Highlights
-
•
VHHs provide many advantages over complete IgG in diagnostics and therapy.
-
•
Toxins and viruses are more efficiently neutralized by multivalent VHHs.
-
•
Camelids could be a source of broadly neutralizing antibodies (bNAbs) to treat zoonotic diseases.
Keywords: Nanobodies, VHH, Diagnostic, Therapeutic, Zoonosis
Abstract
Camelids produce both conventional heterotetrameric antibodies and homodimeric heavy-chain only antibodies. The antigen-binding region of such homodimeric heavy-chain only antibodies consists of one single domain, called VHH. VHHs provide many advantages over conventional full-sized antibodies and currently used antibody-based fragments (Fab, scFv), including high specificity, stability and solubility, and small size, allowing them to recognize unusual antigenic sites and deeply penetrate tissues. Since their discovery, VHHs have been used extensively in diagnostics and therapy. In recent decades, the number of outbreaks of diseases transmissible from animals to humans has been on the rise. In this review, we evaluate the status of VHHs as diagnostic and therapeutic biomolecular agents for the detection and treatment of zoonotic diseases, such as bacterial, parasitic, and viral zoonosis. VHHs show great adaptability to inhibit or neutralize pathogenic agents for the creation of multifunctional VHH-based diagnostic and therapeutic molecules against zoonotic diseases.
1. Introduction
The generation of monoclonal antibody (mAb) triggered a revolution in biotechnology. Therapeutic mAbs belong to the fastest-growing branch of biotechnology. However, the large size of the molecules may hinder efficient tissue penetration. This obstacle can be overcome using Fab or single-chain Fv (scFv) fragments, as they are three to six times smaller than full-sized antibodies. However, cloning these fragments, consisting of heavy and light chain variable regions linked by disulfide bridges or a linker, is challenging and they are not always efficiently expressed [1].
An alternative approach to avoid these pitfalls is the use of functional fragments of heavy chain antibodies, which are present in the serum of animals belonging to the Camelidae family. They interact with the antigen by virtue of one single variable domain referred to as VHHs, single-domain antibodies (sdAbs), or nanobodies™ (trademark of Ablynx). They combine the advantages of both immunoglobulins and small molecules and provide an alternative to conventional antibodies and binders derived from alternative scaffolds. Sharks also produce a unique heavy-chain only immunoglobulin that does not associate with light chains, referred to as IgNAR. V domains of IgNAR are often applied in biotechnological and biomedical fields (for a review [2]).
The Camelidae family (order: Artiodactyla) consists of six species: dromedary (Camelus dromedarius), Bactrian camel (Camelus bactrianus), llama (Lama glama), guanaco (Lama guanicoe), alpaca (Vicugna pacos), and vicuna (Vicugna vicugna). Animals belonging to this family are well adapted to live in harsh environments, such as deserts or high altitudes [3].
2. Structure and sequence of camel heavy chain antibodies
Ungar-Warom et al. [4] and Azwai et al [5] have isolated low molecular weight Ig-like proteins from dromedary serum. However, the detailed characterization and demonstration of the potential usefulness of these proteins stemmed from the work of the Hamers laboratory in the Free University of Brussels [6]. The authors demonstrated that these unusual proteins are “heavy-chain only antibodies’’ (HCAbs), devoid of light chain. These antibodies form homodimers and interact with antigens by virtue of only one single variable domain, referred to as VHH (VH domain of heavy-chain antibodies) to distinguish it from conventional VH [7]. In contrast to conventional antibodies, HCAbs do not possess the CH1 domain [6].
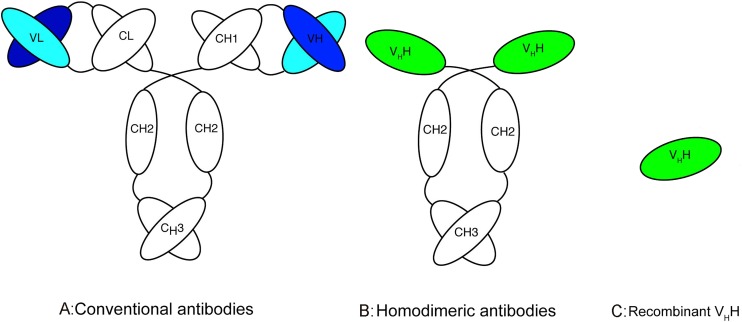
The active antigen-binding fragment of heavy chain antibodies can be cloned and expressed in the form of VHH, which consists of only one domain (Fig. 1 ).
Fig. 1.
Schematic diagram of different types of antibodies (adapted from [23]). A The common structure of an IgG antibody, which composed of two heavy and two light chains, B the structure of homodimeric camelid antibody only composed of heavy chains, C recombinant antibody-binding domain (VHH).
The percentage of HCAbs in the bloodstream of camelids varies greatly among species. It can reach a relatively high level in camels, ranging from 50% to 80%, whereas the maximum level is 45% in South American camelid species [8].
Despite the absence of light chains, the heavy-chain antibodies exhibit a broad antigen-binding repertoire. They exhibit specific characteristics, such as the substitution of three to four hydrophobic residues (which interact with the VL in conventional antibodies) by more hydrophilic amino acids. VH possess the conserved Val37, Gly44, Leu45 and Trp47 while in VHH, these amino acids are often substituted (Val37Phe or Val37Tyr, Gly44Glu, Leu45Arg and Trp47Gly) [[9], [10], [11]]. Furthermore, the complementarity determining regions (CDRs) of VHHs, and especially CDR3, are statistically longer than those of conventional VH-VL antibodies [12].
3. Unique properties of VHH fragments and their use in biotechnology
Over the last decades, VHHs have received progressively greater interest from the pharmaceutical and biotechnology industries, due to their specific properties. Indeed, VHHs provide the following advantages over conventional antibodies and their recombinant fragments:
-
-
VHHs are weakly immunogenic in humans, because the genes encoding them share high sequence homology with genes belonging to human VH families 3 and 4 [13,14].
-
-
VHHs consist of only one domain. They can thus be easily engineered, cloned, and expressed with high yields using various expression systems [15].
-
-
VHHs are highly soluble and stable, even under denaturing conditions or high temperatures [16].
-
-
The high variability of the length and sequence of VHHs allows them to recognize a variety of protein epitopes, located not only on the surface of a protein [17], but also buried deep in the clefts [18]. VHHs have been shown to recognize a wide range of epitope types, from small haptens [16,19] to the binding sites of enzymes [17,20], and can bind epitopes that cannot be recognized by conventional antibodies [21].
