Abstract
Mucosal epithelial cells are infected by a wide variety of pathogens and determining their response to infection is critical for understanding disease pathogenesis. A protocol was developed for culturing primary epithelial cells from fetal bovine intestine and the cultured cells were evaluated for susceptibility to an enteric viral infection. Immunohistochemical staining for cytokeratin confirmed that 60–75% of cultured cells were epithelial cells. Furthermore, following infection with bovine rotavirus (BRV) over 80% of cells in the ileal and jejunal cultures contained viral protein at 16 h post-infection. The intestinal epithelial cell cultures also contained fibroblasts so a jejunal fibroblast culture was established and infected with BRV. Viral protein was detected in jejunal fibroblasts but viral-induced cytopathology was delayed in fibroblast cultures when compared to epithelial cell cultures. This study describes an effective protocol for culturing bovine epithelial cells from fetal intestine and confirmed that the epithelial cells were susceptible to BRV infection. Ileal and jejunal cultures displayed limited growth following continuous passage but early passage epithelial cells provide competent target cells for studying host cell responses to an enteric viral pathogen.
Keywords: Bovine, Bovine rotavirus, Fibroblasts, Intestinal epithelial cells
1. Introduction
Identifying innate immune responses at mucosal surfaces is very important for understanding disease pathogenesis since the majority of infectious pathogens invade through mucosa. The gastrointestinal tract is a major site of entry for various pathogens and these pathogens must attach and/or penetrate through intestinal epithelium. Therefore, having epithelial cell lines greatly facilitates the analysis of host–pathogen interactions.
Many studies have described (reviewed in Kaeffer, 2002) the establishment of short-term intestinal epithelial cell cultures from mice (Booth et al., 1995b, Macartney et al., 2000, Whitehead et al., 1999), rat (Booth et al., 1995a, Evans et al., 1992, Kaeffer et al., 1997, Kaeffer and Briollais, 1998), rabbit (Vidrich et al., 1988) and humans (Gibson et al., 1989, Panja, 2000, Perreault and Beaulieu, 1998, Perreault and Jean-Francois, 1996, Whitehead et al., 1999). Presently, only four studies have reported the establishment of cell cultures from bovine intestinal cells (Birkner et al., 2004, Follmann et al., 2000, Hoey et al., 2003, Rusu et al., 2005). These studies described the generation of epithelial cell cultures from the colon of fetal cattle and the jejunum and colon of adult cattle. The cell lines established from adult cattle may not be relevant for the study of enteric infection, such as by rotavirus or coronavirus, since these viruses infect enterocytes in the small intestine of young calves.
Previous studies with bovine intestinal epithelial cell cultures did not investigate the susceptibility of these cells to enteric viral infection. Considering the difficulties associated with establishing epithelial cell cultures from the small intestine of cattle, previous studies of enteric pathogens have been performed using heterologous cell lines. For example, studies of bovine rotavirus (BRV) have been performed using mouse, monkey and human cell lines (Bass et al., 1990, Bugarcic and Taylor, 2006, Lee et al., 1998). Although clearly valuable, information obtained using these heterologous cell culture models may not be fully applicable to cattle given known differences in rotavirus strains, species barriers, cellular receptors and factors required for rotavirus uptake, infectivity, replication and pathogenesis (Estes et al., 2001, Lopez and Arias, 2006, Pesavento et al., 2006, Ramig, 2004). Therefore, in order to examine the role of intestinal epithelial cells in pathogen attachment, invasion, and to define molecular mechanisms of disease pathogenesis, autologous host cell cultures are required.
In this study we defined a method to successfully generate bovine jejunal and ileal epithelial cell cultures from fetal intestinal tissue. We also determined that these cultured epithelial cells could be infected by a bovine enteric pathogen. This is the first report to confirm that cultured bovine intestinal epithelial cells are susceptible to BRV infection. The establishment of bovine intestinal epithelial cell cultures provides a homologous system for investigating the molecular and cellular mechanisms of BRV uptake and the response of intestinal epithelial cells during viral infection.
2. Materials and methods
2.1. Animals and collection of intestinal tissues
All animal experiments were conducted in accordance with the Guide to the Care and Use of Experimental Animals, provided by the Canadian Council on Animal Care. Seven Hereford cows, with pregnancies ranging between 110 and 265 days of gestation, were purchased and housed at the Vaccine and Infectious Disease Organization (VIDO) animal facility. Caesarian sections were performed to deliver live fetuses. Fetuses were euthanized by intravenous injection of pentobarbital sodium (Euthanyl, Abbott laboratories, Montreal, Canada) and fetal age was determined by measuring the crown to rump distance (Roberts, 1986). Both jejunum and ileum were aseptically collected from each fetus and placed in ice-cold Hanks’ Balanced Salt Solution (HBSS) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B, and 50 μg/ml gentamicin (HBSS-A). The antibiotics and antimycotic were purchased from Sigma–Aldrich (Canada).
2.2. Defining conditions for intestinal epithelial cell isolation
Previous studies used a variety of tissue preparation methods and dissociation agents to isolate epithelial cells from bovine, human and mouse intestine (Birkner et al., 2004, Follmann et al., 2000, Hoey et al., 2003, Kaeffer, 2002, Rusu et al., 2005). Therefore, we investigated the use of different dissociation agents and digestion protocols to identify the best method for isolating and culturing viable epithelial cells from fetal intestine. Intestine was collected from two 110–130-day-old fetuses, cut into 25–30 mm2 pieces, and placed in teflon-coated 50 ml beakers. Four different epithelial cells dissociation protocols were evaluated: phosphate-buffered saline (PBS) alone; PBS supplemented with 2 mM EDTA; PBS supplemented with 0.25% trypsin (Sigma–Aldrich, Canada); or PBS supplemented with collagenase (100 U/ml; Sigma–Aldrich, Canada). Tissues were incubated at 37 °C and stirred at a low speed for 15–30 min using a small magnetic stir bar before collecting the supernatant and centrifuging at 400 × g for 7 min. Cells were washed twice with PBS. Cells from each tissue digestion condition were divided in half, re-suspended in either DMEM-10 or EGF-10 medium (described below), and cultured in 6-well plates for 5 days at 37 °C in a 5% CO2 atmosphere. Non-adherent cells were removed and discarded and the adherent cells were collected after trypsinization. Cytospins were prepared with the adherent cell fraction resulting from each intestinal cell isolation method and stained to quantify the percentage cytokeratin and vimentin positive cells (procedure described below). The results of these studies were used to define the enzymatic digest conditions used for epithelial cell isolation.
2.3. Establishment of jejunal and ileal epithelial cell cultures
Jejunum and ileum from three fetuses, between 150 and 265 days in age, were cut into 15–20 cm long segments and placed in ice-cold HBSS-A. The intestinal lumen was flushed by using a 30 ml syringe to inject HBSS into one end of the intestine and then both ends of the intestinal segment were ligated with silk suture (Fisher scientific, Canada). Collagenase type II enzyme (Sigma–Aldrich, Canada) solution (800 U/ml) was prepared in 37 °C PBS and injected into the lumen until each ligated segment was distended. Intestinal segments were then placed in beakers containing 37 °C pre-warmed HBSS-A and incubated at 37 °C for 30 min. After digestion, the contents of each intestinal segment were collected and centrifuged at 400 × g for 7 min. Cell pellets were re-suspended in DMEM-10 (DMEM containing 10% fetal bovine serum (FBS) (JRH Biosciences, Kansas, USA)), pelleted and washed twice with DMEM-10, and then re-suspended in epidermal growth factor media (EGF medium) containing 10% FBS (EGF-10). EGF media consisted of DMEM (Invitrogen, Ontario, Canada) supplemented with high glucose, 10 mM HEPES, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B, 50 μg/ml gentamicin, 5 μg/ml apo-transferrin, 25 ng/ml epidermal growth factor, 0.1 μg/ml hydrocortisone, 10 μg/ml bovine insulin, and 1–10% heat-inactivated FBS. All culture supplements were purchased from Sigma–Aldrich, Canada.
The intestinal cell suspension was counted, an aliquot of 4–5 × 106 cells was added to either 25 cm2 or 75 cm2 Primaria flasks (BD Biosciences, Canada), and cultures were incubated at 37 °C in 5% CO2 atmosphere. Following incubation for 48 h, half the culture medium was removed and replaced with fresh EGF-10 medium. Then, 24 h later the culture medium was completely removed, the adherent cells washed once with PBS, and fresh EGF-10 medium was added. The primary intestinal cell cultures were maintained by incrementally decreasing the concentration of FBS in the EGF media from 10 to 1%. Fibroblast growth in the cultures was further inhibited by treating epithelial cultures for 3 min with 1× trypsin/EDTA solution (Invitrogen) diluted 1:5 in versene (0.2 g/L EDTA in PBS). Trypsin–EDTA treatment was previously reported to selectively remove fibroblasts from primary epithelial cell cultures (Al-Yaman and Willenborg, 1984, Hague and Paraskeva, 1996). This procedure was repeated 2–3 times each week until a confluent monolayer of cells was observed and cell number was then expanded by serial passage.
2.4. Immunohistochemical characterization of epithelial cell cultures
Cytokeratin is a part of the epithelial cell cytoskeletal complex present during epithelial cells differentiation (Chandrakasan et al., 1991, Schlage et al., 1998). Vimentin is an intermediate filament present in fibroblasts and other mesenchymal cells (Bernal and Stahel, 1985). Therefore, monoclonal antibodies (MoAbs) specific for cytokeratin and vimentin were used to quantify the percentage of epithelial cells and fibroblasts present in primary intestinal cell cultures. For immunohistochemical (IHC) studies, primary ileal and jejunal cell cultures were incubated with trypsin–EDTA for 5–10 min at 37 °C to detach cells from the plastic. Cells were then washed twice with PBS before preparing cytospins using a cytofuge (Cytospin 3; Thermo Shandon Inc., PA, USA). Cytospins were air-dried, fixed in acetone and stored at 4 °C until stained with MoAbs specific for cytokeratin and vimentin. Briefly, slides were equilibrated at room temperature, rehydrated in PBS (pH 7.2) and then incubated with PBS containing 1% goat serum to block non-specific protein binding. Slides were incubated with either MoAb C6909 (clone K8.13, IgG2a isotype; cytokeratin) or V5255 (clone VIM 13.2, IgM isotype; vimentin) for 1 h. Cytokeratin MoAb C6909 is specific for a cytokeratin peptide present in a large number of cytokeratins namely 1, 5, 6, 7, 8, 10, 11 and 18. MoAbs M9144 (IgG2a isotype) and M5170 (IgM isotype) were used as irrelevant isotype-matched controls. All MoAbs were purchased from Sigma–Aldrich, Canada and used at a final concentration of 1 μg/ml. Slides were washed three times in PBS and then incubated with isotype-specific, biotinylated goat anti-mouse IgG2a or IgM antisera (Caltag laboratories, USA; 1:2000 dilution) for 30 min. Slides were washed 3× and then incubated with PBS containing 0.1% sodium azide and 0.3% hydrogen peroxide (Sigma–Aldrich) to block endogenous peroxidase activity. Antibody labeling was visualized by adding ABC solution (Vactastain Elite ABC Kit, Vector Laboratories Canada Inc., Canada) followed by the addition of diaminobenzene (DAB) substrate. Slides were dehydrated in absolute ethanol, dipped in copper sulphate solution (0.5 M in normal saline), and counterstained with Giemsa stain (Sigma–Aldrich) before drying overnight and mounting cover slips with Cytoseal 60 mounting medium (Stephen Scientific, MI, USA). Immunohistochemical staining was examined and photographed with an Axiovert 200 inverted microscope and camera system (Carl Zeiss Canada Ltd., Canada).
2.5. Screening cells for BVDV infection
The isolation of epithelial cells from bovine fetuses may be associated with the risk of culturing bovine viral diarrhea virus (BVDV) infected cells. Culture supernatant from both ileal and jejunal epithelial cell cultures were submitted to Prairie Diagnostic Services (Saskatoon, SK) for PCR detection of both BVDV1 and BVDV2 using the multiplex PCR methodology described previously by Gilbert et al. (1990).
2.6. BRV infection of intestinal epithelial cells
Jejunal and ileal epithelial cells were transferred to 6-well plates (0.8 × 105 cells/well) and incubated at 37 °C for 24 h. The cells were washed twice with serum-free DMEM and then overlaid with 500 μl of EGF medium with or without trypsin-activated BRV lab strain C-486 (MOI = 30) (Lee et al., 1998). Cultures were incubated for 1 h at 37 °C to allow viral adsorption to cells and during this time the medium was swirled every 15 min to ensure a uniform distribution of virus. After 1 h, 4 ml of serum-free EGF medium was added in each well and plates were incubated for 16 h.
2.7. Measurement of cell viability and detection of viral infected cells
Rotavirus infected cells and mock-infected cells were collected at 16 h post-infection (pi) by detaching cells with trypsin–EDTA treatment. Cells were washed twice with PBS and then used to quantify cell viability and viral infection. Cell viability was measured with the LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes, Eugene, Oregon, USA) following the manufacture's instructions. Briefly, an aliquot of cells was incubated in 70% methanol for 20 min at room temperature to provide a positive control for dead cells. Cells from both BRV infected and mock-infected cultures were re-suspended in 400 μl of 2 μM of calcein AM and 4 μM ethidium homodimer (EthD-1) and incubated for 15 min at room temperature. Stained cells were fixed in 2% formaldehyde-PBS and stored at 4 °C until analyzed using a FACScan flow cytometer and CellQuest software (Becton Dickinson, CA, USA).
To quantify viral infection the infected and uninfected cells were fixed in 10% formalin in PBS for 30 min and then permeabilized by incubating cells for 3 min in 1% Trinitron X-100. Cells were washed two times with PBS and then incubated at 4 °C for 30 min in the presence or absence of rabbit anti-BRV antiserum (Lot 93-72; produced at VIDO; 1:4000 dilution). Following this incubation, cells were washed twice with PBS and then incubated at 4 °C for 30 min with goat anti-rabbit IgG-FITC (Zymed Laboratories, CA, USA; 1:400 dilution). Cells were washed three times with PBS, fixed in 2% formaldehyde-PBS and stored at 4 °C until analyzed by flow cytometry.
2.8. Detection of cytopathic effect (CPE) following infection of epithelial cell cultures with BRV
BRV causes a lytic infection of mucosal epithelial cells (Holland, 1990, Ramig, 2004, Saif and Smith, 1985). Therefore, to determine if cultured epithelial cells displayed a similar response to viral infection both the jejunal and ileal primary epithelial cell cultures (2.5 × 105 cells/well) were infected with trypsin-activated BRV lab strain C-486 (MOI = 50; 12.5 × 106 pfu). BRV infected and mock-infected cell cultures were examined with an inverted light microscope at 24 and 48 h pi to determine if there were focal areas of cell destruction which would be indicative of viral-induced cytolysis. At 48 h pi culture supernatants from both jejunal and ileal cultures were collected and the amount of infectious virus in the culture supernatant was determined with a plaque assay which used MA104 cells (Sabara et al., 1985).
2.9. Establishment of jejunal fibroblast culture and infection with BRV
Fragments of fetal jejunal tissue from an 265-day-old fetus were used to establish a fibroblast culture. The jejunal tissue was cultured continuously in DMEM-10 medium instead of EGF-1 medium to suppress epithelial cell growth. This generated a homogenous culture of spindle-shaped cells which were further characterized by using IHC to analyze expression of cytokeratin and vimentin. The jejunal fibroblast cultures were then used for BRV infection studies to determine if viral proteins and viral-induced cytopathology were present in these cultures.
3. Results
3.1. Conditions for intestinal tissue digestion and epithelial cell culture
Collagenase digestion of intestinal tissue and the culture of epithelial cells for 5 days in EFG-10 media produced the highest percentage of cytokeratin positive cells (∼70%) when compared to PBSA and EDTA tissue digestion protocols which resulted in ∼25% and ∼30% cytokeratin positive cells, respectively. Trypsin (0.25%) digestion of intestinal tissues did not result in the establishment of epithelial cells when using similar culture conditions. Therefore, collagenase digestion of tissues and EGF-10 medium were selected as the conditions for establishing epithelial cultures in subsequent experiments.
3.2. Morphology and growth of primary fetal jejunal and ileal epithelial cell cultures
Fetuses ranging from 180 to 210 days old were used for establishing jejunal and ileal epithelial cell cultures. The majority of intestinal cells isolated by collagenase digest died during the first 3–4 days in primary culture and at the end of this time a small numbers of cells and organoids (undissociated crypt-like cellular aggregates) remained attached to the plastic. During the next 4–5 days of culture, foci of plastic-adherent cells were observed around individual organoids. Three different patterns of cell growth were observed in the primary culture (P-0) of both ileal and jejunal cells. Cell clusters with a cobblestone morphology, characteristic of epithelial cells, were observed throughout jejunal (Fig. 1A) and ileal (Fig. 1E) cultures. Discreet clusters of spindle-shaped, fibroblast-like cells were also observed throughout jejunal (Fig. 1B) and ileal (Fig. 1F) cultures, as well as an intermingling of both cell types in the jejunal (Fig. 1C) and ileal (Fig. 1G) cultures. Thus, the primary intestinal cell cultures appeared to contain a mixture of both epithelial cells and fibroblasts.
Fig. 1.
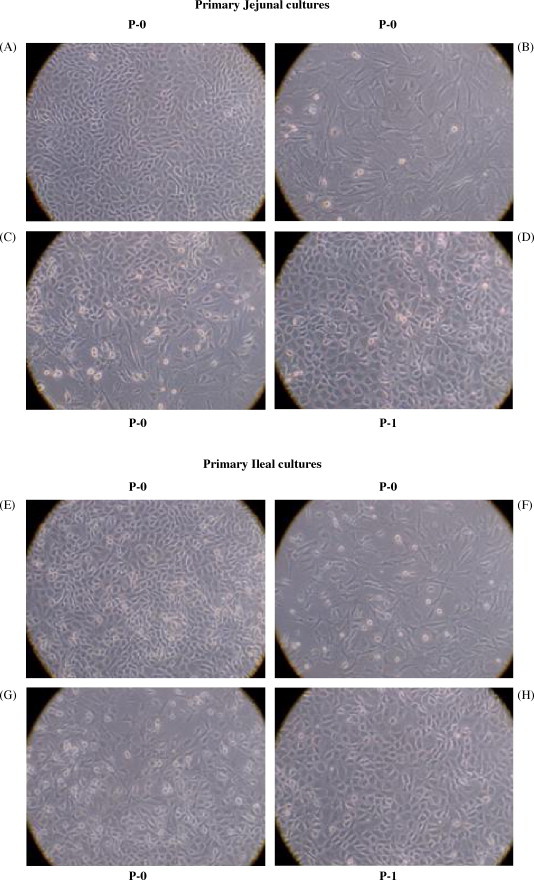
Establishment of epithelial cell cultures from fetal bovine jejunum and ileum. Epithelial cell suspensions obtained from an 180-day-old fetus were cultured in EGF media as described in Section 2. Primary cultures contained foci of both homogeneous and heterogeneous populations of epithelial and fibroblast-like cells. Clusters of cells with ‘cobblestone’ morphology characteristic of epithelial cells were observed in jejunal (A) and ileal (E) primary cultures (P-0). Clusters of spindle-shaped fibroblast-like cells were also present in both the jejunal (B) and ileal (F) primary cultures. An intermingling of epithelial and fibroblasts cells was occasionally observed in both the jejunal (C) and ileal (G) primary cultures. Primary cultures were trypsinized and transferred to new 75 cm2 flasks. In contrast to P-0 cultures, upon further passage secondary cultures (P-1) displayed an homogeneous epithelial cell-like morphology with few fibroblasts visible in either the jejunal (D) or ileal (H) cultures. Magnification 200×.
Earlier studies indicated that a reduction in serum concentration selectively inhibited fibroblast growth without affecting epithelial cell growth and epithelial cell attachment was more resistant to trypsin–EDTA treatment than fibroblast attachment. Therefore, the primary intestinal epithelial cells were maintained in EGF medium with an incremental decrease in FBS from 10 to 1% over a 10-day period. Repeated treatment of the primary cultures with trypsin–EDTA was also used to selectively remove fibroblasts. Immunohistochemical staining of cells obtained following trypsin–EDTA treatment confirmed that the majority of the cells being removed were vimentin positive and cytokeratin negative (data not shown). Thus, the cells being removed were primarily mesenchymal cells and removal of these cells provided additional space for epithelial cell growth. At the end of the 11–12-day primary culture period, there was a confluent monolayer of cells and these cells were split 1:2 and transferred to new flasks (P-1). The transferred cells appeared to be a homogenous population with a cobblestone appearance in both jejunal (Fig. 1D) and ileal (Fig. 1H) cultures. These cultures were confluent within 5–6 days of passage and were maintained by making 1:2 or 1:3 splits with each subsequent passage. Between each passage, cultures were treated once or twice with trypsin–EDTA to selectively remove contaminating fibroblasts. During the early passages (P1-4), both jejunal and ileal cell cultures became confluent within 5–6 days but the time required for cultures to become confluent became progressively longer with subsequent passages. Thus, both the jejunal and ileal epithelial cell cultures could be maintained for approximately 2 months and passaged 7–8 times. No significant difference in intestinal epithelial cell morphology was observed when cultures were established from fetuses ranging in age between 110 and 265 days gestation.
3.3. Immunohistochemical characterization of jejunal and ileal cell cultures
Immunohistochemical staining for cytokeratin was used as a marker for epithelial cells and staining for vimentin was used as a marker for mesenchymal (fibroblast-like) cells. Specific staining for cytokeratin was observed in approximately 55% of the cells collected following the first passage (P1) of both jejunal (Fig. 2B) and ileal cultures (Fig. 2F) established with EGF-10 medium. The expression of cytokeratin and vimentin was re-examined following P3 and P6 and these analyses indicated that the percentage of both cytokeratin and vimentin positive cells continued to increase with each passage (Table 1 ). The percentage of cytokeratin positive cells ranged between 62 and 75% at P-6 which indicated that the low serum culture conditions and repeated treatment with trypsin–EDTA was unable to eliminate mesenchymal cell contamination. These treatments may have been critical, however, to prevent fibroblasts overgrowth of the epithelial cells established with EGF-10 medium during the early culture period.
Fig. 2.
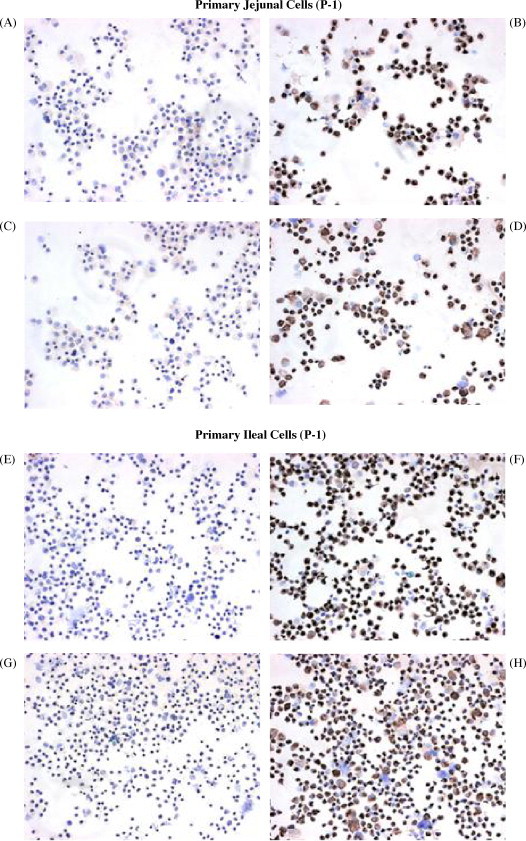
Immunohistochemical (IHC) staining of jejunal and ileal cell cultures. Cytospins from jejunal (A–D) and ileal (E–H) cells (P-1) obtained from a 180-day-old fetus were stained using IgG2a (A and E) and IgM (C and G) isotype control monoclonal antibodies or monoclonal antibodies specific for cytokeratin (B and F) and vimentin (D and H). No visible staining was observed with the isotype control antibodies but both the jejunal (B and D) and ileal (F and H) cytospins were visibly stained for cytokeratin and vimentin.
Table 1.
Immunohistochemical staining of jejunal and ileal cell established in primary cultures with EGF-10 medium and maintained in EGF-1 medium
| Cell type | Cytoplasmic marker | 180 days old fetus % stained cells |
210 days old fetus % stained cells |
||||
|---|---|---|---|---|---|---|---|
| P-1a | P-3 | P-6 | P-1 | P-3 | P-6 | ||
| Jejunal cells | Cytokeratin | 55 | 55 | 67 | NTb | 55 | 75 |
| Vimentin | 55 | 80 | 86 | NT | 80 | 85 | |
| Ileal cells | Cytokeratin | 55 | 65 | 74 | NT | 55 | 62 |
| Vimentin | 65 | 85 | 86 | NT | 45 | 87 | |
P: passage.
NT: not tested.
An alternative approach was evaluated for establishing epithelial cell cultures from the intestine of three fetuses (∼240–265 days old). Jejunal and ileal intestinal cell suspensions were obtained with collagenase digestion as described earlier but the primary cell cultures were initiated in EGF-1 medium. As observed previously, the majority of cells died during the first 48–72 h in culture with a small number of organoids attached to the plastic. The surviving cells were maintained in EGF-1 media throughout the study and within 8–9 days there was an outgrowth of cells from individual organoids. The development of a confluent monolayer in these primary cultures required approximately 18 days which was a significant delay when compared to previous primary cultures established with EGF-10 medium. The majority of cells in the cultures established with EGF-1 medium displayed cobblestone morphology but the cultures were still treated with a 1:5 diluted trypsin–EDTA at room temperature for 2–3 min to remove fibroblast-like cells. Three days later, the cultures were again confluent and cells were collected for immunohistochemical staining and passage to new flasks. Approximately 91% of the jejunal cells (Fig. 3B) and 85% of the ileal cells (Fig. 3F) stained visibly for cytokeratin. In contrast, only 48% of the jejunal cells (Fig. 3D) and 46% of the ileal cells (Fig. 3H) stained visibly for vimentin. These results and previous IHC results (Table 1) suggested that cultured epithelial cells contained both cytokeratin and vimentin. Furthermore, establishing primary intestinal cell cultures in EGF-1 provided a selective advantage for epithelial cells but the growth of these cells was much slower when compared to cultures established with EGF-10 medium.
Fig. 3.
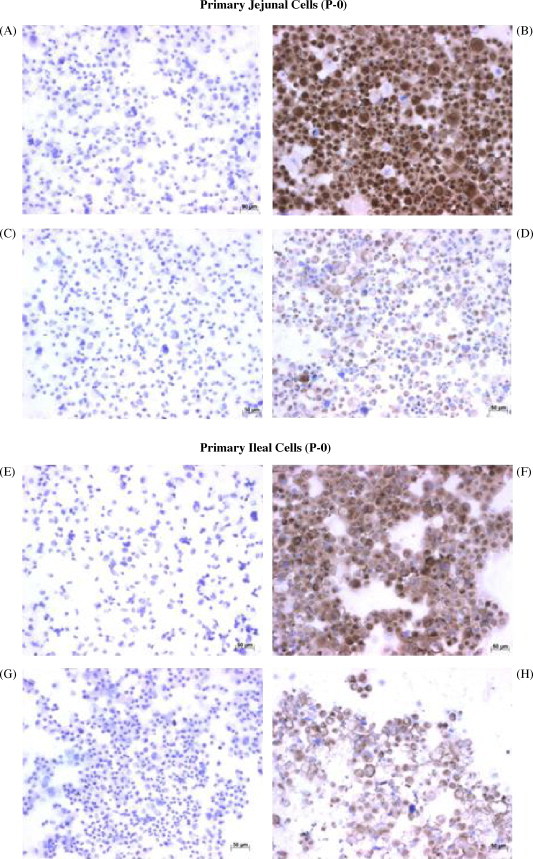
Immunohistochemical (IHC) staining of primary bovine fetal jejunal and ileal cell cultures established in EGF-1 media. Cytospins prepared with jejunal and ileal cells (P-0) obtained from a 265-day-old fetus and cultured in EGF-1 media were stained with mouse IgG2a (A and E) and IgM (C and G) isotype control antibodies and with mouse anti-cytokeratin (B and F) and anti-vimentin (D and H) MoAbs to determine the percentage of cytokeratin and vimentin positive cells.
3.4. Establishment of a fetal jejunal fibroblast culture
Fetal jejunal fibroblast cultures were established using jejunal tissue fragments cultured in DMEM-10 medium. IHC staining of these cells following passage 7 (P7) revealed that 1–2% of cells stained visibly for cytokeratin (Fig. 4B) and 98–99% of cells stained visibly for vimetin (Fig. 4D), indicating that a relatively pure fibroblast culture had been established. Furthermore, the absence of cytokeratin staining in fibroblasts provided evidence that cytokeratin was specific to cultured epithelial cells.
Fig. 4.
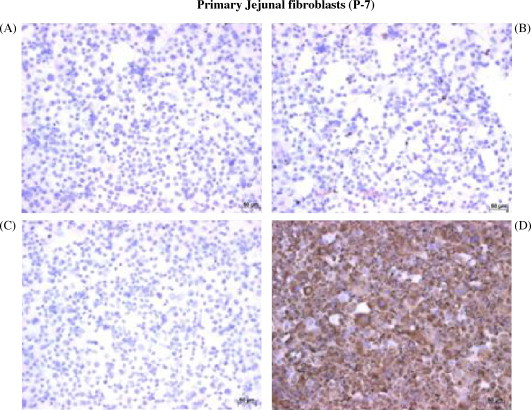
Immunohistochemical characterization of fibroblasts cultured from fetal bovine jejunum. Cytospins were prepared with jejunal fibroblasts obtained from a 265-day-old fetus and cultured in DMEM-10 media (P-7). Cytospins were stained with IgG2a (A) and IgM (C) isotype control monoclonal antibodies or with cytokeratin- (B) and vimentin-specific (D) MoAbs to detect cytokeratin and vimentin positive cells.
3.5. BRV infection of primary fetal jejunal and ileal epithelial cell cultures
Bovine jejunal and ileal epithelial cells were infected with BRV at an MOI of 30 for 16 h to determine if these cultures were competent for BRV infection and to determine the effect of viral infection on cell viability. Second passage (P2) cells were used for these studies and cell viability was evaluated through a combination of ethidium bromide (EB) and calcein staining. Methanol treatment of mock-infected and rotavirus-infected ileal epithelial cells provided a positive control for detection of cell death with EB staining (Fig. 5A and B). EB staining of mock-infected and BRV-infected ileal cells revealed that 75.8% of viral-infected cells were viable at 16 h pi (Fig. 5D). IHC staining with anti-BRV antisera revealed specific staining for viral protein in approximately 88% of ileal (Fig. 6D) and 83% of jejunal cells (Fig. 6H). These results indicated that the majority of cells in both jejunal and ileal cell cultures contained a detectable level of viral protein at 16 h pi.
Fig. 5.
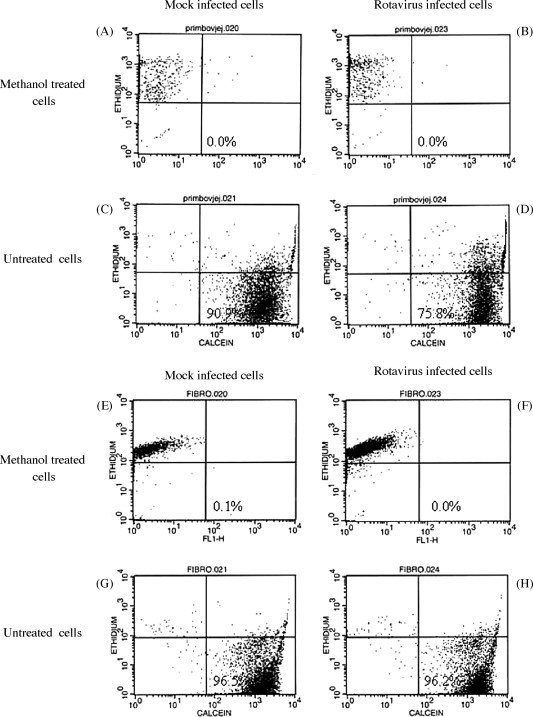
Viability of ileal epithelial cells and jejunal fibroblast cells following BRV infection. Ileal epithelial cells (A–D) were used after the second passage (P-2) and jejunal fibroblasts (E–H), obtained from a 265-day-old fetus, were used following the 6th passage (P-6). Cell cultures were either mock infected or BRV infected (MOI = 30) for 16 h prior to assaying cell viability. Staining with calcein-AM and ethidium homodimer (EthD-1) was used to identify live and dead cells, respectively. Cells fixed with 70% methanol was used as positive control for dead ileal epithelial (A and B) and fibroblast (E and F) cells.
Fig. 6.
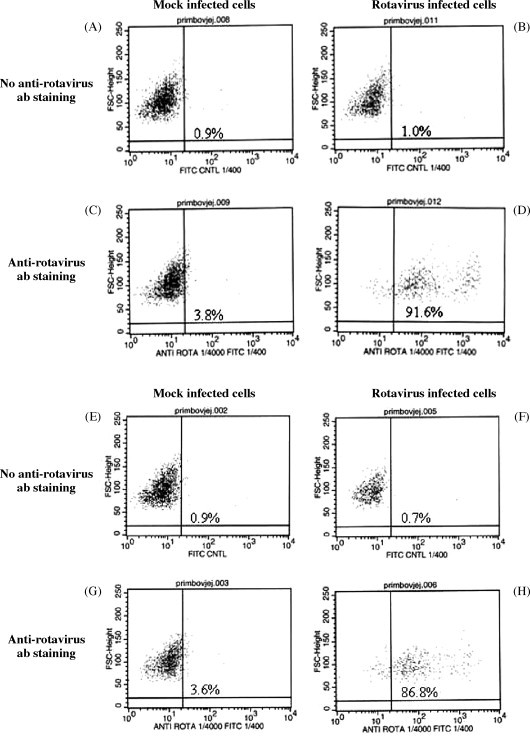
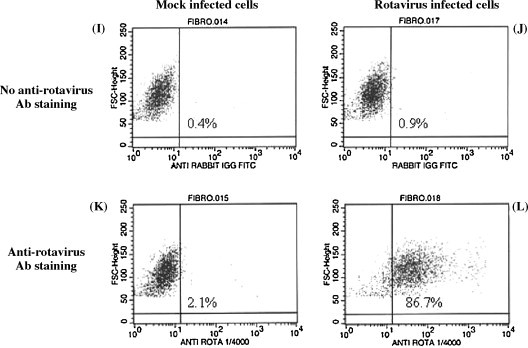
The presence of BRV protein in infected jejunal and ileal epithelial cell cultures and jejunal fibroblast cultures. Mock-infected or rotavirus infected ileal (A–D) and jejunal (E–H) epithelial cells and jejunal fibroblasts (I–L) obtained from a 265-day-old fetus were stained with a rabbit anti-rotavirus serum followed by staining with a goat anti-rabbit Ig-FITC. Cells were analyzed with flow cytometry to determine the percentage of cells expressing a detectable level of viral protein.
Cytokeratin staining indicated that only 50–60% of jejunal and ileal cells used for the viral infection studies were epithelial cells (Table 1). Therefore, the high percentage of viral-infected cells in these cultures suggested that BRV infection may not be restricted to epithelial cells. BRV infection of fibroblasts would not be consistent with the known tropism of BRV for mucosal epithelial cells. To determine if BRV could infect fibroblasts, a jejunal fibroblast culture was infected with BRV (MOI = 30) and cell viability and the presence of BRV protein were analyzed at 16 pi. EB and calcein staining revealed that fibroblast viability was not reduced at 16 h pi (Fig. 5H). When BRV-infected fibroblasts were stained with anti-BRV antisera there was, however, a detectable level of viral protein in approximately 85% cells (Fig. 6L). The staining for viral protein supports the conclusion that BRV could infect fibroblasts but viral replication may have been altered since there was no reduction in fibroblast viability at 16 h pi.
3.6. BRV-induced cytopathology in intestinal epithelial and fibroblast cultures
BRV is a lytic infection and destruction of infected mucosal epithelial cells is an important component of disease pathogenesis. Therefore, it was important to determine if BRV infection resulted in the lysis of the cultured jejunal and ileal epithelial cells. The reduced viability of these cells at 16 h pi (Fig. 5D) indicated that incubation beyond this time point might induce lysis of infected cells. Epithelial cell cultures were infected with BRV (MOI = 50) and checked for a cytopathic effect (CPE) at 24, 48 and 72 h pi. At 24 h pi, CPE was apparent in both jejunal and ileal cell cultures with dead cells present in the culture supernatant (data not shown). There was no visible sign of cell death in jejunal fibroblast cultures at 24 h pi and the cellular monolayer remained intact (data not shown). At 48 h pi, numerous dead cells were present in the culture medium and the cell monolayer was extensively disrupted in both the jejunal (Fig. 7B) and ileal (Fig. 7D) epithelial cell cultures. In contrast, the jejunal fibroblast culture did not show visible signs of CPE until 72 h pi (Fig. 7F). These observations confirmed that BRV infection was lytic within both epithelial and fibroblast cultures but cell lysis was delayed at least 48 h in the fibroblast culture. In the same experiment, supernatants from BRV uninfected and infected (12.5 × 106 pfu/well) jejunal and ileal cultures were collected 48 h pi. Infectious virus recovered in jejunal and ileal culture supernatants were 50 × 106 and 175 × 106 pfu, respectively. These values are a minimal estimate of viral replication since virus present within infected cells was not measured.
Fig. 7.
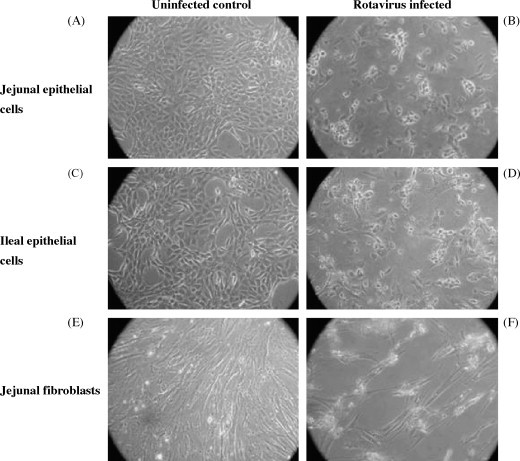
Cytopathic effect (CPE) of BRV infection in jejunal and ileal epithelial cell cultures and jejunal fibroblast cultures obtained from a 265-day-old fetus. Cell cultures were mock infected or infected with BRV (MOI = 50) and then incubated for 72 h. Both jejunal (A and B) and ileal (C and D) epithelial cell cultures displayed extensive CPE at 48 h pi but jejunal fibroblasts (E and F) displayed CPE only at 72 h pi.
4. Discussion
The primary objective of the present study was to establish epithelial cell cultures from fetal small intestine. Epithelial cell cultures have been established from the colon of cattle (Birkner et al., 2004, Follmann et al., 2000, Hoey et al., 2003, Rusu et al., 2005) but these studies did not evaluate epithelial cell susceptibility to infection by enteric pathogens. Therefore, a second objective of the present study was to use BRV as a model pathogen to determine if cultured bovine intestinal epithelial cells were competent target cells for viral infection. To confirm that the response to viral infection was specific we tested the cultured epithelial cells to confirm that they were not infected with either BVDV1 or BVDV2. Protocols were established which supported the isolation and culture of cytokeratin positive cells from both the jejunum and ileum of bovine fetuses. These protocols were applicable irrespective of the various age of fetuses used in the present study. The developmental and differentiation states of the cultured epithelial cells were not, however, checked by special methods such as by electron microscopy, biochemical analysis, enzyme profiling or polarizing cultures on membrane filters. Therefore, we did not determine if fetal age influenced epithelial cell differentiation or function.
A variety of isolation methods, including tissue explants, mechanical isolation by dissection and trituration, chelation (EDTA) and enzymatic digestion, have been used for intestinal epithelial cells (reviewed in Kaeffer, 2002). The main advantage of enzymatic digestion of intestinal tissue is that it may preserve more cell-to-cell interactions, including close contact between pericryptal fibroblasts and epithelial cells. Collagenase and dispase digestion have been reported to provide incomplete disaggregation of cells and result in viable epithelial organoids (Booth et al., 1995b, Evans et al., 1992, Kaeffer, 2002, Macartney et al., 2000). Some of these organoids are presumably derived from crypts which contain epithelial stem cells capable of generating epithelial cells in vitro (Macartney et al., 2000). We consistently observed the outgrowth of epithelial cell foci from intestinal organoids and the incomplete digestion of epithelial tissues with collagenase provided the most consistent generation of epithelial cell cultures (Fig. 1). While standardizing the culture conditions we initially used collagenase at 100 U/ml concentrations but to increase the yield of epithelial cells and crypts, we increased the concentrations of collagenase to 800 U/ml in subsequent experiments. Earlier studies also recommended using more than 100 U collagenase/ml (Rusu et al., 2005; Kaeffer, 2002) when establishing primary bovine and murine intestinal epithelial cultures.
There are two major problems which must be overcome when culturing primary intestinal epithelial cells. These problems include the rapid death of isolated epithelial cells and fibroblast overgrowth of cultured epithelial cells (Kaeffer, 2002). The primary reason for rapid death of isolated epithelial cells may be disruption of epithelial cell contact with the extracellular matrix (ECM) which triggers programmed cell death/apoptosis. This phenomenon is called ‘anoikis’ and intestinal epithelial stem cells are very sensitive to anoikis (Frisch and Francis, 1994, Kaeffer, 2002, Strater et al., 1996). Plastic surfaces coated with laminin, the collagen family of molecules, or metrigel may improve epithelial cell survival, growth, and differentiation (Follmann et al., 2000, Kaeffer, 2002, Macartney et al., 2000, Rusu et al., 2005). Therefore, isolated bovine intestinal cells were cultured in Primaria flasks in which the plastic surface was coated with nitrogen-containing functional groups in addition to negatively charged oxygen-containing groups. Primaria flasks have been reported to improve the attachment and differentiation of a variety of cell types (Chilkoti et al., 1995, Boisclair et al., 2000, Braun et al., 1996, Holgado-Madruga et al., 1997). We observed the outgrowth of epithelial cell foci from organoids when using Primaria flasks (Fig. 1) but there was still extensive cell death during the first 24–48 h of culture. Thus, using Primaria flasks was not sufficient to rescue many of the cells present in the single cell suspension generated by collagenase digestion.
Numerous strategies had been described for the control of fibroblast growth in primary intestinal epithelial cells cultures. These methods include the use of l-valine deficient medium (Hoey et al., 2003, Sordillo et al., 1988), low serum (≤2%) supplementation of growth media (Flint et al., 1994, Macartney et al., 2000), density gradients to enrich crypt epithelium in the original cell suspensions (Follmann et al., 2000, Hoey et al., 2003, Macartney et al., 2000, Rusu et al., 2005), and brief treatment of cultures with trypsin, dispase or pepsin to selectively remove fibroblasts (Hague and Paraskeva, 1996, Radi and Ackermann, 2004). A wide variety of specific nutrients and growth factors such as insulin, insulin-like factor-1, heparin, epidermal growth factor (EGF), colony stimulating factor-1, transferrin, selenium, hydrocortisone, sodium pyruvate have also been used to enhance epithelial cell growth in cultures (Booth et al., 1995a, Chopra et al., 1987, Flint et al., 1994, Kaeffer, 2002, Ramsay et al., 2004). We were able to maintain enriched epithelial cell cultures for a period of 60 days by using a variety of these strategies. The culture medium used in the present study was supplemented with a variety of epithelial cell growth factors but despite the use of these supplements epithelial cell growth slowed after 4–6 passages and was negligible after 8 passages. This growth period exceeded that reported in earlier studies for bovine intestinal epithelial cells (Birkner et al., 2004, Follmann et al., 2000, Hoey et al., 2003, Rusu et al., 2005). The more prolonged growth of fetal intestinal epithelial cells may have been due to the synergistic effects of apo-transferrin, epidermal growth factor, hydrocortisone, and bovine insulin (Kaeffer, 2002, Kedinger et al., 1987, Macartney et al., 2000) and the enhanced growth potential of fetal epithelial cells. It has been reported that murine intestinal crypt stem cells undergo functional changes with age and this may account for the difficulty in establishing primary cultures from young or adult animals (Kaeffer, 2002, Kondo et al., 1984, Martin et al., 1998). We also used fetal intestinal tissues rather than adult intestinal tissue since many enteric pathogens selectively infect the neonate. For example, BRV primarily infects and causes clinical disease and mortality in newborn calves (Saif and Smith, 1985).
In the present study enzyme digested epithelial cells were cultured at the earliest possible time after isolation to minimize anoikis of epithelial cells. The density gradient protocol used by others (Follmann et al., 2000, Hoey et al., 2003, Macartney et al., 2000, Rusu et al., 2005) to enrich cellular aggregates/crypts added significantly more time before cells could be cultured and resulted in relatively low cell yields. Fibroblast contamination of bovine epithelial cultures was also an important factor even when primary cultures were established with crypts or cell aggregates obtained by 2% sorbitol gradient method (Follmann et al., 2000, Hoey et al., 2003). The use of a low percentage of FCS in the medium or l-valine deficient medium were still required to minimize fibroblast growth in these cultures. Earlier studies indicated that the addition of irradiated fibroblasts or conditioned medium was required to support primary epithelial cell growth (Hague and Paraskeva, 1996, Kaeffer, 2002; Perreault and Beaulieu, 1998; Perreault and Jean-Francois, 1996). Other studies also indicated that presence of non-epithelial cell types were required to support the growth and viability of primary epithelial cells (Booth et al., 1995b, Evans et al., 1992, Macartney et al., 2000). Therefore, we did not use the density gradient method in the present intestinal epithelial cell isolation protocol.
In the present study, brief trypsinization and serum deprivation (1% FBS) was used to suppress fibroblast growth in the epithelial cell cultures. Epithelial cell cultures were routinely treated with trypsin–EDTA (1×) solution (diluted 1:5 in versene) for a brief period to selectively remove fibroblasts. We did not observe any morphological changes in the trypsin-treated and non-treated cell cultures but cannot exclude possible effects of trypsin–EDTA treatment on the epithelial cells. Epithelial cells were, however, incubated with media containing FBS immediately after trypsin treatment which effectively neutralizes trypsin activity. It is important to note that the bovine jejunal fibroblast cultures which were not periodically treated with trypsin–EDTA displayed a similar level of BRV infection as the bovine jejunal and ileal epithelial cultures (Fig. 6). This suggests that mild trypsin–EDTA treatment did not have a significant effect on epithelial cell infection by rotavirus. Furthermore, rotavirus infection of epithelial cells requires that rotavirus be cultured in the presence of trypsin or the virus must be treated with trypsin prior to addition to the culture (Sabara et al., 1985, Macartney et al., 2000, Lee et al., 1998, Vijay-Kumar et al., 2005, Cuadras et al., 2002, Bugarcic and Taylor, 2006). These studies indicate that trypsin treatment is required for epithelial cell infection by rotavirus.
Ileal and jejunal cultures established with EGF-1 medium had a high percentage of epithelial cells (80–91%) based on cytokeratin staining but a significant percentage of cells also contained vimentin (Fig. 2, Fig. 3). It has been previously reported that intestinal epithelial cells cultured from cattle and other species may contain both cytokeratin and vimentin (Kaeffer et al., 1993, Kedinger et al., 1987, Rusu et al., 2005) despite the apparent absence of vimentin in mucosal epithelial cells. Consistent with these findings, we observed that the percentage of vimentin containing cells increased in both jejunal and ileal cultures as the passage number increased (Table 1). Previous studies reported that level of cytokeratin within each cell or the percentage of cytokeratin positive cells may decrease when intestinal epithelial cells are cultured (Blay and Brown, 1984, Whitehead et al., 1993). There was not, however, a significant decrease in the percentage of cytokeratin positive cells in either the ileal and jejunal cultures (Table 1). The presence of both cytokeratin and vimentin in epithelial cells observed in the present study might be a normal biological phenomenon in cultured intestinal epithelial cells but the absence of cytokeratin staining in bovine jejunal fibroblasts cultures indicated that cytokeratin is unique to epithelial cells. Collectively, these observations indicate that monitoring cytokeratin may provide the most specific method to determine purity of epithelial cell cultures.
In well established rotavirus-culture systems an MOI of 5–10 is frequently used for infection studies. These culture systems frequently use either the monkey kidney cell line MA 104 or immortalized human intestinal cell lines (Bugarcic and Taylor, 2006, Bass et al., 1990, Bass et al., 1992, Rollo et al., 1999). In some studies, an MOI of 20–30 was used for rotavirus infection of human epithelial cell lines (Vijay-Kumar et al., 2005, Cuadras et al., 2002) but a recent rotavirus infection study demonstrated that bovine and porcine epithelial cell lines were 5–10-fold less susceptible to rotavirus infection than MA 104 or human Caco-2 cells (Ciarlet et al., 2002). It was concluded that a high MOI should be used to successfully infect bovine epithelial cultures. In the present study we used a BRV lab-adapted strain C486, in contrast to the wild-type strains used by others, to infect the cultured bovine intestinal epithelial cell. This strain has previously been used at a high MOI to infect epithelial cultures (Lee et al., 1998, Sabara et al., 1985). Therefore, we used BRV strain C486 at MOIs of 30 and 50 for infectivity and cytopathic effect (CPE) studies, respectively.
When jejunal and ileal epithelial cultures were incubated with trypsin-activated BRV both cell types were similarly infected with rotavirus (Fig. 6) indicating that both jejunal and ileal cultures were permissive to BRV infection. The epithelial cultures were incubated with BRV for only 16 h based on an earlier rotavirus study conducted with Caco-2 cells (Cuadras et al., 2002). We also observed that the viability of BRV-infected epithelial cells began to decline at 16 h pi (Fig. 5). Rotavirus is a lytic virus and when infected jejunal and ileal epithelial cultures were incubated for a longer period of time extensive CPE was observed (Fig. 7). Furthermore, infectious virus was also recovered in the culture supernatants of infected epithelial cells confirming that viral replication had occurred. These observations are consistent with previous reports for rotavirus infection in a variety of other cell types (Jourdan et al., 1997, Macartney et al., 2000, McNulty et al., 1977, Svensson et al., 1991). Thus, cultured jejunal and ileal epithelial cells appear to function as competent host cells for BRV infection and replication studies.
Both jejunal and ileal epithelial cell cultures contained fibroblasts. Therefore, we investigated whether fibroblasts were also competent host cells for BRV infection. No studies have specifically reported fibroblast infection by BRV and viral tropism is thought to be specific for mucosal epithelial cells (Holland, 1990, Ramig, 2004, