Abstract
Angiotensin-converting enzyme (ACE) 2 is a homolog to the carboxypeptidase ACE, which generates angiotensin II, the main active peptide of renin–angiotensin system (RAS). After the cloning of ACE2 in 2000, three major ACE2 functions have been described so far. First ACE2 has emerged as a potent negative regulator of the RAS counterbalancing the multiple functions of ACE. By targeting angiotensin II ACE2 exhibits a protective role in the cardiovascular system and many other organs. Second ACE2 was identified as an essential receptor for the SARS coronavirus that causes severe acute lung failure. Downregulation of ACE2 strongly contributes to the pathogenesis of severe lung failure. Third, both ACE2 and its homologue Collectrin can associate with amino acid transporters and play essential role in the absorption of amino acids in the kidney and gut. In this review, we will discuss the multiple biological functions of ACE2.
Keywords: ACE2, Amino acid transporter, Acute respiratory distress syndrome, Collectrin, Renin–angiotensin system, SARS
Abbreviations: ACE, Angiotensin-converting enzyme; Ang, Angiotensin; ARDS, Acute respiratory distress syndrome; AT1R, Angiotensin II receptor type 1; AT2R, Angiotensin II receptor type 2; HNF-1, Hepatocyte nuclear factor-1; MODY5, Maturity onset diabetes of the young; RAS, Renin–angiotensin system; SARS, Severe acute respiratory syndrome; SARS-CoV, Severe acute respiratory syndrome coronavirus
1. Introduction
The renin–angiotensin system (RAS) plays a key role in maintaining blood pressure homeostasis, as well as fluid and salt balance in mammals (Skeggs et al., 1980, Inagami, 1994, Weinberg et al., 2000, Turner & Hooper, 2002). Abnormal activation of the RAS has been associated with the pathogenesis of cardiovascular and renal diseases such as hypertension, myocardial infarction and heart failure (Ferrario, 1990, Nicholls et al., 1998, Fleming et al., 2006). The protease renin cleaves angiotensinogen to generate angiotensin (Ang) I. The angiotensin-converting enzyme (ACE) is the critical protease the cleaves Ang I to generate Ang II, which is a key regulator of the RAS and exerts biological functions through two G-protein-coupled receptors, the Ang II receptor type 1 receptor (AT1R) and Ang II receptor type 2 receptor (AT2R) (Skeggs et al., 1980, Corvol et al., 1995). Although there exist alternative Ang II-generating enzymes (such as cathepsins and chymase) (Belova, 2000, Miyazaki & Takai, 2006), it has been thought that ACE is the key and possibly sole enzyme in the regulation of Ang II production in the RAS (Skeggs et al., 1980, Turner & Hooper, 2002).
In 2000, a homologue of ACE, angiotensin-converting enzyme 2 (ACE2), has been discovered (Donoghue et al., 2000, Tipnis et al., 2000). Accumulated evidences indicate that ACE2 negatively regulates the activated renin–angiotensin system by degrading Ang II to the heptapeptide Ang 1–7. Several studies support a counter-regulatory role for Ang 1–7 by opposing many AT1 receptor-mediated actions, especially in vasoconstriction and cellular proliferation (Santos et al., 2005, Ferrario, 2006). Thus, Ang 1–7 has become a key component of the RAS system due to its beneficial effects in the cardiovascular system. In addition to its capacity to generate Ang-(1–7), ACE2 is a multifunctional enzyme and its beneficial effects may be a result of its ability to act on other vasoactive peptides (Vickers et al., 2002).
Intriguingly, peptidase-independent actions of ACE2 have been recently elucidated. In particular, ACE2 has been identified as an essential receptor for SARS coronavirus infections as well as a protective molecule against lethal lung failure in SARS (Li et al., 2003, Kuba et al., 2005). Interestingly, SARS receptor function of ACE2 is independent of its catalytic activities for Ang II degradation, whereas ACE2-mediated Ang II degradation is still important for lung protection from SARS pathogenesis (Kuba et al., 2005). Furthermore, ACE2 and its homolog Collectrin have been identified as essential molecules required for expression of neutral amino acid transporters on the cell surface of epithelial cells. Collectrin might also have a role in insulin secretion in pancreatic β-cells and/or growth of islet cells. In this review we will discuss the ‘classical’ functions of ACE2 in regulating the renin–angiotensin system through its peptidase activity and its new, peptidase-independent functions.
2. ACE family molecules
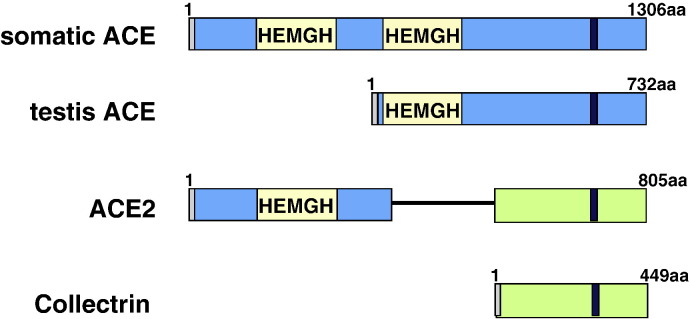
ACE was termed a ‘hypertensin-converting enzyme’ when it was initially isolated in 1956 (Skeggs et al., 1980). The human ACE gene, located on chromosome 17, encodes a 180 kDa protein with two homologous domains. Each domain has an active zinc-binding motif, His-Glu-X-X-His (HEXXH motif), which is found in many peptidases (Skeggs et al., 1980, Soubrier et al., 1988) (Fig. 1 ). ACE is a type-I transmembrane glycoprotein, which anchored to the plasma membrane through a single carboxy-terminal transmembrane domain. In humans, two distinct ACE isoenzymes have been described, an abundant somatic form found on the endothelial surface of the lungs and on brush-border membranes of kidneys, intestine, placenta and choroid plexus, and the germinal form of ACE found only in the testis (Turner and Hooper, 2002). Both ACE isoforms are membrane-bound protein and, at the cell surface, they function as ectoenzymes which hydrolyze circulating peptides. ACE can be cleaved from the cell surface and thereby act as a soluble enzyme. However, the biological significance of soluble ACE remains unclear.
Fig. 1.
Domain structures of ACE, ACE2 and Collectrin. Each protein is a type I integral protein with a signal peptide, depicted in gray, and a transmembrane domain shown in black. The zinc-binding motif (HEMGH) repeats two times in ACE and once in ACE2, and is located within the homology region denoted by the yellow box. Regions of homology between ACE2 and Collectrin are denoted in green. The numbers refer to the amino acids in each human protein.
ACE2 consists of 805 amino acids and is type I transmembrane glycoprotein with a single extracellular catalytic domain. The human ACE2 gene has been cloned and mapped to the X chromosome (Crackower et al., 2002). Like ACE, ACE2 has two domains: the amino-terminal catalytic domain and the carboxy-terminal domain (Fig. 1). The catalytic domain has one active site – the zinc metallo-peptidase domain – and shows 41.8% sequence identity with the amino domain of ACE (Donoghue et al., 2000, Tipnis et al., 2000, Turner & Hooper, 2002). ACE2's carboxy-terminal domain shows 48% sequence identity with Collectrin (Zhang et al., 2001) (Fig. 1), a noncatalytic protein recently shown to have a critical role in amino acid re-absorption in the kidney (Danilczyk et al., 2006, Malakauskas et al., 2007, Verrey et al., 2009), pancreatic beta cell proliferation (Akpinar et al., 2005), and possibly insulin exocytosis (Fukui et al., 2005).
3. ACE2 functions
Early studies observed tissue localization of ACE2 predominantly in the heart, kidneys and testes, and at a lower level in a wide variety of tissues, particularly the colon and lung (Tipnis et al., 2000), while later studies also indicated the significance of ACE2 in other organs like liver and intestines (Komatsu et al., 2002, Hamming et al., 2004). In the heart, ACE2 is expressed in the endothelium (Tipnis et al., 2000) as wells as cardiomyocytes (Gallagher et al., 2008). In kidney ACE2 distributes to the luminal surface of tubular epithelial cells (Donoghue et al., 2000, Tipnis et al., 2000), and in testes, to the adult Leydig cells (Douglas et al., 2004). ACE2 localization was mapped to the apical surface of epithelial cells, which is in contrast to ACE, which appears to be evenly distributed between the apical and basolateral membranes in polarized cells (Warner et al., 2005). Moreover, the efficacy of a SARS-CoV infection was 10-fold increased when the virus was applied at the apical surface of ACE2-expressing cells in vitro (Tseng et al., 2005).
3.1. Peptidase function of ACE2
ACE and ACE2 both belong to the M2 family (clan MA) of metalloproteases, and have their active site domains exposed to the extracellular surface, facilitating the metabolism of circulating peptides. Both ACE and ACE2 catalyze reactions by utilizing zinc, coordinated by conserved histidines within the active site, to facilitate nucleophilic attack on the carbonyl bond of the substrate by a water molecule, forming a noncovalently bound intermediate. In addition to the two histidines (located within the HEXXH motif), a further glutamate residue is involved in coordinating the zinc ion; this is located 23 amino acids C-terminally to the HEXXH motif in both ACE and ACE2 (Donoghue et al., 2000, Tipnis et al., 2000). Structural analyses for native ACE2 compared with inhibitor (MLN 4760)-bound ACE2 (Dales et al., 2002) revealed a large ‘hinge-bending’ motion, in which the catalytic subdomains I and II of the peptidase domain exhibit open-to-close transitions (Fig. 2 ). This movement is induced by binding of the inhibitor and repositions key residues for catalysis (Towler et al., 2004).
