Abstract
To reveal whether bats serve as an amplifying host for Yokose virus (YOKV), we conducted a serological survey and experimentally infected fruit bats with YOKV isolated from microbats in Japan. YOKV belongs to the Entebbe bat virus group of vector unknown group within the genus Flavivirus and family Flaviviridae. To detect antibodies against YOKV, we developed an enzyme-linked immunosorbent assay (ELISA) using biotinylated anti-bat IgG rabbit sera. Serological surveillance was conducted with samples collected in the Philippines and the sera supplied from Malaysia. One of the 36 samples from the Philippines (2.7%) and 5 of the 26 samples from Malaysia (19%) had detectable ELISA antibodies. In the experimental infections, no clinical signs of disease were observed. Moreover, no significant viral genome amplification was detected. These findings revealed that YOKV replicates poorly in the fruit bat, suggesting that fruit bats do not seem to serve as an amplifying host for YOKV.
Keywords: ELISA, Yokose virus, Bat, Experimental infection, Anti-bat IgG, Chiroptera
Résumé
Afin de vérifier si les chauves-souris servent comme hôte amplificateur pour le virus Yokose (YOKV), nous avons mené des études sérologiques sur des chauves-souris frugivores expérimentalement infectées par YOKV. Le virus Yokose appartient à la famille des virus Entebbe, dont le vecteur est inconnu et appartenant au genre Flavivirus, de la famille des Flaviviridae. Pour détecter les anticorps contre YOKV, nous avons développé une technique « ELISA » utilisant des sérums de lapin anti-IgG de chauves-souris. Notre surveillance sérologique s’est effectuée sur des échantillons en provenance des Philippines et des sérums récoltés en Malaisie. Un prélèvement sur 36 provenant des Philippines (2,7%) et cinq sur 26 provenant de Malaisie (19%) ont développé des anticorps détectés par le test ELISA. En revanche, aucun signe clinique de maladie n’a été développé sur les animaux infectés expérimentalement. En outre, aucune amplification du génome viral n’a été détectée. Il en résulte que YOKV se propage mal chez les chauves-souris frugivores, ce qui suggère que les chauves-souris frugivores ne servent pas comme hôte amplificateur du virus Yokose.
Mots clés: ELISA, Yokose virus, Chauve-souris, Infection expérimentale, IgG anti-chauve-souris, Chiroptères
1. Introduction
Bats, the only mammals capable of flight, display large amounts of diversity and account for 20% of the 4800 mammalian species recorded in the world. During the past decade, bats have been associated with several emerging zoonotic agents including Hendra, Nipah, Lyssa, Ebola, and severe acute respiratory syndrome coronavirus-like viruses [1], [2], [3], [4], [5]. Therefore, bats are thought to be an important reservoir for many mammalian viruses. Specifically, numerous viruses have been isolated from the genus Flavivirus, family Flaviviridae [6], [7], [8], [9]. While the epizootology of these viruses remains unknown, nucleotide sequences for some flaviviruses have been reported [8], [9], [10]. Yokose virus (YOKV) is a flavivirus that has been isolated from a bat in Japan in 1971. YOKV belongs to the Entebbe bat virus group within the genus Flavivirus and family Flaviviridae.
To investigate the possibility that bats serve as a reservoir for Japanese encephalitis virus (JEV) during the winter, Oya et al. attempted to isolate arthropod-borne viruses from bats in Oita Prefecture, Japan [11]. During this 1971 investigation, YOKV was isolated from the long-fingered bat Miniopterus fuliginosus. Recently, Tajima et al. [11] reported the complete nucleotide sequence of YOKV. Kuno and Chang [9] compared the complete nucleotides sequences with those of other flaviviruses. They concluded that YOKV is genetically closer to yellow fever virus or Sepik virus than JEV, and it is most closely related to Entebbe bat virus.