-
-
The small size and basic isolectric point of VHHs allows them to penetrate tissues, pass through barriers, such as the blood-brain barrier [[22], [23], [24], [25], [26]], and bind intracellular antigens.
-
-
VHHs can be efficiently functionalized [[27], [28], [29]] and are widely used for imaging [24,26,30].
There has been an explosion in the number of publications concerning applications of VHHs that cover their use in pharmaceutical development or as biotechnological tools [26,[31], [32], [33], [34], [35]]. Here, we mainly focus on the potential use of VHHs for the diagnosis and the treatment of zoonotic diseases.
4. Zoonotic diseases
Zoonotic diseases (ZDs) are infections that can be naturally spread between vertebrate animals and humans. Several recent outbreaks, such as Ebola and Zika have emphasized the serious impact of these diseases on human health [36]. Their expansion is related to global trade, human migration, and climate change. Up to now, the outbreaks have been contained by the control of human populations in the affected areas, the use of antibiotics, and the development of rapid diagnostic tests. However, the emergence of ZDs is still a major challenge and there is a crucial need for new tools for diagnosis and therapy. Camelid VHHs could offer an attractive possibility for the development of such tools in the future.
4.1. Bacterial zoonosis
4.1.1. Campylobacteriosis
Campylobacteriosis is caused mostly by Campylobacter jejuni or Campylobacter coli. Poultry are naturally infected, without clinical signs, and it is the leading cause of foodborne gastroenteritis in humans worldwide [37].
A VHH that binds C. jejuni flagella was isolated by Riazi et al. [38] and engineered for greater thermal and proteolytic stability. Hussack et al. [39] obtained a highly stable VHH through the use of error-prone polymerase chain reaction and disulfide-bond engineering. This VHH, directed against the flagella, can potently inhibit C. jejuni motility and is being studied for the prevention or significant reduction of C. jejuni colonization in the gastrointestinal tract of chickens. Recently Vanmarsenille et al [40] described the isolation and characterization of 6 VHHs against multiple Campylobacter strains. These VHHs which bind with the major outer membrane protein (MOMP) interacted with 23 C. jejuni isolates and 5 C. coli isolates. They could potentially be used in therapy and as a diagnostic tool.
4.1.2. Escherichia coli
Escherichia coli is a facultative gram-negative bacterium that belongs to the family Enterobacteriaceae. Although it is normally commensal in nature and animals, many strains are food and waterborne zoonotic pathogens. Shiga toxin (Stx)-producing E. coli (STEC) bacteria (which include enterohemorrhagic E. coli [EHEC]) cause no discernible disease in their animal reservoirs; however, diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome (HUS) are common in humans [41]. The major virulence determinants of STEC are mainly caused by the Shiga toxins Stx1 and Stx2 [42].
VHHs that neutralize Stx1 and/or Stx2 have recently been obtained [[43], [44], [45]]. The VHH 2vb27 specific of Stx2 was unable to give protection against a lethal dose of toxin in mice while a trivalent molecule (two copies of VHH 2vb27 and one copy of anti-albumin VHH) presented an extended half-life and was able to neutralize the in vivo effect of toxins in mouse models [43]. Tremblay et al [44] showed that VHH heterodimers, containing two linked neutralizing VHHs, generally neutralized Stx much more efficiently than a pool of individual monomers. Moreover co-administration of an effector Ab substantially improved the ability of Stx toxin-neutralizing VHHs to prevent death or kidney damage in mice following challenge with Stx1 or Stx2.
4.1.3. Listeriosis
Listeriosis is a bacterial infection caused by the gram-positive bacterium Listeria monocytogenes. The major source of infection is contaminated food. The disease primarily affects elderly people, immunocompromised patients, pregnant women, causing abortion, and newborns [46].
VHHs specific for the invasin, internalin B, of L. monocytogenes have been isolated [47,48] and can inhibit Listeria invasion in vitro. A crystal structure between one VHH and internalin B shows that the VHH competes with c-met, the target cell receptor of this invasin, explaining the protective effect [48]. Two VHHs that specifically bind to three L. monocytogenes serotypes (1/2a, 1/2b, and 4b) have been isolated from a non-immune library [49]. A sandwich ELISA was developed using a mAb for antibody capture and one VHH, L5-79 for detection, with a detection limit of 1 × 104 colony forming units (CFU)/ml of bacteria in milk.
4.1.4. Anthrax
Bacillus anthracis, the causative organism of anthrax, is a spore-forming Gram-positive bacillus commonly found in the soil of endemic areas. Herbivores can be infected while grazing. Recently, anthrax was detected in Siberia after degradation of the permafrost due to global warming [50]. B. anthracis is also one of the most important biological warfare agents, because of its pathogenic nature and spore-forming capacity [51]. The bioterrorist events in the United States in 2001 revealed that treatment with antibiotics is not always sufficient to prevent patient deaths, due to the effects of toxins produced by the bacteria. Neutralizing mAbs may therefore be of great therapeutic value, complementing antibiotic treatment to prevent the toxin-dependent symptoms of anthrax [52].
Anthrax is caused by a toxin consisting of protective antigen (PA), lethal factor (LF), and edema factor (EF). Several VHHs directed against the three components of the toxin have been shown to be efficient against Anthrax disease [53,54]. Gene therapy with an adenoviral vector expressing a bispecific VHH, consisting of two linked VHHs targeting different PA-neutralizing epitopes, was tested in mice, and found to protect them from anthrax toxin challenge and anthrax spore infection [55].
4.2. Parasitic zoonosis
4.2.1. Taeniasis
Taeniasis occurs in the human host, after ingestion of undercooked pork infected with cysticerci. Cysticercosis is caused by the larval stage of the pork tapeworm Taenia solium. Humans are the definitive host, harboring the adult tapeworm in the intestine, but both pigs and humans can be infected with the cysticerci [56].
The mAbs obtained thus far are not genus-specific, preventing definitive diagnosis of infection by T. solium [57]. Indeed, specific binders are needed. Deckers et al. [58] have isolated VHHs specific for a glycoprotein of T. solium that do not cross react with other Taenia species and a sandwich ELISA has been developed. This serodiagnostic test could be helpful in pigs for epidemiological studies and monitoring the efficacy of control programs.
4.2.2. Trypanosomiasis
African trypanosomiasis (AT), or sleeping sickness, is mainly caused by a unicellular flagellated protozoan parasite, Trypanosoma brucei gambiense, belonging to the genus Trypanosoma. AT affects mainly remote rural areas and its distribution coincides mostly with the habitat of the hematophagous insect vector, i.e., the tsetse fly (Glossina sp) [59].
