Abstract
As for most biological processes, the immune response to microbial infections has to be tightly controlled to remain beneficial for the host. Inflammation is one of the major consequences of the host's immune response. For its orchestration, this process requires a fine-tuned interplay between interleukins, endothelial cells and various types of recruited immune cells. Suppressors of cytokine signalling (SOCS) proteins are crucially involved in the complex control of the inflammatory response through their actions on various signalling pathways including the JAK/STAT and NF-κB pathways. Due to their cytokine regulatory functions, they are frequent targets for exploitation by infectious agents trying to escape the host's immune response. This review article aims to summarize our current knowledge regarding SOCS family members in the different mammalian species studied so far, and to display their complex molecular interactions with microbial pathogens.
Keywords: CIS, SOCS, Infectious diseases, Cytokines, Mammals
1. Introduction
Cytokines are essential mediators of cell-to-cell communications. By activating cell surface receptor complexes, they regulate processes that are involved in the growth, the differentiation, and the defense of various cells. Although lacking catalytic domains, members of the cytokine receptor superfamily mediate ligand-dependent activation of protein tyrosine phosphorylation (Yasukawa et al., 2000). Cytokines induce homo- or hetero-dimerization and activation of their cognate receptors thereby triggering the oligomerization and activation of members of the cytosolic janus kinase (JAK) family (Yasukawa et al., 2000). The activated JAKs subsequently phosphorylate further signalling targets including signal transduction and activators of transcription (STAT) proteins.
Each member of the JAK family contains (i) a conserved catalytic kinase domain that can be phosphorylated upon ligand stimulation, and (ii) an enzymatically inactive pseudo-kinase domain at the carboxyl terminus that regulates the activity of the catalytic domain (Shuai and Liu, 2003). Through their amino-terminal regions, activated JAK proteins physically and selectively interact with cytokine receptors (Dalpke et al., 2008) thereby allowing the phosphorylation of specific tyrosine residues in these proteins. The phosphorylated tyrosine residues in turn serve as docking sites for members of the STAT protein family (Darnell, 1997, Vinkemeier et al., 1998). STATs are subsequently phosphorylated by JAKs, triggering thus their dimerization and release from the receptor complex. Next, the STAT dimers translocate to the nucleus where they induce the transcription of cytokine-responsive genes (Darnell, 1997, Levy and Darnell, 2002). The signalling events outlined above were originally found to be initiated by type I interferons (IFNs); however, it is known today that this pathway can be activated by a large number of cytokines, growth factors and hormones.
This signalling response must be tightly regulated, especially with respect to its duration and intensity. Regulation of the innate immune response is critical, since an excessive inflammatory reaction can be deleterious (Liu et al., 1998, Yasukawa et al., 2000). Negative regulation of JAK/STAT signalling is carried out by a number of factors. Among them suppressors of cytokine signalling (SOCS) were shown to play a prominent role (Endo et al., 1997, Naka et al., 1997, Starr et al., 1997, Yoshimura et al., 1995). SOCS 1–7 and cytokine-inducible SH2 protein (CIS) are the eight members of this family of intracellular proteins that are expressed following cellular activation by various molecular stimuli including cytokines and pathogen-associated molecular patterns (Kubo et al., 2003, Yoshimura et al., 2007, Yoshimura et al., 2012).
2. The SOCS family
SOCS proteins range in size from 198 to 581 amino-acids. CIS, SOCS1, and SOCS3 are the best characterized so far (Alexander, 2002). All SOCS proteins share the same basic structure and are composed of three functional domains as depicted in Fig. 1A (Hilton et al., 1998, Yoshimura et al., 2007). The most conserved feature is the central Src homology 2 (SH2) domain, which is involved in both the recognition and binding of cognate phosphotyrosine motifs (Hilton et al., 1998, Yoshimura et al., 2007) (Fig. 1A). This domain specifies the target of each SOCS/CIS protein to execute its regulatory function (Sasaki et al., 2000). Upstream (towards the N-terminus) of the central SH2 domain is a variable region with an extended SH2 sub-domain (ESS) that contributes to the physical interaction with the substrate. Located downstream of the SH2 domain is a C-terminal 40-amino-acid domain termed SOCS box (Bullock et al., 2007, Hilton et al., 1998, Yasukawa et al., 2000, Yoshimura et al., 2007) (Fig. 1A). The SOCS box interacts with elongin B, elongin C, cullin 5, and with the RING-finger-domain-only protein to recruit E2 ubiquitin-transferase (Kamizono et al., 2001, Kamura et al., 2004). This functional complex ubiquitinylates the target proteins and hence marks them for proteasomal degradation (Kamizono et al., 2001, Kamura et al., 2004). The SOCS box also stabilizes SOCS proteins, probably by sheltering them from proteasome-mediated turnover (Piessevaux et al., 2009). The later function also provides protection from functional interferences between different SOCS proteins.
Fig. 1.
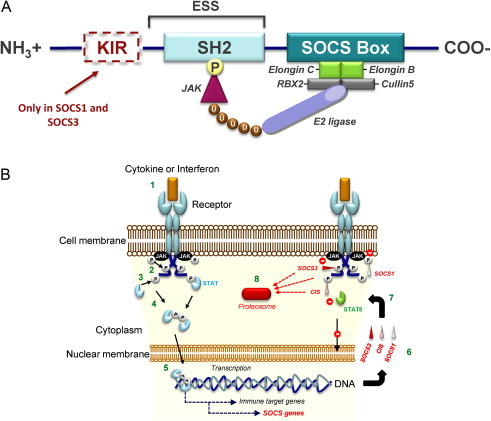
(A) Structure of SOCS proteins. All SOCS proteins have (i) a central SH2 domain, (ii) an amino-terminal end domain of variable length including an extended SH2 sub-domain (ESS) and (iii) a carboxy-terminal SOCS box. SOCS1 and SOCS3 contain an additional amino-terminal kinase inhibitory region known as KIR. The SH2 domain of each SOCS determines its target-specificity, through binding phosphorylated (P) tyrosine residues that are specific to each substrate, such as JAK proteins. The SOCS box interacts with a complex containing elongin B, elongin C, cullin5, RING-box-2 (RBX2), and E2 ligase (also known as E2 ubiquitin-conjugating enzyme). This complex keeps the bound substrate close to the ubiquitinating machinery, thus facilitating its ubiquitination (U) and driving it towards proteosomal degradation. The KIR domain functions as a pseudosubstrate that inhibits the kinase activity of the SOCS-associated proteins. (B) Mechanism of suppression of the JAK/STAT pathway by SOCS1, SOCS3, and CIS. The cytokine or interferon stimulation of their cell surface receptors (1) activates receptor-associated JAK proteins by their phosphorylation (P). Then, activated JAKs phosphorylate receptor cytoplasmic domains (2). Recruited STATs are consequently activated by JAK phosphorylation (3). This phosphorylation enables their dimerization (4). Dimerized they can enter the nucleus and trigger as transcription factor complex the expression of various target genes including SOCS genes (5). Various SOCS proteins such as SOCS1, SOCS3 and CIS are produced (6). They can (7), as SOCS1 but also SOCS3, inhibit the JAK activity with their kinase inhibitory region. They can also, as SOCS3, compete with recruited STAT proteins for shared phosphotyrosine residues or specifically, as CIS, bind the phosphorylated tyrosine residues of cytokine receptors through the SH2 domain consequently masking the STAT5 docking site. Moreover, their SOCS box mediates ubiquitination and degradation of bound receptor components (8). Consecutively to their actions transcription factor complexes cannot anymore form and access the nucleus.
Cytokine receptor signalling is controlled by SOCS at three different levels (Fig. 1B and Fig. 2 ). Firstly, the small N-terminal kinase inhibitory region (KIR) that is specific to SOCS1 and SOCS3 can prevent the action of JAKs. The KIR acts via a competitive mechanism that is due to its sequence homology with the pseudosubstrate inhibitory region of JAKs (Fig. 1B). Interaction of the KIR with the JAK activation loop thus blocks the catalytic activity of JAKs (Kubo et al., 2003, Sasaki et al., 1999, Yasukawa et al., 1999). Secondly, SOCS proteins can suppress cytokine signalling by competing with downstream signal transducers through their interaction with the phosphorylated domains of the receptor (Fig. 1B) (Ram and Waxman, 1999). Thirdly, their SOCS box mediates the transfer of their protein targets to the ubiquitination machinery and thus assigns them for proteasomal degradation (Fig. 1B) (Kamizono et al., 2001, Kamura et al., 2004, Verdier et al., 1998). Like other SOCS-box-containing factors, SOCS proteins also function as E3 ubiquitin ligases and directly mediate the degradation of associated proteins (Fig. 1B) (Yoshimura et al., 2007).
Fig. 2.
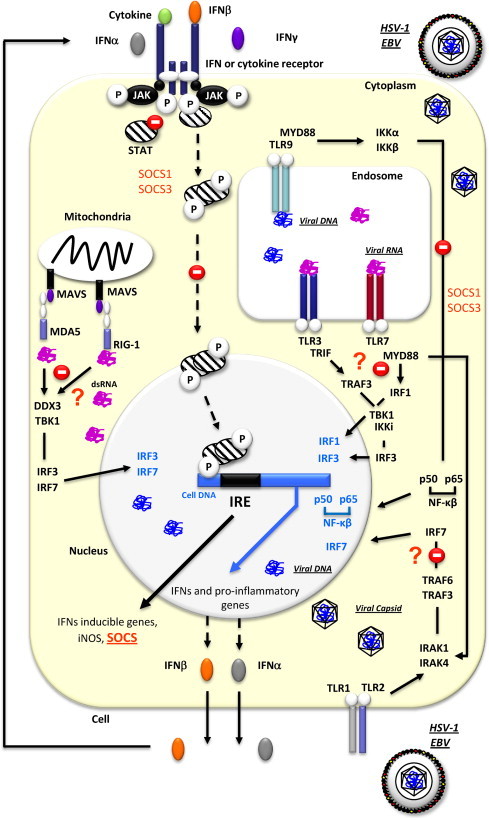
Overview of established and potential pathways targeted by SOCS proteins in a cell infected by HSV-1 and/or EBV. After HSV-1 infection and the stimulation of several pathogen recognition receptors (MDA5, RIG-1, TLR1/TLR2, TLR3, TLR7, TLR9), IFNβ is transcriptionally activated following the stimulation of various signalling pathways. SOCS proteins can act at different levels (inhibition of the JAK activity, competition with recruited STAT proteins for shared phosphotyrosine residues, phosphorylation of receptor tyrosine residues, ubiquitination and degradation of bound protein components) in the cell as indicated by stop signs. Question marks (?) indicate pathways that are possibly targeted by SOCS to modulate the anti-viral immune response. DDX3, Dead box protein 3; dsRNA, double stranded ribonucleic acid; EBV, Epstein-Barr Virus; HSV-1, Herpes Simplex Virus type 1; IFN, interferon; IKKα, I kappa-B kinase-alpha; IKKβ, I kappa-B kinase-beta; IKKi, kinase I kappa B kinase i; iNOS, inducible nitric oxide synthase; IRE, interferon response element; IRAK, IL1-Receptor-associated kinase; IRF, IFN-regulatory factor; JAK, janus kinase; MAVS, mitochondrial antiviral signalling protein; MDA5, melanoma differentiation-associated gene 5; MYD88, myeloid differentiation primary-response protein 88; NF-kβ, Nuclear factor kappa-B; P, phosphorylated tyrosine; p50, p50 subunit of NF-κB; TBK1, TANK-binding kinase 1; TRIF, TIR-containing adaptator inducing interferon-β;TLR, toll like receptor; TRAF, Tumor necrosis factor receptor (TNFR)-associated factor; RIG-1, Retinoic acid-inducible gene I; STAT, signal transduction and activators of transcription.
Since their first discovery in 1997 (Starr et al., 1997), SOCS proteins have been described as important regulators of the JAK/STAT pathway. Shortly after, they have also been reported to be involved in allergy, tumorigenesis, and inflammatory diseases (Yasukawa et al., 2000). SOCS proteins are generally not highly expressed at homeostasis and have short half-lives (typically 1–2 h) (Delgado-Ortega et al., 2011, Haan et al., 2003). They are encoded by cytokine-inducible genes that are rapidly transcribed after exposure of cells to cytokines (Matsumoto et al., 1999, Naka et al., 1997). As outlined later in this article, SOCS proteins also have the ability to regulate other signal transduction pathways such as the NF-κB, MAPK or JNK/p38 pathways (Frobose et al., 2006, He and Stephens, 2006, Ryo et al., 2003).
SOCS proteins have been found in numerous vertebrate species including man (Homo sapiens), chimpanzee (Pan troglodytes), Sumatran orangutan (Pongo abelii), rhesus monkey (Macaca mulatta), mouse (Mus musculus), rat (Rattus norvegicus), Chinese hamster (Cricetulus griseus), Guinea pig (Cavia porcellus), dog (Canis lupus familiaris), giant panda (Ailuropoda melanoleuca), cattle (Bos taurus), sheep (Ovis aries), pig (Sus scrofa), horse (Equus caballus), African elephant (Loxondota africana), rabbit (Oryctelagus cuniculus), chicken (Gallus gallus), turkey (Meleagris gallopavo), zebrafish (Taeniopygia guttata), bony fish (Osteichthyes spp.) and even in some invertebrates such as the Chinese mitten crab (Eriocheir sinensis) and the fruitfly (Drosophila melanogaster) (Bruel et al., 2010, Cheeseman et al., 2008, Rincon et al., 2007, Sandra et al., 2005, Starr et al., 1997, Stec and Zeidler, 2011, Wang et al., 2011, Winkelman et al., 2008, Yan et al., 2011, Zhang et al., 2010). The polypeptide sequences of SOCS proteins are highly conserved between the different species, especially among mammals. For example, mouse and rat SOCS1 share 95–99% amino acid (aa) identity with their human orthologue, while human and porcine SOCS4, SOCS5 and SOCS6 share 93, 96.6, and 92.3% aa identity, respectively (Bruel et al., 2010, Starr et al., 1997). In a recent study, Zhang et al. (2010) even showed a significant sequence similarity (56%) between SOCS2 orthologues from species as distantly related as E. sinensis and H. sapiens. These percentages were even higher when considering only the domains critical for the structure and the function of the protein.
2.1. SOCS proteins and their established immunomodulatory functions
So far, vertebrate SOCS protein functions have only been studied in mammals and bony fishes. In the following, we will focus on the established roles of mammalian SOCS proteins as immunomodulatory factors and their implication in the pathogenesis of infectious diseases (see Section 3 “Role of SOCS proteins during microbial infections”). The current knowledge on SOCS protein functions in bony fishes has recently been summarized in an excellent review article by Wang et al. (2011).
2.1.1. CIS
CIS is a 28.6 kDa protein (referring to the human prototype) which binds through its SH2 domain to phosphorylated tyrosine residues of activated cytokine receptors and does not seem to physically interact with JAKs (Alexander, 2002, Matsumoto et al., 1999). CIS inhibits cytokine signalling by blocking binding sites that are otherwise used to recruit and to activate STATs (Matsumoto et al., 1999). CIS is closely related to SOCS2; it inhibits the signal transduction of erythropoietin, IL2, IL3, growth hormone (GH) and prolactin, by binding the STAT5 sites of the respective receptor (Verdier et al., 1998, Yoshimura, 2005). Transgenic mice over-expressing CIS exhibited growth defects, impaired mammary gland development and reduced numbers of both natural killer (NK) and NKT cells similarly to STAT5a knock-out (KO) and/or STAT5b KO mice (Matsumoto et al., 1999). This phenotype is consistent with the notion that CIS plays a specific role in the regulation of the STAT5-mediated cytokine response (Matsumoto et al., 1999). However, mice lacking the CIS gene are phenotypically normal (Marine et al., 1999a).
CIS aa sequences of different mammalian species display a high degree of conservation especially when the two functional regions – SH2 domain and SOCS box – are considered (Fig. 3 ). The sequence of the SH2 domain is fully conserved between the mammalian species given in Fig. 3, except for the pig where the available aa sequence (ensemble.org ENSSSCG00000011414) appears to be truncated and divergent to the commonly described isoforms one and two. This sequence needs to be confirmed and rapid amplification of cDNA-ends by polymerase chain reaction using the conserved part of the sequence should provide insights. The SOCS boxes of bovine or canine CIS differ by two mismatches from those of primates and the mouse, which are virtually identical to one another. When comparing the aa sequences of mammalian and avian (chicken and turkey) CIS proteins, only slight differences are appreciated in the SH2 domain while their SOCS box sequences are more distantly related (Fig. 3).
Fig. 3.
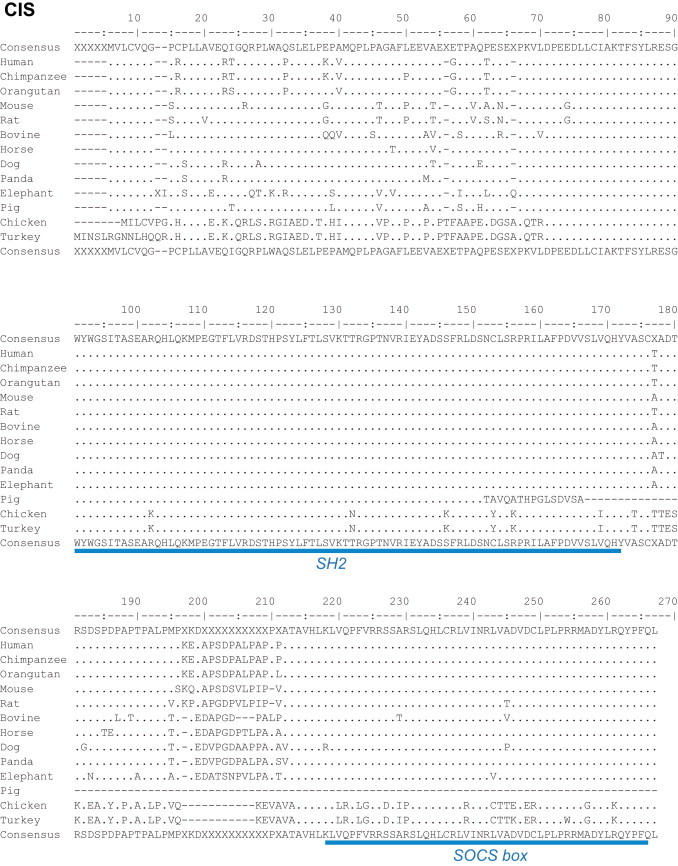
Alignments of CIS proteins from different mammalian and avian species. Consensus sequences are presented above and below the alignments. Below the bottom consensus sequence are indicated, in blue, the main regions of the CIS protein (SH2 and SOCS box). Alignments were performed using Emma and Showalign in the EMBOSS software suite.
It has been shown that variant alleles of multiple CIS polymorphisms impact the susceptibility to major infectious diseases in humans (Khor et al., 2010) suggesting an important role of CIS in immunity against various pathogens. It is likely that this holds true also in other mammals.
2.1.2. SOCS1
SOCS1 is a 23.4 kDa protein (human prototype) that can be induced by various factors acting on or through a signal transduction pathway which is either STATs-dependent or -independent. These factors include cytokines, insulin, LPS, and CpG DNA (Dalpke et al., 2001, Kawazoe et al., 2001, Stoiber et al., 1999). SOCS1 strongly regulates macrophage activation through the JAK/STAT and the TLR/NF-κB pathways (Ryo et al., 2003, Yoshimura et al., 2007). To suppress the TLR/NF-κB pathway, SOCS1 binds to the p65 subunit of NF-κB, thereby facilitating its ubiquitin-mediated proteolysis (Ryo et al., 2003). This mode of action is particularly important for the regulation of inflammatory processes, septic shock and innate/adaptive immune responses. SOCS1 can also block other signalling factors such as the insulin receptor substrate 1 (IRS1) and IL1 receptor-associated kinase (IRAK) (Fujimoto and Naka, 2003, Kawazoe et al., 2001). Moreover, SOCS1 may also inhibit the activity of IL4, IL6, and IFNγ (Fujimoto and Naka, 2003). Observations in mice lacking SOCS1 suggest that this protein plays a key role in the negative regulation of signalling initiated by IFNγ (Morita et al., 2000). SOCS1 also has role in the control of viral and parasitic infections by means of its capacity to modulate the sensitivity of cells to both IFN type I (IFNα and IFNβ) and type II (IFNγ) (Yoshimura et al., 2007, Zimmermann et al., 2006). SOCS1 (as well as SOCS2 and SOCS3) mRNA expression was found to be up-regulated after administration of another type I IFN, IFNτ, in the uterus of cyclic ewes, suggesting an additional role for SOCS proteins in the negative regulation of fertility-related IFNτ signalling in ruminant dams (Sandra et al., 2005). Finally, SOCS1 has a pivotal function in T lymphocyte development and regulation and is therefore highly expressed in the thymus throughout all thymocyte developmental stages (Marine et al., 1999b, Trop et al., 2001).
The SOCS1 aa sequence is highly conserved between different mammalian species (Fig. 4 ). For example, there is 100% identity between human and porcine SOCS1 aa sequences in all the major functional regions of the protein (KIR, ESS, SH2, and SOCS box). Unsurprisingly, sequence differences between mammal species are most frequently observed outside of the functional regions. More prominent aa sequence differences are observed between galliform birds (chicken and turkey) and mammals, especially regarding the length of the Poly-Ser region and the sequence of the SH2 domain (Fig. 4). The high level of identity between mammalian SOCS1 amino acid sequences suggests a conserved functional mechanism of SOCS1 activity in response to microbial infections. Despite fundamental differences in the aa sequences of mammalian and avian SOCS1, it appears as if a strong cytokine (IFN type I) regulatory activity of SOCS1 is also conserved in the chicken (S. Trapp, unpublished data).
Fig. 4.
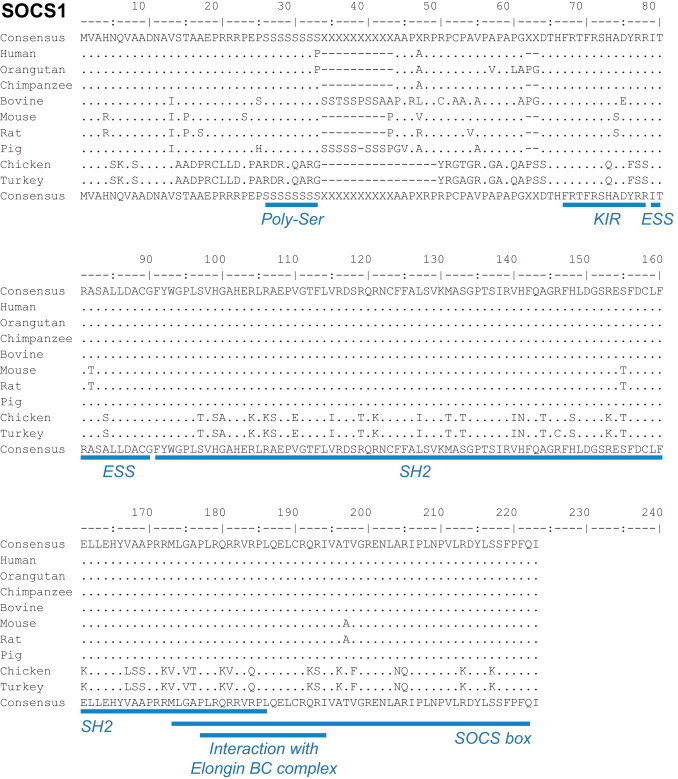
Alignments of SOCS1 proteins from different mammalian and avian species. Consensus sequences are presented above and below the alignments. Below the bottom consensus sequence are listed, in blue, the main regions of the SOCS1 protein (Poly-Ser, KIR, ESS, SH2, SOCS box and the region interacting with Elongin BC complex). Alignments were performed using Emma and Showalign in the EMBOSS software suite.
2.1.3. SOCS3
SOCS3, a 24.8 kDa protein (human prototype), is the second best characterized member of the SOCS family. Through its KIR domain, which is shared with SOCS1 (see Fig. 1), SOCS3 can block the catalytic site of JAKs as a pseudo substrate (Sasaki et al., 1999). In the presence of LPS and following TLR4 stimulation, SOCS3 expression is strongly induced in macrophages (Yasukawa et al., 2003a). SOCS3 is a key regulator of the divergent activities of pro-inflammatory cytokines such as IL6 and anti-inflammatory cytokines such as IL10. This differential regulation is achieved through its selective binding to the GP130 receptor of IL6, while it does not bind the GP130 receptor of IL10 (Yasukawa et al., 2003a). SOCS3 specifically binds the phosphorylated tyrosine 757 (pY757) of the GP130 receptor (Nicholson et al., 2000, Schmitz et al., 2000), which is also the target of the signalling factor SH2-domain containing protein tyrosine phosphatase 2 (SHP2) (Nicholson et al., 2000, Schmitz et al., 2000). SOCS3 also competes with SHP2 further downstream in the pathway (Alexander, 2002). In some acute or chronic pathological conditions, SOCS3 can also suppress inflammatory responses in which IL6 or other pro-inflammatory cytokines are involved. By reducing the expression of IL6 and IL23, SOCS3 acts as a negative regulator of Th17-cell differentiation, which is involved in inflammatory diseases (Chen et al., 2006).
The aa sequence of SOCS3 is highly conserved in mammals (Fig. 5 ). Remarkably, only a small number of differences are observed between mammalian species and the chicken (Fig. 5). This high degree of conservation suggests a common immunomodulatory role of SOCS3 in most vertebrate species. This assumption is also supported by a recent study on SOCS3 cytokine regulatory activities in the turbot (Scophthalmus maximus) (Zhang et al., 2011a). Therefore, it is likely that most of the observations made in the mouse model are also valid in other mammalian species, including farm and companion animals. Only recently, a link between nutritional Vitamin D deficiency, a down-regulation of SOCS3, and the onset of cardiac hypertrophy and inflammatory processes in epicardial adipose tissue has been established in a Yucatan mini pig model (Gupta et al., 2012). The lesions that developed in the mini pigs receiving a 12-month hypercholesterolemic diet deprived of Vitamin D were clearly associated with a decrease in the (mRNA/protein) expression of the Vitamin D receptor and SOCS3 in cardiomyocytes and epicardial adipose tissue (Gupta et al., 2012). This observation highlights the crucial function of SOCS3 in the development or prevention of pathological conditions in all mammalian species.
Fig. 5.
