresis produced by administration of an AT1R antagonist in the obese Zucker rat was abolished by an AT2R antagonist (Hakam & Hussain, 2005). The AT2R has since been shown to inhibit the Na+-K+-ATPase via an NO/cGMP/protein kinase G pathway (Hakam and Hussain, 2006a, Hakam and Hussain, 2006b), thus providing a possible mechanism responsible for AT2R-mediated natriuresis and diuresis in this model.
In addition, in the diabetic kidney, there is significant upregulation of various angiogenic cytokines, such as VEGF, that is mediated via AT1R and may thus contribute to the progression of renal injury (Pupilli et al., 1999, Tamarat et al., 2002, Rizkalla et al., 2005). A link between AT2R and VEGF expression, albeit in a non-diabetic context has also been made (Rizkalla et al., 2003, Sarlos et al., 2003). In the STZ-induced model of diabetes, there is increased gene and protein expression of VEGF and its receptor (VEGF-R2) in the kidney. Chronic AT1R or AT2R blockade attenuated expression of cytokines and their receptors (Rizkalla et al., 2005). It should also be noted that there was increased apoptosis involving tubular and interstitial cells of the cortex and medulla of the kidney in STZ-treated rats (Kumar et al., 2004). Losartan and PD123319 inhibited apoptosis in the kidney to similar extents and there was no additive effect using both antagonists. Therefore it is possible that AT1R and AT2R signalling pathways can both lead to apoptosis and induction of angiogenic cytokines in the diabetic kidney.
There is evidence that Ang II and VEGF and their cognate receptors participate in retinal angiogenesis and ischaemic retinopathies such as diabetic retinopathy (see Wilkinson-Berka, 2004). The AT2R has been identified in several ocular tissues including the retina and has been found to predominate in the developing retina (Sarlos et al., 2003) consistent with the view that the AT2R influences cell growth and differentiation in organ development. There was an increase in AT2R expression and AT2R blockade decreased retinal angiogenesis as well as the levels of VEGF, its receptor VEGF-R2 and angiopoietin-2 similarly to that seen in the diabetic kidney (Sarlos et al., 2003). Zhang et al. (2004) showed that blockade of the AT2R in STZ-treated rats decreased VEGF expression, thereby suggesting that VEGF expression is modulated by both AT1 and AT2 receptors and implicating these receptor subtypes in retinal diseases such as diabetic retinopathy. Thus blockade of the AT2R as well as the AT1R may confer end-organ protection in various retinal diseases.
Limited information is available regarding AT2R function in other non-renal tissues from diabetic animals or in the clinical setting. Increased expression of AT2R in normal (Sechi et al., 1994) and cardiomyopathic (Li et al., 2005a) hearts from diabetic rats have been reported. AT2R-mediated relaxation was also enhanced in aortic tissue obtained from STZ-treated rats compared with controls, and was associated with increased AT2R density, but not AT1R, in this vessel (Arun et al., 2004). Recently, it was shown that 1-year treatment of diabetic hypertensive patients with valsartan, but not atenolol, caused an upregulation of AT2R in gluteal resistance arteries that was associated with improved AT2R-mediated vasorelaxation in these arteries (Savoia et al., 2007). Furthermore, in line with earlier in vivo vascular AT2R function (see Section 3.1), Howes' group has recently examined small-artery vascular stiffness and in fact found that there was increased functional expression of vascular AT2R in patients with insulin resistance (Brillante et al., 2008).
3.7. Atherosclerosis and neointimal formation
Ang II is recognized as a potent mediator in the pathogenesis of atherosclerosis (Daugherty et al., 2000), with AT1-receptor activation by Ang II promoting vascular inflammation, cellular proliferation and oxidative stress which are considered key stages in the initiation and progression of the disease (Dzau, 2001, Daugherty and Cassis, 2004). The role of Ang II in atherosclerosis is evident in a number of studies using transgenic animal models and human tissues, that demonstrate an increase in AT1-receptor density in hypercholesterolemia (Nickenig and Bohm, 1997, Warnholtz et al., 1999), as well as increased expression of ACE in atherosclerotic lesions (Diet et al., 1996, Fukuhara et al., 2000). Furthermore, when the AT1R gene was deleted from 2 widely used models of atherosclerosis, i.e. apolipoprotein E (ApoE)-deficient mice or LDL-receptor-deficient mice, both types of double knockouts exhibited markedly reduced atherosclerotic lesions (Wassmann et al., 2004, Daugherty et al., 2004), suggesting a primary role for AT1R in the pathogenesis of atherosclerosis. Indeed, recent studies using pharmacological intervention of the RAS have demonstrated beneficial effects in the progression of atherosclerosis and associated cardiovascular outcomes (see (Dzau, 2001, Daugherty and Cassis, 2004).
In the same study that used AT1R/LDL-receptor double knockouts, AT2R/LDL-receptor double knockout mice were also reported as a secondary aim. The authors reported that these mice do not exhibit an increase in atherosclerosis (Daugherty et al., 2004), however, no data was presented to support such a statement. By contrast, the AT2R gene was deleted from ApoE-deficient mice independently by 2 groups (Iwai et al., 2005, Sales et al., 2005), with both studies concluding that AT2R do in fact exert an anti-atherosclerotic effect.
Iwai et al. (2005) demonstrated that AT2R/ApoE-double knockout (DKO) mice fed a high cholesterol diet display exaggerated atherosclerotic lesion development, together with increased NADPH oxidase activity and superoxide production, when compared with ApoE knockout mice. It is well known that Ang II can induce expression of NADPH oxidase and subsequently increase superoxide production via activation of the AT1-receptor (Griendling et al., 1994). These results suggest inhibitory modulation of oxidative stress via the AT2R which is consistent with in vitro data using cultured endothelial cells in which AT2R blockade with PD123319 enhanced superoxide formation (Sohn et al., 2000). These findings also have further implications in terms of the progression of atherosclerosis, as decreased oxidative stress (typically superoxide anion production) increases the bioavailability of the potent anti-atherosclerotic vasodilator NO (Cai & Harrison, 2000). In addition, the anti-atherosclerotic effect of the AT1R antagonist, valsartan was markedly reduced in AT2R/ApoE-DKO compared with ApoE-single knockout mice (Iwai et al., 2005), suggesting that AT2R contribute to the beneficial effect of AT1R blockade and are counter-regulatory to AT1R in the setting of atherosclerosis.
Using a separately derived AT2R/ApoE-DKO, Sales et al. (2005) also published data investigating the effect of AT2R on atherosclerotic disease progression. Although this study did not show increases in lesion size in AT2R/ApoE-DKO mice, they demonstrated striking differences in plaque composition, cellular proliferation and apoptosis over time. Typically, there is a reduction in macrophage, smooth muscle, lipid and collagen content of plaques with disease progression together with increased apoptosis. However, AT2R deletion ameliorated this process suggesting that AT2R influences the cellular composition of the atherosclerotic lesion. Importantly, in ApoE-deficient mice, AT2R were co-localised with intimal smooth muscle cells and macrophages, and were upregulated following a high cholesterol diet. The authors noted a temporal association of AT2R expression and reduced smooth muscle cell content during lesion development, emphasizing an anti-inflammatory and antiproliferative role for AT2R (Sales et al., 2005). Increased AT2R expression in atherosclerotic plaques in the same ApoE-deficient mouse model was recently confirmed (Johansson et al., 2005, Vinh et al., 2008b), and increased AT2R and ACE2 immunoreactivity was found in atherosclerotic plaques in rabbits, often co-localised with macrophages (Zulli et al., 2006). Interestingly, Dandapat et al. (2008) have recently reported that over-expression of the AT2R in LDL-receptor knockout mice, abrogated atherosclerotic lesion development, and was associated with a reduction in oxidative stress and other factors involved in the pro-inflammatory cascade. Furthermore, using the same mouse model, Hu et al. (2008) have additionally provided evidence of AT2R-mediated regulation of collagen deposition in atherosclerotic plaques which involved reduced MMP expression, decreased macrophage deposition and increased superoxide dismutase. Indeed these studies strongly support previous studies reporting anti-oxidative and anti-inflammatory effects mediated via the AT2R, both of which are vital processes that contribute to atherosclerotic lesion formation.
Consistent with the anti-atherosclerotic role of AT2R deduced from knockout studies, Daugherty et al. (2001) had previously demonstrated that Ang II-induced atherosclerosis and aneurysm formation in male ApoE-KO mice was significantly potentiated with AT2R blockade. However, Johansson et al. (2005) were unable to reproduce this potentiation of lesion formation in the same model but using female mice. The authors suggested gender differences may have accounted for the disparity, as it has been demonstrated that male hormones may protect against Ang II-mediated lesion development (Henriques et al., 2004). Alternatively, the ability of oestrogen to increase AT2R expression may play a role (Kintscher & Unger, 2005), which illustrates important gender differences that are emerging with respect to AT2R expression and function (see Section 3.9). On close inspection of the same study, Johansson et al. (2005) reported that the incidence of abdominal aortic aneurysm increased from 11% with Ang II treatment alone, to 50% when treated with both Ang II and PD123319 in female ApoE−/− mice on a standard diet. These results are consistent with the protective effect of the AT2R on aneurysm formation (Daugherty et al., 2001), perhaps indicating different mechanisms involved with atherosclerosis and aneurysm formation.
Recent studies from our own laboratory have revealed striking differences between the effects of chronic administration of either Ang II or Ang IV in ApoE-deficient mice fed a high fat diet. As expected, Ang II increased superoxide production and lesion formation in ApoE-deficient mice and worsened aortic endothelial function in wild type mice. By contrast, Ang IV caused vasoprotection in ApoE-deficient mice since there was a marked improvement in endothelial-mediated vasorelaxation that was accompanied by increased eNOS expression and reduced superoxide activity. Moreover, this action of Ang IV to increase NO biovailability was mediated by both AT2R- and AT4R-dependent mechanisms (Vinh et al., 2008a, Vinh et al., 2008b).
The anti-growth effects of AT2R have also been reported for neointimal formation following vascular injury. The RAS has been implicated in neointimal formation mainly via the activation of the AT1R (Kim et al., 1995, Viswanathan et al., 1992). Nakajima et al. (1995) first reported AT2R involvement in neointimal formation in rats by transfecting an AT2R expression vector into the balloon-injured rat carotid artery and observed that over-expression of AT2R attenuated neointimal formation. Similarly, cuff-induced inflammation and subsequent neointimal formation in the femoral artery was substantially enhanced in AT2R knockout mice compared with wild type (Akishita et al., 2000a, Wu et al., 2001), consistent with an increased AT2R expression in neointima of wild type mice (Suzuki et al., 2002). It has also been demonstrated that AT2R knockout mice show decreased apoptosis in the neointima which is consistent with the increased neointimal formation observed following cuff placement (Suzuki et al., 2002). Furthermore, the protective effects of AT1R blockade using valsartan were decreased in AT2R knockout mice, suggesting involvement of AT2R stimulation in the protection associated with AT1R blockade (Wu et al., 2001). More recently, the effects of a sartan (valsartan) have been compared with an ACE inhibitor (benazepril) on vascular remodeling following carotid artery balloon injury. Interestingly, both RAS inhibitors caused anti-remodelling of balloon-injured rat carotid arteries, but by different molecular mechanisms since only valsartan caused increased AT2R expression together with increased cGMP production (Barker et al., 2006).
Thus, with a few minor exceptions (Daugherty et al., 2004, Johansson et al., 2005), AT2R exerts vasoprotective effects and substantial anti-remodelling of atherosclerotic lesions and neointimal formation.
3.8. Aging
Aging is associated with specific cardiovascular changes including cardiac hypertrophy and fibrosis, vascular stiffening and endothelial dysfunction. These cardiovascular adaptations are seen in both elderly normotensive and hypertensive subjects (Lakatta & Levy, 2003), indicating that modifications are more than a mere consequence of confounding cardiovascular complications such as arterial hypertension, decreased aortic compliance and atherosclerosis, which are particularly prevalent in the elderly population. Circulating levels of Ang II are reduced by aging whereas angiotensinogen, ACE, and AT1R are upregulated in both the heart and vasculature (Heymes et al., 1994, Heymes et al., 1998, Wang et al., 2003). AT1R blockade and ACE inhibition have been shown to be effective in preventing or regressing age-associated effects on cardiovascular remodelling and function in humans and animals (Burrell and Johnston, 1997, Basso et al., 2005).
Importantly, although cardiac AT2R expression is relatively low in the adult rat heart (Busche et al., 2000), expression may be upregulated in certain disease states which are particularly common in the elderly, such cardiac fibrosis (Tsutsumi et al., 1998), hypertrophy (Lopez et al., 1994), and heart failure (Ohkubo et al., 1997). Surprisingly, only study has investigated the influence of senescence on AT2R expression in the heart. Heymes et al. (1998) found augmented AT2R expression in both the left and right ventricles of aged rats, as well as in freshly isolated cardiomyocytes from aged rats. Moreover, there was a correlation between AT2R expression and fibrosis in the cardiac tissue. Considering the demonstrated involvement of AT2R stimulation to the cardioprotective effects of AT1R inhibition in adults, it is tempting to speculate that the AT2R may also play an important role in the regulation of cardiovascular aging. Indeed, we have shown that AT2R contribute to the effects of AT1R inhibition in 20 month old WKY rats, since beneficial actions of candesartan cilexetil administration on both aortic remodeling and cardiac fibrosis were completely abolished by simultaneous PD123319 treatment (Jones et al., 2004).
In terms of vascular AT2R, the vasoconstrictor response of human isolated coronary microarteries to Ang II was potentiated by PD123319, as seen in in vitro animal studies (Zwart et al., 1998, Hannan et al., 2003), and the magnitude of this effect was positively correlated with the age of the donor (Batenburg et al., 2004). This finding suggests increased functional effects of vascular AT2R with aging, at least in coronary vasculature. More recently, Pinaud et al. (2007) found increased AT2R expression in mesenteric arteries that was predominantly localised to the medial smooth muscle layer since confocal imaging of fluorescent Ang II in the presence of candesartan revealed a decrease of AT2R in the endothelium. Strikingly, in these vessels, the AT2R phenotype was that of superoxide-mediated contraction, as it was blocked by antioxidant treatment (Pinaud et al., 2007). The effect of aging on aortic AT2R localisation has also recently been determined. Like in mesenteric arteries, there was a marked increase in AT2R immunofluorescence, predominantly in the media while AT2R expression appeared to be preserved in the endothelium (Widdop et al., 2008).
3.9. Gender
Gender differences exist in AT2R expression in the vasculature and kidney. AT2R expression in the kidney was generally higher in female, normotensive mice and rats compared with male counterparts; these changes were abolished by ovariectomy and oestrogen upregulated AT2R in various tissue compartments of the kidney in mice (Armando et al., 2002) and rats (Baiardi et al., 2005). This gender effect was particularly evident in the renal vasculature of the rat since AT2R were only detected in arcuate arteries and veins from female rats (Baiardi et al., 2005). An increased ratio of AT2R/AT1R was also found in kidneys and vasculature of female SHR compared to male SHR. This effect was due to reciprocal changes in AT1R (decreased) and AT2R (increased) in the kidneys whereas AT1R, but not AT2R, was downregulated in aortic and mesenteric vessels of female SHR, in an oestrogen-sensitive manner (Silva-Antonialli et al., 2004).
Gender-specific modulation of cardiovascular disease, and the treatment thereof, represents an important area for future research (Regitz-Zagrosek, 2006). Indeed, losartan reduced BP more effectively within 24 h in female SHR than male SHR (Silva-Antonialli et al., 2000). Moreover, the same group has recently reported that losartan increased vascular AT2R expression after either 24 hour- or 15 day-treatment in female, but not male, SHR, which was associated with the restoration of endothelial function (de P Rodrigues et al., 2006). In addition, (Okumura et al., 2005) reported that the upregulation of vascular AT2R, but not AT1R, following cuff injury was greater in female mice, and was associated with a greater reduction in neointimal formation as well as in indices of inflammation and oxidative stress in females versus males. Moreover, valsartan caused greater vessel anti-remodelling in wild type females whereas this sex difference was less marked in AT2R knockouts (Okumura et al., 2005). Recently, we have made the remarkable finding that chronic low-dose Ang II infusion lowered BP in conscious normotensive female, but not male, rats. This effect occurred via AT2R-dependent mechanisms and involved enhanced renal AT2R expression (Sampson et al., 2008).
