Abstract
Objectives:
Brain maturation differs depending on the area of the brain and sex. Girls show an earlier peak in maturation of the prefrontal cortex. Although differences between adult females and males with schizophrenia have been widely studied, there has been less research in girls and boys with psychosis. The purpose of this study was to examine differences in verbal and visual memory, verbal working memory, auditory attention, processing speed, and cognitive flexibility between boys and girls.
Methods:
We compared a group of 80 boys and girls with first-episode psychosis to a group of controls.
Results:
We found interactions between group and sex in verbal working memory (p = 0.04) and auditory attention (p = 0.01). The female controls showed better working memory (p = 0.01) and auditory attention (p = 0.001) than males. However, we did not find any sex differences in working memory (p = 0.91) or auditory attention (p = 0.93) in the psychosis group.
Conclusions:
These results are consistent with the presence of sex-modulated cognitive profiles at first presentation of early-onset psychosis.
Keywords: Adolescents: schizophrenia, gender differences, cognitive neuroscience, psychosis
Introduction
Early-onset psychotic disorder is associated with more disability and impairment than adult onset of the disease.1,2 It is known that schizophrenia can lead to delays in brain maturation and cognition.3 White et al.4 reported cessation of development in specific cognitive domains following the onset of schizophrenia. This arrested development was more evident in adolescence, and children with psychosis exhibited worse motor function than adults; however, control children had better motor function than control adults.
Despite many studies on differences in cognitive function between males and females, consensus is still lacking. Bozikas et al.5 found that the degree of cognitive impairment is similar for male and female patients with schizophrenia. Similarly, Hoff et al.6 reported that females with schizophrenia performed better than males with schizophrenia in visual memory, but the difference did not remain after adjusting for symptom severity. Roesch-Ely et al.7 found no difference in working memory between females and males in either controls or patients with schizophrenia, whereas females with schizophrenia performed worse in attention tasks than males with schizophrenia. Goldstein et al.8 reported that females with schizophrenia were less vulnerable to particular cognitive deficits than males, especially in verbal processing. The explanations for these differences in results include the following: 1) differences in age at onset, 2) differences in severity of negative and disorganization symptoms in males with psychosis, and 3) disease chronicity.9 In addition, the inclusion of different categories of psychosis (affective, schizophrenia, and other psychoses) could play a role in this lack of consensus about sex differences in cognitive functions. Fitzgerald et al.10 compared early-onset affective psychosis and early-onset schizophrenia (EOS), and patients with schizophrenia appeared to have more generalized impairment across cognitive functions. However, Zabala et al.,11 in a sample of adolescent psychosis, found no differences in terms of cognitive function impairment among schizophrenia, affective psychosis, and other psychoses.
One way of solving this limitation is to study subjects with similar ages at onset, e.g., in early-onset psychosis (EOP). A study with this design could also explore how psychosis modifies brain function. Sex differences in brain development appear to be age-dependent, and prefrontal cortex maturation has been shown to peak earlier in girls than in boys.12 This difference in maturation between sexes could explain differences in the neuropsychological functioning between boys and girls.13
The following factors were considered: first, schizophrenia is associated with delayed brain maturation4,14; second, the prefrontal cortex is the area of the brain that matures latest12; and third, girls show an earlier peak of prefrontal cortex maturation and score better on working memory tasks than boys.15,16 We hypothesized that schizophrenia might modify brain maturation, affecting those areas of the brain that develop later, such as the prefrontal cortex. We would therefore expect to find sex differences in prefrontal cortex-related cognitive function (specifically, working memory) in control subjects with normal brain development; however, we would expect to find no sex differences in working memory associated with the prefrontal cortex as a consequence of delayed brain development in subjects with schizophrenia.
The purpose of this study was to examine differences in cognitive function between boys and girls in a group of adolescents with first-episode psychosis compared to a control group.
Material and methods
Subjects
The sample comprised 80 patients with first-episode EOP, aged 9-17 years, who had been admitted to five Andalusian Child and Adolescent Mental Health Units (Jaen, Granada, Almeria, Cordoba, and Seville), recruited from June 2006 to December 2008. The sample included 25 subjects (31%) diagnosed with affective psychosis and 55 (69%) with non-affective psychosis (Table 1).
