Abstract
Background
Many smokers give up smoking on their own, but materials that provide a structured programme for smokers to follow may increase the number who quit successfully.
Objectives
The aims of this review were to determine the effectiveness of different forms of print‐based self‐help materials that provide a structured programme for smokers to follow, compared with no treatment and with other minimal contact strategies, and to determine the comparative effectiveness of different components and characteristics of print‐based self‐help, such as computer‐generated feedback, additional materials, tailoring of materials to individuals, and targeting of materials at specific groups.
Search methods
We searched the Cochrane Tobacco Addiction Group Trials Register, ClinicalTrials.gov, and the International Clinical Trials Registry Platform (ICTRP). The date of the most recent search was March 2018.
Selection criteria
We included randomised trials of smoking cessation with follow‐up of at least six months, where at least one arm tested print‐based materials providing self‐help compared with minimal print‐based self‐help (such as a short leaflet) or a lower‐intensity control. We defined 'self‐help' as structured programming for smokers trying to quit without intensive contact with a therapist.
Data collection and analysis
We extracted data in accordance with standard methodological procedures set out by Cochrane. The main outcome measure was abstinence from smoking after at least six months' follow‐up in people smoking at baseline. We used the most rigorous definition of abstinence in each study and biochemically validated rates when available. Where appropriate, we performed meta‐analysis using a random‐effects model.
Main results
We identified 75 studies that met our inclusion criteria. Many study reports did not include sufficient detail to allow judgement of risk of bias for some domains. We judged 30 studies (40%) to be at high risk of bias for one or more domains.
Thirty‐five studies evaluated the effects of standard, non‐tailored self‐help materials. Eleven studies compared self‐help materials alone with no intervention and found a small effect in favour of the intervention (n = 13,241; risk ratio (RR) 1.19, 95% confidence interval (CI) 1.03 to 1.37; I² = 0%). We judged the evidence to be of moderate certainty in accordance with GRADE, downgraded for indirect relevance to populations in low‐ and middle‐income countries because evidence for this comparison came from studies conducted solely in high‐income countries and there is reason to believe the intervention might work differently in low‐ and middle‐income countries. This analysis excluded two studies by the same author team with strongly positive outcomes that were clear outliers and introduced significant heterogeneity. Six further studies of structured self‐help compared with brief leaflets did not show evidence of an effect of self‐help materials on smoking cessation (n = 7023; RR 0.87, 95% CI 0.71 to 1.07; I² = 21%). We found evidence of benefit from standard self‐help materials when there was brief contact that did not include smoking cessation advice (4 studies; n = 2822; RR 1.39, 95% CI 1.03 to 1.88; I² = 0%), but not when self‐help was provided as an adjunct to face‐to‐face smoking cessation advice for all participants (11 studies; n = 5365; RR 0.99, 95% CI 0.76 to 1.28; I² = 32%).
Thirty‐two studies tested materials tailored for the characteristics of individual smokers, with controls receiving no materials, or stage‐matched or non‐tailored materials. Most of these studies used more than one mailing. Pooling studies that compared tailored self‐help with no self‐help, either on its own or compared with advice, or as an adjunct to advice, showed a benefit of providing tailored self‐help interventions (12 studies; n = 19,190; RR 1.34, 95% CI 1.20 to 1.49; I² = 0%) with little evidence of difference between subgroups (10 studies compared tailored with no materials, n = 14,359; RR 1.34, 95% CI 1.19 to 1.51; I² = 0%; two studies compared tailored materials with brief advice, n = 2992; RR 1.13, 95% CI 0.86 to 1.49; I² = 0%; and two studies evaluated tailored materials as an adjunct to brief advice, n = 1839; RR 1.72, 95% CI 1.17 to 2.53; I² = 10%). When studies compared tailored self‐help with non‐tailored self‐help, results favoured tailored interventions when the tailored interventions involved more mailings than the non‐tailored interventions (9 studies; n = 14,166; RR 1.42, 95% CI 1.20 to 1.68; I² = 0%), but not when the two conditions were contact‐matched (10 studies; n = 11,024; RR 1.07, 95% CI 0.89 to 1.30; I² = 50%). We judged the evidence to be of moderate certainty in accordance with GRADE, downgraded for risk of bias.
Five studies evaluated self‐help materials as an adjunct to nicotine replacement therapy; pooling three of these provided no evidence of additional benefit (n = 1769; RR 1.05, 95% CI 0.86 to 1.30; I² = 0%). Four studies evaluating additional written materials favoured the intervention, but the lower confidence interval crossed the line of no effect (RR 1.20, 95% CI 0.91 to 1.58; I² = 73%). A small number of other studies did not detect benefit from using targeted materials, or find differences between different self‐help programmes.
Authors' conclusions
Moderate‐certainty evidence shows that when no other support is available, written self‐help materials help more people to stop smoking than no intervention. When people receive advice from a health professional or are using nicotine replacement therapy, there is no evidence that self‐help materials add to their effect. However, small benefits cannot be excluded. Moderate‐certainty evidence shows that self‐help materials that use data from participants to tailor the nature of the advice or support given are more effective than no intervention. However, when tailored self‐help materials, which typically involve repeated assessment and mailing, were compared with untailored materials delivered similarly, there was no evidence of benefit.
Available evidence tested self‐help interventions in high‐income countries, where more intensive support is often available. Further research is needed to investigate effects of these interventions in low‐ and middle‐income countries, where more intensive support may not be available.
Plain language summary
Do printed self‐help materials help people to quit smoking?
Background
We reviewed the evidence showing how effective printed self‐help materials are in helping people to quit smoking. We looked for studies of any type of printed self‐help that gave structured support and advice about quitting. This could include any booklets, leaflets, or information sheets that set out some kind of structured programme that someone could follow to help them quit smoking. We also included self‐help in audio or video format, but we did not include internet programmes or other formats. We were interested in the number of people who were not smoking for at least six months from the time they were given the self‐help materials. Studies had to include people who smoked, but those people did not need to be currently trying to quit smoking.
Study characteristics
We searched electronic databases for studies that investigated printed self‐help. We ran our most recent search in March 2018, and so far we have found 75 studies. Most studies took place in North America or Europe and were carried out with adults, although they did not require that people wanted to quit smoking to join. Studies delivered self‐help materials in person or by post, some all at once, and some spread out over the length of the study. In most studies, self‐help was the only support people were given, but some studies tested self‐help given with other kinds of support to test whether there was any extra benefit from written self‐help. Some studies gathered information about individual smokers, so they could tailor self‐help to better help them.
Key results
Eleven studies including over 13,000 people provided evidence of a small benefit of printed self‐help materials when provided on their own. Our confidence in this evidence was only moderate, because these studies took place in high‐income countries, which makes them less relevant to people from lower‐income countries, who might benefit differently. When people used self‐help as well as receiving face‐to‐face advice on how to stop smoking (11 studies), there was no extra benefit compared with the effect of that advice without printed self‐help.
Thirty‐two studies provided written self‐help that was individually tailored, comparing it with either non‐tailored self‐help or nothing. Evidence based on ten studies including nearly 15,000 people showed that tailored self‐help was more helpful than nothing. Our confidence in this evidence is moderate, because some of these studies might have had problems in the ways they were carried out that could have affected the results.
Conclusions
When no other support is available, written self‐help materials help more people to stop smoking compared with getting no help at all. People were more likely to make successful quit attempts when they were also given face‐to‐face support or nicotine replacement therapy, but printed self‐help did not make these people more likely to quit.
Self‐help materials that were tailored to help individual people are more effective than no help at all. However, tailoring these materials often involves more contact with the research team, and when we compared tailored self‐help with regular self‐help that involved the same amount of contact, we did not find a difference in quit rates.
The studies we found looked at self‐help given to people in high‐income countries, where more intensive support is often available. More research is needed to find out how well self‐help works for people in low‐ and middle‐income countries, where more intensive support is less available.
Summary of findings
Summary of findings for the main comparison. Print‐based self‐help compared to no materials for smoking cessation.
| Print‐based self‐help compared to no materials for smoking cessation | ||||||
| Patient or population: people who smoke; not selected for interest in quitting smoking Settings: community ‐ materials provided without personal contact Intervention: print‐based self‐help materials Comparison: no materials | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No materials | Print‐based self‐helpmaterials | |||||
| Abstinence ‐ non‐tailored self‐help Follow‐up: 6+ months | Moderate‐risk population1 | RR 1.19 (1.03 to 1.37) | 13,241 (11 studies) | ⊕⊕⊕⊝ moderate2,3 | No evidence of effect detected in other studies where the controls received other materials (n = 6), or wher all participants had personal contact (n = 5) or received brief advice (n = 11) | |
| 50 per 1000 | 60 per 1000 (52 to 69) | |||||
| Abstinence ‐ individually tailored self‐help Follow‐up: 6+ months | Moderate‐risk population1 | RR 1.34 (1.19 to 1.51) | 14,359 (10 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 50 per 1000 | 81 per 1000 (71 to 91) | |||||
| CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Control group success rate based on average across studies. Low rate reflects intervention in participants not selected on basis of motivation to quit. All studies conducted in high‐income countries. 2Most studies at high or unclear risk of bias, but no evidence of differential effect based on risk of bias. Not downgraded. 3Downgraded one level for indirectness: indirectly relevant to populations in low‐ and middle‐income countries because evidence for this comparison came from studies conducted solely in high‐income countries, and there is reason to believe the intervention might work differently in low‐ and middle‐income countries. 4Downgraded one level for risk of bias: all but one study at high or unclear risk of bias. One study at low risk of bias was small with wide confidence intervals.
Background
Description of the condition
The World Health Organization has identified tobacco use as the leading behavioural risk factor for preventable premature death (WHO 2012). Globally, tobacco smoking is currently estimated to cause the death of about seven million people a year (WHO 2017). More than 80% of tobacco‐related deaths are projected to occur in low‐ and middle‐ income countries (WHO 2012). Adverse health effects from tobacco use include cardiovascular disease, respiratory disease, and cancer.
Description of the intervention
The aim of self‐help interventions is to provide some of the benefits of intensive behavioural interventions without the need to attend treatment sessions. Such materials can be disseminated and used on a much wider scale than therapist‐delivered treatment. They therefore represent a bridge between the clinical approach to smoking cessation oriented towards individuals and public health approaches that target populations (Curry 1993). Self‐help programmes were first developed as written materials, primarily delivered in print, but other formats such as videos and audiotapes have also been used. New technologies enable delivery of information and support via the internet and mobile phones; separate Cochrane Reviews have evaluated these self‐help formats (Taylor 2017; Whittaker 2016).
How the intervention might work
Self‐help materials provide structured programmes and advice aimed at helping people to quit smoking by following the programmes therein. These materials and programmes can have a theoretical basis or can be tailored to the individuals trying to quit. Printed self‐help materials represent a low‐cost intervention with potentially wide reach.
Why it is important to do this review
Behavioural strategies to aid smoking cessation range from very brief interventions, such as advice from a physician, to intensive multi‐component programmes. There is good evidence supporting the effectiveness of brief, therapist‐delivered interventions, such as physician advice (Stead 2013a), as well as the additional effect of more intensive behavioural interventions, such as group therapy (Stead 2017), individual counselling (Lancaster 2017), and telephone counselling (Stead 2013b). However, a major limitation of therapist‐delivered behavioural interventions is that they reach only a small proportion of smokers. Most successful quitters give up on their own (Lee 2007). Methods to support otherwise unaided quit attempts therefore have the potential to help a far greater proportion of the smoking population. This is especially the case in lower‐income countries, where more intensive cessation support may not be available.
Previous reviews and versions of this review have found evidence of a small but significant effect of print‐based self‐help interventions. However, new theories and technologies have led to continued interest and research in this field. In particular, the ability to tailor materials based on individual characteristics through computer‐based algorithms. Such personalisation is the focus of most new research in this field.
The aim of this review is to summarise existing evidence for print‐based, video, and audiotape forms of self‐help interventions in promoting smoking cessation.
Objectives
The aims of this review were to determine the effectiveness of different forms of print‐based self‐help materials that provide a structured programme for smokers to follow, compared with no treatment and with other minimal contact strategies, and to determine the comparative effectiveness of different components and characteristics of print‐based self‐help, such as computer‐generated feedback, additional materials, tailoring of materials to individuals, and targeting of materials at specific groups.
Methods
Criteria for considering studies for this review
Types of studies
We sought randomised controlled trials with a minimum follow‐up of six months, where at least one arm comprised a print‐based self‐help intervention without repeated face‐to‐face therapist contact compared with another print‐based self‐help intervention or with a minimal control. We included studies that allocated participants to treatment via a quasi‐randomised method, but, where appropriate, we used sensitivity analysis to determine whether inclusion of these studies altered the results.
Types of participants
We included any smokers except pregnant smokers and adolescent smokers. Separate Cochrane Reviews have evaluated interventions in pregnant smokers (Coleman 2015; Chamberlain 2017), and in adolescent smokers (Fanshawe 2017).
Types of interventions
We defined a 'self‐help intervention' as any manual or programme designed to be used by individuals to assist a quit attempt not aided by health professionals, counsellors, or group support. This review primarily covers written materials such as booklets and leaflets, but information could also have been provided via audio or video or a similar medium. Separate reviews cover interventions designed to be delivered via the internet, or via mobile phone (Taylor 2017; Whittaker 2016). Materials could be aimed at smokers in general; could target particular populations of smokers, for example, those of different ages or ethnic groups; or could be tailored to individual smoker characteristics. We did not include brief leaflets on the health effects of smoking ‐ we considered them to be a control intervention if compared with a more substantial manual. We considered interventions with a single session of minimal face‐to‐face contact for the purpose of supplying the self‐help programme materials as self‐help alone. Where a face‐to‐face meeting included discussion of programme content, we categorised this as brief advice in addition to self‐help materials. We excluded interventions that provided repeated sessions of advice in addition to self‐help materials. Separate Cochrane Reviews cover telephone counselling or hotlines as adjuncts to self‐help materials (Stead 2013b), and interventions aimed at relapse prevention (Hajek 2013).
Types of outcome measures
We used sustained abstinence, or point prevalence, where available. We included studies that used self‐report of cessation alone or biochemically validated cessation.
Search methods for identification of studies
We identified studies included in previous reviews and meta‐analyses, and we searched the Cochrane Tobacco Addiction Review Group Specialised Register of controlled trials for additional studies, using the terms self‐help*, manual*, booklet*, or pamphlet* in the title or abstract, or as a keyword (Appendix 1). We conducted the most recent search of the Register in March 2018. At the time of the search, the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL; 2018, Issue 1) in the Cochrane Library; MEDLINE (via OVID) to update 20180209; Embase (via OVID) to week 201807; and PsycINFO (via OVID) to update 20180212. See the Cochrane Tobacco Addiction Group website for full search strategies and a list of other resources searched.
Data collection and analysis
Two review authors (JLB and JMOM) extracted data. Information extracted included details of the intervention, population recruited, method of randomisation, completeness of follow‐up, way in which cessation was defined, and whether self‐reported cessation was validated.
We summarised individual study results as a risk ratio (RR), calculated as: (number of quitters in intervention group/number randomised to intervention group)/(number of quitters in control group/number randomised to control group). Where appropriate, we performed meta‐analysis using a Mantel‐Haenszel random‐effects method to estimate a pooled risk ratio with 95% confidence intervals (CI). We estimated statistical heterogeneity between studies using the I² statistic (Higgins 2003). Values between 30% and 60% may suggest moderate heterogeneity, and values over 75% represent considerable heterogeneity (Higgins 2011).
We categorised studies according to the amount of face‐to‐face contact provided to both treatment and comparison intervention groups, whether or not any written materials were given to the comparison group, and whether the material was individually tailored. Comparison tables included the following.
Non‐tailored self‐help materials versus no treatment or a leaflet only, without face‐to‐face contact.
Non‐tailored self‐help materials versus no treatment or a leaflet only, with face‐to‐face contact.
Non‐tailored self‐help materials and brief advice versus brief advice alone.
Individually tailored materials versus no materials.
Individually tailored versus standard or stage‐matched materials.
Self‐help materials plus nicotine replacement therapy (NRT) versus NRT alone.
A Cochrane Review on individual counselling covered any studies comparing self‐help to individual counselling (Lancaster 2017). The Cochrane Review on group therapy for smoking cessation covered self‐help versus group counselling (Stead 2017). Self‐help plus NRT versus self‐help alone is a test of the efficacy of NRT; a Cochrane Review on NRT versus control covers this topic (Hartmann‐Boyce 2018).
Comparison tables also addressed the following enhancements and adjuncts to self‐help.
Tailored self‐help programmes versus non‐tailored programmes, or no‐intervention controls.
Targeted materials versus standard materials.
Provision of additional materials.
Different self‐help programmes or different media formats (e.g. audio, video) compared to each other.
We define 'tailored materials' as those that make use of participant characteristics to provide individualised programmes. We also include in this category interventions providing individual written feedback in addition to standard materials. We define 'targeted materials' as those tailored for a broadly defined category of smokers, for example, women with young children, older smokers, or smokers at a particular stage of change (Kreuter 2000).
Earlier versions of this review included telephone counselling and relapse prevention interventions. A separate Cochrane Review evaluated the use of proactive telephone counselling or provision of telephone hotlines as an adjunct to self‐help materials (Stead 2013b), so we did not include in this review studies that compare only these interventions. Likewise, Hajek 2013 evaluated interventions aiming to prevent relapse, so we have no longer included them in this review.
'Summary of findings' table
We created a 'Summary of findings' table for our primary outcomes, in accordance with standard Cochrane methods. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of evidence for each outcome.
Results
Description of studies
For the present update of this review, we identified 491 potentially relevant records new to the Tobacco Addiction Group Specialised Register since the last update; three new studies met the inclusion criteria (Figure 1). The review now includes 75 studies of self‐help methods. We treated one study with a factorial design as two studies for data entry purposes (Killen 1997; Killen 1997 +NP). Thirty‐four of the included studies compared standard self‐help materials with no intervention or provided standard materials as an adjunct to advice. The other studies compared targeted or tailored self‐help methods or compared other variations of programmes. Some studies used multiple interventions, testing the effects of different types of information or of increasing amounts of material. Studies of self‐help materials were carried out in a range of settings. Some studies provided the materials without face‐to‐face contact or any additional motivating strategy. Some studies tested the use of materials for people who had called quitlines (self‐help materials were the main form of support offered) or the use of materials as an adjunct to counselling (Strecher 2005). In healthcare settings, studies more frequently provided self‐help materials as an adjunct to brief advice to quit. Some studies described as testing self‐help materials included relatively high levels of face‐to‐face support, although less than in formal counselling programmes. Most studies did not specify an interest in quitting as a selection criterion.
1.
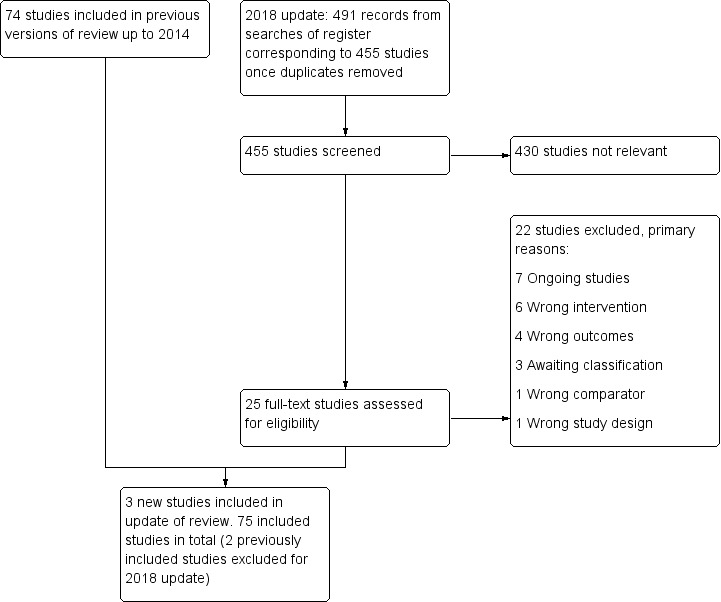
Flow diagram for 2018 update.
The content and format of the self‐help programmes varied. The most frequently used materials were the American Lung Association (ALA) cessation manual: Freedom from Smoking in 20 days, and the maintenance manual: A Lifetime of Freedom from Smoking. Most other programmes were not named or described fully. Materials have tended to become more complex over time and to incorporate more techniques from behaviour therapy approaches. Most recent studies have used computerised expert systems to provide tailored materials judged to be relevant to the characteristics of each smoker, using baseline data. We specified that materials should contain a structured programme for quitting. When it was not clear whether the materials provided met these criteria, we performed sensitivity analysis to determine the effects of including or excluding these studies.
Fraser 2014 factorially tested combinations of ‘on’ and ‘off’ versions of five interventions: the National Cancer Institute website versus a ‘lite’ website, telephone counselling versus no counselling, a self‐help manual versus a brief brochure, motivational email messages versus no messages, and nicotine replacement therapy (NRT) versus no NRT. We were interested in two comparisons from this study. The first compared the arm comprising the ‘off’ version of each intervention except the self‐help manual with the arm comprising the ‘off’ version of every intervention. The second compared the arm comprising the ‘off’ version of each intervention except the self‐help manual and NRT with the arm comprising the ‘off’ version of each intervention except NRT. Unfortunately the study report did not report abstinence rates for these comparisons, and when we contacted the study author team, we received no response. As such, we were unable to include this study in the relevant meta‐analyses.
Further details on each of the included studies can be found in the Characteristics of included studies tables. Details of 79 studies excluded at full‐text stage can be found in the Characteristics of excluded studies tables. The most common reasons for listing studies as excluded are that study authors used self‐help materials as the control, and follow‐up was too short ‐ typically only one month. We previously included Killen 1990 and Fortmann 1995 in this review, but we excluded them from this update on the grounds that they are studies of relapse prevention and are now included in another Cochrane Review (Hajek 2013). Details of seven ongoing studies and three studies for which there was insufficient information to include or exclude are presented in the Characteristics of ongoing studies and Characteristics of studies awaiting classification tables respectively.
Non‐tailored self‐help materials compared to no intervention (Comparisons 1 and 2)
Non‐tailored materials without face‐to‐face contact
We identified 20 studies that sent non‐tailored self‐help materials to smokers without any personal contact. Thirteen of these sent no materials to the comparison control group (Cuckle 1984; Ledwith 1984; Lando 1991; Gritz 1992; Pallonen 1994; Curry 1995; Humerfelt 1998; Dijkstra 1999; Schofield 1999; Becona 2001a; Becona 2001b; Lennox 2001; Willemsen 2006). In the other seven studies, the control group received a brief leaflet (Davis 1984; Cummings 1988; Orleans 1991; Lichtenstein 2000; Lichtenstein 2008; Fraser 2014; Parekh 2014). In 11 studies, participants responded to promotion of smoking cessation programmes or volunteered for a trial. One of these recruited only smokers who were not planning to quit in the next six months (Dijkstra 1999). Two studies sent unsolicited materials to smokers in health maintenance organisations (Gritz 1992; Curry 1995). One sent either tailored or non‐tailored letters from a physician to general practice patients who had answered a questionnaire about smoking behaviour (Lennox 2001); we compared the standard letter with the non‐intervention control in this comparison. One study addressed smoking, diet, physical activity, and weight, so only a subgroup of participants smoked; the control group received information on other health behaviours (Parekh 2014). One study sent a booklet and a personally addressed letter from a consultant to smokers or recent quitters discharged from hospital (Schofield 1999). Three studies targeted factors that might motivate interest in quitting. One of these used a community survey to identify young (aged 30 to 45 years) male smokers with reduced forced expiratory volume in one second (FEV₁) or asbestos exposure. The intervention consisted of self‐help materials accompanied by a letter from a respiratory physician, which drew attention to the individual's higher risk of smoking‐related lung disease and advised quitting (Humerfelt 1998). Two recruited households via a utility bill enclosure offering radon testing, and provided a leaflet (Lichtenstein 2000) or video (Lichtenstein 2008) that highlighted the synergistic impact of radon and smoking and advised on quitting or not smoking indoors. The comparison groups received a standard leaflet about the risks of radon that did not emphasise quitting. Fraser 2014 recruited people visiting a quit smoking website. All but four studies used a single mailing of materials. Becona 2001a and one arm of Becona 2001b sent six weekly mailings; Pallonen 1994 sent stage of change‐based manuals at six‐monthly intervals; one arm of Parekh 2014 received a second assessment and mailing after three months.
Fraser 2014 comprised 32 arms testing combinations of ‘on’ and ‘off’ versions of five interventions and included a comparison of self‐help materials with a minimal brochure. However, because of insufficient data, we were unable to include this study in the analysis.
Non‐tailored materials with brief contact
We identified four studies in which investigators gave non‐tailored self‐help materials personally to participants, but not in the context of formal advice to stop smoking. One study gave the control group health education materials without a specific focus on tobacco use, and intended to give the intervention group a single telephone call (Resnicow 1997). In the other studies, controls received no intervention (Prue 1983; Campbell 1986; Betson 1998). Three studies recruited in outpatient clinics (Prue 1983; Campbell 1986; Betson 1998); the last of these probably included some telephone contact for the self‐help group, although the extent of this is unclear. Resnicow 1997 recruited in healthcare, church, and public housing settings.
Non‐tailored materials and advice versus advice alone
Eleven studies assessed non‐tailored self‐help materials as an adjunct to brief advice about stopping smoking given by a healthcare worker. Three of these studies gave some written materials to the control group. Lando 1988 prescribed nicotine gum to both arms and gave instructions on its use. A doctor alone gave advice in six studies, and a doctor, nurse, or both gave advice in four. In Davies 1992, student nurses advised two smokers each ‐ one before and one after training ‐ to deliver a self‐help manual. Hollis 1993 provided self‐help participants with additional advice from a nurse, as well as a physician message. In a study of physician advice that used a complete factorial design, some participants received structured advice with or without materials, and some received brief advice ‐ we have combined the two levels of advice (Thompson 1988). Kottke 1989 randomised physicians to a workshop with or without a supply of self‐help materials for their patients.
We did not identify any studies that directly compared standard self‐help materials with brief advice.
Tailored self‐help materials (Comparisons 3 and 4)
Thirty‐two studies used materials tailored to the characteristics of individual smokers. Only two of these provided any face‐to‐face contact as part of the baseline intervention (Lipkus 1999; Meyer 2012). Four recruited people who had called a quitline. Borland 2003 recruited only those callers seeking written materials without counselling. Borland 2004 provided brief counselling to some participants before recruitment, and Strecher 2005 and Sutton 2007 ensured that all participants received counselling during their initial call. Just under half of the remaining studies included volunteers who were likely to have been seeking help to quit. Fifteen recruited a mix of people, some of whom were not interested in immediate quit attempts (Velicer 1999; Curry 1995; Lennox 2001; Prochaska 2001a; Prochaska 2001b; Etter 2004; Aveyard 2003; Prochaska 2004; Prochaska 2005; de Vries 2008; Schumann 2008; Meyer 2012; van der Aalst 2012; Gilbert 2013; Parekh 2014). Dijkstra 1999 specifically recruited people not interested in quitting, and Meyer 2016 recruited people not interested in quitting in the next six months. Four studies evaluated multiple risk factor interventions, so only a subgroup of participants smoked (Prochaska 2004; Prochaska 2005; de Vries 2008; Parekh 2014).
Ten studies compared tailored materials with no intervention (Dijkstra 1998b; Prochaska 2001a; Prochaska 2001b; Etter 2004; Prochaska 2004; Prochaska 2005; Meyer 2008; Schumann 2008; Hoving 2010; Meyer 2016). Some of the 19 studies testing the incremental effect of tailoring over standard materials confounded the tailoring with additional contact, so we grouped these studies according to whether or not the number of mailings was matched. Ten studies matched contacts (Burling 1989; Owen 1989; Velicer 1999; Becona 2001a; Lennox 2001; Strecher 2005; Velicer 2006; Sutton 2007; de Vries 2008; van der Aalst 2012). Among studies with additional contacts, some provided the same materials initially but then provided additional tailored materials to the intervention group; six tailored all materials (Curry 1991; Prochaska 1993; Curry 1995; Aveyard 2003; Borland 2003; Gilbert 2013), and three tailored materials only in part (Ledwith 1984; Dijkstra 1999; Borland 2004). Two studies tested tailored materials as an adjunct to advice (Lipkus 1999; Meyer 2012). Webb 2013 compared a placebo tailored intervention (tailoring was not actually conducted, but materials were constructed to suggest it had been) with a standard, non‐tailored intervention.
The method used for obtaining information, the theoretical basis for tailoring materials, the materials provided, and the number of contacts, all varied, and are reported in more detail in the Characteristics of included studies tables. Ten studies tailored materials based only on information provided at baseline (Ledwith 1984; Owen 1989; Dijkstra 1998a; Curry 1995; Lennox 2001; Strecher 2005; Sutton 2007; de Vries 2008; Hoving 2010; van der Aalst 2012), whereas the others sent further materials based on further assessments. Of those interventions that reported the theoretical basis for tailoring, stage of change was by far the most commonly used model, with 15 interventions modelling material on this theory (Pederson 1983; Velicer 1999; Lennox 2001; Prochaska 2001a; Prochaska 2001b; Aveyard 2003; Borland 2003; Etter 2004; Prochaska 2004; Prochaska 2005; Velicer 2006; Meyer 2008; Schumann 2008; Meyer 2012; Meyer 2016). Sutton 2007 tailored materials based on social‐cognitive and perspectives of change theories, and two studies based their intervention on the I‐change model (Dijkstra 1998a; Hoving 2010). Most tailored interventions provided materials at multiple time points.
