Abstract
Background
Tropical Australia has a high incidence of nocardiosis, with high rates of intrinsic antimicrobial resistance. Linezolid, the only antimicrobial to which all local Nocardia species are susceptible, has been recommended in empirical combination treatment regimens for moderate–severe Nocardia infections at Royal Darwin Hospital (RDH) since 2014. We report the safety and efficacy of linezolid use for nocardiosis in this setting.
Methods
We identified cases through a retrospective review of all RDH Nocardia isolates from December 2014 to August 2018 and included 5 linezolid-treated cases from a previous cohort. Laboratory, demographic, and clinical data were included in the primary analysis of safety and treatment outcomes.
Results
Between 2014 and 2018, Nocardia was isolated from 35 individuals; 28 (80%) had clinically significant infection and 23 (82%) received treatment. All isolates were linezolid-susceptible. Safety and efficacy were assessed for 20 patients receiving linezolid-containing regimens and 8 receiving nonlinezolid regimens. Median linezolid induction therapy duration was 28 days. Common adverse effects in those receiving linezolid were thrombocytopenia (45%) and anemia (40%). Adverse events prompted discontinuation of trimethoprim-sulfamethoxazole more often than linezolid (40% vs 20%). Linezolid therapeutic drug monitoring was used in 1 patient, with successful dose reduction and outcome. There was no difference in 30-day survival between those treated with linezolid (90%) vs no linezolid (87%). One Nocardia-attributed death occurred during linezolid therapy.
Conclusions
Linezolid is safe and efficacious in empirical treatment for moderate to severe nocardiosis in a monitored hospital setting, with 100% drug susceptibility and no difference in adverse events or outcomes compared with nonlinezolid regimens.
Keywords: antimicrobial resistance, linezolid, Nocardia, prophylaxis, therapy
Nocardia is a genus of aerobic actinomycetes found in soil, organic matter, and water, with multiple species causing localized or disseminated infection in humans [1]. Reported cases of human disease from Nocardia have increased substantially in the past 2 decades, in association with an increasing population of immunocompromised hosts and improved methods for detection and identification of Nocardia species in the clinical laboratory [1]. The incidence of Nocardia appears higher in tropical regions, with 2.02/100 000 people reported in northern Australia [2] compared with 0.47–0.87/100 000 people in temperate areas of Canada, Spain, and South Australia [1]. Infection usually arises from inhalation or direct inoculation of skin and soft tissues, manifesting as cutaneous, pulmonary, central nervous system (CNS), or disseminated disease [3]. Case fatality rates are high, with 1-year mortality ranging from 14% to 66% [4, 5].
Despite rising incidence, optimal Nocardia treatment regimens have not been established, and there have been no randomized controlled trials of firstline agents. The relative disease burden due to different Nocardia species varies between regions, with each species also differing in intrinsic antimicrobial susceptibilities [1, 2, 6]. In our previous 1997–2014 Nocardia case series, we found unexpectedly high rates of inherent resistance to commonly recommended antimicrobials, with deaths associated with empirical use of antimicrobials to which Nocardia was resistant [2]. Linezolid was the only antimicrobial to which isolates were universally susceptible; in contrast, 89%, 89%, 60%, and 48% of isolates were susceptible to trimethoprim-sulfamethoxazole, amikacin, ceftriaxone, and imipenem, respectively. Due to the documented high risk of severe disease and a 1-year mortality of 31% [2], linezolid was introduced into local practice in 2014 as part of empirical combination induction treatment regimens for patients with moderate to severe or disseminated disease, pending the results of referral laboratory antimicrobial susceptibility testing.
Linezolid is the first antimicrobial agent demonstrated to be active in vitro against essentially all Nocardia species globally [7]. It is an oxazolidinone with good oral bioavailability and an ability to cross the blood–brain barrier, so is an appealing alternative for empirical therapy. Despite these potential advantages, there is a paucity of robust clinical data on linezolid use for nocardiosis. Clinical cure with linezolid use has been reported in a number of individual case reports, particularly for CNS disease, in addition to a number of small case series [8, 9]. Barriers to the use of linezolid primarily involve its previous expense and concerns around potential drug toxicity, with myelosuppression, peripheral neuropathy, lactic acidosis, and optic neuritis being the most commonly reported adverse effects [8].
The objective of this study was to assess the safety and outcomes of linezolid use in combination therapy for moderate to severe Nocardia infections at Royal Darwin Hospital in the Northern Territory of Australia.
METHODS
Design and Setting
This retrospective study was conducted at RDH (a 400-bed tertiary referral center servicing a population of around 170 000 people in the tropical north of Australia. Ethics approval was provided by the Human Research Ethics Committee of the Northern Territory Department of Health (HREC 2018–3142).
Study Population
We conducted a search of the RDH microbiology database (LABTrack) from December 2014 to August 2018 to identify individuals from whom Nocardia had been isolated. Cases were excluded if they were <18 years of age or had not resided in the NT for the previous 6 months. Cases were classified as clinically significant when the isolation of Nocardia species from a clinical specimen was associated with clinical disease necessitating treatment based on review by an infectious diseases physician. Immunosuppression was defined as a history of a solid organ or hematological malignancy on chemotherapy, HIV infection, solid organ or bone marrow transplant, or high-dose corticosteroid therapy (defined as ≥4 weeks of prednisolone >20 mg/d or equivalent) or other immunosuppressive medication for autoimmune disease. Induction therapy was defined as the initial intensive treatment used in Nocardia infections. This was often combination therapy while susceptibility results were pending.