Table 1. Demographic and clinical characteristics.
| Psychosis (n=80) | Controls (n=39) | p-value | |
|---|---|---|---|
| Sex | |||
| Male | 47 (59) | 18 (46) | 0.19 |
| Female | 33 (41) | 21(54) | 0.20 |
| Facility | |||
| Hospital Universitario Virgen Macarena (Seville) | 7 (9) | ||
| Hospital Universitario Reina Sofía (Cordoba) | 12 (15) | ||
| Complejo Hospitalario Torrecárdenas (Almeria) | 12 (15) | ||
| Hospital Universitario Virgen de las Nieves (Granada) | 10 (12) | 15 (39) | |
| Hospital Neurotraumatológico (Jaen) | 39 (49) | 24 ( 61) | |
| Diagnosis | |||
| Affective psychosis | 25 (31) | - | |
| Depressive disorder with psychotic symptoms | 10 (12.5) | ||
| Bipolar disorder, manic episode with psychotic symptoms | 15 (18.5) | ||
| Non-affective psychosis | 55 (69) | ||
| Psychotic disorder not otherwise specified | 22 (27) | ||
| Schizophreniform disorder | 2 (2.5) | ||
| Schizophrenia | 30 (37.5) | ||
| Other psychotic disorders | 1 (1) | ||
| Father’s education | |||
| No formal education | 31 (39) | 5 (13) | 0.009 |
| Primary or secondary education diploma | 43 (54) | 32 (82) | |
| University | 6 (7) | 2 (5) | |
| Mother’s education | |||
| No formal education | 33 (41) | 3 (8) | 0.001 |
| Primary or secondary education diploma | 37 (46) | 30 (77) | |
| University | 10 (12) | 6 (15) | |
| Age, mean (SD) | 16.5 (3.3) | 16.5 (1.8) | 0.10 |
| IQ, mean (SD) | 73.3 (17.0) | 93.8 (8.5) | 0.001 |
| GAF, mean (SD) | 57.8 (16.3) | ||
| Antipsychotic dose as chlorpromazine equivalents (mg), mean (SD) | 261.7 (163.3) | - | |
| PANSS, mean (SD) | |||
| PANSS-positive | 19.5 (7.5) | - | |
| PANSS-negative | 18.3 (9.5) | ||
| PANSS-general | 32.7 (10.0) | ||
| Cognitive tasks, mean (SD) | |||
| Verbal memory | |||
| Immediate (WMS-III) | 27.7 (7.8) | 35.8 (4.7) | 0.0001 |
| Delayed (WMS-III) | 6.6 (2.7) | 9.4 (1.4) | 0.0001 |
| Recognition (WMS-III) | 21.7 (2.5) | 23.6 (0.6) | 0.001 |
| Simple Rey-Osterrieth Complex figure test | 12.0 (7.7) | 20.3 (11.3) | 0.0001 |
| Verbal working memory (backward digit span) | 4.9 (1.8) | 6.5 (1.7) | 0.0001 |
| Attention (digit span) | 7.5 (1.9) | 9.0 (2.0) | 0.0001 |
| Processing speed (digit symbol-coding) | 46.8 (12.2) | 62.1 (14.1) | 0.0001 |
| Flexibility (Stroop Word-Color Test) | 31.8 (11.1) | 41.5 (10.7) | 0.0001 |
Data presented as n (%), unless otherwise specified.
GAF = Global Assessment of Functioning Scale; IQ = intelligence quotient; PANSS = Positive and Negative Syndrome Scale; SD = standard deviation; WMS-III = Wechsler Memory Scale - Third Edition.
First-episode psychosis was defined as presence of the following symptoms for at least 1 week: hallucinations, delusions, severe thought disorder, psychomotor disorder, or bizarre behavior. The exclusion criteria were a history of head trauma with loss of consciousness for > 1 hour, intelligence quotient (IQ) < 70, or a major neurological or somatic disorder with neurological components.
Patients were diagnosed after a full psychiatric examination using the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) semi-structured interview.17 A senior clinician (MR-V) trained in K-SADS made the diagnosis without knowledge of any other study results (such as neuropsychological testing). The diagnosis was confirmed 12 months later by the same clinician. Cognitive assessment was performed by a trained neuropsychologist (MDSM). Although blinding this investigator to patient status was not possible, he did not conduct the diagnostic interviews. Cognitive assessment was performed at 4 or 8 weeks from recruitment for outpatients or at the end of inpatient care (4 weeks after discharge).
Absence of previous psychotic symptoms or intake of antipsychotic medication was confirmed following a systematic assessment of the patients and their families, who acted as collateral informants.
Thirty-nine healthy controls were recruited from general practitioners (GP). The inclusion criteria were: age 9 to 17 years, IQ > 70, no psychiatric disorder as measured by the K-SADS, and no neurological disorder (DSM-IV).
The local clinical research ethics committees in Jaen, Cordoba, Seville, Almeria, and Granada approved the research protocol. All patients and their legal guardians provided written informed consent.
Neuropsychological evaluation
Several studies have demonstrated that psychotic patients exhibit cognitive impairment in auditory attention, verbal memory, auditory and verbal memory, processing speed, and executive function.18,19 The neuropsychological battery employed in this study was designed to address the following domains: IQ, verbal memory, verbal working memory, auditory attention, processing speed, premorbid IQ, executive function, and visual memory.