Self‐help and nicotine replacement therapy (NRT) compared to NRT alone (Comparison 5)
Lando 1988 tested non‐tailored self‐help materials as an adjunct to nicotine replacement. Fraser 2014 comprised 32 arms testing combinations of ‘on’ and ‘off’ versions of five interventions and included a comparison of self‐help materials plus NRT with a minimal brochure plus NRT. However, because of insufficient data, we were unable to include the study in our analysis. We also excluded ICRF 1994 from this comparison because both groups received written materials that we classified as self‐help. For this update, we excluded a study previously included in the review for this comparison because the intervention was targeted at relapse prevention (Fortmann 1995).
Two studies tested tailored/targeted self‐help materials as an adjunct to NRT. One study, published as an abstract (Orleans 2000), used a guide targeted for older smokers and seven age‐tailored computer‐generated mailings as an adjunct to nicotine patches. The control group received a fact sheet on patch‐assisted quitting. Velicer 2006 tested a single tailored letter as an adjunct to stage‐based manuals and nicotine patches for people identified as ready to quit.
Other enhancements or adjuncts to self‐help materials (Comparison 6)
Additional written materials
Four studies examined the effect of further mailings of non‐tailored materials. Owen 1989 compared a quit kit and five‐day cessation plan with a staged correspondence course. McFall 1993 tested the American Lung Association (ALA) manual: Freedom from Smoking in 20 Days, used in conjunction with a televised programme, compared with additional maintenance newsletters and with the manual or programme alone. Cuckle 1984 mailed the materials six months after the quit kit. Brandon 2016 tested two programmes of additional written materials compared with a single mailing. One arm received 19 mailings over 18 months from baseline, and one received eight mailings over 12 months. We added each arm to the meta‐analysis separately.
Additional video
Killen 1997 tested a video as an additional component. Using a factorial design, investigators also tested the effect of nicotine patches, and because there was evidence of an interaction between the NRT and the self‐help condition, we entered the patch and placebo arms separately.
Materials targeted at particular populations of smokers
Five studies compared a manual targeted at a particular population with a standard one. Davis 1992 compared a programme intended for mothers of young children with ALA or National Cancer Institute (NCI) materials. Orleans 1998 compared a guide addressing the quitting needs and barriers of African American smokers with a standard guide that was mailed to smokers calling the NCI Cancer Information Service. Prochaska 1993 provided manuals tailored to smokers' stage of change compared to standard materials. We excluded another study of manuals tailored to older smokers because no long‐term follow‐up has been reported (Rimer 1994). Nollen 2007 compared culturally sensitive materials with standard materials for African American smokers, who also received nicotine patches and two phone calls.
Comparisons between different types of self‐help materials
We identified eight studies that compared different types of self‐help materials that were neither tailored nor personalised, or were delivered over different time periods (Glasgow 1981; Omenn 1988; ICRF 1994; Berman 1995; Becona 2001b; Sykes 2001; Clark 2004; Smith 2004). Two of these studies compared three different sets of materials (Glasgow 1981; Omenn 1988). ICRF 1994 compared a standard 16‐page booklet with a larger manual containing more information about quitting with the use of nicotine patches. Berman 1995 compared two types of materials for smokers volunteering for heart health screening and smoking cessation. Becona 2001b compared a manual with a weekly mailing of six booklets, both based on the same cognitive‐behavioural approach. Sykes 2001 compared a cognitive‐behavioural programme consisting of a handbook, reduction cards, a progress chart, and an audiotape that summarised the programme and provided relaxation music, with a leaflet developed by the UK Health Education Authority, both used as an adjunct to a single introductory session in a group format. Clark 2004 tested a handout listing internet sites providing useful resources, compared with standard self‐help materials. Smith 2004 compared a 44‐page booklet produced by the Canadian Cancer Society with a single‐page advice pamphlet. These were tested using a factorial design, along with telephone counselling of two different intensities (which we collapsed for this review).
Risk of bias in included studies
Figure 2 shows risk of bias judgements for each included study. We have summarised judgements by domain below. We judged 30 studies to be at high risk of bias in at least one domain, 37 to be at unclear risk of bias in at least one domain and not high in any domain, and eight studies to be at low risk of bias across all domains.
2.
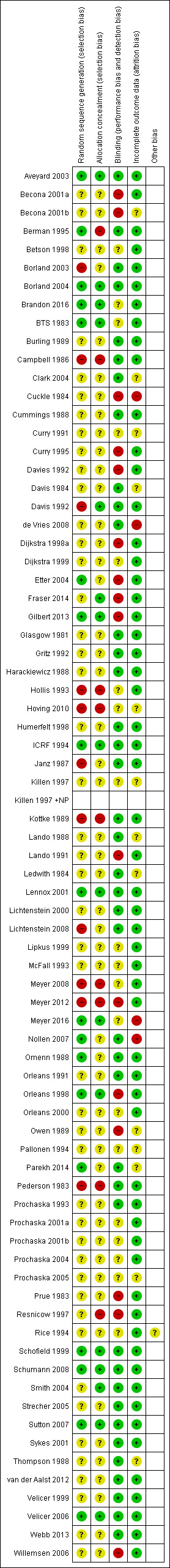
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only 11 study reports provided full descriptions of randomisation and allocation concealment methods that we judged to have low risk of bias (BTS 1983; ICRF 1994;Schofield 1999; Lennox 2001; Aveyard 2003; Borland 2004; Smith 2004; Velicer 2006; Sutton 2007; Schumann 2008; Gilbert 2013). Most studies did not explicitly describe the way in which the randomisation sequence was generated or concealed until participant enrolment. Many studies provided no face‐to‐face contact with participants, and the likelihood of biased allocation was probably low. Four studies used a pseudo‐random method of allocation by day or week of attendance (Pederson 1983; Campbell 1986; Davis 1992; Meyer 2008), and Hollis 1993 used numbers in the patient record. Borland 2003 shuffled questionnaires, and Hoving 2010 used the pre‐printed colour on the questionnaire to determine allocation. Kottke 1989 randomised physicians to intervention groups, and two studies randomised households (Lichtenstein 2000; Lichtenstein 2008). Three studies randomised by recruitment site (Berman 1995; Resnicow 1997; Meyer 2012). For some studies, we judged that the method of generating the allocation could have led to selection bias in the recruitment or assignment of participants. Excluding studies that we judged to be at risk of bias due to an inadequate method of allocation did not alter the conclusions from any meta‐analysis.
Blinding
For this update, we assessed performance and detection bias based on blinding of participants and personnel and on whether biochemical validation was used. Forty‐two studies provided details of blinding, biochemical validation, and/or description of interventions of similar intensity that led us to judge them to be at low risk of bias in this domain. Fifteen studies described procedures that we judged to place the results at high risk of bias in this domain (Prue 1983; Cuckle 1984; Owen 1989; Lando 1991; Davies 1992; Curry 1995; Resnicow 1997; Dijkstra 1998a; Orleans 1998; Becona 2001a; Becona 2001b; Etter 2004; Willemsen 2006; Meyer 2012; Gilbert 2013; Fraser 2014), and the remainder did not provide sufficient detail with which to judge; hence we judged them to be at unclear risk of performance and detection bias.
Twenty‐three studies undertook biochemical validation of all self‐reports of quitting, or provided sufficient data to adjust quit rates for the level of misreport in a sample (Glasgow 1981; BTS 1983; Cuckle 1984; Campbell 1986; Harackiewicz 1988; Omenn 1988; Burling 1989; Kottke 1989; Curry 1991; Orleans 1991; Davies 1992; Hollis 1993; ICRF 1994; Killen 1997; Humerfelt 1998; Schofield 1999; Sykes 2001; Becona 2001a; Lennox 2001; Aveyard 2003; Clark 2004; Nollen 2007; Webb 2013). In three cases, a significant other confirmed quitting (Prue 1983; Cummings 1988; Davis 1992). Amongst those that did not report fully biochemically verified quit rates, 15 studies used self‐reported abstinence at a single follow‐up point (Pederson 1983; Prue 1983; Janz 1987; Thompson 1988; Owen 1989; Lando 1991; Resnicow 1997; Orleans 1998; Dijkstra 1999; Lipkus 1999; de Vries 2008; Hoving 2010 (GP arms only); Gilbert 2013; Meyer 2012; Parekh 2014 (single arms only)). In the other studies without validation, participants classified as non‐smokers had reported sustained abstinence or had been abstinent at one or more points before final follow‐up.
Incomplete outcome data
Some reports give quit rates based only on those people contacted at follow‐up. In this review, we have followed the methods of the Cochrane Tobacco Addiction Review Group in reporting analyses based on the total number randomised wherever possible, with dropouts and participants lost to follow‐up classified as smokers. It has been argued in population‐based studies that it may be pessimistic, and may introduce bias, to classify all dropouts as continuing smokers if those data are missing at random (Velicer 1999). We have noted in the 'Risk of bias' tables the number of dropouts by group, and whether the data used in this review included all randomised participants. Where the proportion of dropouts was high and differed across treatment conditions, we performed sensitivity analyses to assess whether excluding dropouts would affect the conclusions. It should be noted that if the proportion of dropouts is similar across conditions, including losses as treatment failures does not affect the risk ratio. A large majority of included studies reported sufficiently similar losses to follow‐up across arms that we judged them to be at low risk of attrition bias. Thirteen did not provide sufficient detail with which to judge, and four reported data on loss to follow‐up that led us to place these studies at high risk of bias in this domain: Nollen 2007 successfully followed up on less than half of participants; two studies reported follow‐up substantially different between intervention and control arms (Cuckle 1984; Meyer 2016); and de Vries 2008 reported only participants who provided data at final follow‐up.
Measures of abstinence
Studies reported a range of measures of abstinence. A minimum follow‐up period of six months was required for inclusion in this review, but 47 of 75 (62.7%) followed up on participants for 12 months or longer. Thirty‐four of these required abstinence to be sustained for a period. Studies that used strict criteria for self‐reported sustained abstinence, with validation at one or more follow‐up points, tended to report lower quit rates for both experimental and comparison interventions. In minimal contact programmes, researchers often reported that obtaining saliva samples for biochemical validation was a problem. Participants may have declined to provide samples for reasons unrelated to their smoking status. Validated quit rates therefore may be particularly low and are likely to underestimate success rates if all those for whom samples are not available are classified as smokers. Measures using abstinence from the first follow‐up may underestimate the long‐term effect of having access to the self‐help materials, which may prompt a quit attempt some time after they were supplied. Studies with long follow‐up that use only point prevalence abstinence rates may show a trend toward increasing quit rates as more smokers make attempts over time.
Effects of interventions
See: Table 1
Studies varied in the amount of face‐to‐face contact given with both experimental and comparison interventions, and in whether or not control materials were given to smokers in the comparison group. In considering the effects of self‐help, we grouped studies by these categories. For Comparison 1, we calculated a pooled risk ratio (RR) separately for each level of personal contact with subgroups for the type of control. For Comparison 2, we used the same study data and pooled all subgroups to estimate an overall pooled RR from all studies comparing self‐help with no self‐help.
Non‐tailored self‐help materials compared to no intervention (Comparisons 1 and 2)
Non‐tailored materials without face‐to‐face contact
Thirteen studies with a total of over 15,500 participants provided standard non‐tailored self‐help manuals or materials by post; control groups received no materials. Substantial heterogeneity (I² = 71%) was attributable to the inclusion of two studies conducted in Spain that showed very strong effects (Analysis 1.2) (Becona 2001a; Becona 2001b). Both studies enrolled treatment‐seeking smokers, and those in the control group knew they would be offered treatment after six months ‐ a possible disincentive to making an unaided quit attempt. Quit rates were also very high in the intervention groups (16% and 25%). We have therefore excluded these studies from this meta‐analysis and calculated a pooled estimated effect for the other 11 studies, amongst which we found no evidence of heterogeneity (I² = 0%). Our meta‐analysis also excluded Fraser 2014, for which we were unable to access sufficient data; however, the study report suggests that no significant effect was detected for a standard self‐help brochure compared to no intervention. Amongst the studies included in our meta‐analysis, the control quit rate ranged from 1% to 11%, with an average of 5%, and the intervention quit rate ranged from 2% to 10%. The pooled risk ratio favoured self‐help interventions, although the confidence interval (CI) only narrowly excluded 1.0 (n = 13,241; risk ratio (RR) 1.19, 95% CI 1.03 to 1.37; I² = 0%; Analysis 1.1.1/Analysis 2.1.1).
1.2. Analysis.
Comparison 1 Non‐tailored self‐help vs no self‐help, pooled by amount of contact, Outcome 2 Neither group had face‐to‐face contact (Becona studies only).
1.1. Analysis.
Comparison 1 Non‐tailored self‐help vs no self‐help, pooled by amount of contact, Outcome 1 Neither group had face‐to‐face contact (long‐term abstinence).
2.1. Analysis.
Comparison 2 Non‐tailored self‐help vs no self‐help, pooling all studies, Outcome 1 Long‐term abstinence.
Six studies provided controls with some form of written materials and did not show benefit of more structured materials (n = 7023; RR 0.87, 95% CI 0.1 to 1.07; I² = 21%; Analysis 1.1.2/Analysis 2.1.2). Pooling these two subgroups does not demonstrate benefit of self‐help materials (n = 20,264; RR 1.05, 95% CI 0.92 to 1.20; I² = 26%).
Non‐tailored materials with brief contact
Four studies including almost 3000 participants delivered materials in person rather than by post. The average control group quit rate was 4.7%. Results show no evidence of heterogeneity, and whilst we failed to find evidence of a significant effect of self‐help materials given with face‐to‐face contact when subgrouped based on whether or not controls received some written materials, the pooled result of all four studies did provide evidence of an effect (n = 2822; RR 1.39, 95% CI 1.03 to 1.88; I² = 0%; Analysis 1.3).
1.3. Analysis.
Comparison 1 Non‐tailored self‐help vs no self‐help, pooled by amount of contact, Outcome 3 Both groups had face‐to‐face contact (long‐term abstinence).
Non‐tailored materials and advice versus advice alone
Eleven studies with a total of over 5000 participants tested self‐help materials as an adjunct to face‐to‐face advice from a healthcare provider. We noted little heterogeneity and found no evidence that the additional self‐help materials significantly increased quit rates (n = 5365; RR 0.99, 95% CI 0.76 to 1.28; I² = 32%; Analysis 1.4). Whether or not the control group received materials did not affect the estimate. Control group quit rates ranged from 2% to 25% with an average of 7%. As would be expected, this is higher than the rates seen in control groups that received no intervention.
1.4. Analysis.
Comparison 1 Non‐tailored self‐help vs no self‐help, pooled by amount of contact, Outcome 4 Both groups had face‐to‐face contact with advice (long‐term abstinence).
Overall effect of non‐tailored self‐help, alone or as adjunct to advice
When we pooled 32 studies of self‐help materials compared to no self‐help, irrespective of the level of contact and support common to the control group, the point estimate showed a small benefit of the intervention but the confidence interval included the possibility of no difference (n = 28,451; RR 1.06, 95% CI 0.95 to 1.19; I² = 25%; Analysis 2.1;Figure 3). (Note: the estimate excludes Becona 2001a, Becona 2001b, and Fraser 2014. Betson 1998 contributes data to two subgroups.)
3.
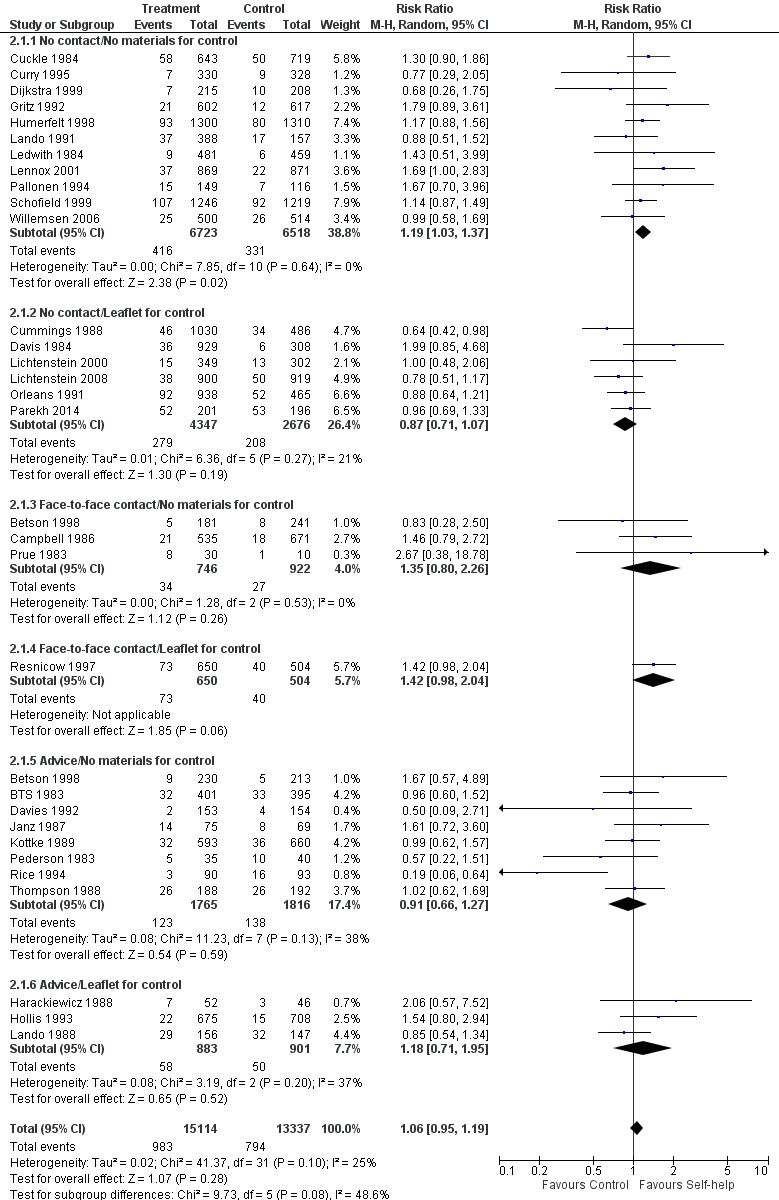
Forest plot of comparison: 2 Self‐help vs no self‐help, pooling all studies, outcome: 2.1 Long‐term abstinence.
Tailored self‐help materials (Comparisons 3 and 4)
Participants in 10 studies receiving tailored self‐help materials had higher quit rates than those receiving no materials at all (n = 14,359; RR 1.34, 95% CI 1.19 to 1.51; I² = 0%; Analysis 3.1.1). Control group quit rates were 4% to 7%. Estimates were largely based on sustained but self‐reported abstinence, with the exception of Prochaska 2005, which reported only point prevalence quit rates. In two studies comparing tailored materials with brief advice (n = 2992), the risk ratio favoured the self‐help intervention but confidence intervals were wide (RR 1.13, 95% CI 0.86 to 1.49; I² = 0%; Analysis 3.1.2). In the two studies evaluating tailored self‐help as an adjunct to brief advice, the risk ratio favoured the self‐help intervention, and confidence intervals excluded no effect (n = 1839; RR 1.72, 95% CI 1.17 to 2.53; I² = 10%; Analysis 3.1.3). When we pooled these three groups of studies comparing tailored self‐help materials with no self‐help, the overall result favoured self‐help interventions with confidence intervals excluding no effect (n = 19,190; RR 1.34, 95% CI 1.20 to 1.49; I² = 0%).
3.1. Analysis.
Comparison 3 Tailored self‐help vs no self‐help, Outcome 1 Long‐term abstinence.
In other studies of tailored materials, the control groups received standard self‐help materials. Ten studies that matched intervention and control groups for number of contacts did not detect a benefit (n = 11,024; RR 1.07, 95% CI 0.89 to 1.30; I² = 50%; Analysis 4.1.1; Figure 4). We noted some evidence of heterogeneity (I² = 50%), largely contributed by Becona 2001a where there was a significant effect of weekly feedback reports. Sutton 2007 included some recent quitters for whom the effect of intervention was smaller, but restricting inclusion to those still smoking at enrolment had little impact on the pooled estimate. Velicer 1999 showed an almost significant effect based on numbers randomised. Excluding dropouts from the denominators increased the estimated effect a little because more people were lost from the expert system intervention groups. This study also tested different numbers of tailored versus non‐tailored mailings but did not detect a consistent dose‐response effect (data not reported).
4.1. Analysis.
Comparison 4 Tailored self‐help vs non‐tailored self‐help, Outcome 1 Long‐term abstinence.
4.
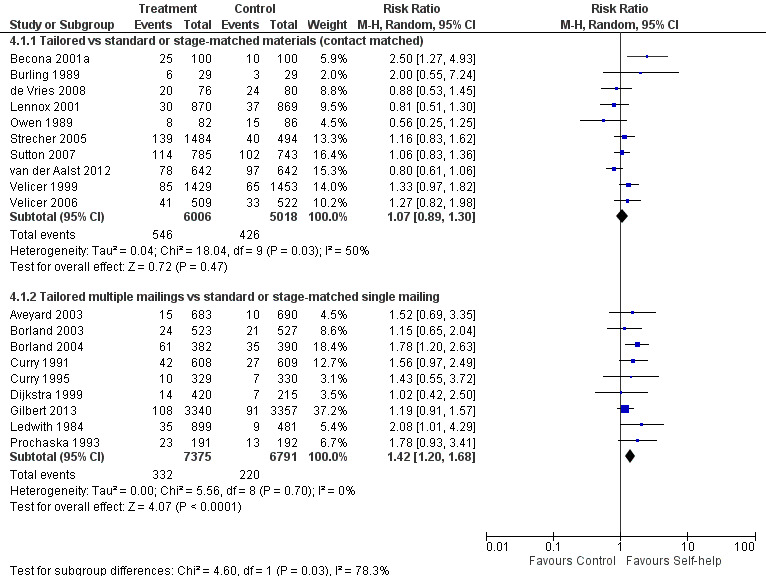
Tailored self‐help materials: Long‐term abstinence.
Nine studies that compared tailored materials with a non‐tailored control and in which the tailored arms received multiple contacts and the non‐tailored arms received a single contact found consistent results in favour of multiple tailored materials. Although none of these studies individually had statistically significant results, the pooled estimate suggests benefit of the intervention (n = 14,166; RR 1.42, 95% CI 1.20 to 1.68; I² = 0%; Analysis 4.1.2; Figure 4).
Webb 2013 detected a benefit of 'placebo tailoring' (n = 424; RR 1.98, 95% CI 1.18 to 3.31; analysis not shown), suggesting that the actual content of the tailored message may be less important than the perception that it is individualised.
Self‐help materials plus nicotine replacement therapy (NRT) compared to NRT alone (Comparison 5)
Studies that specifically examined self‐help materials in addition to NRT did not show any evidence of additional benefit from these materials over the relatively high quit rates achieved with use of NRT (n = 1769; RR 1.05, 95% CI 0.86 to 1.30; I² = 0%; Analysis 5.1). The control group quit rate was over 20% in two of the three studies. Results show no difference between the two studies using standard materials and the two using tailored materials. Due to insufficient data, we were unable to include another relevant study in this analysis (Fraser 2014); however, this study also did not detect a statistically significant benefit of standard self‐help as an adjunct to NRT.
5.1. Analysis.
Comparison 5 Self‐help plus NRT vs NRT alone, Outcome 1 Long‐term abstinence.
Other enhancements or adjuncts to self‐help materials (Comparison 6)
Additional written materials
Pooled results from four studies of additional written materials favoured the intervention, but the lower confidence interval crossed the line of no effect and there was substantial heterogeneity (RR 1.20, 95% CI 0.91 to 1.58; I² = 73%; Analysis 6.1.1). We compared the two arms of Brandon 2016 with control separately in the meta‐analysis. However, when compared with each other the results favoured the standard mailings arm over the intensive mailings arm (RR 1.22, 95% CI 1.02 to 1.47). Cuckle 1984 did not send further materials until six months after sending the initial 'quit kit', but excluding this study does not affect the estimate. We excluded one previously included study from this update because the intervention was given for relapse prevention (Killen 1990). Another Cochrane Review included this study (Hajek 2013).
6.1. Analysis.
Comparison 6 Other enhancements/adjuncts to self‐help materials, Outcome 1 Long‐term abstinence.
Additional video
Killen 1997 used a video as an adjunct to written materials and did not detect a significant overall benefit (n = 424; RR 0.73, 95% CI 0.35 to 1.51; I² = 38%; Analysis 6.1.2). There was a non‐significantly lower quit rate in the active nicotine patch group amongst those who received the video as well as written materials.
Materials targeted at particular populations of smokers
Five studies of materials targeted at specific populations failed to show evidence of significant benefit compared to standard materials (n = 3101; RR 1.12, 95% CI 0.90 to 1.38; I² = 0%; Analysis 6.1.3). Two studies provided telephone counselling to callers to quitlines before sending them materials (Davis 1992; Orleans 1998 (in which the counselling was also tailored)), and Nollen 2007 provided all participants with nicotine replacement therapy. These common components may have contributed to the success in quitting among all groups and limited the potential to detect effects of small differences in adjunct materials.
Comparisons between different types of self‐help material
We did not perform meta‐analysis of this heterogeneous group of studies. Glasgow 1981 (n = 88) compared two different manuals and found no evidence of a difference because of very wide confidence intervals (RR 3.00, 95% CI 0.13 to 67.51). Omenn 1988 (n = 243) compared a multiple‐component manual with a quitters' guide and starter programme, finding no evidence of difference in quit rates (RR 1.30, 95% CI 0.46 to 3.71). ICRF 1994 (n = 1686) also found no significant difference in outcome between those given a longer or a shorter booklet in conjunction with either a nicotine or placebo patch and nurse support (RR 1.02, 95% CI 0.81, 1.27). Berman 1995 compared two types of materials for 348 smoking participants volunteering for heart health screening and cessation. There was no significant difference in quit rates (RR 0.72, 95% CI 0.37 to 1.43). Clark 2004 (n = 171) did not detect the hypothesised benefit of a list of internet resources over standard material. These results favoured the standard materials but with wide confidence intervals (RR 0.45, 95% CI 0.14 to 1.40). Becona 2001b (n = 482) compared weekly mailings with a single manual and detected no significant difference (RR 0.88, 95% CI 0.64 to 1.19). Smith 2004 (n = 632) compared a 44‐page booklet with a pamphlet when used as adjuncts for motivated quitters receiving an extended telephone counselling session and one of two intensities of follow‐up counselling. The effect estimate favoured the longer booklet but the confidence intervals were wide, including the line of no effect (RR 3.26, 95% CI 0.98 to 10.85). Sykes 2001 (n = 260) showed a statistically significant effect after six months with a more than three‐fold increase in the chance of quitting when comparing a cognitive‐behavioural self‐help programme with a standard leaflet, with both used as an adjunct to a single introductory session in a group format (RR 3.45, 95% CI 1.44 to 8.26). This finding was sustained at 12 months' follow‐up (Marks 2002; RR 3.77, 95% CI 1.59 to 8.96).
Discussion
Summary of main results
We defined 'self‐help materials' as those providing a structured approach to smoking cessation. Using this definition, we found moderate evidence that such materials, used on their own and compared with no intervention, marginally but significantly increased the number of people able to quit smoking (Table 1). The point estimate was higher for tailored materials than for non‐tailored materials, but we found no significant differences between studies comparing tailored and non‐tailored materials directly. For non‐tailored materials, the certainty of the result was moderate because whilst the intervention was compared with a no‐intervention control, the evidence came from studies conducted in high‐income countries, where more intensive forms of support are readily available. For tailored materials, where the confidence intervals did exclude the possibility of no effect, certainty was moderate due to risk of bias in the included studies.
Providing non‐tailored self‐help materials in addition to advice from a healthcare professional did not improve the outcome. However, tailored self‐help as an adjunct to brief advice did provide additional benefit, but this comparison included only two studies. We found little evidence of an effect of self‐help materials given in addition to nicotine replacement therapy (NRT).
Overall completeness and applicability of evidence
Self‐help programmes provide information on how to quit smoking but do not provide the sense of being supported nor the interactive elements of more sophisticated behavioural programmes. In many high‐income countries, people know how to quit smoking or can find advice easily; therefore, in these contexts, it is possible that self‐help interventions are less effective than might be the case where such information is not generally known or easily available. The studies included in this review overwhelmingly represent populations with this knowledge and with access to more intensive stop‐smoking support (of the 75 included studies, 74 were conducted in high‐income countries according to the World Bank definition; the one outlier was conducted in Hong Kong). This review, therefore, can inform decisions as to whether print‐based self‐help should be used in developed countries, but paradoxically it cannot tell us about the population that these interventions are now most likely to benefit ‐ people who do not have other support available. By the 2020s or early 2030s, the World Health Organization estimates that more than 7 million tobacco‐related deaths will occur in low‐ and middle‐income countries each year. In light of this, even a very modest effect size could have significant public health impact when applied at a population level. Further research conducted outside of high‐income countries is therefore needed to determine whether print‐based self‐help interventions still have a role to play. Without this, the evidence is incomplete and may not be applicable to the most relevant population.
