Summary
Objectives
Though respiratory viruses are thought to cause substantial morbidity globally in children aged <5 years, the incidence of severe respiratory virus infections in children is unknown in India where 20% of the world's children live.
Methods
During August 2009–July 2011, prospective population-based surveillance was conducted for hospitalizations of children aged <5 years in a rural community in Haryana State. Clinical data and respiratory specimens were collected. Swabs were tested by RT-PCR for influenza and parainfluenza viruses, respiratory syncytial virus (RSV), human metapneumovirus, coronaviruses, and adenovirus. Average annual hospitalization incidence was calculated using census data and adjusted for hospitalizations reported to occur at non-study hospitals according to a comunity healthcare utilization survey.
Results
Of 245 hospitalized children, respiratory viruses were detected among 98 (40%), of whom 92 (94%) had fever or respiratory symptoms. RSV accounted for the highest virus-associated hospitalization incidence (34.6/10,000, 95% CI 26.3–44.7) and 20% of hospitalizations. There were 11.8/10,000 (95% CI 7.9–18.4) influenza-associated hospitalizations (7% of hospitalizations). RSV and influenza virus detection peaked in winter (November–February) and rainy seasons (July), respectively.
Conclusion
Respiratory viruses were associated with a substantial proportion of hospitalizations among young children in a rural Indian community. Public health research and prevention in India should consider targeting RSV and influenza in young children.
Keywords: Respiratory infections, Hospitalization, Respiratory syncytial viruses, Influenza, Parainfluenza, Adenovirus, Coronavirus, Human metapneumovirus
Introduction
Acute respiratory infections are recognized as an important cause of mortality, hospitalization, and healthcare utilization in young children globally.1, 2, 3 Respiratory virus infections are increasingly recognized as major contributors to the burden of severe acute respiratory illness in many countries due to expanding global surveillance and the advent of improved molecular diagnostic testing for respiratory viruses.4, 5, 6, 7, 8 In studies of respiratory virus detection among children hospitalized with respiratory illness from different parts of the world, RSV and influenza are frequently associated with a substantial proportion of hospitalizations.9, 10, 11, 12, 13, 14 These findings are of public health importance because effective and safe influenza vaccines are available but not widely used in many countries, and development of respiratory syncytial virus (RSV) vaccines continues to be an area of intense study although no licensed RSV vaccine is available.12, 15, 16 Understanding the incidence of respiratory virus-associated severe illness and the timing of respiratory virus circulation is critical to inform research priorities and policy decisions about introduction of available respiratory virus vaccines for children.
India is the second most populous country in the world with 20% of the world's population of children aged <5 years, and almost a third of global pneumonia cases among children aged <5 years are thought to occur in India.3 Public health policies that effectively address causes of severe respiratory illness among children in India would have a substantial impact on global child morbidity and mortality. However, the burden of respiratory virus-associated severe illness among young children in India is unknown. In addition, the few studies that evaluated respiratory viral etiologies of severe illness among children in India were conducted before the advent of newer and more sensitive diagnostic tests, including tests to detect more recently discovered viruses.17, 18, 19, 20 Using data from population-based surveillance of approximately 9500 children for hospitalizations for acute medical illness in rural northern India and concomitant testing for respiratory viruses by real-time reverse transcription polymerase chain reaction (rRT-PCR), we estimate the incidence of respiratory virus-associated hospitalizations among children aged <5 years. We also describe the timing of virus circulation and clinical presentation of children hospitalized with predominant viruses.
Patients and methods
Study setting
The Comprehensive Rural Health Services Project (CRHSP), Ballabgarh study site includes a 323 square kilometer area in Haryana State, about 40 km south of New Delhi. The climate is temperate with a defined colder winter season during November–March and rainy season during July–September. As part of the CRHSP, a Health and Demographic Surveillance System (HDSS) was maintained under which all residents of the CRHSP study site were enrolled in a computerized database with unique identification numbers. The HDSS tracked major events such as births, deaths, marriages and migrations. During the study period, the CRHSP study site included a population of approximately 88,000 persons including approximately 9500 children aged <5 years in 28 villages.
The main providers of inpatient care to the CRHSP population are a government-funded secondary level facility with 60 beds that provides outpatient and inpatient care and serves 15–20% of the CRHSP population, two other government-funded secondary level facilities, and a large number of private health facilities (ranging in size from 5 to 35 beds) that provide inpatient and outpatient health services. Health facilities are largely situated outside CRHSP villages within a range of 3–20 km and largely accessible by two/three wheelers. Most facilities have the resources to care for patients requiring supplemental oxygen but transfer patients requiring mechanical ventilation and intensive level to tertiary care facilities outside the district.
Hospitalized patient enrollment
Hospitalized patients were enrolled from the three secondary level facilities and 18 private facilities in Ballabgarh and Faridabad towns where patients from the CRHSP area were likely to seek inpatient care corroborated by health utilization survey. Patients were eligible for enrollment if they were residents of the CRHSP area and were being hospitalized overnight with any acute medical illness, excluding hospitalizations for the following conditions assumed to be unlikely to be related to respiratory infection: trauma, diarrhea without fever, elective surgery, accidental poisonings, elective blood transfusions, or orthopedic or ophthalmologic conditions. During August 2009–July 2011, patients were prospectively enrolled in the study as previously described.21 Data on demographic characteristics, medical history, and clinical symptoms were obtained by interview of patients' caregivers. Data on clinical signs were abstracted from the medical record using a standardized data collection form. Respiratory specimen samples were collected by study doctors from enrolled patients within 24 h of admission using polyester swabs.