Intelligence quotient (IQ) was estimated with the complete WISC-IV (≤ 17 years).20 Verbal memory was measured with the Wechsler Memory Scale – Third Edition (WMS-III), covering logical memory (immediate), logical memory (delayed), and logical memory (recognition)21; for verbal working memory, the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) backward digit span test was used.18 Auditory attention was measured with the WISC-IV digit span test20; processing speed, by digit symbol coding (WISC-IV).20 Premorbid IQ was measured with the vocabulary subtest of the WISC-IV.20 With regard to executive function, inhibition was assessed with the Stroop Word-Color Test, and the variable of interest was the number of incongruent-color words read by the subject.22 Finally, the Spanish version of the Rey Complex Figure Test (RCF-II)23 with a previously copied figure was used to evaluate visual memory. The variables were the direct scores showing accuracy and detail of figure reproduction.
Global functioning, antipsychotic medication, and psychopathology
All antipsychotic doses were converted to chlorpromazine equivalents.24
Global functioning was evaluated with the Global Assessment of Functioning (GAF) scale.25 Psychopathology was assessed with the Spanish version of the Positive and Negative Syndrome Scale (PANSS),26 which provides a standardized method for assessing 30 psychiatric items on a seven-point scale. The negative, positive, and general psychopathology factors of the PANSS were calculated.
Data analysis
The sociodemographic variables of interest (sex, parent education) were analyzed using the chi-square test, whereas continuous variables were compared with Student’s t-test for independent samples. All were checked for normality (Kolmogorov-Smirnov test) and homoscedasticity (Levene’s test) prior to analysis. All variables were found to have a normal distribution.
To compare patients and controls, z scores for the entire sample were calculated using the mean and standard deviation (SD) of the raw test data of the healthy control group for each version of the cognitive test used.25 All z scores were computed so that higher values indicated better performance. Following z score standardization, tests for normality in the control group were explored to identify the non-normally distributed test variables. We used the raw test data to explore sex-group interactions.
Differences in cognitive domains between the two groups were found by Student’s t-test. A linear model analysis was used to analyze the effect of sex on the sample, as well as to test for interaction between sex and group. A second analysis was adjusted for maternal and paternal education, age, and premorbid IQ. These results are presented with and without Bonferroni correction for multiple tests.27 This correction was used to adjust the level of significance for each test by dividing the critical level of significance, 0.05 (5%), by the number of significance tests performed. In our case, eight cognitive tests were performed; thus, the new critical level of significance after Bonferroni’s correction was 0.006 (0.05/8). All analyses were conducted using SPSS version 15.0 statistical package.
Results
Sample description
Eighty subjects with psychosis and 39 healthy controls were recruited. There were no differences in sex between the two groups (p = 0.19). Fifty-five subjects (69%) in the psychosis group were diagnosed with non-affective psychosis. There were no significant differences in age between the two groups (psychosis group = 15.8±2.6, and control group = 16.5±3.3; p = 0.10). However, differences in IQ between the two groups were significant (psychosis group = 73.3±17.0; control group = 93.8±8.5; p = 0.0001). The parents of the patients in the psychosis group had less formal education. Twenty-four (36%) of the fathers and 28 (42%) of the mothers of the psychosis group had had no formal education. These differences were significant both for fathers (p = 0.03) and for mothers (p = 0.001) (Table 1). All of the patients received antipsychotics. The mean dose of the antipsychotic medication was 261.7±163.3 chlorpromazine equivalents, and the duration of antipsychotic treatment was less than 6 months.
We found no difference in age, IQ, GAF, PANSS score, or chlorpromazine dose between girls and boys in the psychosis group. There was a higher ratio of female patients (14 [42%]) than male patients with affective psychosis (11 [23%]; p = 0.07) (Table 2).
Table 2. Demographic and clinical characteristics of study patients and controls.