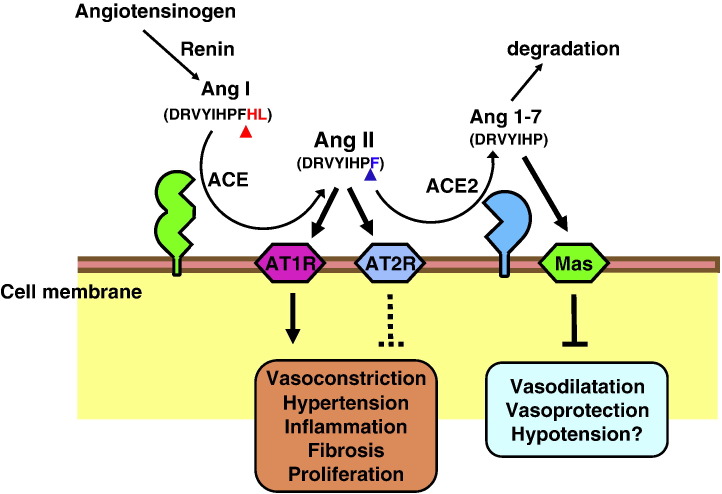
Fig. 2.
Schematic diagram of the role of ACE2 in the renin–angiotensin system. Angiotensin I (Ang I; DRVYIHPFHL) serves as a substrate for ACE, a dipeptidyl carboxypeptidase, and is converted to Angiotensin II (Ang II; DRVYIHPF), the main active peptide of the classical RAS. ACE2 catalyzes and inactivates Angiotensin II and produces the vasodilator peptide Angiotensin 1–7 (Ang 1–7; DRVYIHPF), which binds the Mas receptor and/or is degraded to inactive peptides. Red arrowheads indicate the ACE cleavage site; blue arrowheads show the ACE2 cleavage sites. It should be noted that ACE2 is an unspecific protease and can cleave multiple additional substrates such as Apelin.
Despite the similarities, ACE and ACE2 function differently; ACE releases a C-terminal dipeptide from its substrate (dipeptidylpeptidase), whereas ACE2 cleaves a single amino acid (monocarboxypeptidase) (Donoghue et al., 2000, Tipnis et al., 2000). ACE2 catalyzes peptides with a substrate preference for hydrolysis between proline and a hydrophobic or basic C-terminal residue (Vickers et al., 2002). While ACE converts Ang I to the potent vasoconstrictor Ang II (Corvol et al., 1995), ACE2 cleaves Ang I to generate the presumably inactive Ang 1–9 peptide (Donoghue et al., 2000, Tipnis et al., 2000), which then can be converted to the vasodilator peptide Ang 1–7 by ACE or other peptidases (Donoghue et al., 2000). Importantly, ACE2 directly metabolizes Ang II to generate Ang 1–7 with higher efficiency than converting Ang I to Ang 1–9 (Vickers et al., 2002). The resolution of the ACE2 crystal structure revealed that these substrate specificity differences are a result of the smaller binding pocket in ACE2 due to arginine-273 making a salt-bridge with the C-terminus of the substrate, whereas in ACE this residue is substituted by a smaller glutamine residue (Towler et al., 2004). Although there were already known Ang 1–7-forming enzymes, such as neprilysin (Yamamoto et al., 1992), prolyl endopeptidase 24.26 (Nozaki et al., 1992), and thimet oligopeptidase (Chappell et al., 2000), the identification of ACE2 has added further support to the biological significance of the Ang 1–7 (Donoghue et al., 2000, Tipnis et al., 2000, Vickers et al., 2002). This peptide has been shown to interact with the G-protein-coupled receptor Mas to mediate its vasoprotective effects (Santos et al., 2003, Keidar et al., 2007, Raizada & Ferreira, 2007, Alenina et al., 2008). ACE2 also acts on the C-terminus of the peptides Apelin-13 and Apelin-36 and cleaves an amino acid out from them with high catalytic efficiency in vitro (Vickers et al., 2002). Apelin is synthesized as 77 amino-acid preprohormone, which is processed into the 36 amino acid peptide apelin-36; further proteolytic cleavage generates Apelin-13 (Tatemoto et al., 1998, Lee et al., 2006). Systemic administration of Apelin-13 prompts hypotension in rats and mice (Tatemoto et al., 2001, Ishida et al., 2004). Interestingly, modification of the C-terminal residue of Apelin-13 (F13A) lost its hypotensive action and further antagonized the effect of wild type Apelin-13 (Lee et al., 2005), implicating putative roles of ACE2 in metabolism of Apelin peptides.
The requirement of chloride ions for the hydrolysis of Ang I by ACE has long been recognized (Skeggs et al., 1956). Similarly, ACE2 activity is also regulated by chloride ions (Vickers et al., 2002). However, the presence of chloride ions increases the hydrolysis of Ang I by ACE2, but inhibits cleavage of the vasoconstrictor Ang II (Guy et al., 2003). It has been proposed that chloride binding induces subtle changes in the conformation of the active site, which either facilitate or hinder substrate binding (Ehlers and Riordan, 1991). An increase in [Cl−] above 100 mM which is the physiological concentration in human plasma increases Ang I and decreases Ang II cleavage by ACE2 and increases Ang I cleavage by ACE (Rushworth et al., 2008). This would have the effect of increasing the localized concentration of the vasoconstrictor Ang II in the kidney, where both ACE and ACE2 have high levels of expression and extracellular chloride ion levels fluctuate.
3.2. Inhibitors and activators of ACE2 catalytic activity
A broad range of ACE inhibitors, such as captopril and lisinopril does not affect the activity of ACE2 (Donoghue et al., 2000, Tipnis et al., 2000), whereas ACE2 activity is inhibited by the dipeptide Pro-Phe (Guy et al., 2003), and specific ACE2 inhibitors, such as the peptide analogues DX600 (Huang et al., 2003) and MLN 4760 ((S,S)-2-[1-carboxy-2-[3-(3,5-dichlorobenzyl)-3H-imidazol4-yl]-ethylamino]-4-methylpentanoicacid) have been developed (Dales et al., 2002). MLN 4760 was the first rationally designed inhibitor of ACE2, based on the C-terminal dipeptide of Ang I (His-Leu), and has high potency (K i = 0.44 nM) and specificity. A counter-regulatory axis of the RAS by ACE2 prompted researchers to investigate the impact of ACE2 delivery on cardiovascular diseases in animal models (Katovich et al., 2005). ACE2 treatment by means of gene therapy or recombinant protein indeed improved hypertension, atherosclerosis, and kidney diseases (Diez-Freire et al., 2006, Dong et al., 2008, Lovren et al., 2008, Oudit et al., 2010). In silico conformation-based drug screening identified two compounds of ACE2 activator (a xanthenone and resorcinolnaphthalein) that moderately enhance ACE2 activity (Hernandez Prada et al., 2008). However, it is not clear how specific these compounds are.
3.3. Peptidase-independent functions of ACE2
Although ACE2 functions as a peptidase to catalyze Ang II cleavage, recent studies demonstrate that transmembrane domain of ACE2 has also biological functions. In 2003, the epidemics of SARS threatened the world, and ACE2 was identified as a functional receptor for a causative pathogen, the SARS coronavirus (Fig. 4 ) (Li et al., 2003, Kuba et al., 2005). Cells expressing catalytic inactive mutants of ACE2 are still permissive for SARS-CoV infection, indicating that the peptidase actions of ACE2 are not necessary for SARS entry into host cells. Consistent with the biological result, structural analyses demonstrated that SARS Spike protein contacts the tip of subdomain I of the ACE2 catalytic domain but does not affect the subdomain II nor occludes the peptidase active site (Fig. 2) (Li et al., 2005). Upon ligation of SARS-CoV to ACE2, the ectodomain of ACE2 is cleaved while the transmembrane domain is internalized for further virus particle-host cell fusions (Fig. 3) (Inoue et al., 2007, Haga et al., 2008, Wang et al., 2008). Thus, although detailed mechanisms are still elusive, the transmembrane domain of ACE2 is implicated to the traffic of SARS-CoV-receptor complex from cell membrane to the cytoplasm in SARS-CoV infections.
Fig. 4.
Interaction of ACE2 with the B0AT1 amino acid transporter. ACE2 interacts with the B0AT1 amino acid transporter (SLC6A19) which is required for polarized surface expression of the transporter in gut epithelial cells. Whether ACE2 cleavage contributes to supplying neutral amino acids for the B0AT1 is not known yet.