Previous phylogenetic analyses of the genus Flavivirus have revealed that flaviviruses can be divided into three groups: mosquito-borne, tick-borne, and unknown vector groups [12], [13]. Although YOKV is classified into the Entebbe bat virus group of vector unknown group, conserved sequence element 1 (CS1) in the 3′-untranslated region of YOKV is similar to that of mosquito-borne viruses, which are known to display highly conserved CS regions [11], [14]. Therefore, Tajima et al. [11] suggested that YOKV belongs to the mosquito-borne virus group. Moreover, previous reports indicated that Entebbe bat virus can replicate in mosquito cells in vitro [15], and experimental infection of Entebbe bat virus using frugivorous and insectivorous bats showed no viral growth in bats [16]. Since the initial isolation with YOKV in 1971, there have been no additional reports on the isolation or antibody detection of YOKV from bats or mosquitoes. Therefore, to determine whether bats serve as a natural or amplifying host for YOKV, we conducted a serological survey and experimental infection studies in bats with YOKV.
To detect antibodies against YOKV, we developed an ELISA using biotinylated anti-bat IgG rabbit sera. In this system, polyclonal anti-bat IgG rabbit sera were used as described in a previous paper [17]. The developed anti-bat IgG reacts only with bat IgG but not with IgG of other mammalian species. Therefore, this ELISA detects bat-specific IgG antibodies. Using the conventional ELISA, a serological survey was performed on bat serum samples collected from the Philippines and Malaysia.
2. Materials and methods
2.1. Cell culture and virus growth
Vero cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal calf serum (FCS), penicillin, and streptomycin. The Oita-36 strain of YOKV was kindly provided by Dr. T. Takasaki (National Institute of Infectious Diseases). The virus was grown in Vero cells on 700-cm2 roller bottles. Infection was performed at a multiplicity of 0.005 TCID50/cell with an inoculum containing 5 ml of serum-free maintenance medium (-SMM) in DMEM, 2.95 g triptose phosphate broth, 5 g l-glutamine–Na, 1 g glucose, 0.5 g yeast extract, 0.292 g l-glutamine/L, penicillin, and streptomycin. Following virus adsorption at 37 °C for 1 h, cells were washed with -SMM and 100 ml of -SMM was added per bottle. Four days after infection, ten 100-ml bottles of infectious fluid were harvested and cellular debris was removed by low-speed centrifugation (2000 × g, 15 min, 4 °C). The resulting supernatant was collected, and half was used for virus purification while the remainder was used for virus inactivation.
2.2. Virus purification
Virus purification was performed according to the procedures outlined for JEV [18], [19]. Briefly, YOKV was precipitated from the supernatant by incubation with PEG6000 (10 mM) and NaCl (400 mM). After an overnight incubation at 4 °C, the mixture was centrifuged (5000 × g, 60 min, 4 °C) and the pellet containing the virus was resuspended in 1 ml Tris–saline–EDTA buffer (TEN buffer). Virions were further purified on 12 ml of a continuous 10–60% sucrose gradient. The gradients were centrifuged in an RPS40T rotor (Hitachi, Tokyo, Japan) at 100,000 × g, for 2 h at 4 °C. Fifteen fractions were collected from the bottom of each tube. Each fraction was assayed by using both ELISA and titration method. Peak fractions that demonstrated the highest ELISA reactivity were collected and used as ELISA antigens.
2.3. Virus inactivation and purification
Virus inactivation was performed using 37% formalin at a final concentration of 0.1%, according to the procedures for JEV [18], [19]. The inactivated virus was purified as described above. The peak fractions were collected and dialyzed with PBS. The inactivated virus was filtered through a 0.22 μm low protein-binding (GV-type) filter unit, and stored at 4 °C until it was used as a bat immunogen.
2.4. Immunizations and blood sampling
Two Leschenault's rousette bats (Rousettus leschenaulti) were immunized with inactivated and purified virus. One milliliter of the inactivated virus was inoculated intraperitoneally twice at 4-week intervals to obtain positive sera containing anti-YOKV antibodies. Serum samples were collected at days 14 and 28 postinoculation. Briefly, a small vein on the patagium was cut with a scalpel for blood sampling, and filter papers were soaked with approximately 50 μl of blood. The filter papers were transferred into Eppendorf tubes and eluted in 200 μl PBS by centrifugation (3000 rpm, 10 min, 4 °C). After discarding the filter papers, eluates were centrifuged again and the supernatants were stored as serum samples. These samples were estimated to be equal to a 1:5 sera dilution. On day 28 after collecting sera, two bats were inoculated with a secondary injection. On day 35 after the first inoculation, the two bats were anesthetized using a 1.5 mg intraperitoneal injection of ketamine hydrochloride and were euthanized via intracardiac exsanguination.