| Psychosis | Controls | |||||
|---|---|---|---|---|---|---|
| Boys (n=47) | Girls (n=33) | p-value | Boys (n=18) | Girls (n=21) | p-value | |
| Age (years) | 16.1 (2.6) | 15.3 (2.6) | 0.20 | 16.5 (3.7) | 16.5 (3.1) | 0.98 |
| IQ | 72.3 (17.6) | 75.7 (16) | 0.38 | 95.5 (7.4) | 95.2 (9.8) | 0.34 |
| GAF | 56.6 (15.0) | 59.9 (18.1) | 0.46 | - | - | |
| Chlorpromazine equivalents (mg) | 262.6 (173.3) | 260.5 (152) | 0.96 | - | - | |
| PANSS | ||||||
| PANSS-positive | 20.0 (8.6) | 19.1 (6.8) | 0.75 | - | - | |
| PANSS-negative | 18.7 (10.2) | 18.0 (9.1) | 0.80 | - | - | |
| PANSS-general | 31.5 (9.9) | 33.8 (10.3) | 0.49 | - | - | |
| Affective psychosis, n (%) | 12 (23) | 14 (42) | 0.07 | - | - | |
| Father’s education, n (%) | ||||||
| No formal education | 21 (45) | 10 (30) | 0.33 | 3 (17) | 2 (9) | 0.79 |
| Primary or secondary education diploma | 22 (47) | 21 (64) | 14 (78) | 18 (86) | ||
| University | 4 (8) | 2 (6) | 1 (6) | 1 (5) | ||
| Mother’s education, n (%) | ||||||
| No formal education | 19 (40) | 14 (42) | 0.08 | 2 (11) | 1 (5) | 0.63 |
| Primary or secondary education diploma | 19 (40) | 18 (55) | 14 (78) | 16 (76) | ||
| University | 9 (20) | 1 (3) | 2 (11) | 4 (19) | ||
Data presented as mean (standard deviation), unless otherwise specified.
GAF = Global Assessment of Functioning Scale; IQ = intelligence quotient; PANSS = Positive and Negative Syndrome Scale.
Effect of group (psychosis vs. control) and sex on cognitive function
Table 1 summarizes group raw scores on the individual cognitive tests. The psychotic patients were significantly more impaired than the healthy control subjects on every scale.
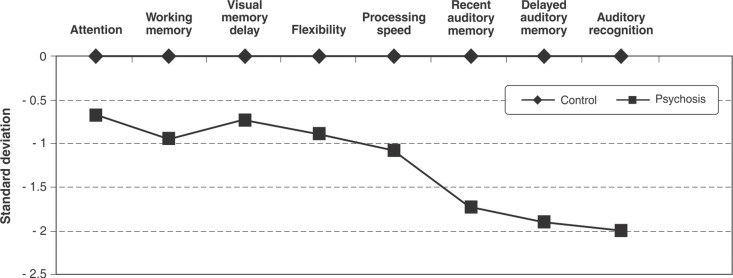
The z score profile on the cognitive battery is presented in Figure 1. There was a clear difference in performance pattern across groups. The percentages of patients and controls that performed one and two SDs below the mean of the controls are presented in Table 3.
Figure 1. Neuropsychological profile stratified by group.
Table 3. Percentage of patients and healthy controls who performed one and two standard deviations (SDs) below the mean of the control group.
| Psychosis (n=80) | Controls (n=39) | |||
|---|---|---|---|---|
| -1 SD* | -2 SD | -1 SD* | -2 SD | |
| Verbal memory: Immediate (WMS-III) | 22.5 (18) | 23 (29) | 1 (2.5) | 4 (10) |
| Verbal memory: Delayed (WMS-III) | 8 (10) | 30 (37) | 3 (8) | 1 (3) |
| Verbal memory: Recognition (WMS-III) | 0 (0) | 27 (33) | 0 (0) | 3 (8) |
| Visual memory SROCFT: Copy score | 17 (21.5) | 0 (0) | 0 (0) | 0 (0) |
| Verbal working memory: Backward digit span | 18 (22.5) | 18 (22.5) | 0 (0) | 6 (15) |
| Auditory attention: Digit span | 12 (15) | 4 (5) | 2 (10) | 1 (2.5) |
| Processing speed: Digit symbol-coding | 9 (11) | 3 (4) | 0 (0) | 0 (0) |
| Flexibility: Stroop Word-Color Test | 21 (26) | 12 (15) | 3 (8) | 1 (3) |
Data presented as n (%).
SD = standard deviation; SROCFT = Simple Rey-Osterrieth Complex Figure Test; WMS-III = Wechsler Memory Scale - Third Edition.
≥ -1 SD and < -2 SD.
Differences between girls and boys overall and within sex groups separately for each cognitive test are displayed in Table 4. In the overall sample (psychosis and controls), girls outperformed boys only in the digit span trials (7.5±1.7 vs. 8.6±2.2; p = 0.001). In the backward digit span, girls performed better than boys, but the difference was not significant (5.1±1.7 vs. 5.7±2.1; p = 0.06). A similar trend was also found for immediate verbal memory (p = 0.06).