Multiple VHHs have already been generated against the parasite (for a review, [60]). Trypanolytic VHH An46 disturbs the endocytic machinery of the parasite in the flagellar pocket of the parasite. Nontrypanolytic VHH An33 was made more potent by linking the nontoxic prodrug cephalosporin mustard (CCM) onto the highly toxic PDM at the surface of the parasite [61]. Linking this VHH to apolipoprotein l-I resulted in an immunotoxin that lyses almost all trypanosomes [18]. Another approach is to couple pentamidine, a first-line antitrypanosomiasis, to VHH An33 to effectively target the drug to the parasite. In vivo, a ten-fold lower dose than the minimal full curative dose of free pentamidin incorporated into this conjugate cured all infected mice, wheras a 100-fold lower dose cured 60% of them [62]. Parasite development in the tsetse fly and subsequent spread of the parasite can be controlled through the expression of trypanolytic VHHs in genetically modified tsetse fly symbionts [63]. In addition, VHHs that target the paraflagellar rod protein of varioius trypanosomes have been described, but are mainly useful as diagnostic markers of trypanosiomasis [64]. A VHH (Nb474) directed against T. congolese aldolase (TcoALD) has been developped for a sandwich immunoassay. This VHH is highly specific and did not recognize other trypanosomes such as T. brucei brucei, T. vivax and T. evansi [65,66].
4.3. Viral zoonosis
4.3.1. Influenza
Influenza A viruses (IAV), members of the RNA family Orthomyxoviridae, consist of up to 144 subtypes, depending on the variation/combination of the surface glycoproteins, hemagglutinin and neuraminidase. IAV are further classified as human, swine (SIV), bat, equine, or avian influenza viruses (AIV). SIV and AIV are transmitted from pigs or birds to humans, respectively, mostly via direct contact with infected animals. The infection in humans ranges from mild self-limiting respiratory-like illness to death. However, pandemic outbreaks remain unpredictable, as illustrated by the 2009 H1N1 virus (also named Mexican flu) and H5N1 virus. Occasional zoonotic infections with these viruses and their high propensity to reassort with SIV have earmarked them as a major pandemic threat [67].
Several VHHs specific for influenza viruses have been raised against the nucleoprotein [68] and M2 ion channel protein [69] of Influenza A, and the neuraminidase [70] and hemaglutinin [71] of H5N1. Most of these VHHs can neutralize influenza viruses. Ploegh et al. [57] exploited the ability of VHHs to bind the intracellular nucleoprotein protein to block viral replication, leading to the possibility of creating new therapeutic molecules to prevent viral escape due to antigenic variation. An alternative approach has been to create multivalent VHHs to increase their antiviral potential: dimers made by the fusion of two neutralizing VHHs [68,72] or a VHH–fused to an immunoglobulin Fc region [68]. In vitro antiviral potency was increased from 1 to 2 logs relative to the monomeric VHH.
4.3.2. Rabies
The rabies virus (RABV) belongs to the genus Lyssavirus of the RNA family Rhabdoviridae, within the order Mononegavirales. Despite the availability of a vaccine against rabies virus (RABV), rabies continues to claim 55,000 human lives per year, mostly in developing countries in Asia and Africa [73,74]. The vaccine is mostly used therapeutically as a post-exposure treatment. For infection combined with a seriously bleeding injury, the WHO recommends complementing vaccination with local instillation of human or equine rabies immunoglobulins (RIG) to neutralize the RABV load in situ. There is currently a critical shortage worldwide and the WHO is exploring alternative approaches, such as cocktails of human or humanized neutralizing mAbs [75].
Neutralizing anti-RABV VHHs directed against glycoproteins have been raised from a VHH phage library generated from the immunization of a llama with inactivated rabies vaccine (genotype 1, Sanofi Pasteur MSD) [71]. The IC50s of the CVS-11 (genotype 1) strain ranged from 7 to 325 nM. These VHHs were fused to an anti-albumin VHH to extend its serum half-life and were able to neutralize the virus at picomolar doses [76]. A combined treatment based on VHH and vaccine (Rabipur, Novartis) acted synergistically to protect mice in an intranasal rabies infection model [77]. However, the principal difficulty of an antiviral approach against rabies resides in the specific neurotropism of RABV, which makes it not readily accessible once it has accessed the CNS. At this stage, only molecules capable of crossing the blood brain barrier and penetrating into neurons would be able to inhibit the infection.
4.3.3. Foot-and-mouth disease
Foot-and-mouth disease (FMD) is a contagious viral disease that affects cattle, swine, sheep, and approximately 70 wildlife species (including llama and camel), with a potential for rapid spread between susceptible animals. The disease has been identified worldwide whereever livestock are raised. In the last 20 years, there have been massive outbreaks of FMD in countries formerly free of the disease, such as the United Kingdom in 2001 [78] and Taiwan in 1997 [79]. Seven antigenically distinct serotypes of FMD viruses have been identified: O, A, C, Asia 1, SAT1, SAT2, and SAT3 [80]. Emergency vaccination can be used as an effective control measure for FMD outbreaks in FMD-free regions, such as the European Union, but the development of novel antiviral therapies that confer rapid protection against FMD is still needed. Moreover, it is important to develop a rapid diagnostic test for identification of the various serotypes of the viruses involved in FMD outbreaks.
VHHs directed against serotype O have been raised. They have provided only limited protection to pigs. Trimers consisting of two VHHs specific for FMDV and one VHH specific for porcine Ig have been constructed to increase their potency and half-life. These trimers provided better protection to pigs and delayed FMD transmission [81]. Specific VHHs raised against FMD Asia 1 virus have also been obtained. They have been used to develop diagnostic assays by conjugating them to either quantum dots [82] or carboxyl-magnetic beads [83].
5. Conclusion
In this review, we provide evidence for the possible use of VHHs as valuable biomolecules for the diagnosis and treatment of ZDs, including bacterial, parasitic, and viral zoonosis. VHHs provide many advantages over conventional antibodies and currently used antibody based fragments. The ease of high-level production, small size, and high stability make VHHs extremely reliable for genetic and chemical modification, such as the production of VHH-based fusion proteins to increase the persistence of VHHs in serum or confer additional functions. Construction of multivalent VHHs consisting of two or more linked VHHs targeting various epitopes leads to an increase in their ability to neutralize toxins [43,44] and viruses [68,72,76,81], suggesting the development of these approaches for VHHs targeting other viruses not yet tested.