Healthcare utilization survey
During August–December 2010, study investigators visited all houses in the 28 villages of the CRHSP area to conduct a healthcare utilization survey using a standardized questionnaire that was field testing in previous years; 90% of households in the area completed the survey. The survey asked whether any member of the household had been admitted to a hospital for an overnight stay during the preceding year (using a reference period of August 1, 2009–July 31, 2010). For each reported hospitalization, the survey asked about the location and reason for hospitalization, and field workers attempted to validate reports with any available documentation related to the hospitalization.
Respiratory sample collection and testing
Combined throat and nasal swabs were collected from each participant (nasal swabs alone in infants). The swabs were transported in viral transport media on ice to the All India Institute of Medical Sciences (AIIMS) laboratory within 24 h. Samples were then divided into aliquots for respiratory virus detection by rRT-PCR. Testing for influenza viruses and influenza virus subtyping was conducted at AIIMS using US Centers for Disease Control and Prevention (CDC) protocols22 and after laboratory staff received CDC-sponsored training and the laboratory underwent quality control assessment by CDC. Testing for non-influenza respiratory viruses was also conducted at AIIMS using highly sensitive rRT-PCR assays for respiratory syncytial virus (RSV), human metapneumovirus (hMPV), human parainfluenza viruses 1–3 (PIV 1–3), adenovirus, and human coronavirus 229E using CDC protocols23, 24, 25; protocol are available from CDC upon request.
Data analysis
Baseline characteristics, clinical symptoms and signs, and length of hospitalization were compared between children with and without respiratory virus detection using bivariate analysis. Frequencies of clinical symptoms and signs and median length of hospitalization were also calculated for children with RSV and influenza, the most commonly detected viruses. Tachypnea was defined based on Integrated Management of Childhood Illness criteria for fast breathing as ≥60 breaths per minute in children aged <2 months, ≥50 in children aged 2–11 months, and ≥40 in children aged 1–5 years.26 Hypoxia was defined as an oxygen saturation of <92% by pulse oximetry at admission. Chi-squared test or Fisher's exact test was used to calculate p-values for categorical variables and the Wilcoxon test was used to compare continuous variables (SAS, version 9.2, SAS Institute Inc., Cary, NC).
Since all children hospitalized with acute medical illness were enrolled, the proportion of respiratory virus detections among children with and without fever or key respiratory signs or symptoms was evaluated. Fever was defined as either measured temperature >38.0 Celsius at admission or parental report of fever because antipyretic use was common in the study community. Key respiratory symptoms or signs were defined as parental report of cough or fast breathing or physician exam findings of tachypnea, crepitations, wheezing, nasal flaring, chest indrawing, grunting, or stridor. Inclusion of nasal discharge did not change the proportion of children meeting criteria for key respiratory symptoms or signs. All children, regardless of symptoms or signs, were included in all subsequent analyses.
Using data from the healthcare utilization survey, the proportion of hospitalizations among children in the study community that occurred at study facilities was calculated as reported hospitalizations at study facilities divided by total reported hospitalizations; 95% confidence limits were calculated using the Wald method. Average annual cumulative incidences of hospitalizations associated with detection of each respiratory virus were calculated for children aged <1 year, 1–4 years, and <5 years. Incidences were also calculated for children aged <6 months for RSV and influenza since maternal immunization with RSV and influenza vaccines has been proposed for protection of children in this age group.27, 28, 29 Incidences unadjusted for healthcare utilization patterns were calculated by dividing total hospitalizations at study facilities by child-years of observation calculated from January 2010 population estimates. Incidences were then adjusted for missed hospitalizations occurring at non-study facilities by dividing the unadjusted incidence by the proportion of hospitalizations among HDSS area residents occurring at study facilities.
Human subjects review
Written informed consent was obtained from the parent/legal guardian of each participant prior to enrollment. The study protocol was reviewed and approved by the Institutional Review Boards (IRB) of the Indian Council of Medical Research and AIIMS. The Human Research Protection Office of the U.S. Centers for Disease Control and Prevention approved reliance on the AIIMS IRB in accordance with 45 CFR 46.114.
Results
During August 2009–July 2011, 245 children aged <5 years were hospitalized with acute medical illness at study hospitals, of whom, 68 (28%) were aged <6 months, 176 (72%) were male, and 4 (2%) had ≥1 underlying medical conditions (Table 1 ). Respiratory viruses were detected among 98 (40%), including 50 (20%) with RSV and 17 (7%) with influenza viruses (9 with influenza A and 8 with influenza B). Eight of 98 (8%) children with respiratory virus detection had co-detection of two respiratory viruses. Children with and without respiratory virus detection did not differ significantly with respect to age, sex, or presence of underlying conditions. The median days from symptom onset to respiratory specimen collection among children with and without respiratory virus detection was the same (4, IQR 3–7 vs. 4 IQR 2–7, p 0.22).
Table 1.