2.5. Sample collection for the serological survey
Bat serum samples were collected in the Philippines and Malaysia. Insectivorous bats were collected from two sites in the Philippines (Fig. 1 ). Sites were chosen on the basis of a previous survey [20]. All captured bats were anesthetized by administering a 15 mg/kg intraperitoneal injection of ketamine hydrochloride. We measured the size of each bat (weight, forearm length, head and body length, ear length, and tail length) and examined additional morphological features (uropatagium, tragus, and nose-leaf). Species were identified based on gross morphology according to the classification criteria for chiropterans [21] (Table 1 ).
Fig. 1.
Map of the Philippines outlining bat collection sites from two locations. 1 = Calbang, Laguna; 2 = Santa Rosa, Laguna.
Table 1.
Bat species and collecting sites
| Bat species | The Philippines (Laguna) |
Malaysia | Total | |
|---|---|---|---|---|
| Calubang | Santa Rosa | |||
| Collecting cite | ||||
| Taphozous melanopogon | 1/31 | 1/31 | ||
| Scotophilus kuhlii | 0/5 | 0/5 | ||
| Unidentified species (fruit bats) | 5/26 | 5/26 | ||
| Total | 1/31 | 0/5 | 6/62 | |
*The number of antibody positive sera/the number of sera tested.
Blood was obtained by cardiac puncture and stored at 4 °C until centrifugation. Sera were frozen at −20 °C during transport and later stored in a −80 °C freezer upon arrival. Fruit bat sera collected in Malaysia were kindly supplied by Dr. A. Rayari (University of Malaysia, Sarawak) and Dr. T. Imada (JICA project leader at the Veterinary Research Institute).
2.6. ELISA
ELISAs were performed according to the procedures described for JEV [22]. To standardize the reagents used for the labeled avidin–biotin enzyme-linked immunosorbent assay (LAB-ELISA), antigen and antibody concentrations were determined by checkerboard titration. Briefly, YOKV antigen was diluted with coating buffer at a concentration of 15 μg/ml and ELISA plates were coated with 100 μl/well of the antigen solution. After washing the plates, 50 μl of serum from each sample was appropriately diluted with blocking buffer (PBS, pH 7.4, containing 0.5% Tween 20 and 5% chicken serum) and delivered to each well. The plates were then kept at 37 °C for 1 h. After washing the plates, biotin-labeled anti-bat IgG rabbit serum was diluted in blocking buffer at 1:3200 and 50 μl of the diluted solution was added to each well. Anti-bat IgG rabbit serum [17] was biotin-labeled by the methods described by Chang et al. [23]. The plates were incubated at 37 °C for 30 min. After washing the plates, horseradish peroxidase-labeled avidin (Sigma, St. Louis, MO, USA) was diluted in blocking buffer at 1:1000 and 50 μl of the diluted solution was added to each well. After incubation at 37 °C for 30 min, the plates were washed and 100 μl of prepared TMB substrate solution (TMB Microwell Peroxidase Substrate; Kappel, USA) was added to each well. Following incubation for 5 min at room temperature, 100 μl of stop solution (1 M H2SO4) was added to each well and OD values were determined.
Minor nonspecific reactions were observed in the control wells without serum and antigen. In the control wells without antigen, some of the OD values were slightly higher than those with the antigen; however, the values were all lower than 0.06 and neared 0. The cutoff point was estimated as the sum of the average OD values of the control wells without serum and antigen and an additional factor of 0.1 in accordance with the procedures for JEV [22]. According to the above criteria, when antiserum was used at the highest dilution in a checkerboard titration (1:100), the end point titer of antigen was determined at 1:800 (a putative 1 unit). We examined 10 negative control samples at a dilution of 1:25–1:1600 using 1:100 diluted antigen (as a putative 8 units). A cutoff value of 0.23 was set according to the following calculation: mean each wells plus (S.D.) × 3. According to these criteria, when the antiserum was used at a dilution of 1:100 in the checkerboard titration, the end point titer of antigen was determined at 1:800. Therefore, the YOKV antigen was used at 1:100 (8 units) for the LAB-ELISA assay.