Presently available anti-viral therapeutic mAbs, at various stages of pre-clinical evaluation, mostly target surface antigens that are often diverse or variable in sequence (e.g. HIV gp160, influenza HA): their efficiency thus requires challenging protein engineering efforts to obtain antibodies that are either broadly neutralizing or robust against viral escape through antigenic variation (drift). An alternative is the use of “broadly neutralizing antibodies (bNAb)”, which are antibodies found in infected mammals able to neutralize most strains of a given highly antigenically variable pathogen. bNAbs have been isolated from infected humans against HIV [84], influenza [85], and dengue viruses [86]. Camelids could also be a source of bNAbs. Indeed antibodies against MERS-Coronaviruses (MERS-CoV) [[87], [88], [89]], Crimean Congo hemorrhagic fever virus (CCHFV) [90,91], Rift Valley fever (RFV) [90], Toxoplasma gondii, and Rickettsia sp. [92] have been found in the sera of infected camels, whereas antibodies against rabies virus, vesicular stomatitis virus, and FMD virus have been detected in llamas [93] and could lead to the possible isolation of specific broadly neutralizing VHHs.
Many neutralizing VHHs that bind to different sites on the same target, including hidden antigenic sites, can be isolated from immunized or infected camelids. These VHHs can be engineered in various ways to improve their diagnostic and/or therapeutic properties and efficacy. In addition, VHHs readily and rapidly penetrate tissues, even the brain, and it is likely that specific VHH-based constructs will be developed that can neutralize agents involved in brain infections, such as influenza [68], Zika, or rabies virus. Overall, VHHs or VHH-based molecules are potentially valuable diagnostic and therapeutic reagents to treat ZDs.
References
- 1.Ehsani P., Meunier A., Nato F., Jafari A., Nato A., Lafaye P. Expression of anti human IL-4 and IL-6 scFvs in transgenic tobacco plants. Plant Mol. Biol. 2003;52 doi: 10.1023/A:1023902407855. [DOI] [PubMed] [Google Scholar]
- 2.Dooley H., Flajnik M.F. Antibody repertoire development in cartilaginous fish. Dev. Comp. Immunol. 2006;30:43–56. doi: 10.1016/j.dci.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Fowler M.E. 2nd ed. 1998. Medicine and Surgery of South American Camelids. [Google Scholar]
- 4.Ungar-Warom H., Elias E., Gluckman A., Trainin Z. Dromedary IgG: purification, characterization and quantitation in sera of dams and new- borns. Isr. J. Vet. Med. 1987;43 [Google Scholar]
- 5.Azwai S.M., Carter S.D., Woldehiwet Z. The isolation and characterization of camel (Camelus dromedarius) immunoglobulin classes and subclasses. J. Comp. Pathol. 1993;109:187–195. doi: 10.1016/s0021-9975(08)80262-9. http://www.ncbi.nlm.nih.gov/pubmed/8245233 (Accessed May 17, 2018) [DOI] [PubMed] [Google Scholar]
- 6.Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hammers C., Songa E.B., Bendahman N., Hammers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 7.Arbabi Ghahroudi M., Desmyter A., Wyns L., Hamers R., Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997;414:521–526. doi: 10.1016/S0014-5793(97)01062-4. [DOI] [PubMed] [Google Scholar]
- 8.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen V.K., Desmyter A., Muyldermans S. Functional heavy-chain antibodies in Camelidae. Adv. Immunol. 2001;79:261–296. doi: 10.1016/s0065-2776(01)79006-2. http://www.ncbi.nlm.nih.gov/pubmed/11680009 (accessed November 2, 2017) [DOI] [PubMed] [Google Scholar]
- 10.Li T., Vandesquille M., Bay S., Dhenain M., Delatour B., Lafaye P. Selection of similar single domain antibodies from two immune VHH libraries obtained from two alpacas by using different selection methods. Immunol. Lett. 2017;188 doi: 10.1016/j.imlet.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Wesolowski J., Alzogaray V., Reyelt J., Unger M., Juarez K., Urrutia M., Cauerhff A., Danquah W., Rissiek B., Scheuplein F., Schwarz N., Adriouch S., Boyer O., Seman M., Licea A., Serreze D.V., Goldbaum F.A., Haag F., Koch-Nolte F. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 2009;198 doi: 10.1007/s00430-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muyldermans S. Single domain camel antibodies: current status. J. Biotechnol. 2001;74:277–302. doi: 10.1016/s1389-0352(01)00021-6. http://www.ncbi.nlm.nih.gov/pubmed/11526908 (Accessed 2 November 2017) [DOI] [PubMed] [Google Scholar]
- 13.Cortez-Retamozo V., Lauwereys M., Hassanzadeh Gh G., Gobert M., Conrath K., Muyldermans S., De Baetselier P., Revets H. Efficient tumor targeting by single-domain antibody fragments of camels. Int. J. Cancer. 2002;98:456–462. doi: 10.1002/ijc.10212. http://www.ncbi.nlm.nih.gov/pubmed/11920600 (Accessed 17 May 2018) [DOI] [PubMed] [Google Scholar]
- 14.Deschacht N., De Groeve K., Vincke C., Raes G., De Baetselier P., Muyldermans S. A novel promiscuous class of camelid single-domain antibody contributes to the antigen-binding repertoire. J. Immunol. 2010;184:5696–5704. doi: 10.4049/jimmunol.0903722. [DOI] [PubMed] [Google Scholar]
- 15.Harmsen M.M., De Haard H.J. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 2007;77:13–22. doi: 10.1007/s00253-007-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladenson R.C., Crimmins D.L., Landt Y., Ladenson J.H. Isolation and characterization of a thermally stable recombinant anti-caffeine heavy-chain antibody fragment. Anal. Chem. 2006;78:4501–4508. doi: 10.1021/ac058044j. [DOI] [PubMed] [Google Scholar]
- 17.Desmyter A., Decanniere K., Muyldermans S., Wyns L. Antigen specificity and high affinity binding provided by one single loop of a camel single-domain antibody. J. Biol. Chem. 2001;276:26285–26290. doi: 10.1074/jbc.M102107200. [DOI] [PubMed] [Google Scholar]