Baseline characteristics and virus detection among children hospitalized with acute medical illness at study facilities, Comprehensive Rural Health Services Project, Ballabgarh, India, August 2009–July 2011, N = 245.
| Total (n = 245) |
Virus negative (n = 147) |
Virus positive (n = 98) |
p-Value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age group | |||||||
| <6 months | 68 | 28% | 36 | 24% | 32 | 33% | 0.09 |
| 6–11 months | 68 | 28% | 48 | 33% | 20 | 20% | |
| 1–4 years | 109 | 44% | 63 | 42% | 46 | 47% | |
| Male | 176 | 72% | 101 | 69% | 75 | 77% | 0.18 |
| ≥1 underlying medical conditiona | 4 | 2% | 3 | 2% | 1 | 1% | 0.53 |
| Time from symptom onset to specimen collection (days)b | |||||||
| 0–3 days | 91/220 | 41% | 56/128 | 44% | 35/92 | 38% | 0.36 |
| 3–6 days | 61/220 | 28% | 35/128 | 27% | 26/92 | 28% | |
| >6 days | 68/220 | 31% | 37/128 | 29% | 31/92 | 34% | |
| Respiratory virus detection | |||||||
| RSV | 50 | 20% | – | – | 50 | 51% | |
| Influenza | 17 | 7% | – | – | 17 | 17% | |
| Adenovirus | 13 | 5% | – | – | 13 | 13% | |
| Coronavirus | 13 | 5% | – | – | 13 | 13% | |
| Parainfluenza | 10 | 4% | – | – | 10 | 10% | |
| Human metapneumovirus | 3 | 1% | – | – | 3 | 3% | |
Includes 1 child with asthma and chronic diarrhea, 2 children with chronic diarrhea, and 1 child with a seizure disorder.
Denominators indicate patients for whom dates of symptom onset were available.
Of the 245 children hospitalized with acute medical illness, 223 (91%) had reported or measured fever or key respiratory symptoms or signs (i.e. cough, fast breathing, tachypnea, crepitations, wheezing, nasal flaring, chest indrawing, grunting, or stridor). Respiratory viruses were detected among 92 of 233 (41%) children with fever or key respiratory symptoms or signs and 6 of 22 (27%) children without. Of the 6 children with respiratory virus detection without fever or key respiratory symptoms or signs, 4 had RSV and 2 had adenovirus; all had vomiting and diarrhea.
Among the 98 children with respiratory virus-associated illness, history of fever (82%) and cough (69%) were the most commonly reported symptoms. Parental report of cough, nasal discharge or congestion, and fast breathing and exam findings of hypoxia, crepitations, wheezing, and increased work of breathing were more common in children with respiratory virus-associated hospitalizations than those without (Table 2 ); the median length of hospitalization was similar in both groups (4 vs. 3 days, p = 0.05). None of the children died. A larger proportion of children with RSV detection compared to children with other respiratory viruses had a reported history of fast breathing (51% vs. 26%, p = 0.01) and exam findings of increased work of breathing (32% vs. 6%, p < 0.01), crepitations (52% vs. 21%, p < 0.01), and wheezing (40% vs. 10%, p < 0.01).
Table 2.
Clinical presentation and duration of hospitalization of children with respiratory virus-associated hospitalizations, Comprehensive Rural Health Services Project, Ballabgarh, India, August 2009–July 2011.
| Virus negative (n = 147) |
Virus positivea (n = 98) |
p-Valueb | RSV (n = 50) |
Influenza (n = 17) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Symptoms | |||||||||
| Fever (reported) | 117 | 80% | 81 | 83% | 0.7 | 39 | 78% | 16 | 94% |
| Cough | 80 | 54% | 72 | 73% | <0.01 | 40 | 80% | 15 | 88% |
| Nasal congestion/nasal discharge | 33 | 22% | 38 | 39% | <0.01 | 20 | 40% | 8 | 47% |
| Fast breathing | 28 | 19% | 37 | 38% | <0.01 | 25 | 50% | 5 | 29% |
| Vomiting | 101 | 69% | 44 | 45% | <0.01 | 19 | 38% | 9 | 53% |
| Inability/refusal to feed | 42 | 29% | 29 | 30% | 0.86 | 18 | 36% | 5 | 29% |
| Diarrhea | 89 | 61% | 47 | 48% | 0.05 | 21 | 42% | 7 | 41% |
| Lethargy | 21 | 14% | 14 | 14% | 0.97 | 7 | 14% | 3 | 18% |
| Seizures | 6 | 4% | 5 | 5% | 0.71 | 3 | 6% | 2 | 12% |
| Signs | |||||||||
| Fever (measured) | 21 | 14% | 19 | 19% | 0.29 | 9 | 18% | 3 | 18% |
| Hypoxia (oxygen saturation <92%) | 24/141 | 17% | 27/95 | 28% | 0.04 | 17/48 | 35% | 4/16 | 25% |
| Tachypnea | 26 | 18% | 20 | 20% | 0.59 | 11 | 22% | 3 | 18% |
| Crepitations | 23 | 16% | 36 | 37% | <0.01 | 26 | 52% | 6 | 35% |
| Wheezing | 21 | 14% | 25 | 26% | 0.03 | 20 | 40% | 3 | 18% |
| Nasal flaring/grunting/chest indrawing | 9 | 6% | 19 | 19% | <0.01 | 16 | 32% | 2 | 12% |
| Stridor | 6 | 4% | 9 | 9% | 0.1 | 7 | 14% | 0 | 0% |
| Median length of stay (IQR, days) | 3 | (3–4) | 4 | (3–5) | 0.05 | 4 | (3–5) | 4 | (3–4) |
Positive for any respiratory virus (RSV, influenza, adenovirus, coronavirus, parainfluenza virus, or human metapneumovirus).