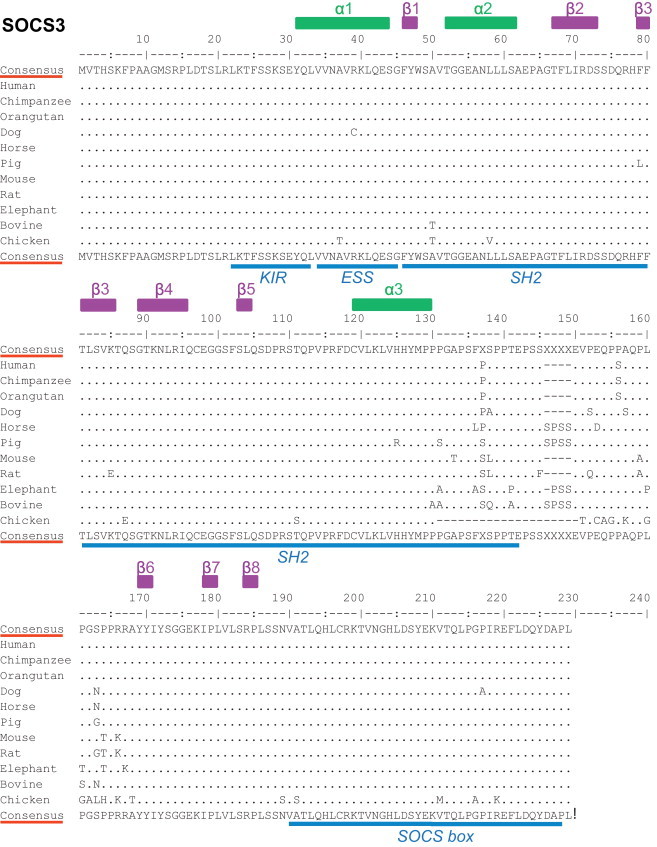
Alignments of SOCS3 proteins from different mammalian and avian species. Consensus sequences are presented above and below the alignments. Above the top consensus sequence are mentioned the positions of α helix (green) and β sheets (purple). Below the bottom consensus sequence are listed, in blue, the main regions of the SOCS3 protein (KIR, ESS, SH2, and SOCS box). Alignments were performed using Emma and Showalign in the EMBOSS software suite. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article).
2.1.4. Other SOCS family members
The mechanistically actions of the other SOCS proteins have been far less studied. SOCS2 is a 22 kDa protein (human prototype) that regulates the GH signalling pathway through binding the GH receptors and inhibiting the GH-mediated activation of STAT5b (Metcalf et al., 2000, Yoshimura et al., 2005). This is reflected by the observation that SOCS2-deficient mice were 30–40% heavier at 3 months of age, compared with control mice (Metcalf et al., 2000).
SOCS4 and SOCS5, with a size of 50.6 and 61.2 kDa, respectively (human prototypes) share distinct sequence homologies (Hilton et al., 1998). The central SH2 domains of SOCS4 and SOCS5 are virtually identical, while the N-terminal regions and the biological functions of these two proteins are less conserved (Hilton et al., 1998). It has been shown that hyper-methylation in the promoter region of SOCS4 in gastric cancer cells co-regulates other factors involved in tumor growth (Kobayashi et al., 2012).
Being highly expressed in lymphoid organs, SOCS5 appears to prevent Th2 differentiation in mice by inhibiting IL4 signalling (Seki et al., 2002, Yoshimura et al., 2005). It is expressed in Th1 cells, and interacts with the alpha chain of IL4 receptor, irrespective of tyrosine phosphorylation (Seki et al., 2002). By reducing IL4-induced activation of STAT6, this interaction efficiently inhibits Th2 polarization (Seki et al., 2002). Until today, several studies have now demonstrated an implication of SOCS5 and other SOCS proteins in the control of Th polarization and its relevance to disease development (Knosp and Johnston, 2012).
SOCS6, a 59.5 kDa protein (human prototype), binds to insulin receptors and inhibits the activation of ERK1/2, protein kinase B and IRS1 (Fujimoto and Naka, 2003, Mooney et al., 2001). SOCS6 can also bind to IRS2, IRS4 and the p85 subunit of PI3 kinase (Krebs et al., 2002) which are all involved in insulin signalling. SOCS6-deficient mice exhibit slight growth retardation (Krebs et al., 2002). SOCS7 (581 aa in size for the human prototype) is prominently expressed in the mouse brain where it seems to be of major importance. Indeed, mice lacking the gene coding for SOCS7 succumbed to the development of a hydrocephalus after 15 weeks of life (Krebs et al., 2004).
Some evidence suggests that SOCS proteins may also interfere with each-other and compete by triggering their mutual degradation. For example, SOCS2 appears to enhance the degradation of SOCS1 and SOCS3 and possibly CIS as well (Piessevaux et al., 2009). On the other hand, SOCS proteins can also exert synergistic effects that potentiate their different inhibitory activities (Palmer and Restifo, 2009).
3. Role of SOCS proteins during microbial infections
As mentioned earlier, cytokine signalling needs to be finely tuned through a tight control of signal transduction. Taking advantage of the key role of SOCS proteins in the immune responses regulation, a number of microorganisms have developed sophisticated strategies to hijack the SOCS system (Table 1 ) in order to inhibit immune defense-signalling pathways. Furthermore, it has been shown that immune evasion provided by SOCS functions within specific cell types is critical for determining the susceptibility of a cell to infection (Akhtar and Benveniste, 2011).
Table 1.
Microorganisms have developed multiple strategies to hijack the SOCS system in order to inhibit immune defense-signalling pathways.
| Microorganisms | SOCS proteins | Species involved | Functions |
|---|---|---|---|
| Bacteria | |||
| Chlamydia pneumoniae | SOCS1 | Mouse | SOCS1 is induced by infection in a STAT1 and IFNα/β-dependent manner and may protect the host from inflammatory disease (Yang et al., 2008) |
| Listeria monocytogenes | SOCS3 | Human | Decreases IFNγ production (Stoiber et al., 2001) |
| Lactobacillus rhamnosus | SOCS2/SOCS3 | Human | Inhibits JAK2 phosphorylation, alters p38-MAPK signalling (Lee et al., 2010, Latvala et al., 2011) |
| Mycobacterium spp. | SOCS1/SOCS3 | Human/mouse-cattle | Inhibits IL12 production by DCs, inhibits IFNγ signalling (Vazquez et al., 2006, Srivastava et al., 2009, Srivastava et al., 2011). Up-regulation of IL10 and SOCS3, prevent clearance? (Weiss et al., 2005) |
| Pasteurella multocida | SOCS1 | Human | Increases levels of tyrosine kinase JAK2, hyperactivity of JAK/STAT (Hildebrand et al., 2010) |
| Streptococcus thermophilus | SOCS2/SOCS3 | Human | Inhibits JAK2 phosphorylation, alters p38-MAPK signalling (Latvala et al., 2011) |
| Protozoa | |||
| Cryptosporidium parvum | CIS/SOCS4 | Human | Regulates STAT3–STAT6 phosphorylation, down regulates miR-98 and let-7 expression (Hu et al., 2009, Hu et al., 2010) |
| Entamoeba histolytica | SOCS2 | Pig | Potentially regulates IFNγ response (Bruel et al., 2010) |
| Leishmania donovani | SOCS3 | Human | Inhibits IFNγ signalling (Nandan and Reiner, 1995, Ray et al., 2000, Bertholet et al., 2003) |
| Leishmania major | SOCS1 | Mouse | Inhibits IFNγ signalling (Alexander et al., 1998, Bullen et al., 2003) |
| Toxoplasma gondii | CIS/SOCS1/SOCS3 | Mouse | Impairs macrophage activation by IFNγ, inhibits the up-regulation of MCH-II and ICAM1 and reduces iNOS induction, impairs IL12 production (Zimmermann et al., 2006, Mirpuri and Yarovinsky, 2012, Stutz et al., 2012) |
| Virus | |||
| Coxsackievirus | SOCS1/SOCS3 | Human | Impairs IFNβ and IFNγ, impairs CT-1 signalling through gp130 receptor (Yasukawa et al., 2003b, Yajima et al., 2006) |
| EBV | SOCS1/SOCS3 | Human | Alters NF-κB signal cascade and p38-MAPK signalling (Lo et al., 2006) |
| HBV | SOCS1/SOCS3 | Human | Suppression of STAT1, impairs IFNα signalling by suppression of STAT1 and blocking the TLR9/IRF-7 pathway (Xu et al., 2009, Koeberlein et al., 2010) |
| HCV | SOCS1/SOCS3/SOCS7 | Human | Regulates T and B cell functions, impairs production of IL12, inhibits phosphorylation and nuclear translocation of STAT1, degrades insulin receptor substrate 1 (Bode et al., 2003, Yao et al., 2008, Frazier et al., 2010, Pazienza et al., 2010, Ni et al., 2011, Zhang et al., 2011b) |
| HIV-1 | SOCS1/SOCS2/SOCS3 | Human | Impairs IFNγ signalling and IL12 production. Attenuates IFNβ signalling (Ryo et al., 2008, Cheng et al., 2009, Yadav et al., 2009, Akhtar et al., 2010, Miller et al., 2011) |
| HSV-1 | SOCS1/SOCS3 | Human/mouse | Inhibits IFNα, IFNβ, and IFNγ signalling (Sato et al., 2000, Yokota et al., 2001, Yokota et al., 2004, Yokota et al., 2005, Li et al., 2011) |
| IAV | SOCS1/SOCS3 | Human/mouse/pig | Inhibits IFNα and IFNβ signalling through RIG-1/MAVS/IFNAR1 pathway (Pothlichet et al., 2008, Jia et al., 2010, Huang et al., 2011). SOCS3 decreases susceptibility of pigs to H5N1 avian influenza virus (Nelli et al., 2012) |
| MuHV-4 | Viral SOCS-box (ORF73) | Mouse | Inhibition of NF-κB pathway (Rodrigues et al., 2009) |
| PRRSV | SOCS1 | Pig | Potentially regulates IFNγ response (Wysocki et al., 2012, Zhou et al., 2011) |
| RSV | CIS/SOCS1/SOCS3 | Human | Impairs type I and type II IFNs inhibiting STAT1 and STAT2 phosphorylation (Zhao et al., 2007, Moore et al., 2008) |
| SARS Co-V | SOCS3 | Human | Enhancement of IL6 signalling by a lower induction of SOCS3 and dysfunction of STAT3 (Okabayashi et al., 2006) |
| TBEV | SOCS1/SOCS3 | Mouse | Potentially limits cytokine response (Mansfield et al., 2010) |
| WNV | SOCS1/SOCS3 | Mouse | Potentially limits cytokine response (Mansfield et al., 2010) |
3.1. Bacteria
3.1.1. Mycobacterium spp.
Mycobacteria can drive and shape the immune response to establish a persistent host cell infection. The outcome of infection is determined by a critical balance between anti- and pro-inflammatory responses (Kirschner et al., 2010). During Mycobacterium spp. infections, pro-inflammatory cytokine signals production is regulated by dendritic cells (DCs) (Flynn and Chan, 2001). Compared to uninfected cells, human macrophages infected with Mycobacterium avium showed a reduced response to IFNγ with diminished phosphorylation of STAT1 (Vazquez et al., 2006). The expressions of SOCS3 and SOCS1 in the infected macrophages were elevated at both the mRNA and protein levels, an over-expression that was directly correlated with the unresponsiveness of the cells to IFNγ (Vazquez et al., 2006). The up-regulation of SOCS1 triggered by M. tuberculosis (Mtb) binding to DC-specific ICAM-3 grabbing non-integrin related 1 (DC-SIGNR1) protein in DCs and peripheral blood monocytes was also reported to be different in healthy and diseased patients (Srivastava et al., 2009). Patients with a productive form of tuberculosis showed an increased expression of SOCS1, which was reduced after chemotherapy (Srivastava et al., 2009). SOCS1 expression was also shown to result in a blockage of IL12 secretion by infected DCs during Mtb infection (Srivastava et al., 2009). In a further study, Srivastava et al. (2011) demonstrated that SOCS1 knock-down in murine T cells significantly improved the ability of Mtb-infected macrophages to clear the infection.
For farm animal species, it has been shown that monocytes obtained from dairy cows that were subclinically infected with M. avium subsp. paratuberculosis (MAP) had up-regulated expression levels of IL10 and SOCS3 within the first 2 h after exposure to bacteria (Weiss et al., 2005). Although the clinical relevance of this observation was not clear, it was postulated by the authors that an up-regulation of IL10 and SOCS3 might have prevented MAP clearance in the infected animals (Weiss et al., 2005).
3.1.2. Other bacteria
The Pasteurella multocida toxin can regulate the JAK/STAT pathway, in an oncogene-like fashion by hijacking host cell pathways and avoiding the host immune defenses. The induction of the STAT-dependent Pim-1 proto-oncogene and subsequent threonine phosphorylation of SOCS1 increases the protein levels of JAK2 (Hildebrand et al., 2010). This disequilibrium leads to a hyperactivity of the JAK/STAT pathway and potential cell transformation (Hildebrand et al., 2010). Similarly, another study has demonstrated a p38 MAPK dependent up-regulation of SOCS3 mRNA/protein levels in murine macrophages in response to continuous stimulation with Listeria monocytogenes that eventually resulted in a decreased IFNγ production (Stoiber et al., 2001). In a murine model of Chlamydia pneumoniae infection, a STAT1 and IFNα/β-dependent induction of SOCS1 has been observed (Yang et al., 2008). The induction of SOCS1 was linked to a moderate inflammatory response and RAG1−/−/SOCS1−/− mice died early after infection showing severe pulmonary inflammation suggesting a pivotal role of SOCS1 in the control of the host's response (Yang et al., 2008).