There is also the potential for ambiguity in the inclusion criteria for this review, as studies do not always report in detail the nature of print‐based interventions, and some interventions may be borderline between print‐based self‐help and printed materials that simply provide information. As such, we may have excluded some studies in which the print‐based intervention did constitute a self‐help intervention as defined in this review on the grounds that it is not clear from the report that the printed materials constituted a structured programme for people to follow to quit smoking. However, whilst there is a risk that we may not have included some eligible studies, we have no reason to suspect that this should result in systematic bias.
Although our searches included clinical trials registries and other sources of unpublished data, we cannot rule out the risk of publication bias. However, we prepared funnel plots for all comparisons with at least 10 studies, and none provided evidence of asymmetry.
Certainty of the evidence
We judged the evidence for our main comparisons to be of moderate certainty in accordance with GRADE. We downgraded the evidence for standard self‐help programmes for being indirectly relevant to populations in low‐ and middle‐income countries because evidence for this comparison came from studies conducted solely in high‐income countries and because of differences between higher‐ and lower‐income countries in literacy rates, availability of support, and prevalence of stop‐smoking messages, there is reason to believe the intervention might work differently. We downgraded the evidence for tailored self‐help materials because of risk of bias. Many study reports did not provide sufficient detail for judgement of risk of bias for some domains. Of the 75 included studies, we judged 30 of them (40%) to be at high risk of bias for one or more domains.
Potential biases in the review process
Our conclusions about the effects of self‐help materials are based on an intention‐to‐treat analysis in which we included all randomised participants, whether or not they received the intended intervention. We also made the assumption that all participants who could not be reached for follow‐up or who declined further participation were still smoking. It has been argued that in minimal contact population‐based studies, participants may be unreachable for reasons unrelated to their smoking status, and that the assumption that they are all smokers leads to unnecessarily conservative quit rates (Hall 2001; Prochaska 2001a). Prochaska, Velicer, and colleagues distinguish between those lost to follow‐up and those who withdraw from the study. We have used numbers randomised in our primary analysis, but we conducted a sensitivity analysis of the effect of using numbers followed up as the denominator. This of course increases the average quit rates in both intervention and control groups, but because dropout rates are typically quite similar across study arms, this has only a small impact on the estimate of relative effect. It has no effect on our conclusions about tailoring.
One reason that it may be difficult to show efficacy for standard self‐help programmes is the level of 'contamination'. Materials encouraging smokers to quit and giving tips are already relatively widely disseminated, so that smokers in a control arm who are motivated to try to give up may well have access to the same kinds of materials that experimental arm smokers have been given. On the other hand, there may be more fundamental reasons why behavioural interventions are more effective when delivered by face‐to‐face contact. Killen has suggested, for example, that the self‐regulatory skills required to withstand the urge to smoke may be better learnt, rehearsed, and retained under the direct supervision of a therapist than through the simple modelling offered by self‐help materials (Killen 1997). Strecher has suggested that the length of generic self‐help manuals and pamphlets may discourage effective use of these materials (Strecher 1994). Meade 1989 suggested that self‐help materials may be too advanced for many readers, and that comprehension can be improved by adjustment of the reading grade level. Tailored materials may have the potential to address these issues.
Agreements and disagreements with other studies or reviews
This review found a small effect in favour of print‐based self‐help compared with no intervention (risk ratio (RR) 1.19, 95% confidence interval (CI) 1.03 to 1.37; I² = 0%). This effect is less pronounced than that found in the Cochrane Review of mobile‐based interventions (Taylor 2017; RR 1.67, 95% CI 1.46 to 1.90; I² = 59%), and it is more pronounced than that found in the Cochrane Review of internet‐based interventions (Whittaker 2016; RR 0.92, 95% CI 0.78 to 1.09; I² = 0%). However, the results of the internet‐based interventions review were based on a comparison with an active control rather than no treatment.
Our review favoured tailored self‐help over no materials (RR 1.34, 95% CI 1.19 to 1.51; I² = 0%). When comparing tailored self‐help to non‐tailored self‐help, results favoured tailored interventions when the tailored interventions contained more mailings than the non‐tailored interventions (RR 1.42, 95% CI 1.20 to 1.68; I² = 0%), but not when the two conditions were contact matched (RR 1.07, 95% CI 0.89 to 1.30; I² = 50%). The Cochrane Review of internet‐based interventions favoured interactive and tailored interventions over non‐active control (RR 1.15, 95% CI 1.01 to 1.30) and favoured an interactive or tailored programme over a non‐tailored internet intervention, but the estimate crossed the null (RR 1.10, 95% CI 0.99 to 1.22; I² = 0%). The review did not comment on any differences in contact between groups, but it is unlikely that contact differed because of the online nature of the interventions. Given the difficulty of obtaining baseline data and the delays involved in mailing printed materials, newer formats for providing self‐help support may have greater potential for providing relevant and timely interventions. Although the evidence from studies is not yet optimal, using the internet to provide individually tailored information and support appears promising and may avoid the limitations of printed self‐help materials. Using mobile phones to deliver text message‐based interventions also shows promise for supporting people who are making quit attempts.
Authors' conclusions
Implications for practice.
Structured self‐help programmes provided in short booklets increase the likelihood of a person stopping smoking successfully in the medium to long term. However, there is no evidence that they add to the effectiveness of brief advice or pharmacological interventions.
Print‐based self‐help that is tailored to the characteristics of individual smokers may be more helpful. However, more modern formats for providing tailored self‐help support, such as the internet and mobile phones, may have greater potential because of advantages in gathering information to tailor by and in delivering support more quickly and flexibly.
Implications for research.
Almost all included studies were conducted in high‐income countries with well‐developed tobacco control policies and high literacy rates. The effect of this climate on the intervention effectiveness is unclear. In low‐ and middle‐income countries, tobacco control policies are often less developed and literacy rates may be lower than in high‐income countries. Future research in low‐ and middle‐ income countries would be useful because the intervention is potentially more cost‐effective than other cessation aids, but the impact of print‐based self‐help interventions in such a context remains unclear.
What's new
| Date | Event | Description |
|---|---|---|
| 9 January 2019 | Amended | Minor change to phrasing of abstract |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 5 September 2018 | New citation required but conclusions have not changed | Conclusions unchanged |
| 5 September 2018 | New search has been performed | Search updated to March 2018. Three new studies included |
| 7 May 2014 | New citation required but conclusions have not changed | JH‐B added as author. Review title changed from "Self‐help interventions for smoking cessation" |
| 7 May 2014 | New search has been performed | Updated with 6 new studies. 'Summary of findings' table added. Risk of bias domains added |
| 28 January 2009 | New search has been performed | Updated with 10 new studies for Issue 2, 2009. No major changes to results |
| 29 October 2008 | Amended | Converted to new review format |
| 28 April 2005 | New citation required and minor changes | Updated for Issue 3, 2005, with 9 new studies. Most studies used tailored interventions and strengthened the evidence that tailored materials are more useful than standard ones |
| 10 April 2002 | New citation required and minor changes | Updated for Issue 3, 2002, with 10 new studies. Most studies used tailored interventions and strengthened the evidence that tailored materials are more useful than standard ones |
| 13 October 1999 | New search has been performed | Updated for Issue 1, 2000, with 4 new trials |
Acknowledgements
Our thanks to Sue Curry, Paul McDonald, and Saul Shiffman for their comments, and to Ann Varady, Joe Rossi, Christine Edwards, Neal Boyd, Virginia Rice, and Tracy Orleans for providing additional data from published or unpublished studies. Our thanks also to Timothy Lancaster and Lindsay Stead, who were the authors of previous versions of this review, and Sandra Wilcox and Lee Bromhead, who reviewed the plain language summary.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care. JHB is also funded in part by the NIHR Oxford Biomedical Research Centre (BRC).
Appendices
Appendix 1. CRS search strategy
#1 (self‐help OR selfhelp OR manual* OR booklet* OR pamphlet*):TI,AB,MH,EMT,KW,KY,XKY #2 (leaflet* or letter* or video*):TI,AB,MH,EMT,KW,KY,XKY #3 #1 OR #2
Data and analyses
Comparison 1. Non‐tailored self‐help vs no self‐help, pooled by amount of contact.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neither group had face‐to‐face contact (long‐term abstinence) | 17 | 20264 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.92, 1.20] |
| 1.1 Control group given no materials | 11 | 13241 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.37] |
| 1.2 Control group given leaflet/pamphlet | 6 | 7023 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.07] |
| 2 Neither group had face‐to‐face contact (Becona studies only) | 2 | 924 | Risk Ratio (M‐H, Random, 95% CI) | 10.91 [5.03, 23.66] |
| 2.1 Control group given no materials | 2 | 924 | Risk Ratio (M‐H, Random, 95% CI) | 10.91 [5.03, 23.66] |
| 3 Both groups had face‐to‐face contact (long‐term abstinence) | 4 | 2822 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.03, 1.88] |
| 3.1 Control group given no materials | 3 | 1668 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.80, 2.26] |
| 3.2 Control group given leaflet/pamphlet | 1 | 1154 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.98, 2.04] |
| 4 Both groups had face‐to‐face contact with advice (long‐term abstinence) | 11 | 5365 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.28] |
| 4.1 Control group given no materials | 8 | 3581 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.66, 1.27] |
| 4.2 Control group given leaflet/pamphlet | 3 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.71, 1.95] |
Comparison 2. Non‐tailored self‐help vs no self‐help, pooling all studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term abstinence | 31 | 28451 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.95, 1.19] |
| 1.1 No contact/No materials for control | 11 | 13241 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.03, 1.37] |
| 1.2 No contact/Leaflet for control | 6 | 7023 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.07] |
| 1.3 Face‐to‐face contact/No materials for control | 3 | 1668 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.80, 2.26] |
| 1.4 Face‐to‐face contact/Leaflet for control | 1 | 1154 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.98, 2.04] |
| 1.5 Advice/No materials for control | 8 | 3581 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.66, 1.27] |
| 1.6 Advice/Leaflet for control | 3 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.71, 1.95] |
Comparison 3. Tailored self‐help vs no self‐help.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term abstinence | 12 | 19190 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.20, 1.49] |
| 1.1 Tailored materials vs no materials | 10 | 14359 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.19, 1.51] |
| 1.2 Tailored materials vs brief advice | 2 | 2992 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.86, 1.49] |
| 1.3 Tailored materials as an adjunct to advice | 2 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.17, 2.53] |
Comparison 4. Tailored self‐help vs non‐tailored self‐help.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term abstinence | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Tailored vs standard or stage‐matched materials (contact matched) | 10 | 11024 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.89, 1.30] |
| 1.2 Tailored multiple mailings vs standard or stage‐matched single mailing | 9 | 14166 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.20, 1.68] |
Comparison 5. Self‐help plus NRT vs NRT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term abstinence | 3 | 1769 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.86, 1.30] |
| 1.1 Non‐tailored materials | 1 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.34] |
| 1.2 Tailored materials | 2 | 1466 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.88, 1.41] |
Comparison 6. Other enhancements/adjuncts to self‐help materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term abstinence | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Additional written materials | 4 | 4741 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.91, 1.58] |
| 1.2 Additional video | 2 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.51] |
| 1.3 Targeted materials vs standard materials | 5 | 3101 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.90, 1.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aveyard 2003.
| Methods | Setting: 65 general practices, UK Recruitment: volunteers from random selection of smoking patients; not selected for motivation | |
| Participants | 2471 smokers, 1373 in relevant arms, > 80% in pre‐contemplation or contemplation, 10% to 14% in preparation 54% female, average age 41, average cpd 20 | |
| Interventions | No face‐to‐face contact ∙ Standard self‐help materials, single mailing ∙ Self‐help manual based on transtheoretical (SoC) model; expert system letter tailored on baseline questionnaire. Further questionnaires at 3 months and 6 months for additional letters (approximately 50% received 3 letters) | |
| Outcomes | Abstinence at 12 months, sustained for 6 months Validation: saliva cotinine < 14.2 ng/mL | |
| Notes | 2 vs 1; tailored self‐help vs standard self‐help | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using minimisation to balance SoC, addiction, and socioeconomic status |
| Allocation concealment (selection bias) | Low risk | Baseline questionnaires read optically and data transferred automatically to the Access database, which performed the minimisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Interventions of similar intensity 12 months: abstinence "was confirmed with salivary cotinine, so that we had unconfirmed and confirmed prevalence of quitting" Confirmed figures used in analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24% of self‐help and 24% of control lost to follow‐up Included in ITT analysis here; sensitivity analyses allowing for differential dropout did not change findings |
Becona 2001a.
| Methods | Setting: community, Spain Recruitment: community volunteers, mainly in contemplation or preparation SoC | |
| Participants | 300 smokers 48% female, average age 37, average cpd 26 | |
| Interventions | ∙ No intervention; treatment offered after 6 months' follow‐up ∙ Standard self‐help pamphlets; 6 mailed weekly with personalised letter ∙ 2 with individual feedback based on weekly reports plus 2 additional 1‐page reports | |
| Outcomes | Abstinence at 6 months or 12 months, sustained since initial quit Validation: CO < 9 ppm | |
| Notes | 2 vs 1, self‐help vs control; excluded from Analysis 2.1 because of heterogeneity; quit rates 16% vs 0% at 6 months 3 vs 2, 12‐month outcome; tailored materials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Wait‐list control (control group participants told treatment would be delayed) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% lost to follow‐up, included in ITT analyses |
Becona 2001b.
| Methods | Setting: community, Spain Recruitment: smokers interested in quitting within 6 months | |
| Participants | 724 smokers, 42% female, average age 37, average cpd 26 | |
| Interventions | ∙ Wait‐list control (followed for only 6 months) ∙ Self‐help manual ∙ Self‐help brochures sent weekly | |
| Outcomes | Abstinence at 12 months (30‐day point prevalence) or 6 months (7‐day point prevalence) Validation: CO at 12 months | |
| Notes | 3 plus 2 vs 1, self‐help vs control; 6 months' follow‐up; excluded from meta‐analysis of Comparison 2 due to heterogeneity 2 vs 3 in Comparison between materials; not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Wait‐list control |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All randomised participants included in ITT analysis, but number followed up not reported |
Berman 1995.
| Methods | Setting: multi‐ethnic community, USA Recruitment: via schools; smokers interested in health screening and cessation | |
| Participants | 348 smokers, 51% female, average age 37 | |
| Interventions | All participants received cardiovascular health screening and educational materials ∙ Freedom from Smoking for You and Your Family, or Spanish equivalent; minimally tailored message at completion of 3 months' telephone follow‐up and tailored letter (group class offered after 6 months' follow‐up) ∙ How to Double Your Quitting Power, or Spanish equivalent | |
| Outcomes | Abstinence at 6 months, continuous (other outcomes also reported, no differences in findings) Validation: attempted unsuccessfully at 12 months | |
| Notes | No non‐self‐help control, so does not contribute to main analysis; no differences at any time point and no definition of 'abstinence' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by school using coin toss |
| Allocation concealment (selection bias) | High risk | Participants enrolled proactively after randomisation, so potential for selection bias Fewer participants in control (179) than experimental (267) conditions |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation attempted but very few participants provided samples; however, interventions of similar intensity differed only by content, so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 218 (62.6%) reached at 12 months' follow‐up |
Betson 1998.
| Methods | Setting: government outpatient clinic, Hong Kong Recruitment: smokers aged < 65 | |
| Participants | 865 smokers, 92% male, 49% smoking > 10 cpd | |
| Interventions | ∙ No intervention ∙ Self‐help materials (Chinese translation of American Cancer Society booklet) ∙ Physician advice (1 minute, based on 4 As) ∙ Physician advice and self‐help booklet | |
| Outcomes | Abstinence at 1 year (sustained from 3 months) Validation: poor response to request for urine specimen, so data based on self‐report | |
| Notes | 2 vs 1, self‐help with face‐to‐face contact 4 vs 3, self‐help as adjunct to advice Full paper provided by Professor Lam | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Table of random numbers used to allocate questionnaires to 4 groups placed in sealed numbered envelopes |
| Allocation concealment (selection bias) | Unclear risk | "Every doctor was given a set of sealed envelopes" Considerable imbalance in numbers in each group; unclear whether this was due to randomisation procedure or selection bias |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Abstract only; unclear if participants were aware of what other arms received, but within comparisons in this review, interventions varied by intensity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36% lost to follow‐up, included in ITT analysis |
Borland 2003.
| Methods | Setting: Quitline, Australia Recruitment: smokers seeking materials or counselling | |
| Participants | 1578 smokers, 1050 in relevant arms, 54% female, modal age 30 to 49, average cpd 23 | |
| Interventions | ∙ Standard self‐help quit‐pack based around SoC ∙ Additional tailored letters at baseline, at 3 months, and at 6 months based on mailed assessments ∙ Additional proactive telephone counselling (not included in this review) Some participants in all groups received brief reactive counselling before enrolment | |
| Outcomes | Abstinence at 1 year (sustained for 9 months) Validation: none | |
| Notes | 2 vs 1, tailored self‐help vs standard self‐help | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Shuffling questionnaires" |
| Allocation concealment (selection bias) | Unclear risk | "no opportunity for interviewers to influence choice of condition", so bias judged unlikely |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Self‐reported outcomes from participants not blinded to treatment condition, but with no personal contact and similar levels of intensity, considered at low risk for differential misreport |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up 78.9% for 1; 76.9% for 2 Losses included in ITT analysis Excluding losses would marginally lower effect size |
Borland 2004.
| Methods | Setting: Quitline, Australia Recruitment: callers wanting written self‐help materials | |
| Participants | 772 baseline smokers (baseline quitters not included in this review), 54% female (all participants), approximately 47% aged < 30, average cpd 19 | |
| Interventions | ∙ Standard self‐help quit pack ∙ Additional tailored letters, based on assessment phone calls; average number 5.7 (SD 4.6) | |
| Outcomes | Abstinence at 12 months (sustained for 6 months) Validation: none | |
| Notes | 2 vs 1, tailored self‐help vs standard self‐help No control for effects of multiple contacts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated ID numbers, even numbers allocated to intervention |
| Allocation concealment (selection bias) | Low risk | ID number generated after agreement to participate obtained |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding not possible because of the nature of the intervention, but "participants in each condition [did] not know about the other condition unless they specifically asked ... (none did)" No blinding or validation of smoking status, but because of low‐contact nature of intervention, differential misreport of smoking unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up 71.3%% for 1; 63.8% for 2 Losses included in ITT analysis Excluding losses would lower effect size |
Brandon 2016.
| Methods |
Setting: USA, population‐based Recruitment: nationwide; smokers called a toll‐free telephone number in response to advertisements in newspapers, radio, cable TV, public transit, and public service announcements |
|
| Participants | 1874 smokers who want to quit, 65.8% female, average age 47.5, average cpd 20.5 | |
| Interventions | ∙ Standard repeated mailings (SRM): a revised version of the Forever Free booklets, sent at baseline, and at 1, 2, 3, 5, 7, 9, and 12 months ∙ Intensive repeated mailings (IRM): same as above with 2 additional booklets (at 15 and 18 months) and 9 brief pamphlets designed to enhance the perception of social support (sent every month without a booklet) ∙ Traditional self‐help (TSH) (control): single self‐help booklet |
|
| Outcomes |
Strictest: 7‐day point prevalence abstinence at 24 months Other follow‐ups: 6, 12, and 18 months Validation: none |
|
| Notes |
Funding: "This work was supported by grant R01CA134347 from the National Cancer Institute. This work has also been supported in part by the Biostatistics and Survey Methods Core Facility at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute‐designated Comprehensive Cancer Center (P30CA76292)" Declaration of interest: "Thomas Brandon has consulted for and received tobacco‐related research support from Pfizer, Inc. The rights to the intervention materials used in this study are owned by Moffitt Cancer Center. In the event that future revenue derives from these products, Moffitt has a revenue‐sharing plan with investigators. No other financial disclosures were reported by the authors of this paper" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Individuals were allocated to the three intervention arms using simple randomization without stratification. Intervention assignment was generated by computer upon entry of screening data into a relational database" |
| Allocation concealment (selection bias) | Low risk | Not described, but no face‐to‐face participant contact with researchers |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear if participants were aware of other group assignments; no objective outcome measure; however, study author states there is "evidence of little benefit derived from inclusion of biochemical verification measures in low‐intensity interventions such as these that have no face‐to‐face contact, and there is little incentive to falsely report abstinence" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout increases from the least to the most intensive intervention: 38% (TSH), 41% (SRM), and 43% (IRM) However, no baseline differences and similar follow‐up rates |
BTS 1983.
| Methods | Setting: hospital chest clinics and inpatient wards, UK Recruitment: patients with smoking‐related conditions | |
| Participants | 748 smokers (in relevant arms), average age 49, average cpd 24 | |
| Interventions | ∙ Brief advice to quit from a physician ∙ Advice and self‐help booklet containing information and advice ∙ Same as second bullet here plus placebo chewing gum (not included in this review) ∙ Same as second bullet here plus nicotine gum (not included in this review) | |
| Outcomes | Sustained abstinence 6 to 12 months (2 months point prevalence) Validation: venous carboxyhaemoglobin and thiocyanate | |
| Notes | 2 vs 1, self‐help vs control. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated; "each physician initially received a balanced block of 12 treatments" |
| Allocation concealment (selection bias) | Low risk | Numbered envelopes, opened after eligibility assessed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Biochemical validation, but possible performance bias in that physicians handing out leaflets were not blind to treatment condition and this may have impacted advice; insufficient detail reported by which to judge |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 48 withdrawals re‐included in this analysis with no impact on effect size |
Burling 1989.
| Methods | Setting: Veterans Administration Medical Center, USA Recruitment: VA employees | |
| Participants | 58 smokers, average age 44, average cpd 27 | |
| Interventions | ∙ American Cancer Society and ALA pamphlets about smoking, a telephone hotline, and a stop‐smoking contest that gave vouchers for a draw, for each day with expired CO < 8 ppm ∙ As bullet above plus use of a computer to enter data on smoking behaviour and smoking a cigarette through a filter attached to the computer; this produced an individualised nicotine fading programme that was explained in an accompanying manual | |
| Outcomes | Abstinence at 6 months Validation: CO < 8 ppm | |
| Notes | 2 vs 1, tailored self‐help vs standard self‐help | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Interventions of similar intensity; biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 dropouts re‐included in denominators for this review |
Campbell 1986.
| Methods | Setting: 2 chest clinics in Scotland, UK Recruitment: smokers attending outpatient clinic (unselected) | |
| Participants | 1206 smokers referred for chest radiography, 44% aged > 50 | |
| Interventions | ∙ Self‐help; 13‐page booklet ∙ No treatment control | |
| Outcomes | Abstinence at 1 year (self‐report of no smoking for 6 months) Validation: expired CO < 10 ppm; non‐attenders classified as smokers | |
| Notes | Face‐to‐face contact but no advice | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random (interventions alternated fortnightly) |
| Allocation concealment (selection bias) | High risk | All smoking patients attending were eligible, so potential for selection bias probably low, but imbalance in age distribution between groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Control group unlikely to know what intervention group was receiving; same amount of personal contact; biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up 74.5% intervention, 74.1% control; losses included in ITT analysis |
Clark 2004.
| Methods | Setting: lung cancer screening centre, USA Recruitment: smokers enrolled in a screening study 1 year previously | |
| Participants | 171 smokers, 21% in pre‐contemplation, 29% female, average age 57, 46% smoked 11 to 20 cpd | |
| Interventions | ∙ List of internet cessation resources; 10 sites with brief descriptions ∙ Self‐help manuals Clearing the Air and Quit Smoking Action Plan | |
| Outcomes | Abstinence at 12 months (7‐day point prevalence) Validation: CO | |
| Notes | Comparison between self‐help interventions; not in meta‐analysis Study authors' hypothesis was that list of internet cessation resources would be superior (OR 0.44, 95% CI 0.12 to 1.43) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation; interventions of similar intensity, so bias judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not reported but all included in ITT analysis |
Cuckle 1984.
| Methods | Setting: community exposed to a 15‐minute TV programme with offer of a smoking quit kit, UK Recruitment: random sample of individuals requesting a kit | |
| Participants | 4492 smokers randomised; results based on 2117 (47%) who replied to a baseline and a follow‐up questionnaire | |
| Interventions | ∙ Control ‐ letter apologising for shortage of kits ∙ Quit kit ∙ Quit kit and additional materials 6 months later | |
| Outcomes | Abstinence at 12 months Saliva cotinine from 66% of quitters; quit rates corrected by the disconfirmation rate found for each group | |
| Notes | 2 vs 1, self‐help vs control 3 vs 2, additional materials |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "One‐third were chosen at random as controls and did not receive a kit" |
| Allocation concealment (selection bias) | Unclear risk | No details given, but no personal contact, so selection bias unlikely |
| Blinding (performance bias and detection bias) All outcomes | High risk | Control group would have expected to receive quit kit and then to be told there was a shortage so they did not get them; could introduce performance bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low response rate in a population‐based study, so only participants who replied to baseline questionnaire and follow‐up questionnaire were included Response rate to baseline questionnaire was 70% in control group compared to 39% for those receiving a kit |
Cummings 1988.
| Methods | Setting; stop smoking hotline, USA Recruitment: callers who accepted offer of a stop smoking booklet and who agreed to follow up | |
| Participants | 1895 smokers, 65% female, average age 42, average cpd 28, 89% had made at least 1 prior quit attempt | |
| Interventions | First 4 groups received booklets of similar length (± 50 pages) and format, differing in precise instructions ∙ High structure (day‐by‐day plan) recommending 'cold turkey' quitting ∙ High structure recommending gradual reduction ∙ Low structure (menu of exercises) with gradual reduction ∙ Low structure, 'cold turkey' Control booklet: 15 pages stressing health effects of smoking | |
| Outcomes | Abstinence from 1 month to 6 months; self‐report by telephone interview with blinded assessors No biochemical validation; confirmation by a significant other | |
| Notes | 1 to 4 vs 5 in main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Confirmation by significant other; booklets of similar length, so differential misreport unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses based on participants reached at 1 month and 6 months' follow‐up; 89% of those randomised Dropout rates similar in all groups |
Curry 1991.
| Methods | Setting: HMO, USA Recruitment: advertisement for study in HMO magazine | |
| Participants | 1217 smokers, average age 44, average cpd 25 | |
| Interventions | Factorial design ∙ Self‐help programme, Breaking Away ∙ Self‐help and up to 3 sets of personalised feedback based on baseline questionnaire and progress reports (intrinsic motivation) ∙ Self‐help and incentives including a prize draw for returning progress reports (extrinsic motivation) ∙ Self‐help and intrinsic and extrinsic motivation | |
| Outcomes | Sustained abstinence at 12 months (7 days at 3 months and 12 months) Validation: saliva cotinine ≤ 10 ng/mL at 12 months for abstainers in locality; correcting for disconfirmation rates did not affect sustained abstinence numbers | |
| Notes | 4 and 2 vs 3 and 1 for effect of personalised feedback (tailoring) Extrinsic motivation did not increase quit rates Aim was to increase use of materials |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; stratified by gender and cpd; no other information |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if control group participants knew the nature of intervention conditions; biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on number lost; all randomised participants included in ITT analysis |
Curry 1995.
| Methods | Setting: HMO, USA Recruitment: smokers identified via a telephone survey of health behaviour in a random sample of HMO members (unselected) | |
| Participants | 1137 smokers, 53% female, average age 41, average cpd 17 | |
| Interventions | No face‐to‐face contact ∙ Control ‐ no materials ∙ Self‐help booklet (Breaking Away) with units to complete, relevant to all stages of readiness to quit ∙ As second bullet above plus feedback based on computer analysis of initial survey; included a handwritten form and a list of relevant parts of booklet ∙ As third bullet above plus up to 3 counsellor‐initiated phone calls (not included in this review) | |
| Outcomes | Sustained abstinence 3 months to 12 months Validation: saliva cotinine requested but not obtained for all participants; disconfirmation rates not significantly different between groups | |
| Notes | 12 months' rather than 21 months' follow‐up used for comparability with other studies; study author confirmed numbers quit
2 vs 1 in self‐help vs control 3 vs 2 in effects of tailoring |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Control group aware that they may be receiving materials or phone calls, which they did not receive; this could introduce performance bias "Collecting saliva cotinine...was challenging because participants had neither explicitly volunteered for a study of smoking behavior nor requested treatment for smoking cessation... nearly one fourth of those contacted refused to provide a sample" Higher disconfirmation in control group but difference not significant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88% provided data at 3 months and at 12 months No difference in response rates across groups Missing counted as smoking in meta‐analysis |
Davies 1992.
| Methods | Setting: community, Ottawa, Canada Recruitment: each of 156 nursing students recruited 2 non‐hospitalised smokers (selected) | |
| Participants | 307 smokers, average age 36, average cpd 20 | |
| Interventions | ∙ List of community resources, delivered during a home visit by a nursing student ∙ Time to Quit (TTQ) self‐help booklet plus list of community resources, delivered by a nursing student after training in the TTQ programme | |
| Outcomes | Abstinence at 9 months Validation: saliva cotinine < 100 ng/mL | |
| Notes | It is unclear what advice was given to the control group Marginal to include because self‐help was confounded by student training, but does not affect meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Nurses knew who would receive more training after delivering control condition and before meeting with intervention participants, introducing likelihood of performance bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up re‐included as smokers for meta‐analysis; 28% lost to follow‐up; similar across groups |
Davis 1984.