Table 4. Mean raw scores on neuropsychological tests for patients in first-episode early-onset psychosis and healthy controls. Interactions between group and sex.
| Psychosis | Control | Group effect | Sex effect | Sex × group | Adjusted sex × group† | |||
|---|---|---|---|---|---|---|---|---|
| Boys(n=47) | Girls(n=33) | Boys(n=18) | Girls(n=21) | |||||
| Verbal memory: Immediate (WMS-III) | 26.4 (8.5) | 29.7 (6.2) | 34.6 (4.2) | 36.8 (4.9) | F1,96 = 27.2 p = 0.0001 d = 0.22 |
F1,96 = 3.5 p = 0.06 d = 0.003 |
F1,96 = 70 p = 0.70 d = 0.001 |
F1,96 = 0.01 p = 0.98 d = 0.001 |
| Verbal memory: Delayed (WMS-III) | 6.3 (2.8) | 7.0 (2.4) | 9.2 (1.3) | 9.6 (1.5) | F1,96 = 28.4 p = 0.0001 d = 0.22 |
F1,96 = 1.3 p = 0.24 d = 0.01 |
F1,96 = 0.12 p = 0.70 d = 0.01 |
F1,96 = 0.86 p = 0.77 d = 0.001 |
| Verbal memory: Recognition (WMS-III) | 22.3 (2.1) | 22.5 (1.7) | 23.8 (0.14) | 23.5 (0.7) | F1,96 = 11.5 p = 0.001 d = 0.10 |
F1,96=0.006 p = 0.98 d = 0.0001 |
F1,96 = 0.76 p = 0.38 d = 0.008 |
F1,96 = 0.17 p = 0.67 d = 0.002 |
| Visual memory SROCFT: Copy score | 11.4 (7.9) | 13.0 (7.5) | 19.1 (5.6) | 21.3 (14.6) | F1,109=19.1 p = 0.0001 d = 0.015 |
F1,109 = 1.0 p = 0.30 d = 0.10 |
F1,109 = 0.83 p = 0.85 d = 0.0001 |
F1,109 = 2.13 p = 0.14 d = 0.02 |
| Verbal working memory: Backward digit span | 4.9 (1.7) | 4.9 (1.9) | 5.7 (1.3)* | 7.1 (1.7)* | F1,115=19.3 p = 0.0001 d = 0.14 |
F1,115 = 3.5 p = 0.06 d = 0.03 |
F1,115 = 4.1 p = 0.04 d = 0.03 |
F1,115 = 7.86 p = 0.005‡ d = 0.06 |
| Auditory attention: Digit span | 7.9 (1.8) | 7.8 (2.0) | 7.8 (1.6)§ | 10.0 (1.9)§ | F1,115=12.5 p = 0.0001 d = 0.09 |
F1,115=12.2 p = 0.001 d = 0.09 |
F1,115 = 5.9 p = 0.01 d = 0.04 |
F1,115 = 6.6 p = 0.01 d = 0.05 |
| Processing speed: Digit symbol-coding | 46.3 (15.3) | 47.6 (10.2) | 58.1 (13.3) | 65.5 (14.3) | F1,115 = 22.5 p = 0.0001 d = 0.19 |
F1,115 = 2.4 p = 0.12 d = 0.002 |
F1,115 = 1.2 p = 0.26 d = 0.001 |
F1,115 = 0.68 p = 0.41 d = 0.006 |
| Flexibility: Stroop Word-Color Test | 33.4 (12.1) | 29.7 (9.4) | 42.4 (9.1) | 40.7 (12.1) | F1,111 = 20.9 p = 0.0001 d = 0.16 |
F1,111 = 1.4 p = 0.23 d = 0.01 |
F1,111 = 0.18 p = 0.67 d = 0.002 |
F1,111 = 0.04 p = 0.81 d = 0.0001 |
SROCFT = Simple Rey-Osterrieth Complex Figure Test; WMS-III = Wechsler Memory Scale - Third Edition.
d = eta squared. Using Cohen’s d criteria (1988): small effect = 0.01; moderate effect = 0.06; large effect = 0.14.
Difference between boys and girls in the control group (p < 0.001).
Adjusted for parent education, age, and premorbid IQ (vocabulary subtest).
The critical level of significance after Bonferroni correction was 0.05/8 = 0.006.
Difference between boys and girls in the control group (p < 0.05).
Considering each group separately, there were sex differences in the control group in the backward and forward digit span tests. Specifically, girls performed better than boys in the backward (p = 0.01) and forward (p = 0.001) digit span tests. In these two measures, there were no differences between boys and girls in the psychosis group (backward digit span, p = 0.9; forward digit span, p = 0.9).
We found a significant interaction effect between sex and group in the forward (p = 0.01) and backward (p = 0.04) digit span tests (Table 4). These interactions remained significant after controlling for vocabulary subtest, age, and paternal and maternal educational level (forward digit span, p = 0.01; backward digit span, p = 0.005). The interaction in the backward digit span test was significant after correction for multiple testing (Bonferroni, p = 0.005). However, interaction in the forward digit span test was no longer significant after correction for multiple testing (Bonferroni, p = 0.08) (Table 4).
