Summary
Objective
Neopterin is generated and released in increased amounts by macrophages upon activation by interferon-γ during Th1-type immune response. The potential usefulness of neopterin in early prognostic information of dengue virus infection was investigated.
Methods
Neopterin concentrations were determined in serum samples from 110 dengue fever (DF) patients. The neopterin levels were compared with those in 50 measles and 40 influenza patients; 155 healthy blood donors served as controls.
Results
In acute sera of DF patients mean neopterin concentration was 48.2 nmol/L, which was higher than that in patients with measles (mean: 36.3 nmol/L) and influenza (18.8 nmol/L) and in healthy controls (6.7 nmol/L; P < 0.001). In the patients with confirmed DF, an early neopterin elevation was detected already at the first day after the onset of symptoms and rose to a maximum level of 54.3 nmol/L 4 days after the onset. Higher increase of neopterin level in DF patients was associated with longer duration of fever and thus predicted the clinical course of the disease.
Conclusions
Neopterin concentrations were found significantly higher in DF patients compared with healthy controls and also with other viral infections (P < 0.001) and may allow early assessment of the severity of DF.
Keywords: Dengue virus, Dengue fever, Neopterin, Prognostic marker, Severe acute respiratory syndrome
Introduction
Dengue is an acute febrile disease resulting from an infection by dengue virus, a group of four antigenically related flaviviruses designated serotypes 1 through 4. Transmission involves ingestion of viremic blood by mosquitoes and subsequent passage to a susceptible human host, the principal vector being Aedes aegypti.1 Dengue virus infections can manifest as dengue fever (DF), an uncomplicated febrile illness, or dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), the severe form.2, 3, 4, 5
Considerable evidence suggests an abnormal immune response and a disturbance in immune regulation as the basis of the pathogenesis.6, 7 Macrophage is an important target of dengue virus infection,2, 8 which is known to produce various cytokines upon stimulation.9, 10 In dengue virus infection, cytokines may be released either directly from virus-infected cells such as monocytes/macrophages or upon interactions of virus-infected cells with other immunocompetent cells such as activated T lymphocytes.9, 10
During immune response, e.g. triggered by a viral infection, 6-d-erythro-neopterin (molecular weight 253.2 Da) is generated and released in increased amounts by human macrophages upon activation by Th1-type cytokine interferon-γ. Accordingly, determination of neopterin concentrations in body fluids is useful for the monitoring of cellular (=Th1-type) immune activation in various diseases such as infections, autoimmune diseases, malignant disorders, and to early detect allograft rejection episodes.11, 12, 13, 14, 15, 16, 17, 18, 19 In particular, increased neopterin concentrations in blood or urine are an early and sensitive indicator for the presence of a broad panel of viral infectious diseases including human immunodeficiency virus type 1 (HIV-1),11, 20 and the degree of neopterin elevation, e.g. in patients with HIV-1 infection, is of predictive value.11, 20 During acute infections with HIV-1, cytomegalovirus or rubella, increased neopterin concentrations were observed before specific antibodies became detectable.15, 21, 22, 23 However, increased neopterin concentrations are not specific for virus infections.
The neopterin level in early stage of DF patients may be a sensitive indicator for estimation of the severity of the diseases. To date there is only one small study on the importance of neopterin in dengue-infected patients available in the scientific literature.24 It shows a correlation between severity of illness and neopterin levels, i.e., highest levels in DHF/DSS. However, the sample size was too small to draw a persuasive conclusion. In the present study, we detected concentration of neopterin in 174 serum samples collected from 110 DF patients confirmed by serology and/or virology and 155 sera from healthy blood donors. Samples were also tested on patients with confirmed influenza virus infection and measles virus infection, two of the most common diseases with fever and similar early symptoms, as other viral infectious disease controls.
Materials and methods
Study subjects and sample collection
We studied 110 DF patients who were admitted to 18 hospitals in Guangzhou from August 15 to November 2, 2002 with contact history (living around the known DF patients, 66.4%), travel history (33.6%) and high fever (≥38.5 °C) for at least 2 days. All these 110 DF patients showed elevated IgM antibodies without detectable IgG antibodies against a dengue virus according to the WHO criteria.25 Diagnosis of DF in 66 patients was also confirmed by virological tests of the virus isolation and/or PCR. All these DF patients were identified to be infected with serotype I dengue virus by serological and/or virological tests. The clinical information of these DF patients is summarized in Table 1.