Data Collection
A case report form developed and validated in our previous 1997–2014 RDH cohort study was used [2]. Demographic, clinical, treatment, and outcome data were collected from medical and laboratory records. Microbiological data (specimen type, Nocardia species, and antimicrobial susceptibility) were obtained from the RDH microbiology laboratory database. Data were entered into a password-protected REDCap electronic database.
Microbiology Methods
A presumptive diagnosis of Nocardia species was made in the RDH microbiology laboratory based on standardized gram and/or modified acid-fast staining findings and colony morphology. Cultured isolates were referred to a reference laboratory for species identification by sequencing of 16S ribosomal ribonucleic acid and secA1 housekeeping genes [10]. At this time, the Matrix-assisted laser desorption / ionization time of flight mass spectrometry (MALDI-TOF MS) was not validated at the reference laboratory for identification of Nocardia isolates, and whole-genome sequencing was not performed on those isolates in which a species could not be identified. Antimicrobial susceptibility testing (AST) was performed by Clinical and Laboratory Standards Institute standardized broth microdilution methods [11].
Statistical Analysis
Data were analyzed using STATA (version 16; StataCorp, College Station, TX, USA). Clinical and epidemiological data were analyzed using mean ± SD for normally distributed variables and median ± interquartile range (IQR) for non–normally distributed variables. For group analyses comparing linezolid vs nonlinezolid treatment, binary data were evaluated with the chi-square or Fisher exact test, and for continuous data the Student t test or Mann-Whitney U test was used.
RESULTS
Study Population
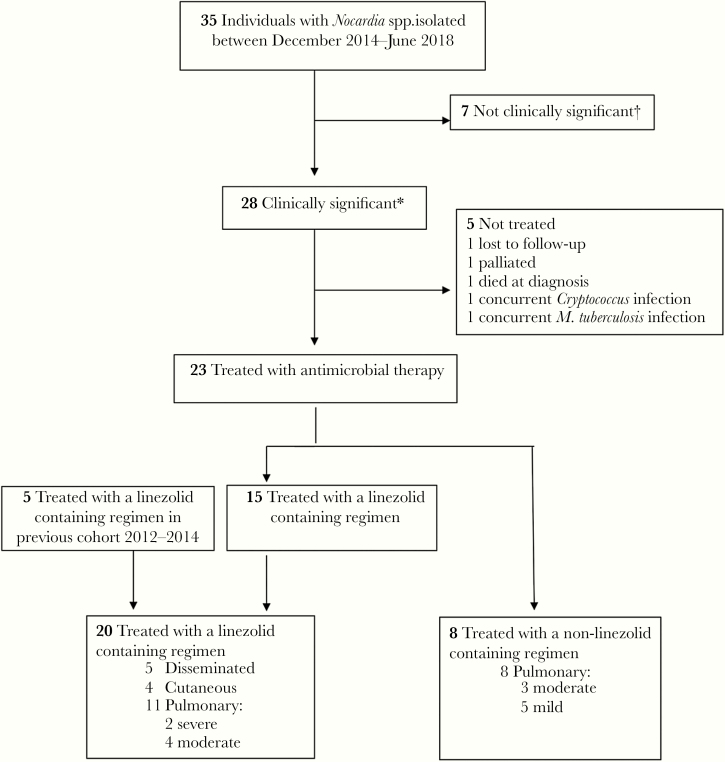
From December 2014 to August 2018, we identified 35 individuals from whom Nocardia spp. were isolated at RDH (Figure 1). Seven (20%) were deemed not clinically significant and were not treated. Of the 28 (80%) patients with clinically significant infection, 23 (82%) were treated with antimicrobial therapy: 15 with a linezolid-containing regimen and 8 with an alternative regimen, none of which were salvage regimens. Five additional patients with nocardiosis treated with linezolid in our previous RDH cohort study between 2012 and 2014 were also included in the analysis [2], resulting in a total of 20 linezolid-treated patients included in the final analysis.
Figure 1.
Study population. aClinically significant: consistent with infection as per an infectious diseases physician. bOf the 7 cases with isolates deemed not clinically significant, none were treated. After 1 year, 2 had died of unrelated causes and 5 had not developed disease.
Demographics and Clinical Manifestation
Baseline characteristics in the linezolid vs nonlinezolid treatment groups are described in Table 1. The median age (IQR) was 62 (52–68) years in the linezolid group and 67 (56–74) years in the nonlinezolid group. The most common underlying risk factor was chronic lung disease, found in 60% and 63% of patients in the linezolid and nonlinezolid groups, respectively. Pulmonary infection was the most common presentation in both groups, including 80% of those in the linezolid group and 88% in the nonlinezolid group. Among patients with clinically significant infections, 6 (21%) had severe disease (disseminated disease [n = 4] or a requirement for intensive care unit admission [n = 2]); all were treated with a linezolid-containing combination treatment regimen. Linezolid was also included in combination treatment regimens for all immunosuppressed (n = 5) patients. None of the linezolid-treated patients had baseline thrombocytopenia (platelet count < 150×103/μL), compared with 30% (2/6) of those treated with an alternative regimen (P = .046), consistent with a clinical avoidance of linezolid in those with thrombocytopenia. No other statistically significant differences between the 2 groups were noted. None of the patients in either group were receiving trimethoprim-sulfamethoxazole prophylaxis for Pneumocystis jiroveci pneumonia (PJP) or melioidosis (caused by Burkholderia pseudomallei) at diagnosis.
Table 1.