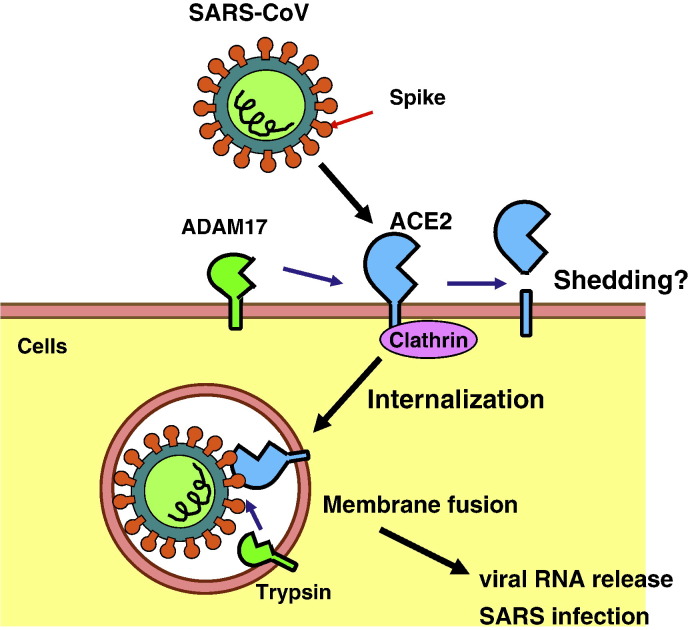
Fig. 3.
Post-translational modifications of ACE2; internalization and shedding. SARS coronavirus (SARS-CoV) binds to and internalizes with ACE2 for its cellular entry in a Clathrin-dependent manner. Membrane fusion is mediated via activation of Spike by proteases, such as trypsin or furin, and viral RNAs are released into cytoplasm, establishing SARS infection. The transmembrane proteinase (ADAM17) cleaves the extracellular juxtamembrane region of ACE2, releasing the catalytically active ectodomain into the extracellular milieu. Whether such ACE2 cleavage contributes to SARS pathogenesis is not known yet.
Expression analyses for rat kidneys isolated Collectrin as an upregulated gene in regenerating collecting ducts. Collectrin shares 47.8% identity with C-terminal end of ACE2; however, unlike ACE2, Collectrin lacks active carboxypeptidase catalytic domains (Fig. 1) (Zhang et al., 2001). The initial report documented that Collectrin localized in the cytoplasm of collecting duct epithelial cells, but further studies indicated the predominant localization of Collectrin in brush borders of the proximal tubular epithelial cells (Danilczyk et al., 2006). Danilczyk et al. also serendipitously identified Collectrin as an essential regulator of neutral amino acid transporters by gene-targeting studies in mice. Excess amounts of neutral amino acids (tyrosine and phenylalanine) appeared in the urine of Collectrin knockout mice. Biochemical studies indicated that Collectrin binds to B0AT1 neutral amino acid transporters and plays critical roles for proper expression of these transporters on cell surface required for amino acid re-absorption in proximal tubules of the kidney (Danilczyk et al., 2006, Malakauskas et al., 2007). Despite its structural similarity, ACE2 does not bind to amino acid transporters in kidneys, but in intestines, where ACE2 is highly expressed and amino acids are absorbed. In the gut, ACE2 does bind to the B0AT1 amino acid transporter and contributes to absorption of neutral amino acids (Camargo et al., 2009). Importantly, the peptidase activity of ACE2 is not necessary for pairing with the amino acid transporter.
4. Regulation of ACE2 expression
4.1. Transcriptional regulation of ACE2
ACE2 was originally cloned using a cDNA library of human failing heart ventricle (Donoghue et al., 2000), and ACE2 mRNA levels changing dynamically depending on the physiological and pathological conditions (Zisman et al., 2003, Kuba et al., 2006, Koitka et al., 2008, Oudit et al., 2009). Although transcriptional regulation of ACE2 is still elusive, accumulating evidences have indicated that inhibition of RAS by ACE inhibitors or AT1 receptor blockers upregulates ACE2 mRNA expression (Ishiyama et al., 2004, Ferrario et al., 2005). For instance, downregulation of ACE2 mRNA levels in rat proteinuric renal injury model was reversed by pharmacological inhibition of the NF-κB transcription factor, which operates downstream of Ang II receptor and cytokine signaling (Takase et al., 2005). Inhibition of mineralcorticoids (or aldosterone) increased ACE2 mRNA in macrophages likely through suppression of oxidative stress, which is associated with Ang II and/or NF-κB signaling (Keidar et al., 2005). Consistently, Interferon-γ and interleukin-4 downregulate expression of ACE2 mRNA levels in epithelial cells (de Lang et al., 2006). Thus, inflammatory signals, including Ang II, cytokines, and NF-κB, are likely to suppress ACE2 transcription.
Our early studies observed the upregulation of hypoxia-inducible genes in the hearts of Ace2 knockout mice (Crackower et al., 2002). Tissue local hypoxia increases ACE2 expression in human and rat myocardial infarction (Burrell et al., 2005), although in another model of rat, myocardial infarction changes in ACE2 mRNA levels were not observed (Ishiyama et al., 2004). ACE2 overexpression counteracts and inhibits hypoxia-induced collagen production by cardiac fibroblasts (Grobe et al., 2007). In pulmonary smooth muscle cells subjected to hypoxia, ACE2 mRNA levels increased during the early stages of hypoxia and decreased to near-baseline levels at the later stages after HIF-1α accumulation (Zhang et al., 2009). Thus, the regulation ACE2 expression under hypoxia is still elusive and may be context or cells/organ-dependent. All-trans retinoic acid has also been shown to elevate ACE2 mRNA levels in spontaneously hypertensive rats (Zhong et al., 2004). Hepatocyte nuclear factor 1β (HNF-1β, TCF2) is a tissue-specific transcription factor whose mutation in humans leads to renal cysts, genital malformations, pancreas atrophy and maturity onset diabetes of the young (MODY5). In cell lines, ACE2 was identified as a direct target gene for HNF-1β, but not HNF-1α (TCF1), and there are multiple HNF-1β binding sites in ACE2 promoter regions (Senkel et al., 2005). The ACE2 homologue Collectrin, located close to ACE2 locus on the X chromosome, is also a target gene for HNF-1 transcription factors; HNF-1α in pancreatic β cells (Fukui et al., 2005) and HNF-1β in kidney epithelial cells (Zhang et al., 2007). Thus, one can speculate that ACE2 and Collectrin gene expression is coordinately regulated by HNF-1 transcription factors.
4.2. ACE2 shedding and internalization
ACE2 was identified as a SARS receptor (Li et al., 2003) and is has been reported that ACE2 as an intact molecule and/or its transmembrane domain are internalized together with SARS-CoV upon infection, since endocytosis is essential for establishment of the virus entry (Blau & Holmes, 2001, Inoue et al., 2007). The internalization can take place even when recombinant SARS Spike protein, the SARS-CoV surface ligand for receptor binding, interacts with ACE2 (Kuba et al., 2005, Wang et al., 2008). Clathrin-dependent and -independent entry of SARS-CoV into target cells has been proposed (Inoue et al., 2007, Wang et al., 2008). However, the role of the cytoplasmic tail of ACE2 is controversial; for instance, deletion of the cytoplasmic tail of ACE2 did not affect SARS-CoV entry (Inoue et al., 2007), whereas it attenuated SARS-CoV entry in another study (Haga et al., 2008). Similar to ACE (Parkin et al., 2004), ACE2 is subject to a juxtamembrane cleavage events (shedding) which releases the catalytically active ectodomain (Lambert et al., 2005). The process can be stimulated by phorbol ester, ionomycin, endotoxin, IL-1β or TNFα. Shedding is mediated by a promiscuous ‘sheddase’, ADAM17 (or TACE, TNF-α converting enzyme; Fig. 3) (Lambert et al., 2005, Jia et al., 2009) and ADAM17-null cells are reduced in ACE2 shedding (Iwata et al., 2009). Moreover, calmodulin-binding sites were identified in the cytoplasmic tail of ACE2 (Lambert et al., 2008), and inhibition of calmodulin increased the release of the ACE2 ectodomain to the culture supernatants (Lambert et al., 2008, Lai et al., 2009). Although the physiological role of ACE2 ectodomain shedding remains elusive as roles for circulating ACE2 and remnant C-terminal domain have yet to be identified, shedding seems to be involved in SARS-CoV cellular entry and replication and an ADAM17 inhibitor suppresses SARS-CoV replication in vitro (Glowacka et al., 2010; Haga et al., 2008).
5. ACE2 as a negative regulator of RAS in the cardiovascular system
5.1. Blood pressure and vascular diseases
The ace2 gene was mapped to a quantitative trait locus (QTL) on the X chromosome in three different rat models of hypertension. These hypertensive rats show reduced ACE2 transcripts and protein expression in heart and kidney (Crackower et al., 2002). Consistently, transgenic ACE2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function (Rentzsch et al., 2008), and neuronal overexpression of ACE2 also attenuates hypertension (Yamazato et al., 2007, Feng et al., 2008). In humans, several studies have shown a strong association of ACE2 polymorphisms to hypertension in female Chinese patients with metabolic syndrome (Zhong et al., 2006) or essential hypertension (Yi et al., 2006, Fan et al., 2007, Niu et al., 2007). Thus, together with biochemical data that ACE2 degrades Ang II to generate Ang 1–7, ACE2 plays a profound role in controlling blood pressure. However, genetic inactivation of ACE2 using homologous recombination results in no apparent alterations in blood pressure in basal levels (Crackower et al., 2002). In another ACE2 knockout mouse line blood pressure was significantly increased following Ang II infusions (Gurley et al., 2006). This is a sharp contrast to spontaneous hypotension observed in ACE knockout mice (Krege et al., 1995), suggesting that, in addition to the Ang II system, ACE2 might regulate blood pressure through other peptide systems, such as bradykinin and/or Apelin. Nevertheless, exogenous supplementation of ACE2 by gene transfer decreased blood pressure in SHR hypertensive rats (Rentzsch et al., 2008), and recombinant ACE2 treatment attenuated Ang II-induced hypertension specifically (Wysocki et al., 2010). Thus, ACE2 is a negative regulator of RAS in blood pressure control. In addition to hypertension, ACE2 gene delivery has also shown beneficial effects on atherosclerosis in animal models, suggesting that ACE2 confers endothelial protection (Dong et al., 2008, Lovren et al., 2008).