Comparison of virus negative and virus positive children using chi-squared test or Fisher's exact test for categorical variables and the Wilcoxon test for continuous variables.
Health utilization and incidence rates of hospitalization
Of 9509 children aged <5 years residing in CRHSP area, 8773 (92%) were included in the health utilization survey, and 75% (95% CI 74–76%) of all acute medical hospitalizations reported on the health utilization survey occurred at study facilities (Table 3 ). During August 2009–July 2010, the unadjusted and adjusted average annual incidences of hospitalization for acute medical illness among children aged <5 years were 128.8/10,000 (95% CI 114.1–146.2) and 169.5/10,000 (95% CI 150.0–192.1), respectively. The highest virus-associated hospitalization incidences were those associated with RSV (adjusted incidence 34.6/10,000, 95% CI 26.3–44.7), followed by influenza viruses (adjusted incidence 11.8/10,000, 95% CI 7.9–18.4) (Table 4 ). Compared to children aged 1–4 years, children aged <1 year had more than 5-fold higher all-cause and RSV-associated hospitalization incidences. Among infants aged <6 months, the adjusted incidences of hospitalization for RSV and influenza were 136.8 hospitalizations/10,000 (95% CI 91.1–207.6) and 17.8 hospitalizations/10,000 (95% CI 6.3–51.9), respectively.
Table 3.
Study population and healthcare utilization survey coverage, Comprehensive Rural Health Services Project, Ballabgarh, India, August 2009–July 2011.
| Age | Population in catchment area |
Population surveyed with HUSa |
% of hospitalizations that occurred at study hospitalsb |
||
|---|---|---|---|---|---|
| n | n | (%) | % | (95%CI)c | |
| 0–5 months | 1064 | 993 | (93%) | 79% | (77–81%) |
| 6–11 months | 848 | 754 | (89%) | 79% | (77–81%) |
| 1–4 years | 7597 | 7025 | (92%) | 73% | (72–74%) |
| Total (<5 years) | 9509 | 8773 | (92%) | 75% | (74–76%) |
HUS: Healthcare utilization survey.
As reported on HUS.
95% CI calculated using the Wald method.
Table 4.
Hospitalizations among children aged <5 years at study hospitals and average unadjusted and adjusteda incidence per 10,000 child-years by respiratory virus,b Comprehensive Rural Health Services Project, Ballabgarh, India, August 2009–July 2011.
| <5 years |
<1 year |
1–4 years |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Hospitalizations | Unadjusted incidence | Adjusted incidence | Hospitalizations | Unadjusted incidence | Adjusted incidence | Hospitalizations | Unadjusted incidence | Adjusted incidence | |
| All-cause | 245 | 128.8 (114.1–146.2) | 169.5 (150.0–192.1) | 138 | 360.9 (306.0–425.3) | 456.8 (387.3–538.0) | 107 | 70.4 (58.9–85.9) | 96.5 (79.5–116.4) |
| Respiratory syncytial virusc | 50 | 26.3 (20.0–34.2) | 34.6 (26.3–44.7) | 30 | 78.5 (55.9–111.8) | 99.3 (69.6–140.5) | 20 | 13.2 (8.9–20.7) | 18.0 (11.0–27.4) |
| Influenzad | 17 | 8.9 (6.8–14.2) | 11.8 (7.9–18.4) | 7 | 18.3 (9.8–37.6) | 23.2 (11.4–46.8) | 10 | 6.6 (4.9–13.2) | 9.0 (5.5–17.8) |
| Adenovirus | 13 | 6.8 (4.2–12.1) | 9.0 (5.3–15.8) | 6 | 15.7 (7.8–35.0) | 19.9 (8.9–44.3) | 7 | 4.6 (2.0–10.9) | 6.3 (2.7–13.7) |
| Coronavirus 229E | 13 | 6.8 (4.2–12.1) | 9.0 (5.3–15.8) | 9 | 23.5 (13.1–45.5) | 29.8 (16.5–57.0) | 4 | 2.6 (1.3–7.2) | 3.6 (1.4–9.6) |
| Parainfluenza | 10 | 5.3 (3.2–9.9) | 6.9 (3.9–11.8) | 5 | 13.1 (6.2–30.8) | 16.6 (7.6–38.0) | 5 | 3.3 (1.3–7.2) | 4.5 (1.4–9.6) |
| Human metapneumovirus | 3 | 1.6 (1.1–5.3) | 2.1 (1.3–6.6) | 2 | 5.2 (1.0–19.3) | 6.6 (1.3–24.1) | 1 | 0.7 (0–3.9) | 0.9 (0–5.5) |
Adjusted to account for hospitalizations that occurred at non-study facilities based on data from healthcare utilization survey.
Children with co-detection of more than 1 respiratory virus were included in the incidence estimate of each respiratory virus detected.
Among infants aged <6 months, the adjusted incidence of hospitalization for RSV was 136.8 hospitalizations/10,000 (95% CI 91.1–207.6).
Among infants aged <6 months, the adjusted incidence of hospitalization for influenza was 17.8 hospitalizations/10,000 (95% CI 6.3–51.9).