Table 1.
Clinical information of DF patients from Guangzhou, China
| Total number | 110 |
| Onset of DF | August 15–November 2, 2002 |
| Gender (Male/Female) | 49/61 |
| Age range (mean ± SD, years) | 2–75 (37 ± 17) |
| Duration of fever (mean ± SD, days) | 6.3 ± 1.8 |
| Living around known DF patients (%) | 73 (66.4) |
| WBC count 109/L (mean ± SD) | 6.3 ± 3.9 |
| Lymphocyte 109/L (mean ± SD) | 0.93 ± 0.53 |
| Specific IgM antibody positive (%) | 110 (100) |
| Virology (virus isolation and/or PCR) positive (%) | 66 (60) |
| Hospitalization (%) | 62 (56.4) |
| DHF/DSS (%) | 0 (0) |
| Death (%) | 0 (0) |
Paired sera were taken from each of 64 DF patients and single sera were taken from 46 DF patients. A total of 174 serum samples from 110 DF patients were collected for antibody and neopterin detections in this study. One-hundred and fifty acute sera were collected within 9 days (4.4 ± 2.0 days, mean ± SD) after the onset of the disease. Serum samples were also collected from 50 measles patients (16 male and 34 female) with an average age of 9.1 ± 9.9 years (mean ± SD, range: 1–35 years) and 40 influenza patients (22 male and 18 female) with an average age of 34.5 ± 28.4 years (mean ± SD, range: 2–84 years), who were confirmed according to China Health Ministry Standards by clinical laboratories of the hospitals, as other viral infectious disease controls. The 50 measles samples were collected within 7 days (3.7 ± 1.5 days, mean ± SD) after the onset of the disease. The 40 influenza samples were also collected within 7 days (3.9 ± 2.0 days, mean ± SD) after the onset of the disease. Serum samples of non-infectious controls were obtained from 155 healthy adults (75 male and 80 female) with an average age of 35.2 ± 12.5 years (mean ± SD, range: 18–57 years) by blood banks of Red Cross in Hong Kong (109 sera) and Guangzhou (46 sera) as control. All serum samples were stored at −20 °C until used. For all patients with informed consent, parts of their blood specimens obtained during routine procedures were anonymously referred to additional analyses within this study.
Isolation and identification of dengue virus
The presence of dengue virus was detected by isolation of the virus from the samples and/or viral RNA by RT–PCR. The virus was isolated in C6/36 cells (a generous gift of Institute for Viral Disease Control and Prevention, China CDC). The serotypes of isolated virus were further identified by immune fluorescence assay (IFA) using specific I–IV monoclonal antibodies (Chemicon Co. Ltd, USA). The viral RNA was also tested by RT–PCR directly from the specimens. Briefly, the viral RNA was extracted from the samples using RNeasy Mini Kit (Qiagen, Chatsworth, CA, USA). The first-strand cDNA was synthesized using RNA H+ Reverse Transcriptase (Life Technologies Inc., MD, USA) and random primers. Subsequently, PCR was carried out using PCR kit (Invitrogen Corp., CA, USA) and a pair of primers to determine the presence of the virus. Serotypes of the virus in positive samples were further identified using a series of primers.
Detection of dengue virus specific antibodies
Dengue virus specific antibodies IgM and IgG were tested by Dengue blot kits (Genelabs Co. Ltd, Singapore) and ELISA kits (IBL Co. Ltd, Germany) according to the instructions of the companies and methods described previously.26, 27
Neopterin assay
Serum neopterin was determined using a commercially available ELISA (ELItest® Neopterin-Screening, BRAHMS Diagnostica, Berlin, Germany) according to the manufacturer's instructions, having a sensitivity of 2 nmol/L neopterin. The reference level for healthy controls was set at 10 nmol/L.28
Statistical analysis
Results are presented as mean ± SD. The plot of neopterin concentrations versus time after admission is presented as means ± SEM. The Mann–Whitney test was used to assess statistically significant differences. Correlation analyses were performed calculating Spearman rank correlation coefficients. The level of significance was set at P < 0.05.