5.2. Heart function control and diseases
The heart is a major organ, which abundantly expresses ACE2, and many literatures have shown its importance in heart failure. ACE2 null mice displayed impaired cardiac contractility which is associated with aging and/or cardiac pressure overload in several different ACE2 knockout mouse lines (Crackower et al., 2002, Yamamoto et al., 2006, Nakamura et al., 2008). Heart contractility impairment was correlated with elevated cardiac and plasma Ang II levels, and double knockout mice of ACE and ACE2 genes or treatment with AT1 receptor blockers reversed the cardiac phenotype in ACE2 knockout mice (Crackower et al., 2002, Yamamoto et al., 2006, Nakamura et al., 2008). Similarly, in myocardial infarction loss of ACE2 accelerates maladaptive left ventricular remodeling (Kassiri et al., 2009). Mechanistically, the age-dependent cardiomyopathy in ACE2 knockout mice is likely mediated by Ang II-induced oxidative stress and inflammation through AT1 receptor downstream PI3K (phosphatidylinositol-3-kinase) signaling (Oudit et al., 2007). Of note, one study reported that their knockout mouse line did not show alterations in cardiac function, although these mice were not investigated under conditions of cardiac like pressure overload (Gurley et al., 2006, Gurley & Coffman, 2008). However, these differences were resolved and appear to be dependent on modifier genes in different mouse background (Oudit et al., 2007). Thus, ACE and ACE2 balance Ang II levels and contribute to maintaining heart functions through AT1 receptor.
In addition to counterbalancing Ang II with ACE, the involvement of Ang 1–7 and Apelin in the actions of ACE2 on controlling heart function has been postulated. Increasing evidences have shown the beneficial effects of Ang 1–7/Mas receptor axis in pathological settings of the heart. Treatment with Ang 1–7 peptide has been shown to improve myocardial performance, cardiac remodeling, and even survival in rodent heart failure models, including ischemia/reperfusion injury, myocardial infarction, hypertension-induced cardiomyopathy (Ferreira et al., 2001, Santos et al., 2004). The nonpeptide AVE-0991, a selective ligand for Mas receptor, has actions similar to the cardioprotective effects of Ang 1–7 peptide (Ferreira et al., 2007), and knockout mice of Mas receptor displayed a reduced heart contractility (Alenina et al., 2008). Although there are accumulating literatures on ACE2-Ang 1–7-Mas axis, most of them are correlative studies on ACE2 relationships with Ang 1–7-Mas system. We still miss direct evidence linking functional causal relationships between ACE2 and Ang 1–7-Mas system. For instance, whether Ang 1–7 peptide or Mas agonist can rescue ACE2 knockout mouse heart phenotypes is not even challenged. A connection of ACE2 and Ang 1–7-Mas system is still elusive. In addition to Ang II, ACE2 also acts on the peptides Apelin-13 and Apelin-36 with high catalytic efficiency in vitro (Vickers et al., 2002). Apelin-13 and Apelin-36 are overlapped C-terminus fragments of 77 amino-acid Apelin preprohormone and are generated from it by proteolytic cleavage (Tatemoto et al., 1998, Lee et al., 2006). Although systemic administration of Apelin peptides prompts hypotension in rats and mice (Tatemoto et al., 2001, Ishida et al., 2004), Apelin knockout mice and APJ (Apelin receptor) knockout mice both showed little alterations in blood pressure at baseline. Interestingly, Apelin knockout mice showed aging- or stress-associated cardiac contractility defects, similar to the heart phenotype of ACE2 knockout mice, implicating a potential in vivo interaction of ACE2 with Apelin (Kuba et al., 2007). Nevertheless, there are no direct evidences of in vivo catalysis of Apelin peptides by ACE2, and further studies are awaited.
In humans, ACE2 gene polymorphisms associate with parameters of left ventricular hypertrophy in men (Lieb et al., 2006), and soluble ACE2 activity is increased in patients with myocardial dysfunction and correlates with disease severity (Epelman et al., 2008), implicating physiological roles of ACE2 in patients. For therapeutic applications of ACE2 using transgenic overexpression of ACE2 in the hearts, the phenotypes are controversial; one study showed that cardiac overexpression of ACE2 protects the heart from ischemia-induced heart failure (Der Sarkissian et al., 2008), whereas in the others overexpression of ACE2 was not beneficial, leading to fatal arrhythmia and severe fibrosis (Donoghue et al., 2003, Masson et al., 2009). The latter harmful effects may be due to the artifacts of transgenic gene overexpression, since utilizing recombinant ACE2 protein for therapeutic models has been recently shown to be beneficial in various disease models (Oudit et al., 2010, Treml et al., 2010, Wysocki et al., 2010). Neuronal control of heart function by ACE2 in brains has also been demonstrated. Injections of the ACE2 inhibitor MLN4760 into the nucleus tractus solitarii reduced the baroreceptor reflex for reflex bradycardia in rats (Diz et al., 2008, Xia & Lazartigues, 2008), suggesting a role for ACE2 and RAS in controlling baroreceptor reflex.
5.3. Kidney diseases
In kidney, ACE2 is abundantly expressed (Crackower et al., 2002), and the activity of ACE2 is even higher in the kidney cortex than in heart tissue (Wysocki et al., 2006). ACE2 has emerged as a protective molecule against kidney diseases in the context of negative regulation of the RAS (Soler et al., 2008, Oudit et al., 2009). For instance, deletion of ACE2 leads to late-onset nephrotic glomerulosclerosis spontaneously (Oudit et al., 2006) and accelerates diabetic kidney injury in Akita diabetic mice (Wong et al., 2007) and in streptozocin-induced diabetic mice (Tikellis et al., 2008). In line with these results, pharmacological ablation of ACE2 by MLN-4760 resulted in increased albuminuria and glomerular pathology in the kidneys of db/db diabetic mice and streptozocin-induced diabetic mice (Ye et al., 2006, Soler et al., 2007). These phenotypes are at least in part dependent on Ang II signaling and can be rescued by AT1 receptor blockade (Ye et al., 2006, Soler et al., 2007). Thus, these data definitely indicate that ACE2 is physiologically renoprotective. In hypertensive SHR rats, with the onset of hypertension, the tubular expression of ACE2 in the kidneys is downregulated compared to control rats. Diabetic nephropathy in experimental models has been shown to alter both glomerular and tubular expressions of ACE2. In a long-term diabetic rat model, renal ACE2 expression is decreased (Tikellis et al., 2003), whereas there is an early increase in ACE2 expression and activity in the kidneys of diabetic db/db (Ye et al., 2004, Wysocki et al., 2006) and Akita (Wong et al., 2007) mice. The highest expression of the ACE2 mRNA found in renal proximal tubules was significantly reduced in the tubules from diabetic rats (Tikellis et al., 2003). Thus, reduced ACE2 expression might contribute to the pathogenesis and progression of kidney diseases (Tikellis et al., 2003, Mizuiri et al., 2008, Reich et al., 2008). Renal expression of ACE2 may play a more profound role in controlling local RAS rather than regulating systemic blood pressure. Therefore, ACE2 counterbalances ACE function, negatively regulates Ang II levels and thereby controls local kidney homeostasis.
6. ACE2 is a SARS receptor - ACE2 in the respiratory system
6.1. ACE2 in lung diseases
Acute respiratory distress syndrome (ARDS) is the most severe form of acute lung injury, which affects approximately one million individuals worldwide/year and has a mortality rate of at least 30–50% (Hudson et al., 1995, Ware, 2006). ARDS can be triggered by multiple diseases such as sepsis, aspiration, trauma, acute pancreatitis, or pneumonias following infections with SARS coronavirus or avian and human influenza viruses. Previous studies of ACE insertion/deletion (I/D) polymorphism correlation with severity of ARDS in humans (Marshall et al., 2002, Jerng et al., 2006) and ACE inhibitor treatments in rodent ARDS models (Raiden et al., 2002) have suggested that the RAS could have a role in ARDS/acute lung injury.