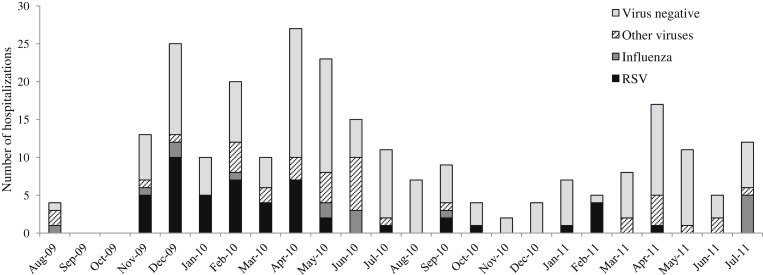
Seasonality of respiratory virus detection
Respiratory viruses were detected almost year-round among children hospitalized with acute medical illnesses (Fig. 1 ). RSV detection occurred from November through May with a longer period of detection during 2009–2010 than during 2011 (Fig. 1). In contrast, influenza virus detection peaked during the rainy season in June–July when influenza accounted for 20–42% of monthly acute medical illness hospitalizations. Detection of other respiratory viruses occurred sporadically throughout the year.
Figure 1.
Number of acute medical illness hospitalizations among children aged <5 years by month and virus, Comprehensive Rural Health Services Project, Ballabgarh, India, August 2009–July 2011.
Discussion
Based on surveillance of approximately 9500 children, this study is the first prospective, population-based study to measure the incidence of respiratory virus-associated hospitalizations among children aged <5 years in rural India. We found that respiratory virus infections were associated with 40% of acute medical illness hospitalizations among children aged <5 years, and one in five children hospitalized for acute medical illness had RSV infection. Consistent with prior studies, we also found that virus-associated hospitalization rates were highest among children aged <1 year. RSV was the predominant virus identified among children aged <1 year. Among children aged 1–4 years, incidence rates of RSV and influenza viruses were similar. RSV and influenza viruses circulated with clearly defined but different seasonality and were infrequently detected among children without fever or respiratory symptoms or signs, similar to prior studies.9, 10, 30
The incidence of RSV-associated hospitalizations in our study community was substantial, with an incidence of 35 per 10,000 child-years among children aged <5 years and 99 per 10,000 child-years among children aged <1 year. Many studies have reported RSV as the most common respiratory virus resulting in hospitalization in young children, but relatively few have estimated the incidence of RSV-associated hospitalizations. RSV-associated hospitalization rates per 10,000 children ranged from 109 to 274 among children aged <1 year in studies from rural Thailand, Indonesia, the United Kingdom, and the United States; 25–30 among children aged <5 years in Hong Kong and the United States; and 194 and 450 among children aged <5 years without and with HIV infection in South Africa. Our estimates are similar to those of prior studies from the United States, Europe and Asia. Given the high incidence of RSV-associated hospitalization in our study, availability of an effective and accessible RSV vaccine for young children in India would profoundly impact hospitalization rates in this age group. Because the incidence of RSV-associated hospitalization is highest in infants aged <6 months in whom maternal antibody interference with immune responses may make vaccination challenging, RSV vaccination of pregnant women has been suggested as another potential vaccination strategy.27, 28, 29 Though licensed RSV vaccines are not currently available, candidate vaccines are under development15, 16 and data on RSV disease burden will be important for policy decisions about RSV vaccine introduction if a licensed vaccine becomes available.
Although we found that influenza viruses were associated with a lower incidence of hospitalization than RSV among children aged <5 years, influenza-associated hospitalizations accounted for 7% of all acute medical hospitalizations and up to 42% of monthly hospitalizations during peak circulation. A similar population-based study conducted in western India during the same time period as ours found similar influenza-associated hospitalization rates among children aged <1 year and higher rates among children aged 1–4 years.31 The contribution of influenza to severe respiratory illness among children in India deserves attention because influenza vaccines are not currently recommended and are not widely available in India. Both traditional inactivated influenza vaccines32, 33, 34 and live attenuated influenza vaccines35, 36, 37, 38 have been shown to be effective against influenza in children in high-income countries. Additional studies are needed to further develop the evidence base for policy makers in India to decide whether to support influenza vaccination recommendations for children. These studies should include data on the incidence of severe influenza in other regions of India, the effectiveness of influenza vaccine among Indian children, and the costs of influenza in India. A study of influenza vaccine effectiveness among children aged <5 years in rural India is underway.39
The timing of respiratory virus circulation varies globally. RSV and influenza viruses circulate during the colder winter months in some but not all temperate countries. However, influenza viruses circulate year-round in some tropical countries and both RSV and influenza virus circulation seems to coincide with increased rainfall in some places.40, 41 India is a large and geographically diverse country with temperate, sub-tropical and tropical climates. In our study, conducted in a temperate area, we found that RSV and influenza circulated during clearly defined but different periods. Studies from western India suggest that influenza virus circulation is less defined there, with peaks during the rainy season but perennial influenza virus circulation.20, 31 Additional studies of influenza virus seasonality in other regions of India are needed to determine optimal timing for influenza vaccination and other prevention measures.