3.10. AT2R clinical context
There is much less clinical data pertaining to the effects of AT2R. Sartans have similar clinical benefit to ACE inhibitors in the majority of cardiovascular diseases. Indeed, large-scale clinical trials have been unable to unequivocally discriminate between these 2 classes of drugs, emphasising the prime importance of disrupting AT1R function. However, this does not necessarily rule out subtle effects of AT2R contributing to the beneficial effects of AT1R antagonists in much the same way that BK may contribute to the effects of ACE inhibition.
One area of uncertainty lies in the use of sartans in high cardiovascular risk patients. The VALUE trial reported a relative increase in first time MI (fatal and non-fatal) in patients on valsartan compared with amlodipine, although there were early differences in BP-reduction between treatment arms which may have contributed to differences in MI rates (Julius et al., 2004). Based on the results of this trial, Verma & Strauss (2004) initiated a debate that is still ongoing, as to the potential harm of sartans for MI in high-risk patients (Verma & Strauss, 2004) (for detailed review, see Strauss and Hall, 2006, Tsuyuki and McDonald, 2006). As explanation for potential differences between sartans and ACE inhibitors, it was argued that the ability of AT1R antagonists to indirectly facilitate AT2R stimulation may contribute to the risk of MI in high-risk patients (Strauss & Hall, 2006). On this point, they provided pre-clinical evidence on the potentially deleterious effects of AT2R stimulation from limited experimental studies (Strauss & Hall, 2006), although a much larger body of evidence, as outlined in this review, supporting a cardioprotective role for AT2R was overlooked. Indeed, results from the recently completed ONTARGET trial, which evaluated the effects of telmisartan either alone, in combination with ramipril, or ramipril alone, in patients with vascular disease or high-risk diabetes, saw no difference in the incidence of the primary endpoints of cardiovascular death, MI, stroke or heart failure in any treatment arm (Yusuf et al., 2008).
In the meantime, the AT2R gene polymorphism -1332G/A has also been linked with cardiovascular pathologies such as LVH and premature coronary artery disease, although the interpretation of these data is inconsistent, since either beneficial or deleterious effects of AT2R have been reported (see Section 3.2). Clinical studies determining forearm vasodilatation or arterial stiffness in small groups of healthy subjects or patients on sartans or with insulin resistance have provided evidence of functional vascular AT2R, but with some variation likely reflecting the heterogeneous patient populations examined (see Section 3.1). Finally, as outlined, there is a growing body of literature from ex vivo human data which implicates a cardio- and vaso-protective effect of AT2R. For example there is a marked upregulation of AT2R that is thought to exert anti-fibrotic effects in human heart failure (see Section 3.3); and vasorelaxation has been directly demonstrated in human isolated coronary (Batenburg et al., 2004) and gluteal (Savoia et al., 2006b) vasculatures.
3.11. Limitations and future directions
There are some limitations in the AT2R research just discussed that still confound interpretations and hamper future progress. In particular, there is a dearth of selective AT2R agonists and antagonists, with CGP42112 and PD123319, respectively, being the gold standards for comparison. While these compounds are excellent tools, studies using other compounds are needed since AT2R knockouts bred on different background strain have most likely contributed to some of the controversial findings regarding AT2R function. In the future, data using non-peptide drugs such as Compound 21, under both acute and chronic conditions, are likely to contribute to the understanding of AT2R function and its potential therapeutic role. Indeed, to date there are no data reporting chronic effects of direct pharmacological stimulation of AT2R to determine definitively any BP and remodelling effects while avoiding potentially confounding issues such as the use of genetically modified animal models, stimulation of AT2R by Ang II during AT1R inhibition, or inference of AT2R function during AT2R blockade.
4. Conclusions
Over the past decade, a variety of effects caused by stimulation of AT2R in the cardiovascular system have been reported. In this review, we have highlighted that many such AT2R effects, and AT2R itself, are upregulated in a range of cardiovascular disease states. Generally, AT2R mediates acute haemodynamic effects but do not always translate into marked changes in basal BP inferred from AT2R knockout and transgenic studies although further studies with AT2R agonists are required. On the other hand, AT2R exert substantial anti-growth and anti-remodelling effects that indirectly contributes ‘beyond BP control’ under chronic conditions. The experimental evidence overwhelmingly indicates that AT2R makes a major contribution to the beneficial structural and remodelling effects of ‘sartans’ although this involvement is difficult to gauge in the clinical context. Often, a pharmacological effect of AT2R is only unmasked during AT1R blockade suggesting crosstalk between ATR which may take the form of either functional, physical or signalling interactions between AT2R and AT1R. Clearly, the level of AT2R expression, relative to AT1R, in different pathological states, together with the relative mixture of angiotensin peptide fragments in the local milieu and the prevailing crosstalk, will determine end-organ response. Thus, there is still much to learn about the AT2R context.
Acknowledgments
The original research findings discussed in this review were supported by Project Grants from the National Health and Medical Research Council (NHMRC) of Australia (ID 143564; 384237 and 436823).
References
- Abadir P.M., Periasamy A., Carey R.M., Siragy H.M. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension. 2006;48:316–322. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- AbdAlla S., Lother H., Abdel-tawab A.M., Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- AbdAlla S., Lother H., el Massiery A., Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- AbdAlla S., Lother H., Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- Achard V., Boullu-Ciocca S., Desbriere R., Nguyen G., Grino M. Renin receptor expression in human adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R274–282. doi: 10.1152/ajpregu.00439.2005. [DOI] [PubMed] [Google Scholar]
- Akasu M., Urata H., Kinoshita A., Sasaguri M., Ideishi M., Arakawa K. Differences in tissue angiotensin II-forming pathways by species and organs in vitro. Hypertension. 1998;32:514–520. doi: 10.1161/01.hyp.32.3.514. [DOI] [PubMed] [Google Scholar]
- Akishita M., Horiuchi M., Yamada H., Zhang L., Shirakami G., Tamura K. Inflammation influences vascular remodeling through AT2 receptor expression and signaling. Physiol Genomics. 2000;2:13–20. doi: 10.1152/physiolgenomics.2000.2.1.13. [DOI] [PubMed] [Google Scholar]
- Akishita M., Ito M., Lehtonen J.Y., Daviet L., Dzau V.J., Horiuchi M. Expression of the AT2 receptor developmentally programs extracellular signal-regulated kinase activity and influences fetal vascular growth. J Clin Invest. 1999;103:63–71. doi: 10.1172/JCI5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akishita M., Iwai M., Wu L., Zhang L., Ouchi Y., Dzau V.J. Inhibitory effect of angiotensin II type 2 receptor on coronary arterial remodeling after aortic banding in mice. Circulation. 2000;102:1684–1689. doi: 10.1161/01.cir.102.14.1684. [DOI] [PubMed] [Google Scholar]
- Albiston A.L., McDowall S.G., Matsacos D., Sim P., Clune E., Mustafa T. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem. 2001;276:48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- Alfakih K., Lawrance R.A., Maqbool A., Walters K., Ball S.G., Balmforth A.J. The clinical significance of a common, functional, X-linked angiotensin II type 2-receptor gene polymorphism (-1332 G/A) in a cohort of 509 families with premature coronary artery disease. Eur Heart J. 2005;26:584–589. doi: 10.1093/eurheartj/ehi013. [DOI] [PubMed] [Google Scholar]
- Alfakih K., Maqbool A., Sivananthan M., Walters K., Bainbridge G., Ridgway J. Left ventricle mass index and the common, functional, X-linked angiotensin II type-2 receptor gene polymorphism (-1332 G/A) in patients with systemic hypertension. Hypertension. 2004;43:1189–1194. doi: 10.1161/01.HYP.0000128532.28165.77. [DOI] [PubMed] [Google Scholar]
- Allen A.M., Zhuo J., Mendelsohn F.A. Localization of angiotensin AT1 and AT2 receptors. J Am Soc Nephrol. 1999;10:S23–29. [PubMed] [Google Scholar]
- Ardaillou R., Chansel D. Synthesis and effects of active fragments of angiotensin II. Kidney Int. 1997;52:1458–1468. doi: 10.1038/ki.1997.476. [DOI] [PubMed] [Google Scholar]
- Armando I., Jezova M., Juorio A.V., Terron J.A., Falcon-Neri A., Semino-Mora C. Estrogen upregulates renal angiotensin II AT(2) receptors. Am J Physiol Renal Physiol. 2002;283:F934–943. doi: 10.1152/ajprenal.00145.2002. [DOI] [PubMed] [Google Scholar]
- Arnold J.M., Yusuf S., Young J., Mathew J., Johnstone D., Avezum A. Prevention of Heart Failure in Patients in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:1284–1290. doi: 10.1161/01.cir.0000054165.93055.42. [DOI] [PubMed] [Google Scholar]
- Arun K.H., Kaul C.L., Poduri R. Tempol augments angiotensin II-induced AT2 receptor-mediated relaxation in diabetic rat thoracic aorta. J Hypertens. 2004;22:2143–2152. doi: 10.1097/00004872-200411000-00017. [DOI] [PubMed] [Google Scholar]
- Asano K., Dutcher D.L., Port J.D., Minobe W.A., Tremmel K.D., Roden R.L. Selective down-regulation of the angiotensin II AT1-receptor subtype in human ventricular myocardium. Circulation. 1997;95:1193–1200. doi: 10.1161/01.cir.95.5.1193. [DOI] [PubMed] [Google Scholar]
- Baiardi G., Macova M., Armando I., Ando H., Tyurmin D., Saavedra J.M. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regulatory Pept. 2005;124:7–17. doi: 10.1016/j.regpep.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Baker K.M., Aceto J.F. Angiotensin II stimulation of protein synthesis and cell growth in chick heart cells. Am J Physiol. 1990;259:H610–H618. doi: 10.1152/ajpheart.1990.259.2.H610. [DOI] [PubMed] [Google Scholar]
- Baker K.M., Booz G.W., Dostal D.E. Cardiac actions of angiotensin II: role of an intracardiac renin–angiotensin system. Annu Rev Physiol. 1992;54:227–241. doi: 10.1146/annurev.ph.54.030192.001303. [DOI] [PubMed] [Google Scholar]
- Barber M.N., Sampey D.B., Widdop R.E. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 1999;34:1112–1116. doi: 10.1161/01.hyp.34.5.1112. [DOI] [PubMed] [Google Scholar]
- Barker T.A., Massett M.P., Korshunov V.A., Mohan A.M., Kennedy A.J., Berk B.C. Angiotensin II type 2 receptor expression after vascular injury: differing effects of angiotensin-converting enzyme inhibition and angiotensin receptor blockade. Hypertension. 2006;48:942–949. doi: 10.1161/01.HYP.0000241061.51003.b7. [DOI] [PubMed] [Google Scholar]
- Bartunek J., Weinberg E.O., Tajima M., Rohrbach S., Lorell B.H. Angiotensin II type 2 receptor blockade amplifies the early signals of cardiac growth response to angiotensin II in hypertrophied hearts. Circulation. 1999;99:22–25. doi: 10.1161/01.cir.99.1.22. [DOI] [PubMed] [Google Scholar]
- Basso N., Paglia N., Stella I., de Cavanagh E.M., Ferder L., del Rosario Lores Arnaiz M. Protective effect of the inhibition of the renin–angiotensin system on aging. Regulatory Pept. 2005;128:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Batenburg W.W., de Bruin R.J., van Gool J.M., Muller D.N., Bader M., Nguyen G. Aliskiren-binding increases the half life of renin and prorenin in rat aortic vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1151–1157. doi: 10.1161/ATVBAHA.108.164210. [DOI] [PubMed] [Google Scholar]
- Batenburg W.W., Garrelds I.M., Bernasconi C.C., Juillerat-Jeanneret L., van Kats J.P., Saxena P.R. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- Batlle M., Roig E., Perez-Villa F., Lario S., Cejudo-Martin P., Garcia-Pras E. Increased expression of the renin–angiotensin system and mast cell density but not of angiotensin-converting enzyme II in late stages of human heart failure. J Heart Lung Transplant. 2006;25:1117–1125. doi: 10.1016/j.healun.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Bautista R., Sanchez A., Hernandez J., Oyekan A., Escalante B. Angiotensin II type AT(2) receptor mRNA expression and renal vasodilatation are increased in renal failure. Hypertension. 2001;38:669–673. doi: 10.1161/hy09t1.096186. [DOI] [PubMed] [Google Scholar]
- Bedecs K., Elbaz N., Sutren M., Masson M., Susini C., Strosberg A.D. Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J. 1997;325:449–454. doi: 10.1042/bj3250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benter I.F., Yousif M.H., Anim J.T., Cojocel C., Diz D.I. Angiotensin-(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with l-NAME. Am J Physiol Heart Circ Physiol. 2006;290:H684–691. doi: 10.1152/ajpheart.00632.2005. [DOI] [PubMed] [Google Scholar]
- Bockaert J., Marin P., Dumuis A., Fagni L. The ‛magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett. 2003;546:65–72. doi: 10.1016/s0014-5793(03)00453-8. [DOI] [PubMed] [Google Scholar]
- Bonnet F., Candido R., Carey R.M., Casley D., Russo L.M., Osicka T.M. Renal expression of angiotensin receptors in long-term diabetes and the effects of angiotensin type 1 receptor blockade. J Hypertens. 2002;20:1615–1624. doi: 10.1097/00004872-200208000-00025. [DOI] [PubMed] [Google Scholar]
- Booz G.W. Cardiac angiotensin AT2 receptor: what exactly does it do? Hypertension. 2004;43:1162–1163. doi: 10.1161/01.HYP.0000128531.39964.c0. [DOI] [PubMed] [Google Scholar]
- Bottari S.P., King I.N., Reichlin S., Dahlstroem I., Lydon N., de Gasparo M. The angiotensin AT2 receptor stimulates protein tyrosine phosphatase activity and mediates inhibition of particulate guanylate cyclase. Biochem Biophys Res Commun. 1992;183:206–211. doi: 10.1016/0006-291x(92)91629-5. [DOI] [PubMed] [Google Scholar]
- Boulanger C.M., Caputo L., Levy B.I. Endothelial AT1-mediated release of nitric oxide decreases angiotensin II contractions in rat carotid artery. Hypertension. 1995;26:752–757. doi: 10.1161/01.hyp.26.5.752. [DOI] [PubMed] [Google Scholar]
- Bouley R., Perodin J., Plante H., Rihakova L., Bernier S.G., Maletinska L. N- and C-terminal structure–activity study of angiotensin II on the angiotensin AT2 receptor. Eur J Pharmacol. 1998;343:323–331. doi: 10.1016/s0014-2999(97)01549-5. [DOI] [PubMed] [Google Scholar]
- Bove C.M., Gilson W.D., Scott C.D., Epstein F.H., Yang Z., Dimaria J.M. The angiotensin II type 2 receptor and improved adjacent region function post-MI. J Cardiovasc Magn Reson. 2005;7:459–464. [Google Scholar]