Baseline Characteristics and Clinical Manifestations
| Treatment Group | Linezolid-Containing | Non-Linezolid-Containing | P Value |
|---|---|---|---|
| No. | 20 (71) | 8 (29) | |
| Age at time of diagnosis, median (IQR) [range], y | 62 (52–68) [23–80] | 67 (56–74) [30–76] | .94 |
| Male sex | 15 (75) | 6 (75) | .99 |
| Urban residence (Darwin/Palmerston) | 13 (65) | 6 (75) | .99 |
| Indigenous ethnicity | 4 (20) | 2/7 (29) | .63 |
| Travel in last 6 mo | 1 (5) | 2 (25) | .18 |
| Hazardous alcohol | 3 (15) | 2 (25) | .60 |
| Diabetes mellitus | 6 (30) | 1 (13) | .63 |
| Chronic kidney disease | 3/15a (20) | 0 (0) | .52 |
| Hemodialysis | 1/15a (7) | 0 (0) | .99 |
| Cancer | 3 (15) | 3 (38) | .31 |
| Chronic lung disease | 12 (60) | 5 (63) | .99 |
| Smoker | 5 (25) | 5 (63) | .09 |
| Organ transplant | 1 (5) | 0 (0) | .99 |
| HIV-positive | 1 (5) | 0 (0) | .99 |
| Hemoglobin level, median (IQR), g/dL | 129 (109–139) | 120 (105–133) | .33 |
| Anemia (Hb < 100 g/dL) | 8 (40) | 2/6 (33) | .99 |
| Platelet count, median (IQR), ×103/μL | 282 (242–389) | 205 (131–245) | .07 |
| Thrombocytopenia (platelets < 150×103/μL) | 0 (0) | 2/6 (33) | .04 |
| Site/focus | |||
| Skin | 5 (25) | 1 (13) | .64 |
| Lung | 16 (80) | 7 (88) | .99 |
| Brain | 2 (10) | 0 (0) | - |
| Bones | 1 (5) | 0 (0) | - |
| Eyes (chorioretinitis) | 1 (5) | 0 (0) | .99 |
| Multiple foci present | 4 (20) | 0 (0) | .29 |
| Severe (ICU admission +/- multiple foci) | 6 (30) | 0 (0) | .14 |
| Immunosuppressed patientb | 5 (25) | 0 (0) | .28 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
aValues are No. (%) unless otherwise specified.
bNo patient was on trimethoprim-sulfamethoxazole prophylaxis.
Microbiology and Susceptibility Profiles
Of the 35 isolates identified, 32 (91%) were sent to a referral laboratory for molecular identification with 16s RNA sequencing and antimicrobial susceptibility testing. Species-level identification was successful in 25 patients, and 9 different species were identified (Table 2). The most common species identified were Nocardia beijingensis (19%) and Nocardia cyriacigeorgica (16%). Antimicrobial susceptibility testing results were available for 30 patients. Overall, 100% of isolates were susceptible to linezolid, 100% to amikacin, and 93% to trimethoprim-sulfamethoxazole. Only 60% and 45% of isolates were susceptible to ceftriaxone and imipenem, respectively. Variability in susceptibility patterns was noted across species.
Table 2.
Nocardia Species Classification and Drug Sensitivity Profiles
| Species | No. of Isolates (% Total) | LZDa | TMP-SMX | AMI | CTX | CLA | IMI | CIP | TOB |
|---|---|---|---|---|---|---|---|---|---|
| N. beijingensis | 6 (19) | 100 | 100 | 100 | 100 | 100 | 50 | 0 | 100 |
| N. cyriacigeorgica | 5 (16) | 100 | 80 | 100 | 60 | 20 | 40 | 0 | 100 |
| N. veteran/elegans | 4 (12) | 100 | 100 | 100 | 75 | 100 | 75 | 0 | 25 |
| N. farcinica | 4 (12)b | 100 | 100 | 100 | 0 | 0 | 0 | 100 | 0 |
| N. brasiliensis | 2 (6) | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 100 |
| N. brevicatena | 1 (3) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| N. nova | 1 (3) | NP | 100 | 100 | 100 | 0 | NP | 0 | 0 |
| N. yamanashiensis | 1 (3) | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 0 |
| N. species (unspeciated) | 8 (25) | 100 | 100 | 100 | 37 | 62 | 37 | 12 | 37 |
| Total | 32c | 29 (100) | 28 (93) | 30 (100) | 18 (60) | 18 (60) | 13 (45) | 4 (14) | 18 (60) |
Abbreviations: AMI, amikacin; AST, Antimicrobial susceptibility testing; CIP, ciprofloxacin; CLA, clarithromycin; CTX, ceftriaxone; IMI, imipenem; NP, not performed; TMP-SMX, trimethoprim-sulfamethoxazole; TOB, tobramycin.
aDrug susceptibilities presented as % susceptible.
bAST available for 2 of 4 isolates.
cAST available for 30 of 32 isolates.