| Methods | Setting: local communities with lung associations, USA Recruitment: media advertisements for American Lung Association (ALA) self‐help materials | |
| Participants | 1237 smokers who completed a questionnaire and paid a refundable deposit | |
| Interventions | No face‐to‐face contact ∙ ALA leaflets (8 leaflets including 2 brief cessation brochures: Me Quit Smoking? Why? and Me Quit Smoking? How?) ∙ Leaflets and maintenance manual: A Lifetime of Freedom from Smoking ∙ Cessation manual: Freedom from Smoking in 20 Days. ∙ Cessation and maintenance manuals | |
| Outcomes | Sustained abstinence at 12 months (point prevalence at all 5 follow‐up points); self‐report in a telephone interview Validation: none | |
| Notes | 2 plus 3 plus 4 vs 1, self‐help vs leaflet only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No biochemical validation used but no personal contact; interventions all of similar intensity, so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on number lost; all randomised participants included in ITT analysis |
Davis 1992.
| Methods | Setting: community, USA Recruitment: advertisements for the Cancer Information Service hotline | |
| Participants | Women smokers with children under 6 calling hotline; results based on 630 of 873 (72%) of those recruited who were followed up at 6 months | |
| Interventions | ∙ Quitting Times, a self‐help guide developed to meet the special needs of women smokers with young children (65 pages in magazine format) ∙ ALA: Freedom from Smoking for You and Your Family ∙ National Cancer Institute: Clearing the Air | |
| Outcomes | Abstinence at 6 months (7‐day point prevalence) Validation: no biochemical validation; confirmation by surrogate; those refusing to give a surrogate were classified as smokers | |
| Notes | Does not contribute to main analysis, 1 vs 2 and 3; impact of targeting to population All 3 guides covered similar topics; no significant differences were found between any of them | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Preassigned list randomized by day of week" |
| Allocation concealment (selection bias) | Low risk | Counsellors who recruited participants during calls were blinded to the self‐help guide that would be received |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All groups received self‐help, so similar levels of intensity "Follow‐up interviews were conducted by trained interviewers who were blinded regarding subject assignment.... Surrogate interviews were conducted to verify the smoking status of those who reported that they had quit smoking..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72% of participants reached at follow‐up; similar for all 3 groups Analyses based on those reached |
de Vries 2008.
| Methods | Setting: community, Netherlands Recruitment: telephone recruitment for a multiple risk factor health promotion intervention | |
| Participants | 156 smokers amongst 2827 participants, of whom 1331 (47%) responded at T4 Baseline all participants: 55% female, average age 49 |
|
| Interventions | ∙ Printed tailored letters on smoking as an identified risk factor (other targets were physical activity, nutrition); half of the group had action planning component in third letter ∙ Printed generic letters | |
| Outcomes | Abstinence at 9 months (not defined) Validation: none | |
| Notes | Effect of tailoring Numbers of smokers at baseline and numbers of quitters provided by study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | Details not given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No biochemical validation but interventions of similar intensity; differential misreport judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only baseline smokers who responded to follow‐up survey included in analysis |
Dijkstra 1998a.
| Methods | Setting: community, Netherlands Recruitment: newspaper adverts; not selected by level of motivation to quit | |
| Participants | 1546 smokers, 59% female, average age 40, average cpd 20.3 | |
| Interventions | No face‐to‐face contact ∙ Letter with information on positive outcomes of quitting (OC) ∙ Letter with information on skills for quitting (SE) ∙ Letter with outcomes and skills information (BO) All letters computer‐generated reports of 4 to 7 pages, personalised and tailored from baseline questionnaire ∙ No information (CO) | |
| Outcomes | 12‐month sustained abstinence at 14 months; self‐report by postal questionnaire Validation: none; participants told that a sample would be tested for CO levels | |
| Notes | 1 and 2 and 3 vs 4 in tailored materials since 2014 (previously in main comparison) Results sensitive to the outcome used; no difference in point prevalences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | No biochemical validation used; control group knew other participants receiving an intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64% responded at 14 months; no differences across groups Attrition predicted by perceived pros of quitting and intention to quit but not different between groups Denominator in meta‐analysis based on all randomised |
Dijkstra 1999.
| Methods | Setting: community, Netherlands Recruitment: newspaper adverts for smokers not planning to quit in the next 6 months (unmotivated volunteers) | |
| Participants | 843 smokers not planning to quit, 63% female, average age 42, average cpd 22 | |
| Interventions | No face‐to‐face contact ∙ Three tailored letters (MT) ∙ Single tailored letter (ST) ∙ Self‐help manual, 48 pages colour (SHG) ∙ No intervention (CO) | |
| Outcomes | Abstinence at 6 months (7‐day point prevalence), self‐report by postal questionnaire Validation: none Primary outcomes for trial: SoC; intention to quit | |
| Notes | 3 vs 4 in self‐help vs control 1 and 2 vs 3 in effects of tailoring |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if control group knew intervention arms receiving additional information; no biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89% responded at 6 months Attrition predicted by years smoking and group Denominator used in meta‐analysis includes all randomised |
Etter 2004.
| Methods | Setting: community, Switzerland Recruitment: mailing to population registers (not selected) | |
| Participants | 2934 smokers aged 15+, 74% pre‐contemplators, 40% tried to quit in previous year, 51% female, average age 36, average cpd 20 | |
| Interventions | ∙ Tailored 8‐page letter plus SoC‐matched booklets; at 2 months, 4 months, 12 months ‐ repeat questionnaire to initiate further letter ∙ No intervention | |
| Outcomes | Abstinence at 24 months (in maintenance stage; quit for > 6 months), 4 weeks; 7‐day abstinence also reported Validation: none | |
| Notes | Tailored self‐help vs nothing; approximately half of group 1 received 1 letter only
Effects at 6 months (Etter Arch Int Med 2001) not sustained at 24 months Relative difference smaller if shorter‐term abstinence used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation: "list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | "...members of the control group received a letter indicating that they had been attributed to that group..." No validation; intervention intensity higher than for control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 14.0% in 1; 10.7% in 2 All non‐responders included in ITT analysis |
Fraser 2014.
| Methods |
Setting: USA, population‐based Recruitment: smokers who entered a website for smokers (smokefree.gov) recruited through their computers |
|
| Participants | 1034 people who smoked and were seeking help to quit, 68% female, average age 39.3, average cpd 19.3 | |
| Interventions | Study comprised 32 arms testing combinations of ‘on’ and ‘off’ versions of 5 interventions: the National Cancer Institute website vs a ‘lite’ website, telephone counselling vs no counselling, a self‐help manual vs a brief brochure, motivational email messages vs no messages, and nicotine replacement therapy (NRT) vs no NRT | |
| Outcomes |
Smoking abstinence: 7‐day point‐prevalence at 7 months Validation: none; self‐report only |
|
| Notes |
Funding: the project was funded through a contract to our university from Matthews Media Group, underwritten by ARRA funding to the National Cancer Institute. Additional funding was provided by the National Cancer Institute (5K05CA139871) Declaration of interests: study authors declared that they have no conflicts of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization occurred immediately after the confirmation call, and participants completing this step were sent an automated email welcoming them to the study" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Follow‐up interviewers were blind as to treatment assignment"; however, "participants [...] were sent an automated email [...] outlining services they would receive"; no biochemical validation of self‐reported abstinence used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Range of response rates across the 5 treatment factors = 76‐81%" |
Gilbert 2013.
| Methods |
Setting: general practices, UK Recruitment: identified via GP records; mailed proactively |
|
| Participants | 6697 current smokers aged 18 to 65 years, 56% female, average age 45, average cpd 18 (excluding 5.4% non‐daily smokers) 47% not planning to quit within 6 months |
|
| Interventions | ∙ Standard, non‐tailored NHS: Stop Smoking Start Living booklet and computer tailored advice; report based on information obtained through baseline assessment questionnaire, letter from GP; follow‐up assessment via mail at 1 month; additional tailored mailing ∙ Standard, non‐tailored NHS: Stop Smoking Start Living booklet |
|
| Outcomes | 3 months' sustained abstinence at 6 months Validation: none |
|
| Notes | 214 participants excluded post randomisation for valid reasons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Blocked randomisation codes were generated externally and given to an independent administrator in sealed envelopes upon receipt of completed questionnaires" |
| Allocation concealment (selection bias) | Low risk | "Participants were accepted into the study before knowledge of the next assignment in the sequence in order to minimise selection bias. Each study participant randomised received the treatment corresponding to the next free study number in the randomised sequence" |
| Blinding (performance bias and detection bias) All outcomes | High risk | No biochemical validation; participants aware of what other condition was receiving "Participants were told that they would be sent some information about quitting, and could be randomly selected to receive additional information based on their answers in the questionnaire" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27% lost intervention, 24% lost control; no significant differences in predictors of dropouts between groups Study authors conducted sensitivity analyses with alternative assumptions about dropouts |
Glasgow 1981.
| Methods | Setting: community, USA Recruitment: media advertisements | |
| Participants | 88 smokers (40 in self‐help conditions) | |
| Interventions | Factorial trial of 3 different self‐help materials, with or without additional group support ∙ Danaher & Lichtenstein manual ∙ Pomerleau & Pomerleau manual ∙ I Quit Kit | |
| Outcomes | Abstinence at 6 months Validation: CO < 15 ppm | |
| Notes | 3 different self‐help conditions and no strong hypothesis about direction of treatment difference between the Danaher & Lichtenstein manual and the Pomerleau & Pomerleau manual, so not used in the meta‐analysis of different programmes No statistical differences between quit rates (also included in group therapy review) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned"; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation used; all groups received materials (differences in content only) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/88 lost to follow‐up; group not specified, so not included as smokers |
Gritz 1992.
| Methods | Setting: HMO, USA Recruitment: members of HMO agreeing to participate in a Preventive Health Behaviour study and completing a baseline survey (unselected ‐ not informed that study was focussed on smoking) | |
| Participants | 1396 female smokers, average age 38, 42% smoked 15 to 24 cpd | |
| Interventions | No face‐to‐face contact; 5 follow‐up interviews in 2 years ∙ Self‐help programme mailed in 6 weekly instalments; manuals tailored to the concerns of female smokers and addressing weight gain, social support, stress, and coping mechanisms ∙ Control ‐ no materials; same schedule of follow‐up phone calls | |
| Outcomes | Sustained abstinence at 1 month, 6 months, 12 months, and 18 months Validation: saliva cotinine < 15 ng/mL, but due to low success in obtaining samples, unadjusted rates used. No difference in disconfirmation rates between intervention and control groups | |
| Notes | The strictest measure of abstinence extracted gives the lowest P value for the difference between groups; all other measures show a smaller difference in quit rates | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Control group participants not aware of the nature of the intervention; participants did not know study was aimed at smoking cessation Biochemical validation conducted; not used due to low success in obtaining samples, but no difference in disconfirmation between groups, suggesting differential misreport unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12.7% lost to follow‐up at 18 months Number in each group at baseline not stated, so losses not included as smokers in meta‐analysis Similar losses across groups, so no effect on estimate |
Harackiewicz 1988.
| Methods | Setting: university campus health centre/medical centre, USA Recruitment: smokers applying for free cessation programme | |
| Participants | 98 smokers in relevant arms, 61% female, average age 35, average cpd 27 for all participants | |
| Interventions | All received advice from a doctor or nurse to quit by using the written materials, which were different for each group ∙ Self‐help manual employing intrinsic motivation approach (Stopping Smoking on Your Own with Nicorette) and nicotine gum ∙ Self‐help manual employing extrinsic motivation approach (The Doctor's Program for Stopping Smoking with Nicorette) and nicotine gum ∙ Intrinsic motivation self‐help manual only ∙ Control ‐ short booklet only, with no motivational element | |
| Outcomes | Sustained abstinence at 12 months (3 months to 12 months) Validation CO < 8 ppm at each visit; saliva thiocyanate < 10 mg/dL at 3 months and 6 months; 2 participants reclassified as smokers | |
| Notes | 3 vs 4 for self‐help compared to control 1 and 2 not used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned"; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation; groups differed in intervention contact but not in intensity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 of 197 participants did not attend any follow‐up and were excluded from analyses "drop‐out rates did not differ according to condition" Other losses assumed to be smoking |
Hollis 1993.
| Methods | Setting: HMO, USA Recruitment: smokers visiting primary care physicians (unselected) | |
| Participants | 2707 smokers (1383 in relevant arms) who received provider advice, average age 40, average cpd 18 | |
| Interventions | All received 30‐second quit smoking advice from the physician ∙ Self‐quit training from a nurse or health counsellor who showed a video, gave a choice of self‐help manuals plus quit kit; 1 follow‐up phone call ∙ Group referral ∙ Choice of group referral or a self‐help kit ∙ Control ‐ provider advice and 2‐page pamphlet from nurse | |
| Outcomes | Abstinence at 12 months (3 months' and 12 months' point prevalence) Validation: saliva cotinine; participants not providing samples counted as smokers | |
| Notes | 1 vs 4, comparison of self‐help vs control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐randomisation (2 random digits in health record number) of smokers receiving provider advice; more allocated to control than to each other condition |
| Allocation concealment (selection bias) | High risk | Allocation not concealed but no evidence of selection bias; baseline characteristics similar |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if participants knew the nature of what other groups were receiving; biochemical validation; 55% of reported quitters provided saliva sample; no differences by group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14% lost to follow‐up at 12 months; response rates not significantly different across treatment groups; all participants included in analysis |
Hoving 2010.
| Methods |
Setting: community, Netherlands Recruitment: 75 general practices (passive recruitment via questionnaire in waiting room), 65 pharmacies (15 passive, 50 active recruitment) |
|
| Participants | 1019 smokers (545 pharmacy, 474 GP); motivated to quit within 6 months; smoked in last 7 days before baseline assessment 56% female, average age 45, average cpd 22 |
|
| Interventions | All participants completed baseline questionnaire ∙ Mailed 5‐ to 7‐page tailored letter, using same tailoring as Dijkstra 1998a (based on I‐change model) ∙ Thank you letter only |
|
| Outcomes | Continuous abstinence from baseline at 3 months and at 12 months in pharmacy group, at 6 months in GP group Validation: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomised based on the colour coding on their questionnaire (blue for experimental group, yellow for control group)" |
| Allocation concealment (selection bias) | High risk | Allocation would not be concealed if anyone was aware of the significance of colour |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only experimental losses to follow‐up reported (63/256 pharmacy, 42/220 GP); unclear how many participants in the control group were lost |
Humerfelt 1998.
| Methods | Setting: community, Norway Recruitment: from participants in a community survey of men aged 30 to 45 years who had increased risk of obstructive lung disease or lung cancer | |
| Participants | 2610 men who smoked with reduced FEV₁ and/or occupational asbestos exposure, average age 37, average cpd 16 | |
| Interventions | ∙ Mailed self‐help pamphlet (15 pages), emphasising behavioural modification techniques in smoking cessation and recommending an early quit date, accompanied by a letter from a respiratory physician advising of high risk status established by the survey ∙ No intervention | |
| Outcomes | Abstinence at 15 months (point prevalence) Validation: participants in 1 geographical area invited for CO measurement (CO < 10 ppm) | |
| Notes | For meta‐analysis, the number of quitters has been adjusted for the validated rate found in the sample tested (63% in intervention/67% in control) Participants who stopped smoking before receiving materials were included Study authors give 12 months' sustained abstinence rates of 5.6% vs 3.5%, but these rates are based on self‐report by responders |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Control group not aware of intervention received by intervention group; biochemical validation conducted in subset of participants; no significant difference in misreport detected (1 intervention, 2 control) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up greater in the intervention group (17%) than in the control group (8%) The probability of responding to the follow‐up questionnaire was inversely related to baseline cpd consumption in the intervention group but not in the control group Losses included as smokers |
ICRF 1994.
| Methods | Setting: primary care, UK Recruitment: patients registered with practice invited to join | |
| Participants | 1686 smokers (over 15 cpd) | |
| Interventions | 2 × 2 factorial design ∙ Nicotine patch and 16‐page Health Education Authority (HEA) pamphlet ∙ Placebo patch and HEA pamphlet ∙ Nicotine patch and 46‐page booklet with more detailed information on cessation with the use of patches All participants seen once by a doctor and 4 times by a nurse | |
| Outcomes | Sustained abstinence at 12 months Validation: salivary cotinine or expired CO | |
| Notes | Comparison between different self‐help materials Not used in meta‐analysis No clinically or statistically significant differences between materials in either patch condition |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation of study numbers to intervention groups |
| Allocation concealment (selection bias) | Low risk | Sequential allocation of study numbers and pre‐coded packages |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation; similar levels of intensity across interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only early abstainers were followed up at 6 months and 12 months; 9.2% lost to follow‐up at 12 weeks All losses included as smokers |
Janz 1987.
| Methods | Setting: 2 outpatient medical clinics, USA Recruitment: all smokers attending and giving informed consent for a study of health practices (unselected) | |
| Participants | 250 smokers, average age 46, average cpd 24 | |
| Interventions | ∙ Control ‐ no intervention; clinic physicians not aware of the study (not included in the review) ∙ Advice from the physician and brief consultation with a nurse ∙ As second bullet above and the Step‐by‐Step Quit Kit | |
| Outcomes | Abstinence at 6 months (ascertainment by telephone by independent interviewer) Validation: none | |
| Notes | 3 vs 2 for effect of self‐help as adjunct to advice Graphed percentages based on numbers followed up It has not been possible to obtain data from the study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐random assignment of half‐day clinic sessions to experiment or control (control does not contribute to this review) Within experiment clinics, participants randomised to manual or no manual condition; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No biochemical validation but interventions tested in this review included same amount of face‐to‐face contact and self‐report collection procedures designed to minimise misreport (research personnel made clear they had no relationship to healthcare team and responses were confidential) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 39 (16%) lost to follow‐up at 6 months "Drop‐out rates did not vary significantly across study groups" Losses not given by group, so not included in meta‐analysis |
Killen 1997.
| Methods | Setting: community, USA Recruitment: advertisements | |
| Participants | 424 smokers, 50% female, average age 42 to 47 years, average cpd 24 | |
| Interventions | 2 × 2 factorial design. All participants received self‐help materials designed to help develop self‐control skills ∙ Self‐help and placebo patch ∙ Self‐help and nicotine patch (21 mg) ∙ As first bullet above and video, watched during initial office visit, and for use at home ∙ As second bullet above and video | |
| Outcomes | Sustained abstinence (6 months and 12 months) Validation: saliva cotinine < 20 ng/mL | |
| Notes | Test of additional materials With evidence of an interaction between nicotine and video conditions, nicotine arms entered separately via a dummy study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in a 2 × 2 fully crossed factorial design; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if participants not receiving video knew other participants were receiving it (and viewing it in a group); biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not specified, but all participants included in denominators |
Killen 1997 +NP.
| Methods | Dummy study to enter results of Killen 1997 arms with nicotine patch | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Kottke 1989.
| Methods | Setting: family practices, USA Recruitment: physicians recruited for trial; target population ‐ all patients seen during the month (unselected) | |
| Participants | 66 physicians, 1653 smoking patients, "2/3rds female, average age slightly over 40 years, just under one pack/day" | |
| Interventions | ∙ Physicians attended 6‐hour workshop ∙ Physicians attended workshop and given copies of Quit and Win for their patients ∙ Physicians received no support, but were asked to advise patients during the study period | |
| Outcomes | Abstinence at 1 year Validation: serum cotinine | |
| Notes | 2 vs 1, effect of self‐help in addition to advice from a trained physician Including 3 in control group does not affect results (RR for trial becomes 1.02 rather than 0.99) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Cluster‐randomised by physician ‐ not by smoker; method not described; potential for imbalance in participant characteristics, but number of participants per physician low |
| Allocation concealment (selection bias) | High risk | Researchers attempted to contact all participants seen by physicians during 1 month |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation used, as with cluster‐randomisation by physician, seems unlikely that control group participants would know what other participants were offered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 87% of smokers identified at baseline were reached at 1 year ‐ similar across groups |
Lando 1988.
| Methods | Setting: family practice or pulmonary specialists, USA Recruitment: physicians' patients wishing to use nicotine gum as a cessation aid | |
| Participants | 304 smokers, 62% female, average age 42, average cpd 31 | |
| Interventions | ∙ Nicotine gum (NG) and experimental self‐help materials emphasising behavioural strategies, as well as correct use of gum ∙ NG and control pamphlet Danger: The Facts About Smoking (American Cancer Society) | |
| Outcomes | Abstinence at 12 months Validation: proportion asked to provide saliva for thiocyanate: 5 discrepant ‐ 2 self‐help, 3 control ‐ but not clear if these were at 6 months or 12 months, so self‐reported outcomes used | |
| Notes | In main comparison with advice and leaflet for control, and in comparison of NG plus self‐help vs NG alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation conducted but not used, but similar levels of intensity and physicians blind to pamphlet condition, so differential misreport judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of number lost to follow‐up |
Lando 1991.
| Methods | Setting: community cardiovascular risk factor screening programme, USA Recruitment: smokers identified from screening programme who agreed to take part | |
| Participants | 570 smokers, approx 50% female, average age 42, average cpd 20 | |
| Interventions | No face‐to‐face contact ∙ Self‐help Quit for Good materials (NCI) ∙ Self‐help Quit and Win materials ‐ a more extensive and structured programme ∙ Wait‐list control | |
| Outcomes | Abstinence 7 months after randomisation (but only 3 to 4 months after receipt of materials) Validation: none | |
| Notes | Items in first and second bullets above treated as self‐help programmes; no difference in results between them Both intervention and control participants likely to have been exposed to simultaneous community Quit and Win contests Study author notes that a number of participants quit between randomisation and receipt of materials |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given; significant differences between intervention and control for sex and education; higher confidence in quitting among controls |
| Blinding (performance bias and detection bias) All outcomes | High risk | Wait‐list control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25 lost to follow‐up, of whom 13 were in control groups Denominators are those followed up |
Ledwith 1984.
| Methods | Setting: community, Scotland, UK Recruitment: newspaper advertisements for a smoker's advice centre | |
| Participants | 1839 smokers responding to offers of advice on stopping smoking | |
| Interventions | No face‐to‐face contact ∙ No advice control ∙ Self‐help leaflet with standard letter ∙ Self‐help leaflet and offer of individual advice upon returning a questionnaire | |
| Outcomes | Abstinence at 12 months (for 10 months or longer ‐ based on self‐report) Validation: attempt to obtain saliva for thiocyanate but not complete; data based on self‐report only | |
| Notes | 2 vs 1, self‐help 3 vs 2, effect of tailored advice Only 34% returned baseline questionnaire to initiate tailored component No information about contents of leaflet Borderline whether this counts as a structured self‐help programme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned at random"; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although attempts to get biochemical validation were unsuccessful, control group was unaware of other treatment assignments; no face‐to‐face contact was given, hence differential misreport was judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16% lost to follow‐up Non‐respondents included as smokers |
Lennox 2001.
| Methods | Setting: general practice, Scotland, UK Recruitment: smokers in general practices who returned questionnaires | |
| Participants | 2610 smokers; no demographic details | |
| Interventions | No face‐to‐face contact ∙ Tailored letter from physician (4 pages) ‐ based on SoC; decisional balance and other indicators from questionnaire ∙ Untailored letter from physician (same format) ‐ included specific behavioural advice on quitting ∙ Control ‐ letter acknowledging questionnaire | |
| Outcomes | Abstinence at 12 months (24 months' data reported but point prevalence, so does not represent a more conservative measure) Validation: saliva cotinine | |
| Notes | 2 vs 3, self‐help, no contact 1 vs 2, tailoring |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | "After the questionnaires were returned, we randomised the participants to the groups" No participant contact; low risk of selection bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Similar intervention intensities; no face‐to‐face contact; biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22% loss to follow‐up; similar across groups; non‐responders counted as smokers |
Lichtenstein 2000.
| Methods | Setting: community, USA Recruitment: via electric utility mailing to identify households with smokers and low radon concentrations | |
| Participants | 1006 smokers in 714 households, average cpd 20 | |
| Interventions | No face‐to‐face contact ∙ Standard Environmental Protection Agency leaflet on risks of radon ∙ Pamphlet highlighting risk of smoking in low concentrations of radon, with tips for quitting, or not smoking indoors ∙ Second bullet above plus up to 2 brief proactive telephone calls. All groups received standard letter with radon results | |
| Outcomes | Abstinence at 12 months, sustained at 3 months and 12 months Validation: none | |
| Notes | 2 vs 1, self‐help vs other control 3 contributes to telephone counselling review (Stead 2013b) Cluster‐randomisation; 54% of smokers lived with another smoker Intraclass correlation for sustained abstinence was .010; analyses did not correct for this |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by household; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Self‐reported outcomes from participants not blinded to treatment condition, but the arms included in this analysis had similar levels of intensity with no personal contact, so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80% of households reached at 3 months and 12 months; no difference across conditions Missing treated as smoking |
Lichtenstein 2008.
| Methods | Setting: community, USA Recruitment: via electric utility mailing with offer of radon test kit to identify households with smokers | |
| Participants | 1364 households with 1821 smokers, ˜ 18 cpd | |
| Interventions | Factorial design crossing ± brief phone counselling with video self‐help materials All households given A Citizen's Guide to Radon and a letter tailored to results of radon level test ∙ Video (15 minutes) explaining risk of smoking and radon combination, encouraging quitting and/or household smoking bans ∙ No video |
|
| Outcomes | Abstinence at 12 months, sustained at 3 months and 12 months Validation: none | |
| Notes | Analyses accounting for clustering of multiple smokers in households reported to yield results generally consistent with simple analyses We were unable to obtain data for arms with and without phone counselling, so the collapsed data contribute to comparisons 1.1.2 and 2.1.2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Responding households sequentially randomised to 4 conditions subject to stratification on radon test status; no true randomisation sequence used |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Self‐reported outcomes from participants not blinded to treatment condition, but all received phone counselling and some self‐help, so performance and detection bias judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83% of households completed 12 months' assessment; 76% completed both 3 months' and 12 months' assessment |
Lipkus 1999.
| Methods | Setting: health centre, USA Recruitment: from telephone survey of patients | |
| Participants | 266 randomised, 160 followed up; low‐income African American smokers, unselected by motivation; 52% female, 49% aged > 50 years | |
| Interventions | ∙ Physician prompts attached to chart (included other screening tests); providers trained to use 4As model ∙ First bullet above plus mailing of tailored print communication around birthday ∙ Second bullet above plus TC |
|
| Outcomes | Abstinence 16 months after last intervention; 30‐day quit Validation: none | |
| Notes | 2 vs 1, tailored self‐help adjunct to advice (3 vs 2 in telephone counselling review) Reported rates based on numbers followed up, not numbers randomised Provider compliance reported to be 48% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if participants were aware of what other participants were receiving; no biochemical validation; self‐help group has more communication than control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40% loss to follow‐up, largely due to disconnected phone numbers "Loss to follow‐up did not appear to be a function of any demographic, psychosocial of smoking pattern, nor was it a function of the intervention smokers received" Losses not included as smokers |
McFall 1993.
| Methods | Setting: community, USA Recruitment: during a TV cessation programme | |
| Participants | Smokers who registered and received the manual or reported viewing at least 1 part of the programme | |
| Interventions | ∙ TV programme and ALA FfS ∙ Maintenance; first bullet above and 10 newsletters over following 6 months | |
| Outcomes | Abstinence at 12 months (24 months' data reported but point prevalence with increase over time, so does not represent a more conservative measure; RR similar) Validation: none | |
| Notes | 2 vs 1, effect of additional materials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if participants were aware of what other participants were receiving; no biochemical validation; maintenance group has more communication than control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24% lost in maintenance condition, 27% in control condition Meta‐analysis includes responders; including losses would yield a less conservative effect |
Meyer 2008.
| Methods | Setting: primary healthcare centres, Germany Recruitment: smoking patients attending practices during 3 study weeks | |
| Participants | Smokers, unselected for motivation; 48% female, average age 34, average cpd 16 | |
| Interventions | ∙ Assessment only control ∙ Up to 3 letters individually tailored to SoC ‐ first used baseline assessment; 3 months and 6 months depended on further assessment; stage‐matched self‐help manuals used ∙ Brief physician advice and self‐help manuals | |
| Outcomes | Abstinence at 24 months (sustained for 6 months) Validation: none | |
| Notes | Analyses in paper allowing for clustering yield slightly larger estimates than use of crude numbers on quitting Different assumptions about losses to follow up did not substantially alter any results Abstinence rates increased over time in all groups Prolonged abstinence at all follow‐ups is very low ‐ not used here; 63% got 3 letters, 21% got 2, and 17% only 1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random and clustered based on time of attendance; fixed sequence of assessment‐only, tailored letters, advice; at least 2 weeks between study weeks |
| Allocation concealment (selection bias) | High risk | Condition known at the time of recruitment All patients screened, so recruitment bias should have been avoided; no evidence of differences in baseline characteristics |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No validation but practice team, practitioner, and follow‐up interviewers all blinded; however unclear if control participants were aware of what intervention participants were receiving |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 56% of intervention group and 64% of control group reached at 24 months; different approaches to missing data did not alter conclusions |
Meyer 2012.