2.7. Neutralization test
Neutralization tests were performed using the microtiter method. One hundred TCID50 of virus was incubated with twofold serially diluted serum samples and added into Vero cells seeded in a 96-well plate. The titer of neutralization antibody was determined based on the highest serum dilution, which completely suppressed a cytopathic effect.
2.8. Experimental infection
Leschenault's rousette bats were obtained from zoos in Japan. These fruit bats were housed in separate cages in an air-conditioned room. The animals were fed several kinds of fruit with free access to water. Serum samples were collected from the orbital sinus under anesthesia by diethylether.
Nine of the seronegative bats were randomly selected for experimental infection and placed in a negative-pressure isolator. The bats were inoculated intraperitoneally with 1 ml of solution containing 107 TCID50/ml of YOKV. The bats were separated into three groups, each containing three bats. One group each was sacrificed by cardiac puncture on days 2, 4, and 7 postinoculation following anesthesia by a 1.5 mg intraperitoneal injection of ketamine hydrochloride. The experiment was conducted according to the Guidelines for the Care and Use of Laboratory Animals, Graduate School of Agriculture and Life Sciences, the University of Tokyo. Serum samples were obtained via whole blood by centrifugation at 3000 rpm for 10 min at 4 °C. Organs (liver, kidney, spleen, lung, and brain) were also collected. During the experimental infection, bats were examined daily for clinical symptoms of infections. Urine and fecal specimens were collected using a clean translucent plastic sheet spread along the bottom of the cage. Virus isolation was attempted from these samples (i.e., organs, serum, urine, and feces). Each sample was homogenized in DMEM as 10% suspensions and assayed for viral titers using TCID50 on Vero cells. Each sample was also tested using RT-PCR to detect YOKV RNA. Finally, serum samples were tested using NT and ELISA.
2.9. Detection of viral genome by RT-PCR
Viral RNA extraction was performed on samples obtained from infected bats using an SV Total RNA Isolation System kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. SuperScript™ One-Step RT-PCR with Platinum Taq (Invitrogen, Carlsbad, CA, USA) was used for RT-PCR. The primer set (5′-ATAAGACAGCCAACCATTGC-3′ and 5′-TATCCGGCAAATCCAATCAC-3′) was targeted to a 320-bp fragment of the envelope gene and designed to be specific to YOKV according to a prior report [24].
3. Results
3.1. Immunization
To obtain sera positive for anti-YOKV antibodies, two bats were immunized with inactivated and purified virus. Antibodies were first detected in Bat 2 with a titer of 1:25 on day 14 postinoculation. Anti-YOKV antibodies reached the peak titer (1:800 in Bat 2 and 1:1600 in Bat 1) a week after the second inoculation on day 35 (Fig. 2 ). On day 35, the bats were killed by intracardiac exsanguination under anesthesia. Neutralizing antibodies were also tested on days 0 and 35. We could not obtain sufficient serum volumes to perform the neutralizing test for days 14 and 28. Neutralizing titers were found to be low on day 35 (1:8 in Bat 2 and 1:16 in Bat 1).
Fig. 2.
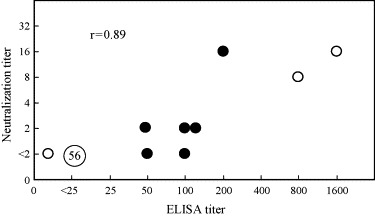
Comparison of YOKV antibody titers in bat serum samples collected from the Philippines and Malaysia (close circle), and immunized bats. Titers were determined using ELISAs and NT tests; two positive and one negative serum samples were collected from immunized and control bats (open circle). The number in the circle indicates the number of serum samples. The correlation coefficient was calculated using the field sample titers.