- Bove C.M., Yang Z., Gilson W.D., Epstein F.H., French B.A., Berr S.S. Nitric oxide mediates benefits of angiotensin II type 2 receptor overexpression during post-infarct remodeling. Hypertension. 2004;43:680–685. doi: 10.1161/01.HYP.0000115924.94236.91. [DOI] [PubMed] [Google Scholar]
- Brassard P., Amiri F., Schiffrin E.L. Combined angiotensin II type 1 and type 2 receptor blockade on vascular remodeling and matrix metalloproteinases in resistance arteries. Hypertension. 2005;46:598–606. doi: 10.1161/01.HYP.0000176744.15592.7d. [DOI] [PubMed] [Google Scholar]
- Brechler V., Reichlin S., De Gasparo M., Bottari S.P. Angiotensin II stimulates protein tyrosine phosphatase activity through a G-protein independent mechanism. Recept Channels. 1994;2:89–98. [PubMed] [Google Scholar]
- Brede M., Hadamek K., Meinel L., Wiesmann F., Peters J., Engelhardt S. Vascular hypertrophy and increased P70S6 kinase in mice lacking the angiotensin II AT(2) receptor. Circulation. 2001;104:2602–2607. doi: 10.1161/hc4601.099401. [DOI] [PubMed] [Google Scholar]
- Brede M., Roell W., Ritter O., Wiesmann F., Jahns R., Haase A. Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension. 2003;42:1177–1182. doi: 10.1161/01.HYP.0000100445.80029.8E. [DOI] [PubMed] [Google Scholar]
- Brenner B.M., Cooper M.E., de Zeeuw D., Keane W.F., Mitch W.E., Parving H.H. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- Brilla C.G., Zhou G., Matsubara L., Weber K.T. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994;26:809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- Brilla C.G., Zhou G., Rupp H., Maisch B., Weber K.T. Role of angiotensin II and prostaglandin E2 in regulating cardiac fibroblast collagen turnover. Am J Cardiol. 1995;76:8D–13D. doi: 10.1016/s0002-9149(99)80485-8. [DOI] [PubMed] [Google Scholar]
- Brillante D.G., Johnstone M.T., Howes L.G. Effects of intravenous PD 123319 on haemodynamic and arterial stiffness indices in healthy volunteers. J Renin-Angiotensin-Aldosterone Syst. 2005;6:102–106. doi: 10.3317/jraas.2005.007. [DOI] [PubMed] [Google Scholar]
- Brillante, D. G., O'Sullivan, A. J., Johnston, M. T., & Howes, L. G. (2008). Arterial stiffness and haemodynamic response to vasoactive medications in patients with insulin resistant syndrome. Clin Sci 114, 139−147. [DOI] [PubMed]
- Brink M., Erne P., de Gasparo M., Rogg H., Schmid A., Stulz P. Localization of the angiotensin II receptor subtypes in the human atrium. J Mol Cell Cardiol. 1996;28:1789–1799. doi: 10.1006/jmcc.1996.0168. [DOI] [PubMed] [Google Scholar]
- Brown L., Wall D., Marchant C., Sernia C. Tissue-specific changes in angiotensin II receptors in streptozotocin-diabetic rats. J Endocrinol. 1997;154:355–362. doi: 10.1677/joe.0.1540355. [DOI] [PubMed] [Google Scholar]
- Burckle C.A., Jan Danser A.H., Muller D.N., Garrelds I.M., Gasc J.M., Popova E. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension. 2006;47:552–556. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- Burrell L.M., Johnston C.I. Angiotensin II receptor antagonists. Potential in elderly patients with cardiovascular disease. Drugs Aging. 1997;10:421–434. doi: 10.2165/00002512-199710060-00003. [DOI] [PubMed] [Google Scholar]
- Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. discussion 22-4. [DOI] [PubMed] [Google Scholar]
- Busche S., Gallinat S., Bohle R.M., Reinecke A., Seebeck J., Franke F. Expression of angiotensin AT(1) and AT(2) receptors in adult rat cardiomyocytes after myocardial infarction. A single-cell reverse transcriptase-polymerase chain reaction study. Am J Pathol. 2000;157:605–611. doi: 10.1016/S0002-9440(10)64571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Campbell D.J. Endogenous angiotensin II levels and the mechanism of action of angiotensin-converting enzyme inhibitors and angiotensin receptor type 1 antagonists. Clin Exp Pharmacol Physiol Suppl. 1996;3:S125–131. [PubMed] [Google Scholar]
- Campbell D.J., Kladis A., Duncan A.M. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension. 1994;23:439–449. doi: 10.1161/01.hyp.23.4.439. [DOI] [PubMed] [Google Scholar]
- Campbell D.J., Kladis A., Valentijn A.J. Effects of losartan on angiotensin and bradykinin peptides and angiotensin-converting enzyme. J Cardiovasc Pharmacol. 1995;26:233–240. doi: 10.1097/00005344-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Campbell D.J., Lawrence A.C., Towrie A., Kladis A., Valentijn A.J. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–773. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- Cao Z., Bonnet F., Candido R., Nesteroff S.P., Burns W.C., Kawachi H. Angiotensin type 2 receptor antagonism confers renal protection in a rat model of progressive renal injury. J Am Soc Nephrol. 2002;13:1773–1787. doi: 10.1097/01.asn.0000019409.17099.33. [DOI] [PubMed] [Google Scholar]
- Cao Z., Dean R., Wu L., Casley D., Cooper M.E. Role of angiotensin receptor subtypes in mesenteric vascular proliferation and hypertrophy. Hypertension. 1999;34:408–414. doi: 10.1161/01.hyp.34.3.408. [DOI] [PubMed] [Google Scholar]
- Cao Z., Kelly D.J., Cox A., Casley D., Forbes J.M., Martinello P. Angiotensin type 2 receptor is expressed in the adult rat kidney and promotes cellular proliferation and apoptosis. Kidney Int. 2000;58:2437–2451. doi: 10.1046/j.1523-1755.2000.00427.x. [DOI] [PubMed] [Google Scholar]
- Capponi A.M. Distribution and signal transduction of angiotensin II AT1 and AT2 receptors. Blood pressure. Supplement. 1996;2:41–46. [PubMed] [Google Scholar]
- Caputo L., Benessiano J., Boulanger C.M., Levy B.I. Angiotensin II increases cGMP content via endothelial angiotensin II AT1 subtype receptors in the rat carotid artery. Arterioscler Thromb Vasc Biol. 1995;15:1646–1651. doi: 10.1161/01.atv.15.10.1646. [DOI] [PubMed] [Google Scholar]
- Carey R.M., Siragy H.M. Newly recognized components of the renin–angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- Carey R.M., Howell N.L., Jin X.H., Siragy H.M. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38:1272–1277. doi: 10.1161/hy1201.096576. [DOI] [PubMed] [Google Scholar]
- Caron A.Z., Arguin G., Guillemette G. Angiotensin IV interacts with a juxtamembrane site on AT(4)/IRAP suggesting an allosteric mechanism of enzyme modulation. Regulatory Pept. 2003;113:9–15. doi: 10.1016/s0167-0115(02)00294-x. [DOI] [PubMed] [Google Scholar]
- Castro C.H., Santos R.A., Ferreira A.J., Bader M., Alenina N., Almeida A.P. Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- Chai S.Y., Fernando R., Peck G., Ye S.Y., Mendelsohn F.A.O., Jenkins T.A. The angiotensin IV/AT4 receptor. Cell Mol Life Sci. 2004;61:2728–2737. doi: 10.1007/s00018-004-4246-1. [DOI] [PubMed] [Google Scholar]
- Chaki S., Inagami T. New signaling mechanism of angiotensin II in neuroblastoma neuro-2A cells: activation of soluble guanylyl cyclase via nitric oxide synthesis. Mol Pharmacol. 1993;43:603–608. [PubMed] [Google Scholar]
- Chapman N., Huxley R., Anderson C., Bousser G., Chalmers J., Colman S. Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history. Stroke. 2004;35:116–121. doi: 10.1161/01.STR.0000106480.76217.6F. [DOI] [PubMed] [Google Scholar]
- Chen S., Patel J.M., Block E.R. Angiotensin IV-mediated pulmonary artery vasorelaxation is due to endothelial intracellular calcium release. Am J Physiol Lung Cell Mol Physiol. 2000;279:L849–856. doi: 10.1152/ajplung.2000.279.5.L849. [DOI] [PubMed] [Google Scholar]
- Cheng H.F., Burns K.D., Harris R.C. Reduced proximal tubule angiotensin II receptor expression in streptozotocin-induced diabetes mellitus. Kidney Int. 1994;46:1603–1610. doi: 10.1038/ki.1994.458. [DOI] [PubMed] [Google Scholar]
- Chou T.C., Yen M.H., Li C.Y., Ding Y.A. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- Ciuffo G.M., Viswanathan M., Seltzer A.M., Tsutsumi K., Saavedra J.M. Glomerular angiotensin II receptor subtypes during development of rat kidney. Am J Physiol. 1993;265:F264–271. doi: 10.1152/ajprenal.1993.265.2.F264. [DOI] [PubMed] [Google Scholar]
- Coleman J.K., Krebs L.T., Hamilton T.A., Ong B., Lawrence K.A., Sardinia M.F. Autoradiographic identification of kidney angiotensin IV binding sites and angiotensin IV-induced renal cortical blood flow changes in rats. Peptides. 1998;19:269–277. doi: 10.1016/s0196-9781(97)00291-x. [DOI] [PubMed] [Google Scholar]
- Cosentino F., Savoia C., De Paolis P., Francia P., Russo A., Maffei A. Angiotensin II type 2 receptors contribute to vascular responses in spontaneously hypertensive rats treated with angiotensin II type 1 receptor antagonists. Am J Hypertens. 2005;18:493–499. doi: 10.1016/j.amjhyper.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Cote F., Do T.H., Laflamme L., Gallo J.M., Gallo-Payet N. Activation of the AT(2) receptor of angiotensin II induces neurite outgrowth and cell migration in microexplant cultures of the cerebellum. J Biol Chem. 1999;274:31686–31692. doi: 10.1074/jbc.274.44.31686. [DOI] [PubMed] [Google Scholar]
- Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Csikos T., Gallinat S., Unger T. Extrarenal aspects of angiotensin II function. Eur J Endocrinol. 1997;136:349–358. doi: 10.1530/eje.0.1360349. [DOI] [PubMed] [Google Scholar]
- Cui T., Nakagami H., Iwai M., Takeda Y., Shiuchi T., Tamura K. ATRAP, novel AT1 receptor associated protein, enhances internalization of AT1 receptor and inhibits vascular smooth muscle cell growth. Biochem Biophys Res Commun. 2000;279:938–941. doi: 10.1006/bbrc.2000.4055. [DOI] [PubMed] [Google Scholar]
- D'Amore A., Black M.J., Thomas W.G. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension. 2005;46:1347–1354. doi: 10.1161/01.HYP.0000193504.51489.cf. [DOI] [PubMed] [Google Scholar]
- Dahlof B., Devereux R.B., Kjeldsen S.E., Julius S., Beevers G., de Faire U. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Dai W., Funk A., Herdegen T., Unger T., Culman J. Blockade of central angiotensin AT1 receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in rats. Stroke. 1999;20:2391–2399. doi: 10.1161/01.str.30.11.2391. [DOI] [PubMed] [Google Scholar]
- Dandapat A., Hu C.P., Chen J., Liu Y., Khan J.A., Remeo F. Over-expression of angiotensin II type 2 receptor (agtr2) decreases collagen accumulation in atherosclerotic plaque. Biochem Biophys Res Commun. 2008;366:871–877. doi: 10.1016/j.bbrc.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Danser A.H., Deinum J. Renin, prorenin and the putative (pro)renin receptor. J Renin-Angiotensin-Aldosterone Syst. 2005;6:163–165. doi: 10.3317/jraas.2005.025. [DOI] [PubMed] [Google Scholar]
- Danser A.H., Derkx F.H., Schalekamp M.A., Hense H.W., Riegger G.A., Schunkert H. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J Hypertens. 1998;16:853–862. doi: 10.1097/00004872-199816060-00017. [DOI] [PubMed] [Google Scholar]
- Danser A.H., van Kats J.P., Admiraal P.J., Derkx F.H., Lamers J.M., Verdouw P.D. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension. 1994;24:37–48. doi: 10.1161/01.hyp.24.1.37. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Cassis L. Angiotensin II-mediated development of vascular diseases. Trends Cardiovasc Med. 2004;14:117–120. doi: 10.1016/j.tcm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Manning M.W., Cassis L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Manning M.W., Cassis L.A. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Rateri D.L., Lu H., Inagami T., Cassis L.A. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- Daviet L., Lehtonen J.Y., Tamura K., Griese D.P., Horiuchi M., Dzau V.J. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J Biol Chem. 1999;274:17058–17062. doi: 10.1074/jbc.274.24.17058. [DOI] [PubMed] [Google Scholar]
- de Gasparo M., Catt K.J., Inagami T., Wright J.W., Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- de P Rodrigues S.F., dos Santos R.A., Silva-Antonialli M.M., Scavone C., Nigro D., Carvalho M.H.C. Differential effect of losartan in female and male spontaneously hypertensive rats. Life Sci. 2006;78:2280–2285. doi: 10.1016/j.lfs.2005.09.049. [DOI] [PubMed] [Google Scholar]
- De Souza A.M., Lopes A.G., Pizzino C.P., Fossari R.N., Miguel N.C.O., Cardozo F.P. Angiotensin II and angiotensin-(1–7) inhibit the inner cortex Na+-ATPase activity through AT2 receptor. Regulatory Pept. 2004;120:167–175. doi: 10.1016/j.regpep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Diep Q.N., Li J.S., Schiffrin E.L. In vivo study of AT(1) and AT(2) angiotensin receptors in apoptosis in rat blood vessels. Hypertension. 1999;34:617–624. doi: 10.1161/01.hyp.34.4.617. [DOI] [PubMed] [Google Scholar]
- Diet F., Pratt R.E., Berry G.J., Momose N., Gibbons G.H., Dzau V.J. Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease. Circulation. 1996;94:2756–2767. doi: 10.1161/01.cir.94.11.2756. [DOI] [PubMed] [Google Scholar]
- Diez-Freire C., Vazquez J., Correa de Adjounian M.F., Ferrari M.F., Yuan L., Silver X. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Dudley D.T., Panek R.L., Major T.C., Lu G.H., Bruns R.F., Klinkefus B.A. Subclasses of angiotensin II binding sites and their functional significance. Mol Pharmacol. 1990;38:370–377. [PubMed] [Google Scholar]
- Duke L.M., Eppel G.A., Widdop R.E., Evans R.G. Disparate roles of AT2 receptors in the renal cortical and medullary circulations of anesthetized rabbits. Hypertension. 2003;42:200–205. doi: 10.1161/01.HYP.0000083341.64034.00. [DOI] [PubMed] [Google Scholar]
- Duke L.M., Evans R.G., Widdop R.E. AT2 receptors contribute to acute blood pressure-lowering and vasodilator effects of AT1 receptor antagonism in conscious normotensive but not hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;288:H2289–2297. doi: 10.1152/ajpheart.01096.2004. [DOI] [PubMed] [Google Scholar]
- Duke L.M., Widdop R.E., Kett M.M., Evans R.G. AT(2) receptors mediate tonic renal medullary vasoconstriction in renovascular hypertension. Br J Pharmacol. 2005;144:486–492. doi: 10.1038/sj.bjp.0706036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzau V.J. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- Esteban V., Ruperez M., Sanchez-Lopez E., Rodriguez-Vita J., Lorenzo O., Demaegdt H. Angiotensin IV activates the nuclear transcription factor-kappaB and related proinflammatory genes in vascular smooth muscle cells. Circ Res. 2005;96:965–973. doi: 10.1161/01.RES.0000166326.91395.74. [DOI] [PubMed] [Google Scholar]
- Falcon B.L., Stewart J.M., Bourassa E., Katovich M.J., Walter G., Speth R.C. Angiotensin II type 2 receptor gene transfer elicits cardioprotective effects in an angiotensin II infusion rat model of hypertension. Physiol Genomics. 2004;19:255–261. doi: 10.1152/physiolgenomics.00170.2004. [DOI] [PubMed] [Google Scholar]
- Falcon B.L., Veerasingham S.J., Sumners C., Raizada M.K. Angiotensin II type 2 receptor-mediated gene expression profiling in human coronary artery endothelial cells. Hypertension. 2005;45:692–697. doi: 10.1161/01.HYP.0000154254.89733.29. [DOI] [PubMed] [Google Scholar]