Discussion
Our results demonstrated a cognitive deficit in EOP patients at first presentation compared to healthy controls. We also found an interaction between group and sex effects on verbal working memory and auditory attention (measured with backward and forward digit span rests). Whereas girls in the control group performed significantly better on verbal working memory and auditory attention tests than control boys, we found no sex differences in these cognitive domains in the psychosis groups. These findings suggest that there are sex-specific cognitive function abnormalities that are already present in patients with first-episode EOP.3,28 Specifically, less impairment in verbal working memory and auditory attention was present only in girls with EOP.
Adolescents at high risk of developing psychosis fail to show typical age-related increases in white matter integrity in tracts linking the prefrontal and posterior cortex. This aberrant development of white matter predicts functional impairment.28 Similarly, Douaud et al.29 showed that schizophrenia in adolescence delays and alters brain maturation. This delay in maturity could explain the lack of sex differences observed in the psychosis groups in our study. However, we were unable to draw a conclusion on the presence of a delay in brain maturity, as longitudinal data would have been required to support such an assertion. As our study is cross-sectional, we can only conclude that sex-specific abnormalities in cognitive function were present.
Bombin et al.30 described the profile of neuropsychological deficits associated with the first episode of EOP. They concluded that healthy controls and EOP patients had improved after 2 years of follow-up on all cognitive measures tested except for the working memory test. The authors also concluded that “cognitive development seems to be arrested early in early-onset psychosis patients compared to their healthy peers,” at least for cognitive functions such as working memory. However, the authors of that study did not disaggregate their results by sex.
Some studies have reported sex differences in the cognitive functions of adults with schizophrenia,28,31,32 suggesting that females perform better than males in executive function, visual working memory, verbal memory, and learning tasks. Similarly, Hafner33 showed, in an adult-onset sample, that male sex is associated with a poor outcome and greater cognitive deficit. However, we did not find such a difference between girls and boys with psychosis. The finding of a worse outcome in adult male patients is often contaminated by the fact that males typically are younger at onset, which is frequently insidious. By studying subjects with similar ages at onset, e.g., in EOS, we were able to overcome this limitation.34,35
The lack of coincidence in studies of cognitive differences between sexes in adult- and adolescent-onset psychosis could be because females who develop psychosis during adolescence probably have a higher load of genetic or environmental factors leading to the development of psychosis despite protective factors such as estrogens.36
Many studies have investigated the difference between females and males in adult-onset schizophrenia; however, we found only one investigation concerning cognitive differences between girls and boys with EOP.37 In that study, boys with psychosis were found to have more overall cognitive impairment than girls. These findings confirmed the possibility of greater developmental problems in patients.31 Our study did not replicate the results of Brickman et al.,37 but rather found that girls with psychosis had a more severe cognitive deficit in such functions as working memory and auditory attention than girls in the control group. Brickman et al.37 found that subjects with EOP showed greater impairment in memory, executive function, and attention. These results are somewhat different from ours. We found greater impairment only in memory and, to a lesser degree, in flexibility and working memory. This incongruence could be explained by differences in medication and clinical state. Our patients were in treatment, while Brickman et al. reported on patients who were treatment-naive and relatively ill.
Taking these differences into account, one might hypothesize that patients with EOP, especially boys, respond better to antipsychotics in terms of attention and flexibility performance. Memory was not affected by antipsychotics, but more research with a prospective design is required to confirm this. Several studies have reported that antipsychotics affect grey matter and working memory. Davidson et al.38 showed that antipsychotic medication improved the cognitive test performance of patients with schizophrenia. Szesko et al.39 reported that the use of antipsychotics might be associated with a subtle loss of GM integrity. Other studies have associated GM integration with working memory performance in schizophrenia patients.40 In this sense, the sex effects found in our study might be explained by differences in antipsychotic doses. However, we found no difference in chlorpromazine-equivalent doses between girls and boys.
This study is clinically relevant in that cognitive deficits are present at the onset of a first episode of psychosis.11 As neurocognition is associated with social functioning,39 some interventions, such as cognitive remediation, focus on specific cognitive domains. Depending on the age at onset and sex, this type of therapy could improve functioning in patients with EOP. Assessments based on systematic longitudinal studies will be necessary to determine the significance of cognitive impairment for prognosis.
This study has some limitations. First, its cross-sectional design precludes the drawing of conclusions regarding cognitive deficit trajectories. Another limitation is the lack of assessment of the duration of untreated psychosis (DUP). Although results are inconsistent, some studies have found an association between a longer DUP and greater severity of cognitive deficits in patients with psychosis.41 There were more female than male patients with affective psychosis in our sample, and some studies have reported better performance in cognition in affective psychosis than in schizophrenia.10 To control for this limitation, we adjusted for sex. Another limitation is that the absence of a nonpsychotic disorder group prevented us from drawing any conclusions regarding the specificity of the cognitive deficits seen in EOP. Finally, although our healthy control sample was matched for age and sex, it was relatively small, and patients were recruited from only two of the healthcare districts covered by the study (Granada and Jaén).
In this study, patients with EOP displayed wide-ranging performance impairments compared to healthy controls. Our findings suggest that there are sex-specific abnormalities in cognitive function in patients with EOP. Specifically, the sex differences observed in control subjects (verbal working memory and auditory attention) were not found between boys and girls in the psychosis group.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
This study was supported by Estancia Formativa de la Junta de Andalucia 2010, Investigacion Biomedica y en Ciencias de Salud en Andalucia 2007 (grant BAE 09/90088).
References
- 1.Schmidt M, Blanz B, Dippe A, Koppe T, Lay B. Course of patients diagnosed as having schizophrenia during first episode occurring under age 18 years. Eur Arch Psychiatry Clin Neurosci. 1995;245:93–100. doi: 10.1007/BF02190735. [DOI] [PubMed] [Google Scholar]
- 2.Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry. 2012;12:150. doi: 10.1186/1471-244X-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schimmelman BG, Shultze-Lutter F. Early detection and the intervention of psychosis in children and adolescent: urgent need for studies. Eur Child Adolesc Psychiatry. 2012;21:239–41. doi: 10.1007/s00787-012-0271-z. [DOI] [PubMed] [Google Scholar]
- 4.White T, Ho BC, Ward J, O´Leary D, Andreasen NC. Neuropsychological performance in first episode adolescent with schizophrenia: a comparison with First- Episode adult and adolescent control subjects. Biol Psychiatry. 2006;60:463–71. doi: 10.1016/j.biopsych.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Bozikas VP, Kosmidis MH, Peltekis A, Giannakou M, Nimatoudis I, Karavatos A, et al. Sex differences in neuropsychological functioning among schizophrenia patients. Aust N Z J Psychiatry. 2010;44:333–41. doi: 10.3109/00048670903489833. [DOI] [PubMed] [Google Scholar]
- 6.Hoff AL, Wieneke M, Faustman WO, Horon R, Sakuma M, Blankfeld H, et al. Sex differences in neuropsychological functioning of first-episode and chronically ill schizophrenic patients. Am J Psychiatry. 1998;155:1437–9. doi: 10.1176/ajp.155.10.1437. [DOI] [PubMed] [Google Scholar]
- 7.Roesch-Ely D, Hornberger E, Weiland S, Hornstein C, Parzer P, Thomas C, et al. Do sex differences affect prefrontal cortex associated cognition in schizophrenia? Schizophr Res. 2009;107:255–61. doi: 10.1016/j.schres.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, et al. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry. 1998;155:1358–64. doi: 10.1176/ajp.155.10.1358. [DOI] [PubMed] [Google Scholar]
- 9.Crow TJ. “Just the facts” of schizophrenia in the context of human evolution: commentary on Keshavan et al. 2011. Schziophr Res. 2011;129:205–7. doi: 10.1016/j.schres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald D, Lucas S, Redoblado MA, Winter V, Brennan J, Anderson J, et al. Cognitive functioning in young people with first episode psychosis: relationship to diagnosis and clinical characteristics. Aust N Z J Psychiatry. 2004;38:501–10. doi: 10.1080/j.1440-1614.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 11.Zabala A, Rapado M, Arango C, Robles O, de la Serna E, González C, et al. Neuropsychological functioning in early-onset first-episode psychosis: comparison of diagnostic subgroups. Eur Arch Psychiatry Clin Neurosci. 2010;260:225–33. doi: 10.1007/s00406-009-0046-9. [DOI] [PubMed] [Google Scholar]
- 12.Marsh R, Gerber AJ, Peterson MD. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:1233–51. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozikas VP, Andreu C. Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust N Z J Psychiatry. 2011;45:93–108. doi: 10.3109/00048674.2010.541418. [DOI] [PubMed] [Google Scholar]
- 14.Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–12. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 15.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speck O, Emst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the funcional organization of the brain for working memory. Neuroreport. 2000;11:2581–5. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- 17.Ulloa RE, Ortiz S, Higuera F, Nogales I, Fresán A, Apiquian R, et al. [Interrater reliability of the Spanish versión of Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime version (K-SADS-PL)]. Actas Esp Psiquiatr. 2006;34:36–40. [PubMed] [Google Scholar]
- 18.de la Serna E, Mayoral M, Baeza I, Arango C, Andrés P, Bombín I, et al. Cognitive functioning in children and adolescents in their first episode of psychosis: differences between previous cannabis users and nonusers. J Nerv Ment Dis. 2010;198:159–62. doi: 10.1097/NMD.0b013e3181cc0d41. [DOI] [PubMed] [Google Scholar]