3.2. Serological survey
Of the 60 bat serum samples collected in the Philippines, 36 had sufficient volumes and quality for analysis. Twenty-six additional samples were supplied by Dr. A. Rayari (University of Malaysia, Sarawak) and Dr. T. Imada (JICA project leader at the Veterinary Research Institute). Sera were screened at a dilution of 1:25 and each sample was tested three times by ELISA. Of the 62 serum samples tested, 6 (9.6%) were determined to be positive in the ELISA assay; ELISA titers of each serum sample were determined. Neutralization tests (NTs) were also conducted using collected bat sera at a concentration of 1:2. Of the 62 samples, five were determined to be positive. Although the titers of these five samples were also determined, they were low.
Yokose antibody titers obtained by ELISA were compared with those obtained by NT (Fig. 2). Close correlations existed between ELISA and NT titers (r = 0.89).
3.3. Cross-reactivity of Japanese encephalitis virus antigen
Serological tests used for flaviviruses, such as ELISAs or fluorescent antibody techniques, have been reported to show high cross-reactivity with other flaviviruses [25], [26]. Therefore, to check the specificity of the YOKV ELISA, an ELISA with the JEV antigen substituted for YOKV antigen was conducted. ELISA titers of the JEV antigen were determined using positive sera from Bat 1. The heterologous titer using the JEV antigen was 1:50 whereas the homologous titer (using the YOKV antigen) was 1:1600. Therefore, we found that the homologous titer was 32 times higher than the heterologous titer. ELISA with the JEV antigen was also conducted using the bat serum samples described in Section 3.2. Titers of all the samples were less than 1:25. Furthermore, the serum samples collected from bats which were obtained from Japan Zoos were also screened by ELISA using the JEV antigen. Titers of all samples were less than 1:25.
3.4. Experimental infection
An ELISA was used to exclude bats that were positive for antibodies against YOKV. Fourteen percent of the fruit bats (Leschenault's rousette bats) collected from several zoos in Japan had antibodies against YOKV (Table 2 ). All of the bats seropositive against YOKV were obtained from zoos in the western portion of Japan. Nine of the seronegative bats were selected and experimentally infected with YOKV. During experimental infection, none of the bats inoculated with YOKV showed clinical signs of infection. Viral particles were not recovered from any of the collected samples. Viral genome amplification was not detected in any of the samples including sera, organs (brain, heart, kidney, liver, lung, and spleen), feces, and urine; however, viral RNA was detected in the liver of one bat that was killed 2 days after inoculation (Table 3 ).
Table 2.
Prevalence of ELISA antibodies in fruit bats obtained from zoos
| ELISA titer | No. of sera |
|---|---|
| 100 | 3 (4.6%) |
| 50 | 3 (4.6%) |
| 25 | 8 (12%) |
| <25 (=neg.) | 50 (78%) |
Table 3.
Results of RT-PCR and virus isolation from sera, tissue samples, urine, and feces
| Days after inoculation | No. bats tested | RT-PCR for YOKV (real time PCR) |
Virus isolation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sera | Liver | Kidney | Lung | Brain | Spleen | Urine and feces | |||
| 2 | 2 | − | − | − | − | − | − | − | − |
| 1 | − | + | − | − | − | − | − | − | |
| 4 | 3 | − | − | − | − | − | − | − | − |
| 7 | 3 | − | − | − | − | − | − | − | − |
(+) Positive; (−) negative.
Sera collected from the infected bats were tested using a NT (Table 4 ). NT titers of six preinoculated serum samples were less than 1:4. For the remaining three samples, NT was not performed due to insufficient volumes of serum. No NT antibodies were detected in sera obtained 2 days after inoculation. On days 4 and 7 postinoculation, NT antibodies were detected in all samples except for one bat killed on day 4, and the NT titers were less than 8.
Table 4.
Neutralizing titers of the bats experimentally infected with YOKV
| Bat no. | Days after inoculation |
|||
|---|---|---|---|---|
| Pre | 2 | 4 | 7 | |
| 1 | <4 | <4 | ||
| 2 | <4 | <4 | ||
| 3 | – | <4 | ||
| 4 | <4 | – | 8 | |
| 5 | <4 | – | <4 | |
| 6 | – | – | 4 | |
| 7 | <4 | – | – | 4 |
| 8 | <4 | – | – | 8 |
| 9 | – | – | – | 4 |
(–) Not tested.