Intriguingly, despite its normal lung structures and functions, ace2 knockout mice exhibited very severe pathology of ARDS/acute lung injury compared with wild type control mice (Imai et al., 2005); enhanced vascular permeability, increased lung edema, neutrophil accumulation, and worsened lung function. AT1 blocker treatment or additional ace gene deficiency on an ace2 knockout background rescues the severe phenotype of ace2 single mutant mice in acute lung injury (Imai et al., 2005). Importantly, treatment with catalytically active, but not enzymatically inactive, recombinant ACE2 protein improved the symptoms of acute lung injury in wild type mice as well as in ace2 knockout mice (Imai et al., 2005). Furthermore, in a recent study of large animals in sepsis ARDS models, recombinant ACE2 protein significantly improved the respiratory failure and increased the oxygen levels of ARDS in pigs (Treml et al., 2010). Thus, in animal models of acute lung injury, ACE, Ang II, and AT1 receptor function as lung injury-promoting factors, while the negative regulation of Ang II levels by ACE2 protects from lung injury (Imai et al., 2005). Moreover, in other lung injury models like bleomycin-induced lung fibrosis and monocrotaline-induced pulmonary hypertension, ACE2 has recently been shown to protect from chronic lung injuries, fibrosis, and pulmonary vasoconstriction (Ferreira et al., 2009, Yamazato et al., 2009). These results suggest that ACE2 may serve as an entirely novel therapeutic for chronic lung diseases as well as acute lung injury, and the efficacy of recombinant ACE2 protein or AT1 receptor blockers on lung diseases should be further tested in clinical settings.
6.2. ACE2 as a SARS receptor
During several months of 2003, a newly identified illness termed severe acute respiratory syndrome (SARS) spread rapidly through the world. International cooperative teams swiftly isolated a novel coronavirus as the SARS pathogen (SARS-CoV) and determined the SARS-CoV genome sequence (Marra et al., 2003, Rota et al., 2003). Surprisingly, ACE2 was identified as a functional receptor in vitro by using co-immunoprecipitation techniques (Li et al., 2003), and subsequently, in a mouse SARS infection model with ace2 knockout mice, ACE2 was indeed identified as the essential receptor for SARS infections in vivo (Kuba et al., 2005).
Interaction of SARS-CoV with ACE2 is initiated via trimers of the SARS Spike protein which extends into a hydrophobic pocket of ACE2 catalytic domain (Li et al., 2005). This ACE2–Spike interaction leads to endocytosis of virus particles through internalization with ACE2, induces the fusion of virus and host cells, and establishes SARS-CoV infection. However, this process does not require nor affect the peptidase activity of ACE2, since cells expressing enzymatically inactive ACE2 can still be infected with the SARS-CoV and ACE2 substrates are still accessible to the catalytic pocket of ACE2 when the SARS Spike protein is bound. Furthermore, in the structure of SARS Spike-bound ACE2, the catalytically active site of ACE2 was not blocked by the SARS Spike protein (Li et al., 2005). Therefore, ACE2 functions as a SARS receptor independently of its peptidase activity.
One mystery of SARS-CoV is why, in contrast to the other coronaviruses, such infections trigger severe lung disease with such high mortality. Accumulating evidence indicates that severe SARS infections are dependent on the burden of viral replication as well as on the immunopathologic consequences of the host response (Lau & Peiris, 2005, Perlman & Dandekar, 2005). In addition to the aberrant activation of the immune system, our own studies have implicated a direct involvement of ACE2 in SARS pathogenesis. Intriguingly, both SARS-CoV infection and challenge with recombinant SARS-Spike protein triggered a marked downregulation of ACE2 expression in lungs and in cell culture (Kuba et al., 2005). Thus, SARS-CoV-infected or Spike protein-treated mice resemble ace2 knockout mice. Similar to ace2 mutant mice, Spike-treated wild type mice show markedly more severe pathology in acute lung injury (Kuba et al., 2005). Therefore, downregulation of the SARS receptor ACE2 expression by SARS-CoV infection activates the renin–angiotensin system and contributes to pathogenesis of severe ARDS/acute lung injury in SARS.
6.3. ACE2 in lung progenitor cells
One of the clinical features of SARS patients is the progressive and rapid deterioration of lung function and the obvious loss of lung repair capacity after the viral load has declined (Peiris et al., 2003). Although many studies demonstrated that SARS receptor ACE2-expressing cells are alveolar type-1 or type-2 pneumocytes or vascular endothelial cells, a couple of recent studies suggested that lung epithelial stem/progenitor cells are also important for SARS infection. Mouse Oct-4+ lung epithelial progenitor cells, which arise from in vitro cultures of enzyme-released cells from neonatal lung tissues, express ACE2 and are the target cells for SARS-CoV infection in vitro and support active virus replication leading to their own destruction (Ling et al., 2006). Detailed expression analyses for mouse lungs revealed the highest expression of ACE2 in late embryonic days (E18.5) which declines after birth (Wiener et al., 2007) and further supporting a role for ACE2 in progenitor cells. In SARS-infected human patient lungs, CD34+Oct4+ lung progenitor cells are also positive for SARS-CoV antigens using immunohistochemistry, while mature pneumocytes appear to be negative for SARS-CoV antigens (Chen et al., 2007). Interestingly, in rabbit atherosclerosis models, co-localization of ACE2 in CD34+Oct4+ cells was also observed in the atherosclerotic plaques (Zulli et al., 2006). However, it should be noted that ACE2 expression is downregulated by SARS infection at the transcriptional and post-translational level (Glowacka et al., 2010; Inoue et al., 2007, Haga et al., 2008, Wang et al., 2008), and therefore one would actually expect low ACE2-immunoreactivity in SARS-CoV infected cells. Nevertheless, these observations suggest a potential role of ACE2 for lung stem/progenitor cells, in addition to type-1/type-2 pneumocytes, in the continued destruction of lung tissues and apparent loss in the capacity for lung repair after SARS-CoV infections. Further studies are, however, required.
7. Amino acid transporter partners, ACE2 and Collectrin
7.1. Identification of Collectrin function
Homology searches have shown that ACE2 is a chimeric protein that has emerged from the duplication of two genes: homology with ACE at the catalytic domain and homology with Collectrin in the transmembrane C-terminal domain. The Collectrin gene (gene name transmembrane protein 27; Tmem27) is located in immediate proximity to the ACE2 locus on the X chromosome and both genes share similar transcription factor binding sites. Collectrin was shown to be expressed in β-cells of the pancreas under the transcriptional control of hepatocyte nuclear factor 1 (HNF1), and its function was implicated as insulin exocytosis or β-cell proliferation (Akpinar et al., 2005, Fukui et al., 2005). Surprisingly, using gene-targeting studies of Collectrin, the physiological in vivo function of Collectrin turned out to be a critical binding partner for amino acid transporters. Collectrin physically associates with numerous apical amino acid transporters in the kidney and controls their expression and transport functions (Danilczyk et al., 2006). As a consequence Collectrin mutant mice exhibit a marked defect in re-absorption of amino acids in the proximal tubules of the kidneys. Biochemically, Collectrin binds to B0AT1-family amino acid transporters and controls polarized expression of these transporters on the cell surface (Danilczyk et al., 2006, Malakauskas et al., 2007).
7.2. Hartnup’s disorder genes: ACE2 and Collectrin
Due to the high homology at its transmembrane with Collectrin, ACE2 was also anticipated to play a role in amino acid re-absorption in kidneys. However, this was not the case; rather ACE2 binds to the B0AT1 amino acid transporter in the intestine but not in the kidney (Kowalczuk et al., 2008, Camargo et al., 2009). In ACE2 knockout mice, luminal expression of B0AT1 in the intestine is completely lost, suggesting that ACE2 is essential for expression of B0AT1 in cell surface of the intestine (Camargo et al., 2009). Thus, ACE2 and Collectrin are both binding partners for B0AT1 and contribute to absorption of neutral amino acids in intestine and kidney, respectively, although reasons for tissue-specific bindings of B0AT1 to ACE2 or Collectrin are unknown. Hartnup disorder is a hereditary familial disease, characterized by a pellagra-like light-sensitive rash, cerebellar ataxia, emotional instability, and amino aciduria (Baron et al., 1956). Gene mutations in the B0AT1 (Slc6a19) neutral amino acid transporter were identified as one cause of Hartnup's disorder (Kleta et al., 2004, Seow et al., 2004). Despite crucial roles of ACE2 and Collectrin in expression of this amino acid transporter, gene mutations of ACE2 and Collectrin have not been identified in Hartnup's disease. Among the human Hartnup mutations of B0AT1, the A69T and R240Q missense mutants were shown in vitro defective for activation of transport function by ACE2 and Collectrin. In in vitro Xenopus oocyte expression system the B0AT1 mutants alone show similar levels of amino acid uptake to wild-type one, although the transport rates are low (Seow et al., 2004, Camargo et al., 2009). However, when the mutants were co-expressed with ACE2, transport function was not activated by the binding partners, while the co-expression of ACE2 and B0AT1 dramatically activates transport functions. Thus, mutations in Hartnup disorder patients interfering with the interaction between B0AT1 and ACE2 may explain the disease in some individuals. On the other hand, ACE2 knockout mice were not reported for any phenotypes recapitulating Hartnup disorder. Also in Hartnup patients, for instance, a compound heterozygous for A69T and the original Hartnup mutation IVS8+2T->G showed only seizures but not other symptoms like pellagra or ataxic gaits (Camargo et al., 2009). Therefore, the physiological significance of this impaired activation of the mutants by ACE2 is yet elusive, and further analyses for the role of the mutations would be needed.