Results
Neopterin level raised at early stage of DF patients
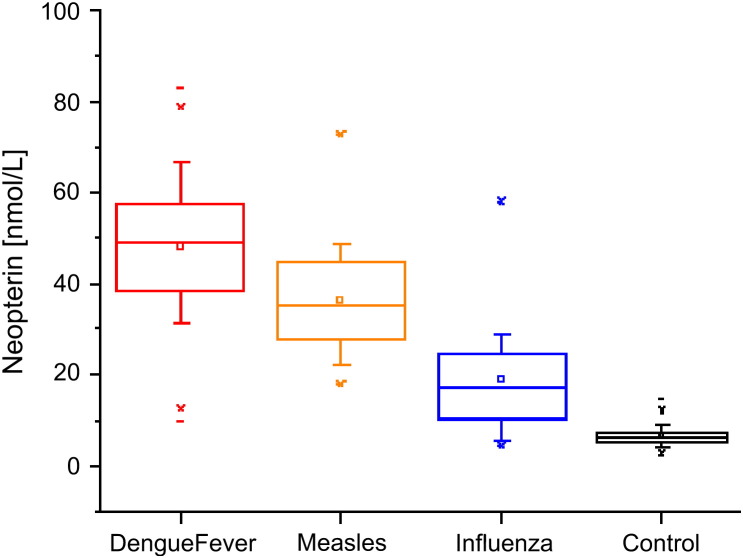
Serum neopterin concentrations raised in DF patients as early as on the first day of the onset of symptoms (>10 nmol/L), reaching a mean concentration of 48.2 ± 14.1 nmol/L (mean ± SD, range 9.9–83.0 nmol/L) in 150 acute sera ( Fig. 1). The mean neopterin concentration of healthy blood donors was 6.7 ± 2.0 nmol/L (mean ± SD, range 2.8 to 14.5 nmol/L). Thus, the mean neopterin concentration in DF patients exceeded that of the controls by 7.19 fold (P < 0.001). The mean concentration of neopterin in DF patients was slightly higher than that in patients with acute measles infection (36.3 ± 11.5 nmol/L) and nearly threefold higher than that in 40 acute influenza sera (18.8 ± 11.9 nmol/L). The difference between the groups is statistically significant (P < 0.05, Mann–Whitney test).
Figure 1.
Box plots of serum neopterin concentrations in 150 acute dengue fever sera with a mean concentration of 48.2 ± 14.1 nmol/L (mean ± SD), 50 acute measles sera (36.3 ± 11.5 nmol/L), 40 acute influenza sera (18.8 ± 11.9 nmol/L) and 155 healthy sera (6.7 ± 2.0 nmol/L) as control. The small squares (□) denote the means and the lines inside the boxes denote medians. The difference between the groups is statistically significant (P < 0.05).
Release kinetics of neopterin
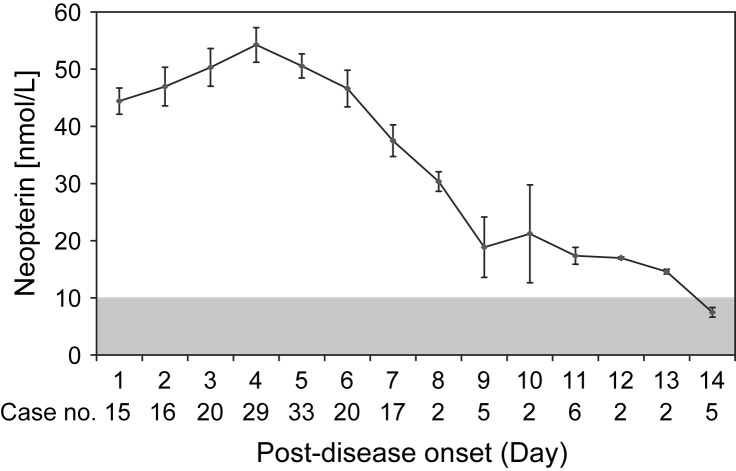
As shown in Fig. 2, the neopterin concentration was elevated on day 1 of the onset (44.4 ± 2.3 nmol/L, mean ± SEM) and reached the peak value of 54.3 ± 3.0 nmol/L on day 4. The serum neopterin concentrations remained at high levels (30 nmol/L) up to day 8 of the onset, but returned to normal level (<10 nmol/L) after 14 days of onset.