Treatment Regimens
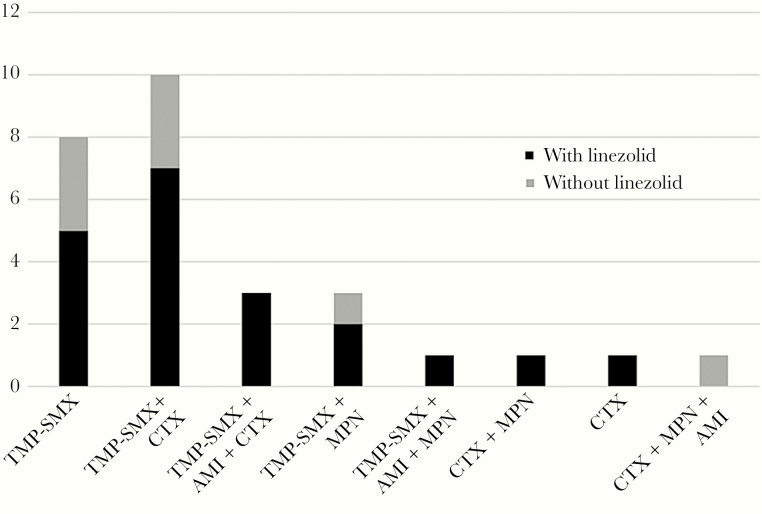
There were 23 patients who commenced empirical treatment before susceptibility results were available, including 17 (74%) receiving a linezolid-containing regimen. Of the 5 patients who only commenced treatment once susceptibility results were available, 3 were treated with linezolid. Figure 2 demonstrates the heterogeneity of induction regimens used with and without linezolid. The most common induction regimen (7/28; 25%) was a combination of linezolid, trimethoprim-sulfamethoxazole, and ceftriaxone. Twenty-five (89%) patients were treated with a combination therapy induction regimen; the remaining 3 patients were treated with trimethoprim-sulfamethoxazole monotherapy.
Figure 2.
Induction regimens. Abbreviations: AMI, amikacin; CTX, ceftriaxone; MPN, meropenem; TMP-SMX, trimethoprim-sulfamethoxazole.
The median duration of linezolid use (range) was 28 (8–50) days. Of those receiving linezolid, 90% were also concurrently treated with trimethoprim-sulfamethoxazole. Within this group, a higher proportion required permanent discontinuation of trimethoprim-sulfamethoxazole due to adverse events (40%) compared with linezolid (20%). Of those treated with a linezolid-containing regimen, 80% completed their planned duration of linezolid induction therapy, including 60% without interruptions (Table 3).
Table 3.
Treatment and Related Adverse Events
| Treatment Group | Linezolid-Containing (n = 20) | Non-Linezolid-Containing (n = 8)b | P Value |
|---|---|---|---|
| Induction treatment | |||
| Duration of linezolid, median (IQR), d | 28 (21–37) [8–50] | - | - |
| Any TMP-SMX-containing regimen | 18 (90) | 7 (88) | .99 |
| TMP-SMX dose reduced | 9 (45) | 1 (13) | .19 |
| TMP-SMX ceased | 8 (40) | 2 (25) | .67 |
| Linezolid ceased | 4 (20) | - | - |
| Completed induction therapy | 16 (80) | 6 (75) | .99 |
| Uninterrupted | 12 (60) | 5 (63) | .99 |
| Interrupted | 4 (20) | 1 (13) | .99 |
| Complications associated with induction treatment | |||
| Thrombocytopenia (<150×103/μL) | 9 (45) | 2/6 (33) | .67 |
| Thrombocytopenia (<50×103/μL) | 1 (5) | 0/6 (0) | .99 |
| Thrombocytopenia, median (IQR), d to onset | 19 (14–47) | - | |
| Anemia (Hb < 100 g/dL) | 8 (40) | 2/6 (33) | .99 |
| Anemia, median (IQR) [range], d to onset | 21 (12–36) [6–54] | 29 (20–38) [20–38] | .51 |
| Acute kidney injury (modified KDIGO criteriaa) | 7 (35) | 2/6 (33) | .99 |
| Acute kidney injury attributed to TMP-SMX | 6/7 (86) | 2/2 (100) | .99 |
| Neutropenia (<2.0 g/dL) | 3 (15) | 2/6 (33) | .55 |
| Neutropenia, median (IQR), d to onset | 23 (11–25) | 21 (14–28) | .58 |
| Neutropenia attributed to TMP-SMX | 3/3 (100) | 2/2 (100) | .99 |
| Peripheral neuropathy | 1 (5) | 0 (0) | .99 |
Abbreviations: IQR, interquartile range; TMP-SMX, trimethoprim-sulfamethoxazole.
Values are No. (%) or no./No. (%) unless otherwise specified.
aSerum creatinine ≥1.5 × baseline.
bOnly 6/8 patients in the non-linezolid-containing group had laboratory monitoring available.
Treatment Outcomes
Among those treated with linezolid, 30-day and 1-year survival were 90% and 85%, respectively, comparable to survival in those not treated with linezolid (Table 4). One of the 2 deaths in the linezolid group was partially attributed to Nocardia infection 8 days after diagnosis. This patient had a history of severe interstitial lung disease and died secondary to multifactorial respiratory failure, having received 8 days of linezolid combined with meropenem and trimethoprim-sulfamethoxazole (Table 5). The other death was from a pulmonary embolus after 14 days of LZD combination therapy. In total, there were 6 severe and/or disseminated infections, with individual clinical manifestations, treatment, and outcomes described in Table 5. Within the whole cohort, there were no relapses in the linezolid-treated patients and 1 relapse in the nonlinezolid group, in a patient treated with 3 months of combination therapy with ceftriaxone, meropenem, and amikacin, despite the isolate testing susceptible to both ceftriaxone and amikacin (Table 4). Follow-up was for a median (range) of 12 (1–36) months.
Table 4.
Outcomes
| Treatment Group | Linezolid- Containing (n = 20), No. (%) | Non-Linezolid- Containing (n = 8), No. (%) | P Value |
|---|---|---|---|
| Cure | 17 (85) | 6 (75) | .61 |
| Relapse | 0 (0) | 1 (13) | .29 |
| 30-d survival | 18 (90) | 7 (87) | .99 |
| 1-y survival | 17 (85) | 7 (87) | .99 |
| Primary cause of death | |||
| Nocardia infection | 1 (5) | 0 (0) | .99 |
| Cancer | 1 (5) | 1 (12) | .50 |
| Pulmonary embolism | 1 (5) | 0 (0) | .99 |
Table 5.