In another study aiming to explore how various probiotic or non-pathogenic bacteria strains can modulate the immune system, Latvala et al. (2011) showed that Lactobacillus rhamnosus and Streptococcus thermophilus can up-regulate SOCS3 gene expression in human macrophages, both directly via the p38-MAPK signalling pathway in the absence of protein synthesis, and indirectly via bacteria-induced IL10 production. Similarly, in a previous study designed to assess the anti-inflammatory effects of L. plantarum, L. rhamnosus and L. acidophilus against Helicobacter pylori-associated gastritis, the authors assigned a beneficial role to SOCS2 and SOCS3 in the control of the infection (Lee et al., 2010). In fact, the authors demonstrated that a co-culture of H. pylori and probiotic bacteria with a human gastric carcinoma cell line induced the expressions of SOCS2 and SOCS3 with subsequent anti-inflammatory effects through STAT1/STAT3 activation and JAK2 inactivation (Lee et al., 2010). To summarize, there is accumulating evidence that bacterial pathogens takes advantage of the powerful SOCS protein functions to modulate the immune and/or inflammatory response of the respective host. Importantly, this biological feature bears the potential to be targeted by pro- or metaphylactic treatments.
3.2. Parasites
3.2.1. Cryptosporidium parvum
Cryptosporidium parvum is a major cause of acute gastrointestinal diseases in a wide range of mammalian hosts (Petry et al., 2010). In man, the outcome of intestinal cryptosporidiosis largely depends on the immune status. In immuno-compromised patients, gastrointestinal symptoms progressively worsen until the patient's death (Colford et al., 1996). Despite the low tissue-invasive potential of C. parvum, both humoral and cell-mediated responses are activated by the host to control the invasion, the reproduction and/or the survival of the parasite (Deng et al., 2004, Kasper and Buzoni-Gatel, 2001). The constitutive expression of TLRs and intracellular Nod-like receptors by intestinal epithelial cells permit the recognition of the parasite and the immediate activation of the innate immune response (Petry et al., 2010). In a study aiming to decipher the regulation of TLR/NF-κB signalling in human cholangiocytes exposed to C. parvum, Hu et al. (2009) found that two small endogenous microRNAs, miR-98 and let-7, were involved in the regulation of CIS protein expression via translational suppression. C. parvum infection in turn increased CIS expression in a TLR4/MyD88 dependent manner and down-regulated both miR-98 and let-7 expression. Consequentially, this down-regulation resulted in a decrease of CIS translational suppression (Hu et al., 2009). Furthermore, the same group found that C. parvum induced the expression of SOCS4 in human biliary epithelial cells to regulate the phosphorylation of STAT3 and STAT6 (Hu et al., 2010).
3.2.2. Leishmania donovani
Caused by the protozoan Leishmania donovani, leishmaniasis threatens millions of people living in or traveling to tropical and subtropical regions (den Boer et al., 2011). L. donovani adopts an intracellular life-cycle, which allows it to escape the humoral antibody response. However, Leishmania parasites lack the machinery required for active cellular invasion, and therefore infections are preferentially restricted to macrophages (Rittig and Bogdan, 2000). Inside its host cell, L. donovani exists in a non-flagellated amastigote form and needs to protect itself from toxic antimicrobial molecules produced by activated macrophages (Bogdan et al., 1996). Infection with L. donovani promastigotes was shown to impair IFNγ mediated tyrosine phosphorylation and subsequently influence the JAK/STAT pathway in both human mononuclear phagocytes and human promonocytic U937 cells (Nandan and Reiner, 1995, Ray et al., 2000). In another study, it has been demonstrated that SOCS3 functions provide potential means for the suppression of macrophage activation during the initiation of the intracellular infection by down-regulating the expression of the IFNγRα chain (Bertholet et al., 2003). The obvious link between SOCS3 over-expression and IFNγRα down-regulation could be explained by the fact that SOCS3 can interact with members of the ubiquitinylation protein family and thus may target receptors to proteosomal degradation (Bertholet et al., 2003).
In agreement with these data, an earlier study demonstrated that killing of the closely related parasite L. major by SOCS1-knockout macrophages was improved upon IFNγ and LPS stimulation (Alexander et al., 1998). Moreover, mice possessing only one copy of the SOCS1 gene endured a worse clinical outcome with increased lesion severity and an overwhelming cytokine activity (Bullen et al., 2003).
3.2.3. Toxoplasma gondii and Entamoeba histolytica
Toxoplasma gondii, an obligate intracellular parasite from the phylum Apicomplexa (Munoz et al., 2011) invades nucleated cells of warm-blooded vertebrates, in which it multiplies. Its multiplication in both phagocytic and non-phagocytic cells induces the production of IL12 and a strong IFNγ cell-mediated immune response (Sacks and Sher, 2002). In macrophages, T. gondii has developed different mechanisms to inhibit both the primary macrophage activation and the antiparasitic actions of type II IFNs. One of these mechanisms is the inhibition of IFNγ signal transduction, as determined by the reduction of IFNγ-mediated MHC class II up-regulation and reduced expression of the inducible nitric oxide synthase (iNOS) (Luder et al., 2003, Luder et al., 2001). T. gondii suppresses the IFNγ-mediated activation of murine macrophages, through the induction of SOCS1 and CIS and subsequent impairment of STAT1 tyrosine phosphorylation (Zimmermann et al., 2006). It was further shown that a virulent genotype I strain of T. gondii inhibited the up-regulation of MHC-class II and ICAM1 after IFNγ stimulation and reduced the production NO radicals, by inducing both SOCS1 and CIS expression (Stutz et al., 2012). In the latter study, SOCS1 induction was dependent on p38-MAPK signalling, egr2 and egr1 transcription factors activity together with an active cell penetration. Only recently, a role for STAT3 and SOCS3 in the context of T. gondii infection has also been evidenced (Mirpuri and Yarovinsky, 2012). The authors showed here that an abolishment of SOCS3 feedback regulation of IL6 signalling resulted in higher susceptibility of the respective KO mice to T. gondii infections due to an impaired IL12 production by inflammatory cells (Mirpuri and Yarovinsky, 2012).
A recent study from the group of F. Meurens demonstrated an induction of SOCS2 mRNA in porcine intestinal cells that were co-cultured with the human parasite Entamoeba histolytica (Bruel et al., 2010). This gastro-intestinal parasite drives the T auxiliary response towards Th2 and/or Th17 orientations, which are less suitable to allow full recovery from the infection (Guo et al., 2008). The SOCS2 induction could be associated with a subsequent inhibition of the Th1 response consequently helping the parasite to better resist to the host's immune response.
3.3. Viruses
3.3.1. Hepatitis C virus
Early after their discovery, SOCS proteins revealed their capacity to inhibit the signal transduction of type I IFNs (Song and Shuai, 1998). Hepatitis C virus (HCV) was the first virus for which the exploitation of SOCS protein functions was demonstrated (Akhtar and Benveniste, 2011, Bode et al., 2003). The flavivirus infects hepatocytes and may eventually cause liver cirrhosis and the onset of hepatocellular carcinoma (Alter, 1997). With its capacity to establish persistent, lifelong infection, HCV has been identified as an effective combatant of the host antiviral immune response (Choo et al., 1989). Like many other RNA viruses, HCV has developed strategies to impair the host's antiviral type I IFN response (Raglow et al., 2011). In chronically HCV-infected patients, Th1 and cytotoxic responses are decreased in the liver and peripheral blood (Thimme et al., 2001). Some studies revealed functional interactions of the HCV core protein with gC1qR, a complement receptor (Frazier et al., 2010, Ni et al., 2011, Yao et al., 2008). Triggered by this interaction, HCV differentially regulates T and B lymphocyte functions through exploitation of programmed death-1 (PD-1) and SOCS1 functions (Frazier et al., 2010, Ni et al., 2011, Yao et al., 2008). The cross talk between SOCS1 and PD1 in human blood derived monocytes/macrophages was further shown to suppress expression of IL12 through the JAK/STAT pathway (Zhang et al., 2011b). The HCV core protein also induces expression of SOCS3 mRNA in human hepatoma cells and inhibits activation, tyrosine phosphorylation, and nuclear translocation of STAT1, thereby counteracting the antiviral activity of IFNs (Bode et al., 2003). Furthermore, it has been demonstrated that the HCV core protein (genotype 3a) can modulate the expression of SOCS7, which is involved in the development of insulin resistance (Pazienza et al., 2010). This expression appeared to be STAT3 independent and to be regulated by peroxisome proliferator-activated receptor gamma activity (Pazienza et al., 2010). In conclusion, HCV employs several strategies to escape the host's immune response by hijacking SOCS functions. Importantly, this effective immune evasion strategy may explain the non-responsiveness of a substantial number (20–50%) of HCV-infected patients to antiviral treatments with pegylated interferon (PEG-IFN).
3.3.2. Herpesviridae
Members of the Herpesviridae family have developed various mechanisms to escape host defenses. They can selectively block the synthesis and the functions of cellular factors, degrade cellular proteins, and abrogate signalling of the host immune response (Roizman and Taddeo, 2007). Herpesviruses can accomplish a lifelong infection by establishing latency upon primary infection (Roizman, 1996).
During primary infection, Herpes Simplex Virus type 1 (HSV-1) productively infects muco-epithelial cells of the respiratory or genital tract (Whitley and Roizman, 2001). Subsequently, the virus establishes latency in trigeminal or sacral ganglia until possible re-activation. Upon HSV-1 infection, IFNβ is transcriptionally activated following the stimulation of several pathogen recognition receptors and various signalling cascades including TLR3/IFN-regulatory factor 3 (IRF-3) and NF-κB pathways (Paludan et al., 2011) (Fig. 2). IFNβ then triggers the activity the of JAK/STAT pathway with a subsequent type I IFN and SOCS induction (Sato et al., 2000). One strategy used by HSV-1 to escape host defenses and increase its capacity to replicate and/or to persist in the host is the induction of SOCS3 expression, which in turn suppresses the signal transduction activated by IFNβ (Yokota et al., 2001, Yokota et al., 2005, Yokota et al., 2004) (Fig. 2). SOCS3 expression reaches maximal levels at 1–2 h post-HSV-1 infection, and its induction is determinant to allow robust viral replication during acute infection. Indeed, viral replication was found to be reduced in cells unable to express SOCS3 in response to HSV-1, or when SOCS3 expression was inhibited through addition of the JAK2 inhibitor WHI-P131 (Yokota et al., 2005). A similar mechanism of resistance to IFN involving the induction of SOCS protein expression was observed in a keratinocyte cell line treated with pJAK2, a peptide inhibitor of SOCS1. This cell line, originally refractory to IFNγ anti-herpesviral treatment, started to respond to the treatment after the application of a SOCS1 antagonist indicating that HSV-1 induction of SOCS1 is one of the mechanisms of its resistance to an IFNγ-induced antiviral state (Frey et al., 2009). More recently, IFNλ-induced suppression of SOCS1 was shown to diminish HSV-1 replication in astrocytes and neurons, highlighting the requirement of SOCS1 for HSV-1 replication in cells from the central nervous system (Li et al., 2011).
Epstein-Barr virus (EBV), a lymphotropic human gammaherpesvirus, is also able to induce SOCS1 and SOCS3 expression after activation of STAT and NF-κB signalling cascades in EBV-transformed nasopharyngeal epithelial cells (Lo et al., 2006). In addition, EBV latent infection induced the suppression of p38-MAPK activities (Lo et al., 2006). Altogether, these findings suggest that EBV can manipulate several anti-viral signalling pathways in a SOCS1/3-dependent fashion. Murid herpesvirus-4 (MuHV-4) was recently shown to inhibit NF-κB activity in latently infected centroblasts of the germinal centre (Rodrigues et al., 2009). Remarkably, this inhibition, which was critical for the establishment of the persistent infection, involved the action of an unconventional SOCS box motif present in an MuHV-4-encoded protein instead of an exploitation of endogenous SOCS protein functions, illustrating yet another viral strategy to employ SOCS-related activities (Rodrigues et al., 2009).
3.3.3. Human immunodeficiency virus
Human immunodeficiency virus (HIV) encodes 15 distinct proteins (Frankel and Young, 1998) among which are two regulatory proteins: transcriptional transactivator (Tat) and the regulator of virion gene expression (Rev) (Peterlin and Trono, 2003). The tropism of HIV for Th cells, macrophages, DCs and microgial cells is determined at the level of viral entry by the concomitant use of CD4 as a primary receptor and co-receptors that are both strain- and target-specific (Peterlin and Trono, 2003). In order to suppress antiviral innate immunity, the HIV Tat protein induces the expression of different members of the SOCS protein family (Akhtar et al., 2010, Miller et al., 2011, Ryo et al., 2008, Yadav et al., 2009). Tat was shown to induce SOCS3-mediated antagonism of IFNβ signalling in macrophages both upstream, at the level of STAT1 and STAT2 activation, and downstream, at the level of expression of the type I IFN effector proteins, dsRNA protein kinase and interferon-induced exonuclease IGS20 (Akhtar et al., 2010). In addition, Tat was shown to induce SOCS2 to subvert type II IFN signalling in human monocytes (Cheng et al., 2009). Strikingly, over-expression of SOCS1 mRNA was also observed in PBMCs after HIV infection (Miller et al., 2011, Ryo et al., 2008). Ryo et al. (2008) also showed that, by physically binding to HIV Gag and facilitating the intracellular trafficking and stability of this viral protein, SOCS1 acts as a crucial host factor for productive HIV replication.