- 18.Baral T.N., Magez S., Stijlemans B., Conrath K., Vanhollebeke B., Pays E., Muyldermans S., De Baetselier P. Experimental therapy of African trypanosomiasis with a nanobody-conjugated human trypanolytic factor. Nat. Med. 2006;12:580–584. doi: 10.1038/nm1395. [DOI] [PubMed] [Google Scholar]
- 19.Spinelli S., Frenken L.G., Hermans P., Verrips T., Brown K., Tegoni M., Cambillau C. Camelid heavy-chain variable domains provide efficient combining sites to haptens. Biochemistry. 2000;39:1217–1222. doi: 10.1021/bi991830w. http://www.ncbi.nlm.nih.gov/pubmed/10684599 (Accessed 2 November 2017) [DOI] [PubMed] [Google Scholar]
- 20.Desmyter A., Transue T.R., Ghahroudi M.A., Thi M.H., Poortmans F., Hamers R., Muyldermans S., Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat. Struct. Biol. 1996;3:803–811. doi: 10.1038/nsb0996-803. http://www.ncbi.nlm.nih.gov/pubmed/8784355 (Accessed 2 November 2017) [DOI] [PubMed] [Google Scholar]
- 21.Lafaye P., Achour I., England P., Duyckaerts C., Rougeon F. Single-domain antibodies recognize selectively small oligomeric forms of amyloid β, prevent Aβ-induced neurotoxicity and inhibit fibril formation. Mol. Immunol. 2009;46:695–704. doi: 10.1016/j.molimm.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Abulrob A., Zhang J., Tanha J., MacKenzie R., Stanimirovic D. Single domain antibodies as blood-brain barrier delivery vectors. Int. Congr. Ser. 2005;1277:212–223. doi: 10.1016/j.ics.2005.02.024. [DOI] [Google Scholar]
- 23.Perruchini C., Pecorari F., Bourgeois J.-P., Duyckaerts C., Rougeon F., Lafaye P. Llama VHH antibody fragments against GFAP: better diffusion in fixed tissues than classical monoclonal antibodies. Acta Neuropathol. 2009;118:685–695. doi: 10.1007/s00401-009-0572-6. http://www.ncbi.nlm.nih.gov/pubmed/19597828 [DOI] [PubMed] [Google Scholar]
- 24.Li T., Bourgeois J.-P., Celli S., Glacial F., Le Sourd A.-M., Mecheri S., Weksler B., Romero I., Couraud P.-O., Rougeon F., Lafaye P. Cell-penetrating anti-GFAP VHH and corresponding fluorescent fusion protein VHH-GFP spontaneously cross the blood-brain barrier and specifically recognize astrocytes: application to brain imaging. FASEB J. 2012;26:3969–3979. doi: 10.1096/fj.11-201384. [DOI] [PubMed] [Google Scholar]
- 25.Li T., Vandesquille M., Koukouli F., Dudeffant C., Youssef I., Lenormand P., Ganneau C., Maskos U., Czech C., Grueninger F., Duyckaerts C., Dhenain M., Bay S., Delatour B., Lafaye P. Camelid single-domain antibodies: a versatile tool for in vivo imaging of extracellular and intracellular brain targets. J. Control. Release. 2016;243:1–10. doi: 10.1016/j.jconrel.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Vandesquille M., Li T., Po C., Ganneau C., Lenormand P., Dudeffant C., Czech C., Grueninger F., Duyckaerts C., Delatour B., Dhenain M., Lafaye P., Bay S. Chemically-defined camelid antibody bioconjugate for the magnetic resonance imaging of Alzheimer’s disease. MAbs. 2017 doi: 10.1080/19420862.2017.1342914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popp M.W., Antos J.M., Grotenbreg G.M., Spooner E., Ploegh H.L. Sortagging: a versatile method for protein labeling. Nat. Chem. Biol. 2007;3:707–708. doi: 10.1038/nchembio.2007.31. [DOI] [PubMed] [Google Scholar]
- 28.Massa S., Vikani N., Betti C., Ballet S., Vanderhaegen S., Steyaert J., Descamps B., Vanhove C., Bunschoten A., van Leeuwen F.W.B., Hernot S., Caveliers V., Lahoutte T., Muyldermans S., Xavier C., Devoogdt N. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: a versatile strategy for multiple molecular imaging modalities. Contrast Media Mol. Imaging. 2016;11:328–339. doi: 10.1002/cmmi.1696. [DOI] [PubMed] [Google Scholar]
- 29.Massa S., Xavier C., De Vos J., Caveliers V., Lahoutte T., Muyldermans S., Devoogdt N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug. Chem. 2014;25:979–988. doi: 10.1021/bc500111t. [DOI] [PubMed] [Google Scholar]
- 30.Traenkle B., Rothbauer U. Under the Microscope: Single-Domain Antibodies for Live-Cell Imaging and Super-Resolution Microscopy. Front. Immunol. 2017;8:1030. doi: 10.3389/fimmu.2017.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nosenko M.A., Atretkhany K.-S.N., Mokhonov V.V., Efimov G.A., Kruglov A.A., Tillib S.V., Drutskaya M.S., Nedospasov S.A. VHH-based bispecific antibodies targeting cytokine production. Front. Immunol. 2017;8:1073. doi: 10.3389/fimmu.2017.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch K., Kalusche S., Torres J.L., Stanfield R.L., Danquah W., Khazanehdari K., von Briesen H., Geertsma E.R., Wilson I.A., Wernery U., Koch-Nolte F., Ward A.B., Dietrich U. Selection of nanobodies with broad neutralizing potential against primary HIV-1 strains using soluble subtype C gp140 envelope trimers. Sci. Rep. 2017;7:8390. doi: 10.1038/s41598-017-08273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Sapienza G., Rossotti M.A., Tabares-da Rosa S. Single-domain antibodies as versatile affinity reagents for analytical and diagnostic applications. Front. Immunol. 2017;8:977. doi: 10.3389/fimmu.2017.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassanzadeh-Ghassabeh G., Devoogdt N., De Pauw P., Vincke C., Muyldermans S. Nanobodies and their potential applications. Nanomedicine. 2013;8:1013–1026. doi: 10.2217/nnm.13.86. [DOI] [PubMed] [Google Scholar]
- 35.Tarr A.W., Lafaye P., Meredith L., Damier-Piolle L., Urbanowicz R.A., Meola A., Jestin J.-L., Brown R.J.P., Mckeating J.A., Rey F.A., Ball J.K., Krey T. An alpaca nanobody inhibits hepatitis C virus entry and cell-to-cell transmission. Hepatology. 2013;58 doi: 10.1002/hep.26430. [DOI] [PubMed] [Google Scholar]
- 36.Quaglio G., Goerens C., Putoto G., Rübig P., Lafaye P., Karapiperis T., Dario C., Delaunois P., Zachariah R. Ebola: Lessons learned and future challenges for Europe. Lancet Infect. Dis. 2016;16:259–263. doi: 10.1016/S1473-3099(15)00361-8. [DOI] [PubMed] [Google Scholar]