8. Conclusions
Studies of ACE2 knockout mice and gene transfer or administration of recombinant ACE2 protein have demonstrated that ACE2 is an in vivo negative regulator of the both systemic and local effects of the RAS systems through peptidase activity. The carboxypeptidase activity of ACE2 appears to limit the availability of Ang II, generates counter-regulatory vasodilator peptides such as Ang 1–7, and may even act on other peptide systems such as Apelin and dynorphin. Importantly, ACE2 was also identified as a key SARS receptor and plays a protective role in SARS-mediated ARDS. These findings are now providing the opportunity to develop a potential novel medicine for the treatment of ARDS, an often lethal condition that can develop in emerging infectious lung diseases such as avian influenza (H5N1) or the swine flu (H1N1). During the course of studying ACE2 function, we also made an entirely unexpected finding: ACE2 and Collectrin are essential for expression of amino acid transporters in the gut and kidney, respectively.
The discovery and functional characterization of ACE2 have shown that the RAS system is far more complex and that this system is tightly connected to other peptide systems and even amino acid transport functions. An understanding of such complexity and molecular crosstalks will not only broaden our knowledge on the evolution and biology of cardiovascular systems, but will also offer a chance to develop new and refined therapeutic strategies for various diseases.
Acknowledgments
KK is supported by KAKEN, Japan Heart Foundation, Kato Nambyo Foundation and Mishima Kaiun Foundation. YI is supported by KAKEN, Takeda Foundation and Uehara Foundation. JMP is supported by IMBA, the Austrian Ministry of Science and Education, and Advanced ERC grant, and EuGeneHeart.
References
- Akpinar P., Kuwajima S., Krutzfeldt J., Stoffel M. Tmem27: a cleaved and shed plasma membrane protein that stimulates pancreatic beta cell proliferation. Cell Metab. 2005;2:385–397. doi: 10.1016/j.cmet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Alenina N., Xu P., Rentzsch B., Patkin E.L., Bader M. Genetically altered animal models for Mas and angiotensin-(1–7) Exp Physiol. 2008;93:528–537. doi: 10.1113/expphysiol.2007.040345. [DOI] [PubMed] [Google Scholar]
- Baron D.N., Dent C.E., Harris H., Hart E.W., Jepson J.B. Hereditary pellagra-like skin rash with temporary cerebellar ataxia, constant renal amino-aciduria, and other bizarre biochemical features. Lancet. 1956;271:421–428. doi: 10.1016/s0140-6736(56)91914-6. [DOI] [PubMed] [Google Scholar]
- Belova L.A. Angiotensin II-generating enzymes. Biochemistry (Mosc) 2000;65:1337–1345. doi: 10.1023/a:1002848402911. [DOI] [PubMed] [Google Scholar]
- Blau D.M., Holmes K.V. Human coronavirus HCoV-229E enters susceptible cells via the endocytic pathway. Adv Exp Med Biol. 2001;494:193–198. doi: 10.1007/978-1-4615-1325-4_31. [DOI] [PubMed] [Google Scholar]
- Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. discussion 322-364. [DOI] [PubMed] [Google Scholar]
- Camargo S.M., Singer D., Makrides V., Huggel K., Pos K.M., Wagner C.A. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M.C., Gomez M.N., Pirro N.T., Ferrario C.M. Release of angiotensin-(1–7) from the rat hindlimb: influence of angiotensin-converting enzyme inhibition. Hypertension. 2000;35:348–352. doi: 10.1161/01.hyp.35.1.348. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chan V.S., Zheng B., Chan K.Y., Xu X., To L.Y. A novel subset of putative stem/progenitor CD34+Oct-4+ cells is the major target for SARS coronavirus in human lung. J Exp Med. 2007;204:2529–2536. doi: 10.1084/jem.20070462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol P., Williams T.A., Soubrier F. Peptidyl dipeptidase A: angiotensin I-converting enzyme. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Dales N.A., Gould A.E., Brown J.A., Calderwood E.F., Guan B., Minor C.A., Gavin J.M., Hales P., Kaushik V.K., Stewart M. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J Am Chem Soc. 2002;124:11852–11853. doi: 10.1021/ja0277226. [DOI] [PubMed] [Google Scholar]
- Danilczyk U., Sarao R., Remy C., Benabbas C., Stange G., Richter A., Arya S., Pospisilik J.A., Singer D., Camargo S.M. Essential role for collectrin in renal amino acid transport. Nature. 2006;444:1088–1091. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- de Lang A., Osterhaus A.D., Haagmans B.L. Interferon-gamma and interleukin-4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells. Virology. 2006;353:474–481. doi: 10.1016/j.virol.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Sarkissian S., Grobe J.L., Yuan L., Narielwala D.R., Walter G.A., Katovich M.J., Raizada M.K. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- Diez-Freire C., Vazquez J., Correa de Adjounian M.F., Ferrari M.F., Yuan L., Silver X., Torres R., Raizada M.K. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- Diz D.I., Garcia-Espinosa M.A., Gegick S., Tommasi E.N., Ferrario C.M., Ann Tallant E., Chappell M.C., Gallagher P.E. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol. 2008;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Zhang C., Feng J.B., Zhao Y.X., Li S.Y., Yang Y.P., Dong Q.L., Deng B.P., Zhu L., Yu Q.T. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1270–1276. doi: 10.1161/ATVBAHA.108.164715. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Wakimoto H., Maguire C.T., Acton S., Hales P., Stagliano N., Fairchild-Huntress V., Xu J., Lorenz J.N., Kadambi V. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35:1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Douglas G.C., O'Bryan M.K., Hedger M.P., Lee D.K., Yarski M.A., Smith A.I., Lew R.A. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- Ehlers M.R., Riordan J.F. Angiotensin-converting enzyme: zinc- and inhibitor-binding stoichiometries of the somatic and testis isozymes. Biochemistry. 1991;30:7118–7126. doi: 10.1021/bi00243a012. [DOI] [PubMed] [Google Scholar]
- Epelman S., Tang W.H., Chen S.Y., Van Lente F., Francis G.S., Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin–angiotensin–aldosterone system. J Am Coll Cardiol. 2008;52:750–754. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Wang Y., Sun K., Zhang W., Yang X., Wang S., Zhen Y., Wang J., Li W., Han Y. Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of Captopril in women. Clin Pharmacol Ther. 2007;82:187–196. doi: 10.1038/sj.clpt.6100214. [DOI] [PubMed] [Google Scholar]
- Feng Y., Yue X., Xia H., Bindom S.M., Hickman P.J., Filipeanu C.M., Wu G., Lazartigues E. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin II-mediated pressor and drinking responses and is associated with angiotensin II type 1 receptor downregulation. Circ Res. 2008;102:729–736. doi: 10.1161/CIRCRESAHA.107.169110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M. The renin–angiotensin system: importance in physiology and pathology. J Cardiovasc Pharmacol. 1990;15(Suppl 3):S1–S5. [PubMed] [Google Scholar]
- Ferrario C.M. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferreira A.J., Jacoby B.A., Araujo C.A., Macedo F.A., Silva G.A., Almeida A.P., Caliari M.V., Santos R.A. The nonpeptide angiotensin-(1–7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H1113–H1119. doi: 10.1152/ajpheart.00828.2006. [DOI] [PubMed] [Google Scholar]
- Ferreira A.J., Santos R.A., Almeida A.P. Angiotensin-(1–7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001;38:665–668. doi: 10.1161/01.hyp.38.3.665. [DOI] [PubMed] [Google Scholar]
- Ferreira A.J., Shenoy V., Yamazato Y., Sriramula S., Francis J., Yuan L., Castellano R.K., Ostrov D.A., Oh S.P., Katovich M.J. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I., Kohlstedt K., Busse R. The tissue renin–angiotensin system and intracellular signalling. Curr Opin Nephrol Hypertens. 2006;15:8–13. doi: 10.1097/01.mnh.0000196146.65330.ea. [DOI] [PubMed] [Google Scholar]
- Fukui K., Yang Q., Cao Y., Takahashi N., Hatakeyama H., Wang H., Wada J., Zhang Y., Marselli L., Nammo T. The HNF-1 target collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab. 2005;2:373–384. doi: 10.1016/j.cmet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Gallagher P.E., Ferrario C.M., Tallant E.A. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008;295:H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka, I., Bertram, S., Herzog, P., Pfefferle, S., Steffen, I., Muench, M. O., et al. (2010). Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol, 84, 1198–1205. [DOI] [PMC free article] [PubMed]
- Grobe J.L., Der Sarkissian S., Stewart J.M., Meszaros J.G., Raizada M.K., Katovich M.J. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci (Lond) 2007;113:357–364. doi: 10.1042/CS20070160. [DOI] [PubMed] [Google Scholar]
- Gurley S.B., Allred A., Le T.H., Griffiths R., Mao L., Philip N., Haystead T.A., Donoghue M., Breitbart R.E., Acton S.L. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest. 2006;116:2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley S.B., Coffman T.M. Angiotensin-converting enzyme 2 gene targeting studies in mice: mixed messages. Exp Physiol. 2008;93:538–542. doi: 10.1113/expphysiol.2007.040014. [DOI] [PubMed] [Google Scholar]