3.3.4. Influenza A virus
Influenza viruses have developed several strategies to down-regulate type I IFN signalling. One of these involves NF-κB-dependent activation of SOCS3 expression, which negatively affects STAT phosphorylation (Pauli et al., 2008). Influenza virus infection activates anti-viral signalling primarily through retinoic acid-inducible gene I (RIG-I), an intracellular sensor of viral RNA genomes and replication (Kato et al., 2006). IAV was shown to induce, in human respiratory epithelial cells, the expression of SOCS1 and SOCS3, and both proteins seemed to differentially regulate type I IFN signalling (Pothlichet et al., 2008). The IAV-induced up-regulation of SOCS1 and SOCS3 appears to be TLR3-independent, but requires a RIG-I/mitochondrial antiviral signalling protein (MAVS)/IFNAR1-dependent pathway (Huang et al., 2011, Pothlichet et al., 2008). Pothlichet et al. (2008) proposed three potential mechanisms by which SOCS1 and SOCS3 may counteract anti-IAV signalling: (i) modulation of JAK activity, (ii) competition with STAT for binding to IFNAR1, and/or (iii) proteasomal degradation of SOCS-flagged antiviral cellular proteins including RIG/MAVS/IFNAR signalling elements.
The non-structural protein NS1 of an H5N1 avian influenza virus was shown to reduce the IFN-inducible phosphorylation of STAT proteins, resulting in decreased formation of downstream STAT/DNA complexes (Jia et al., 2010). NS1-mediated inhibition of IFN-inducible signalling involved a reduction of both IFNAR1 and IFNAR2 gene expression, which was likely responsible for the observed decrease in IFN-inducible STAT phosphorylation and DNA binding (Jia et al., 2010). Strikingly, NS1 expression also induced an up-regulation of SOCS1 and SOCS3 (Jia et al., 2010) leading to an efficient abrogation of type I IFN signalling. Very recently a study aiming at elucidating the mechanisms behind the differential susceptibility of pigs and humans to a highly pathogen strain of H5N1 avian influenza virus (humans developing cytokine storm or hypercytokinemia when infected while pigs had barely no symptoms) has shown a potential central role of SOCS3 in this difference of susceptibility between mammal hosts (Nelli et al., 2012). Authors concluded that SOCS3 in pig cells is able to confer resistance against the establishment of hypercytokinemia by moderating the pro-inflammatory response (Nelli et al., 2012).
3.3.5. Respiratory syncytial virus
Respiratory syncytial virus (RSV) is a paramyxovirus causing important respiratory pathologies in young children (Falsey and Walsh, 2000, Staat, 2002). RSV infects and replicates in the airway epithelium and in lung alveolar macrophages (Mellow et al., 2004, Wang et al., 2003). Infection of epithelial cells leads to the expression of host genes involved in the establishment of an interferon antiviral state. RSV infection has been shown to inhibit the type I IFN-JAK/STAT pathway (Ramaswamy et al., 2004). RSV nonstructural proteins NS1 and NS2 prevent the antiviral effect of type I IFN by specifically inhibiting the phosphorylation and activation of STAT2 (Bossert et al., 2003, Spann et al., 2004). A study using a type II alveolar cell line suggested an important role of SOCS1 in the regulation of the type I IFN response to RSV infection, highlighting the possibility that NS1/NS2 could in part mediate type I IFN antagonism through the induction of SOCS1 (Moore et al., 2008). In macrophage-like U937 cells, RSV also induced rapid expression of SOCS1, SOCS3 and CIS mRNA, which were associated with the inhibition of IFNα-induced STAT1 and STAT2 phosphorylations (Zhao et al., 2007).
3.3.6. Other viruses
Studies were conducted in various viral infection models to clarify the link between SOCS proteins and the inflammatory responses regulation during viral infection. Caco2 (human colorectal adenocarcinoma) cells infected with severe acute respiratory syndrome-coronavirus (SARS-CoV) were found to express lower levels of SOCS3 mRNAs than after RSV infection, along with higher levels of IL6 expression (Okabayashi et al., 2006). The reduction of SOCS3 expression in SARS-CoV infected Caco2 cells allowed prolonged and increased IL6-signalling when compared to RSV-infected, and may thus explain the increased severity of inflammation in SARS-CoV infections (Okabayashi et al., 2006).
Regarding the Picornaviridae family members, it was shown that increased levels of SOCS1 and SOCS3 expression in mice have severe effects on the development of coxsackievirus-mediated cardiac injury by favoring viral replication (Yasukawa et al., 2003b). Furthermore, SOCS3 was shown to prevent STAT3 phosphorylation in murine cardiomyocites infected with coxsackievirus B3, resulting in the impairment of the protection through CT-1 signalling via the gp130 receptor (Yajima et al., 2006). Next to HCV, other flaviviruses, namely the arthropod-borne viruses West Nile virus (WNV) and tick-borne encephalitis virus (TBEV), were shown to up-regulate SOCS1 and SOCS3 expression, notably in brain tissues from experimentally infected mice (Mansfield et al., 2010). Here, SOCS1 and SOCS3 may play a role in the pathogenesis of flavivrus induced encephalitis by temporarily preventing neurotoxic cytokine signalling and eventually favoring viral spread and the onset severe neurological disorders (Mansfield et al., 2010).
Hepatitis B virus (HBV) was also described to induce the expression of SOCS proteins (Bock et al., 2008, Ko et al., 2008). SOCS3 over-expression, detected in liver biopsies of chronically HBV-infected patients, was accompanied by a significant suppression of STAT1 (Koeberlein et al., 2010). Despite the over-expression of SOCS3, the same authors also observed a constitutive activation of STAT3 that clearly contributed to the development of severe inflammatory liver diseases (Koeberlein et al., 2010). Another study, in which plasmacytoid DCs were treated with the HBV surface antigen (HBsAg), showed that HBsAg also impairs IFNα signalling by blocking the TLR9/IRF-7/IFNα pathway through an up-regulation of SOCS1 (Xu et al., 2009).
Recently an induction of SOCS1 in response to porcine reproductive and respiratory syndrome virus (PRRSV) infection of porcine alveolar macrophages and lung tissue has been observed suggesting also a role for SOCS in PRRSV infection (Wysocki et al., 2012, Zhou et al., 2011).
Collectively, these studies show that numerous phylogenetically distinct viruses take advantage of SOCS protein functions to warrant sufficient replication in their respective hosts, and it seems likely that this strategy is also adopted by a wide array of veterinary viruses, notably those that lack the coding capacities to harbor various pathogenicity factors in their genomes.
4. SOCS proteins as therapeutic targets
Given the close relationships of SOCS proteins with several infectious agents, the manipulation of SOCS functions might be useful to set up new pro- or metaphylactic approaches for the prevention and control of infectious and inflammatory diseases. Three strategies involving SOCS proteins have been proposed in the literature such as: (i) over-expression of SOCS proteins, (ii) small-molecule antagonists of SOCS signalling, and (iii) down-regulation of SOCS gene expression (Yoshimura et al., 2007).
In the first approach, exogenous expression of SOCS1 using viral vectors has been successfully used to limit host inflammatory response (Mahller et al., 2008, Sakurai et al., 2008). In one of these studies using mice with experimentally-induced arthritis, periarticular injection of a recombinant adenovirus carrying the SOCS3 cDNA dramatically reduced the severity of arthritis and joint swelling compared with control groups (Shouda et al., 2001).
The second approach is based on the development and the use of small-molecule SOCS antagonists. Waiboci et al. (2007) have designed a peptide mimicking the phosphorylated JAK2 activation loop (synthetic peptide pJAK2[1001–1013], LPQDKEYYKVKEP) that exhibits anti-infectious activity (Lucet et al., 2006). Peptide pJAK2[1001–1013] increased IFNγ activity and activation site promoter activity, blocked SOCS1 induced inhibition of STAT3 phosphorylation in IL6-treated cells, and enhanced Ag-specific proliferation (Waiboci et al., 2007). In another study, Frey et al. (2009), in an effort to find a therapeutic alternative to conventional HSV-1 drug treatments, evaluated the synergy between IFNγ and peptide pJAK2[1001–1013] in keratinocytes infected with HSV-1. The competition for SOCS1 binding between pJAK2[1001–1013] and its natural counterpart induced a strong antiviral state against HSV-1 in a dose-dependent manner. In a follow-up study, the same peptide was shown to inhibit the replication of vaccinia virus and encephalomyocarditis virus in cultured cells, and to protect mice challenged with a lethal dose of both viruses (Ahmed et al., 2010).
Using the third approach, Song et al. (2006) showed that SOCS1-silencing in DCs by siRNAs allowed these cells to better evoke anti-HIV-1 antibody and T cell responses in mice. Knock-down of SOCS1 also dramatically enhanced the ability to generate HIV-1-envelope-specific memory T cell and B cell responses (Song et al., 2006). Furthermore, in order to improve therapeutic and prophylactic vaccine efficiencies, a co-immunization approach with vectors encoding HIV gp140CF and SOCS1-specific siRNA was applied. The authors found that SOCS1-silencing enhanced the effectiveness of DNA vaccination as evidenced by an increased production of gp120-specific antibodies, and better CTL and CD4+ T cell responses in immunized mice (Song et al., 2006). Moreover, Subramanya et al. (2010) reported that targeting the SOCS1 siRNA to DCs through their linkage to a DC-specific fusion peptide enhanced the induction of co-stimulatory molecules and DC production of cytokines in HIV-infected individuals. The SOCS1-silenced DCs stimulated primary CD8+ T cell responses against various antigens in vitro (Subramanya et al., 2010). A similar approach was tested to improve anti-tumor therapy strategies (Shen et al., 2004). In this study, the authors showed that a vaccination protocol using SOCS1-silenced DCs strongly increased antigen-specific, anti-tumor immunity (Shen et al., 2004). SOCS1 silencing probably allowed antigen-presenting immunogenic DCs to persistently stimulate antigen-specific T cells in the murine vaccine recipients (Shen et al., 2004). The authors of this study postulated an inactivation of T regulatory cells by the SOCS1 silenced DCs through an enhancement of DC maturation and the production of pro-inflammatory cytokines (Shen et al., 2004). Only recently, it has also been demonstrated that treatment with IL7 could improve the immune response to persistent infections caused by HIV, HBV and HCV by targeting SOCS3 (Pellegrini et al., 2011). While SOCS3 impaired T cell functions and promoted T cell exhaustion favoring viral persistence, IL7 treatment decreased the amount of SOCS3 within T cells and enabled early virus clearance (Pellegrini et al., 2011, Rincon et al., 2007). For the future, it would be interesting to further evaluate the experimental approach of down-regulating SOCS in order to improve the host's response to antimicrobial vaccines. This approach would be particularly relevant in large animal species which have been demonstrated as valuable models for the study of human infectious diseases, such as the pig (flu, tuberculosis and chlamydiosis) (Meurens et al., 2012) and cattle (RSV infections).
5. Conclusion
SOCS proteins are key regulators of the host immune response, and their functions are hijacked by several pathogens in order to promote their survival and/or increase their propagation. There is no doubt that in the near future, investigators will discover additional roles for SOCS proteins and further implications of these fascinating proteins in the pathogenesis of other infectious diseases. Further studies on SOCS proteins are particularly required in animal species, notably in farm and companion animals, because of the paucity of data outside the field of human health. Due to their central regulatory role, SOCS proteins also appear as ideal targets for the development of novel therapeutic approaches and vaccines both in animal and human health.