Figure 2.
Serum neopterin concentrations (mean ± SEM) in 174 sera of 110 DF patients after the onset of symptoms; gray area indicates normal range of neopterin levels (<10 nmol/L).
Higher neopterin level associated with longer fever period
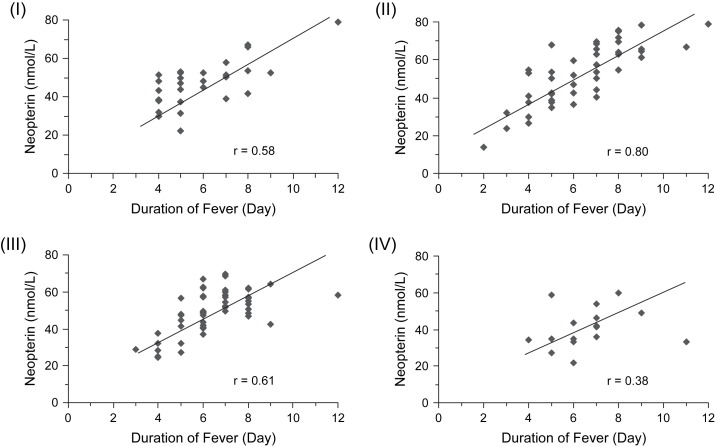
To analyze the clinical significance of increased neopterin in early stage of DF patients, we compared the patients' neopterin level on different days (1–8 days) after the onset and their fever period. Higher neopterin concentrations in patients were associated with longer duration of fever, and neopterin concentrations determined within the first two days predicted the length of fever episodes developing thereafter ( Fig. 3). The patients with longer fever period also appeared to have severer course of the disease, including higher fever and longer hospitalized period ( Table 2).
Figure 3.
Relationship between serum neopterin concentrations detected 1–2 days (I), 3–4 days (II), 5–6 days (III) and 7–8 days (IV) after the onset and the duration of fever of these DF patients (Spearman rank correlation coefficients are shown, all P < 0.01).
Table 2.
Relationship of neopterin level and the disease severity of DF patients
| Groupsa | No. of patients | Neopterin (nmol/L) |
Highest feverb |
Duration of fever (days)b |
Hospitalizationb |
|
|---|---|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean days (±SD) | No. (%) | Mean days (±SD) | ||
| 1 | 57 | 58.8 (±9.1) | 39.1 °C (±0.2) | 7.2 (±1.7) | 45 (78.9) | 8.7 (±1.5) |
| 2 | 53 | 38.2 (±8.2) | 38.4 °C (±0.3) | 5.4 (±1.5) | 17 (32.1) | 6.6 (±1.2) |
The 110 DF patients were separated into two groups based on their serum neopterin levels: Higher (group 1) or lower (group 2) than the mean value of neopterin tested in the blood samples collected on the same day after the onset of the disease in this study cohort.
P < 0.001.
Discussion
The present study shows increase of serum neopterin concentrations in DF patients as early as the first day after the onset of symptoms, and there is also a significant difference in neopterin concentrations between acute DF sera and 155 healthy blood donors. Neopterin concentrations were found to be higher in acute DF sera compared with those in acute measles sera and acute influenza sera. Dengue virus primarily infects human monocytes and macrophages regardless of the stage of cell differentiation. After dengue virus infection, the monocytes and macrophages secreted multiple innate cytokines and chemokines.29 The kinetics of neopterin concentrations based on the 110 DF patients indicates that an early neopterin elevation can be detected as early as 1 day after the onset of symptoms and quickly reaches its peak level 4 days after the onset. The neopterin concentration maintained at high level up to 8 days after the onset, and there were no false negative results within this period. The data confirm and extend results of an earlier pilot study in patients with Dengue virus infection.24 The observation also fits well to data obtained from other virus infections such as HIV,21, 23 rubella22 or also Ebola30 and it agrees with results from, e.g., the experimental infection of rhesus macaques with simian immunodeficiency virus (SIV).31 Data available primarily from studies with HIV-1 infection20, 32 imply that a predictive value of neopterin concentrations might exist also in patients with DF, those with lower neopterin concentrations being more likely to recover than others. Notably, behavior of neopterin concentrations in acute DF patients is similar to severe acute respiratory syndrome (SARS), cytomegalovirus (CMV) or rubella infection,15, 22, 33 but differs considerably from patients with HIV-1 or animals with SIV infection21, 23, 31: like in DF patients, SARS, acute CMV and rubella infection causes a sharp increase of neopterin concentrations which is followed by seroconversion and a drop of neopterin concentrations into the range of healthy controls.