- Guy J.L., Jackson R.M., Acharya K.R., Sturrock E.D., Hooper N.M., Turner A.J. Angiotensin-converting enzyme-2 (ACE2): comparative modeling of the active site, specificity requirements, and chloride dependence. Biochemistry. 2003;42:13185–13192. doi: 10.1021/bi035268s. [DOI] [PubMed] [Google Scholar]
- Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci USA. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Prada J.A., Ferreira A.J., Katovich M.J., Shenoy V., Qi Y., Santos R.A., Castellano R.K., Lampkins A.J., Gubala V., Ostrov D.A. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- Huang L., Sexton D.J., Skogerson K., Devlin M., Smith R., Sanyal I., Parry T., Kent R., Enright J., Wu Q.L. Novel peptide inhibitors of angiotensin-converting enzyme 2. J Biol Chem. 2003;278:15532–15540. doi: 10.1074/jbc.M212934200. [DOI] [PubMed] [Google Scholar]
- Hudson L.D., Milberg J.A., Anardi D., Maunder R.J. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagami T. The renin–angiotensin system. Essays Biochem. 1994;28:147–164. [PubMed] [Google Scholar]
- Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida J., Hashimoto T., Hashimoto Y., Nishiwaki S., Iguchi T., Harada S., Sugaya T., Matsuzaki H., Yamamoto R., Shiota N. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Iwata M., Silva Enciso J.E., Greenberg B.H. Selective and specific regulation of ectodomain shedding of angiotensin-converting enzyme 2 by tumor necrosis factor alpha-converting enzyme. Am J Physiol Cell Physiol. 2009;297:C1318–C1329. doi: 10.1152/ajpcell.00036.2009. [DOI] [PubMed] [Google Scholar]
- Jerng J.S., Yu C.J., Wang H.C., Chen K.Y., Cheng S.L., Yang P.C. Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome*. Crit Care Med. 2006;13 doi: 10.1097/01.CCM.0000206107.92476.39. [DOI] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Tan P., Shi L., Hickey M., Gakhar L., Chappell M.C., Wohlford-Lenane C., McCray P.B., Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiri Z., Zhong J., Guo D., Basu R., Wang X., Liu P.P., Scholey J.W., Penninger J.M., Oudit G.Y. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- Katovich M.J., Grobe J.L., Huentelman M., Raizada M.K. Angiotensin-converting enzyme 2 as a novel target for gene therapy for hypertension. Exp Physiol. 2005;90:299–305. doi: 10.1113/expphysiol.2004.028522. [DOI] [PubMed] [Google Scholar]
- Keidar S., Gamliel-Lazarovich A., Kaplan M., Pavlotzky E., Hamoud S., Hayek T., Karry R., Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97:946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- Keidar S., Kaplan M., Gamliel-Lazarovich A. ACE2 of the heart: from angiotensin I to angiotensin (1–7) Cardiovasc Res. 2007;73:463–469. doi: 10.1016/j.cardiores.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kleta R., Romeo E., Ristic Z., Ohura T., Stuart C., Arcos-Burgos M., Dave M.H., Wagner C.A., Camargo S.R., Inoue S. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat Genet. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- Koitka A., Cooper M.E., Thomas M.C., Tikellis C. Angiotensin converting enzyme 2 in the kidney. Clin Exp Pharmacol Physiol. 2008;35:420–425. doi: 10.1111/j.1440-1681.2008.04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T., Suzuki Y., Imai J., Sugano S., Hida M., Tanigami A., Muroi S., Yamada Y., Hanaoka K. Molecular cloning, mRNA expression and chromosomal localization of mouse angiotensin-converting enzyme-related carboxypeptidase (mACE2) DNA Seq. 2002;13:217–220. doi: 10.1080/1042517021000021608. [DOI] [PubMed] [Google Scholar]
- Kowalczuk S., Broer A., Tietze N., Vanslambrouck J.M., Rasko J.E., Broer S. A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J. 2008;22:2880–2887. doi: 10.1096/fj.08-107300. [DOI] [PubMed] [Google Scholar]
- Krege J.H., John S.W., Langenbach L.L., Hodgin J.B., Hagaman J.R., Bachman E.S., Jennette J.C., O'Brien D.A., Smithies O. Male–female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6:271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Zhang L., Imai Y., Arab S., Chen M., Maekawa Y., Leschnik M., Leibbrandt A., Markovic M., Schwaighofer J. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ Res. 2007;101:e32–e42. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- Lai Z.W., Lew R.A., Yarski M.A., Mu F.T., Andrews R.K., Smith A.I. The identification of a calmodulin-binding domain within the cytoplasmic tail of angiotensin-converting enzyme-2. Endocrinology. 2009;150:2376–2381. doi: 10.1210/en.2008-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., Clarke N.E., Hooper N.M., Turner A.J. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008;582:385–390. doi: 10.1016/j.febslet.2007.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y.L., Peiris J.S. Pathogenesis of severe acute respiratory syndrome. Curr Opin Immunol. 2005;17:404–410. doi: 10.1016/j.coi.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.K., George S.R., O'Dowd B.F. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006;27:190–194. doi: 10.1016/j.tips.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Lee D.K., Saldivia V.R., Nguyen T., Cheng R., George S.R., O'Dowd B.F. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231–236. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb W., Graf J., Gotz A., Konig I.R., Mayer B., Fischer M., Stritzke J., Hengstenberg C., Holmer S.R., Doring A. Association of angiotensin-converting enzyme 2 (ACE2) gene polymorphisms with parameters of left ventricular hypertrophy in men Results of the MONICA Augsburg echocardiographic substudy. J Mol Med. 2006;84:88–96. doi: 10.1007/s00109-005-0718-5. [DOI] [PubMed] [Google Scholar]
- Ling T.Y., Kuo M.D., Li C.L., Yu A.L., Huang Y.H., Wu T.J., Lin Y.C., Chen S.H., Yu J. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc Natl Acad Sci USA. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C., Levitt K.S., Oudit G.Y., Al-Omran M., Stewart D.J. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- Malakauskas S.M., Quan H., Fields T.A., McCall S.J., Yu M.J., Kourany W.M., Frey C.W., Le T.H. Aminoaciduria and altered renal expression of luminal amino acid transporters in mice lacking novel gene collectrin. Am J Physiol Renal Physiol. 2007;292:F533–F544. doi: 10.1152/ajprenal.00325.2006. [DOI] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Marshall R.P., Webb S., Bellingan G.J., Montgomery H.E., Chaudhari B., McAnulty R.J., Humphries S.E., Hill M.R., Laurent G.J. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- Masson R., Nicklin S.A., Craig M.A., McBride M., Gilday K., Gregorevic P., Allen J.M., Chamberlain J.S., Smith G., Graham D. Onset of experimental severe cardiac fibrosis is mediated by overexpression of Angiotensin-converting enzyme 2. Hypertension. 2009;53:694–700. doi: 10.1161/HYPERTENSIONAHA.108.122333. [DOI] [PubMed] [Google Scholar]
- Miyazaki M., Takai S. Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. J Pharmacol Sci. 2006;100:391–397. doi: 10.1254/jphs.cpj06008x. [DOI] [PubMed] [Google Scholar]
- Mizuiri S., Hemmi H., Arita M., Ohashi Y., Tanaka Y., Miyagi M., Sakai K., Ishikawa Y., Shibuya K., Hase H. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Koibuchi N., Nishimatsu H., Higashikuni Y., Hirata Y., Kugiyama K., Nagai R., Sata M. Candesartan ameliorates cardiac dysfunction observed in angiotensin-converting enzyme 2-deficient mice. Hypertens Res. 2008;31:1953–1961. doi: 10.1291/hypres.31.1953. [DOI] [PubMed] [Google Scholar]
- Nicholls M.G., Richards A.M., Agarwal M. The importance of the renin–angiotensin system in cardiovascular disease. J Hum Hypertens. 1998;12:295–299. doi: 10.1038/sj.jhh.1000638. [DOI] [PubMed] [Google Scholar]
- Niu W., Qi Y., Hou S., Zhou W., Qiu C. Correlation of angiotensin-converting enzyme 2 gene polymorphisms with stage 2 hypertension in Han Chinese. Transl Res. 2007;150:374–380. doi: 10.1016/j.trsl.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Sato N., Iida T., Hara K., Fukuyama K., Epstein W.L. Prolyl endopeptidase purified from granulomatous inflammation in mice. J Cell Biochem. 1992;49:296–303. doi: 10.1002/jcb.240490313. [DOI] [PubMed] [Google Scholar]
- Oudit G.Y., Herzenberg A.M., Kassiri Z., Wong D., Reich H., Khokha R., Crackower M.A., Backx P.H., Penninger J.M., Scholey J.W. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.Y., Imai Y., Kuba K., Scholey J.W., Penninger J.M. The role of ACE2 in pulmonary diseases—relevance for the nephrologist. Nephrol Dial Transplant. 2009;24:1362–1365. doi: 10.1093/ndt/gfp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.Y., Kassiri Z., Patel M.P., Chappell M., Butany J., Backx P.H., Tsushima R.G., Scholey J.W., Khokha R., Penninger J.M. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Oudit G.Y., Liu G.C., Zhong J., Basu R., Chow F.L., Zhou J., Loibner H., Janzek E., Schuster M., Penninger J.M. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin E.T., Turner A.J., Hooper N.M. Secretase-mediated cell surface shedding of the angiotensin-converting enzyme. Protein Pept Lett. 2004;11:423–432. doi: 10.2174/0929866043406544. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiden S., Nahmod K., Nahmod V., Semeniuk G., Pereira Y., Alvarez C., Giordano M., Geffner J.R. Nonpeptide antagonists of AT1 receptor for angiotensin II delay the onset of acute respiratory distress syndrome. J Pharmacol Exp Ther. 2002;303:45–51. doi: 10.1124/jpet.102.037382. [DOI] [PubMed] [Google Scholar]