References
- Ahmed C.M., Dabelic R., Martin J.P., Jager L.D., Haider S.M., Johnson H.M. Enhancement of antiviral immunity by small molecule antagonist of suppressor of cytokine signaling. J. Immunol. 2010;185:1103–1113. doi: 10.4049/jimmunol.0902895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar L.N., Benveniste E.N. Viral exploitation of host SOCS protein functions. J. Virol. 2011;85:1912–1921. doi: 10.1128/JVI.01857-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar L.N., Qin H., Muldowney M.T., Yanagisawa L.L., Kutsch O., Clements J.E., Benveniste E.N. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J. Immunol. 2010;185:2393–2404. doi: 10.4049/jimmunol.0903563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J., Coombs G.H., Mottram J.C. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 1998;161:6794–6801. [PubMed] [Google Scholar]
- Alexander W.S. Suppressors of cytokine signalling (SOCS) in the immune system. Nat. Rev. Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- Alter M.J. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- Bertholet S., Dickensheets H.L., Sheikh F., Gam A.A., Donnelly R.P., Kenney R.T. Leishmania donovani-induced expression of suppressor of cytokine signaling 3 in human macrophages: a novel mechanism for intracellular parasite suppression of activation. Infect. Immun. 2003;71:2095–2101. doi: 10.1128/IAI.71.4.2095-2101.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C.T., Toan N.L., Koeberlein B., Song le H., Chin R., Zentgraf H., Kandolf R., Torresi J. Subcellular mislocalization of mutant hepatitis B X proteins contributes to modulation of STAT/SOCS signaling in hepatocellular carcinoma. Intervirology. 2008;51:432–443. doi: 10.1159/000209672. [DOI] [PubMed] [Google Scholar]
- Bode J.G., Ludwig S., Ehrhardt C., Albrecht U., Erhardt A., Schaper F., Heinrich P.C., Haussinger D. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003;17:488–490. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Gessner A., Solbach W., Rollinghoff M. Invasion, control and persistence of Leishmania parasites. Curr. Opin. Immunol. 1996;8:517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- Bossert B., Marozin S., Conzelmann K.K. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol. 2003;77:8661–8668. doi: 10.1128/JVI.77.16.8661-8668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel T., Guibon R., Melo S., Guillen N., Salmon H., Girard-Misguich F., Meurens F. Epithelial induction of porcine suppressor of cytokine signaling 2 (SOCS2) gene expression in response to Entamoeba histolytica. Dev. Comp. Immunol. 2010;34:562–571. doi: 10.1016/j.dci.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Bullen D.V., Baldwin T.M., Curtis J.M., Alexander W.S., Handman E. Persistence of lesions in suppressor of cytokine signaling-1-deficient mice infected with Leishmania major. J. Immunol. 2003;170:4267–4272. doi: 10.4049/jimmunol.170.8.4267. [DOI] [PubMed] [Google Scholar]
- Bullock A.N., Rodriguez M.C., Debreczeni J.E., Songyang Z., Knapp S. Structure of the SOCS4-ElonginB/C complex reveals a distinct SOCS box interface and the molecular basis for SOCS-dependent EGFR degradation. Structure. 2007;15:1493–1504. doi: 10.1016/j.str.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J.H., Lillehoj H.S., Lamont S.J. Reduced nitric oxide production and iNOS mRNA expression in IFN-gamma-stimulated chicken macrophages transfected with iNOS siRNAs. Vet. Immunol. Immunopathol. 2008;125:375–380. doi: 10.1016/j.vetimm.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.M., Tato C., Yoshimura A., Hennighausen L., O'Shea J.J. Selective regulatory function of SOCS3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.M., Li J.C., Lin S.S., Lee D.C., Liu L., Chen Z., Lau A.S. HIV-1 transactivator protein induction of suppressor of cytokine signaling-2 contributes to dysregulation of IFN{gamma} signaling. Blood. 2009;113:5192–5201. doi: 10.1182/blood-2008-10-183525. [DOI] [PubMed] [Google Scholar]
- Choo Q.L., Kuo G., Weiner A.J., Overby L.R., Bradley D.W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Colford J.M., Jr., Tager I.B., Hirozawa A.M., Lemp G.F., Aragon T., Petersen C. Cryptosporidiosis among patients infected with human immunodeficiency virus. Factors related to symptomatic infection and survival. Am. J. Epidemiol. 1996;144:807–816. doi: 10.1093/oxfordjournals.aje.a009015. [DOI] [PubMed] [Google Scholar]
- Dalpke A., Heeg K., Bartz H., Baetz A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213:225–235. doi: 10.1016/j.imbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Dalpke A.H., Opper S., Zimmermann S., Heeg K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J. Immunol. 2001;166:7082–7089. doi: 10.4049/jimmunol.166.12.7082. [DOI] [PubMed] [Google Scholar]
- Darnell J.E., Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Delgado-Ortega M., Melo S., Meurens F. Expression of SOCS1-7 and CIS mRNA in porcine tissues. Vet. Immunol. Immunopathol. 2011;144:493–498. doi: 10.1016/j.vetimm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- den Boer M., Argaw D., Jannin J., Alvar J. Leishmaniasis impact and treatment access. Clin. Microbiol. Infect. 2011;17:1471–1477. doi: 10.1111/j.1469-0691.2011.03635.x. [DOI] [PubMed] [Google Scholar]
- Deng M., Rutherford M.S., Abrahamsen M.S. Host intestinal epithelial response to Cryptosporidium parvum. Adv. Drug Deliv. Rev. 2004;56:869–884. doi: 10.1016/j.addr.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Endo T.A., Masuhara M., Yokouchi M., Suzuki R., Sakamoto H., Mitsui K., Matsumoto A., Tanimura S., Ohtsubo M., Misawa H., Miyazaki T., Leonor N., Taniguchi T., Fujita T., Kanakura Y., Komiya S., Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Walsh E.E. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.L., Chan J. Tuberculosis: latency and reactivation. Infect. Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A.D., Young J.A. HIV-1: fifteen proteins and an RNA. Annu. Rev. Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- Frazier A.D., Zhang C.L., Ni L., Ma C.J., Zhang Y., Wu X.Y., Atia A.N., Yao Z.Q., Moorman J.P. Programmed death-1 affects suppressor of cytokine signaling-1 expression in T cells during hepatitis C infection. Viral Immunol. 2010;23:487–495. doi: 10.1089/vim.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey K.G., Ahmed C.M., Dabelic R., Jager L.D., Noon-Song E.N., Haider S.M., Johnson H.M., Bigley N.J. HSV-1-induced SOCS-1 expression in keratinocytes: use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J. Immunol. 2009;183:1253–1262. doi: 10.4049/jimmunol.0900570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobose H., Ronn S.G., Heding P.E., Mendoza H., Cohen P., Mandrup-Poulsen T., Billestrup N. Suppressor of cytokine signaling-3 inhibits interleukin-1 signaling by targeting the TRAF-6/TAK1 complex. Mol. Endocrinol. 2006;20:1587–1596. doi: 10.1210/me.2005-0301. [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Naka T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003;24:659–666. doi: 10.1016/j.it.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Guo X., Stroup S.E., Houpt E.R. Persistence of Entamoeba histolytica infection in CBA mice owes to intestinal IL-4 production and inhibition of protective IFN-gamma. Mucosal Immunol. 2008;1:139–146. doi: 10.1038/mi.2007.18. [DOI] [PubMed] [Google Scholar]
- Gupta G.K., Agrawal T., Delcore M.G., Mohiuddin S.M., Agrawal D.K. Vitamin D deficiency induces cardiac hypertrophy and inflammation in epicardial adipose tissue in hypercholesterolemic swine. Exp. Mol. Pathol. 2012;93:82–90. doi: 10.1016/j.yexmp.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan S., Ferguson P., Sommer U., Hiremath M., McVicar D.W., Heinrich P.C., Johnston J.A., Cacalano N.A. Tyrosine phosphorylation disrupts elongin interaction and accelerates SOCS3 degradation. J. Biol. Chem. 2003;278:31972–31979. doi: 10.1074/jbc.M303170200. [DOI] [PubMed] [Google Scholar]
- He F., Stephens J.M. Induction of SOCS-3 is insufficient to confer IRS-1 protein degradation in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2006;344:95–98. doi: 10.1016/j.bbrc.2006.03.142. [DOI] [PubMed] [Google Scholar]
- Hildebrand D., Walker P., Dalpke A., Heeg K., Kubatzky K.F. Pasteurella multocida Toxin-induced Pim-1 expression disrupts suppressor of cytokine signalling (SOCS)-1 activity. Cell. Microbiol. 2010;12:1732–1745. doi: 10.1111/j.1462-5822.2010.01504.x. [DOI] [PubMed] [Google Scholar]
- Hilton D.J., Richardson R.T., Alexander W.S., Viney E.M., Willson T.A., Sprigg N.S., Starr R., Nicholson S.E., Metcalf D., Nicola N.A. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc. Natl. Acad. Sci. U. S. A. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Zhou R., Liu J., Gong A.Y., Chen X.M. MicroRNA-98 and let-7 regulate expression of suppressor of cytokine signaling 4 in biliary epithelial cells in response to Cryptosporidium parvum infection. J. Infect. Dis. 2010;202:125–135. doi: 10.1086/653212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Zhou R., Liu J., Gong A.Y., Eischeid A.N., Dittman J.W., Chen X.M. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J. Immunol. 2009;183:1617–1624. doi: 10.4049/jimmunol.0804362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zaas A.K., Rao A., Dobigeon N., Woolf P.J., Veldman T., Oien N.C., McClain M.T., Varkey J.B., Nicholson B., Carin L., Kingsmore S., Woods C.W., Ginsburg G.S., Hero A.O., 3rd Temporal dynamics of host molecular responses differentiate symptomatic and asymptomatic influenza a infection. PLoS Genet. 2011;7:e1002234. doi: 10.1371/journal.pgen.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D., Rahbar R., Chan R.W., Lee S.M., Chan M.C., Wang B.X., Baker D.P., Sun B., Peiris J.S., Nicholls J.M., Fish E.N. Influenza virus non-structural protein 1 (NS1) disrupts interferon signaling. PLoS ONE. 2010;5:e13927. doi: 10.1371/journal.pone.0013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamizono S., Hanada T., Yasukawa H., Minoguchi S., Kato R., Minoguchi M., Hattori K., Hatakeyama S., Yada M., Morita S., Kitamura T., Kato H., Nakayama K., Yoshimura A. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J. Biol. Chem. 2001;276:12530–12538. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- Kamura T., Maenaka K., Kotoshiba S., Matsumoto M., Kohda D., Conaway R.C., Conaway J.W., Nakayama K.I. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18:3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L.H., Buzoni-Gatel D. Ups and downs of mucosal cellular immunity against protozoan parasites. Infect. Immun. 2001;69:1–8. doi: 10.1128/IAI.69.1.1-8.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J., Yamaguchi O., Otsu K., Tsujimura T., Koh C.S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawazoe Y., Naka T., Fujimoto M., Kohzaki H., Morita Y., Narazaki M., Okumura K., Saitoh H., Nakagawa R., Uchiyama Y., Akira S., Kishimoto T. Signal transducer and activator of transcription (STAT)-induced STAT inhibitor 1 (SSI-1)/suppressor of cytokine signaling 1 (SOCS1) inhibits insulin signal transduction pathway through modulating insulin receptor substrate 1 (IRS-1) phosphorylation. J. Exp. Med. 2001;193:263–269. doi: 10.1084/jem.193.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor C.C., Vannberg F.O., Chapman S.J., Guo H., Wong S.H., Walley A.J., Vukcevic D., Rautanen A., Mills T.C., Chang K.C., Kam K.M., Crampin A.C., Ngwira B., Leung C.C., Tam C.M., Chan C.Y., Sung J.J., Yew W.W., Toh K.Y., Tay S.K., Kwiatkowski D., Lienhardt C., Hien T.T., Day N.P., Peshu N., Marsh K., Maitland K., Scott J.A., Williams T.N., Berkley J.A., Floyd S., Tang N.L., Fine P.E., Goh D.L., Hill A.V. CISH and susceptibility to infectious diseases. N. Engl. J. Med. 2010;362:2092–2101. doi: 10.1056/NEJMoa0905606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner D.E., Young D., Flynn J.L. Tuberculosis: global approaches to a global disease. Curr. Opin. Biotechnol. 2010;21:524–531. doi: 10.1016/j.copbio.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knosp C.A., Johnston J.A. Regulation of CD4+ T-cell polarization by suppressor of cytokine signalling proteins. Immunology. 2012;135:101–111. doi: 10.1111/j.1365-2567.2011.03520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E., Kim S.J., Joh J.W., Park C.K., Park J., Kim D.H. CpG island hypermethylation of SOCS-1 gene is inversely associated with HBV infection in hepatocellular carcinoma. Cancer Lett. 2008;271:240–250. doi: 10.1016/j.canlet.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Kobayashi D., Nomoto S., Kodera Y., Fujiwara M., Koike M., Nakayama G., Ohashi N., Nakao A. Suppressor of cytokine signaling 4 detected as a novel gastric cancer suppressor gene using double combination array analysis. World J. Surg. 2012;36:362–372. doi: 10.1007/s00268-011-1358-2. [DOI] [PubMed] [Google Scholar]