4. Discussion
We developed an ELISA system using biotin-labeled anti-bat-IgG rabbit serum to detect antibodies against YOKV in bat sera and conducted serological surveys using this system. One of the 36 samples collected from the Philippines and five of the 26 samples from Malaysia had detectable antibodies against YOKV. These results suggest that YOKV is distributed not only in Japan, but also in other Asian countries. The possibility exists that antibodies detected in this survey were against other flaviviruses, since antibodies against flaviviruses have been reported to show high cross-reactivity with other flaviviral antigens [26]. Thus, we conducted an ELISA in which we substituted the JEV antigen to confirm assay specificity. The ELISA using the JEV antigen was found to react with the positive serum against YOKV from the immunized bat; however, the titer against JEV was much lower than the homologous titer using YOKV antigen. We also tested field samples using an ELISA with the JEV antigen, and all samples were negative (data not shown). Moreover, ELISA and NT antibody titers from both field samples and positive sera from immunized bats showed a close correlation. These data suggest that the antibody detected in this survey was specific to YOKV.
Serological surveys of several viruses have been conducted using bat sera collected in the field; however, most of the surveys were performed using a NT or fluorescent antibody tests [2], [3], [4], [5], [27]. Obtaining sufficient volumes of blood necessary for these serological tests is difficult, particularly in smaller species including microbats. Moreover, these assays are not suitable for testing large numbers of samples at one time. Therefore, an ELISA is a powerful tool that can be helpful in serological surveys of infected bats. However, no known conventional ELISAs are available, except assays using protein G or competitive techniques with monoclonal antibodies [1], [28]. In this study, we demonstrated that a conventional ELISA using biotin-labeled anti-bat IgG rabbit sera was able to detect antibodies in bat sera obtained from field studies.
From complete nucleotide sequence analyses of YOKV, Tajima et al. [11] suggested that YOKV belongs to the mosquito-borne group. Thus, to reveal whether bats serve as an amplifying host for YOKV, we conducted an experimental infection study of YOKV in bats and examined viral growth and pathogenicity. Since YOKV was originally isolated from a species of microbat, M. fuliginosus, it would be preferable to conduct experiments on viral characteristics of YOKV not only with fruit bats but also with M. fuliginosus or other microbats. However, maintaining and feeding microbats, especially insectivorous bats, is difficult. Therefore, we conducted our experimental infection studies on the fruit bat R. leschenaultia. Prior to experimental infection, we performed an ELISA to exclude fruit bats that had been previously exposed to (i.e., were positive for) antibodies against YOKV. Fourteen percent of the fruit bats collected from several zoos in the western portion of Japan were positive for YOKV antibodies. These positive sera against YOKV were also tested using an ELISA with the JEV antigen. All samples were negative. Given that Leschenault's rousette bats have been bred in each zoo, and reared separately in open-air cages, it seems likely that these antibody positive bats had been exposed to YOKV at the zoos themselves. Although no accounts of viral isolation or antibody detection of YOKV have been reported since the initial one in 1971, YOKV appears to be present in Japan.
No clinical signs of disease were observed in fruit bats following viral infection. Moreover, significant viral genome amplification was not detected in any of the samples, except for one liver sample obtained from a virus-inoculated bat killed at day 2 postinoculation. No viral particles were isolated from any of the samples and antibody responses were low. These results reveal that YOKV replicates poorly in Leschenault's rousette bats, and might suggest that fruit bats do not serve as an amplifying host for YOKV. Our results from the serological field survey demonstrate a low prevalence of YOKV in bats from the Philippines and Malaysia, further supporting this suggestion. YOKV may have additional amplifying hosts besides bats, such as mosquitoes. To confirm the viral pathogenicity in microbats and also the relationship between YOKV and mosquitoes, further studies are needed. Although no cases of YOKV infection have been reported in other animals, a single human case of febrile illness, possibly caused by Sepik virus, has been published [29]. Interestingly, Sepik virus exhibits high nucleotide sequence similarities with YOKV. Further studies are necessary to more fully elucidate the pathogenicity of YOKV.