Cases of Severe Nocardia Infection
| Case | Sex/ Age, y | Indigenous | Clinical Manifestation | Comorbidities | ICU | Nocardia sp. | Antimicrobial Therapy and Duration, d | Inappropriate Treatment (Nonsusceptible) | Survival | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M 79 | N | Pulmonary and CNS | ILD, T2DM | Yes | N. farcinia | LZD 8 CTX 3 MPN 8 TMP-SMX 8 | N/Aa | No | Respiratory failure— likely ILD-related |
| 2 | M 82 | Y | Pulmonary, CNS, chorioretinitis | T2DM | No | N. beijingensis | LZD 28 CTX 90 AMI 28 TMP-SMX 365 | - | Yes | - |
| 3 | M 61 | N | Pulmonary, CNS | Asthma | Yes | N. beijingensis | LZD 28 CTX 28 TMP-SMX 365 | - | Yes | - |
| 4 | M 64 | N | Pulmonary and cutaneous | NSCLC, CLL | No | N. species (unspeciated) | LZD 28 CTX 7 AMI 14 TMP-SMX 90 | CTX | Yes | - |
| 5 | F 63 | N | Pulmonary, CNS | COPD, SCLC | No | N. farcinia N. cyriacigeorgica | LZD 14 MPN 2 CTX 14 TMP-SMX 14 | CTX MPN | No | PE |
| 6 | M 37 | Y | Pulmonary | COPD | Yes | N. species (unspeciated) | LZD 21 MPN 28 AMI 7 TMP-SMX 180 | MPN | Yes | - |
Abbreviations: AMI, amikacin; CLL, chronic lymphocytic leukemia; COPD, chronic obstructive pulmonary disease; CTX, ceftriaxone; ILD, interstitial lung disease; LZD, linezolid; MPN, meropenem; NSCLC, non–small cell lung cancer; SCLC, small cell lung cancer; T2DM, type 2 diabetes mellitus; TMP-SMX, trimethoprim-sulfamethoxazole.
aNo susceptibilities available.
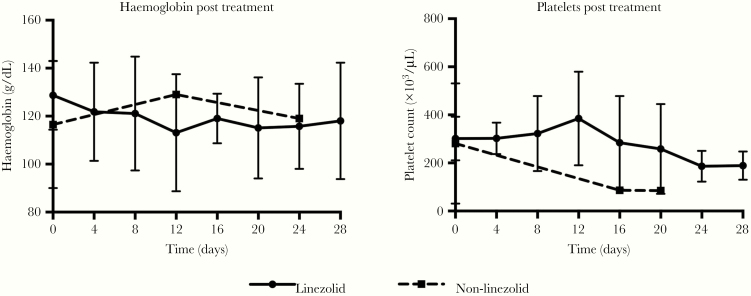
Thrombocytopenia was the most common adverse effect attributed to linezolid treatment, developing in 9 (45%) patients. However, treatment cessation was only required in a single case, after a reduction in platelet count from 380×103/µL to 89×103/µL after 21 days of therapy (Table 3). Anemia was common in both groups and occurred in 8 (40%) of linezolid-treated patients. However, severe anemia requiring linezolid cessation was only documented in a single patient (1/20; 5%) with a hemoglobin decrease from 107 g/dL to 63 g/dL after 14 days of treatment. The median time to development of clinically significant thrombocytopenia (platelet count < 150×103/μL) and anemia (Hb < 100 g/dL) for those receiving linezolid-containing regimens was ~3 weeks (Table 3). There was no difference in the trends of these hematological parameters between the 2 groups on logistic regression (Figure 3). Acute kidney injury was also common (35% in the linezolid group and 33% in the nonlinezolid group), and in each group kidney injury was attributed solely to trimethoprim-sulfamethoxazole, improving in all cases with cessation of this antibiotic. There was 1 case of moderate sensory peripheral neuropathy with the loss of pinprick sensation to above the ankles in the linezolid group, in a patient with type 2 diabetes mellitus and hazardous alcohol use, diagnosed after 28 days of therapy; the patient initially improved after cessation of linezolid therapy but was then lost to follow-up. There were no cases of lactic acidosis or optic neuritis.
Figure 3.
Hemoglobin and platelets during treatment. aMean ± SD.
Linezolid therapeutic drug monitoring (TDM) was used in a single patient receiving hemodialysis in the context of a failed renal transplant. In this case, thrombocytopenia developed after 14 days of treatment, with linezolid subsequently ceased. However, due to subsequent development of adverse effects from trimethoprim-sulfamethoxazole and an unfavorable isolate susceptibility profile (only susceptible to linezolid, trimethoprim-sulfamethoxazole, amikacin, and ciprofloxacin), linezolid was re-introduced. Plasma drug levels were performed, and an area under the curve (AUC)–to–minimum inhibitory concentration (MIC) ratio was calculated by the Begg formula utilizing minimum (Cmin) and peak (Cmax) concentrations [12]. The MIC of the isolate was 4, and the aim was a minimum AUC-to-MIC ratio of 80 based on TDM data for Staphylococcus aureus and vancomycin-resistant enterococci with linezolid [13]. The dose was reduced to 400 mg twice daily, and the patient completed induction treatment with no further complications.
DISCUSSION
We describe the largest case series of Nocardia infections treated with linezolid to date and demonstrate that linezolid is effective and at least as safe as other firstline treatments. With linezolid being the only antimicrobial to which all Nocardia isolates in our region are susceptible, these findings support inclusion of linezolid in empirical induction treatment regimens for moderate to severe nocardiosis.
There has been increased recognition of antimicrobial resistance in Nocardia infections to traditional firstline antibiotics, including from 2% to 42% for trimethoprim-sulfamethoxazole [14, 15]. Studies of in vitro antimicrobial susceptibilities have indicated that 98%–100% of Nocardia isolates worldwide are susceptible to linezolid [7, 16], consistent with previous findings from our setting [2]. Antimicrobial susceptibility to injectable antimicrobials commonly used in empiric Nocardia treatment regimens, such as ceftriaxone and imipenem, vary geographically and by Nocardia species [17]. In a previous study from our setting, only modest susceptibility to ceftriaxone (60%) and imipenem (45%) was found [2]. In contrast, a recent study from temperate Australia reported lower susceptibility to ceftriaxone (44%) and higher susceptibility to imipenem (92%), albeit in a cohort with markedly different species composition, with Nocardia nova being the most common species isolated [6]. Due to taxonomic changes and differences in identification methods, it can be difficult to compare regions; however, it is clear that in tropical Australia, high rates of antimicrobial resistance are present, and guidelines should reflect the local epidemiology [10]. At hospitals without reference laboratory capacity, it can take up to 4 weeks for results to be obtained from isolates referred for susceptibility testing. In such settings, particularly where severe disease is common, it is important to choose empiric treatment regimens with a high likelihood of susceptibility to at least 2 antimicrobials. Our findings provide support for the new 2019 Australian Therapeutic Guidelines for empirical treatment of Nocardia (Box 1) [18].
Box 1. . 2019 Australian Therapeutic Guidelines Recommendations for Empirical Treatment of Nocardia Infections.
| Milda | Moderatea | Severea | |
|---|---|---|---|
| Empiric treatment for Nocardia infection | TMP-SMX 160/800 mg (child 1 mo or older 4 + 20 mg/kg up to 160 + 800 mg) orally, every 12 h | TMP-SMX 160/800 mg (adult >60 kg: 320 + 1600 mg; adult 40 to 60 kg: 240 + 1200 mg; child 1 mo or older: 6 + 30 mg/kg up to 240 + 1200 mg) orally, every 12 h PLUS EITHER ceftriaxone 2 g (child 1 mo or older: 50 mg/kg up to 2 g) IV, daily OR linezolid 600 mg orally, every 12 h (child younger than 12 y: 10 mg/kg up to 600 mg orally, every 8 h) |
TMP-SMX 320/1600 mg (child 1 mo or older: 8 + 40 mg/kg up to 320 + 1600 mg) IV or orally, every 12 h PLUS linezolid 600 mg IV or orally, every 12 h (child younger than 12 y: 10 mg/kg up to 600 mg IV or orally, every 8 h) PLUS EITHER amikacin (adult and child) 20 mg/kg IV daily or 10 mg/kg IV, every 12 h OR imipenem 500 mg (child 15 mg/kg up to 500 mg) IV, every 6 h OR meropenem 2 g (child 40 mg/kg up to 2 g) IV, every 8 h |
Abbreviations: IV, intravenous; TMP-SMX, trimethoprim-sulfamethoxazole.
aMild: nonsevere disease in immunocompetent patients with localized cutaneous disease. Moderate: nonsevere dis-ease in immunocompetent patients with more extensive cutaneous disease, immunocompromised patients with cuta-neous disease, or any patient with mild to moderate pulmonary disease. Severe: central nervous system disease with brain abscess, disseminated disease, and severe pneumonia. Adapted with permission from Mandell et al., eds. [19].
Linezolid is an appealing choice for empirical treatment for moderate to severe disease given its near-universal susceptibility profile. It also has excellent central nervous system (CNS) penetration [19], has high oral bioavailability (minimizing complications from intravenous access), and is now off-patent, reducing previous concerns about high cost [20]. As the use of trimethoprim-sulfamethoxazole monotherapy for nocardiosis involving the CNS has been associated with high mortality (50%) in previous studies, a number of current guidelines recommend the addition of an injectable agent [21, 22]. Although amikacin has an excellent susceptibility profile, its use is limited by irreversible aminoglycoside toxicity and stringent monitoring requirements [15]. When susceptibility of traditional firstline agents cannot be relied upon, the empiric use of linezolid is a more logical choice. Despite this, historically linezolid has been used infrequently for nocardiosis due to concerns around drug-related adverse events. There have been 2 previous case series demonstrating safe and successful linezolid use for nocardiosis to date [8, 9]. One, solely in 15 solid organ transplant recipients, reported high rates of myelosuppression (though this was likely to be multifactorial and as a result of the use of concomitant medications) [9]. In the current series, only 25% of patients were immunosuppressed, a group in whom linezolid may be less well tolerated. In our current study, discontinuation of linezolid due to adverse events was much less common (20%) than discontinuation of trimethoprim-sulfamethoxazole due to adverse events (40%). In addition, the median time to development of anemia and/or thrombocytopenia was ~3 weeks, by which time susceptibility results should be imminently available to guide a switch to eradication therapy. No patients in this study had baseline thrombocytopenia, so these results cannot be extrapolated to that population. All linezolid-related adverse events encountered during our study were reversible, apart from 1 case of peripheral neuropathy with underlying risk factors for neuropathic complications. It is notable that all patients with severe and/or disseminated infection were treated with linezolid, and despite this, cure rates and 30-day survival rates were still comparable to the nonlinezolid treatment group. These high survival rates may also reflect the low rates of immunosuppression in this cohort, and it must be noted that linezolid was given in combination, so although these results support the efficacy of linezolid, further dedicated research is required.
There have been case reports of linezolid use for up to 9 months in Nocardia infection [23]. Recent World Health Organisation guidelines for drug-resistant tuberculosis (TB) include linezolid as an option for inclusion in long treatment regimens for drug-resistant TB, albeit at a lower dose (600 mg once daily, or 300 mg daily if adverse events are experienced [24]) than typically used for Nocardia [25]. Conventional linezolid dosing for Nocardia induction therapy is 600 mg twice daily, raising the question as to whether therapeutic drug monitoring (TDM) has a role in optimizing time in the therapeutic window while limiting toxicity. Linezolid TDM has been used in drug-resistant tuberculosis to limit long-term adverse effects mediated by mitochondrial toxicity [24, 26]. There is wide individual variation in the relationships between measured serum linezolid concentrations and the minimum concentrations (Cmin) and 24-hour AUC measurements associated with the development of hematological toxicity [27, 28]. In addition, patients with renal impairment are more likely to have high plasma linezolid concentrations, but the recommended dose is the same [29, 30]. TDM is now available for linezolid and may allow clinicians to tailor dosing and therefore reduce toxicity and prolong treatment courses [31]. Some studies have suggested improvement in long-term outcomes with TDM of linezolid treatment, although this has not been reported for Nocardia infections specifically [32]. In some cases of drug-resistant tuberculosis, with TDM, doses have been reduced to <300 mg without compromising efficacy [33]. We used TDM to guide reintroduction of linezolid in a patient receiving hemodialysis after initial discontinuation for linezolid-related side effects; TDM-guided dose reduction did not compromise clinical cure, and side effects did not recur. For patients who develop dose-dependent toxicity, TDM should be considered to reduce linezolid doses while ensuring efficacy.
In this study, we identified 5 immunosuppressed patients with clinically significant nocardiosis, none of whom were receiving prophylaxis with trimethoprim-sulfamethoxazole for PJP or melioidosis at diagnosis [34]. The dosage of trimethoprim-sulfamethoxazole used for PJP prophylaxis (800/160 mg thrice weekly) is not adequate to prevent Nocardia infection [35]. However, the dosage used for melioidosis prophylaxis is higher, at 800/160 mg daily. From 2015, daily trimethoprim-sulfamethoxazole prophylaxis for melioidosis has been recommended for all hemodialysis and immunocompromised patients in our setting [36]. We speculate that the reduction in the proportion of Nocardia cases occurring in immunocompromised patients in this 2014–2018 study (18%) compared with our previous 1997–2014 cohort study (36%) may be a reflection of wider routine use of higher-dose trimethoprim-sulfamethoxazole prophylaxis for melioidosis in chemotherapy and hemodialysis populations. This study also highlights the incidence of nocardiosis in patients for whom chemoprophylaxis is not recommended because of risk factors including chronic lung disease, hazardous alcohol, and diabetes.
The limitations of this study include its retrospective nature. Although being a single-center study in a particular geographic region may limit its generalizability, the patient population was unselected and included a range of nonsevere and severe manifestations. Although numbers were relatively small, it is still the largest series to date of linezolid use in nocardiosis. These results support the empirical use of linezolid for the treatment of moderate to severe nocardiosis and have informed and support the 2019 Australian Therapeutic Guidelines recommendations for empirical treatment of Nocardia infections (Box 1) [18]. Further studies are needed to assess the safety of longer treatment courses of linezolid and the role of TDM to guide dosage.
Acknowledgments
We thank colleagues in the Infectious Diseases Department and Clinical Microbiology Laboratory at Royal Darwin Hospital.
Financial support. This work was supported by the National Health and Medical Research Council of Australia (Fellowships to N.M.A. [1135820] and M.J.G. [1138860]).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Transparency declarations. None to declare.
References
- 1. Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog 2018; 114:369–84. [DOI] [PubMed] [Google Scholar]
- 2. McGuinness SL, Whiting SE, Baird R, et al. Nocardiosis in the tropical Northern Territory of Australia, 1997–2014. Open Forum Infect Dis 2016; 3(4):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sorell TC MItchell DH, Iredell JR, Chen SC. Nocardia spp. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 8th ed.Philadelphia: Elsevier; 2016:2853. [Google Scholar]
- 4. Peleg AY, Husain S, Qureshi ZA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis 2007; 44:1307–14. [DOI] [PubMed] [Google Scholar]
- 5. Mamelak AN, Obana WG, Flaherty JF, Rosenblum ML. Nocardial brain abscess: treatment strategies and factors influencing outcome. Neurosurgery 1994; 35:622–31. [DOI] [PubMed] [Google Scholar]
- 6. Paige EK, Spelman D. Nocardiosis: 7-year experience at an Australian tertiary hospital. Intern Med J 2019; 49:373–9. [DOI] [PubMed] [Google Scholar]
- 7. Brown-Elliott BA, Ward SC, Crist CJ, et al. In vitro activities of linezolid against multiple Nocardia species. Antimicrob Agents Chemother 2001; 45:1295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moylett EH, Pacheco SE, Brown-Elliott BA, et al. Clinical experience with linezolid for the treatment of Nocardia infection. Clin Infect Dis 2003; 36:313–8. [DOI] [PubMed] [Google Scholar]