- Koeberlein B., zur Hausen A., Bektas N., Zentgraf H., Chin R., Nguyen L.T., Kandolf R., Torresi J., Bock C.T. Hepatitis B virus overexpresses suppressor of cytokine signaling-3 (SOCS3) thereby contributing to severity of inflammation in the liver. Virus Res. 2010;148:51–59. doi: 10.1016/j.virusres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Krebs D.L., Metcalf D., Merson T.D., Voss A.K., Thomas T., Zhang J.G., Rakar S., O’Bryan M.K., Willson T.A., Viney E.M., Mielke L.A., Nicola N.A., Hilton D.J., Alexander W.S. Development of hydrocephalus in mice lacking SOCS7. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15446–15451. doi: 10.1073/pnas.0406870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs D.L., Uren R.T., Metcalf D., Rakar S., Zhang J.G., Starr R., De Souza D.P., Hanzinikolas K., Eyles J., Connolly L.M., Simpson R.J., Nicola N.A., Nicholson S.E., Baca M., Hilton D.J., Alexander W.S. SOCS-6 binds to insulin receptor substrate 4, and mice lacking the SOCS-6 gene exhibit mild growth retardation. Mol. Cell. Biol. 2002;22:4567–4578. doi: 10.1128/MCB.22.13.4567-4578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M., Hanada T., Yoshimura A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- Latvala S., Miettinen M., Kekkonen R.A., Korpela R., Julkunen I. Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin. Exp. Immunol. 2011;165:94–103. doi: 10.1111/j.1365-2249.2011.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Paek N.S., Kwon O.S., Hahm K.B. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: a novel mechanism. J. Gastroenterol. Hepatol. 2010;25:194–202. doi: 10.1111/j.1440-1746.2009.06127.x. [DOI] [PubMed] [Google Scholar]
- Levy D.E., Darnell J.E., Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Li J., Hu S., Zhou L., Ye L., Wang X., Ho J., Ho W. Interferon lambda inhibits herpes simplex virus type I infection of human astrocytes and neurons. Glia. 2011;59:58–67. doi: 10.1002/glia.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.D., Gaffen S.L., Goldsmith M.A. JAK/STAT signaling by cytokine receptors. Curr. Opin. Immunol. 1998;10:271–278. doi: 10.1016/s0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- Lo A.K., Lo K.W., Tsao S.W., Wong H.L., Hui J.W., To K.F., Hayward D.S., Chui Y.L., Lau Y.L., Takada K., Huang D.P. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia. 2006;8:173–180. doi: 10.1593/neo.05625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucet I.S., Fantino E., Styles M., Bamert R., Patel O., Broughton S.E., Walter M., Burns C.J., Treutlein H., Wilks A.F., Rossjohn J. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107:176–183. doi: 10.1182/blood-2005-06-2413. [DOI] [PubMed] [Google Scholar]
- Luder C.G., Algner M., Lang C., Bleicher N., Gross U. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int. J. Parasitol. 2003;33:833–844. doi: 10.1016/s0020-7519(03)00092-4. [DOI] [PubMed] [Google Scholar]
- Luder C.G., Walter W., Beuerle B., Maeurer M.J., Gross U. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1alpha. Eur. J. Immunol. 2001;31:1475–1484. doi: 10.1002/1521-4141(200105)31:5<1475::AID-IMMU1475>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mahller Y.Y., Sakthivel B., Baird W.H., Aronow B.J., Hsu Y.H., Cripe T.P., Mehrian-Shai R. Molecular analysis of human cancer cells infected by an oncolytic HSV-1 reveals multiple upregulated cellular genes and a role for SOCS1 in virus replication. Cancer Gene Ther. 2008;15:733–741. doi: 10.1038/cgt.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K.L., Johnson N., Cosby S.L., Solomon T., Fooks A.R. Transcriptional upregulation of SOCS 1 and suppressors of cytokine signaling 3 mRNA in the absence of suppressors of cytokine signaling 2 mRNA after infection with West Nile virus or tick-borne encephalitis virus. Vector Borne Zoonotic Dis. 2010;10:649–653. doi: 10.1089/vbz.2009.0259. [DOI] [PubMed] [Google Scholar]
- Marine J.C., McKay C., Wang D., Topham D.J., Parganas E., Nakajima H., Pendeville H., Yasukawa H., Sasaki A., Yoshimura A., Ihle J.N. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell. 1999;98:617–627. doi: 10.1016/s0092-8674(00)80049-5. [DOI] [PubMed] [Google Scholar]
- Marine J.C., Topham D.J., McKay C., Wang D., Parganas E., Stravopodis D., Yoshimura A., Ihle J.N. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Seki Y., Kubo M., Ohtsuka S., Suzuki A., Hayashi I., Tsuji K., Nakahata T., Okabe M., Yamada S., Yoshimura A. Suppression of STAT5 functions in liver, mammary glands, and T cells in cytokine-inducible SH2-containing protein 1 transgenic mice. Mol. Cell. Biol. 1999;19:6396–6407. doi: 10.1128/mcb.19.9.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellow T.E., Murphy P.C., Carson J.L., Noah T.L., Zhang L., Pickles R.J. The effect of respiratory synctial virus on chemokine release by differentiated airway epithelium. Exp. Lung Res. 2004;30:43–57. doi: 10.1080/01902140490252812. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Greenhalgh C.J., Viney E., Willson T.A., Starr R., Nicola N.A., Hilton D.J., Alexander W.S. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature. 2000;405:1069–1073. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- Meurens F., Summerfield A., Nauwynck H., Saif L., Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.C., Schlaepfer E., Baenziger S., Crameri R., Zeller S., Byland R., Audige A., Nadal D., Speck R.F. HIV interferes with SOCS-1 and -3 expression levels driving immune activation. Eur. J. Immunol. 2011;41:1058–1069. doi: 10.1002/eji.201041198. [DOI] [PubMed] [Google Scholar]
- Mirpuri J., Yarovinsky F. IL-6 signaling SOCS critical for IL-12 host response to Toxoplasma gondii. Future Microbiol. 2012;7:13–16. doi: 10.2217/fmb.11.147. [DOI] [PubMed] [Google Scholar]
- Mooney R.A., Senn J., Cameron S., Inamdar N., Boivin L.M., Shang Y., Furlanetto R.W. Suppressors of cytokine signaling-1 and -6 associate with and inhibit the insulin receptor. A potential mechanism for cytokine-mediated insulin resistance. J. Biol. Chem. 2001;276:25889–25893. doi: 10.1074/jbc.M010579200. [DOI] [PubMed] [Google Scholar]
- Moore E.C., Barber J., Tripp R.A. Respiratory syncytial virus (RSV) attachment and nonstructural proteins modify the type I interferon response associated with suppressor of cytokine signaling (SOCS) proteins and IFN-stimulated gene-15 (ISG15) Virol. J. 2008;5:116. doi: 10.1186/1743-422X-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Naka T., Kawazoe Y., Fujimoto M., Narazaki M., Nakagawa R., Fukuyama H., Nagata S., Kishimoto T. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor alpha-induced cell death in fibroblasts. Proc Natl Acad Sci U S A. 2000;97:5405–5410. doi: 10.1073/pnas.090084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M., Liesenfeld O., Heimesaat M.M. Immunology of Toxoplasma gondii. Immunol. Rev. 2011;240:269–285. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- Naka T., Narazaki M., Hirata M., Matsumoto T., Minamoto S., Aono A., Nishimoto N., Kajita T., Taga T., Yoshizaki K., Akira S., Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- Nandan D., Reiner N.E. Attenuation of gamma interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: selective inhibition of signaling through Janus kinases and Stat1. Infect. Immun. 1995;63:4495–4500. doi: 10.1128/iai.63.11.4495-4500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelli R.K., Dunham S.P., Kuchipudi S.V., White G.A., Baquero-Perez B., Chang P., Ghaemmaghami A., Brookes S.M., Brown I.H., Chang K.C. Mammalian innate resistance to highly pathogenic avian influenza H5N1 virus infection is mediated through reduced proinflammation and infectious virus release. J. Virol. 2012;86:9201–9210. doi: 10.1128/JVI.00244-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Ma C.J., Zhang Y., Nandakumar S., Zhang C.L., Wu X.Y., Borthwick T., Hamati A., Chen X.Y., Kumaraguru U., Moorman J.P., Yao Z.Q. PD-1 modulates regulatory T cells and suppresses T-cell responses in HCV-associated lymphoma. Immunol. Cell Biol. 2011;89:535–539. doi: 10.1038/icb.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S.E., De Souza D., Fabri L.J., Corbin J., Willson T.A., Zhang J.G., Silva A., Asimakis M., Farley A., Nash A.D., Metcalf D., Hilton D.J., Nicola N.A., Baca M. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi T., Kariwa H., Yokota S., Iki S., Indoh T., Yokosawa N., Takashima I., Tsutsumi H., Fujii N. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J. Med. Virol. 2006;78:417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D.C., Restifo N.P. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009;30:592–602. doi: 10.1016/j.it.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan S.R., Bowie A.G., Horan K.A., Fitzgerald K.A. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 2011;11:143–154. doi: 10.1038/nri2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli E.K., Schmolke M., Wolff T., Viemann D., Roth J., Bode J.G., Ludwig S. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 2008;4:e1000196. doi: 10.1371/journal.ppat.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazienza V., Vinciguerra M., Andriulli A., Mangia A. Hepatitis C virus core protein genotype 3a increases SOCS-7 expression through PPAR-{gamma} in Huh-7 cells. J. Gen. Virol. 2010;91:1678–1686. doi: 10.1099/vir.0.020644-0. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Calzascia T., Toe J.G., Preston S.P., Lin A.E., Elford A.R., Shahinian A., Lang P.A., Lang K.S., Morre M., Assouline B., Lahl K., Sparwasser T., Tedder T.F., Paik J.H., DePinho R.A., Basta S., Ohashi P.S., Mak T.W. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Peterlin B.M., Trono D. Hide, shield and strike back: how HIV-infected cells avoid immune eradication. Nat. Rev. Immunol. 2003;3:97–107. doi: 10.1038/nri998. [DOI] [PubMed] [Google Scholar]
- Petry F., Jakobi V., Tessema T.S. Host immune response to Cryptosporidium parvum infection. Exp. Parasitol. 2010;126:304–309. doi: 10.1016/j.exppara.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Piessevaux G., Lella V., Riviere M., Stieber D., Dreze P., Szpirer J., Szpirer C. Contrasting epistatic interactions between rat quantitative trait loci controlling mammary cancer development. Mamm. Genome. 2009;20:43–52. doi: 10.1007/s00335-008-9155-4. [DOI] [PubMed] [Google Scholar]
- Pothlichet J., Chignard M., Si-Tahar M. Cutting edge: innate immune response triggered by influenza A virus is negatively regulated by SOCS1 and SOCS3 through a RIG-I/IFNAR1-dependent pathway. J. Immunol. 2008;180:2034–2038. doi: 10.4049/jimmunol.180.4.2034. [DOI] [PubMed] [Google Scholar]
- Raglow Z., Thoma-Perry C., Gilroy R., Wan Y.J. The interaction between HCV and nuclear receptor-mediated pathways. Pharmacol. Ther. 2011;132:30–38. doi: 10.1016/j.pharmthera.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram P.A., Waxman D.J. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J. Biol. Chem. 1999;274:35553–35561. doi: 10.1074/jbc.274.50.35553. [DOI] [PubMed] [Google Scholar]
- Ramaswamy M., Shi L., Monick M.M., Hunninghake G.W., Look D.C. Specific inhibition of type I interferon signal transduction by respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2004;30:893–900. doi: 10.1165/rcmb.2003-0410OC. [DOI] [PubMed] [Google Scholar]
- Ray M., Gam A.A., Boykins R.A., Kenney R.T. Inhibition of interferon-gamma signaling by Leishmania donovani. J. Infect. Dis. 2000;181:1121–1128. doi: 10.1086/315330. [DOI] [PubMed] [Google Scholar]
- Rincon G., Young A.E., Bannasch D.L., Medrano J.F. Characterization of variation in the canine suppressor of cytokine signaling-2 (SOCS2) gene. Genet. Mol. Res.: GMR. 2007;6:144–151. [PubMed] [Google Scholar]
- Rittig M.G., Bogdan C. Leishmania-host-cell interaction: complexities and alternative views. Parasitol. Today. 2000;16:292–297. doi: 10.1016/s0169-4758(00)01692-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues L., Filipe J., Seldon M.P., Fonseca L., Anrather J., Soares M.P., Simas J.P. Termination of NF-kappaB activity through a gammaherpesvirus protein that assembles an EC5S ubiquitin-ligase. EMBO J. 2009;28:1283–1295. doi: 10.1038/emboj.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. Herpesviridae. In: Fields B.N., Knip D.M., Howley P.M., editors. Fields Virology. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 2220–2230. [Google Scholar]
- Roizman, B., Taddeo, B., 2007. The strategy of herpes simplex virus replication and takeover of the host cell. In: Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K. (Eds.), Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge (Chapter 13).