- 19.Robles O, Zabala A, Bombín I, Parellada M, Moreno D, Ruiz-Sancho A, et al. Cognitive efficacy of quetiapine and olanzapine in early-onset first-episode psychosis. Schizophr Bull. 2011;37:405–15. doi: 10.1093/schbul/sbp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corral S, Arribas D, Santamaría P, Sueiro MJ, Pereãa J. WISC-IV, Escala de Inteligencia de Weschler para Niãos. Cuarta Edición. Adaptación Española. Madrid: TEA Ediciones; 2007. [Google Scholar]
- 21.Pereãa J, Seisdedos N, Corral S, Arribas D, Santamaría P, Sueiro M. WMS-III, Escala de Memoria de Weschler. Adaptación Espaãola. Madrid: TEA Ediciones; 2004. [Google Scholar]
- 22.Golden C J. Spanish translation and adaptation. 5th ed. Madrid: TEA Ediciones; 2007. Stroop color and word test. [Google Scholar]
- 23.Rey A. The rey complex figure test. Spanish Adaptation. Madrid: TEA Ediciones; 1980. [Google Scholar]
- 24.Fuller A, Sajatovic M. Drug handbook for psychiatry. 5th ed. Hudson: Lexi Comp; 2005. [Google Scholar]
- 25.Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–75. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- 26.Peralta Martín V, Cuesta Zorita MJ. [Validation of positive and negative symptom scale (PANSS) in a sample of Spanish schizophrenic patients]. Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1994;22:171–7. [PubMed] [Google Scholar]
- 27.Sedgwick P. Multiple hypothesis testing and Bonferroni’s correction. BMJ. 2014;349:g6284. doi: 10.1136/bmj.g6284. [DOI] [PubMed] [Google Scholar]
- 28.Seidman LJ, Goldstein JM, Goodman JM, Koren D, Turner WM, Faraone SV, et al. Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relationship to attention and verbal ability. Biol Psychiatry. 1997;42:104–15. doi: 10.1016/S0006-3223(96)00300-9. [DOI] [PubMed] [Google Scholar]
- 29.Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK, et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132:2437–48. doi: 10.1093/brain/awp126. [DOI] [PubMed] [Google Scholar]
- 30.Bombin I, Mayoral M, Castro-Fornieles J, Gonzalez-Pinto A, de la Serna E, Rapado-Castro M, et al. Neuropsychological evidence for abnormal neurodevelopment associated with early-onset psychoses. Psychol Med. 2013;43:757–68. doi: 10.1017/S0033291712001535. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein JM, Seidman LJ, Santangelo S, Knapp PH, Tsuang MT. Are schizophrenic men at higher risk for developmental deficits than schizophrenic women? Implications for adult neuropsychological functions. J Psychiatr Res. 1994;28:483–98. doi: 10.1016/0022-3956(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 32.Halari R, Kumari V, Mehrotra R, Wheeler M, Hines T, Sharma T. The relationship of sex hormones and cortisol with cognitive functioning in schizophrenia. J Psychopharmacol. 2004;18:366–74. doi: 10.1177/026988110401800307. [DOI] [PubMed] [Google Scholar]
- 33.Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 34.McClellan J, Prezbindowski A, Breiger D, McCurry C. Neurpsychological functioning in early onset psychotic disorders. Schizophr Res. 2004;68:21–6. doi: 10.1016/S0920-9964(03)00058-6. [DOI] [PubMed] [Google Scholar]
- 35.Frongou S. Neurocognition in early-onset schizophrenia. Child Adolesc Psychiatr Clin N Am. 2013;22:715–26. doi: 10.1016/j.chc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Wu YC, Hill RA, Gogos A, van den Buuse M. Sex differences and the role of estrogen in animal models of schizophrenia: interaction with BDNF. Neuroscience. 2013;239:67–83. doi: 10.1016/j.neuroscience.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Brickman AM, Buchsmaum MS, Bloom R, Bokhoven P, Paul-Odouard R, Haznedar MM, et al. Neurppsychological functioning in first-break, never medicated adolescents with psychosis. J Nerv Ment Dis. 2004;192:615–22. doi: 10.1097/01.nmd.0000138229.29157.3e. [DOI] [PubMed] [Google Scholar]
- 38.Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, et al. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST). Am J Psychiatry. 2009;166:675–82. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- 39.Szesko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, et al. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neurpsychopharmacology. 2014;39:1324–31. doi: 10.1038/npp.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goghari VM, Macdonald AW, Sponheim SR. Relationship between prefrontal gray matter volumes and working memory performance in schizophrenia: a family study. Schizophr Res. 2014;1:113–21. doi: 10.1016/j.schres.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho BC, Alicata D, Ward J, Moser DJ, O'Leary DS, Arndt S, et al. Untreated initial psychosis: relation to cognitive deficits and brain morphology in first-episode schizophrenia. Am J Psychiatry. 2003;160:142–148. doi: 10.1176/appi.ajp.160.1.142. [DOI] [PubMed] [Google Scholar]