| Methods |
Setting: 151 general practices, Germany Recruitment: smoking patients attending practice |
|
| Participants | 3215 patients (113 excluded), age 18+, who reported any tobacco smoking within last 6 months 44% female, average cpd not stated, average age 41, 38% pre‐contemplators |
|
| Interventions | ∙ Brief advice from practitioner (10 minutes) plus stage of change‐specific self‐help manuals ∙ Two individually tailored computer‐generated letters based on stage of change, plus self‐help manuals as per first bullet above ∙ 1 plus 2 |
|
| Outcomes | Abstinence at 12 months, self‐reported as prolonged for previous 6 months Validation: none |
|
| Notes | 3 vs 1 used as test of individually tailored self‐help as adjunct to advice 2 vs 1 in Analysis 4.1; direct comparison of tailored materials and advice |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Cluster‐randomised by practice Practices randomly assigned before recruitment Study authors note: "randomization was seriously undermined by obviously different mechanisms of patient selection for each study condition" |
| Allocation concealment (selection bias) | High risk | Practices not blind to condition when patients recruited; differential recruitment rates by condition |
| Blinding (performance bias and detection bias) All outcomes | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30% dropout in group 1, 21% in group 2, 29% in group 3 Study authors report that sensitivity analyses regarding assumptions about participants lost to follow‐up showed "same patterns of results" |
Meyer 2016.
| Methods |
Setting: Germany, population‐based Recruitment: nationwide random sample of general population using a random digit‐dialling procedure |
|
| Participants | 1462 daily smokers with no intention to quit in the next 6 months; 48.5% female, average age 39.4, average cpd 19.8 | |
| Interventions | ∙ Abstinence Intervention ‐ 3 computer‐tailored counselling letters and self‐help manuals that targeted smoking abstinence, sent just after baseline and at 3 and 6 months. Letters were tailored according to the principles of the transtheoretical model (TTM) of behaviour change and were generated by a fully automated computer expert system ∙ Reduction Intervention ‐ 3 counselling letters and self‐help manuals that targeted reduced smoking, sent at baseline and at 3 and 6 months. Letters were tailored in the same way as in the abstinence intervention, but specifically for reduction of smoking as the target behaviour ∙ Minimal assessment ‐ assessment only |
|
| Outcomes |
Strictest: 6 months' continuous abstinence at 24 months Other: 12 months Validation: none |
|
| Notes |
Funding: "Funding was gained from the German Federal Ministry of Education and Research (grant no. 01EB0120, 01EB0420, 01EE1406F) and the Social Ministry of the State of Mecklenburg‐Vorpommern (grant no. IX311a 406.68.43.05)" Declaration of interest: "The Project is part of the German research network EARLINT (EARLy substance use INTervention) and was supported by the research consortium on addiction, AERIAL. Funding was gained from the German Federal Ministry of Education and Research (grant no. 01EB0120, 01EB0420, 01EE1406F) and the Social Ministry of the State of Mecklenburg‐Vorpommern (grant no. IX311a 406.68.43.05). None of the authors have other relevant financial disclosures" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomized to the three study conditions via a computer‐based procedure. To increase the power of the comparisons between both intervention groups, we used a disproportional randomization algorithm (Dumville et al., 2006), setting the allocation probability to 36.8% for each intervention group and 26.4% for the assessment‐only control group" |
| Allocation concealment (selection bias) | Low risk | Not specified, but no face‐to‐face contact with researchers |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not specified, but no objective measure of results |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The number of participants lost to follow‐up rate was significantly higher (at month 12: chi2‐test, df = 2, p < 0.001; at month 24: chi2‐ test, df = 2, p < 0.001) in the intervention groups (at month 12: 23% reduction group, […] at month 24: […] 27% abstinence group) compared with the assessment‐only control group (at month 12: 9%; at month 24: 16%)" |
Nollen 2007.
| Methods | Setting: hospital, USA Recruitment: smokers visiting hospital, interested in quitting in next 6 months | |
| Participants | 500 African American smokers; 60% female, average age 43, average cpd 20 | |
| Interventions | All participants received 8 weeks on nicotine patch and 2 phone calls ∙ Standard materials ‐ ALA FfS plus How to Quit video ∙ Culturally sensitive guide Pathways to Freedom: Winning the Fight Against Tobacco, and Harlem Health Connection's Kick‐It video (40 minutes) targeted to African Americans | |
| Outcomes | Abstinence at 6 months (30‐day point prevalence) Validation: CO < 10 ppm | |
| Notes | Study ID was Ahluwalia 1999 until publication of full report; minor change to results; comparison between targeted and untargeted materials; significantly more participants used targeted materials (68.8% vs 59.6%) but no difference was detected in salience or in perceived materials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes computer‐generated by study statistician in blocks of 20 |
| Allocation concealment (selection bias) | Unclear risk | Described as investigator blinded, but no explicit statement provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation used; interventions of similar intensity |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 66% lost to follow‐up at 6 months, included in ITT analysis; no evidence of differential loss by group. |
Omenn 1988.
| Methods | Setting: single worksite (13,000 workers, 9 employers), USA Recruitment: worksite volunteers | |
| Participants | 243 smokers with preference for a self‐help programme | |
| Interventions | Only self‐help format conditions considered in this review ∙ Multiple‐component programme ∙ Relapse prevention programme ∙ Minimal treatment programme (American Cancer Society Quitter's Guide; 7‐day plan) | |
| Outcomes | Abstinence at 12 months Validation: saliva cotinine ≤ 35 ng/mL | |
| Notes | Comparison between self‐help materials; not in meta‐analysis No clinical or statistically significant differences between quit rates in the 3 groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "nurses at aid stations using randomized assignment lists generated by research centre, within preference for format" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation; interventions of similar intensities |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At least 89% followed up in each arm; non‐respondents counted as smokers |
Orleans 1991.
| Methods | Setting: HMO, USA Recruitment: largely through publicity in HMO magazine | |
| Participants | 2021 smokers; 63% female, average age 44, average cpd 26 | |
| Interventions | ∙ Free & Clear ‐ 28‐page guide incorporating nicotine fading and standard behavioural abstinence and relapse prevention techniques. Also, a Quit Kit and ALA publication, A Lifetime of Freedom from Smoking ∙ Same materials as first bullet above plus 2 copies of a social support guide to be given to "allies" ∙ Same materials as second bullet above plus TC plus quitline ∙ Control ‐ Referral guide describing available self‐help guides and local resources, plus NCI publication, Clearing the Air | |
| Outcomes | Abstinence at 16 months for over 6 months by blinded telephone interview Validation: saliva cotinine < 10 ng/mL, or thiocyanate < 2400 μmol/L for gum users | |
| Notes | 1 plus 2 vs 4, effect of self‐help alone (3 assessed in TC review) By 16 months, 59% of participants in the control group reported that they had used an additional treatment method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not stated; stratified by living alone/not; advice to quit in last 12 months/not and nicotine content of cigarette brand |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All arms included in this review received written material at similar levels of intensity. Biochemical validation in sample at 16 months "to improve the veracity of smoking self‐report, all follow‐up questionnaires and interviews began with a reminder that the subjects might be asked for a saliva specimen for nicotine assessment, creating a sort of 'bogus pipeline'" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 6% at 16 months; did not differ across treatment groups Analyses based on respondents, including losses, would marginally increase estimated effect |
Orleans 1998.
| Methods | Setting: Community, USA Recruitment: African American smokers calling a Cancer Information Service telephone counselling line in response to a targeted campaign | |
| Participants | 1422 African American smokers; average age not stated, 62% in 20 to 39‐year age group, median cpd 20 | |
| Interventions | ∙ 36‐page Pathways to Freedom guide and tailored TC. Guide used African American models and addressed specific obstacles ∙ Standard guide Clearing the Air and standard NCI TC | |
| Outcomes | Abstinence at 6 months, 7‐day point prevalence, telephone questionnaire (12 months' abstinence also assessed in a sample of 445 smokers) Validation: none | |
| Notes | Test of population targeting; counselling was also different for the 2 groups At 12 months, results showed significant differences (15.0% vs 8.8% for the sample selected for follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by last digit of caller's contact phone number; risk of bias probably low |
| Allocation concealment (selection bias) | Low risk | Presumably recruited before phone number and thus allocation known, so risk of bias probably low |
| Blinding (performance bias and detection bias) All outcomes | High risk | Self‐reported outcomes from participants not blinded to treatment condition; intervention includes personal contact with tailoring in one group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 37% lost to follow‐up at 6 months No differential dropout Meta‐analysis includes non‐responders as smokers |
Orleans 2000.
| Methods | Setting: community, USA Recruitment: smokers aged > 65 using nicotine patch | |
| Participants | 720 smokers; "mostly female", average age 72, average cpd 22 | |
| Interventions | All participants had filled a prescription for nicotine patch ∙ Clear Horizons guide for older smokers plus 7 personalised tailored computer‐generated mailings over 6 months ∙ Fact sheet on patch‐assisted quitting | |
| Outcomes | Abstinence at 12 months; 7‐day point prevalence Validation: none (limited information in abstract) | |
| Notes | Follow‐up rates supplied by N Boyd Considered with other studies testing self‐help adjuncts to pharmacotherapy, not with other tailored studies "Tailored messages were based on past research identifying the factors associated with general quitting success and with patch assisted quitting among older smokers" Not based on individual characteristics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Limited information in abstract; unclear if biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 21% experiment, 23% control; not significantly different Non‐responders included as smokers |
Owen 1989.
| Methods | Setting: community, Australia Recruitment: advertisements for smokers wishing to quit | |
| Participants | 208 smokers; average age 42, average cpd 28 | |
| Interventions | ∙ Quit Kit along with apology that course was full. Kit included a 5‐day cessation plan ∙ Self‐help programme in 4 mailed parts ∙ As in second bullet above, but personalised with additional text based on registration form (option to send for additional materials) | |
| Outcomes | Abstinence at 9 months (point prevalence) Validation: some cotinine assays but no correction for a possible 15% misreport level | |
| Notes | First intervention listed above meets criteria for basic self‐help, so 2 vs 1 for effect of additional materials and 3 vs 2 for effect of personalised materials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Control group (1) received notice that course was full; could introduce performance bias by artificially decreasing control group quit rates |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12% lost to follow‐up at 9 months; similar between groups Non‐respondents included as smokers |
Pallonen 1994.
| Methods | Setting: community cardiovascular risk factor study, Finland Recruitment: male smokers identified via survey | |
| Participants | 165 male smokers who were classified as pre‐contemplators or contemplators according to the SoC model; average age 52 years, average cpd 19 | |
| Interventions | ∙ Self‐help: five 10 to 20‐page self‐help manuals matched to SoC; mailed after each 6‐month assessment ∙ Usual care and annual telephone assessment | |
| Outcomes | Sustained abstinence at 2 years (point prevalence) Validation: none | |
| Notes | Included in main analysis although targeted materials Demoninators are smokers for whom complete follow‐up data were available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in 2:1 ratio, but prepared smokers in treatment condition then offered clinic, so groups were not balanced by SoC |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear if control participants knew the nature of the intervention; no biochemical validation; different intensities of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 37% lost to follow‐up by 2 years and not re‐included in MA, as group not given Study authors report sensitivity analysis of effect of excluding people with incomplete follow‐up and state that bias was not introduced |
Parekh 2014.
| Methods |
Setting: 21 general practices, Australia Recruitment: letters to patients identified via practice records |
|
| Participants | Approximately 400 people who completed a baseline health behaviour questionnaire and were not non‐smokers (˜ 14% of participants). Aged 18 to 70 years, had consulted in previous 6 months, 69% female, average age 46.9 years (all participants) | |
| Interventions | ∙ Single Intervention ‐ feedback on combined health score and personalised computer‐tailored advice (addressing smoking, diet, physical activity, and BMI), plus 1‐page health promotion information sheets for each behaviour that did not meet national guidelines ∙ Dual intervention ‐ as in first bullet above plus additional assessment and computerised feedback at 3 months ∙ Dual control ‐ as in first bullet above but without combined health score, and addressing other health behaviours (immunisation, protection behaviour, non‐smoking policies in home, screening; none of the items in the first bullet above) ∙ As per the third bullet above plus additional assessment and computerised feedback at 3 months |
|
| Outcomes | Abstinence at 12 months; self‐reported | |
| Notes | Multiple risk factor intervention; only a minority of participants were smokers Intervention tailored to health risks; smoking materials not individually tailored Numbers of smokers at baseline and follow‐up estimated from percentages Dual and single arms combined in comparison 1.1.2/2.1.2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “permuted block procedure stratified by GP” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “participants were blinded to the group to which they were randomized” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported only for all participants ‐ not for smokers |
Pederson 1983.
| Methods | Setting: respiratory specialist outpatient clinic, USA Recruitment: all smokers attending (unselected) | |
| Participants | 75 smokers; average age 52, average cpd 25 | |
| Interventions | ∙ Advice to quit, and effects of smoking on present health, from respiratory specialist ∙ Advice and self‐help manual, Break the Smoking Habit: A Behavioral Program for Giving Up Cigarettes (Pomerleau & Pomerleau) | |
| Outcomes | Abstinence at 6 months (self‐report of no smoking for 3 months via telephone interview) Validation: none | |
| Notes | Due to quasi‐random allocation, a sensitivity analysis of the effect of excluding this study is reported in the discussion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐random assignment by week of attendance; possibility of baseline differences |
| Allocation concealment (selection bias) | High risk | Not concealed, so risk of bias present, although all eligible patients at a clinic were supposed to be recruited, thus avoiding selection bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No biochemical validation but self‐report not given to physician; control group not aware of intervention content; equal amounts of physician contact in both groups, so differential misreport judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 lost in intervention arm, 1 in control arm; included as non‐responders in MA |
Prochaska 1993.
| Methods | Setting: community, USA Recruitment: advertisements for volunteers to test self‐help materials | |
| Participants | 756 smokers (93 pre‐contemplation, 435 contemplation, 228 preparation; 569 in relevant arms); average age 43, average cpd 27 | |
| Interventions | ∙ Standard self‐help ‐ ALA FfS, A Lifetime of Freedom from Smoking, 50 Most Often Asked Questions ∙ Targeted manuals ‐ 5 covering pre‐contemplation, contemplation, action, maintenance, and relapse. Participants were sent manual for their SoC and subsequent ones, except for relapse, which was sent following an assessment at which relapse occurred ∙ Tailored Interactive ‐ in addition to manuals, participants were sent personalised reports in response to questionnaires ∙ Counsellor telephone calls ‐ same as third bullet above with short calls at 0, 1, 3, and 6 months (not included in this review) | |
| Outcomes | Sustained abstinence at 18 months (12 months and 18 months) Validation: none; participants were asked for names of significant others but these were not contacted | |
| Notes | 2 vs 1 targeting, 3 vs 2 tailoring Numbers randomised and quit rates as shown on graphs obtained from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not stated; stratified by SoC |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Bogus pipeline" approach; names of significant others asked for but not contacted Similar intensities across interventions (all received manuals) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition at each assessment averaged 5.5% ‐ not significantly different across conditions Non‐respondents included as smokers in meta‐analysis |
Prochaska 2001a.
| Methods | Setting: managed care organisation, USA Recruitment: smokers identified by survey of members; 85% recruited to a study | |
| Participants | 1447 smokers (967 at 18 months' follow‐up); 56% female, average age 38, average cpd 20 | |
| Interventions | ∙ Assessment only (completed questionnaires on 4 occasions) ∙ Expert system ‐ tailored 2 to 3‐page report at 0 months, 3 months, and 6 months, and SoC‐matched manual ∙ As second bullet above plus telephone counselling ∙ As third bullet above plus computer for scheduled cigarette reduction | |
| Outcomes | Abstinence at 18 months, sustained for 6 months (other measures of abstinence also reported) Validation: none | |
| Notes | 2 vs 1, tailoring. 3 contributes to telephone counselling review; 4 not included Arm 2 is also evaluated in Velicer 1999 results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants not blinded to treatment condition; treatment more intensive than control, and no information on blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | MA includes losses to follow‐up and refusals Study author analysis suggests ITT analysis is biased Sensitivity analysis (comparison 99) tests impact on outcome |
Prochaska 2001b.
| Methods | Setting: community, USA Recruitment: random digit dialling; 80% of smokers reached recruited | |
| Participants | 4144 smokers (2571 at 24‐month follow up); 55% female, average age 41 years, average cpd 20 | |
| Interventions | ∙ Assessment only (questioned at 6‐month intervals) ∙ Expert System; see Prochaska 2001a | |
| Outcomes | Abstinence at 24 months, sustained for 6 months (other measures of abstinence also reported) Validation: none | |
| Notes | 2 vs 1, tailoring | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information on blinding provided; no validation; interventions at different levels of intensity, so differential misreport judged possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of loss to follow‐up but slightly higher refusal in intervention arm Non‐respondents included as smokers in meta‐analysis Sensitivity analysis (comparison 99) tests impact on outcome |
Prochaska 2004.
| Methods | Setting: community, USA Recruitment: parents of ninth grade students in a separate study; at risk for one of the targeted health behaviours | |
| Participants | 711 smokers from total of 2460 participants; 75% female (full sample), average age 43 years (full), average cpd 18, 41% at pre‐contemplation phase, 41% contemplators, 18% in preparation | |
| Interventions | ∙ Assessment only (completed questionnaires on 3 occasions) ∙ Expert system ‐ tailored 3 to 5‐page report at 0 months, 6 months, and 12 months and manual | |
| Outcomes | Abstinence at 24 months sustained for 6 months (other measures of abstinence also reported) Validation: none | |
| Notes | 2 vs 1, tailoring | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information on blinding provided; no validation; interventions at different levels of intensity, so differential misreport judged possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Slightly higher loss to follow‐up in Intervention (45%) than in control (40%) All participants included in this meta‐analysis |
Prochaska 2005.
| Methods | Setting: community, USA Recruitment: primary care patients proactively recruited by phone; at risk for one of the targeted health behaviours | |
| Participants | 1211 smokers from total of 5407 participants; 70% female (full sample), average age 45 years (full), average cpd 17, 31% at pre‐contemplation phase, 46% contemplators, 23.5% in preparation | |
| Interventions | ∙ Assessment only (completed questionnaires on 3 occasions) ∙ Expert system ‐ tailored 3 to 5‐page report at 0 months, 6 months, and 12 months and manual | |
| Outcomes | Abstinence at 24 months; point prevalence Validation: none | |
| Notes | 2 vs 1, tailoring Sustained abstinence also an outcome "same pattern of results" but details not reported Number of smokers by group at baseline not reported; data requested |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Telephone assessors blinded but unclear if participants knew the nature of the other arm; no validation; interventions at different levels of intensity, so differential misreport judged possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 35% loss to follow‐up at 24 months Insufficient data to include non‐respondents in meta‐analysis, but no interaction between missing data and intervention |
Prue 1983.
| Methods | Setting: Veterans Administration Medical Center outpatient clinic, USA Recruitment: smokers who could not attend clinic sessions referred to smoking treatment programme (selected) | |
| Participants | 40 smokers (likely to be predominantly male); average age 45 years, average cpd 32 | |
| Interventions | ∙ Self‐help programme (Pomerleau & Pomerleau) preceded by brand fading schedule; also telephone calls from psychologists ∙ Wait‐list control | |
| Outcomes | Point prevalence abstinence at 6 months' follow‐up (wait‐list treated after 6 months) Validation: significant other only | |
| Notes | This is a minimal contact programme rather than a strict self‐help one; marginal for inclusion; very small impact on meta‐analysis effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described; unbalanced group size |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Wait‐list control; performance bias possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analyses, but number lost to follow‐up not reported |
Resnicow 1997.
| Methods | Setting: predominantly African American community in USA Recruitment: in healthcare, church, and public housing settings; presented as 'health promotion' ‐ not smoking cessation | |
| Participants | 650 smokers who completed follow‐up interviews recruited in treatment channels and 504 in control channels (attrition similar between groups) Average age 45 years, average cpd 16 | |
| Interventions | ∙ Self‐help kit including Kick It guide, video, and aids; bimonthly mailings and single booster telephone call ∙ Health education materials not exclusively addressing smoking, and a cholesterol education video | |
| Outcomes | Point prevalence at 6 months Validation: none | |
| Notes | Less than a third of intervention group received telephone call Post hoc analysis reported significantly higher quit rates amongst call than no call group Multi‐variate analysis controlling for intracluster correlation gives OR of quitting in treatment group as 1.36 (95% CI 0.87 to 2.11) compared to OR 1.42 (95% CI 0.98 to 2.04) from figures used in meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised; stratified by type of site before recruitment of smokers; method of sequence generation not reported |
| Allocation concealment (selection bias) | High risk | Allocation known at time of recruitment; unclear whether this introduced high risk of bias; all participants received smoking cessation materials |
| Blinding (performance bias and detection bias) All outcomes | High risk | No biochemical validation and differential levels of contact between groups (including additional phone call); differential misreport judged possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition similar between treatment (7.5%) and control (6.8%) conditions Non‐respondents did not differ on baseline characteristics; not included in meta‐analysis denominators |
Rice 1994.
| Methods | Setting: hospital clinic, USA Recruitment: by health professional and self‐referral | |
| Participants | 406 smokers with a cardiovascular health problem | |
| Interventions | ∙ Self‐help materials ‐ Smokeless 6 booklet programme and individual nurse counselling ∙ Self‐help materials and group meetings ∙ Self‐help alone ‐ prompted to open envelope containing booklets on same schedule as other groups ∙ Advice to quit from nurse only | |
| Outcomes | Abstinence at 12 months Validation: saliva thiocyanate tested but rates not corrected for misreport | |
| Notes | 3 vs 4, self‐help vs control 1 and 2 not used in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not stated Stratified by sex, smoking history, and history of cardiovascular incident |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Biochemical validation conducted but not reported; unclear if participants knew what other arms were receiving; arms involved differing levels of intensity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% did not provide data at final follow‐up and were counted as smokers in final analysis; 12 died before follow‐up and were not included in final outcome figures |
| Other bias | Unclear risk | Differential non‐participation by experimental group assignments |
Schofield 1999.
| Methods | Setting: hospital, Australia Recruitment: smokers discharged from hospital (unselected) | |
| Participants | 2465 smokers or recent quitters (excludes 1693 randomised but lost at 12‐month follow‐up) No differential dropout; 59% followed up in each arm; no demographic data |
|
| Interventions | ∙ Self‐help 31‐page SoC‐based booklet + personally addressed letter from consultant stating health risks and urging to quit ∙ Usual care | |
| Outcomes | Abstinence at 12 months and at 6 months
Validation: urine cotinine ≤ 50 ng/mL or CO ≤ 8 ppm for sample Refusers (22% in each group) classified as smokers |
|
| Notes | Self‐help; no contact Study authors reported benefit for subgroup for whom quitting was highly relevant to diagnosis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised: "alternately allocated to intervention or control conditions by computer" |
| Allocation concealment (selection bias) | Low risk | Smokers identified at time of admission and allocation determined at that time Mailing of materials done by medical records office |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Control group does not appear to have been aware of intervention condition; biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some people discovered to be ineligible at follow‐up and excluded Loss to follow‐up 41% ‐ identical in each group Meta‐analysis based on eligible respondents |
Schumann 2008.
| Methods | Setting: community, Germany Recruitment: from participants in a general population health examination survey | |
| Participants | 847 smokers (ex‐smokers in study not included here); 46% female (full sample), average age 44 years (full), average cpd 15 Controls more likely to be in preparation (32% vs 20%) and to have past year quit attempt |
|
| Interventions | ∙ Assessment only (completed questionnaires on 3 occasions) ∙ Expert system ‐ tailored 3 to 4‐page letter and 8 to 26‐page SoC‐matched booklet at 0 months, 3 months, and 6 months | |
| Outcomes | Abstinence at 24 months; sustained 18 months' follow‐up (other measures of abstinence also reported) Validation: none | |
| Notes | Tailoring ‐ 67% got 3 letters, 21% 2, 13% only 1 72% reported reading some materials |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was assigned a unique computer‐generated random number between 0 and 1; the data file was sorted by ascending random numbers; participants were then consecutively assigned to the 3 study conditions |
| Allocation concealment (selection bias) | Low risk | No opportunity to alter allocation or exclude |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although no biochemical validation, written contact only; participants in control group do not appear to have known of intervention; all participants engaged in long‐term questionnaires re smoking status, so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Somewhat greater loss in intervention (34%) than in control (27%) Meta‐analysis includes those lost as smokers Study authors report that generalized estimation equation gave similar results |
Smith 2004.
| Methods | Setting: 10 communities, Canada Recruitment: volunteers intending to quit | |
| Participants | 632 smokers (423 in relevant arms); 61% female, average age 42 years, 61% had prior use of NRT | |
| Interventions | Factorial design comparing 2 intensities of TC and 2 types of print materials ∙ Booklet (Canadian Cancer Society (CCS) ‐ One Step at a Time ‐ 44 pages) ∙ Pamphlet (CCS How to Quit Smoking ‐ single page) TC conditions collapsed; booklet‐only control group not used in the review | |
| Outcomes | Abstinence at 12 months, sustained at 3 months' and 6 months' follow‐up Validation: none | |
| Notes | No non‐self‐help control; comparison between materials Results not reported by group; "no significant interactions or main effects" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; stratified by community; method not described |
| Allocation concealment (selection bias) | Low risk | Centralised sequential envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Self‐reported outcomes from participants not blinded to treatment condition, but no difference in personal contact between intervention arms, so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Collapsing across telephone counselling groups, significantly more participants receiving print only were available for follow‐up at 12 months (73%) than those receiving telephone counselling (62%). Those not available for follow‐up were considered smokers for the intention‐to‐treat analyses" |
Strecher 2005.
| Methods | Setting: community, USA Recruitment: telephone callers to NCI Cancer Information Service, interested in quitting | |
| Participants | 1978 smokers; 70% female, average age 41 years, 46% smoked > pack/d, FTND 5.9 | |
| Interventions | All participants received approx 15 minutes of telecounselling Control ‐ single untailored 24‐page booklet (Clearing the Air) Intervention 1 ‐ single 8‐page tailored booklet, addressing motives and barriers cited by smoker Intervention 2 ‐ single untailored 24‐page booklet (Clearing the Air); multiple tailored materials (booklet, 2 newsletters, letter) at 5 months, 8 months, 12 months; tailored on baseline data Intervention 3 ‐ single untailored 24‐page booklet (Clearing the Air); multiple re‐tailored materials (same components and schedule as Intervention 2; used data from 5‐month follow up for re‐tailoring) | |
| Outcomes | Abstinence at 12 months (7‐day point prevalence, but had also reported abstinence at 5 months' follow‐up) Validation: none | |
| Notes | To derive numbers quit, assumed equal numbers in each condition 2 plus 3 plus 4 vs 1 in tailored vs untailored Slightly more evidence of effect when multiple compared to single (3 plus 4 vs 1 plus 2), and also for re‐tailored materials amongst subgroup who were quit at 5 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although no biochemical validation, all participants received same telecounselling and were unaware of other treatment conditions, so risk judged to be low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only respondents at 5 months eligible for 12 months' follow‐up 56% loss at 12 months (but includes those smoking at 5 months); no difference by condition Losses included as smokers |
Sutton 2007.
| Methods | Setting: community, UK Recruitment: callers to UK Quitline (smokers planning to quit in next 30 days or who quit in last 14 days) | |
| Participants | 1506 including 344 (23%) recent quitters; 66% female, average age 38, average cpd 21 | |
| Interventions | All participants received telephone counselling and Quit information pack ∙ Standard letter ∙ Tailored 3‐page letter (based on social‐cognitive theory and perspectives on change model. Aimed to encourage and support smokers. Medium‐ or high‐dependence smokers advised to talk to their GP about cessation products) | |
| Outcomes | Abstinence at 6 months; self‐reported as sustained for 3 months Validation: none | |
| Notes | Tailoring; subgroup of baseline smokers showed larger effect of intervention, but effect was still not significant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was effected by dividing days randomly within each of a series of consecutive 56‐day blocks into two equal sets, with allocation to group depending on which day the participant called the Quitline" |
| Allocation concealment (selection bias) | Low risk | "Randomization was carried out by a member of the research team who had no direct contact with the counsellors or the participants. Counsellors were unaware of which condition the participant was allocated to and would have remained blind unless the participant had happened to mention during a subsequent telephone conversation that they had or had not received a tailored letter" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although no biochemical validation, participants received same telephone counselling and one‐off written material, so risk of differential misreport judged to be low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up non‐significantly higher in control (24.4%) than in intervention (20.8%) Losses treated as smoking |
Sykes 2001.
| Methods | Setting: cessation clinic, UK Recruitment: community volunteers interested in quitting | |
| Participants | 260 smokers, high proportion low socioeconomic status; 64% female, average age not stated, average cpd 25 | |
| Interventions | ∙ Quit for Life ‐ cognitive‐behavioural manual, audiotape; gradual reduction pre‐quit day; stresses psychological addiction ∙ Stopping Smoking Made Easier ‐ leaflet, SoC‐based; abrupt quit | |
| Outcomes | Abstinence at 12 months (Sykes 2001 reports 6 months) Validation: CO < 9 ppm | |
| Notes | Comparison between self‐help materials; does not contribute to MA 1‐year data from Marks 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised by orientation group attended; method not described |
| Allocation concealment (selection bias) | Unclear risk | Although potential for selection bias, "the receptionist was unaware of which intervention each group of participants would receive" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation; similar intensity of interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15% loss to follow‐up at 1 year; similar across groups |
Thompson 1988.
| Methods | Setting: HMO, USA Recruitment: consecutive attenders (unselected) | |
| Participants | 379 smokers (in relevant arms); average cpd not stated, 68% smoked > 15 cpd | |
| Interventions | Complete factorial design of 3 interventions A ‐ Physician advice ‐ structured and interactive, 3 to 5 minutes B ‐ Self‐help materials (NCI Calling It Quits and Why Do You Smoke?; and a personalised follow‐up letter) C ‐ Referral to group cessation classes Control ‐ brief advice only | |
| Outcomes | Abstinence at 8 months to 9 months by telephone survey Validation: none | |
| Notes | A plus B and B vs A and Control in self‐help plus advice vs advice only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "physician used a randomized folder placed in the patient chart"; unclear when and how randomisation schedule was generated |
| Allocation concealment (selection bias) | Unclear risk | Participants enrolled before visiting physician, so selection bias by physician was avoided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although no biochemical validation, participants were never aware that smoking cessation was the study target, so risk of performance bias and differential misreport were judged to be low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8% lost to follow‐up, but not clear by what arm; not included in final analyses |
van der Aalst 2012.
| Methods |
Setting: community, Belgium and the Netherlands Recruitment: subgroup of participants enrolled in lung cancer screening trial; identified via population registry |
|
| Participants | 1284 currently smoking male participants of lung cancer screening trial, 50 to 75 years old, smokers of > 15 cpd for > 25 years or > 10 cpd for > 30 years 100% male, average age 57, average cpd 18, 55% not planning to quit within 6 months |
|
| Interventions | ∙ Computer‐tailored smoking cessation advice via mail (one‐off), sent only to participants who completed questionnaire after randomisation ∙ Standard brochure (35 pages; Smoking Cessation, Why and How) |
|
| Outcomes | Continuous abstinence at 2 years (prolonged; point prevalence also reported) Validation: none |
|
| Notes | Participants had to return questionnaire before receiving tailored brochure – only 23% did so (147/642) In this subset, quit rates were slightly higher (14.3% prolonged as compared to 12.5% in total intervention group) but were still less than in control group and no significant difference |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not blinded, but at assessment, majority of participants were unaware of which they had been assigned to; differential misreport judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 84% intervention and 85% control followed up at 2 years |
Velicer 1999.
| Methods | Setting: managed care organisation, USA Recruitment: smokers identified by survey of members; 85% recruited to study | |
| Participants | 2882 smokers in a managed care organisation; average age 38 years, average cpd 20 | |
| Interventions | ∙ Interactive expert system ‐ generated 2 to 4‐page reports based on SoC model and stage‐based manuals; 4 different levels of contact ‐ 1, 2, 3, and 4 occasions at 3‐month intervals ∙ Stage‐based manuals only; same 4 levels of contact | |
| Outcomes | Abstinence at 18 months, sustained for 6 months (other measures of abstinence also reported) Validation: none | |
| Notes | 1 vs 2, tailoring No evidence of a dose response to the number of contacts in either condition Expert system conditions were better than stage‐based at each contact level, so these were collapsed in meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not blinded or biochemically validated, but given similar intensity of both conditions; performance bias and differential misreport judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors report numbers refusing follow‐up and numbers not reached Size and significance of results are sensitive to whether or not those lost to follow‐up or refusing to respond are included in the denominator as continued smokers Including all non‐responders in denominator gives a more conservative estimate and is done in the meta‐analysis |
Velicer 2006.
| Methods | Setting: community, USA Recruitment: proactive approach to smokers at Veterans Administration Medical Center | |
| Participants | 2054 smokers (1031 in relevant arms); 23% female, average age 51 years, 40% pre‐contemplators, 40% contemplators, 20% preparers | |
| Interventions | ∙ Stage‐based self‐help manuals; participants sent manual for current stage and for next stage on ∙ As first bullet above plus 6 weeks nicotine patch if in appropriate stage; reassessed for NRT eligibility at 6 months and 10 months ∙ As second bullet plus 1 expert system feedback report (see Prochaska trials) ∙ As third bullet plus regular automated telephone counselling | |
| Outcomes | Abstinence at 30 months; sustained for 6 months Validation: none | |
| Notes | 3 vs 2 for tailored adjunct to targeted self‐help In NRT groups, 350 (67%) received NRT at baseline and 448 (86%) received NRT at some point |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generator |
| Allocation concealment (selection bias) | Low risk | Allocation done after completion of survey Randomised participants who did not return consent form were excluded from further analyses |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Self‐reported outcomes from participants not blinded to treatment condition, but intensity did not differ substantially by condition, so differential misreport judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 39% lost including 8% refused by 30 months; no significant differences between groups Different treatments of missing data reported not to have altered pattern of results |
Webb 2013.
| Methods |
Setting: community, USA Recruitment: community volunteers |
|
| Participants | 424 current smokers of ≥ 5 cpd, 57% female, average age 42 years, average cpd 19.7 | |
| Interventions | Both groups received 2 priming phone calls explaining benefits of intervention and encouraging use of materials. In intervention, priming call explicitly stated materials were tailored ∙ 4 self‐help booklets with placebo tailoring, covering cigarette smoking, cessation, and relapse prevention, based on cognitive‐behavioural techniques ∙ 4 standard self‐help booklets covering same materials |
|
| Outcomes | 1 month sustained abstinence at 6 months Validation: CO ≤ 8 ppm for locals |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Biochemical validation used; interventions of similar intensities |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29% lost in intervention group, 25% lost in control group |
Willemsen 2006.
| Methods | Setting: community, Netherlands Recruitment: smokers identified from a market research database; willing to participate in evaluation of an ‘information aid’ | |
| Participants | 1014 smokers 'intending to quit'; 46% female, modal age 35 to 44 years, modal cpd 18 to 22, 86% daily smokers | |
| Interventions | ∙ Mailed Decision Aid: Starter’s Kit, including information about all major available treatment methods, classified into known effective and unknown; samples of materials and information on how to obtain them; video with descriptions of quitting experiences ∙ No intervention | |
| Outcomes | Sustained abstinence at 6 months (quit for longer than ˜ 4 months) Validation: none | |
| Notes | Self‐help vs control; aid had no effect on prolonged abstinence outcome used in meta‐analysis but did have an effect on point prevalence abstinence Aim of intervention was to increase use of efficacious aids, but it had no effect Study authors note that aid "did not contain any concrete self‐help information that the smokers might have put into practice" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants in control group aware that it was a trial of self‐help materials; never received materials, which could have artificially lowered control group quit rate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11.8% lost at 6 months; intervention participants more likely to be missing at 2 weeks' but not at 6 months' follow‐up Losses included as smokers |
4As: Ask, Advise, Assist, Arrange. ALA FfS: American Lung Association Freedom from Smoking programme. CCS: Canadian Cancer Society. CI: confidence interval. CO: carbon monoxide. cpd: cigarettes per day. FEV₁: forced expiratory volume in one second. FTND: Fagerstrom Test for Nicotine Dependence. GP: general practitioner. HEA: Health Education Authority. HMO: health maintenance organisation. ITT: intention to treat. MA: meta‐analysis. NCI: National Cancer Institute. NG: nicotine gum. NRT: nicotine replacement therapy. OR: odds ratio. ppm: parts per million. RR: risk ratio. SES: socioeconomic status. SoC: stage of change. TTM: transtheoretical model. TTQ: time to quit. VA: Veterans Administration.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ainsworth 2013 | Not a self‐help intervention; intervention print‐based, but aimed at faith leaders to effect change in their communities |
| Armitage 2008a | Follow‐up only 2 months |
| Armitage 2008b | Follow‐up only 1 month; intervention borderline for inclusion |
| Arnold 2009 | Follow‐up only 1 month |
| Balanda 1999 | Follow‐up only 1 month after provision of 1 of 2 self‐help guides to quitline callers; no differences found between groups |
| Bansal‐Travers 2010 | Only 1 month's follow‐up; all participants received NRT and counselling |
| Barnett 2015 | Intervention group also received counselling |
| Brandon 2000 | Only recent quitters recruited; included in Cochrane Review of relapse prevention (Hajek 2013) |
| Brandon 2004 | Only recent quitters recruited; included in Cochrane Review of relapse prevention (Hajek 2013) |
| Brandon 2012 | Relapse prevention intervention |
| Brown 1992 | Both arms received S‐H materials; test of telephone counselling; included in Cochrane Review of telephone counselling (Stead 2013b) |
| Burling 2000 | Evaluated an internet‐based intervention; previously included in review but not in meta‐analysis; falls within scope of separate Cochrane protocol (Koshy 2008) |
| Carré 2008 | Short follow‐up; not primarily directed at cessation |
| Conway 2004 | Intervention targeted at relapse prevention (see Edwards 1999) |
| Curry 1988 | Compares self‐help materials with a relapse prevention approach vs abstinence‐based approach; now included in relapse prevention review (Hajek 2013) |
| Dijkstra 1998b | Follow‐up only 4 months (6 weeks from last contact for multiple tailored letters condition) Study compared combinations of tailored letters and a self‐help guide for a population of smokers not planning to quit |
| Dijkstra 2001 | Follow‐up only 3 months; compares different types of information in self‐help materials |
| Dijkstra 2005 | Not a structured S‐H intervention; outcome is "quitting activity" at 4 months. Participants were students recruited to evaluate smoking cessation messages |
| Dijkstra 2006 | Outcome is change in stage ‐ not abstinence |
| Dijkstra 2009 | Field study testing function of disengagement beliefs; numbers abstinent not reported |
| Edwards 1999 | Intervention directed at relapse prevention in female naval recruits required to quit smoking during basic training; included in review of relapse prevention interventions (Hajek 2013) |
| Emmons 2013 | Does not test self‐help; self‐help served as control for more intensive intervention |
| Etter 2007 | Intervention provided information about additives in cigarettes; focus on motivating rather than assisting quitting |
| Fortmann 1995 | Excluded from 2018 update because study of relapse prevention |
| Garcia 2000 | Trial of group therapy‐based interventions; self‐help manuals provided in addition to group therapy to test effect of therapist contact; included in Cochrane Group Therapy Review (Stead 2017) |
| Gritz 1988 | No control group |
| Hall 2003 | Smoking cessation not an outcome |
| Jeffery 1982 | No long‐term follow‐up; control was a group programme |
| Jeffery 1990 | Compared the offer of a self‐help programme at a nominal cost vs the same programme for a USD60 payment, refundable if successful. Rate of recruitment to the incentive programme very low (9 participants, 0.09% of households randomly assigned to receive the incentive option) |
| Johs 2003 | No long‐term follow‐up |
| Jordan 1999 | Only 3 months' follow‐up planned; comparison of an internet‐based programme vs ALA printed manuals; 54 participants |
| Killen 1990 | Excluded from 2018 update because study of relapse prevention |
| Kreuter 1996 | Intervention provided single page of cessation information for participants who were smokers (22%) and interested in quitting; not a self‐help intervention by the criteria for this review (neither standard nor enhanced feedback increased quit rates over control) |
| Kreuter 2012 | Print materials not designed as self‐help; intention to increase number of people taking up referrals to specialist service |
| Lenert 2004 | Not randomised; used consecutive series of participants |
| Lipkus 2004 | Self‐help was the control condition |
| McBride 1999 | Intervention included 3 proactive telephone calls in addition to provision of self‐help materials; no effect of the intervention was found |
| McDonald 2003 | Unpublished study; insufficient data to include |
| McDonnell 2011 | Does not test self‐help; self‐help served as control for more intensive intervention |
| McMahon 2000 | Tested incentives and social support as adjuncts to self‐help; included in Cochrane Review of support (Park 2004) |
| Meade 1989 | Compared smokers' ability to understand materials written at different grade levels; cessation was not an outcome |
| Moore 2002 | Participants were pregnant women |
| Murphy 2005 | Only 3 months' follow‐up; marginal to classify as self‐help intervention; provided information on access to pharmacotherapy and cessation support |
| Naughton 2012 | Does not test self‐help; self‐help served as control for more intensive intervention |
| NCT00714467 | Experimental variable is partner support ‐ not self‐help |
| NCT01566994 | No suitable control group for comparison |
| O'Hara 1993 | Follow‐up only 3 weeks after receipt of materials |
| Ossip‐Klein 1991 | Both arms received self‐help materials; test of hotline availability; included in Cochrane Review of telephone counselling (Stead 2013b) |
| Ossip‐Klein 1997 | Both arms received self‐help materials; test of telephone counselling; included in Cochrane Review of telephone counselling (Stead 2013b) |
| Pallonen 1998 | Intervention targeted for adolescents; 2 self‐help computer‐based interventions compared; included in a Cochrane Review of cessation interventions for adolescents and young people (Fanshawe 2017) |
| Pederson 1981 | Although this is described as a trial of behavioural self‐help manuals, treatment conditions included an introductory meeting and 2 further group meetings |
| Rimer 1994 | No long‐term follow‐up data reported in full |
| Russell 1979 | Leaflet used as an adjunct to physician advice did not meet study criteria for a structured self‐help intervention Smokers given the leaflet were also warned that they would be followed up Study found a non‐significant increase in the quit rate amongst participants who were given the leaflet in addition to advice, but including it would not alter the results of the MA, which found no effect of materials as an adjunct to advice |
| Sallis 1986 | Only 2 months' follow‐up; then wait‐list control offered treatment |
| Senesael 2013 | Multiple risk factor intervention recruiting only 7 smokers; unclear if smoking intervention met inclusion criteria |
| Shi 2013 | Does not test self‐help; self‐help served as control for more intensive intervention |
| Shiffman 2000 | Only 6 weeks' follow‐up; tested materials tailored to individual smokers, in addition to nicotine gum; compared to gum and standard written materials |
| Shiffman 2001 | Only 6 weeks' follow‐up; tested materials tailored to individual smokers, in addition to nicotine patches; compared to patches and standard written materials |
| Sims 2013 | Does not test self‐help; self‐help served as control for more intensive intervention |
| Song 2012 | Relapse prevention intervention |
| Stanczyk 2013 | Web‐based intervention |
| Strecher 1994 | Did not meet review criteria for self‐help materials Compared health letters tailored to individual recipient's smoking behaviour vs no intervention (Study 2) or a standardised health letter from a physician (an adaptation of NCI Quit for Good pamphlet addressing general benefits of and barriers to quitting smoking) (Study 1) Study 1 had less than 6 months' follow‐up |
| Strecher 2000 | Participants were pregnant women |
| Strecher 2005b | Short follow‐up |
| Strecher 2008 | Did not meet review criteria for self‐help materials; Web‐based programme |
| Te Poel 2009 | Web‐based intervention |
| Travis 2004 | Short follow‐up; self‐help was an adjunct to telephone counselling |
| Travis 2009 | Only 3 months' follow‐up |
| Ussher 2011 | Uncontrolled evaluation |
| Webb 2005 | Smoking status not a measured outcome |
| Webb 2007 | Smoking status not a measured outcome |
| Webb 2008 | Only 3 months' follow‐up |
| Webb 2009 | Only 3 months' follow‐up |
| Webb 2010 | Outcomes included risk perceptions, readiness to quit smoking, and smoking‐related knowledge ‐ not smoking cessation |
| Weissfeld 1991 | 'Self‐help' condition received several individual counselling sessions |
| Wetter 2011 | All groups received multiple group counselling sessions |
| Willemsen 1995 | Not a randomised trial |
| Windsor 1989 | All groups received the same self‐help intervention; differed on additional support or incentives |
| Zhu 1996 | All arms received self‐help materials; test of telephone counselling; included in Cochrane Review of telephone counselling (Stead 2013b) |
ALA: American Lung Association. MA: meta‐analysis. NCI: National Cancer Institute. NRT: nicotine replacement therapy.
Characteristics of studies awaiting assessment [ordered by study ID]
Campos 2014.
| Methods | Prospective randomised study |
| Participants | 90 people who smoke hospitalised in the University Hospital Antonio Pedro, Brazil 61.1% male, average age 51.1 ± 12.2 years |
| Interventions | ∙ Brief intervention (n = 45) ∙ Intensive intervention with presentation of an educational video produced by researchers (n = 45) |
| Outcomes | Smoking abstinence assessed by telephone in first, third, and sixth month after discharge and confirmed by carbon monoxide measurement |
| Notes | Could not find contact details of study author team |
Oh 2013.
| Methods | Prospective randomised controlled study |
| Participants | 58 active smokers who presented to an academic otolaryngology clinic |
| Interventions | ∙ Received a free copy of the book, Allen Carr’s The Easy Way to Stop Smoking ∙ Received only the name of the book and author Both cohorts received physician‐directed cessation counselling |
| Outcomes | Follow‐up phone calls were conducted at 2 weeks and 6 months to assess smoking status, whether control group participants bought the book, and how many pages of the book were read |
| Notes | Study authors contacted for further details but no response yet |
Pereira 2017.
| Methods | Prospective randomised study |
| Participants | Hypertensive, diabetic, and chronic renal patients with high cardiovascular risk Sixty‐six people who smoke, 64.7% female |
| Interventions | ∙ Intervention group ‐ using an interactive and educational video about smoking ∙ Control group ‐ using a basic approach to smoking |
| Outcomes | Unclear if smoking abstinence was reported |
| Notes | Could not find contact details of study author team |
Characteristics of ongoing studies [ordered by study ID]
JPRN‐UMIN000008750.
| Trial name or title | A randomized study of bibliotherapy for smoking cessation with and without focusing on cognitive elements |
| Methods | Randomised controlled trial |
| Participants | People who smoke (target sample size: 1000) |
| Interventions | ∙ Provide 1 bibliotherapy booklet focussed on cognitive elements ∙ Provide 1 conventional bibliotherapy booklet |
| Outcomes | Smoking status followed up at 1 year |
| Starting date | 23/08/2012 |
| Contact information | Takeshi Isomur; atakeshiisomura@gmail.com |
| Notes | Funding: self‐funded |
NCT01544010.
| Trial name or title | Optimal TTM tailoring for population cessation (STAR) |
| Methods | Randomised controlled trial |
| Participants | 3006 people who smoke |
| Interventions | ∙ Assessment only control group ∙ Stage‐tailored manual ∙ Stage‐tailored feedback report ∙ Moderate TTM‐tailored feedback report ∙ Full TTM‐tailored feedback report ∙ Enhanced TTM+Addiction‐tailored feedback report |
| Outcomes | Self‐reported smoking abstinence at 24 months |
| Starting date | February 2009 |
| Contact information | Colleen A. Redding; credding@uri.edu |
| Notes | Study presumed complete. Study author team contacted for results, but no response received |
NCT02276664.
| Trial name or title | Capitalizing on a teachable moment to promote smoking cessation |
| Methods | Randomised controlled trial |
| Participants | People who have smoked at least 1 cigarette over the past week |
| Interventions | ∙ Self‐help intervention ∙ Usual care |
| Outcomes | Self‐reported 7‐day abstinence up to 9 months |
| Starting date | 20/10/2014 |
| Contact information | Thomas Brandon; Thomas.Brandon@moffitt.org |
| Notes |
NCT02416011.
| Trial name or title | Smoking cessation self‐help for dual users of tobacco cigarettes and e‐cigarettes |
| Methods | Randomised controlled trial |
| Participants | Adult current dual users of tobacco cigarettes and e‐cigarettes |
| Interventions | ∙ Assessment only ∙ Generic self‐help ∙ Targeted self‐help |
| Outcomes | Smoking abstinence at 24 months |
| Starting date | 31/03/2015 |
| Contact information | Thomas Brandon; Thomas.Brandon@moffitt.org |
| Notes | Funding: this work is supported by the National Institute on Drug Abuse of the National Institutes of Health (R01DA037961) |
NCT02611076.
| Trial name or title | Smoking‐cessation: a Spanish‐language clinical trial |
| Methods | Randomised controlled trial |
| Participants | People who currently smoke and are monolingual Spanish, or bilingual Spanish‐English, and prefer receiving educational health materials in Spanish |
| Interventions | ∙ Stop Smoking for Good Intervention in Spanish (10 booklets distributed over 18 months, plus additional monthly contacts via 9 supportive pamphlets) ∙ Usual care |
| Outcomes | Smoking cessation rates based on 7‐day point prevalence abstinence at 24 months |
| Starting date | 15/10/2015 |
| Contact information | Vani N. Simmons; Vani.simmons@moffitt.org |
| Notes |
NCT02945787.
| Trial name or title | Spanish‐language smoking cessation trial |
| Methods | Randomised controlled trial |
| Participants | People who currently smoke and are monolingual Spanish‐speaking, or bilingual Spanish‐English, and prefer receiving educational health materials in Spanish |
| Interventions | ∙ Extended self‐help (Spanish‐language version of the Stop Smoking for Good: 11 Stop Smoking for Good booklets and 9 supportive My Story pamphlets) ∙ Usual care |
| Outcomes | Smoking cessation rates based on 7‐day point prevalence abstinence at 24 months |
| Starting date | 24/10/2016 |
| Contact information | Thomas Brandon; Thomas.Brandon@moffitt.org |
| Notes |
NCT03064971.
| Trial name or title | Enhancing quitline services for African American smokers |
| Methods | Randomised controlled trial |
| Participants | People who currently smoke and self‐identify as Black/African American |
| Interventions | ∙ Standard care plus Pathways to Freedom DVD ∙ Standard care plus standard smoking cessation DVD ∙ Standard care only |
| Outcomes | Cotinine‐verified 7‐day point prevalence abstinence at 6 months |
| Starting date | 22/05/2017 |
| Contact information | Monica Webb Hooper; monica.hooper@case.edu |
| Notes |
Funding: Monica Webb Hooper, PhD, was supported by a Research Scholars Grant from the American Cancer Society (15–154‐01‐CPPB) Declaration of interests: one study author declares that she is employed by Alere Wellbeing and has no other competing interests. Remaining study authors declare that they have no competing interests |
TTM: transtheoretical model.
Differences between protocol and review
Studies testing telephone counselling as an adjunct to print‐based self‐help or interventions for preventing smoking relapse are no longer included in this review as they are included in other Cochrane Reviews.
As of the 2018 update of this review, we conducted meta‐analyses using a random‐effects model in accordance with new guidance from the Cochrane Tobacco Addiction Group.
Contributions of authors
In the most recent update, JLB conducted searches for studies, and JLB and JMOM performed study screening and data extraction. JLB updated the text and meta‐analyses with oversight from JHB. All review authors reviewed, commented on, and approved the final manuscript.
Sources of support
Internal sources
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK.
National Institute for Health Research (NIHR) School for Primary Care Research, UK.
External sources
National Institute for Health Research (NIHR) Cochrane Programme Grant, UK.
National Institute for Health Research (NIHR) Community Healthcare Medtech and In Vitro Diagnostics Cooperative (MIC), UK.
NIHR Biomedical Research Centre, Oxford, UK.
Declarations of interest
JLB: none known.
JMOM: none known.
JHB: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Aveyard 2003 {published data only}
- Aveyard P, Griffin C, Lawrence T, Cheng KK. A controlled trial of an expert system and self‐help manual intervention based on the stages of change versus standard self‐help materials in smoking cessation. Addiction 2003;98(3):345‐54. [DOI] [PubMed] [Google Scholar]
Becona 2001a {published data only}
- Becona E, Vazquez FL. Effectiveness of personalized written feedback through a mail intervention for smoking cessation: a randomized‐controlled trial in Spanish smokers. Journal of Consulting and Clinical Psychology 2001;69(1):33‐40. [PubMed] [Google Scholar]
Becona 2001b {published data only}
- Becona E, Vasquez FL. Comparison of the efficacy of a self‐help smoking cessation by mail with a manual in one‐time intervention or weekly intervention. Psiquis 2001;22:41‐50. [Google Scholar]
Berman 1995 {published data only}
- Berman BA, Gritz ER, Braxton Owens H, Nisenbaum R. Targeting adult smokers through a multi‐ethnic public school system. Journal of Cancer Education 1995;10:91‐101. [DOI] [PubMed] [Google Scholar]
Betson 1998 {published and unpublished data}
- Betson CL, Lam TH, Chung TWH, Chung SF. A randomized controlled trial of smoking cessation in Government out‐patient clinics in Hong Kong (Abstract OS 321). Proceedings of the 10th World Conference on Tobacco or Health; Aug 24‐28; Beijing, China. 1997:113.
Borland 2003 {published data only}
- Borland R, Balmford J, Segan C, Livingston P, Owen N. The effectiveness of personalized smoking cessation strategies for callers to a Quitline service. Addiction 2003;98(6):837‐46. [DOI] [PubMed] [Google Scholar]
Borland 2004 {published data only}
- Borland R, Balmford J, Hunt D. The effectiveness of personally tailored computer‐generated advice letters for smoking cessation. Addiction 2004;99(3):369‐77. [DOI] [PubMed] [Google Scholar]
Brandon 2016 {published data only (unpublished sought but not used)}
- Brandon TH, Simmons VN, Sutton SK, Unrod M, Harrell PT, Meade CD, et al. Extended self‐help for smoking cessation: a randomized controlled trial. American Journal of Preventive Medicine 2016;51(1):54‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unrod M, Simmons VN, Sutton SK, Meltzer LR, Harrell PT, Meade CD, et al. A randomized clinical trial of self‐help intervention for smoking cessation: research design, interventions, and baseline data. Contemporary Clinical Trials 2014;38(2):284‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 1983 {published data only}
- British Thoracic Society. Comparison of four methods of smoking withdrawal in patients with smoking related diseases. Report by a subcommittee of the Research Committee of the British Thoracic Society. BMJ 1983;286:595‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burling 1989 {published data only}
- Burling TA, Marotta J, Gonzalez R, Moltzen JO, Eng AM, Schmidt GA, et al. Computerized smoking cessation program for the worksite: treatment outcome and feasibility. Journal of Consulting and Clinical Psychology 1989;57:619‐22. [DOI] [PubMed] [Google Scholar]
Campbell 1986 {published data only}
- Campbell IA, Hansford M, Prescott RJ. Effect of a "stop smoking" booklet on smokers attending for chest radiography: a controlled study. Thorax 1986;41:369‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2004 {published data only}
- Clark MM, Cox LS, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Effectiveness of smoking cessation self‐help materials in a lung cancer screening population. Lung Cancer 2004;44(1):13‐21. [DOI] [PubMed] [Google Scholar]
Cuckle 1984 {published data only}
- Cuckle HS, Vunakis H. The effectiveness of a postal smoking cessation 'kit'. Community Medicine 1984;6:210‐5. [DOI] [PubMed] [Google Scholar]
Cummings 1988 {published data only}
- Cummings KM, Emont SL, Jaen C, Sciandra R. Format and quitting instructions as factors influencing the impact of a self‐administered quit smoking program. Health Education Quarterly 1988;15:199‐216. [DOI] [PubMed] [Google Scholar]
Curry 1991 {published data only}
- Curry SJ, Wagner EH, Grothaus LC. Evaluation of intrinsic and extrinsic motivation interventions with a self‐help smoking cessation program. Journal of Consulting and Clinical Psychology 1991;59:318‐24. [DOI] [PubMed] [Google Scholar]
Curry 1995 {published data only}
- Curry SJ, McBride C, Grothaus LC, Louie D, Wagner EH. A randomized trial of self‐help materials, personalized feedback, and telephone counselling with nonvolunteer smokers. Journal of Consulting and Clinical Psychology 1995;63:1005‐114. [DOI] [PubMed] [Google Scholar]
Davies 1992 {published data only}
- Davies BL, Matte Lewis L, O'Connor AM, Dulberg CS, Drake ER. Evaluation of the "Time to Quit" self‐help smoking cessation program. Canadian Journal of Public Health 1992;83:19‐23. [PubMed] [Google Scholar]
Davis 1984 {published data only}
- Davis AL, Faust R, Ordentlich M. Self‐help smoking cessation and maintenance programs: a comparative study with 12‐month follow‐up by the American Lung Association. American Journal of Public Health 1984;74:1212‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1992 {published data only}
- Davis SW, Cummings KM, Rimer BK, Sciandra R. The impact of tailored self‐help smoking cessation guides on young mothers. Health Education Quarterly 1992;19:495‐504. [DOI] [PubMed] [Google Scholar]
de Vries 2008 {published and unpublished data}
- Smeets T, Kremers SP, Brug J, Vries H. Effects of tailored feedback on multiple health behaviors.[erratum appears in Ann Behav Med. 2007 Jul‐Aug;34(1):104]. Annals of Behavioral Medicine 2007;33(2):117‐23. [DOI] [PubMed] [Google Scholar]
- Vries H, Kremers SP, Smeets T, Brug J, Eijmael K. The effectiveness of tailored feedback and action plans in an intervention addressing multiple health behaviors. American Journal of Health Promotion 2008;22(6):417‐25. [DOI] [PubMed] [Google Scholar]
Dijkstra 1998a {published data only}
- Dijkstra A, Devries H, Roijackers J. Long‐term effectiveness of computer‐generated tailored feedback in smoking cessation. Health Education Research 1998;13:207‐14. [DOI] [PubMed] [Google Scholar]
- Dijkstra A, Devries H, Roijackers J, vanBreukelen G. Individualized interventions in smoking cessation: a field experimental trial. Gedrag and Gezondheid Tijdschrift voor Psychologie and Gezondheid 1996;24:314‐22. [Google Scholar]
- Dijkstra A, Vries H, Roijackers J, Breukelen G. Tailored interventions to communicate stage‐matched information to smokers in different motivational stages. Journal of Consulting and Clinical Psychology 1998;66(3):549‐57. [DOI] [PubMed] [Google Scholar]
Dijkstra 1999 {published data only}
- Dijkstra A, Devries H, Roijackers J. Targeting smokers with low readiness to change with tailored and nontailored self‐help materials. Preventive Medicine 1999;28:203‐11. [DOI] [PubMed] [Google Scholar]
Etter 2004 {published data only}
- Etter JF, Perneger TV. Effectiveness of a computer‐tailored smoking cessation program. 11th World Conference on Tobacco or Health 6‐11 August Chicago Abstract book. Chicago, Illinois, USA, 2000; Vol. 1:159.
- Etter JF, Perneger TV. Effectiveness of a computer‐tailored smoking cessation program: a randomized trial. Archives of Internal Medicine 2001;161(21):2596‐601. [DOI] [PubMed] [Google Scholar]
- Etter JF, Perneger TV. Post‐intervention effect of a computer tailored smoking cessation programme. Journal of Epidemiology & Community Health 2004;58:849‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraser 2014 {published data only (unpublished sought but not used)}
- Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population‐based interventions for smoking cessation: a MOST trial. Translational Behavioral Medicine 2014;4:382‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2013 {published data only}
- Bennett K, Gilbert H, Sutton S. Computer‐tailored smoking cessation advice matched to reading ability: perceptions of participants from the ESCAPE trial. Patient Education and Counseling 2015;98:1577‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K, Gilbert H, Sutton S. Computer‐tailored smoking cessation advice matched to reading ability: perceptions of participants from the ESCAPE trial. Patient Education and Counseling 2015;98:1577‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H, Leurent B, Sutton S, Morris R, Alexis‐Garsee C, Nazareth I. Factors predicting recruitment to a UK wide primary care smoking cessation study (the ESCAPE trial). Family Practice 2012;29(1):110‐7. [CENTRAL: 814656; CRS: 9400123000012702; EMBASE: 2012054404; PUBMED: 21624941] [DOI] [PubMed] [Google Scholar]
- Gilbert H, Nazareth I, Sutton S, Morris R, Godfrey C. Effectiveness of computer‐tailored Smoking Cessation Advice in Primary Care (ESCAPE): a randomised trial. Trials 2008;9:23. [CENTRAL: 707723; CRS: 9400123000005322; EMBASE: 2008278924] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert HM, Leurent B, Sutton S, Alexis‐Garsee C, Morris RW, Nazareth I. ESCAPE: a randomised controlled trial of computer‐tailored smoking cessation advice in primary care. Addiction (Abingdon, England) 2013;108(4):811‐9. [CENTRAL: 920999; CRS: 9400107000000699; PEER: Reviewed Journal: 2013‐09671‐028; PUBMED: 23072513] [DOI] [PubMed] [Google Scholar]
- Gilbert HM, Sutton SR, Leurent B, Alexis‐Garsee C, Morris RW, Nazareth I. Characteristics of a population‐wide sample of smokers recruited proactively for the ESCAPE trial. Public Health 2012;126(4):308‐16. [CRS: 9400123000012586; EMBASE: 2012173870; PUBMED: 22385924] [DOI] [PubMed] [Google Scholar]
- Wu Q, Parrott S, Godfrey C, Gilbert H, Nazareth I, Leurent B, et al. Cost‐effectiveness of computer‐tailored smoking cessation advice in primary care: a randomized trial (ESCAPE). Nicotine & Tobacco Research 2014;16:270‐8. [DOI] [PubMed] [Google Scholar]
Glasgow 1981 {published data only}
- Glasgow RE, Schafer L, O'Neill HK. Self‐help books and amount of therapist contact in smoking cessation programs. Journal of Consulting and Clinical Psychology 1981;49:659‐67. [DOI] [PubMed] [Google Scholar]
Gritz 1992 {published data only}
- Gritz ER, Berman BA, Bastani R, Wu M. A randomized trial of a self‐help smoking cessation intervention in a nonvolunteer female population: testing the limits of the public health model. Health Psychology 1992;11:280‐9. [DOI] [PubMed] [Google Scholar]
- Hudmon KS, Gritz ER, Clayton S, Nisenbaum R. Eating orientation, postcessation weight gain, and continued abstinence among female smokers receiving an unsolicited smoking cessation intervention. Health Psychology 1999;18:29‐36. [DOI] [PubMed] [Google Scholar]
Harackiewicz 1988 {published data only}
- Harackiewicz JM, Blair LW, Sansone C, Epstein JA, Stuchell RN. Nicotine gum and self‐help manuals in smoking cessation: an evaluation in a medical context. Addictive Behaviors 1988;13:319‐30. [DOI] [PubMed] [Google Scholar]
- Harackiewicz JM, Sansone C, Blair LW, Epstein JA, Manderlink G. Attributional processes in behavior change and maintenance: smoking cessation and continued abstinence. Journal of Consulting and Clinical Psychology 1987;55:372‐8. [DOI] [PubMed] [Google Scholar]
Hollis 1993 {published data only}
- Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse‐assisted counseling for smokers in primary care. Annals of Internal Medicine 1993;118:521‐5. [DOI] [PubMed] [Google Scholar]
Hoving 2010 {published data only}
- Hoving C, Mudde AN, Dijk F, Vries H. Effectiveness of a smoking cessation intervention in Dutch pharmacies and general practices. Health Education 2010;110(1):17‐29. [CENTRAL: 782925; CRS: 9400123000005944] [Google Scholar]
Humerfelt 1998 {published data only}
- Humerfelt S, Eide GE, Kvale G, Aaro LE, Gulsvik A. Effectiveness of postal smoking cessation advice: a randomized controlled trial in young men with reduced FEV1 and asbestos exposure. European Respiratory Journal 1998;11:284‐90. [DOI] [PubMed] [Google Scholar]
- Humerfelt S, Eide GE, Kvale G, Aaro LE, Gulsvik A. Predictors of stopping smoking in a community of young adults [abstract]. European Respiratory Journal 1995;8 Suppl 19:11S. [Google Scholar]
- Humerfelt S, Eide GE, Kvale G, Aaro LE, Gulsvik A. Smoking cessation after a doctor's postal advice to quit smoking among high risk male smokers in a community [abstract]. European Respiratory Journal 1995;8 Suppl 19:112S. [Google Scholar]
- Humerfelt S, Kvale G, Aaro LE, Gulsvik A. Success rates of smoking cessation after a doctor's postal advice to quit smoking in a randomized trial among men with asbestos exposure and reduced FEV1 from a population survey [abstract]. European Respiratory Journal 1994;7 Suppl 18:255s. [Google Scholar]
ICRF 1994 {published data only}
- Imperial Cancer Research Fund General Practice Research Group. Effectiveness of a nicotine patch in helping people stop smoking: results of a randomised trial in general practice. BMJ 1993;306:1304‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Randomised trial of nicotine patches in general practice: results at one year. BMJ 1994;308:1476‐7. [PMC free article] [PubMed] [Google Scholar]
Janz 1987 {published data only}
- Janz NK, Becker MH, Kirscht JP, Eraker SA, Billi JE, Woolliscroft JO. Evaluation of a minimal‐contact smoking cessation intervention in an outpatient setting. American Journal of Public Health 1987;77:805‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Killen 1997 {published data only}
- Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self‐help video for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 1997;65:663‐72. [DOI] [PubMed] [Google Scholar]
Killen 1997 +NP {published data only}
- Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self‐help video for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 1997;65:663‐72. [DOI] [PubMed] [Google Scholar]
Kottke 1989 {published data only}
- Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomized trial to increase smoking intervention by physicians. Doctors Helping Smokers, Round I. JAMA 1989;261:2101‐6. [PubMed] [Google Scholar]
Lando 1988 {published data only}
- Lando HA, Kalb EA, McGovern PG. Behavioral self‐help materials as an adjunct to nicotine gum. Addictive Behaviors 1988;13:181‐4. [DOI] [PubMed] [Google Scholar]
Lando 1991 {published data only}
- Lando HA, Pirie PL, McGovern PG, Pechacek TF, Swim J, Loken B. A comparison of self‐help approaches to smoking cessation. Addictive Behaviors 1991;16:183‐93. [DOI] [PubMed] [Google Scholar]
Ledwith 1984 {published data only}
- Ledwith F. Immediate and delayed effects of postal advice on stopping smoking. Health Bulletin 1984;42:332‐44. [PubMed] [Google Scholar]
- Ledwith F. Immediate and delayed effects of postal advice on stopping smoking. Proceedings of the 5th World Conference on Smoking and Health, July 10‐15 1983, Winnipeg. Ottawa: Canadian Council on Smoking and Health, 1984; Vol. 1:383‐7. [PubMed]
Lennox 2001 {published data only}
- Lennox AS, Osman LM, Reiter E, Robertson R, Friend J, McCann I, et al. Cost effectiveness of computer tailored and non‐tailored smoking cessation letters in general practice: randomised controlled trial. BMJ 2001;322(7299):1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E, Robertson R, Osman LM. Lessons from a failure: generating tailored smoking cessation letters. Artificial Intelligence 2003;144:41‐58. [Google Scholar]
Lichtenstein 2000 {published data only}
- Lee ME, Lichtenstein E, Andrews JA, Glasgow RE, Hampson SE. Radon‐smoking synergy: a population‐based behavioral risk reduction approach. Preventive Medicine 1999;29:222‐7. [DOI] [PubMed] [Google Scholar]
- Lichtenstein E, Andrews JA, Lee ME, Glasgow RE, Hampson SE. Using radon risk to motivate smoking reduction: evaluation of written materials and brief telephone counselling. Tobacco Control 2000;9:320‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichtenstein 2008 {published data only}
- Hampson SE, Andrews JA, Barckley M, Lichtenstein E, Lee ME. Personality traits, perceived risk, and risk‐reduction behaviors: a further study of smoking and radon. Health Psychology 2006;25(4):530‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein E, Boles SM, Lee ME, Hampson SE, Glasgow RE, Fellows J. Using radon risk to motivate smoking reduction II: randomized evaluation of brief telephone counseling and a targeted video. Health Education Research 2008;23(2):191‐201. [DOI] [PubMed] [Google Scholar]
- Lichtenstein E, Lee ME, Boles SM, Foster L, Hampson SE. Using radon risk to motivate smoking reduction: replication and extension (PO3 21). Society for Research on Nicotine and Tobacco 8th Annual Meeting February 20‐23 Savannah, Georgia. 2002.
Lipkus 1999 {published data only}
- Lipkus IM, Lyna PR, Rimer BK. Using tailored interventions to enhance smoking cessation among African‐Americans at a community health center. Nicotine & Tobacco Research 1999;1(1):77‐85. [DOI] [PubMed] [Google Scholar]
McFall 1993 {published data only}
- McFall SL, Michener A, Rubin D, Flay BR, Mermelstein RJ, Burton D, et al. The effects and use of maintenance newsletters in a smoking cessation intervention. Addictive Behaviors 1993;18:151‐8. [DOI] [PubMed] [Google Scholar]
Meyer 2008 {published data only}
- Haug S, Meyer C, Ulbricht S, Gross B, Rumpf HJ, John U. Need for cognition as a predictor and a moderator of outcome in a tailored letters smoking cessation intervention. Health Psychology 2010;29(4):367‐73. [CENTRAL: 761578; CRS: 9400123000005761; PUBMED: 20658823] [DOI] [PubMed] [Google Scholar]
- Haug S, Meyer C, Ulbricht S, Schorr G, Ruge J, Rumpf HJ, et al. Predictors and moderators of outcome in different brief interventions for smoking cessation in general medical practice. Patient Education and Counselling 2010;78(1):57‐64. [DOI] [PubMed] [Google Scholar]
- Klein G, Ulbricht S, Haug S, Gross B, Rumpf HJ, John U, et al. Effects of practitioner‐delivered brief counseling and computer‐generated tailored letters on cigarettes per day among smokers who do not quit ‐ a randomized controlled trial. Drug and Alcohol Dependence 2010;112(1‐2):81‐9. [DOI] [PubMed] [Google Scholar]
- Meyer C, Ulbricht S, Baumeister SE, Schumann A, Ruge J, Bischof G, et al. Proactive interventions for smoking cessation in general medical practice: a quasi‐randomized controlled trial to examine the efficacy of computer‐tailored letters and physician‐delivered brief advice. Addiction 2008;103(2):294‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann A, John U, Ulbricht S, Ruge J, Bischof G, Meyer C. Computer‐generated tailored feedback letters for smoking cessation: theoretical and empirical variability of tailoring. International Journal of Medical Informatics 2008;77(11):715‐22. [DOI] [PubMed] [Google Scholar]
- Schumann A, John U, Ulbricht S, Ruge J, Bischof G, Meyer C. Variability of tailoring of a smoking cessation intervention based on the transtheoretical model. Addictive Behaviors 2007;32(12):3083‐7. [DOI] [PubMed] [Google Scholar]
- Ulrich JU, Ulbricht S, Goeze C, Meyer C. Brief intervention to motivate smokers to quit in primary medical care. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(1 Suppl 1):S40. [CENTRAL: 835566; CRS: 9400123000011714] [Google Scholar]
Meyer 2012 {published data only}
- Meyer C, Ulbricht S, Gross B, Kastel L, Wittrien S, Klein G, et al. Adoption, reach and effectiveness of computer‐based, practitioner delivered and combined smoking interventions in general medical practices: a three‐arm cluster randomized trial. Drug and Alcohol Dependence 2012;121(1‐2):124‐32. [CENTRAL: 814655; CRS: 9400123000012692; EMBASE: 2012037444; PUBMED: 21924563] [DOI] [PubMed] [Google Scholar]
- Ulrich JU, Ulbricht S, Goeze C, Meyer C. Brief intervention to motivate smokers to quit in primary medical care. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(1 Suppl 1):S40. [CENTRAL: 835566; CRS: 9400123000011714] [Google Scholar]
Meyer 2016 {published data only}
- Meyer C, Ulbricht S, Haug S, Broda A, Bischof G, Rumpf H‐J, et al. Motivating smokers to quit using computer‐generated letters that target either reduction or cessation: a population‐based randomized controlled trial among smokers who do not intend to quit. Drug and Alcohol Dependence 2016;166:177‐86. [DOI] [PubMed] [Google Scholar]
Nollen 2007 {published data only}
- Ahluwalia JS, Kimber R, Mayo MS, Ahluwalia HK, Choi WS, Schmelsle KH, et al. African American smokers interested and eligible for a smoking cessation clinical trial: predictors of not returning for randomization. Annals of Epidemiology 2002;12:206‐12. [DOI] [PubMed] [Google Scholar]
- Ahluwalia JS, Richter KP, Mayo MS, Resnicow K. Quit for Life: a randomized trial of culturally sensitive materials for smoking cessation in African Americans. Journal of General Internal Medicine 1999;14 Suppl 2:6. [Google Scholar]
- Catley D, Ahluwalia JS, Resnicow K, Nazir N. Depressive symptoms and smoking cessation among inner‐city African Americans using the nicotine patch. Nicotine & Tobacco Research 2003;5(1):61‐8. [PubMed] [Google Scholar]
- Nollen N, Ahluwalia JS, Mayo MS, Richter K, Choi WS, Okuyemi KS, et al. A randomized trial of targeted educational materials for smoking cessation in African Americans using transdermal nicotine. Health Education & Behavior 2007;34(6):911‐27. [DOI] [PubMed] [Google Scholar]
Omenn 1988 {published data only}
- Omenn GS, Thompson B, Sexton M, Hessol N, Breitenstein B, Curry S, et al. A randomized comparison of worksite‐sponsored smoking cessation programs. American Journal of Preventive Medicine 1988;4:261‐7. [PubMed] [Google Scholar]
- Thompson B, Omenn G, Sexton M, Breitenstein B, Hessol N, Curry S, et al. Worksite smoking cessation: a test of two programs. Progress in Clinical and Biological Research 1987;248:93‐100. [PubMed] [Google Scholar]
Orleans 1991 {published data only}
- Orleans CT, Schoenbach VJ, Wagner EH, Quade D, Salmon MA, Pearson DC, et al. Self‐help quit smoking interventions: effects of self‐help materials, social support instructions, and telephone counseling. Journal of Consulting and Clinical Psychology 1991;59:439‐48. [DOI] [PubMed] [Google Scholar]
- Schoenbach VJ, Orleans CT, Wagner EH, Quade D, Salmon MAP, Porter CQ. Characteristics of smokers who enroll and quit in self‐help programs. Health Education Research 1992;7:369‐80. [Google Scholar]
Orleans 1998 {published data only}
- Orleans CT, Boyd NR, Bingler R, Sutton C, Fairclough D, Heller D, et al. A self‐help intervention for African American smokers: tailoring Cancer Information Service counseling for a special population. Preventive Medicine 1998;27:S61‐S70. [DOI] [PubMed] [Google Scholar]
Orleans 2000 {published and unpublished data}
- Orleans CT, Boyd NR, Noll E, Crosette L, Glassman B. Computer tailored intervention for older smokers using transdermal nicotine. Tobacco Control 2000;9 Suppl 1:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Owen 1989 {published data only}
- Owen N, Ewins AL, Lee C. Smoking cessation by mail: a comparison of standard and personalized correspondence course formats. Addictive Behaviors 1989;14:355‐63. [DOI] [PubMed] [Google Scholar]
Pallonen 1994 {published data only}
- Pallonen UE, Leskinen L, Prochaska JO, Willey CJ, Kaariainen R, Salonen JT. A 2‐year self‐help smoking cessation manual intervention among middle‐aged Finnish men: an application of the transtheoretical model. Preventive Medicine 1994;23:507‐14. [DOI] [PubMed] [Google Scholar]
Parekh 2014 {published data only}
- Parekh S, King D, Boyle FM, Vandelanotte C. Randomized controlled trial of a computer‐tailored multiple health behaviour intervention in general practice: 12‐month follow‐up results. International Journal of Behavioral Nutrition and Physical Activity 2014;11(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh S, Vandelanotte C, King D, Boyle FM. Improving diet, physical activity and other lifestyle behaviours using computer‐tailored advice in general practice: a randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2012;9:58. [CRS: 9400123000016867] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pederson 1983 {published data only}
- Pederson LL, Wood T, Lefcoe NM. Use of a self‐help smoking cessation manual as an adjunct to advice from a respiratory specialist. International Journal of the Addictions 1983;18:777‐82. [DOI] [PubMed] [Google Scholar]
Prochaska 1993 {published and unpublished data}
- Norman SB, Norman GJ, Rossi JS, Prochaska JO. Identifying high‐ and low‐success smoking cessation subgroups using signal detection analysis. Addictive Behaviors 2006;31:31‐41. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Clemente CC, Velicer WF, Rossi JS. Standardized, individualized, interactive, and personalized self‐help programs for smoking cessation. Health Psychology 1993;12:399‐405. [DOI] [PubMed] [Google Scholar]
Prochaska 2001a {published data only}
- Prochaska JO, Velicer WF, Fava JL, Ruggiero L, Laforge RG, Rossi JS, et al. Counselor and stimulus control enhancements of a stage‐matched expert system intervention for smokers in a managed care setting. Preventive Medicine 2001;32:23‐32. [DOI] [PubMed] [Google Scholar]
- Sun X, Prochaska JO, Velicer WF, Laforge RG. Transtheoretical principles and processes for quitting smoking: a 24‐month comparison of a representative sample of quitters, relapsers, and non‐quitters. Addictive Behaviors 2007;32(12):2707‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer WF, Redding CA, Sun X, Prochaska JO. Demographic variables, smoking variables, and outcome across five studies. Health Psychology 2007;26(3):278‐87. [DOI] [PubMed] [Google Scholar]
Prochaska 2001b {published data only}
- Prochaska JO, Velicer WF, Fava JL, Rossi JS, Tsoh JY. Evaluating a population‐based recruitment approach and a stage‐based expert system intervention for smoking cessation. Addictive Behaviors 2001;26:583‐602. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Redding CA, Sun X, Prochaska JO. Demographic variables, smoking variables, and outcome across five studies. Health Psychology 2007;26(3):278‐87. [DOI] [PubMed] [Google Scholar]
Prochaska 2004 {published data only}
- Prochaska JO, Velicer WF, Rossi JS, Redding CA, Greene GW, Rossi SR, et al. Multiple risk expert systems interventions: impact of simultaneous stage‐matched expert system interventions for smoking, high‐fat diet, and sun exposure in a population of parents. Health Psychology 2004;23:503‐16. [DOI] [PubMed] [Google Scholar]
Prochaska 2005 {published data only}
- Prochaska JO, Velicer WF, Redding C, Rossi JS, Goldstein M, DePue J, et al. Stage‐based expert systems to guide a population of primary care patients to quit smoking, eat healthier, prevent skin cancer, and receive regular mammograms. Preventive Medicine 2005;41:406‐16. [DOI] [PubMed] [Google Scholar]
Prue 1983 {published data only}
- Prue DM, Davis CJ, Martin JE, Moss RA. An investigation of a minimal contact brand fading program for smoking treatment. Addictive Behaviors 1983;8:307‐10. [DOI] [PubMed] [Google Scholar]
Resnicow 1997 {published data only}
- Resnicow K, Royce J, Vaughan R, Orlandi MA, Smith M. Analysis of a multicomponent smoking cessation project: what worked and why. Preventive Medicine 1997;26:373‐81. [DOI] [PubMed] [Google Scholar]
- Resnicow K, Vaughan R, Futterman R, Weston RE, Royce J, Parms C, et al. A self‐help smoking cessation program for inner‐city African Americans: results from the Harlem health connection project. Health Education & Behavior 1997;24:201‐17. [DOI] [PubMed] [Google Scholar]
Rice 1994 {published and unpublished data}
- Rice VH, Fox DH, Lepczyk M, Sieggreen M, Mullin M, Jarosz P, et al. A comparison of nursing interventions for smoking cessation in adults with cardiovascular health problems. Heart & Lung 1994;23:473‐86. [PubMed] [Google Scholar]
Schofield 1999 {published data only}
- Schofield PE, Hill DJ, Johnston CI, Streeton JA. The effectiveness of a directly mailed smoking cessation intervention to Australian discharged hospital patients. Preventive Medicine 1999;29(6 Pt 1):527‐34. [DOI] [PubMed] [Google Scholar]
Schumann 2008 {published data only}
- Schumann A, John U, Baumeister SE, Ulbricht S, Rumpf HJ, Meyer C. Computer‐tailored smoking cessation intervention in a general population setting in Germany: outcome of a randomized controlled trial. Nicotine & Tobacco Research 2008;10:371‐9. [DOI] [PubMed] [Google Scholar]
- Schumann A, John U, Rumpf HJ, Hapke U, Meyer C. Changes in the 'stages of change' as outcome measures of a smoking cessation intervention: a randomized controlled trial. Preventive Medicine 2006;43(2):101‐6. [DOI] [PubMed] [Google Scholar]
- Schumann A, John U, Ulbricht S, Ruge J, Bischof G, Meyer C. Computer‐generated tailored feedback letters for smoking cessation: theoretical and empirical variability of tailoring. International Journal of Medical Informatics 2008;77(11):715‐22. [DOI] [PubMed] [Google Scholar]
- Schumann A, John U, Ulbricht S, Ruge J, Bischof G, Meyer C. Variability of tailoring of a smoking cessation intervention based on the transtheoretical model. Addictive Behaviors 2007;32(12):3083‐7. [DOI] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith PM, Cameron R, McDonald PW, Kawash B, Madill C, Brown KS. Telephone counseling for population‐based smoking cessation. American Journal of Health Behavior 2004;28(3):231‐41. [DOI] [PubMed] [Google Scholar]
Strecher 2005 {published data only}
- Strecher VJ, Marcus A, Bishop K, Fleisher L, Stengle W, Levinson A, et al. A randomized controlled trial of multiple tailored messages for smoking cessation among callers to the cancer information service. Journal of Health Communication 2005;10 Suppl 1:105‐18. [DOI] [PubMed] [Google Scholar]
Sutton 2007 {published data only}
- Gilbert H, Sutton S. Does adding tailored feedback to telephone counselling improve quit rates? (POS1‐033). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004:43.
- Sutton S, Gilbert H. Effectiveness of individually tailored smoking cessation advice letters as an adjunct to telephone counselling and generic self‐help materials: randomized controlled trial. Addiction 2007;102(6):994‐1000. [DOI] [PubMed] [Google Scholar]
Sykes 2001 {published data only}
- Marks DF, Sykes CM. Randomized controlled trial of cognitive behavioural therapy for smokers living in a deprived area of London: outcome at one‐year follow‐up. Psychology, Health and Medicine 2002;7:17‐24. [Google Scholar]
- Sykes CM, Marks DF. Effectiveness of a cognitive behaviour therapy self‐help programme for smokers in London, UK. Health Promotion International 2001;16:255‐60. [DOI] [PubMed] [Google Scholar]
Thompson 1988 {published data only}
- Thompson RS, Michnich ME, Friedlander L, Gilson B, Grothaus LC, Storer B. Effectiveness of smoking cessation interventions integrated into primary care practice. Medical Care 1988;26:62‐76. [DOI] [PubMed] [Google Scholar]
van der Aalst 2012 {published data only}
- Aalst CM, Koning HJ, Bergh KA, Willemsen MC, Klaveren RJ. The effectiveness of a computer‐tailored smoking cessation intervention for participants in lung cancer screening: a randomised controlled trial. Lung Cancer (Amsterdam, Netherlands) 2012;76(2):204‐10. [CENTRAL: 830557; CRS: 9400123000012567; EMBASE: 2012198268; PUBMED: 22054915] [DOI] [PubMed] [Google Scholar]
Velicer 1999 {published data only}
- Velicer WF, Prochaska JO. An expert system intervention for smoking cessation. Patient Education & Counseling 1999;36:119‐29. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Prochaska JO, Fava JL, Laforge RG, Rossi JS. Interactive versus noninteractive interventions and dose‐response relationships for stage‐matched smoking cessation programs in a managed care setting. Health Psychology 1999;18(1):21‐8. [DOI] [PubMed] [Google Scholar]
Velicer 2006 {published data only}
- Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X, et al. Evaluating nicotine replacement therapy and stage‐based therapies in a population‐based effectiveness trial. Journal of Consulting and Clinical Psychology 2006;74(6):1162‐72. [DOI] [PubMed] [Google Scholar]
Webb 2013 {published data only}
- Webb Hooper M, Rodriguez De Ybarra D, Baker EA. The effect of placebo tailoring on smoking cessation: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2013;81(5):800‐9. [CENTRAL: 875034; CRS: 9400126000000237; EMBASE: 2013603080; PUBMED: 23544680] [DOI] [PubMed] [Google Scholar]
Willemsen 2006 {published data only}
- Willemsen MC, Wiebing M, Emst A, Zeeman G. Helping smokers to decide on the use of efficacious smoking cessation methods: a randomized controlled trial of a decision aid. Addiction 2006;101(3):441‐9. [DOI] [PubMed] [Google Scholar]
- Willemsen MC, Wiebing MA, Ernst AJ, Zeeman G. A self‐help quit kit to motivate smokers to undergo treatment: a randomised controlled trial. Society for Research on Nicotine and Tobacco 11th Annual Meeting. 20‐23 March 2005; Prague, Czech Republic. 2005.
References to studies excluded from this review
Ainsworth 2013 {published data only}
- Ainsworth H, Shah S, Ahmed F, Amos A, Cameron I, Fairhurst C, et al. Muslim communities learning about second‐hand smoke (MCLASS): study protocol for a pilot cluster randomised controlled trial. Trials 2013;14:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armitage 2008a {published data only}
- Armitage CJ, Arden MA. How useful are the stages of change for targeting interventions? Randomized test of a brief intervention to reduce smoking. Health Psychology 2008;27(6):789‐98. [CENTRAL: 754030; CRS: 9400123000005686; EMBASE: 2008603965] [DOI] [PubMed] [Google Scholar]
Armitage 2008b {published data only}
- Armitage CJ. A volitional help sheet to encourage smoking cessation: a randomized exploratory trial. Health Psychology 2008;27:557‐66. [DOI] [PubMed] [Google Scholar]
Arnold 2009 {published data only}
- Arnold J, Goodacre S, Bath P, Price J. Information sheets for patients with acute chest pain: randomised controlled trial. BMJ (Clinical Research Ed.) 2009;338:b541. [CENTRAL: 682280; CRS: 9400123000005097; EMBASE: 2009155221; PUBMED: 19246544] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balanda 1999 {published data only}
- Balanda KP, Lowe JB, OConnor Fleming ML. Comparison of two self‐help smoking cessation booklets. Tobacco Control 1999;8:57‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bansal‐Travers 2010 {published data only}
- Bansal‐Travers M, Cummings KM, Hyland A, Brown A, Celestino P. Educating smokers about their cigarettes and nicotine medications. Health Education Research 2010;25(4):678‐86. [CENTRAL: 759258; CRS: 9400123000005725; PUBMED: 20064838] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnett 2015 {published data only}
- Barnett PG, Wong W, Jeffers A, Hall SM, Prochaska JJ. Cost‐effectiveness of smoking cessation treatment initiated during psychiatric hospitalization: analysis from a randomized, controlled trial. Journal of Clinical Psychiatry 2015;76(10):e1285‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandon 2000 {published data only}
- Brandon TH, Collins BN, Juliano LM, Lazev AB. Preventing relapse among former smokers: a comparison of minimal interventions through telephone and mail. Journal of Consulting and Clinical Psychology 2000;68:103‐13. [DOI] [PubMed] [Google Scholar]
Brandon 2004 {published data only}
- Brandon TH, Meade CD, Herzog TA, Chirikos TN, Webb MS, Cantor AB. Efficacy and cost‐effectiveness of a minimal intervention to prevent smoking relapse: dismantling the effects of amount of content versus contact. Journal of Consulting and Clinical Psychology 2004;72(5):797‐808. [DOI] [PubMed] [Google Scholar]
Brandon 2012 {published data only}
- Brandon TH, Simmons VN, Meade CD, Quinn GP, Lopez Khoury EN, Sutton SK, et al. Self‐help booklets for preventing postpartum smoking relapse: a randomized trial. American Journal of Public Health 2012;102(11):2109‐15. [CENTRAL: 867762; CRS: 9400123000017587; EMBASE: 22994170; PUBMED: 22994170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1992 {published data only}
- Brown S, Hunt G, Owen N. The effect of adding telephone contact to self‐instructional smoking‐cessation materials. Behavior Change 1992;9:216‐22. [Google Scholar]
Burling 2000 {published data only}
- Burling AS, Burling TA. A work in progress: effectiveness of a comprehensive internet‐delivered interactive multimedia stop smoking program. Presented at the 34th Annual Convention of the Association for the Advancement of Behavior Therapy, New Orleans, LA, November 2000.
Carré 2008 {published data only}
- Carré PC, Roche N, Neukirch F, Radeau T, Perez T, Terrioux P, et al. The effect of an information leaflet upon knowledge and awareness of COPD in potential sufferers. A randomized controlled study. Respiration; International Review of Thoracic Diseases 2008;76(1):53‐60. [CENTRAL: 647637; CRS: 9400123000004868; PUBMED: 18253024] [DOI] [PubMed] [Google Scholar]
Conway 2004 {published data only}
- Conway TL, Woodruff SI, Edwards CC, Elder JP, Hurtado SL, Hervig LK. Operation Stay Quit: evaluation of two smoking relapse prevention strategies for women after involuntary cessation during US Navy recruit training. Military Medicine 2004;169(3):236‐42. [DOI] [PubMed] [Google Scholar]
Curry 1988 {published data only}
- Curry SJ, Marlatt GA, Gordon J, Baer JS. A comparison of alternative theoretical approaches to smoking cessation and relapse. Health Psychology 1988;7:545‐56. [DOI] [PubMed] [Google Scholar]
Dijkstra 1998b {published data only}
- Dijkstra A, Vries H, Roijackers J, Breukelen G. Tailoring information to enhance quitting in smokers with low motivation to quit: three basic efficacy questions. Health Psychology 1998;17:513‐9. [DOI] [PubMed] [Google Scholar]
Dijkstra 2001 {published data only}
- Dijkstra A, Vries H. Do self‐help interventions in health education lead to cognitive changes, and do cognitive changes lead to behavioural change?. British Journal of Health Psychology 2001;6:121‐34. [DOI] [PubMed] [Google Scholar]
Dijkstra 2005 {published data only}
- Dijkstra A. Working mechanisms of computer‐tailored health education: evidence from smoking cessation. Health Education Research 2005;20(5):527‐39. [DOI] [PubMed] [Google Scholar]
Dijkstra 2006 {published data only}
- Dijkstra A, Conijn B, Vries H. A field experiment on stages of change for smoking cessation: the effects of matched and mismatched information. Psychologie & Gezondheid 2007;35(1):5‐16. [Google Scholar]
- Dijkstra A, Conijn B, Vries H. A match‐mismatch test of a stage model of behaviour change in tobacco smoking. Addiction 2006;101(7):1035‐43. [DOI] [PubMed] [Google Scholar]
Dijkstra 2009 {published data only}
- Dijkstra A. Disengagement beliefs in smokers: do they influence the effects of a tailored persuasive message advocating smoking cessation?. Psychology & Health 2009;24(7):791‐804. [CENTRAL: 752163; CRS: 9400123000005663; PUBMED: 20205027] [DOI] [PubMed] [Google Scholar]
Edwards 1999 {published data only}
- Edwards CC, Woodruff SI, Conway TL. Operation Stay Quit: preventing smoking relapse among US Navy women. American Journal of Health Behavior 1999;23:352‐5. [Google Scholar]
Emmons 2013 {published data only}
- Emmons KM, Puleo E, Sprunck‐Harrild K, Ford J, Ostroff JS, Hodgson D, et al. Partnership for health‐2, a web‐based versus print smoking cessation intervention for childhood and young adult cancer survivors: randomized comparative effectiveness study. Journal of Medical Internet Research 2013;15(11):e218. [CRS: 9400126000000377; PUBMED: 24195867] [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2007 {published data only}
- Etter JF. Informing smokers on additives in cigarettes: a randomized trial. Patient Education & Counseling 2007;66(2):188‐91. [DOI] [PubMed] [Google Scholar]
Fortmann 1995 {published and unpublished data}
- Fortmann SP, Killen JD. Nicotine gum and self‐help behavioral treatment for smoking relapse prevention ‐ resuIts from a trial using population‐based recruitment. Journal of Consulting and Clinical Psychology 1995;63:460‐8. [DOI] [PubMed] [Google Scholar]
Garcia 2000 {published data only}
- Garcia MP, Becona E. Evaluation of the amount of therapist contact in a smoking cessation program. Spanish Journal of Psychology 2000;3(1):28‐36. [DOI] [PubMed] [Google Scholar]
Gritz 1988 {published data only}
- Gritz ER, Marcus AC, Berman BA, Read LL, Kanim LEA, Reeder SJ. Evaluation of a worksite self‐help smoking cessation program for registered nurses. American Journal of Health Promotion 1988;3(2):26‐35. [DOI] [PubMed] [Google Scholar]
Hall 2003 {published data only}
- Hall S, Bishop AJ, Marteau TM. Increasing readiness to stop smoking in women undergoing cervical screening: evaluation of two leaflets. Nicotine & Tobacco Research 2003;5(6):821‐6. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Hall S. Motivating smoking cessation in women undergoing cervical screening: evaluation of two leaflets. International Journal of Cancer 2002;Suppl 13:469. [Google Scholar]
Jeffery 1982 {published data only}
- Jeffery RW, Danaher BG, Killen J, Farquhar JW, Kinnier R. Self‐administered programs for health behavior change: smoking cessation and weight reduction by mail. Addictive Behaviors 1982;7:57‐63. [DOI] [PubMed] [Google Scholar]
Jeffery 1990 {published data only}
- Jeffery RW, Hellerstedt WL, Schmid TL. Correspondence programs for smoking cessation and weight control: a comparison of two strategies in the Minnesota Heart Health Program. Health Psychology 1990;9:585‐98. [DOI] [PubMed] [Google Scholar]
Johs 2003 {published data only}
- Johs Artisensi JL. The effect of web‐based support as an adjunct to a self‐help smoking cessation program. Dissertation Abstracts International: Section B: The Sciences and Engineering 2003;63(9‐B):4138. [Google Scholar]
Jordan 1999 {published data only}
- Jordan JC, Reynolds RV, Myers DL, Tobin TL, Jones JR, Hall LK, et al. Comparison of internet delivered and printed self‐help smoking cessation programs with hospital employees. Society for Research on Nicotine and Tobacco Fifth Annual Meeting March 5‐7 San Diego CA. 1999.
Killen 1990 {published data only}
- Fortmann SP, Killen JD, Telch MJ, Newman B. Minimal contact treatment for smoking cessation. A placebo controlled trial of nicotine polacrilex and self‐directed relapse prevention: initial results of the Stanford Stop Smoking Project. JAMA 1988;260:1575‐80. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self‐guided behavioral treatments for smoking relapse prevention. Journal of Consulting and Clinical Psychology 1990;58:85‐92. [DOI] [PubMed] [Google Scholar]
Kreuter 1996 {published data only}
- Kreuter MW, Strecher VJ. Do tailored behavior change messages enhance the effectiveness of health risk appraisal? Results from a randomized trial. Health Education Research 1996;11:97‐105. [DOI] [PubMed] [Google Scholar]
Kreuter 2012 {published data only}
- Kreuter MW, Eddens KS, Alcaraz KI, Rath S, Lai C, Caito N, et al. Use of cancer control referrals by 2‐1‐1 callers: a randomized trial. American Journal of Preventive Medicine 2012;43(6 Suppl 5):S425‐34. [CRS: 9400107000000806; PEER: Reviewed Journal: 2012‐31259‐006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lenert 2004 {published data only}
- Lenert L, Munoz RF, Perez JE, Bansod A. Automated e‐mail messaging as a tool for improving quit rates in an internet smoking cessation intervention. Journal of the American Medical Informatics Association 2004;11(4):235‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipkus 2004 {published data only}
- Lipkus IM, McBride CM, Pollak KI, Schwartz‐Bloom RD, Tilson E, Bloom PN. A randomized trial comparing the effects of self‐help materials and proactive telephone counseling on teen smoking cessation. Health Psychology 2004;23(4):397‐406. [DOI] [PubMed] [Google Scholar]
McBride 1999 {published data only}
- McBride CM, Scholes D, Grothaus LC, Curry SJ, Ludman E, Albright J. Evaluation of a minimal self‐help smoking cessation intervention following cervical cancer screening. Preventive Medicine 1999;29(2):133‐8. [DOI] [PubMed] [Google Scholar]
McDonald 2003 {published data only}
- McDonald PW, Jessup L, Stephen Brown K, Ahmed R, Filsinger S. Are cessation interventions based on stage of change really more effective? (POS4‐27). Society for Research on Nicotine and Tobacco 9th Annual Meeting February 19‐22 New Orleans, Louisiana. 2003.
McDonnell 2011 {published data only}
- McDonnell DD, Kazinets G, Lee HJ, Moskowitz JM. An internet‐based smoking cessation program for Korean Americans: results from a randomized controlled trial. Nicotine & Tobacco Research 2011;13(5):336‐43. [CENTRAL: 831359; CRS: 9400123000011568; 135; PUBMED: 21330285] [DOI] [PubMed] [Google Scholar]
- McDonnell DD, Lee HJ, Kazinets G, Moskowitz JM. Online recruitment of targeted populations: lessons learned from a smoking cessation study among Korean Americans. Social Marketing Quarterly 2010;16(3):2‐22. [CENTRAL: 794867; CRS: 9400123000006106] [Google Scholar]
McMahon 2000 {published data only}
- McMahon SD, Jason LA. Social support in a worksite smoking intervention. A test of theoretical models. Behavior Modification 2000;24:184‐201. [DOI] [PubMed] [Google Scholar]
Meade 1989 {published data only}
- Meade CD, Byrd JC, Lee M. Improving patient comprehension of literature on smoking. American Journal of Public Health 1989;79(10):1411‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2002 {published data only}
- Moore L, Campbell R, Whelan A, Mills N, Lupton P, Misselbrook E, et al. Self help smoking cessation in pregnancy: cluster randomised controlled trial. BMJ 2002;325(7377):1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2005 {published data only}
- Murphy JM, Mahoney MC, Cummings KM, Hyland AJ, Lawvere S. A randomized trial to promote pharmacotherapy use and smoking cessation in a Medicaid population (United States). Cancer Causes and Control 2005;16(4):373‐82. [DOI] [PubMed] [Google Scholar]
Naughton 2012 {published data only}
- Naughton F, Prevost AT, Gilbert H, Sutton S. Randomized controlled trial evaluation of a tailored leaflet and SMS text message self‐help intervention for pregnant smokers (MiQuit). Nicotine & Tobacco Research 2012;14(5):569‐77. [CENTRAL: 832524; CRS: 9400123000014482; EMBASE: 2012254938; 334; PUBMED: 22311960] [DOI] [PubMed] [Google Scholar]
NCT00714467 {published data only}
- NCT00714467. Expert system and family assisted interventions for chinese smokers. ClinicalTrials.gov/show/NCT00714467 2004.
NCT01566994 {published data only}
- NCT01566994. Comparing population cessation services. ClinicalTrials.gov/show/NCT01566994 2010.
O'Hara 1993 {published data only}
- O'Hara P, Gerace TA, Elliott LL. Effectiveness of self‐help smoking cessation guides for firefighters. Journal of Occupational Medicine 1993;35:795‐9. [DOI] [PubMed] [Google Scholar]
Ossip‐Klein 1991 {published data only}
- Ossip Klein DJ, Giovino GA, Megahed N, Black PM, Emont SL, Stiggins J, et al. Effects of a smoker's hotline: results of a 10‐county self‐help trial. Journal of Consulting and Clinical Psychology 1991;59:325‐32. [DOI] [PubMed] [Google Scholar]
Ossip‐Klein 1997 {published data only}
- Ossip Klein DJ, Carosella AM, Krusch DA. Self‐help interventions for older smokers. Tobacco Control 1997;6(3):188‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pallonen 1998 {published data only}
- Pallonen UE, Velicer WF, Prochaska JO, Rossi JS, Bellis JM, Tsoh JY, et al. Computer‐based smoking cessation interventions in adolescents: description, feasibility, and six‐month follow‐up findings. Substance Use and Misuse 1998;33(4):935‐65. [DOI] [PubMed] [Google Scholar]
Pederson 1981 {published data only}
- Pederson LL, Baldwin N, Lefcoe NM. Utility of behavioral self‐help manuals in a minimal‐contact smoking cessation program. International Journal of Addiction 1981;16:1233‐9. [DOI] [PubMed] [Google Scholar]
Rimer 1994 {published data only}
- Rimer BK, Orleans CT. Tailoring smoking cessation for older adults. Cancer 1994;74(7 Suppl):2051‐4. [DOI] [PubMed] [Google Scholar]
- Rimer BK, Orleans CT, Fleisher L, Cristinzio S. Does tailoring matter? The impact of a tailored guide on ratings and short‐term smoking‐related outcomes for older smokers. Health Education Research 1994;9:69‐84. [DOI] [PubMed] [Google Scholar]
Russell 1979 {published data only}
- Russell MAH, Wilson C, Taylor C, Baker CD. Effect of general practitioners' advice against smoking. British Medical Journal 1979;2:231‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sallis 1986 {published data only}
- Sallis JF, Hill RD, Killen JD, Telch MJ, Flora JA, Girard J, et al. Efficacy of self‐help behavior modification materials in smoking cessation. American Journal of Preventive Medicine 1986;2:342‐4. [PubMed] [Google Scholar]
Senesael 2013 {published data only}
- Senesael E, Borgermans L, Vijver E, Devroey D. Effectiveness of a quality improvement intervention targeting cardiovascular risk factors: are patients responsive to information and encouragement by mail or post?. Vascular Health and Risk Management 2013;9:13‐20. [CENTRAL: 862212; CRS: 9400107000001662; PUBMED: 23426275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2013 {published data only}
- Shi HJ, Jiang XX, Yu CY, Zhang Y. Use of mobile phone text messaging to deliver an individualized smoking behaviour intervention in Chinese adolescents. Journal of Telemedicine and Telecare 2013;19(5):282‐7. [CRS: 9400126000000380; PUBMED: 24163238] [DOI] [PubMed] [Google Scholar]
Shiffman 2000 {unpublished data only}
- Shiffman S, Gitchell J, Strecher V, et al. Real‐world efficacy of computer‐tailored smoking cessation material as a supplement to nicotine replacement [Abstract]. 10th World Congress on Smoking or Health, Beijing, China, August. 1997.
- Shiffman S, Paty JA, Rohay JM, Dimarino ME, Gitchell J. The efficacy of computer‐tailored smoking cessation material as a supplement to nicotine polacrilex gum therapy. Archives of Internal Medicine 2000;160:1675‐81. [DOI] [PubMed] [Google Scholar]
Shiffman 2001 {unpublished data only}
- Shiffman S, Paty J, Rohay J, Marino M, Strecher V. The efficacy of computer‐tailored smoking cessation material as a supplement to nicotine patch therapy. Society for Research on Nicotine and Tobacco; 1998 August; Copenhagen, Denmark.
- Shiffman S, Paty JA, Rohay JM, Marino ME, Gitchell JG. The efficacy of computer‐tailored smoking cessation material as a supplement to nicotine patch therapy. Drug & Alcohol Dependence 2001;64(1):35‐46. [DOI] [PubMed] [Google Scholar]
Sims 2013 {published data only}
- Sims TH, McAfee T, Fraser DL, Baker TB, Fiore MC, Smith SS. Quitline cessation counseling for young adult smokers: a randomized clinical trial. Nicotine & Tobacco Research 2013;15(5):932‐41. [CRS: 9400123000018624; EMBASE: 2013286046] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2012 {published data only}
- Blyth A, Maskrey V, Notley C, Barton GR, Brown TJ, Aveyard P, et al. Effectiveness and economic evaluation of self‐help educational materials for the prevention of smoking relapse: randomised controlled trial. Health Technology Assessment 2015;19(59):1‐70. [CNO: CN‐01170619] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Holland R, Barton GR, Bachmann M, Blyth A, Maskrey V, et al. Self‐help materials for the prevention of smoking relapse: study protocol for a randomized controlled trial. Trials 2012;13:69. [CENTRAL: 855130; CRS: 9400123000017475; EMBASE: 2012625152; 316; PUBMED: 22647290] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Maskrey V, Blyth A, Brown TJ, Barton GR, Aveyard P, et al. Differences in longer‐term smoking abstinence after treatment by specialist or nonspecialist advisors: secondary analysis of data from a relapse prevention trial. Nicotine & Tobacco Research 2016;18(5):1061‐6. [CNO: CN‐01215184] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanczyk 2013 {published data only}
- Stanczyk NE, Crutzen R, Bolman C, Muris J, Vries H. Influence of delivery strategy on message‐processing mechanisms and future adherence to a Dutch computer‐tailored smoking cessation intervention. Journal of Medical Internet Research 2013;15(2):e28. [CENTRAL: 873085; CRS: 9400107000001436; PEER: Reviewed Journal: 2013‐26866‐003; PUBMED: 23388554] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strecher 1994 {published data only}
- Strecher VJ, Kreuter M, Boer DJ, Kobrin S, Hospers HJ, Skinner CS. The effects of computer‐tailored smoking cessation messages in family practice settings. Journal of Family Practice 1994;39:262‐70. [PubMed] [Google Scholar]
Strecher 2000 {published data only}
- Strecher VJ, Bishop KR, Bernhardt J, Thorp JM, Cheuvront B, Potts P. Quit for keeps: tailored smoking cessation guides for pregnancy and beyond. Tobacco Control 2000;9 Suppl 3:78‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strecher 2005b {published data only}
- Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web‐based computer‐tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction 2005;100:682‐8. [DOI] [PubMed] [Google Scholar]
Strecher 2008 {published data only}
- Strecher VJ, McClure JB, Alexander GL, Chakraborty B, Nair VN, Konkel JM, et al. Web‐based smoking‐cessation programs: results of a randomized trial. American Journal of Preventive Medicine 2008;34(5):373‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Te Poel 2009 {published data only}
- Poel F, Bolman C, Reubsaet A, Vries H. Efficacy of a single computer‐tailored e‐mail for smoking cessation: results after 6 months. Health Education Research 2009;24(6):930‐40. [CENTRAL: 733113; CRS: 9400123000005498; PUBMED: 19574405] [DOI] [PubMed] [Google Scholar]
Travis 2004 {published data only}
- Travis HE, Lawrance K. Effectiveness of self‐help smoking cessation interventions for college smokers: a randomized controlled trial (POS2‐032). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
Travis 2009 {published data only}
- Travis HE, Lawrance KA. Randomized controlled trial examining the effectiveness of a tailored self‐help smoking‐cessation intervention for postsecondary smokers. Journal of American College Health 2009;57(4):437‐44. [CENTRAL: 683030; CRS: 9400123000005106; PUBMED: 19114383] [DOI] [PubMed] [Google Scholar]
Ussher 2011 {published data only}
- Ussher M, Chambers M, Adams R, Croghan E, Murray R. Evaluation of a nationally disseminated self‐help intervention for smoking cessation ('Quit Kit'). Tobacco Control 2011;20(5):380‐2. [CRS: 9400123000012921; EMBASE: 21415063; 31695] [DOI] [PubMed] [Google Scholar]
Webb 2005 {published data only}
- Webb MS, Simmons VN, Brandon TH. Tailored interventions for motivating smoking cessation: using placebo tailoring to examine the influence of expectancies and personalization. Health Psychology 2005;24(2):179‐88. [DOI] [PubMed] [Google Scholar]
Webb 2007 {published data only}
- Webb MS, Hendricks PS, Brandon TH. Expectancy priming of smoking cessation messages enhances the placebo effect of tailored interventions. Health Psychology 2007;26(5):598‐609. [DOI] [PubMed] [Google Scholar]
Webb 2008 {published data only}
- Webb MS. Does one size fit all African American smokers? The moderating role of acculturation in culturally specific interventions. Psychology of Addictive Behaviors 2008;22(4):592‐6. [CENTRAL: 665144; CRS: 9400123000004966; PUBMED: 19071987] [DOI] [PubMed] [Google Scholar]
Webb 2009 {published data only}
- Webb MS. Culturally specific interventions for African American smokers: an efficacy experiment. Journal of the National Medical Association 2009;101(9):927‐35. [CENTRAL: 749773; CRS: 9400123000005621; EMBASE: 2009543758; PUBMED: 19806851] [DOI] [PubMed] [Google Scholar]
Webb 2010 {published data only}
- Webb MS, Baker EA, Rodríguez de Ybarra D. Effects of culturally specific cessation messages on theoretical antecedents of behavior among low‐income African American smokers. Psychology of Addictive Behaviors 2010;24(2):333‐41. [CENTRAL: 762147; CRS: 9400123000005767; PUBMED: 20565159] [DOI] [PubMed] [Google Scholar]
Weissfeld 1991 {published data only}
- Weissfeld JL, Holloway JL. Treatment for cigarette smoking in a department of Veterans Affairs outpatient clinic. Archives of Internal Medicine 1991;151:973‐7. [PubMed] [Google Scholar]
Wetter 2011 {published data only}
- Wetter DW, McClure JB, Cofta‐Woerpel L, Costello TJ, Reitzel LR, Businelle MS, et al. A randomized clinical trial of a palmtop computer‐delivered treatment for smoking relapse prevention among women. Psychology of Addictive Behaviors 2011;25(2):365‐71. [CENTRAL: 800597; CRS: 9400123000011588; PUBMED: 21500879] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willemsen 1995 {published data only}
- Willemsen MC, Vries H. Evaluation of a smoking cessation intervention for Dutch employees consisting of self help methods and a group programme. Tobacco Control 1995;4:351‐64. [Google Scholar]
Windsor 1989 {published data only}
- Windsor RA, Lowe JB. Behavioral impact and cost analysis of a worksite self‐help smoking cessation program. Progress in Clinical and Biological Research 1989;293:231‐42. [PubMed] [Google Scholar]
Zhu 1996 {published data only}
- Zhu SH, Stretch V, Balabanis M, Rosbrook BP, Sadler G, Pierce JP. Telephone counseling for smoking cessation ‐ effects of single‐session and multiple‐session interventions. Journal of Consulting and Clinical Psychology 1996;64:202‐11. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Campos 2014 {published data only (unpublished sought but not used)}
- Campos ACF, Martins LVO, Yano RN, Alvim RNT, Silva EN, Silva VA, et al. Comparison of two strategies for smoking cessation in hospitalized patients. European Heart Journal 2014;35:903. [Google Scholar]
Oh 2013 {published data only (unpublished sought but not used)}
- Oh A, Luginbuhl A, Curry J, Cognetti DM. Prospective randomized control trial using best‐selling smoking cessation book. Otolaryngology ‐ Head and Neck Surgery 2013;2 Suppl:188. [Google Scholar]
Pereira 2017 {published data only (unpublished sought but not used)}
- Pereira LS, Madureira MF, Lamas MFM, Brum DA, Sobreira NP, Pereira NS, et al. Making the decision to quit smoking: impact of interactive and educational video applied in population with multimorbidities. 22nd World Congress on Heart Disease International Academy of Cardiology Annual Scientific Sessions. 2017; Vol. 137:234.
References to ongoing studies
JPRN‐UMIN000008750 {published data only}
- JPRN‐UMIN000008750. A randomized study of bibliotherapy for smoking cessation with and without focusing on cognitive elements. https://upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000010276&type=summary&language=E 2012.
NCT01544010 {unpublished data only}
- Redding CA, Prochaska JO, Rossi JS, Kobayashi H, Yin HQ, Paiva AL, et al. Levels of transtheoretical model tailoring for cessation: randomized trial outcomes. Annals of Behavioral Medicine 2014;47:S238. [Google Scholar]
NCT02276664 {published data only}
- NCT02276664. Capitalizing on a teachable moment to promote smoking cessation. clinicaltrials.gov/ct2/show/NCT02276664 2014.
NCT02416011 {published data only}
- Meltzer LR, Simmons VN, Sutton SK, Drobes DJ, Quinn GP, Meade CD, et al. A randomized controlled trial of a smoking cessation self‐help intervention for dual users of tobacco cigarettes and E‐cigarettes: intervention development and research design. Contemporary Clinical Trials 2017;60:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02611076 {published data only}
- NCT02611076. Smoking‐cessation: a Spanish‐language clinical trial. clinicaltrials.gov/ct2/show/NCT02611076 2015.
NCT02945787 {published data only}
- NCT02945787. Spanish‐language smoking cessation trial. clinicaltrials.gov/ct2/show/NCT02945787 2016.
NCT03064971 {published data only}
- NCT03064971. Enhancing quitline services for African American smokers. https://ClinicalTrials.gov/show/NCT03064971 2017.
Additional references
Chamberlain 2017
- Chamberlain C, O'Mara‐Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2015
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi‐Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD010078.pub2] [DOI] [PubMed] [Google Scholar]
Curry 1993
- Curry SJ. Self‐help interventions for smoking cessation. Journal of Consulting and Clinical Psychology 1993;61:790‐803. [DOI] [PubMed] [Google Scholar]
Fanshawe 2017
- Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone‐Banks J, Hartmann‐Boyce J. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD003289.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2013
- Hajek P, Stead LF, West R, Jarvis M, Hartmann‐Boyce J, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD003999.pub4] [DOI] [PubMed] [Google Scholar]
Hall 2001
- Hall SM, Delucchi KL, Velicer WF, Kahler CW, Ranger‐Moore J, Hedeker D, et al. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine & Tobacco Research 2001;3:193‐202. [DOI] [PubMed] [Google Scholar]
Hartmann‐Boyce 2018
- Hartmann‐Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD000146.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Greene S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Koshy 2008
- Koshy E, Marlais M, Civljak M. Internet‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007078] [DOI] [PubMed] [Google Scholar]
Kreuter 2000
- Kreuter MW, Skinner CS. Tailoring: what's in a name?. Health Education Research 2000;15:1‐4. [DOI] [PubMed] [Google Scholar]
Lancaster 2017
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PubMed] [Google Scholar]
Lee 2007
- Lee C‐W, Kahende J. Factors associated with successful smoking cessation in the United States, 2000. American Journal of Public Health 2007;97(8):1503‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marks 2002
- Marks DF, Sykes CM. Randomized controlled trial of cognitive behavioural therapy for smokers living in a deprived area of London: outcome at one‐year follow‐up. Psychology, Health and Medicine 2002;7:17‐24. [Google Scholar]
Park 2004
- Park EW, Schultz JK, Tudiver FG, Campbell T, Becker LA. Enhancing partner support to improve smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD002928.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2013a
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2013b
- Stead LF, Hartmann‐Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD002850.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2017
- Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001007.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2017
- Taylor GMJ, Dalili M, Semwal M, Civljak M, Sheikh A, Car J. Internet‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD007078.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittaker 2016
- Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD006611.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
WHO 2017
- World Health Organization. WHO Report on the Global Tobacco Epidemic: Monitoring Tobacco Use and Prevention Policies. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
References to other published versions of this review
Hartmann‐Boyce 2014
- Hartmann‐Boyce J, Lancaster T, Stead LF. Print‐based self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001118.pub3] [DOI] [PubMed] [Google Scholar]
Lancaster 1998
- Lancaster T, Stead LF. Self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 1998, Issue 4. [Google Scholar]
Lancaster 2000
- Lancaster T, Stead LF. Self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI] [PubMed] [Google Scholar]
Lancaster 2002
- Lancaster T, Stead LF. Self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001118] [DOI] [PubMed] [Google Scholar]
Lancaster 2005a
- Lancaster T, Stead LF. Self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001118.pub2] [DOI] [PubMed] [Google Scholar]