- Ryo A., Suizu F., Yoshida Y., Perrem K., Liou Y.C., Wulf G., Rottapel R., Yamaoka S., Lu K.P. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell. 2003;12:1413–1426. doi: 10.1016/s1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- Ryo A., Tsurutani N., Ohba K., Kimura R., Komano J., Nishi M., Soeda H., Hattori S., Perrem K., Yamamoto M., Chiba J., Mimaya J., Yoshimura K., Matsushita S., Honda M., Yoshimura A., Sawasaki T., Aoki I., Morikawa Y., Yamamoto N. SOCS1 is an inducible host factor during HIV-1 infection and regulates the intracellular trafficking and stability of HIV-1 Gag. Proc. Natl. Acad. Sci. U. S. A. 2008;105:294–299. doi: 10.1073/pnas.0704831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D., Sher A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- Sakurai H., Tashiro K., Kawabata K., Yamaguchi T., Sakurai F., Nakagawa S., Mizuguchi H. Adenoviral expression of suppressor of cytokine signaling-1 reduces adenovirus vector-induced innate immune responses. J. Immunol. 2008;180:4931–4938. doi: 10.4049/jimmunol.180.7.4931. [DOI] [PubMed] [Google Scholar]
- Sandra O., Bataillon I., Roux P., Martal J., Charpigny G., Reinaud P., Bolifraud P., Germain G., Al-Gubory K.H. Suppressor of cytokine signalling (SOCS) genes are expressed in the endometrium and regulated by conceptus signals during early pregnancy in the ewe. J. Mol. Endocrinol. 2005;34:637–644. doi: 10.1677/jme.1.01667. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Yasukawa H., Shouda T., Kitamura T., Dikic I., Yoshimura A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J. Biol. Chem. 2000;275:29338–29347. doi: 10.1074/jbc.M003456200. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Yasukawa H., Suzuki A., Kamizono S., Syoda T., Kinjyo I., Sasaki M., Johnston J.A., Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999;4:339–351. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- Sato M., Suemori H., Hata N., Asagiri M., Ogasawara K., Nakao K., Nakaya T., Katsuki M., Noguchi S., Tanaka N., Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Weissenbach M., Haan S., Heinrich P.C., Schaper F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J. Biol. Chem. 2000;275:12848–12856. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- Seki Y., Hayashi K., Matsumoto A., Seki N., Tsukada J., Ransom J., Naka T., Kishimoto T., Yoshimura A., Kubo M. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13003–13008. doi: 10.1073/pnas.202477099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Evel-Kabler K., Strube R., Chen S.Y. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat. Biotechnol. 2004;22:1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- Shouda T., Yoshida T., Hanada T., Wakioka T., Oishi M., Miyoshi K., Komiya S., Kosai K., Hanakawa Y., Hashimoto K., Nagata K., Yoshimura A. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J. Clin. Invest. 2001;108:1781–1788. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K., Liu B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- Song M.M., Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J. Biol. Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- Song X.T., Evel-Kabler K., Rollins L., Aldrich M., Gao F., Huang X.F., Chen S.Y. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3:e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann K.M., Tran K.C., Chi B., Rabin R.L., Collins P.L. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected] J. Virol. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V., Manchanda M., Gupta S., Singla R., Behera D., Das G., Natarajan K. Toll-like receptor 2 and DC-SIGNR1 differentially regulate suppressors of cytokine signaling 1 in dendritic cells during Mycobacterium tuberculosis infection. J. Biol. Chem. 2009;284:25532–25541. doi: 10.1074/jbc.M109.006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V., Vashishta M., Gupta S., Singla R., Singla N., Behera D., Natarajan K. Suppressors of cytokine signaling inhibit effector T cell responses during Mycobacterium tuberculosis infection. Immunol. Cell Biol. 2011;89:786–791. doi: 10.1038/icb.2011.1. [DOI] [PubMed] [Google Scholar]
- Staat M.A. Respiratory syncytial virus infections in children. Semin. Respir. Infect. 2002;17:15–20. doi: 10.1053/srin.2002.31688. [DOI] [PubMed] [Google Scholar]
- Starr R., Willson T.A., Viney E.M., Murray L.J., Rayner J.R., Jenkins B.J., Gonda T.J., Alexander W.S., Metcalf D., Nicola N.A., Hilton D.J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Stec W.J., Zeidler M.P. Drosophila SOCS proteins. J. Signal Transduct. 2011;2011:894510. doi: 10.1155/2011/894510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber D., Kovarik P., Cohney S., Johnston J.A., Steinlein P., Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J. Immunol. 1999;163:2640–2647. [PubMed] [Google Scholar]
- Stoiber D., Stockinger S., Steinlein P., Kovarik J., Decker T. Listeria monocytogenes modulates macrophage cytokine responses through STAT serine phosphorylation and the induction of suppressor of cytokine signaling 3. J. Immunol. 2001;166:466–472. doi: 10.4049/jimmunol.166.1.466. [DOI] [PubMed] [Google Scholar]
- Stutz A., Kessler H., Kaschel M.E., Meissner M., Dalpke A.H. Cell invasion and strain dependent induction of suppressor of cytokine signaling-1 by Toxoplasma gondii. Immunobiology. 2012;217:28–36. doi: 10.1016/j.imbio.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanya S., Armant M., Salkowitz J.R., Nyakeriga A.M., Haridas V., Hasan M., Bansal A., Goepfert P.A., Wynn K.K., Ladell K., Price D.A.N.M., Kan-Mitchell J., Shankar P. Enhanced induction of HIV-specific cytotoxic T lymphocytes by dendritic cell-targeted delivery of SOCS-1 siRNA. Mol. Ther.: J. Am. Soc. Gene Ther. 2010;18:2028–2037. doi: 10.1038/mt.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R., Oldach D., Chang K.M., Steiger C., Ray S.C., Chisari F.V. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trop S., De Sepulveda P., Zuniga-Pflucker J.C., Rottapel R. Overexpression of suppressor of cytokine signaling-1 impairs pre-T-cell receptor-induced proliferation but not differentiation of immature thymocytes. Blood. 2001;97:2269–2277. doi: 10.1182/blood.v97.8.2269. [DOI] [PubMed] [Google Scholar]
- Vazquez N., Greenwell-Wild T., Rekka S., Orenstein J.M., Wahl S.M. Mycobacterium avium-induced SOCS contributes to resistance to IFN-gamma-mediated mycobactericidal activity in human macrophages. J. Leukoc. Biol. 2006;80:1136–1144. doi: 10.1189/jlb.0306206. [DOI] [PubMed] [Google Scholar]
- Verdier F., Chretien S., Muller O., Varlet P., Yoshimura A., Gisselbrecht S., Lacombe C., Mayeux P. Proteasomes regulate erythropoietin receptor and signal transducer and activator of transcription 5 (STAT5) activation. Possible involvement of the ubiquitinated CIS protein. J. Biol. Chem. 1998;273:28185–28190. doi: 10.1074/jbc.273.43.28185. [DOI] [PubMed] [Google Scholar]
- Vinkemeier U., Moarefi I., Darnell J.E., Jr., Kuriyan J. Structure of the amino-terminal protein interaction domain of STAT-4. Science. 1998;279:1048–1052. doi: 10.1126/science.279.5353.1048. [DOI] [PubMed] [Google Scholar]
- Waiboci L.W., Ahmed C.M., Mujtaba M.G., Flowers L.O., Martin J.P., Haider M.I., Johnson H.M. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J. Immunol. 2007;178:5058–5068. doi: 10.4049/jimmunol.178.8.5058. [DOI] [PubMed] [Google Scholar]
- Wang S.Z., Rosenberger C.L., Bao Y.X., Stark J.M., Harrod K.S. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J. Immunol. 2003;171:1051–1060. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- Wang T., Gorgoglione B., Maehr T., Holland J.W., Vecino J.L., Wadsworth S., Secombes C.J. Fish suppressors of cytokine signaling (SOCS): gene discovery, modulation of expression and function. J. Signal Transduct. 2011;2011:905813. doi: 10.1155/2011/905813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D.J., Evanson O.A., Souza C.D. Expression of interleukin-10 and suppressor of cytokine signaling-3 associated with susceptibility of cattle to infection with Mycobacterium avium subsp paratuberculosis. Am. J. Vet. Res. 2005;66:1114–1120. doi: 10.2460/ajvr.2005.66.1114. [DOI] [PubMed] [Google Scholar]
- Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- Winkelman L.A., Lucy M.C., Elsasser T.H., Pate J.L., Reynolds C.K. Short communication: suppressor of cytokine signaling-2 mRNA increases after parturition in the liver of dairy cows. J. Dairy Sci. 2008;91:1080–1086. doi: 10.3168/jds.2007-0433. [DOI] [PubMed] [Google Scholar]
- Wysocki M., Chen H., Steibel J.P., Kuhar D., Petry D., Bates J., Johnson R., Ernst C.W., Lunney J.K. Identifying putative candidate genes and pathways involved in immune responses to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Anim. Genet. 2012;43:328–332. doi: 10.1111/j.1365-2052.2011.02251.x. [DOI] [PubMed] [Google Scholar]
- Xu Y., Hu Y., Shi B., Zhang X., Wang J., Zhang Z., Shen F., Zhang Q., Sun S., Yuan Z. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol. Immunol. 2009;46:2640–2646. doi: 10.1016/j.molimm.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Yadav A., Fitzgerald P., Sajadi M.M., Gilliam B., Lafferty M.K., Redfield R., Reid W. Increased expression of suppressor of cytokine signaling-1 (SOCS-1): a mechanism for dysregulated T helper-1 responses in HIV-1 disease. Virology. 2009;385:126–133. doi: 10.1016/j.virol.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T., Yasukawa H., Jeon E.S., Xiong D., Dorner A., Iwatate M., Nara M., Zhou H., Summers-Torres D., Hoshijima M., Chien K.R., Yoshimura A., Knowlton K.U. Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation. 2006;114:2364–2373. doi: 10.1161/CIRCULATIONAHA.106.642454. [DOI] [PubMed] [Google Scholar]
- Yan G., Zhang G., Fang X., Zhang Y., Li C., Ling F., Cooper D.N., Li Q., Li Y., van Gool A.J., Du H., Chen J., Chen R., Zhang P., Huang Z., Thompson J.R., Meng Y., Bai Y., Wang J., Zhuo M., Wang T., Huang Y., Wei L., Li J., Wang Z., Hu H., Yang P., Le L., Stenson P.D., Li B., Liu X., Ball E.V., An N., Huang Q., Fan W., Zhang X., Wang W., Katze M.G., Su B., Nielsen R., Yang H., Wang X. Genome sequencing and comparison of two nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nat. Biotechnol. 2011;29:1019–1023. doi: 10.1038/nbt.1992. [DOI] [PubMed] [Google Scholar]
- Yang T., Stark P., Janik K., Wigzell H., Rottenberg M.E. SOCS-1 protects against Chlamydia pneumoniae-induced lethal inflammation but hampers effective bacterial clearance. J. Immunol. 2008;180:4040–4049. doi: 10.4049/jimmunol.180.6.4040. [DOI] [PubMed] [Google Scholar]
- Yao Z.Q., Prayther D., Trabue C., Dong Z.P., Moorman J. Differential regulation of SOCS-1 signalling in B and T lymphocytes by hepatitis C virus core protein. Immunology. 2008;125:197–207. doi: 10.1111/j.1365-2567.2008.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H., Misawa H., Sakamoto H., Masuhara M., Sasaki A., Wakioka T., Ohtsuka S., Imaizumi T., Matsuda T., Ihle J.N., Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H., Ohishi M., Mori H., Murakami M., Chinen T., Aki D., Hanada T., Takeda K., Akira S., Hoshijima M., Hirano T., Chien K.R., Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- Yasukawa H., Sasaki A., Yoshimura A. Negative regulation of cytokine signaling pathways. Annu. Rev. Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- Yasukawa H., Yajima T., Duplain H., Iwatate M., Kido M., Hoshijima M., Weitzman M.D., Nakamura T., Woodard S., Xiong D., Yoshimura A., Chien K.R., Knowlton K.U. The suppressor of cytokine signaling-1 (SOCS1) is a novel therapeutic target for enterovirus-induced cardiac injury. J. Clin. Invest. 2003;111:469–478. doi: 10.1172/JCI16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Yokosawa N., Kubota T., Suzutani T., Yoshida I., Miura S., Jimbow K., Fujii N. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and janus kinases during an early infection stage. Virology. 2001;286:119–124. doi: 10.1006/viro.2001.0941. [DOI] [PubMed] [Google Scholar]
- Yokota S., Yokosawa N., Okabayashi T., Suzutani T., Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virology. 2005;338:173–181. doi: 10.1016/j.virol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Yokota S., Yokosawa N., Okabayashi T., Suzutani T., Miura S., Jimbow K., Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J. Virol. 2004;78:6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A. Negative regulation of cytokine signaling. Clin. Rev. Allergy Immunol. 2005;28:205–220. doi: 10.1385/CRIAI:28:3:205. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Naka T., Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Nishinakamura H., Matsumura Y., Hanada T. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res. Ther. 2005;7:100–110. doi: 10.1186/ar1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Ohkubo T., Kiguchi T., Jenkins N.A., Gilbert D.J., Copeland N.G., Hara T., Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Suzuki M., Sakaguchi R., Hanada T., Yasukawa H. SOCS, inflammation, and autoimmunity. Front. Immunol. 2012;3:20. doi: 10.3389/fimmu.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Xiao Z.Z., Sun L. Suppressor of cytokine signaling 3 inhibits head kidney macrophage activation and cytokine expression in Scophthalmus maximus. Dev. Comp. Immunol. 2011;35:174–181. doi: 10.1016/j.dci.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ma C.J., Ni L., Zhang C.L., Wu X.Y., Kumaraguru U., Li C.F., Moorman J.P., Yao Z.Q. Cross-talk between programmed death-1 and suppressor of cytokine signaling-1 in inhibition of IL-12 production by monocytes/macrophages in hepatitis C virus infection. J. Immunol. 2011;186:3093–3103. doi: 10.4049/jimmunol.1002006. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhao J., Zhang H., Gai Y., Wang L., Li F., Yang J., Qiu L., Song L. The involvement of suppressors of cytokine signaling 2 (SOCS2) in immune defense responses of Chinese mitten crab Eriocheir sinensis. Dev. Comp. Immunol. 2010;34:42–48. doi: 10.1016/j.dci.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Zhao D.C., Yan T., Li L., You S., Zhang C. Respiratory syncytial virus inhibits interferon-alpha-inducible signaling in macrophage-like U937 cells. J. Infect. 2007;54:393–398. doi: 10.1016/j.jinf.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Zhou P., Zhai S., Zhou X., Lin P., Jiang T., Hu X., Jiang Y., Wu B., Zhang Q., Xu X., Li J.P., Liu B. Molecular characterization of transcriptome-wide interactions between highly pathogenic porcine reproductive and respiratory syndrome virus and porcine alveolar macrophages in vivo. Int. J. Biol. Sci. 2011;7:947–959. doi: 10.7150/ijbs.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S., Murray P.J., Heeg K., Dalpke A.H. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-gamma signaling. J. Immunol. 2006;176:1840–1847. doi: 10.4049/jimmunol.176.3.1840. [DOI] [PubMed] [Google Scholar]