- Faure S., Chapot R., Tallet D., Javellaud J., Achard J.M., Oudart N. Cerebroprotective effect of angiotensin IV in experimental ischemic stroke in the rat mediated by AT(4) receptors. J Physiol Pharmacol. 2006;57:329–342. [PubMed] [Google Scholar]
- Feldman D.L., Jin L., Xuan H., Contrepas A., Zhou Y., Webb R.L. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130–136. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- Feldt S., Batenburg W.W., Mazak I., Maschke U., Wellner M., Kvakan H. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension. 2008;51:682–688. doi: 10.1161/HYPERTENSIONAHA.107.101444. [DOI] [PubMed] [Google Scholar]
- Feldt S., Maschke U., Dechend R., Luft F.C., Muller D.N. The putative (pro)renin receptor blocker hrp fails to prevent (pro)renin signaling. J Am Soc Nephrol. 2008;19:743–748. doi: 10.1681/ASN.2007091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Chappell M.C., Tallant E.A., Brosnihan K.B., Diz D.I. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Iyer S.N. Angiotensin-(1–7): a bioactive fragment of the renin–angiotensin system. Regulatory Pept. 1998;78:13–18. doi: 10.1016/s0167-0115(98)00134-7. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Trask A.J., Jessup J.A. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A.J., Jacoby B.A., Araujo C.A., Macedo F.A., Silva G.A., Almeida A.P. The nonpeptide angiotensin-(1–7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H1113–1119. doi: 10.1152/ajpheart.00828.2006. [DOI] [PubMed] [Google Scholar]
- Fischer T.A., Singh K., O'Hara D.S., Kaye D.M., Kelly R.A. Role of AT1 and AT2 receptors in regulation of MAPKs and MKP-1 by ANG II in adult cardiac myocytes. Am J Physiol. 1998;275:H906–916. doi: 10.1152/ajpheart.1998.275.3.H906. [DOI] [PubMed] [Google Scholar]
- Fukuhara M., Geary R.L., Diz D.I., Gallagher P.E., Wilson J.A., Glazier S.S. Angiotensin-converting enzyme expression in human carotid artery atherosclerosis. Hypertension. 2000;35:353–359. doi: 10.1161/01.hyp.35.1.353. [DOI] [PubMed] [Google Scholar]
- Gallinat S., Busche S., Schutze S., Kronke M., Unger T. AT2 receptor stimulation induces generation of ceramides in PC12W cells. FEBS Lett. 1999;443:75–79. doi: 10.1016/s0014-5793(98)01675-5. [DOI] [PubMed] [Google Scholar]
- Georgsson J., Rosenstrom U., Wallinder C., Beaudry H., Plouffe B., Lindeberg G. Short pseudopeptides containing turn scaffolds with high AT2 receptor affinity. Bioorganic Med Chem. 2006;14:5963–5972. doi: 10.1016/j.bmc.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Georgsson J., Skold C., Botros M., Lindeberg G., Nyberg F., Karlen A. Synthesis of a new class of druglike angiotensin II C-terminal mimics with affinity for the AT2 receptor. J Med Chem. 2007;50:1711–1715. doi: 10.1021/jm0613469. [DOI] [PubMed] [Google Scholar]
- Georgsson J., Skold C., Plouffe B., Lindeberg G., Botros M., Larhed M. Angiotensin II pseudopeptides containing 1,3,5-trisubstituted benzene scaffolds with high AT2 receptor affinity. J Med Chem. 2005;48:6620–6631. doi: 10.1021/jm050280z. [DOI] [PubMed] [Google Scholar]
- Gigante B., Piras O., De Paolis P., Porcellini A., Natale A., Volpe M. Role of the angiotensin II AT2-subtype receptors in the blood pressure-lowering effect of losartan in salt-restricted rats. J Hypertens. 1998;16:2039–2043. doi: 10.1097/00004872-199816121-00027. [DOI] [PubMed] [Google Scholar]
- Gilbert R.E., Krum H., Wilkinson-Berka J., Kelly D.J. The renin–angiotensin system and the long-term complications of diabetes: pathophysiological and therapeutic considerations. Diabet Med. 2003;20:607–621. doi: 10.1046/j.1464-5491.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- Gilles R., Vingerhoedt N., Howes J., Griffin M., Howes L.G. Increase in systemic blood pressure during intra-arterial PD123319 infusion: evidence for functional expression of angiotensin type 2 receptors in normal volunteers. Blood Press. 2004;13:110–114. doi: 10.1080/08037050310031017. [DOI] [PubMed] [Google Scholar]
- Gohlke P., Pees C., Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–355. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- Goulter A.B., Goddard M.J., Allen J.C., Clark K.L. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2:19. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos T., Morris K., Ferguson P., Weyhenmeyer J. Angiotensin protects cortical neurons from hypoxic-induced apoptosis via the angiotensin type 2 receptor. Brain Res Mol Brain Res. 2002;99:114–124. doi: 10.1016/s0169-328x(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Griendling K.K., Lassegue B., Alexander R.W. Angiotensin receptors and their therapeutic implications. Annu Rev Pharmacol Toxicol. 1996;36:281–306. doi: 10.1146/annurev.pa.36.040196.001433. [DOI] [PubMed] [Google Scholar]
- Griendling K.K., Minieri C.A., Ollerenshaw J.D., Alexander R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Grobe J.L., Mecca A.P., Lingis M., Shenoy V., Bolton T.A., Machado J.M. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7) Am J Physiol Heart Circ Physiol. 2007;292(2):H736–742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- Grobe J.L., Mecca A.P., Mao H., Katovich M.J. Chronic angiotensin-(1–7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H2417–2423. doi: 10.1152/ajpheart.01170.2005. [DOI] [PubMed] [Google Scholar]
- Gross V., Milia A.F., Plehm R., Inagami T., Luft F.C. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J Hypertens. 2000;18:955–961. doi: 10.1097/00004872-200018070-00018. [DOI] [PubMed] [Google Scholar]
- Gross V., Schunck W.H., Honeck H., Milia A.F., Kargel E., Walther T. Inhibition of pressure natriuresis in mice lacking the AT2 receptor. Kidney Int. 2000;57:191–202. doi: 10.1046/j.1523-1755.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- Gross V.A., Obst M.A., Kiss E.C., Janke J.B., Mazak I.B., Shagdarsuren E.B. Cardiac hypertrophy and fibrosis in chronic l-NAME-treated AT2 receptor-deficient mice. J Hypertens. 2004;22:997–1005. doi: 10.1097/00004872-200405000-00023. [DOI] [PubMed] [Google Scholar]
- Groth W., Blume A., Golhlke P., Unger T., Culman J. Chronic pretreatment with candersartan improves recovery from focal cerebral ischemia in rats. J Hypertens. 2003;21:2175–2182. doi: 10.1097/00004872-200311000-00028. [DOI] [PubMed] [Google Scholar]
- Guo D.F., Chenier I., Tardif V., Orlov S.N., Inagami T. Type 1 angiotensin II receptor-associated protein ARAP1 binds and recycles the receptor to the plasma membrane. Biochem Biophys Res Commun. 2003;310:1254–1265. doi: 10.1016/j.bbrc.2003.09.154. [DOI] [PubMed] [Google Scholar]
- Guo P., Nishiyama A., Rahman M., Nagai Y., Noma T., Namba T. Contribution of reactive oxygen species to the pathogenesis of left ventricular failure in Dahl salt-sensitive hypertensive rats: effects of angiotensin II blockade. J Hypertens. 2006;24:1097–1104. doi: 10.1097/01.hjh.0000226200.73065.5d. [DOI] [PubMed] [Google Scholar]
- Gurley S.B., Allred A., Le T.H., Griffiths R., Mao L., Philip N. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest. 2006;116:2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie G.P., Jr. Angiotensin receptors: physiology and pharmacology. Clin Cardiol. 1995;18(III):29–34. doi: 10.1002/clc.4960181507. [DOI] [PubMed] [Google Scholar]
- Haithcock D., Jiao H., Cui X.L., Hopfer U., Douglas J.G. Renal proximal tubular AT2 receptor: signaling and transport. J Am Soc Nephrol. 1999;10(Suppl 11):S69–S74. [PubMed] [Google Scholar]
- Hakam A.C., Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension. 2005;45:270–275. doi: 10.1161/01.HYP.0000151622.47814.6f. [DOI] [PubMed] [Google Scholar]
- Hakam A.C., Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol. 2006;290:F1430–1436. doi: 10.1152/ajprenal.00218.2005. [DOI] [PubMed] [Google Scholar]
- Hakam A.C., Hussain T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not in lean Zucker rats. Hypertension. 2006;47:1117–1124. doi: 10.1161/01.HYP.0000220112.91724.fc. [DOI] [PubMed] [Google Scholar]
- Hakam A.C., Siddiqui A.H., Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006;290:F503–508. doi: 10.1152/ajprenal.00092.2005. [DOI] [PubMed] [Google Scholar]
- Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus A.D.M.E. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan R.E., Davis E.A., Widdop R.E. Functional role of angiotensin II AT2 receptor in modulation of AT1 receptor-mediated contraction in rat uterine artery: involvement of bradykinin and nitric oxide. Br J Pharmacol. 2003;140:987–995. doi: 10.1038/sj.bjp.0705484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan R.E., Widdop R.E. Vascular angiotensin II actions mediated by angiotensin II type 2 receptors. Curr Hypertens Rep. 2004;6:117–123. doi: 10.1007/s11906-004-0086-5. [DOI] [PubMed] [Google Scholar]
- Hansen J.L., Servant G., Baranski T.J., Fujita T., Iiri T., Sheikh S.P. Functional reconstitution of the angiotensin II type 2 receptor and G(i) activation. Circ Res. 2000;87:753–759. doi: 10.1161/01.res.87.9.753. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Maeshima Y., Satoh M., Odawara M., Sugiyama H., Kashihara N. Overexpression of angiotensin type 2 receptor ameliorates glomerular injury in a mouse remnant kidney model. Am J Physiol Renal Physiol. 2004;286:F516–525. doi: 10.1152/ajprenal.00294.2003. [DOI] [PubMed] [Google Scholar]
- Haywood G.A., Gullestad L., Katsuya T., Hutchinson H.G., Pratt R.E., Horiuchi M. AT1 and AT2 angiotensin receptor gene expression in human heart failure. Circulation. 1997;95:1201–1206. doi: 10.1161/01.cir.95.5.1201. [DOI] [PubMed] [Google Scholar]
- Hein L., Barsh G.S., Pratt R.E., Dzau V.J., Kobilka B.K. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- Hein L., Meinel L., Pratt R.E., Dzau V.J., Kobilka B.K. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11:1266–1277. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- Heitsch H., Brovkovych S., Malinski T., Wiemer G. Angiotensin-(1–7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- Henrion D., Kubis N., Levy B.I. Physiological and pathophysiological functions of the AT(2) subtype receptor of angiotensin II: from large arteries to the microcirculation. Hypertension. 2001;38:1150–1157. doi: 10.1161/hy1101.096109. [DOI] [PubMed] [Google Scholar]
- Henriques T.A., Huang J., D'Souza S.S., Daugherty A., Cassis L.A. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- Heymes C., Silvestre J.S., Llorens-Cortes C., Chevalier B., Marotte F., Levy B.I. Cardiac senescence is associated with enhanced expression of angiotensin II receptor subtypes. Endocrinology. 1998;139:2579–2587. doi: 10.1210/endo.139.5.6023. [DOI] [PubMed] [Google Scholar]
- Heymes C., Swynghedauw B., Chevalier B. Activation of angiotensinogen and angiotensin-converting enzyme gene expression in the left ventricle of senescent rats. Circulation. 1994;90:1328–1333. doi: 10.1161/01.cir.90.3.1328. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N., Kapturczak M.H., Block E.R., Patel J.M., Malinski T., Madsen K.M. Angiotensin II-stimulated nitric oxide release from porcine pulmonary endothelium is mediated by angiotensin IV. J Am Soc Nephrol. 1999;10:481–491. doi: 10.1681/ASN.V103481. [DOI] [PubMed] [Google Scholar]
- Hiyoshi H., Yayama K., Takano M., Okamoto H. Angiotensin type 2 receptor-mediated phosphorylation of eNOS in the aortas of mice with 2-kidney, 1-clip hypertension. Hypertension. 2005;45:967–973. doi: 10.1161/01.HYP.0000164571.77710.19. [DOI] [PubMed] [Google Scholar]
- Hiyoshi H., Yayama K., Takano M., Okamoto H. Stimulation of cyclic GMP production via AT2 and B2 receptors in the pressure-overloaded aorta after banding. Hypertension. 2004;43:1258–1263. doi: 10.1161/01.HYP.0000128022.24598.4f. [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Hayashida W., Kambe T., Yamada T., Dzau V.J. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997;272:19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- Hu, C., Dandapat, A., Chen, J., Liu, Y., Hermonat, P. L., Carey, R. M., et al. (2008). Over-expression of angiotensin II type 2 receptor (agtr2) reduces atherogenesis and modulates LOX-1, endothelial nitric oxide synthase and heme-oxygenase-1 expression. Atherosclerosis 199, 288−294. [DOI] [PubMed]
- Huang Y., Wongamorntham S., Kasting J., McQuillan D., Owens R.T., Yu L. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- Huentelman M.J., Grobe J.L., Vazquez J., Stewart J.M., Mecca A.P., Katovich M.J. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- Ichihara A., Hayashi M., Kaneshiro Y., Suzuki F., Nakagawa T., Tada Y. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A., Kaneshiro Y., Takemitsu T., Sakoda M., Suzuki F., Nakagawa T. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension. 2006;47:894–900. doi: 10.1161/01.HYP.0000215838.48170.0b. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Senbonmatsu T., Price E., Jr., Ichiki T., Gaffney F.A., Inagami T. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation. 2001;104:346–351. doi: 10.1161/01.cir.104.3.346. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Senbonmatsu T., Price E., Jr., Ichiki T., Gaffney F.A., Inagami T. Targeted deletion of angiotensin II type 2 receptor caused cardiac rupture after acute myocardial infarction. Circulation. 2002;106:2244–2249. doi: 10.1161/01.cir.0000033826.52681.37. [DOI] [PubMed] [Google Scholar]
- Ichiki T., Labosky P.A., Shiota C., Okuyama S., Imagawa Y., Fogo A. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- Isbell D.C., Voros S., Yang Z., DiMaria J.M., Berr S.S., French B.A. Interaction between bradykinin subtype 2 and angiotensin II type 2 receptors during post-MI left ventricular remodeling. Am J Physiol Heart Circ Physiol. 2007;293(6):H3372–H3378. doi: 10.1152/ajpheart.00997.2007. [DOI] [PubMed] [Google Scholar]
- Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Ito T., Yamakawa H., Bregonzio C., Terron J., Falcon-Neri A., Saavedra J. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002;33:2297–2303. doi: 10.1161/01.str.0000027274.03779.f3. [DOI] [PubMed] [Google Scholar]
- Iwai M., Chen R., Li Z., Shiuchi T., Suzuki J., Ide A. Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation. 2005;112:1636–1643. doi: 10.1161/CIRCULATIONAHA.104.525550. [DOI] [PubMed] [Google Scholar]
- Iwai M., Liu H., Chen R., I. A., Okamoto S., Hata R., Sakanaka M. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation. 2004;110 doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]
- Jacobs L.S., Douglas J.G. Angiotensin II type 2 receptor subtype mediates phospholipase A2-dependent signaling in rabbit proximal tubular epithelial cells. Hypertension. 1996;28:663–668. doi: 10.1161/01.hyp.28.4.663. [DOI] [PubMed] [Google Scholar]
- Jaiswal N., Tallant E.A., Jaiswal R.K., Diz D.I., Ferrario C.M. Differential regulation of prostaglandin synthesis by angiotensin peptides in porcine aortic smooth muscle cells: subtypes of angiotensin receptors involved. J Pharmacol Exp Ther. 1993;265:664–673. [PubMed] [Google Scholar]