Higher neopterin concentration in DF patients was associated with longer fever period and thus reflected severer inflammation occurred in these patients. This shows that neopterin can be an early reference indicator of the severity of DF. Using neopterin to quantify the severity of the inflammation in DF patients, patients can receive proper medical treatment at an early stage, thus producing a good recovery rate, contributing considerable economic gains for both patients and hospitals.
Serum neopterin exhibits age-dependence but not sex-dependence. Neopterin concentrations in healthy controls aged <19 years and between 19 and 75 years are 6.8 ± 3.6 nmol/L (95th percentile: 13.5%) and 5.3 ± 2.7 nmol/L (8.7%).12 In the present study, the neopterin concentrations in healthy controls aged between 2 and 75 years (mean ± S.D.: 6.7 ± 2.0 nmol/L) were comparable to data from the literature (5.3 ± 2.7 nmol/L),12 albeit slightly higher.
In patients with chronic renal failure the concentration of neopterin in serum is very likely to be high and elevates over a longer period of time.34 Thus, caution must be taken when using neopterin for screening of DF in case of renal insufficiency.
To date, there is no commercially available biomarker test approved for use as an aid in the assessment and diagnosis of DF. There have been various reports in the scientific literature describing the ability of various biomarkers to predict or identify DF, with varied success.24, 35, 36, 37, 38 A biomarker panel approach can enhances diagnostic accuracy, particularly when the panel includes protein markers associated with various components of the disease pathophysiology. The application of new biomarkers in studies on dengue diagnosis and pathogenesis represents a significant challenge for the future.
Further studies are required to validate and refine the optimal serum neopterin cut-off values for prognosis, and future work should address relationships with neopterin levels among patients with DF and DHF/DSS, effects of treatment and morbidity outcomes.
Technological advances may further enhance the usefulness of neopterin measurement in acute infection. At present the use of neopterin ELISA allows the results to be available at least 2 h after sampling.
Conclusion
This study reports neopterin as an early marker for assessment of the severity of DF and elevated neopterin level can be detected as early as the first day after the onset of symptoms. Data suggest that neopterin measurement could serve as an early predictor of future course of dengue virus infection.
Acknowledgment
This study was partially supported by the Small Entrepreneur Research Assistance Programme (SERAP; S/P895/05A) and the University Development Fund of the University of Hong Kong (UDF 00503758).
Contributor Information
Reinhard Renneberg, Email: chrenneb@usthk.ust.hk.
Bo-Jian Zheng, Email: bzheng@hkucc.hku.hk.