Acknowledgments
We thank Dr. Tomohiko Takasaki of the National Institute of Infectious Diseases of Japan for providing the Oita-36 strain of YOKV, and the members of the Veterinary Research Department of the Research Institute for Tropical Medicine in the Philippines for their assistance in collecting and processing specimens. This study was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102(39):14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438(7068):575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 3.Philbey A.W., Kirkland P.D., Ross A.D., Davis R.J., Gleeson A.B., Love R.J. An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerg Infect Dis. 1998;4(2):269–271. doi: 10.3201/eid0402.980214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpin K., Young P.L., Field H.E., Mackenzie J.S. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000;81(8):1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 5.Chua K.B. Nipah virus outbreak in Malaysia. J Clin Virol. 2003;26(3):265–275. doi: 10.1016/s1386-6532(02)00268-8. [DOI] [PubMed] [Google Scholar]
- 6.Bres P., Chambon L. Isolation at Dakar of a strain of arborvirus from the salivary glands of the bat (preliminary note) Ann Inst Pasteur. 1963;104:705–712. [PubMed] [Google Scholar]
- 7.Price J.L. Isolation of Rio Bravo and a hitherto undescribed agent, Tamana bat virus, from insectivorous bats in Trinidad, with serological evidence of infection in bats and man. Am J Trop Med Hyg. 1978;27(1):153–161. doi: 10.4269/ajtmh.1978.27.153. [DOI] [PubMed] [Google Scholar]
- 8.Charlier N., Leyssen P., Pleij C.W., Lemey P., Billoir F., Van Laethem K. Complete genome sequence of Montana Myotis leukoencephalitis virus, phylogenetic analysis and comparative study of the 3′ untranslated region of flaviviruses with no known vector. J Gen Virol. 2002;83(8):1875–1885. doi: 10.1099/0022-1317-83-8-1875. [DOI] [PubMed] [Google Scholar]
- 9.Kuno G., Chang G.J. Characterization of Sepik and Entebbe bat viruses closely related to yellow fever virus. Am J Trop Med Hyg. 2006;75(6):1165–1170. [PubMed] [Google Scholar]
- 10.Billoir F., de Chesse R., Tolou H., de Micco P., Gould E.A., de Lamballerie X. Phylogeny of the genus flavivirus using complete coding sequences of arthropod-borne viruses and viruses with no known vector. J Gen Virol. 2000;81(3):781–790. doi: 10.1099/0022-1317-81-3-781. [DOI] [PubMed] [Google Scholar]
- 11.Tajima S., Takasaki T., Matsuno S., Nakayama M., Kurane I. Genetic characterization of Yokose virus, a flavivirus isolated from the bat in Japan. Virology. 2005;332(1):38–44. doi: 10.1016/j.virol.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 12.Kuno G., Chang G.J., Tsuchiya K.R., Karabatsos N., Cropp C.B. Phylogeny of the genus Flavivirus. J Virol. 1998;72(1):73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaunt M.W., Sall A.A., de Lamballerie X., Falconar A.K., Dzhivanian T.I., Gould E.A. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J Gen Virol. 2001;82(8):1867–1876. doi: 10.1099/0022-1317-82-8-1867. [DOI] [PubMed] [Google Scholar]
- 14.Leyssen P., Charlier N., Lemey P., Billoir F., Vandamme A.M., De Clercq E. Complete genome sequence, taxonomic assignment, and comparative analysis of the untranslated regions of the Modoc virus, a flavivirus with no known vector. Virology. 2002;293(1):125–140. doi: 10.1006/viro.2001.1241. [DOI] [PubMed] [Google Scholar]
- 15.Varelas-Wesley I., Calisher C.H. Antigenic relationships of flaviviruses with undetermined arthropod-borne status. Am J Trop Med Hyg. 1982;31(6):1273–1284. doi: 10.4269/ajtmh.1982.31.1273. [DOI] [PubMed] [Google Scholar]