The advent of highly sensitive PCR assays for respiratory viruses allows easier detection of a wider array of viruses but also presents the challenge of interpreting positive test results. Prior studies have shown that some respiratory viruses are frequently detected by PCR among children without fever or respiratory symptoms.9, 10, 30 Respiratory virus detection in these children may reflect acute infection with atypical symptoms or prolonged shedding of virus from a prior infection highlighting the utility of a control group of children without acute illness to aid interpretation of test results. Our study did not have a control group, but the large majority of children with respiratory virus detection had fever or respiratory findings that support the clinical diagnosis of respiratory infection. Our estimates of RSV and influenza-associated hospitalization incidence are supported by prior studies of hospitalized children in which detection of these viruses was rare from control groups comprised of hospitalized children without fever or respiratory symptoms or non-hospitalized children. Though adenoviruses, coronaviruses, human metapneumovirus, and parainfluenza viruses have all been previously implicated in the pathogenesis of severe illness, our hospitalization incidence estimates for these viruses should be interpreted with more caution as detection of these viruses has varied among control groups in other studies.9, 10, 30
Several points should be considered when interpreting our findings. First, our study was conducted at primary and secondary level health facilities without capacity to provide mechanical ventilation and intensive care for critically ill children. Thus, we may have missed hospitalizations of children with more severe illness who were directly admitted to tertiary care hospitals outside the study area. Our study community may also have been too small to evaluate the frequency of rare but severe outcomes such as death. Second, although we attempted to collect the discharge diagnosis of all enrolled children, these data were largely absent from the medical record and therefore could not be used to aid interpretation of respiratory virus testing results. Third, we included testing results for only a select group of respiratory viruses previously identified as being associated with a large proportion of respiratory illnesses in children. Therefore, our results reflect the burden of respiratory virus-associated hospitalizations caused by this select group of viruses. We did not include test results for rhinoviruses because interpretation of rhinovirus detection in the absence of a control group is difficult, although rhinovirus was the most commonly detected virus in children with respiratory illness in some prior studies. Additionally, we did not conduct testing for coronaviruses other than coronavirus 229E, and may have underestimated the incidence of coronavirus-associated hospitalization in the study community. Lastly, respiratory virus circulation and incidence is known to vary by year. Our study was conducted over a two year period and we present average annual cumulative incidences of virus-associated hospitalization. Additional studies of respiratory virus-associated disease burden during additional years will be useful for comparison with our findings.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Role of the funding source
This study was supported in part by cooperative agreements U01 IP000206 from the Centers for Disease Control and Prevention, Atlanta, US. The authors do not have any relevant conflicts of interest to declare.
Acknowledgments
The authors wish to acknowledge the Division of Viral Diseases, Centers for Disease Control and Prevention for providing assay kits used for laboratory testing in this study.
Contributor Information
Shobha Broor, Email: shobha.broor@gmail.com.
Fatimah S. Dawood, Email: fdawood@cdc.gov.
References
- 1.Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012 Jun 9;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [PubMed PMID: 22579125. Epub 2012/05/15. eng] [DOI] [PubMed] [Google Scholar]
- 2.Nair H., Simooes E.A., Rudan I., Gessner B.D., Azziz-Baumgartner E., Zhang J.S. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013 Jan 28 doi: 10.1016/S0140-6736(12)61901-1. [PubMed PMID: 23369797. Epub 2013/02/02. Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker C.L., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013 Apr 20;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [PubMed PMID: 23582727] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011 Dec 3;378(9807):1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [PubMed PMID: 22078723. Epub 2011/11/15. eng] [DOI] [PubMed] [Google Scholar]