References
- 1.Henchal E.A., Putnak J.R. The dengue viruses. Clin Microbiol Rev. 1990;3(4):376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead S.B. Immunological parameters of togavirus disease sydromes. In: Schlesinger R.W., editor. The toga viruses. Academic Press; New York: 1980. pp. 107–173. [Google Scholar]
- 3.Halstead S.B. The XXth century dengue pandemic: need for surveillance and research. World Health Stat Q. 1992;45(2–3):292–298. [PubMed] [Google Scholar]
- 4.Krishnamurti C., Alving B. Effect of dengue virus on procoagulant and fibrinolytic activities of monocytes. Rev Infect Dis. 1989;11(4):S843–S846. doi: 10.1093/clinids/11.supplement_4.s843. [DOI] [PubMed] [Google Scholar]
- 5.Shekhar K.C., Huat O.L. Epidemiology of dengue/dengue hemorrhagic fever in Malaysia–a retrospective epidemiological study 1973–1987. Part I: Dengue hemorrhagic fever (DHF) Asia Pac J Public Health. 1992–93;6(2):15–25. doi: 10.1177/101053959300600203. [DOI] [PubMed] [Google Scholar]
- 6.Halstead S.B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 7.Kurane I., Ennis F.A. Immunopathogenesis of dengue virus infections. In: Gubler D.J., Kuno G., editors. Dengue and dengue haemorrhagic fever. CAB International; London: 1997. pp. 273–290. [Google Scholar]
- 8.Hayes E.B., Gubler D.J. Dengue and dengue hemorrhagic fever. Pediatr Infect Dis J. 1992;11(4):311–317. doi: 10.1097/00006454-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Cosgriff T.M. Mechanisms of disease in Hantavirus infection: pathophysiology of hemorrhagic fever with renal syndrome. Rev Infect Dis. 1991;13:97–107. doi: 10.1093/clinids/13.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Nathan C.F. Secretory products of macrophages. J Clin Invest. 1987;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs D., Hausen A., Reibnegger G., Werner E.R., Dierich M.P., Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol Today. 1988;9:150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs D., Weiss G., Reibnegger G., Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci. 1992;29:307–341. doi: 10.3109/10408369209114604. [DOI] [PubMed] [Google Scholar]
- 13.Hamerlinck F.F. Neopterin: a review. Exp Dermatol. 1999;8:167–176. doi: 10.1111/j.1600-0625.1999.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 14.Widner B., Murr C., Wirleitner B., Mayer C., Baier-Bitterlich G., Fuchs D. The importance of neopterin as a laboratory diagnostic marker of immune activation. Pteridines. 1999;10:101–111. [Google Scholar]
- 15.Jungraithmayr T.C., Reschke M., Grebe S.O., Lange H., Radsak K., Mueller T.F. Assessment of cytomegalovirus infections using neopterin and a new immunoblot. Clin Chim Acta. 2001;310:63–69. doi: 10.1016/s0009-8981(01)00528-9. [DOI] [PubMed] [Google Scholar]
- 16.Reibnegger G., Fuchs D., Fuith L.C., Hausen A., Werner E.R., Werner-Felmayer G. Neopterin as a marker for activated cell-mediated immunity: application in malignant disease. Cancer Detect Prev. 1991;15:483–490. [PubMed] [Google Scholar]
- 17.Murr C., Bergant A., Widschwendter M., Heim K., Schrocksnadel H., Fuchs D. Neopterin is an independent prognostic variable in females with breast cancer. Clin Chem. 1999;45:1998–2004. [PubMed] [Google Scholar]
- 18.Samsonov M.Y., Tilz G.P., Egorova O., Reibnegger G., Balabanova R.M., Nassonov E.L. Serum soluble markers of immune activation and disease activity in systemic lupus erythematosus. Lupus. 1995;4:29–32. doi: 10.1177/096120339500400107. [DOI] [PubMed] [Google Scholar]
- 19.Reibnegger G., Aichberger C., Fuchs D., Hausen A., Spielberger M., Werner E.R. Posttransplant neopterin excretion in renal allograft recipients–a reliable diagnostic aid for acute rejection and a predictive marker of long-term graft survival. Transplantation. 1991;52:58–63. doi: 10.1097/00007890-199107000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Fahey J.L., Taylor J.M., Detels R., Hofmann B., Melmed R., Nishanian P. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 21.Gaines H., von Sydow M.A., von Stedingk L.V., Biberfeld G., Bottiger B., Hansson L.O. Immunological changes in primary HIV-1 infection. AIDS. 1990;4:995–999. doi: 10.1097/00002030-199010000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Zaknun D., Weiss G., Glatzl J., Wachter H., Fuchs D. Neopterin levels during acute rubella in children. Clin Infect Dis. 1993;17:521–522. doi: 10.1093/clinids/17.3.521. [DOI] [PubMed] [Google Scholar]
- 23.Zangerle R., Schoenitzer D., Fuchs D., Most J., Dierich M.P., Wachter H. Reducing HIV transmission by seronegative blood. Lancet. 1992;399:130–131. doi: 10.1016/0140-6736(92)91046-b. [DOI] [PubMed] [Google Scholar]
- 24.Babb K., Carrington C.V., Monteil M.A. A preliminary study of neopterin as a potential marker for severe dengue virus infection. Trans R Soc Trop Med Hyg. 1999;93(4):447–448. doi: 10.1016/s0035-9203(99)90155-4. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization . 2nd ed. World Health Organization; Geneva: 1998. Dengue hemorrhagic fever: diagnosis, treatment, prevention, and control. [Google Scholar]
- 26.Cuzzubbo A.J., Vaughn D.W., Nisalak A., Solomon T., Kalayanarooj S., Aaskov J. Comparison of PanBio Dengue Duo IgM and IgG capture ELISA and venture technologies dengue IgM and IgG dot blot. J Clin Virol. 2000;16(2):135–144. doi: 10.1016/s1386-6532(99)00076-1. [DOI] [PubMed] [Google Scholar]
- 27.Lam S.K., Devine P.L. Evaluation of capture ELISA and rapid immunochromatographic test for the determination of IgM and IgG antibodies produced during dengue infection. Clin Diagn Virol. 1998;10(1):75–81. doi: 10.1016/s0928-0197(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 28.Hönlinger M., Fuchs D., Hausen A., Reibnegger G., Schönitzer D., Werner E.R. Serum-Neopterinbestimmung zur zusätzlichen Sicherung der Bluttransfusion. Erfahrungen an 76.587 Blutspendern. Dtsch Med Wschr. 1989;114:172–176. doi: 10.1055/s-2008-1066571. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y.C., Wang S.Y. Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J Virol. 2002;76:9877–9887. doi: 10.1128/JVI.76.19.9877-9887.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baize S., Leroy E.M., Georges A.J., Georges-Courbot M.C., Capron M., Bedjabaga I. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fendrich C., Luke W., Stahl-Hennig C., Herchenroder O., Fuchs D., Wachter H. Urinary neopterin concentrations in rhesus monkeys after infection with simian immunodeficiency virus (SIVmac 251) AIDS. 1989;3:305–307. doi: 10.1097/00002030-198905000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Kramer A., Biggar R.J., Hampl H., Friedman R.M., Fuchs D., Wachter H. Immunologic markers of progression to acquired immunodeficiency syndrome are time-dependent and illness-specific. Am J Epidemiol. 1992;136:71–80. doi: 10.1093/oxfordjournals.aje.a116422. [DOI] [PubMed] [Google Scholar]
- 33.Zheng B.J., Cao K.Y., Chan C.P.Y., Choi J.W.Y., Leung W., Leung M. Serum neopterin for early assessment of severity of severe acute respiratory syndrome. Clin Immunol. 2005;116(1):18. doi: 10.1016/j.clim.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs D., Stahl-Hennig C., Gruber A., Murr C., Hunsmann G., Wachter H. Neopterin—its clinical use in urinalysis. Kidney Int Suppl. 1994;47:S8–S11. [PubMed] [Google Scholar]
- 35.Mairuhu A.T., Peri G., Setiati T.E., Hack C.E., Koraka P., Soemantri A. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol. 2005;76(4):547–552. doi: 10.1002/jmv.20397. [DOI] [PubMed] [Google Scholar]
- 36.Koraka P., Murgue B., Deparis X., Van Gorp E.C., Setiati T.E., Osterhaus A.D. Elevation of soluble VCAM-1 plasma levels in children with acute dengue virus infection of varying severity. J Med Virol. 2004;72(3):445–450. doi: 10.1002/jmv.20007. [DOI] [PubMed] [Google Scholar]
- 37.Murgue B., Cassar O., Deparis X. Plasma concentrations of sVCAM-1 and severity of dengue infections. J Med Virol. 2001;65(1):97–104. [PubMed] [Google Scholar]
- 38.Bethell D.B., Flobbe K., Cao X.T., Day N.P., Pham T.P., Buurman W.A. Pathophysiologic and prognostic role of cytokines in dengue hemorrhagic fever. J Infect Dis. 1998;177(3):778–782. doi: 10.1086/517807. [DOI] [PubMed] [Google Scholar]