- 16.Simpson D.I., O'Sullivan J.P. Studies on arboviruses and bats (Chiroptera) in East Africa. I. Experimental infection of bats and virus transition attempts in Aedes (Stegomyia) aegypti (Linnaeus) Ann Trop Med Parasitol. 1968;62(4):422–431. doi: 10.1080/00034983.1968.11686579. [DOI] [PubMed] [Google Scholar]
- 17.Omatsu T., Ishii Y., Kyuwa S., Milanda E.G., Terao K., Yoshikawa Y. Molecular evolution inferred from immunological cross-reactivity of immunoglobulin G among Chiroptera and closely related species. Exp Anim. 2003;52(5):425–428. doi: 10.1538/expanim.52.425. [DOI] [PubMed] [Google Scholar]
- 18.Aizawa C., Hasegawa S., Chih-Yuan C., Yoshioka I. Large-scale purification of Japanese encephalitis virus from infected mouse brain for preparation of vaccine. Appl Environ Microbiol. 1980;39(1):54–57. doi: 10.1128/aem.39.1.54-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava A.K., Putnak J.R., Lee S.H., Hong S.P., Moon S.B., Barvir D.A. A purified inactivated Japanese encephalitis virus vaccine made in Vero cells. Vaccine. 2001;19(31):4557–4565. doi: 10.1016/s0264-410x(01)00208-0. [DOI] [PubMed] [Google Scholar]
- 20.Arguin P.M., Murray-Lillibridge K., Miranda M.E., Smith J.S., Calaor A.B., Rupprecht C.E. Serologic evidence of Lyssavirus infections among bats, the Philippines. Emerg Infect Dis. 2002;8(3):258–262. doi: 10.3201/eid0803.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingle NR, Heaney LR. A key to the bats of the Philippine Islands, 1992.
- 22.Chang H.C., Ohkubo Y., Takashima I., Arikawa J., Hashimoto N. Labeled avidin–biotin enzyme-linked immunosorbent assay (LAB-ELISA) for detection of Japanese encephalitis antibody in swine sera. Jpn J Vet Res. 1984;32(2):59–71. [PubMed] [Google Scholar]
- 23.Chang H.C., Takashima I., Arikawa J., Hashimoto N. Biotin-labeled antigen sandwich enzyme-linked immunosorbent assay (BLA-S-ELISA) for the detection of Japanese encephalitis antibody in human and a variety of animal sera. J Immunol Methods. 1984;72(2):401–409. doi: 10.1016/0022-1759(84)90008-5. [DOI] [PubMed] [Google Scholar]
- 24.Mandl C.W., Guirakhoo F., Holzmann H., Heinz F.X., Kunz C. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J Virol. 1989;63(2):564–571. doi: 10.1128/jvi.63.2.564-571.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez M.D., Pierson T.C., McAllister D., Hanna S.L., Puffer B.A., Valentine L.E. Characterization of neutralizing antibodies to West Nile virus. Virology. 2005;336(1):70–82. doi: 10.1016/j.virol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Pugachev K.V., Guirakhoo F., Trent D.W., Monath T.P. Traditional and novel approaches to flavivirus vaccines. Int J Parasitol. 2003;33(5–6):567–582. doi: 10.1016/s0020-7519(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 27.Warrilow D., Harrower B., Smith I.L., Field H., Taylor R., Walker C. Public health surveillance for Australian bat lyssavirus in Queensland, Australia, 2000–2001. Emerg Infect Dis. 2003;9(2):262–264. doi: 10.3201/eid0902.020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashiwazaki Y., Na Y.N., Tanimura N., Imada T. A solid-phase blocking ELISA for detection of antibodies to Nipah virus. J Virol Methods. 2004;121(2):259–261. doi: 10.1016/j.jviromet.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Karabatsos N. Supplement to International Catalogue of Arboviruses including certain other viruses of vertebrates. Am J Trop Med Hyg. 1978;27(2):372–440. doi: 10.4269/ajtmh.1978.27.372. [DOI] [PubMed] [Google Scholar]