- 5.Ren L., Xiang Z., Guo L., Wang J. Viral infections of the lower respiratory tract. Current Infectious Disease Reports. 2012 Jun;14(3):284–291. doi: 10.1007/s11908-012-0258-4. [PubMed PMID: 22456860. Epub 2012/03/30. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011 Apr 9;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [PubMed PMID: 21435708. Epub 2011/03/26. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010 May 1;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [PubMed PMID: 20399493. Pubmed Central PMCID: 2864404. Epub 2010/04/20. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luksic I., Kearns P.K., Scott F., Rudan I., Campbell H., Nair H. Viral etiology of hospitalized acute lower respiratory infections in children under 5 years of age – a systematic review and meta-analysis. Croatian Medical Journal. 2013 Apr;54(2):122–134. doi: 10.3325/cmj.2013.54.122. [PubMed PMID: 23630140. Pubmed Central PMCID: 3641872] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Advani S., Sengupta A., Forman M., Valsamakis A., Milstone A.M. Detecting respiratory viruses in asymptomatic children. Pediatric Infectious Disease Journal. 2012 Dec;31(12):1221–1226. doi: 10.1097/INF.0b013e318265a804. [PubMed PMID: 22739572. Pubmed Central PMCID: 3505556. Epub 2012/06/29. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feikin D.R., Njenga M.K., Bigogo G., Aura B., Aol G., Audi A. Viral and bacterial causes of severe acute respiratory illness among children aged less than 5 years in a high malaria prevalence area of western Kenya, 2007–2010. Pediatric Infectious Disease Journal. 2013 Jan;32(1):e14–e19. doi: 10.1097/INF.0b013e31826fd39b. [PubMed PMID: 22914561. Epub 2012/08/24. eng] [DOI] [PubMed] [Google Scholar]
- 11.Nascimento-Carvalho C.M., Ribeiro C.T., Cardoso M.R., Barral A., Araujo-Neto C.A., Oliveira J.R. The role of respiratory viral infections among children hospitalized for community-acquired pneumonia in a developing country. Pediatric Infectious Disease Journal. 2008 Oct;27(10):939–941. doi: 10.1097/INF.0b013e3181723751. [PubMed PMID: 18756190. Epub 2008/08/30. eng] [DOI] [PubMed] [Google Scholar]
- 12.Schickli J.H., Dubovsky F., Tang R.S. Challenges in developing a pediatric RSV vaccine. Human Vaccines. 2009 Sep;5(9):582–591. doi: 10.4161/hv.9131. [PubMed PMID: 19556888. Epub 2009/06/27. eng] [DOI] [PubMed] [Google Scholar]
- 13.Nicholson K.G., McNally T., Silverman M., Simons P., Stockton J.D., Zambon M.C. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine. 2006 Jan 9;24(1):102–108. doi: 10.1016/j.vaccine.2005.02.004. [PubMed PMID: 16310899. Epub 2005/11/29. eng] [DOI] [PubMed] [Google Scholar]
- 14.O'Callaghan-Gordo C., Bassat Q., Morais L., Diez-Padrisa N., Machevo S., Nhampossa T. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatric Infectious Disease Journal. 2011 Jan;30(1):39–44. doi: 10.1097/INF.0b013e3181f232fe. [PubMed PMID: 20805786. Epub 2010/09/02. eng] [DOI] [PubMed] [Google Scholar]
- 15.Beeler J.A., Eichelberger M.C. Influenza and respiratory syncytial virus (RSV) vaccines for infants: safety, immunogenicity, and efficacy. Microbial Pathogenesis. 2013 Feb;55:9–15. doi: 10.1016/j.micpath.2012.11.013. [PubMed PMID: 23247146. Epub 2012/12/19. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair H., Verma V.R., Theodoratou E., Zgaga L., Huda T., Simoes E.A. An evaluation of the emerging interventions against respiratory syncytial virus (RSV)-associated acute lower respiratory infections in children. BMC Public Health. 2011;11(Suppl. 3):S30. doi: 10.1186/1471-2458-11-S3-S30. [PubMed PMID: 21501449. Pubmed Central PMCID: 3231904. Epub 2011/04/29. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John T.J., Cherian T., Steinhoff M.C., Simoes E.A., John M. Etiology of acute respiratory infections in children in tropical southern India. Reviews of Infectious Diseases. 1991 May-Jun;13(Suppl. 6):S463–S469. doi: 10.1093/clinids/13.supplement_6.s463. [PubMed PMID: 1862277. Epub 1991/05/01. eng] [DOI] [PubMed] [Google Scholar]
- 18.Kabra S.K., Lodha R., Broor S., Chaudhary R., Ghosh M., Maitreyi R.S. Etiology of acute lower respiratory tract infection. Indian Journal of Pediatrics. 2003 Jan;70(1):33–36. doi: 10.1007/BF02722742. [PubMed PMID: 12619950. Epub 2003/03/07. eng] [DOI] [PubMed] [Google Scholar]
- 19.Steinhoff M.C., Padmini B., John T.J., Kingsley J., Pereira S.M. Viral etiology of acute respiratory infections in south Indian children. Indian Journal of Medical Research. 1985 Apr;81:349–353. [PubMed PMID: 2991137. Epub 1985/04/01. eng] [PubMed] [Google Scholar]
- 20.Yeolekar L.R., Damle R.G., Kamat A.N., Khude M.R., Simha V., Pandit A.N. Respiratory viruses in acute respiratory tract infections in Western India. Indian Journal of Pediatrics. 2008 Apr;75(4):341–345. doi: 10.1007/s12098-008-0035-4. [PubMed PMID: 18536887. Epub 2008/06/10. eng] [DOI] [PubMed] [Google Scholar]
- 21.Gupta V., Dawood F.S., Rai S.K., Broor S., Wigh R., Mishra A.C. Validity of clinical case definitions for influenza surveillance among hospitalized patients: results from a rural community in North India. Influenza and Other Respiratory Viruses. 2012 Jul 16 doi: 10.1111/j.1750-2659.2012.00401.x. [PubMed PMID: 22804843. Epub 2012/07/19. Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . 2009. CDC protocol of realtime RTPCR for swine flu influenza A (H1N1)http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf Geneva. [accessed 20.07.09] [Google Scholar]
- 23.Fry A.M., Chittaganpitch M., Baggett H.C., Peret T.C., Dare R.K., Sawatwong P. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS One. 2010;5(11):e15098. doi: 10.1371/journal.pone.0015098. [PubMed PMID: 21152047. Pubmed Central PMCID: 2994907. Epub 2010/12/15. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heim A., Ebnet C., Harste G., Pring-Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. Journal of Medical Virology. 2003 Jun;70(2):228–239. doi: 10.1002/jmv.10382. [PubMed PMID: 12696109] [DOI] [PubMed] [Google Scholar]