- Raizada M.K., Ferreira A.J. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol. 2007;50:112–119. doi: 10.1097/FJC.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- Reich H.N., Oudit G.Y., Penninger J.M., Scholey J.W., Herzenberg A.M. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- Rentzsch B., Todiras M., Iliescu R., Popova E., Campos L.A., Oliveira M.L., Baltatu O.C., Santos R.A., Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension. 2008;52:967–973. doi: 10.1161/HYPERTENSIONAHA.108.114322. [DOI] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Rushworth C.A., Guy J.L., Turner A.J. Residues affecting the chloride regulation and substrate selectivity of the angiotensin-converting enzymes (ACE and ACE2) identified by site-directed mutagenesis. FEBS J. 2008;275:6033–6042. doi: 10.1111/j.1742-4658.2008.06733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.A., Ferreira A.J., Nadu A.P., Braga A.N., de Almeida A.P., Campagnole-Santos M.J., Baltatu O., Iliescu R., Reudelhuber T.L., Bader M. Expression of an angiotensin-(1–7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics. 2004;17:292–299. doi: 10.1152/physiolgenomics.00227.2003. [DOI] [PubMed] [Google Scholar]
- Santos R.A., Frezard F., Ferreira A.J. Angiotensin-(1–7): blood, heart, and blood vessels. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:383–391. doi: 10.2174/156801605774322373. [DOI] [PubMed] [Google Scholar]
- Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de Buhr I., Heringer-Walther S., Pinheiro S.V., Lopes M.T., Bader M. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkel S., Lucas B., Klein-Hitpass L., Ryffel G.U. Identification of target genes of the transcription factor HNF1beta and HNF1alpha in a human embryonic kidney cell line. Biochim Biophys Acta. 2005;1731:179–190. doi: 10.1016/j.bbaexp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Seow H.F., Broer S., Broer A., Bailey C.G., Potter S.J., Cavanaugh J.A., Rasko J.E. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet. 2004;36:1003–1007. doi: 10.1038/ng1406. [DOI] [PubMed] [Google Scholar]
- Skeggs L.T., Dorer F.E., Levine M., Lentz K.E., Kahn J.R. The biochemistry of the renin–angiotensin system. Adv Exp Med Biol. 1980;130:1–27. doi: 10.1007/978-1-4615-9173-3_1. [DOI] [PubMed] [Google Scholar]
- Skeggs L.T., Jr., Kahn J.R., Shumway N.P. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M.J., Wysocki J., Batlle D. Angiotensin-converting enzyme 2 and the kidney. Exp Physiol. 2008;93:549–556. doi: 10.1113/expphysiol.2007.041350. [DOI] [PubMed] [Google Scholar]
- Soler M.J., Wysocki J., Ye M., Lloveras J., Kanwar Y., Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- Soubrier F., Alhenc-Gelas F., Hubert C., Allegrini J., John M., Tregear G., Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci USA. 1988;85:9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase O., Marumo T., Imai N., Hirahashi J., Takayanagi A., Hishikawa K., Hayashi M., Shimizu N., Fujita T., Saruta T. NF-kappaB-dependent increase in intrarenal angiotensin II induced by proteinuria. Kidney Int. 2005;68:464–473. doi: 10.1111/j.1523-1755.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Takayama K., Zou M.X., Kumaki I., Zhang W., Kumano K., Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- Tikellis C., Bialkowski K., Pete J., Sheehy K., Su Q., Johnston C., Cooper M.E., Thomas M.C. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- Tikellis C., Johnston C.I., Forbes J.M., Burns W.C., Burrell L.M., Risvanis J., Cooper M.E. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem. 2004;279:17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treml B., Neu N., Kleinsasser A., Gritsch C., Finsterwalder T., Geiger R., Schuster M., Janzek E., Loibner H., Penninger J. Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit Care Med. 2010;38:596–601. doi: 10.1097/CCM.0b013e3181c03009. [DOI] [PubMed] [Google Scholar]
- Tseng C.T., Tseng J., Perrone L., Worthy M., Popov V., Peters C.J. Apical entry and release of severe acute respiratory syndrome-associated coronavirus in polarized Calu-3 lung epithelial cells. J Virol. 2005;79:9470–9479. doi: 10.1128/JVI.79.15.9470-9479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A.J., Hooper N.M. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol Sci. 2002;23:177–183. doi: 10.1016/s0165-6147(00)01994-5. [DOI] [PubMed] [Google Scholar]
- Verrey F., Singer D., Ramadan T., Vuille-dit-Bille R.N., Mariotta L., Camargo S.M. Kidney amino acid transport. Pflugers Arch. 2009;458:53–60. doi: 10.1007/s00424-009-0638-2. [DOI] [PubMed] [Google Scholar]
- Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware L.B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- Warner F.J., Lew R.A., Smith A.I., Lambert D.W., Hooper N.M., Turner A.J. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem. 2005;280:39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- Weinberg M.S., Weinberg A.J., Zappe D.H. Effectively targetting the renin–angiotensin–aldosterone system in cardiovascular and renal disease: rationale for using angiotensin II receptor blockers in combination with angiotensin-converting enzyme inhibitors. J Renin Angiotensin Aldosterone Syst. 2000;1:217–233. doi: 10.3317/jraas.2000.034. [DOI] [PubMed] [Google Scholar]
- Wiener R.S., Cao Y.X., Hinds A., Ramirez M.I., Williams M.C. Angiotensin converting enzyme 2 is primarily epithelial and is developmentally regulated in the mouse lung. J Cell Biochem. 2007;101:1278–1291. doi: 10.1002/jcb.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D.W., Oudit G.Y., Reich H., Kassiri Z., Zhou J., Liu Q.C., Backx P.H., Penninger J.M., Herzenberg A.M., Scholey J.W. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J., Ye M., Rodriguez E., Gonzalez-Pacheco F.R., Barrios C., Evora K., Schuster M., Loibner H., Brosnihan K.B., Ferrario C.M. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J., Ye M., Soler M.J., Gurley S.B., Xiao H.D., Bernstein K.E., Coffman T.M., Chen S., Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Chappell M.C., Brosnihan K.B., Ferrario C.M. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692–696. doi: 10.1161/01.hyp.19.6.692. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Ohishi M., Katsuya T., Ito N., Ikushima M., Kaibe M., Tatara Y., Shiota A., Sugano S., Takeda S. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- Yamazato M., Yamazato Y., Sun C., Diez-Freire C., Raizada M.K. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- Yamazato Y., Ferreira A.J., Hong K.H., Sriramula S., Francis J., Yamazato M., Yuan L., Bradford C.N., Shenoy V., Oh S.P. Prevention of pulmonary hypertension by Angiotensin-converting enzyme 2 gene transfer. Hypertension. 2009;54:365–371. doi: 10.1161/HYPERTENSIONAHA.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Wysocki J., Naaz P., Salabat M.R., LaPointe M.S., Batlle D. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension. 2004;43:1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- Ye M., Wysocki J., William J., Soler M.J., Cokic I., Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- Yi L., Gu Y.H., Wang X.L., An L.Z., Xie X.D., Shao W., Ma L.Y., Fang J.R., An Y.D., Wang F. Association of ACE, ACE2 and UTS2 polymorphisms with essential hypertension in Han and Dongxiang populations from north-western China. J Int Med Res. 2006;34:272–283. doi: 10.1177/147323000603400306. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wada J., Hida K., Tsuchiyama Y., Hiragushi K., Shikata K., Wang H., Lin S., Kanwar Y.S., Makino H. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J Biol Chem. 2001;276:17132–17139. doi: 10.1074/jbc.M006723200. [DOI] [PubMed] [Google Scholar]
- Zhang R., Wu Y., Zhao M., Liu C., Zhou L., Shen S., Liao S., Yang K., Li Q., Wan H. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wada J., Yasuhara A., Iseda I., Eguchi J., Fukui K., Yang Q., Yamagata K., Hiesberger T., Igarashi P. The role for HNF-1beta-targeted collectrin in maintenance of primary cilia and cell polarity in collecting duct cells. PLoS ONE. 2007;2:e414. doi: 10.1371/journal.pone.0000414. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhong J., Yan Z., Liu D., Ni Y., Zhao Z., Zhu S., Tepel M., Zhu Z. Association of angiotensin-converting enzyme 2 gene A/G polymorphism and elevated blood pressure in Chinese patients with metabolic syndrome. J Lab Clin Med. 2006;147:91–95. doi: 10.1016/j.lab.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.C., Huang D.Y., Yang Y.M., Li Y.F., Liu G.F., Song X.H., Du K. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;11:11. doi: 10.1161/01.HYP.0000146400.57221.74. [DOI] [PubMed] [Google Scholar]
- Zisman L.S., Keller R.S., Weaver B., Lin Q., Speth R., Bristow M.R., Canver C.C. Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- Zulli A., Burrell L.M., Widdop R.E., Black M.J., Buxton B.F., Hare D.L. Immunolocalization of ACE2 and AT2 receptors in rabbit atherosclerotic plaques. J Histochem Cytochem. 2006;54:147–150. doi: 10.1369/jhc.5C6782.2005. [DOI] [PubMed] [Google Scholar]