- Jandeleit-Dahm K., Burrell L.M., Johnston C.I., Koch K.M. Elevated vascular angiotensin converting enzyme mediates increased neointima formation after balloon injury in spontaneously hypertensive rats. J Hypertens. 1997;15:643–650. doi: 10.1097/00004872-199715060-00011. [DOI] [PubMed] [Google Scholar]
- Jankowski V., Vanholder R., van der Giet M., Henning L., Tolle M., Schonfelder G. Detection of angiotensin II in supernatants of stimulated mononuclear leukocytes by matrix-assisted laser desorption ionization time-of-flight/time-of-flight mass analysis. Hypertension. 2005;46:591–597. doi: 10.1161/01.HYP.0000177436.09733.d4. [DOI] [PubMed] [Google Scholar]
- Jensen C., Herold P., Brunner H.R. Aliskiren: the first renin inhibitor for clinical treatment. Nat Rev Drug Discov. 2008;7:399–410. doi: 10.1038/nrd2550. [DOI] [PubMed] [Google Scholar]
- Jin X.Q., Fukuda N., Su J.Z., Lai Y.M., Suzuki R., Tahira Y. Angiotensin II type 2 receptor gene transfer downregulates angiotensin II type 1a receptor in vascular smooth muscle cells. Hypertension. 2002;39:1021–1027. doi: 10.1161/01.hyp.0000016179.52601.b4. [DOI] [PubMed] [Google Scholar]
- Jin X.H., McGrath H.E., Gildea J.J., Siragy H.M., Felder R.A., Carey R.M. Renal interstitial guanosine cyclic 3′, 5′-monophosphate mediates pressure–natriuresis via protein kinase G. Hypertension. 2004;43:1133–1139. doi: 10.1161/01.HYP.0000123574.60586.7d. [DOI] [PubMed] [Google Scholar]
- Jin X.H., Siragy H.M., Carey R.M. Renal interstitial cGMP mediates natriuresis by direct tubule mechanism. Hypertension. 2001;38:309–316. doi: 10.1161/01.hyp.38.3.309. [DOI] [PubMed] [Google Scholar]
- Johannesson P., Erdelyi M., Lindeberg G., Frandberg P.A., Nyberg F., Karlen A. AT2-selective angiotensin II analogues containing tyrosine-functionalized 5,5-bicyclic thiazabicycloalkane dipeptide mimetics. J Med Chem. 2004;47:6009–6019. doi: 10.1021/jm049651m. [DOI] [PubMed] [Google Scholar]
- Johansson M.E., Wickman A., Fitzgerald S.M., Gan L.M., Bergstrom G. Angiotensin II, type 2 receptor is not involved in the angiotensin II-mediated pro-atherogenic process in ApoE−/− mice. J Hypertens. 2005;23:1541–1549. doi: 10.1097/01.hjh.0000174078.95745.77. [DOI] [PubMed] [Google Scholar]
- Johnston C.I. Franz Volhard Lecture. Renin–angiotensin system: a dual tissue and hormonal system for cardiovascular control. J Hypertens Suppl. 1992;10:S13–S26. [PubMed] [Google Scholar]
- Jones E.S., Black M.J., Widdop R.E. Angiotensin AT2 receptor contributes to cardiovascular remodelling of aged rats during chronic AT1 receptor blockade. J Mol Cell Cardiol. 2004;37:1023–1030. doi: 10.1016/j.yjmcc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Julius S., Kjeldsen S.E., Weber M., Brunner H.R., Ekman S., Hansson L. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- Kalinyak J.E., Sechi L.A., Griffin C.A., Don B.R., Tavangar K., Kraemer F.B. The renin–angiotensin system in streptozotocin-induced diabetes mellitus in the rat. J Am Soc Nephrol. 1993;4:1337–1345. doi: 10.1681/ASN.V461337. [DOI] [PubMed] [Google Scholar]
- Kang J., Posner P., Sumners C. Angiotensin II type 2 receptor stimulation of neuronal K+ currents involves an inhibitory GTP binding protein. Am J Physiol. 1994;267:C1389–C1397. doi: 10.1152/ajpcell.1994.267.5.C1389. [DOI] [PubMed] [Google Scholar]
- Kaschina E., Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- Katwa L.C., Campbell S.E., Tyagi S.C., Lee S.J., Cicila G.T., Weber K.T. Cultured myofibroblasts generate angiotensin peptides de novo. J Mol Cell Cardiol. 1997;29:1375–1386. doi: 10.1006/jmcc.1997.0376. [DOI] [PubMed] [Google Scholar]
- Keidar S., Gamliel-Lazarovich A., Kaplan M., Pavlotzky E., Hamoud S., Hayek T. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97:946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- Keidar S., Kaplan M., Pavlotzky E., Coleman R., Hayek T., Hamoud S. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004;109:2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- Kijima K., Matsubara H., Murasawa S., Maruyama K., Mori Y., Ohkubo N. Mechanical stretch induces enhanced expression of angiotensin II receptor subtypes in neonatal rat cardiac myocytes. Circ Res. 1996;79:887–897. doi: 10.1161/01.res.79.4.887. [DOI] [PubMed] [Google Scholar]
- Kim M.P., Zhou M., Wahl L.M. Angiotensin II increases human monocyte matrix metalloproteinase-1 through the AT2 receptor and prostaglandin E2: implications for atherosclerotic plaque rupture. J Leukoc Biol. 2005;78:195–201. doi: 10.1189/jlb.1204715. [DOI] [PubMed] [Google Scholar]
- Kim S., Kawamura M., Wanibuchi H., Ohta K., Hamaguchi A., Omura T. Angiotensin II type 1 receptor blockade inhibits the expression of immediate-early genes and fibronectin in rat injured artery. Circulation. 1995;92:88–95. doi: 10.1161/01.cir.92.1.88. [DOI] [PubMed] [Google Scholar]
- Kintscher U., Unger T. Does the AT2-receptor mediate anti-atherosclerotic actions? J Hypertens. 2005;23:1469–1470. doi: 10.1097/01.hjh.0000174611.49274.1e. [DOI] [PubMed] [Google Scholar]
- Kokje R.J., Wilson W.L., Brown T.E., Karamyan V.T., Wright J.W., Speth R.C. Central pressor actions of aminopeptidase-resistant angiotensin II analogs: challenging the angiotensin III hypothesis. Hypertension. 2007;49:1328–1335. doi: 10.1161/HYPERTENSIONAHA.107.087130. [DOI] [PubMed] [Google Scholar]
- Kostenis E., Milligan G., Christopoulos A., Sanchez-Ferrer C.F., Heringer-Walther S., Sexton P.M. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- Kramar E.A., Harding J.W., Wright J.W. Angiotensin II- and IV-induced changes in cerebral blood flow. Roles of AT1, AT2, and AT4 receptor subtypes. Regulatory Pept. 1997;68:131–138. doi: 10.1016/s0167-0115(96)02116-7. [DOI] [PubMed] [Google Scholar]
- Kumar D., Zimpelmann J., Robertson S., Burns K.D. Tubular and interstitial cell apoptosis in the streptozotocin-diabetic rat kidney. Nephron Exp Nephrol. 2004;96:e77–88. doi: 10.1159/000076749. [DOI] [PubMed] [Google Scholar]
- Kurisu S., Ozono R., Oshima T., Kambe M., Ishida T., Sugino H. Cardiac Angiotensin II Type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension. 2003;41:99–107. doi: 10.1161/01.hyp.0000050101.90932.14. [DOI] [PubMed] [Google Scholar]
- Laflamme L., Gasparo M., Gallo J., Payet M., Gallo-Payet N. Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108-15 cells. J Biol Chem. 1996;271:22729–22735. doi: 10.1074/jbc.271.37.22729. [DOI] [PubMed] [Google Scholar]
- Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Lako-Futo Z., Szokodi I., Sarman B., Foldes G., Tokola H., Ilves M. Evidence for a functional role of angiotensin II type 2 receptor in the cardiac hypertrophic process in vivo in the rat heart. Circulation. 2003;108:2414–2422. doi: 10.1161/01.CIR.0000093193.63314.D9. [DOI] [PubMed] [Google Scholar]
- Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara Lda S., Cavalcante F., Axelband F., De Souza A.M., Lopes A.G., Caruso-Neves C. Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1–7) Biochem J. 2006;395:183–190. doi: 10.1042/BJ20051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Mustafa T., McDowall S.G., Mendelsohn F.A.O., Brennan M., Lew R.A. Structure–activity study of LVV-hemorphin-7: angiotensin AT4 receptor ligand and inhibitor of insulin-regulated aminopeptidase. J Pharmacol Exp Ther. 2003;305:205–211. doi: 10.1124/jpet.102.045492. [DOI] [PubMed] [Google Scholar]
- Lehtonen J.Y., Horiuchi M., Daviet L., Akishita M., Dzau V.J. Activation of the de novo biosynthesis of sphingolipids mediates angiotensin II type 2 receptor-induced apoptosis. J Biol Chem. 1999;274:16901–16906. doi: 10.1074/jbc.274.24.16901. [DOI] [PubMed] [Google Scholar]
- Lemos V.S., Silva D.M., Walther T., Alenina N., Bader M., Santos R.A. The endothelium-dependent vasodilator effect of the nonpeptide Ang(1–7) mimic AVE 0991 is abolished in the aorta of mas-knockout mice. J Cardiovasc Pharmacol. 2005;46:274–279. doi: 10.1097/01.fjc.0000175237.41573.63. [DOI] [PubMed] [Google Scholar]
- Leung P.S., Chan H.C., Fu L.X., Wong P.Y. Localization of angiotensin II receptor subtypes AT1 and AT2 in the pancreas of rodents. J Endocrinol. 1997;153:269–274. doi: 10.1677/joe.0.1530269. [DOI] [PubMed] [Google Scholar]
- Levy D., Garrison R.J., Savage D.D., Kannel W.B., Castelli W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- Lew R.A., Mustafa T., Ye S., McDowall S.G., Chai S.Y., Albiston A.L. Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP) J Neurochem. 2003;86:344–350. doi: 10.1046/j.1471-4159.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- Lewis E.J., Hunsicker L.G., Clarke W.R., Berl T., Pohl M.A., Lewis J.B. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- Li C., Cao L., Zeng Q., Liu X., Zhang Y., Dai T. Taurine may prevent diabetic rats from developing cardiomyopathy also by downregulating angiotensin II type2 receptor expression. Cardiovasc Drugs Ther. 2005;19:105–112. doi: 10.1007/s10557-005-0443-x. [DOI] [PubMed] [Google Scholar]
- Li H., Gao Y., Grobe J.L., Raizada M.K., Katovich M., Sumners C. Potentiation of the antihypertensive action of losartan by peripheral over expression of the angiotensin II (Ang II) type 2 receptor. Am J Physiol Heart Circ Physiol. 2006;292(2):H727–H735. doi: 10.1152/ajpheart.00938.2006. [DOI] [PubMed] [Google Scholar]
- Li J., Culman J., Hortnagl H., Zhao Y., Gerova N., Timm M. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 2005;10 doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.C., Widdop R.E. AT2 receptor-mediated vasodilatation is unmasked by AT1 receptor blockade in conscious SHR. Br J Pharmacol. 2004;142:821–830. doi: 10.1038/sj.bjp.0705838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen P., Petrov V., Rumilla K., Fagard R. Transforming growth factor-beta 1 promotes contraction of collagen gel by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Findings Exp Clin Pharmacol. 2003;25:79–86. doi: 10.1358/mf.2003.25.2.723680. [DOI] [PubMed] [Google Scholar]
- Lijnen P.J., Petrov V.V., Fagard R.H. Induction of cardiac fibrosis by angiotensin II. Methods Findings Exp Clin Pharmacol. 2000;22:709–723. doi: 10.1358/mf.2000.22.10.802287. [DOI] [PubMed] [Google Scholar]
- Liu Y.H., Yang X.P., Sharov V.G., Nass O., Sabbah H.N., Peterson E. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokuta A.J., Cooper C., Gaa S.T., Wang H.E., Rogers T.B. Angiotensin II stimulates the release of phospholipid-derived second messengers through multiple receptor subtypes in heart cells. J Biol Chem. 1994;269:4832–4838. [PubMed] [Google Scholar]
- Loot A.E., Roks A.J., Henning R.H., Tio R.A., Suurmeijer A.J., Boomsma F. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- Lopez-Ilasaca M., Liu X., Tamura K., Dzau V.J. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol Biol Cell. 2003;14:5038–5050. doi: 10.1091/mbc.E03-06-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.J., Lorell B.H., Ingelfinger J.R., Weinberg E.O., Schunkert H., Diamant D. Distribution and function of cardiac angiotensin AT1- and AT2-receptor subtypes in hypertrophied rat hearts. Am J Physiol. 1994;267:H844–H852. doi: 10.1152/ajpheart.1994.267.2.H844. [DOI] [PubMed] [Google Scholar]
- Lorenzo O., Ruiz-Ortega M., Suzuki Y., Ruperez M., Esteban V., Sugaya T. Angiotensin III activates nuclear transcription factor-kappaB in cultured mesangial cells mainly via AT(2) receptors: studies with AT(1) receptor-knockout mice. J Am Soc Nephrol. 2002;13:1162–1171. doi: 10.1681/ASN.V1351162. [DOI] [PubMed] [Google Scholar]
- Loufrani L., Henrion D., Chansel D., Ardaillou R., Levy B.I. Functional evidence for an angiotensin IV receptor in rat resistance arteries. J Pharmacol Exp Ther. 1999;291:583–588. [PubMed] [Google Scholar]
- Lu H., Rateri D.L., Feldman D.L.R.J., Jr., Fukamizu A., Ishida J., Oesterling E.G. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Zhu Y., Wong P.T. Neuroprotective effects of candesartan against cerebral ischemia in spontaneously hypertensive rats. Neuroreport. 2005;16:1963–1967. doi: 10.1097/01.wnr.0000187636.13147.cd. [DOI] [PubMed] [Google Scholar]
- Lucius R., Gallinat S., Busche S., Rosenstiel P., Unger T. Beyond blood pressure: new roles for angiotensin II. Cell Mol Life Sci. 1999;56:1008–1019. doi: 10.1007/s000180050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucius R., Gallinat S., Rosenstiel P., Herdegen T., Sievers J., Unger T. The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J Exp Med. 1998;188:661–670. doi: 10.1084/jem.188.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Nishimura H., Fogo A., Kon V., Inagami T., Ichikawa I. Accelerated fibrosis and collagen deposition develop in the renal interstitium of angiotensin type 2 receptor null mutant mice during ureteral obstruction. Kidney Int. 1998;53:937–944. doi: 10.1111/j.1523-1755.1998.00893.x. [DOI] [PubMed] [Google Scholar]
- Masaki H., Kurihara T., Yamaki A., Inomata N., Nozawa Y., Mori Y. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101:527–535. [Google Scholar]
- Matrougui K., Levy B.I., Henrion D. Tissue angiotensin II and endothelin-1 modulate differently the response to flow in mesenteric resistance arteries of normotensive and spontaneously hypertensive rats. Br J Pharmacol. 2000;130:521–526. doi: 10.1038/sj.bjp.0703371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrougui K., Loufrani L., Heymes C., Levy B.I., Henrion D. Activation of AT(2) receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension. 1999;34:659–665. doi: 10.1161/01.hyp.34.4.659. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sugaya T., Murasawa S., Nozawa Y., Mori Y., Masaki H. Tissue-specific expression of human angiotensin II AT1 and AT2 receptors and cellular localization of subtype mRNAs in adult human renal cortex using in situ hybridization. Nephron. 1998;80:25–34. doi: 10.1159/000045121. [DOI] [PubMed] [Google Scholar]
- Metcalfe B.L., Huentelman M.J., Parilak L.D., Taylor D.G., Katovich M.J., Knot H.J. Prevention of cardiac hypertrophy by angiotensin II type-2 receptor gene transfer. Hypertension. 2004;43:1233–1238. doi: 10.1161/01.HYP.0000127563.14064.FD. [DOI] [PubMed] [Google Scholar]
- Mezzano S., Droguett A., Burgos M.E., Ardiles L.G., Flores C.A., Aros C.A. Renin–angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int Suppl. 2003:S64–S70. doi: 10.1046/j.1523-1755.64.s86.12.x. [DOI] [PubMed] [Google Scholar]
- Mifune M., Sasamura H., Nakazato Y., Yamaji Y., Oshima N., Saruta T. Examination of angiotensin II type 1 and type 2 receptor expression in human kidneys by immunohistochemistry. Clin Exp Hypertens (NY) 2001;23:257–266. doi: 10.1081/ceh-100102664. [DOI] [PubMed] [Google Scholar]
- Mifune M., Sasamura H., Shimizu-Hirota R., Miyazaki H., Saruta T. Angiotensin II type 2 receptors stimulate collagen synthesis in cultured vascular smooth muscle cells. Hypertension. 2000;36:845–850. doi: 10.1161/01.hyp.36.5.845. [DOI] [PubMed] [Google Scholar]