- 25.Dare R.K., Fry A.M., Chittaganpitch M., Sawanpanyalert P., Olsen S.J., Erdman D.D. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. Journal of Infectious Diseases. 2007 Nov 1;196(9):1321–1328. doi: 10.1086/521308. [PubMed PMID: 17922396] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. 2nd ed. 2013. WHO Guidelines Approved by the Guidelines Review Committee. Geneva. [PubMed] [Google Scholar]
- 27.Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E. Effectiveness of maternal influenza immunization in mothers and infants. New England Journal of Medicine. 2008 Oct 9;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [PubMed PMID: 18799552. Epub 2008/09/19. eng] [DOI] [PubMed] [Google Scholar]
- 28.Englund J., Glezen W.P., Piedra P.A. Maternal immunization against viral disease. Vaccine. 1998 Aug-Sep;16(14–15):1456–1463. doi: 10.1016/s0264-410x(98)00108-x. [PubMed PMID: 9711788. Epub 1998/08/26. eng] [DOI] [PubMed] [Google Scholar]
- 29.van Drunen Littel-van den Hurk S., Mapletoft J.W., Arsic N., Kovacs-Nolan J. Immunopathology of RSV infection: prospects for developing vaccines without this complication. Reviews in Medical Virology. 2007 Jan-Feb;17(1):5–34. doi: 10.1002/rmv.518. [PubMed PMID: 17004293. Epub 2006/09/28. eng] [DOI] [PubMed] [Google Scholar]
- 30.Singleton R.J., Bulkow L.R., Miernyk K., DeByle C., Pruitt L., Hummel K.B. Viral respiratory infections in hospitalized and community control children in Alaska. Journal of Medical Virology. 2010 Jul;82(7):1282–1290. doi: 10.1002/jmv.21790. [PubMed PMID: 20513097. Epub 2010/06/01. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chadha M.S., Hirve S., Dawood F.S., Lele P., Deoshatwar A., Sambhudas S. Burden of seasonal and pandemic influenza-associated hospitalization during and after 2009 A(H1N1)pdm09 pandemic in a rural community in India. PLoS One. 2013;8(5):e55918. doi: 10.1371/journal.pone.0055918. [PubMed PMID: 23690913. Pubmed Central PMCID: 3654970] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenberg K.W., Szilagyi P.G., Fairbrother G., Griffin M.R., Staat M., Shone L.P. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003–2004 and 2004–2005 influenza seasons. Pediatrics. 2008 Nov;122(5):911–919. doi: 10.1542/peds.2007-3304. [PubMed PMID: 18977968. Epub 2008/11/04. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuler C.M., Iwamoto M., Bridges C.B., Marin M., Neeman R., Gargiullo P. Vaccine effectiveness against medically attended, laboratory-confirmed influenza among children aged 6 to 59 months, 2003–2004. Pediatrics. 2007 Mar;119(3):e587–e595. doi: 10.1542/peds.2006-1878. [PubMed PMID: 17332179. Epub 2007/03/03. eng] [DOI] [PubMed] [Google Scholar]
- 34.Szilagyi P.G., Fairbrother G., Griffin M.R., Hornung R.W., Donauer S., Morrow A. Influenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Archives of Pediatrics & Adolescent Medicine. 2008 Oct;162(10):943–951. doi: 10.1001/archpedi.162.10.943. [PubMed PMID: 18838647. Epub 2008/10/08. eng] [DOI] [PubMed] [Google Scholar]
- 35.Belshe R.B., Gruber W.C. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatric Infectious Disease Journal. 2000 May;19(Suppl. 5):S66–S71. doi: 10.1097/00006454-200005001-00010. [PubMed PMID: 10821474. Epub 2000/05/23. eng] [DOI] [PubMed] [Google Scholar]
- 36.Bracco Neto H., Farhat C.K., Tregnaghi M.W., Madhi S.A., Razmpour A., Palladino G. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine-naive children. Pediatric Infectious Disease Journal. 2009 May;28(5):365–371. doi: 10.1097/INF.0b013e31819219b8. [PubMed PMID: 19395948. Epub 2009/04/28. eng] [DOI] [PubMed] [Google Scholar]
- 37.Tam J.S., Capeding M.R., Lum L.C., Chotpitayasunondh T., Jiang Z., Huang L.M. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatric Infectious Disease Journal. 2007 Jul;26(7):619–628. doi: 10.1097/INF.0b013e31806166f8. [PubMed PMID: 17596805. Epub 2007/06/29. eng] [DOI] [PubMed] [Google Scholar]
- 38.Vesikari T., Fleming D.M., Aristegui J.F., Vertruyen A., Ashkenazi S., Rappaport R. Safety, efficacy, and effectiveness of cold-adapted influenza vaccine-trivalent against community-acquired, culture-confirmed influenza in young children attending day care. Pediatrics. 2006 Dec;118(6):2298–2312. doi: 10.1542/peds.2006-0725. [PubMed PMID: 17142512. Epub 2006/12/05. eng] [DOI] [PubMed] [Google Scholar]
- 39.Sullender W., Fowler K., Krishnan A., Gupta V., Moulton L.H., Lafond K. Design and initiation of a study to assess the direct and indirect effects of influenza vaccine given to children in rural India. Vaccine. 2012 Jul 27;30(35):5235–5239. doi: 10.1016/j.vaccine.2012.06.002. [PubMed PMID: 22709952. Epub 2012/06/20. eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azziz Baumgartner E., Dao C.N., Nasreen S., Bhuiyan M.U., Mah E.M.S., Al Mamun A. Seasonality, timing, and climate drivers of influenza activity worldwide. Journal of Infectious Diseases. 2012 Sep 15;206(6):838–846. doi: 10.1093/infdis/jis467. [PubMed PMID: 22829641. Epub 2012/07/26. eng] [DOI] [PubMed] [Google Scholar]
- 41.Shek L.P., Lee B.W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatric Respiratory Reviews. 2003 Jun;4(2):105–111. doi: 10.1016/s1526-0542(03)00024-1. [PubMed PMID: 12758047. Epub 2003/05/22. eng] [DOI] [PubMed] [Google Scholar]