- 9. De La Cruz O, Minces LR, Silveira FP. Experience with linezolid for the treatment of nocardiosis in organ transplant recipients. J Infect 2015;70:44–51. [DOI] [PubMed] [Google Scholar]
- 10. Conville PS, Brown-Elliott BA, Smith T, Zelazny AM. The complexities of Nocardia taxonomy and identification. J Clin Microbiol 2018; 56:e01419–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CLSI. Susceptibility testing of mycobacteria, Nocardia spp. and other aerobic actinomycetes; approved standard. In: Woods G, ed. CLSI Document M24. 3rd ed Wayne, PA: Clinical and Laboratory Standards; Institute; 2018:45–52. [PubMed] [Google Scholar]
- 12. Begg EJ, Barclay ML, Duffull SB. A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol 1995; 39:605–9. [PMC free article] [PubMed] [Google Scholar]
- 13. Pea F, Furlanut M, Cojutti P, et al. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother 2010; 54:4605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown-Elliott BA, Biehle J, Conville PS, et al. Sulfonamide resistance in isolates of Nocardia spp. from a US multicenter survey. J Clin Microbiol 2012; 50:670–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uhde KB, Pathak S, McCullum I Jr, et al. Antimicrobial-resistant Nocardia isolates, United States, 1995–2004. Clin Infect Dis 2010; 51:1445–8. [DOI] [PubMed] [Google Scholar]
- 16. Zhao P, Zhang X, Du P, et al. Susceptibility profiles of Nocardia spp. to antimicrobial and antituberculotic agents detected by a microplate Alamar Blue assay. Sci Rep 2017; 7:43660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Restrepo A, Clark NM; Infectious Diseases Community of Practice of the American Society of Transplantation Nocardia infections in solid organ transplantation: guidelines from the infectious diseases community of practice of the American Society of Transplantation. Clin Transplant 2019; 33:e13509. [DOI] [PubMed] [Google Scholar]
- 18.Mandell GL, Bennett JE, Dolin R, eds.; Antibiotic Expert Group. Nocardiosis. eTG Complete. 2019 Available at: https://tgldcdp.tg.org.au/viewTopic? topicfile=nocardiosis&guidelineName=Antibiotic&topicNavigation=navigate Topic#toc_d1e78. Accessed 6 January 2019. [Google Scholar]
- 19. Myrianthefs P, Markantonis SL, Vlachos K, et al. Serum and cerebrospinal fluid concentrations of linezolid in neurosurgical patients. Antimicrob Agents Chemother 2006; 50:3971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mwandia G, Polenakovik H. Nocardia spp. pneumonia in a solid organ recipient: role of linezolid. Case Rep Infect Dis 2018; 2018:1749691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lerner PI. Nocardiosis. Clin Infect Dis 1996; 22:891–903; quiz 904–5. [DOI] [PubMed] [Google Scholar]
- 22. Clark NM, Reid GE; AST Infectious Diseases Community of Practice Nocardia infections in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):83–92. [DOI] [PubMed] [Google Scholar]
- 23. Pea F, Cojutti P, Pagotto A, et al. Successful long-term treatment of cerebral nocardiosis with unexpectedly low doses of linezolid in an immunocompromised patient receiving complex polytherapy. Antimicrob Agents Chemother 2012; 56:3438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolhuis MS, Akkerman OW, Sturkenboom MGG, et al. Linezolid-based regimens for multidrug-resistant tuberculosis (TB): a systematic review to establish or revise the current recommended dose for TB treatment. Clin Infect Dis 2018; 67:327–35. [DOI] [PubMed] [Google Scholar]
- 25. WHO. WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 26. Song T, Lee M, Jeon HS, et al. Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMed 2015; 2:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cattaneo D, Alffenaar JW, Neely M. Drug monitoring and individual dose optimization of antimicrobial drugs: oxazolidinones. Expert Opin Drug Metab Toxicol 2016; 12:533–44. [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto K, Shigemi A, Takeshita A, et al. Analysis of thrombocytopenic effects and population pharmacokinetics of linezolid: a dosage strategy according to the trough concentration target and renal function in adult patients. Int J Antimicrob Agents 2014; 44:242–7. [DOI] [PubMed] [Google Scholar]
- 29. Nukui Y, Hatakeyama S, Okamoto K, et al. High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J Antimicrob Chemother 2013; 68:2128–33. [DOI] [PubMed] [Google Scholar]
- 30. Crass RL, Cojutti PG, Pai MP, Pea F. Reappraisal of linezolid dosing in renal impairment to improve safety. Antimicrob Agents Chemother 2019; 63:e00605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cattaneo D, Orlando G, Cozzi V, et al. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with gram-positive infections. Int J Antimicrob Agents 2013; 41:586–9. [DOI] [PubMed] [Google Scholar]
- 32. Pea F, Viale P, Cojutti P, et al. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother 2012; 67:2034–42. [DOI] [PubMed] [Google Scholar]
- 33. Bolhuis M. Treatment of multidrug-resistant tuberculosis using therapeutic drug monitoring: first experiences with sub-300 mg linezolid dosages using in-house made capsules. Eur Respir J 2019; 54:0903–1936. [DOI] [PubMed] [Google Scholar]
- 34. Marshall C. Opportunistic Infetions Prevention in Patients on Immunosupression TEHS Guideline. Darwin, Northern Territory: Royal Darwin Hospital; 2017. [Google Scholar]
- 35. Hemmersbach-Miller M, Stout JE, Woodworth MH, et al. Nocardia infections in the transplanted host. Transpl Infect Dis 2018; 20:e12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Majoni SW, Hughes JT, Heron B, Currie BJ. Trimethoprim+sulfamethoxazole reduces rates of melioidosis in high-risk hemodialysis patients. Kidney Int Rep 2018; 3:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]