- Min L.J., Cui T.X., Yahata Y., Yamasaki K., Shiuchi T., Liu H.W. Regulation of collagen synthesis in mouse skin fibroblasts by distinct angiotensin II receptor subtypes. Endocrinology. 2004;145:253–260. doi: 10.1210/en.2003-0673. [DOI] [PubMed] [Google Scholar]
- Miura S., Karnik S.S. Ligand-independent signals from angiotensin II type 2 receptor induce apoptosis. EMBO J. 2000;19:4026–4035. doi: 10.1093/emboj/19.15.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Karnik S.S., Saku K. Constitutively active homo-oligomeric angiotensin II type 2 receptor induces cell signaling independent of receptor conformation and ligand stimulation. J Biol Chem. 2005;280:18237–18244. doi: 10.1074/jbc.M500639200. [DOI] [PubMed] [Google Scholar]
- Miyata N., Park F., Li X.F., Cowley A.W., Jr. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- Moeller I., Clune E.F., Fennessy P.A., Bingley J.A., Albiston A.L., Mendelsohn F.A. Up regulation of AT4 receptor levels in carotid arteries following balloon injury. Regulatory Pept. 1999;83:25–30. doi: 10.1016/s0167-0115(99)00047-6. [DOI] [PubMed] [Google Scholar]
- Mogi M., Iwai M., Horiuchi M. Emerging concepts of regulation of angiotensin II receptors. new players and targets for traditional receptors. Arterioscler Thromb Vasc Biol. 2007;27(12):2532–2539. doi: 10.1161/ATVBAHA.107.144154. [DOI] [PubMed] [Google Scholar]
- Mogi M., Jian-Mei L., Iwanami J., Min L., Tsukuda K., Iwai M. Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension. 2006;48:141–148. doi: 10.1161/01.HYP.0000229648.67883.f9. [DOI] [PubMed] [Google Scholar]
- Morrissey J.J., Klahr S. Effect of AT2 receptor blockade on the pathogenesis of renal fibrosis. Am J Physiol. 1999;276:F39–F45. doi: 10.1152/ajprenal.1999.276.1.F39. [DOI] [PubMed] [Google Scholar]
- Morsing P. Candesartan: a new-generation angiotensin II AT1 receptor blocker: pharmacology, antihypertensive efficacy, renal function, and renoprotection. J Am Soc Nephrol. 1999;10(Suppl 11):S248–S254. [PubMed] [Google Scholar]
- Mukoyama M., Horiuchi M., Nakajima M., Pratt R.E., Dzau V.J. Characterization of a rat type 2 angiotensin II receptor stably expressed in 293 cells. Mol Cell Endocrinol. 1995;112:61–68. doi: 10.1016/0303-7207(95)03586-v. [DOI] [PubMed] [Google Scholar]
- Muller D.N., Fischli W., Clozel J.P., Hilgers K.F., Bohlender J., Menard J. Local angiotensin II generation in the rat heart: role of renin uptake. Circ Res. 1998;82:13–20. doi: 10.1161/01.res.82.1.13. [DOI] [PubMed] [Google Scholar]
- Muller D.N., Klanke B., Feldt S., Cordasic N., Hartner A., Schmieder R.E. (Pro)renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension. 2008;51:676–681. doi: 10.1161/HYPERTENSIONAHA.107.101493. [DOI] [PubMed] [Google Scholar]
- Munzenmaier D.H., Greene A.S. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension. 1996;27:760–765. doi: 10.1161/01.hyp.27.3.760. [DOI] [PubMed] [Google Scholar]
- Muthalif M.M., Benter I.F., Uddin M.R., Harper J.L., Malik K.U. Signal transduction mechanisms involved in angiotensin-(1–7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther. 1998;284:388–398. [PubMed] [Google Scholar]
- Nahmias C., Cazaubon S.M., Briend-Sutren M.M., Lazard D., Villageois P., Strosberg A.D. Angiotensin II AT2 receptors are functionally coupled to protein tyrosine dephosphorylation in N1E-115 neuroblastoma cells. Biochem J. 1995;306:87–92. doi: 10.1042/bj3060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Hutchinson H.G., Fujinaga M., Hayashida W., Morishita R., Zhang L. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen G. Renin/prorenin receptors. Kidney Int. 2006;69:1503–1506. doi: 10.1038/sj.ki.5000265. [DOI] [PubMed] [Google Scholar]
- Nguyen G., Delarue F., Burckle C., Bouzhir L., Giller T., Sraer J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickenig G., Bohm M. Regulation of the angiotensin AT1 receptor expression by hypercholesterolemia. Eur J Med Res. 1997;2:285–289. [PubMed] [Google Scholar]
- Nio Y., Matsubara H., Murasawa S., Kanasaki M., Inada M. Regulation of gene transcription of angiotensin II receptor subtypes in myocardial infarction. J Clin Invest. 1995;95:46–54. doi: 10.1172/JCI117675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H., Yerkes E., Hohenfellner K., Miyazaki Y., Ma J., Hunley T.E. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell. 1999;3:1–10. doi: 10.1016/s1097-2765(00)80169-0. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Ito T., Saavedra J. Angiotensin II AT1 blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke. 2000;31:2478–2486. doi: 10.1161/01.str.31.10.2478. [DOI] [PubMed] [Google Scholar]
- Nora E.H., Munzenmaier D.H., Hansen-Smith F.M., Lombard J.H., Greene A.S. Localization of the ANG II type 2 receptor in the microcirculation of skeletal muscle. Am J Physiol. 1998;275:H1395–H1403. doi: 10.1152/ajpheart.1998.275.4.h1395. [DOI] [PubMed] [Google Scholar]
- Nouet S., Amzallag N., Li J.M., Louis S., Seitz I., Cui T.X. Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor-interacting protein, ATIP. J Biol Chem. 2004;279:28989–28997. doi: 10.1074/jbc.M403880200. [DOI] [PubMed] [Google Scholar]
- Nussberger J., Aubert J.F., Bouzourene K., Pellegrin M., Hayoz D., Mazzolai L. Renin inhibition by aliskiren prevents atherosclerosis progression: comparison with irbesartan, atenolol, and amlodipine. Hypertension. 2008;51:1306–1311. doi: 10.1161/HYPERTENSIONAHA.108.110932. [DOI] [PubMed] [Google Scholar]
- Ohkubo N., Matsubara H., Nozawa Y., Mori Y., Murasawa S., Kijima K. Angiotensin type 2 receptors are reexpressed by cardiac fibroblasts from failing myopathic hamster hearts and inhibit cell growth and fibrillar collagen metabolism. Circulation. 1997;96:3954–3962. doi: 10.1161/01.cir.96.11.3954. [DOI] [PubMed] [Google Scholar]
- Oishi Y., Ozono R., Yano Y., Teranishi Y., Akishita M., Horiuchi M. Cardioprotective role of AT2 receptor in postinfarction left ventricular remodeling. Hypertension. 2003;41:814–818. doi: 10.1161/01.HYP.0000048340.53100.43. [DOI] [PubMed] [Google Scholar]
- Okumura M., Iwai M., Ide A., Mogi M., Ito M., Horiuchi M. Sex difference in vascular injury and the vasoprotective effect of valsartan are related to differential AT2 receptor expression. Hypertension. 2005;46:577–583. doi: 10.1161/01.HYP.0000178564.14464.80. [DOI] [PubMed] [Google Scholar]
- Oro C., Qian H., Thomas W.G. Type 1 angiotensin receptor pharmacology: signaling beyond G proteins. Pharmacol Ther. 2007;113:210–226. doi: 10.1016/j.pharmthera.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozono R., Wang Z.Q., Moore A.F., Inagami T., Siragy H.M., Carey R.M. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- Padia S.H., Howell N.L., Siragy H.M., Carey R.M. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- Padia S.H., Kemp B.A., Howell N.L., Fournie-Zaluski M.C., Roques B.P., Carey R.M. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension. 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- Padia S.H., Kemp B.A., Howell N.L., Siragy H.M., Fournie-Zaluski M.C., Roques B.P. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension. 2007;49:625–630. doi: 10.1161/01.HYP.0000254833.85106.4d. [DOI] [PubMed] [Google Scholar]
- Parker S.B., Wade S.S., Prewitt R.L. Pressure mediates angiotensin II-induced arterial hypertrophy and PDGF-A expression. Hypertension. 1998;32:452–458. doi: 10.1161/01.hyp.32.3.452. [DOI] [PubMed] [Google Scholar]
- Parving H.H., Lehnert H., Brochner-Mortensen J., Gomis R., Andersen S., Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- Patel J.M., Martens J.R., Li Y.D., Gelband C.H., Raizada M.K., Block E.R. Angiotensin IV receptor-mediated activation of lung endothelial NOS is associated with vasorelaxation. Am J Physiol. 1998;275:L1061–L1068. doi: 10.1152/ajplung.1998.275.6.L1061. [DOI] [PubMed] [Google Scholar]
- Paul M., Poyan Mehr A., Kreutz R. Physiology of local renin–angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Phoon S., Howes L.G. Role of angiotensin type 2 receptors in human forearm vascular responses of normal volunteers. Clin Exp Pharmacol Physiol. 2001;28:734–736. doi: 10.1046/j.1440-1681.2001.03511.x. [DOI] [PubMed] [Google Scholar]
- Phoon S., Howes L.G. Forearm vasodilator response to angiotensin II in elderly women receiving candesartan: role of AT(2)-receptors. J Renin-Angiotensin-Aldosterone Syst. 2002;3:36–39. doi: 10.3317/jraas.2002.006. [DOI] [PubMed] [Google Scholar]
- Pilz B., Shagdarsuren E., Wellner M., Fiebeler A., Dechend R., Gratze P. Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double-transgenic rats.[see comment] Hypertension. 2005;46:569–576. doi: 10.1161/01.HYP.0000179573.91016.3f. [DOI] [PubMed] [Google Scholar]
- Pinaud F., Bocquet A., Dumont O., Retailleau K., Baufreton C., Andriantsitohaina R. Paradoxical role of angiotensin II type 2 receptors in resistance arteries of old rats. Hypertension. 2007;50:96–102. doi: 10.1161/HYPERTENSIONAHA.106.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro S.V., Simoes e Silva A.C., Sampaio W.O., de Paula R.D., Mendes E.P., Bontempo E.D. Nonpeptide AVE 0991 is an angiotensin-(1–7) receptor Mas agonist in the mouse kidney. Hypertension. 2004;44:490–496. doi: 10.1161/01.HYP.0000141438.64887.42. [DOI] [PubMed] [Google Scholar]
- Pueyo M.E., Arnal J.F., Rami J., Michel J.B. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol. 1998;274:C214–C220. doi: 10.1152/ajpcell.1998.274.1.C214. [DOI] [PubMed] [Google Scholar]
- Pupilli C., Lasagni L., Romagnani P., Bellini F., Mannelli M., Misciglia N. Angiotensin II stimulates the synthesis and secretion of vascular permeability factor/vascular endothelial growth factor in human mesangial cells. J Am Soc Nephrol. 1999;10:245–255. doi: 10.1681/ASN.V102245. [DOI] [PubMed] [Google Scholar]
- Rajapakse N.W., Eppel G.A., Widdop R.E., Evans R.G. ANG II type 2 receptors and neural control of intrarenal blood flow. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1669–R1676. doi: 10.1152/ajpregu.00183.2006. [DOI] [PubMed] [Google Scholar]
- Rajapakse N.W., Sampson A.K., Eppel G.A., Evans R.G. Angiotensin II and nitric oxide in neural control of intrarenal blood flow. Am J Physiol Regul Integr Comp Physiol. 2005;289:R745–R754. doi: 10.1152/ajpregu.00477.2004. [DOI] [PubMed] [Google Scholar]
- Reaux A., Fournie-Zaluski M.C., Llorens-Cortes C. Angiotensin III: a central regulator of vasopressin release and blood pressure. Trends Endocrinol Metabol. 2001;12:157–162. doi: 10.1016/s1043-2760(01)00381-2. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006;5:425–438. doi: 10.1038/nrd2032. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V., Friedel N., Heymann A., Bauer P., Neuss M., Rolfs A. Regulation, chamber localization, and subtype distribution of angiotensin II receptors in human hearts. Circulation. 1995;91:1461–1471. doi: 10.1161/01.cir.91.5.1461. [DOI] [PubMed] [Google Scholar]
- Reinecke K., Lucius R., Reinecke A., Rickert U., Herdengen T., Unger T. Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kB. FASEB J. 2003;17:2094–2096. doi: 10.1096/fj.02-1193fje. [DOI] [PubMed] [Google Scholar]
- Reudelhuber T.L. The continuing saga of the AT2 receptor: a case of the good, the bad, and the innocuous. Hypertension. 2005;46:1261–1262. doi: 10.1161/01.HYP.0000193498.07087.83. [DOI] [PubMed] [Google Scholar]
- Reudelhuber T.L. A place in our hearts for the lowly angiotensin 1–7 peptide? Hypertension. 2006;47:811–815. doi: 10.1161/01.HYP.0000209020.69734.73. [DOI] [PubMed] [Google Scholar]
- Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihal C.S., Nishimura R.A., Hatle L.K., Bailey K.R., Tajik A.J. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation. 1994;90:2772–2779. doi: 10.1161/01.cir.90.6.2772. [DOI] [PubMed] [Google Scholar]
- Rizkalla B., Forbes J.M., Cao Z., Boner G., Cooper M.E. Temporal renal expression of angiogenic growth factors and their receptors in experimental diabetes: role of the renin–angiotensin system. J Hypertens. 2005;23:153–164. doi: 10.1097/00004872-200501000-00026. [DOI] [PubMed] [Google Scholar]
- Rizkalla B., Forbes J.M., Cooper M.E., Cao Z. Increased renal vascular endothelial growth factor and angiopoietins by angiotensin II infusion is mediated by both AT1 and AT2 receptors. J Am Soc Nephrol. 2003;14:3061–3071. doi: 10.1097/01.asn.0000099374.58607.c9. [DOI] [PubMed] [Google Scholar]
- Rogg H., de Gasparo M., Graedel E., Stulz P., Burkart F., Eberhard M. Angiotensin II-receptor subtypes in human atria and evidence for alterations in patients with cardiac dysfunction. Eur Heart J. 1996;17:1112–1120. doi: 10.1093/oxfordjournals.eurheartj.a015008. [DOI] [PubMed] [Google Scholar]
- Rosenstrom U., Skold C., Lindeberg G., Botros M., Nyberg F., Hallberg A. Synthesis and AT2 receptor-binding properties of angiotensin II analogues. J Pept Res. 2004;64:194–201. doi: 10.1111/j.1399-3011.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- Rosenstrom U., Skold C., Plouffe B., Beaudry H., Lindeberg G., Botros M. New selective AT2 receptor ligands encompassing a gamma-turn mimetic replacing the amino acid residues 4–5 of angiotensin II act as agonists. J Med Chem. 2005;48:4009–4024. doi: 10.1021/jm0491492. [DOI] [PubMed] [Google Scholar]
- Roulston C.L., Lawrence A.J., Jarrott B., Widdop R.E. Localization of AT(2) receptors in the nucleus of the solitary tract of spontaneously hypertensive and Wistar Kyoto rats using [125I] CGP42112: upregulation of a non-angiotensin II binding site following unilateral nodose ganglionectomy. Brain Res. 2003;968:139–155. doi: 10.1016/s0006-8993(03)02231-5. [DOI] [PubMed] [Google Scholar]
- Rowe B.P., Saylor D.L., Speth R.C., Absher D.R. Angiotensin-(1–7) binding at angiotensin II receptors in the rat brain. Regulatory Pept. 1995;56:139–146. doi: 10.1016/0167-0115(95)00010-9. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M., Esteban V., Suzuki Y., Ruperez M., Mezzano S., Ardiles L. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int Suppl. 2003:S21–S26. doi: 10.1046/j.1523-1755.64.s86.5.x. [DOI] [PubMed] [Google Scholar]
- Saavedra J.M., Benicky J., Zhou J. Mechanisms of the anti-ischemic effect of angiotensin II AT(1) receptor antagonists in the brain. Cell Mol Neurobiol. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0. [DOI] [PubMed] [Google Scholar]
- Saito S., Hirata Y., Emori T., Imai T., Marumo F. Angiotensin II activates endothelial constitutive nitric oxide synthase via AT1 receptors. Hypertens Res Clin Exp. 1996;19:201–206. doi: 10.1291/hypres.19.201. [DOI] [PubMed] [Google Scholar]
- Sales V.L., Sukhova G.K., Lopez-Ilasaca M.A., Libby P., Dzau V.J., Pratt R.E. Angiotensin type 2 receptor is expressed in murine atherosclerotic lesions and modulates lesion evolution. Circulation. 2005;112:3328–3336. doi: 10.1161/CIRCULATIONAHA.105.541714. [DOI] [PubMed] [Google Scholar]
- Salomone L.J., Howell N.L., McGrath H.E., Kemp B.A., Keller S.R., Gildea J.J. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–161. doi: 10.1161/01.HYP.0000251881.89610.ee. [DOI] [PubMed] [Google Scholar]
- Sampson, A. K., Moritz, K. M., Jones, E. S., Flower, R. L., Widdop, R. E., & Denton, K. M. (2008). Enhanced AT2R-vasodilator mechanisms mediate Ang II-induced decreases in arterial pressure in female rats. Hypertension 52, 666−671. [DOI] [PubMed]
- Santos R.A.S., Ferreira A.J. Angiotensin-(1–7) and the renin–angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122–128. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- Santos R.A., Campagnole-Santos M.J., Andrade S.P. Angiotensin-(1–7): an update. Regulatory Pept. 2000;91:45–62. doi: 10.1016/s0167-0115(00)00138-5. [DOI] [PubMed] [Google Scholar]
- Santos R.A., Castro C.H., Gava E., Pinheiro S.V., Almeida A.P., Paula R.D. Impairment of in vitro and in vivo heart function in angiotensin-(1–7) receptor MAS knockout mice. Hypertension. 2006;47:996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de Buhr I. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardinia M.F., Hanesworth J.M., Krishnan F., Harding J.W. AT4 receptor structure-binding relationship: N-terminal-modified angiotensin IV analogues. Peptides. 1994;15:1399–1406. doi: 10.1016/0196-9781(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Sarlos S., Rizkalla B., Moravski C.J., Cao Z., Cooper M.E., Wilkinson-Berka J.L. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003;163:879–887. doi: 10.1016/S0002-9440(10)63448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C., Ebrahimian T., He Y., Gratton J.P., Schiffrin E.L., Touyz R.M. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cGMP-dependent protein kinase. J Hypertens. 2006;24:2417–2422. doi: 10.1097/01.hjh.0000251902.85675.7e. [DOI] [PubMed] [Google Scholar]
- Savoia C., Tabet F., Yao G., Schiffrin E.L., Touyz R.M. Negative regulation of RhoA/Rho kinase by angiotensin II type 2 receptor in vascular smooth muscle cells: role in angiotensin II-induced vasodilation in stroke-prone spontaneously hypertensive rats. J Hypertens. 2005;23:1037–1045. doi: 10.1097/01.hjh.0000166845.49850.39. [DOI] [PubMed] [Google Scholar]
- Savoia C., Touyz R.M., Volpe M., Schiffrin E.L. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2006 doi: 10.1161/01.HYP.0000253968.95136.b8. [DOI] [PubMed] [Google Scholar]
- Savoia C., Touyz R.M., Volpe M., Schiffrin E.L. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007;49:341–346. doi: 10.1161/01.HYP.0000253968.95136.b8. [DOI] [PubMed] [Google Scholar]
- Schefe J.H., Menk M., Reinemund J., Effertz K., Hobbs R.M., Pandolfi P.P. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- Scheuer D.A., Perrone M.H. Angiotensin type 2 receptors mediate depressor phase of biphasic pressure response to angiotensin. Am J Physiol. 1993;264:R917–R923. doi: 10.1152/ajpregu.1993.264.5.R917. [DOI] [PubMed] [Google Scholar]
- Schmieder R.E., Erdmann J., Delles C., Jacobi J., Fleck E., Hilgers K. Effect of the angiotensin II type 2-receptor gene (+1675 G/A) on left ventricular structure in humans. J Am Coll Cardiol. 2001;37:175–182. doi: 10.1016/s0735-1097(00)01063-9. [DOI] [PubMed] [Google Scholar]
- Schrader J., Luders S., Kulschewski A., Berger J., Zidek W., Treib J. The ACCESS study. Evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke. 2003;34:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- Schrader J., Luders S., Kulschewski A., Hammersen F., Plate K., Berger J. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- Sechi L.A., Griffin C.A., Schambelan M. The cardiac renin–angiotensin system in STZ-induced diabetes. Diabetes. 1994;43:1180–1184. doi: 10.2337/diab.43.10.1180. [DOI] [PubMed] [Google Scholar]
- Senbonmatsu T., Ichihara S., Price E., Jr., Gaffney F.A., Inagami T. Evidence for angiotensin II type 2 receptor-mediated cardiac myocyte enlargement during in vivo pressure overload. J Clin Invest. 2000;106:R25–R29. doi: 10.1172/JCI10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senbonmatsu T., Saito T., Landon E.J., Watanabe O., Price E., Jr., Roberts R.L. A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. EMBO J. 2003;22:6471–6482. doi: 10.1093/emboj/cdg637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senni M., Tribouilloy C.M., Rodeheffer R.J., Jacobsen S.J., Evans J.M., Bailey K.R. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- Seyedi N., Xu X., Nasjletti A., Hintze T.H. Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension. 1995;26:164–170. doi: 10.1161/01.hyp.26.1.164. [DOI] [PubMed] [Google Scholar]
- Shanmugam S., Llorens-Cortes C., Clauser E., Corvol P., Gasc J.M. Expression of angiotensin II AT2 receptor mRNA during development of rat kidney and adrenal gland. Am J Physiol. 1995;268:F922–F930. doi: 10.1152/ajprenal.1995.268.5.F922. [DOI] [PubMed] [Google Scholar]
- Shenoy U.V., Richards E.M., Huang X.C., Sumners C. Angiotensin II type 2 receptor-mediated apoptosis of cultured neurons from newborn rat brain. Endocrinology. 1999;140:500–509. doi: 10.1210/endo.140.1.6396. [DOI] [PubMed] [Google Scholar]
- Shibasaki Y., Mori Y., Tsutumi Y., Masaki H., Sakamoto K., Murasawa S. Differential kinetics of circulating angiotensin IV and II after treatment with angiotensin II type 1 receptor antagonist and their plasma levels in patients with chronic renal failure. Clin Nephrol. 1999;51:83–91. [PubMed] [Google Scholar]
- Shibouta Y., Inada Y., Ojima M., Wada T., Noda M., Sanada T. Pharmacological profile of a highly potent and long-acting angiotensin II receptor antagonist, 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylic acid (CV-11974), and its prodrug, (+/−)-1-(cyclohexyloxycarbonyloxy)-ethyl 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylate (TCV-116) J Pharmacol Exp Ther. 1993;266:114–120. [PubMed] [Google Scholar]
- Shiota N., Miyazaki M., Okunishi H. Increase of angiotensin converting enzyme gene expression in the hypertensive aorta. Hypertension. 1992;20:168–174. doi: 10.1161/01.hyp.20.2.168. [DOI] [PubMed] [Google Scholar]
- Silva-Antonialli M.M., Fortes Z.B., Carvalho M.H., Scivoletto R., Nigro D. Sexual dimorphism in the response of thoracic aorta from SHRs to losartan. Gen Pharmacol. 2000;34:329–335. doi: 10.1016/s0306-3623(00)00078-1. [DOI] [PubMed] [Google Scholar]
- Silva-Antonialli M.M., Tostes R.C., Fernandes L., Fior-Chadi D.R., Akamine E.H., Carvalho M.H. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62:587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Simon G., Illyes G., Csiky B. Structural vascular changes in hypertension: role of angiotensin II, dietary sodium supplementation, blood pressure, and time. Hypertension. 1998;32:654–660. doi: 10.1161/01.hyp.32.4.654. [DOI] [PubMed] [Google Scholar]
- Siragy H.M., Carey R.M. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragy H.M., Carey R.M. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragy H.M., Carey R.M. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- Siragy H.M., de Gasparo M., Carey R.M. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000;35:1074–1077. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- Siragy H.M., de Gasparo M., El-Kersh M., Carey R.M. Angiotensin-converting enzyme inhibition potentiates angiotensin II type 1 receptor effects on renal bradykinin and cGMP. Hypertension. 2001;38:183–186. doi: 10.1161/01.hyp.38.2.183. [DOI] [PubMed] [Google Scholar]
- Siragy H.M., Inagami T., Carey R.M. NO and cGMP mediate angiotensin AT2 receptor-induced renal renin inhibition in young rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1461–R1467. doi: 10.1152/ajpregu.00014.2007. [DOI] [PubMed] [Google Scholar]
- Siragy H.M., Inagami T., Ichiki T., Carey R.M. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci U S A. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn H.Y., Raff U., Hoffmann A., Gloe T., Heermeier K., Galle J. Differential role of angiotensin II receptor subtypes on endothelial superoxide formation. Br J Pharmacol. 2000;131:667–672. doi: 10.1038/sj.bjp.0703566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelings U.M., Kaschina E., Unger T. The AT2 receptor—a matter of love and hate. Peptides. 2005;26:1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Stoll M., Steckelings U.M., Paul M., Bottari S.P., Metzger R., Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95:651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M.H., Hall A.S. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114:838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- Strawn W.B., Ferrario C.M., Tallant E.A. Angiotensin-(1–7) reduces smooth muscle growth after vascular injury. Hypertension. 1999;33:207–211. doi: 10.1161/01.hyp.33.1.207. [DOI] [PubMed] [Google Scholar]
- Stroth U., Meffert S., Gallinat S., Unger T. Angiotensin II and NGF differentially influence microtubule proteins in PC12W cells: role of the AT2 receptor. Brain Res Mol Brain Res. 1998;53:187–195. doi: 10.1016/s0169-328x(97)00298-2. [DOI] [PubMed] [Google Scholar]
- Su J.Z., Fukuda N., Jin X.Q., Lai Y.M., Suzuki R., Tahira Y. Effect of AT2 receptor on expression of AT1 and TGF-beta receptors in VSMCs from SHR. Hypertension. 2002;40:853–858. doi: 10.1161/01.hyp.0000042096.17141.b1. [DOI] [PubMed] [Google Scholar]
- Sugawara T.H.K., Oda M., Shoji H., Omae T., Mizoi K. Candesartan reduces superoxide production after global cerebral ischemia. Neuropharmacol Neurotox. 2005;16:325–328. doi: 10.1097/00001756-200503150-00004. [DOI] [PubMed] [Google Scholar]
- Sugino H., Ozono R., Kurisu S., Matsuura H., Ishida M., Oshima T. Apoptosis is not increased in myocardium overexpressing type 2 angiotensin II receptor in transgenic mice. Hypertension. 2001;37:1394–1398. doi: 10.1161/01.hyp.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Suzuki J., Iwai M., Nakagami H., Wu L., Chen R., Sugaya T. Role of angiotensin II-regulated apoptosis through distinct AT1 and AT2 receptors in neointimal formation. Circulation. 2002;106:847–853. doi: 10.1161/01.cir.0000024103.04821.86. [DOI] [PubMed] [Google Scholar]
- Suzuki J., Matsubara H., Urakami M., Inada M. Rat angiotensin II (type 1A) receptor mRNA regulation and subtype expression in myocardial growth and hypertrophy. Circ Res. 1993;73:439–447. doi: 10.1161/01.res.73.3.439. [DOI] [PubMed] [Google Scholar]
- Swanson G.N., Hanesworth J.M., Sardinia M.F., Coleman J.K., Wright J.W., Hall K.L., Miller-Wing A.V., Stobb J.W., Cook V.I., Harding E.C. Discovery of a distinct binding site for angiotensin II (3–8), a putative angiotensin IV receptor. Regulatory Pept. 1992;40:409–419. doi: 10.1016/0167-0115(92)90527-2. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- Tamarat R., Silvestre J.S., Durie M., Levy B.I. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab Investig. 2002;82:747–756. doi: 10.1097/01.lab.0000017372.76297.eb. [DOI] [PubMed] [Google Scholar]
- Tea B.S., Der Sarkissian S., Touyz R.M., Hamet P., deBlois D. Proapoptotic and growth-inhibitory role of angiotensin II type 2 receptor in vascular smooth muscle cells of spontaneously hypertensive rats in vivo. Hypertension. 2000;35:1069–1073. doi: 10.1161/01.hyp.35.5.1069. [DOI] [PubMed] [Google Scholar]
- Tejera N., Gomez-Garre D., Lazaro A., Gallego-Delgado J., Alonso C., Blanco J. Persistent proteinuria up-regulates angiotensin II type 2 receptor and induces apoptosis in proximal tubular cells. Am J Pathol. 2004;164:1817–1826. doi: 10.1016/S0002-9440(10)63740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W.G. Regulation of angiotensin II type 1 (AT1) receptor function. Regulatory Pept. 1999;79:9–23. doi: 10.1016/s0167-0115(98)00140-2. [DOI] [PubMed] [Google Scholar]
- Thone-Reineke C., Steckelings U.M., Unger T. Angiotensin receptor blockers and cerebral protection in stroke. J Hypertens Suppl. 2006;24:S115–S121. doi: 10.1097/01.hjh.0000220416.07235.37. [DOI] [PubMed] [Google Scholar]
- Thorup C., Kornfeld M., Goligorsky M.S., Moore L.C. AT1 receptor inhibition blunts angiotensin II-stimulated nitric oxide release in renal arteries. J Am Soc Nephrol. 1999;10(Suppl 11):S220–S224. [PubMed] [Google Scholar]
- Thorup C., Kornfeld M., Winaver J.M., Goligorsky M.S., Moore L.C. Angiotensin-II stimulates nitric oxide release in isolated perfused renal resistance arteries. Pflugers Arch Eur J Physiol. 1998;435:432–434. doi: 10.1007/s004240050535. [DOI] [PubMed] [Google Scholar]
- Timmermans P.B., Wong P.C., Chiu A.T., Herblin W.F. Nonpeptide angiotensin II receptor antagonists. Trends Pharmacol Sci. 1991;12:55–62. doi: 10.1016/0165-6147(91)90498-h. [DOI] [PubMed] [Google Scholar]
- Timmermans P.B., Wong P.C., Chiu A.T., Herblin W.F., Benfield P., Carini D.J. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Touyz R.M., Berry C. Recent advances in angiotensin II signaling. Braz J Med Biol Res. 2002;35:1001–1015. doi: 10.1590/s0100-879x2002000900001. [DOI] [PubMed] [Google Scholar]
- Touyz R.M., Endemann D., He G., Li J.S., Schiffrin E.L. Role of AT2 receptors in angiotensin II-stimulated contraction of small mesenteric arteries in young SHR. Hypertension. 1999;33:366–372. doi: 10.1161/01.hyp.33.1.366. [DOI] [PubMed] [Google Scholar]
- Touyz R.M., He G., El Mabrouk M., Schiffrin E.L. p38 Map kinase regulates vascular smooth muscle cell collagen synthesis by angiotensin II in SHR but not in WKY. Hypertension. 2001;37:574–580. doi: 10.1161/01.hyp.37.2.574. [DOI] [PubMed] [Google Scholar]
- Touyz R.M., Tabet F., Schiffrin E.L. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–866. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- Tsurumi Y., Tamura K., Tanaka Y., Koide Y., Sakai M., Yabana M. Interacting molecule of AT1 receptor, ATRAP, is colocalized with AT1 receptor in the mouse renal tubules. Kidney Int. 2006;69:488–494. doi: 10.1038/sj.ki.5000130. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y., Matsubara H., Masaki H., Kurihara H., Murasawa S., Takai S. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi Y., Matsubara H., Ohkubo N., Mori Y., Nozawa Y., Murasawa S. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res. 1998;83:1035–1046. doi: 10.1161/01.res.83.10.1035. [DOI] [PubMed] [Google Scholar]
- Tsuyuki R.T., McDonald M.A. Angiotensin receptor blockers do not increase risk of myocardial infarction. Circulation. 2006;114:855–860. doi: 10.1161/CIRCULATIONAHA.105.594978. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S., Matoba T., Eguchi S., Inagami T. Angiotensin II type 2 receptor inhibits cell proliferation and activates tyrosine phosphatase. Hypertension. 1996;28:916–918. doi: 10.1161/01.hyp.28.5.916. [DOI] [PubMed] [Google Scholar]
- Unger T., Chung O., Csikos T., Culman J., Gallinat S., Gohlke P. Angiotensin receptors. J Hypertens Supplement. 1996;14:S95–S103. [PubMed] [Google Scholar]
- Varagic J., Susic D., Frohlich E.D. Coronary hemodynamic and ventricular responses to angiotensin type 1 receptor inhibition in SHR: interaction with angiotensin type 2 receptors. Hypertension. 2001;37:1399–1403. doi: 10.1161/01.hyp.37.6.1399. [DOI] [PubMed] [Google Scholar]
- Vazquez E., Coronel I., Bautista R., Romo E., Villalon C.M., Avila-Casado M.C. Angiotensin II-dependent induction of AT(2) receptor expression after renal ablation. Am J Physiol Renal Physiol. 2005;288:F207–F213. doi: 10.1152/ajprenal.00216.2004. [DOI] [PubMed] [Google Scholar]
- Verma S., Strauss M. Angiotensin receptor blockers and myocardial infarction. BMJ. 2004;329:1248–1249. doi: 10.1136/bmj.329.7477.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Vincent J.M., Kwan Y.W., Chan S.L., Perrin-Sarrado C., Atkinson J., Chillon J.M. Constrictor and dilator effects of angiotensin II on cerebral arterioles. Stroke. 2005;36:2691–2695. doi: 10.1161/01.STR.0000190002.79052.bf. [DOI] [PubMed] [Google Scholar]
- Vinh, A., Widdop, R. E., Drummond, G. R., & Gaspari, T. A. (2008a). Chronic angiotensin IV treatment reverses endothelial dysfunction in ApoE-deficient mice. Cardiovasc Res 77, 178−187. [DOI] [PubMed]
- Vinh, A., Widdop, R. E., Chai, S. Y., & Gaspari, T. A. (2008b). Angiotensin IV-evoked vasoprotection is conserved in advanced atheroma. Atherosclerosis 200, 37−44. [DOI] [PubMed]
- Virdis A., Neves M.F., Amiri F., Viel E., Touyz R.M., Schiffrin E.L. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- Viswanathan M., Saavedra J.M. Expression of angiotensin II AT2 receptors in the rat skin during experimental wound healing. Peptides. 1992;13:783–786. doi: 10.1016/0196-9781(92)90187-8. [DOI] [PubMed] [Google Scholar]
- Viswanathan M., Stromberg C., Seltzer A., Saavedra J.M. Balloon angioplasty enhances the expression of angiotensin II AT1 receptors in neointima of rat aorta. J Clin Invest. 1992;90:1707–1712. doi: 10.1172/JCI116043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bohlen und Halbach O., Walther T., Bader M., Albrecht Genetic deletion of angiotensin AT2 receptor leads to increased cell numbers in different brain structures of mice. Regulatory Pept. 2001;99:209–216. doi: 10.1016/s0167-0115(01)00258-0. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W., Thomas G.D., Schwartz R., Cassis L.A., Osborne-Lawrence S., Hahner L. C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation. 2007;115:1020–1028. doi: 10.1161/CIRCULATIONAHA.106.664854. [DOI] [PubMed] [Google Scholar]
- Voros S., Yang Z., Bove C.M., Gilson W.D., Epstein F.H., French B.A. Interaction between AT1 and AT2 receptors during postinfarction left ventricular remodeling. Am J Physiol Heart Circ Physiol. 2006;290:H1004–H1010. doi: 10.1152/ajpheart.00886.2005. [DOI] [PubMed] [Google Scholar]
- Walters P.E., Gaspari T.A., Widdop R.E. Angiotensin III acts as a vasodepressor agent via AT2R. Hypertension. 2003;42:443. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- Walters P.E., Gaspari T.A., Widdop R.E. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005;45:960–966. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- Walther T., Olah L., Harms C., Maul B., Bader M., Hortanagl H. Ischemic injury in experimental stroke depends on angiotensin II. Stroke. 2002;16:169–176. doi: 10.1096/fj.01-0601com. [DOI] [PubMed] [Google Scholar]
- Wan Y., Wallinder C., Johansson B., Holm M., Mahalingam A.K., Wu X. First reported nonpeptide AT1 receptor agonist (L-162,313) acts as an AT2 receptor agonist in vivo. J Med Chem. 2004;47:1536–1546. doi: 10.1021/jm031031i. [DOI] [PubMed] [Google Scholar]
- Wan Y., Wallinder C., Plouffe B., Beaudry H., Mahalingam A.K., Wu X. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- Wang H., Gallinat S., Li H.W., Sumners C., Raizada M.K., Katovich M.J. Elevated blood pressure in normotensive rats produced by ‛knockdown’ of the angiotensin type 2 receptor. Exp Physiol. 2004;89:313–322. doi: 10.1113/expphysiol.2004.027359. [DOI] [PubMed] [Google Scholar]
- Wang M., Takagi G., Asai K., Resuello R.G., Natividad F.F., Vatner D.E. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- Wang Z.Q., Millatt L.J., Heiderstadt N.T., Siragy H.M., Johns R.A., Carey R.M. Differential regulation of renal angiotensin subtype AT1A and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension. 1999;33:96–101. doi: 10.1161/01.hyp.33.1.96. [DOI] [PubMed] [Google Scholar]
- Warnecke C., Kaup D., Marienfeld U., Poller W., Yankah C., Grafe M. Adenovirus-mediated overexpression and stimulation of the human angiotensin II type 2 receptor in porcine cardiac fibroblasts does not modulate proliferation, collagen I mRNA expression and ERK1/ERK2 activity, but inhibits protein tyrosine phosphatases. J Mol Med. 2001;79:510–521. doi: 10.1007/s001090100243. [DOI] [PubMed] [Google Scholar]
- Warnecke C., Mugrauer P., Surder D., Erdmann J., Schubert C., Regitz-Zagrosek V. Intronic ANG II type 2 receptor gene polymorphism 1675 G/A modulates receptor protein expression but not mRNA splicing. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1729–R1735. doi: 10.1152/ajpregu.00385.2005. [DOI] [PubMed] [Google Scholar]
- Warner F.J., Lew R.A., Smith A.I., Lambert D.W., Hooper N.M., Turner A.J. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem. 2005;280:39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- Warnholtz A., Nickenig G., Schulz E., Macharzina R., Brasen J.H., Skatchkov M. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin–angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- Wassmann S., Czech T., van Eickels M., Fleming I., Bohm M., Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- Weber K.T. Extracellular matrix remodeling in heart failure: a role for de novo angiotensin II generation. Circulation. 1997;96:4065–4082. doi: 10.1161/01.cir.96.11.4065. [DOI] [PubMed] [Google Scholar]
- Weber K.T., Sun Y., Katwa L.C. Myofibroblasts and local angiotensin II in rat cardiac tissue repair. Int J Biochem Cell Biol. 1997;29:31–42. doi: 10.1016/s1357-2725(96)00116-1. [DOI] [PubMed] [Google Scholar]
- Wehbi G.J., Zimpelmann J., Carey R.M., Levine D.Z., Burns K.D. Early streptozotocin-diabetes mellitus downregulates rat kidney AT2 receptors. Am J Physiol Renal Physiol. 2001;280:F254–F265. doi: 10.1152/ajprenal.2001.280.2.F254. [DOI] [PubMed] [Google Scholar]
- Whaley-Connell A., Habibi J., Cooper S.A., DeMarco V.G., Hayden M.R., Stump C.S. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab. 2008;295:E103–E109. doi: 10.1152/ajpendo.00752.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton J., Morgan K., Rutherford R.A., Catravas J.D., Chester A., Whitehead B.F. Differential distribution of angiotensin AT2 receptors in the normal and failing human heart. J Pharmacol Exp Ther. 1998;284:323–336. [PubMed] [Google Scholar]
- Wheeler-Schilling T.H., Kohler K., Sautter M., Guenther E. Angiotensin II receptor subtype gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. Eur J Neurosci. 1999;11:3387–3394. doi: 10.1046/j.1460-9568.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- Whitebread S., Mele M., Kamber B., de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;163:284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- Widdop R.E., Jones E.S., Hannan R.E., Gaspari T.A. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol. 2003;140:809–824. doi: 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdop R.E., Matrougui K., Levy B.I., Henrion D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- Widdop R.E., Vinh A., Henrion D., Jones E.S. Vascular angiotensin AT2 receptors in hypertension and ageing. Clin Exp Pharmacol Physiol. 2008;35:386–390. doi: 10.1111/j.1440-1681.2008.04883.x. [DOI] [PubMed] [Google Scholar]
- Wiemer G., Dobrucki L.W., Louka F.R., Malinski T., Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1–7) on the endothelium. Hypertension. 2002;40:847–852. doi: 10.1161/01.hyp.0000037979.53963.8f. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka J.L. Diabetes and retinal vascular disorders: role of the reninangiotensin system. Exp Rev Mol Med. 2004;6:1–18. doi: 10.1017/S1462399404008129. [DOI] [PubMed] [Google Scholar]
- Wright J.W., Krebs L.T., Stobb J.W., Harding J.W. The angiotensin IV system: functional implications. Front Neuroendocrinol. 1995;16:23–52. doi: 10.1006/frne.1995.1002. [DOI] [PubMed] [Google Scholar]
- Wright J.W., Miller-Wing A.V., Shaffer M.J., Higginson C., Wright D.E., Hanesworth J.M. Angiotensin II(3–8) (ANG IV) hippocampal binding: potential role in the facilitation of memory. Brain Res Bull. 1993;32:497–502. doi: 10.1016/0361-9230(93)90297-o. [DOI] [PubMed] [Google Scholar]
- Wright J.W., Stubley L., Pederson E.S., Kramar E.A., Hanesworth J.M., Harding J.W. Contributions of the brain angiotensin IV-AT4 receptor subtype system to spatial learning. J Neurosci. 1999;19:3952–3961. doi: 10.1523/JNEUROSCI.19-10-03952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.W., Tamura-Myers E., Wilson W.L., Roques B.P., Llorens-Cortes C., Speth R.C. Conversion of brain angiotensin II to angiotensin III is critical for pressor response in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R725–R733. doi: 10.1152/ajpregu.00326.2002. [DOI] [PubMed] [Google Scholar]
- Wruck C.J., Funke-Kaiser H., Pufe T., Kusserow H., Menk M., Schefe J.H. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25:57–64. doi: 10.1161/01.ATV.0000150662.51436.14. [DOI] [PubMed] [Google Scholar]
- Wu L., Iwai M., Nakagami H., Chen R., Suzuki J., Akishita M. Effect of angiotensin II type 1 receptor blockade on cardiac remodeling in angiotensin II type 2 receptor null mice. Arterioscler Thromb Vasc Biol. 2002;22:49–54. doi: 10.1161/hq0102.102277. [DOI] [PubMed] [Google Scholar]
- Wu L., Iwai M., Nakagami H., Li Z., Chen R., Suzuki J. Roles of angiotensin II type 2 receptor stimulation associated with selective angiotensin II type 1 receptor blockade with valsartan in the improvement of inflammation-induced vascular injury. Circulation. 2001;104:2716–2721. doi: 10.1161/hc4601.099404. [DOI] [PubMed] [Google Scholar]
- Wysocki J., Ye M., Soler M.J., Gurley S.B., Xiao H.D., Bernstein K.E. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- Xu J., Carretero O.A., Liu Y.H., Shesely E.G., Yang F., Kapke A. Role of AT2 receptors in the cardioprotective effect of AT1 antagonists in mice. Hypertension. 2002;40:244–250. doi: 10.1161/01.hyp.0000029095.23198.ad. [DOI] [PubMed] [Google Scholar]
- Yamada H., Akishita M., Ito M., Tamura K., Daviet L., Lehtonen J.Y. AT2 receptor and vascular smooth muscle cell differentiation in vascular development. Hypertension. 1999;33:1414–1419. doi: 10.1161/01.hyp.33.6.1414. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Ohishi M., Katsuya T., Ito N., Ikushima M., Kaibe M. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- Yan X., Price R.L., Nakayama M., Ito K., Schuldt A.J.T., Manning W.J. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;285:H2179–H2187. doi: 10.1152/ajpheart.00361.2003. [DOI] [PubMed] [Google Scholar]
- Yang Z., Bove C.M., French B.A., Epstein F.H., Berr S.S., DiMaria J.M. Angiotensin II type 2 receptor overexpression preserves left ventricular function after myocardial infarction. Circulation. 2002;106:106–111. doi: 10.1161/01.cir.0000020014.14176.6d. [DOI] [PubMed] [Google Scholar]
- Yayama K., Hiyoshi H., Imazu D., Okamoto H. Angiotensin II stimulates endothelial NO synthase phosphorylation in thoracic aorta of mice with abdominal aortic banding via type 2 receptor. Hypertension. 2006;48:958–964. doi: 10.1161/01.HYP.0000244108.30909.27. [DOI] [PubMed] [Google Scholar]
- Yayama K., Horii M., Hiyoshi H., Takano M., Okamoto H., Kagota S. Up-regulation of angiotensin II type 2 receptor in rat thoracic aorta by pressure-overload. J Pharmacol Exp Ther. 2004;308:736–743. doi: 10.1124/jpet.103.058420. [DOI] [PubMed] [Google Scholar]
- You D., Loufrani L., Baron C., Levy B.I., Widdop R.E., Henrion D. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation. 2005;111:1006–1011. doi: 10.1161/01.CIR.0000156503.62815.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S., Teo K.K., Pogue J., Dyal L., Copland I., Schumacher H. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- Zhang X., Lassila M., Cooper M.E., Cao Z. Retinal expression of vascular endothelial growth factor is mediated by angiotensin type 1 and type 2 receptors. Hypertension. 2004;43:276–281. doi: 10.1161/01.HYP.0000113628.94574.0f. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Biermann T., Luther C., Unger T., Culman J., Gohlke P. Contribution of bradykinin and nitric oxide to AT2 receptor-mediated differentiation in PC12 W cells. J Neurochem. 2003;85:759–767. doi: 10.1046/j.1471-4159.2003.01719.x. [DOI] [PubMed] [Google Scholar]
- Zhou J., Pavel J., Macova M., Yu Z.X., Imboden H., Ge L. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]
- Zhu M., Gelband C.H., Moore J.M., Posner P., Sumners C. Angiotensin II type 2 receptor stimulation of neuronal delayed-rectifier potassium current involves phospholipase A2 and arachidonic acid. J Neurosci. 1998;18:679–686. doi: 10.1523/JNEUROSCI.18-02-00679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.C., Zhu Y.Z., Lu N., Wang M.-J., Wang Y.-X., Yao T. Role of angiotensin AT1 and AT2 receptors in cardiac hypertrophy and cardiac remodelling. Clin Exp Pharmacol Physiol. 2003;30:911–918. doi: 10.1111/j.1440-1681.2003.03942.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y.Z., Chimon G.N., Zhu Y.C., Lu Q., Li B., Hu H.Z. Expression of angiotensin II AT2 receptor in the acute phase of stroke in rats. Neuroreport. 2000;11:1191–1194. doi: 10.1097/00001756-200004270-00009. [DOI] [PubMed] [Google Scholar]
- Zhuo J., Dean R., MacGregor D., Alcorn D., Mendelsohn F.A. Presence of angiotensin II AT2 receptor binding sites in the adventitia of human kidney vasculature. Clin Exp Pharmacol Physiol Suppl. 1996;3:S147–S154. [PubMed] [Google Scholar]
- Zhuo J., Ohishi M., Mendelsohn F.A. Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene transgenic rat kidney. Hypertension. 1999;33:347–353. doi: 10.1161/01.hyp.33.1.347. [DOI] [PubMed] [Google Scholar]
- Zimmerman B.G., Dunham E.W. Tissue renin–angiotensin system: a site of drug action? Annu Rev Pharmacol Toxicol. 1997;37:53–69. doi: 10.1146/annurev.pharmtox.37.1.53. [DOI] [PubMed] [Google Scholar]
- Zisman L.S., Keller R.S., Weaver B., Lin Q., Speth R., Bristow M.R. Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- Zulli A., Burrell L.M., Widdop R.E., Black M.J., Buxton B.F., Hare D.L. Immunolocalization of ACE2 and AT2 receptors in rabbit atherosclerotic plaques. J Histochem Cytochem. 2006;54:147–150. doi: 10.1369/jhc.5C6782.2005. [DOI] [PubMed] [Google Scholar]
- Zwart A.S., Davis E.A., Widdop R.E. Modulation of AT1 receptor-mediated contraction of rat uterine artery by AT2 receptors. Br J Pharmacol. 1998;125:1429–1436. doi: 10.1038/sj.bjp.0702210. [DOI] [PMC free article] [PubMed] [Google Scholar]