- 37.Helmy Y.A., El-Adawy H., Abdelwhab E.M. A Comprehensive Review of Common Bacterial, Parasitic and Viral Zoonoses at the Human-Animal Interface in Egypt. Pathog. (Basel, Switzerland) 2017;6:33. doi: 10.3390/pathogens6030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riazi A., Strong P.C.R., Coleman R., Chen W., Hirama T., van Faassen H., Henry M., Logan S.M., Szymanski C.M., Mackenzie R., Ghahroudi M.A. Pentavalent single-domain antibodies reduce Campylobacter jejuni motility and colonization in chickens. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussack G., Riazi A., Ryan S., van Faassen H., MacKenzie R., Tanha J., Arbabi-Ghahroudi M. Protease-resistant single-domain antibodies inhibit Campylobacter jejuni motility. Protein Eng. Des. Sel. 2014;27:191–198. doi: 10.1093/protein/gzu011. [DOI] [PubMed] [Google Scholar]
- 40.Vanmarsenille C., Díaz Del Olmo I., Elseviers J., Hassanzadeh Ghassabeh G., Moonens K., Vertommen D., Martel A., Haesebrouck F., Pasmans F., Hernalsteens J.-P., De Greve H. Nanobodies targeting conserved epitopes on the major outer membrane protein of Campylobacter as potential tools for control of Campylobacter colonization. Vet. Res. 2017;48(86) doi: 10.1186/s13567-017-0491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García A., Fox J.G., Besser T.E. Zoonotic enterohemorrhagic Escherichia coli: A One Health perspective. ILAR J. 2010;51:221–232. doi: 10.1093/ilar.51.3.221. http://www.ncbi.nlm.nih.gov/pubmed/21131723 (accessed November 2, 2017) [DOI] [PubMed] [Google Scholar]
- 42.Karmali M.A., Petric M., Lim C., Fleming P.C., Arbus G.S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. http://www.ncbi.nlm.nih.gov/pubmed/3886804 (accessed November 2, 2017) [DOI] [PubMed] [Google Scholar]
- 43.Mejías M.P., Hiriart Y., Lauché C., Fernández-Brando R.J., Pardo R., Bruballa A., Ramos M.V., Goldbaum F.A., Palermo M.S., Zylberman V. Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS) Sci. Rep. 2016;6:24913. doi: 10.1038/srep24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tremblay J.M., Mukherjee J., Leysath C.E., Debatis M., Ofori K., Baldwin K., Boucher C., Peters R., Beamer G., Sheoran A., Bedenice D., Tzipori S., Shoemaker C.B. A single VHH-based toxin-neutralizing agent and an effector antibody protect mice against challenge with Shiga toxins 1 and 2. Infect. Immun. 2013;81:4592–4603. doi: 10.1128/IAI.01033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo A.W.H., Moonens K., De Kerpel M., Brys L., Pardon E., Remaut H., De Greve H. The molecular mechanism of Shiga toxin Stx2e neutralization by a single-domain antibody targeting the cell receptor-binding domain. J. Biol. Chem. 2014;289:25374–25381. doi: 10.1074/jbc.M114.566257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramaswamy V., Cresence V.M., Rejitha J.S., Lekshmi M.U., Dharsana K.S., Prasad S.P., Vijila H.M. Listeria--review of epidemiology and pathogenesis. J. Microbiol. Immunol. Infect. 2007;40:4–13. http://www.ncbi.nlm.nih.gov/pubmed/17332901 (accessed November 2, 2017) [PubMed] [Google Scholar]
- 47.King M.T., Huh I., Shenai I.A., Brooks T.M., BrooksHuh C.L. Structural basis of VHH-mediated neutralization of the food-borne pathogen Listeria monocytogenes. J. Biol. Chem. 2018;293:13626–13635. doi: 10.1074/jbc.RA118.003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gene R.W., Kumaran J., Aroche C., van Faassen H., Hall J.C., MacKenzie C.R., Arbabi-Ghahroudi M. High affinity anti-Internalin B VHH antibody fragments isolated from naturally and artificially immunized repertoires. J. Immunol. Methods. 2015;416:29–39. doi: 10.1016/j.jim.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Tu Z., Chen Q., Li Y., Xiong Y., Xu Y., Hu N., Tao Y. Identification and characterization of species-specific nanobodies for the detection of Listeria monocytogenes in milk. Anal. Biochem. 2016;493:1–7. doi: 10.1016/j.ab.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 50.Revich B.A., Podolnaya M.A. Thawing of permafrost may disturb historic cattle burial grounds in East Siberia. Glob. Health Action. 2011;4:8482. doi: 10.3402/gha.v4i0.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atlas R.M. Responding to the threat of bioterrorism: a microbial ecology perspective--the case of anthrax. Int. Microbiol. 2002;5:161–167. doi: 10.1007/s10123-002-0084-x. [DOI] [PubMed] [Google Scholar]
- 52.Brossier F., Lévy M., Landier A., Lafaye P., Mock M. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 2004;72:6313–6317. doi: 10.1128/IAI.72.11.6313-6317.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moayeri M., Leysath C.E., Tremblay J.M., Vrentas C., Crown D., Leppla S.H., Shoemaker C.B. A heterodimer of a VHH (variable domains of camelid heavy chain-only) antibody that inhibits anthrax toxin cell binding linked to a VHH antibody that blocks oligomer formation is highly protective in an anthrax spore challenge model. J. Biol. Chem. 2015;290:6584–6595. doi: 10.1074/jbc.M114.627943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vrentas C.E., Moayeri M., Keefer A.B., Greaney A.J., Tremblay J., O’Mard D., Leppla S.H., Shoemaker C.B. A diverse set of single-domain antibodies (VHHs) against the Anthrax toxin lethal and edema factors provides a basis for construction of a bispecific agent that protects against Anthrax infection. J. Biol. Chem. 2016;291:21596–21606. doi: 10.1074/jbc.M116.749184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moayeri M., Tremblay J.M., Debatis M., Dmitriev I.P., Kashentseva E.A., Yeh A.J., Cheung G.Y.C., Curiel D.T., Leppla S., Shoemaker C.B. Adenoviral expression of a bispecific VHH-Based neutralizing agent that targets protective antigen provides prophylactic protection from Anthrax in mice. Clin. Vaccine Immunol. 2016;23:213–218. doi: 10.1128/CVI.00611-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White A.C. Neurocysticercosis: a major cause of neurological disease worldwide. Clin. Infect. Dis. 1997;24:101–113. doi: 10.1093/clinids/24.2.101. http://www.ncbi.nlm.nih.gov/pubmed/9114131 quiz 114–5 (Accessed 3 November 2017) [DOI] [PubMed] [Google Scholar]
- 57.Dorny P., Brandt J., Zoli A., Geerts S. Immunodiagnostic tools for human and porcine cysticercosis. Acta Trop. 2003;87:79–86. doi: 10.1016/s0001-706x(03)00058-5. http://www.ncbi.nlm.nih.gov/pubmed/12781381 (Accessed 3 November 2017) [DOI] [PubMed] [Google Scholar]
- 58.Deckers N., Saerens D., Kanobana K., Conrath K., Victor B., Wernery U., Vercruysse J., Muyldermans S., Dorny P. Nanobodies, a promising tool for species-specific diagnosis of Taenia solium cysticercosis. Int. J. Parasitol. 2009;39:625–633. doi: 10.1016/j.ijpara.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Welburn S.C., Molyneux D.H., Maudlin I. Beyond Tsetse--implications for research and control of human African Trypanosomiasis epidemics. Trends Parasitol. 2016;32:230–241. doi: 10.1016/j.pt.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Stijlemans B., De Baetselier P., Caljon G., Van Den Abbeele J., Van Ginderachter J.A., Magez S. Nanobodies as tools to understand, diagnose, and treat African Trypanosomiasis. Front. Immunol. 2017;8:724. doi: 10.3389/fimmu.2017.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stijlemans B., Conrath K., Cortez-Retamozo V., Van Xong H., Wyns L., Senter P., Revets H., De Baetselier P., Muyldermans S., Magez S. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies. African trypanosomes as paradigm. J. Biol. Chem. 2004;279:1256–1261. doi: 10.1074/jbc.M307341200. [DOI] [PubMed] [Google Scholar]
- 62.Arias J.L., Unciti-Broceta J.D., Maceira J., Del Castillo T., Hernández-Quero J., Magez S., Soriano M., García-Salcedo J.A. Nanobody conjugated PLGA nanoparticles for active targeting of African Trypanosomiasis. J. Control. Release. 2015;197:190–198. doi: 10.1016/j.jconrel.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 63.De Vooght L., Caljon G., De Ridder K., Van Den Abbeele J. Delivery of a functional anti-trypanosome Nanobody in different tsetse fly tissues via a bacterial symbiont, Sodalis glossinidius. Microb. Cell Fact. 2014;13:156. doi: 10.1186/s12934-014-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Obishakin E., Stijlemans B., Santi-Rocca J., Vandenberghe I., Devreese B., Muldermans S., Bastin P., Magez S. Generation of a Nanobody Targeting the Paraflagellar Rod Protein of Trypanosomes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Odongo S., Sterckx Y.G.J., Stijlemans B., Pillay D., Baltz T., Muyldermans S., Magez S. An Anti-proteome Nanobody Library Approach Yields a Specific Immunoassay for Trypanosoma congolense Diagnosis Targeting Glycosomal Aldolase. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004420. e0004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinto J., Odongo S., Lee F., Gaspariunaite V., Muyldermans S., Magez S., Sterckx Y.G.-J. Structural basis for the high specificity of a Trypanosoma congolense immunoassay targeting glycosomal aldolase. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005932. e0005932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roose K., Fiers W., Saelens X. Pandemic preparedness: toward a universal influenza vaccine. Drug News Perspect. 2009;22:80–92. doi: 10.1358/dnp.2009.22.2.1334451. [DOI] [PubMed] [Google Scholar]
- 68.Ashour J., Schmidt F.I., Hanke L., Cragnolini J., Cavallari M., Altenburg A., Brewer R., Ingram J., Shoemaker C., Ploegh H.L. Intracellular expression of camelid single-domain antibodies specific for influenza virus nucleoprotein uncovers distinct features of its nuclear localization. J. Virol. 2015;89:2792–2800. doi: 10.1128/JVI.02693-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei G., Meng W., Guo H., Pan W., Liu J., Peng T., Chen L., Chen C.-Y. Potent neutralization of influenza A virus by a single-domain antibody blocking M2 ion channel protein. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cardoso F.M., Ibañez L.I., Van den Hoecke S., De Baets S., Smet A., Roose K., Schepens B., Descamps F.J., Fiers W., Muyldermans S., Depicker A., Saelens X. Single-domain antibodies targeting neuraminidase protect against an H5N1 influenza virus challenge. J. Virol. 2014;88:8278–8296. doi: 10.1128/JVI.03178-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hultberg A., Temperton N.J., Rosseels V., Koenders M., Gonzalez-Pajuelo M., Schepens B., Ibañez L.I., Vanlandschoot P., Schillemans J., Saunders M., Weiss R.A., Saelens X., Melero J.A., Verrips C.T., van Gucht S., de Haard H.J. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS One. 2011;6:1–12. doi: 10.1371/journal.pone.0017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ibañez L.I., De Filette M., Hultberg A., Verrips T., Temperton N., Weiss R.A., Vandevelde W., Schepens B., Vanlandschoot P., Saelens X. Nanobodies With In Vitro Neutralizing Activity Protect Mice Against H5N1 Influenza Virus Infection. J. Infect. Dis. 2011;203:1063–1072. doi: 10.1093/infdis/jiq168. [DOI] [PubMed] [Google Scholar]
- 73.Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M., Barrat J., Blanton J.D., Briggs D.J., Cleaveland S., Costa P., Freuling C.M., Hiby E., Knopf L., Leanes F., Meslin F.-X., Metlin A., Miranda M.E., Müller T., Nel L.H., Recuenco S., Rupprecht C.E., Schumacher C., Taylor L., Vigilato M.A.N., Zinsstag J., Dushoff J. Global alliance for rabies control partners for rabies prevention, estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003709. e0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schnell M.J., McGettigan J.P., Wirblich C., Papaneri A. The cell biology of rabies virus: using stealth to reach the brain. Nat. Rev. Microbiol. 2010;8:51–61. doi: 10.1038/nrmicro2260. [DOI] [PubMed] [Google Scholar]
- 75.Müller T., Dietzschold B., Ertl H., Fooks A.R., Freuling C., Fehlner-Gardiner C., Kliemt J., Meslin F.X., Franka R., Rupprecht C.E., Tordo N., Wanderler A.I., Kieny M.P. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000542. e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terryn S., Francart A., Lamoral S., Hultberg A., Rommelaere H., Wittelsberger A., Callewaert F., Stohr T., Meerschaert K., Ottevaere I., Stortelers C., Vanlandschoot P., Kalai M., Van Gucht S. Protective effect of different anti-rabies virus VHH constructs against rabies disease in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terryn S., Francart A., Rommelaere H., Stortelers C., Van Gucht S. Post-exposure Treatment with anti-rabies VHH and vaccine significantly improves protection of mice from lethal rabies infection. PLoS Negl. Trop. Dis. 2016;10:1–15. doi: 10.1371/journal.pntd.0004902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knowles N.J., Samuel A.R., Davies P.R., Kitching R.P., Donaldson A.I. Outbreak of foot-and-mouth disease virus serotype O in the UK caused by a pandemic strain. Vet. Rec. 2001;148:258–259. http://www.ncbi.nlm.nih.gov/pubmed/11292084 (accessed November 3, 2017) [PubMed] [Google Scholar]
- 79.P.C. Yang, R.M. Chu, W.B. Chung, H.T. Sung, Epidemiological characteristics and financial costs of the 1997 foot-and-mouth disease epidemic in Taiwan., Vet. Rec. 145 (n.d.) 731–734. http://www.ncbi.nlm.nih.gov/pubmed/10972111 (accessed November 3, 2017). [DOI] [PubMed]
- 80.Knowles N.J., Samuel A.R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003;91:65–80. doi: 10.1016/s0168-1702(02)00260-5. http://www.ncbi.nlm.nih.gov/pubmed/12527438 (Accessed November 3, 2017) [DOI] [PubMed] [Google Scholar]
- 81.Harmsen M.M., Fijten H.P.D., Engel B., Dekker A., Eblé P.L. Passive immunization with llama single-domain antibody fragments reduces foot-and-mouth disease transmission between pigs. Vaccine. 2009;27:1904–1911. doi: 10.1016/j.vaccine.2009.01.110. [DOI] [PubMed] [Google Scholar]
- 82.Wang D., Yang S., Yin S., Shang Y., Du P., Guo J., He J., Cai J., Liu X. Characterization of single-domain antibodies against Foot and Mouth Disease Virus (FMDV) serotype O from a camelid and imaging of FMDV in baby hamster kidney-21 cells with single-domain antibody-quantum dots probes. BMC Vet. Res. 2015;11:120. doi: 10.1186/s12917-015-0437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang S., Yin S., Shang Y., Wang D., Ma W., He J., Guo J., Cai J., Liu X. Specific detection of foot-and-mouth disease serotype Asia 1 virus by carboxyl-magnetic beads conjugated with single-domain antibody. BMC Biotechnol. 2015;15:83. doi: 10.1186/s12896-015-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scheid J.F., Mouquet H., Feldhahn N., Seaman M.S., Velinzon K., Pietzsch J., Ott R.G., Anthony R.M., Zebroski H., Hurley A., Phogat A., Chakrabarti B., Li Y., Connors M., Pereyra F., Walker B.D., Wardemann H., Ho D., Wyatt R.T., Mascola J.R., Ravetch J.V., Nussenzweig M.C. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 85.Corti D., Cameroni E., Guarino B., Kallewaard N.L., Zhu Q., Lanzavecchia A. Tackling influenza with broadly neutralizing antibodies. Curr. Opin. Virol. 2017;24:60–69. doi: 10.1016/j.coviro.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rouvinski A., Guardado-Calvo P., Barba-Spaeth G., Duquerroy S., Vaney M.-C., Kikuti C.M., Navarro Sanchez M.E., Dejnirattisai W., Wongwiwat W., Haouz A., Girard-Blanc C., Petres S., Shepard W.E., Desprès P., Arenzana-Seisdedos F., Dussart P., Mongkolsapaya J., Screaton G.R., Rey F.A. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature. 2015;520:109–113. doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- 87.Meyer B., Müller M.A., Corman V.M., Reusken C.B.E.M., Ritz D., Godeke G.-J., Lattwein E., Kallies S., Siemens A., van Beek J., Drexler J.F., Muth D., Bosch B.-J., Wernery U., Koopmans M.P.G., Wernery R., Drosten C. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg. Infect. Dis. 2014;20:552–559. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perera R.A., Wang P., Gomaa M.R., El-Shesheny R., Kandeil A., Bagato O., Siu L.Y., Shehata M.M., Kayed A.S., Moatasim Y., Li M., Poon L.L., Guan Y., Webby R.J., Ali M.A., Peiris J.S., Kayali G. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.36.20574. http://www.ncbi.nlm.nih.gov/pubmed/24079378 pii=20574. (accessed November 3, 2017) [DOI] [PubMed] [Google Scholar]
- 89.Reusken C.B.E.M., Haagmans B.L., Müller M.A., Gutierrez C., Godeke G.-J., Meyer B., Muth D., Raj V.S., Smits-De Vries L., Corman V.M., Drexler J.-F., Smits S.L., El Tahir Y.E., De Sousa R., van Beek J., Nowotny N., van Maanen K., Hidalgo-Hermoso E., Bosch B.-J., Rottier P., Osterhaus A., Gortázar-Schmidt C., Drosten C., Koopmans M.P.G. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013;13:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mariner J.C., Morrill J., Ksiazek T.G. Antibodies to hemorrhagic fever viruses in domestic livestock in Niger: Rift Valley fever and Crimean-Congo hemorrhagic fever. Am. J. Trop. Med. Hyg. 1995;53:217–221. doi: 10.4269/ajtmh.1995.53.217. http://www.ncbi.nlm.nih.gov/pubmed/7573699 (Accessed November 6, 2017) [DOI] [PubMed] [Google Scholar]
- 91.Suliman H.M., Adam I.A., Saeed S.I., Abdelaziz S.A., Haroun E.M., Aradaib I.E. Crimean Congo hemorrhagic fever among the one-humped camel (Camelus dromedaries) in Central Sudan. Virol. J. 2017;14:147. doi: 10.1186/s12985-017-0816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mentaberre G., Gutiérrez C., Rodríguez N.F., Joseph S., González-Barrio D., Cabezón O., de la Fuente J., Gortazar C., Boadella M. A transversal study on antibodies against selected pathogens in dromedary camels in the Canary Islands, Spain. Vet. Microbiol. 2013;167:468–473. doi: 10.1016/j.vetmic.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 93.Rivera H., Madewell B.R., Ameghino E. Serologic survey of viral antibodies in the Peruvian alpaca (Lama pacos) Am. J. Vet. Res. 1987;48:189–191. http://www.ncbi.nlm.nih.gov/pubmed/3826854 (Accessed November 6, 2017) [PubMed] [Google Scholar]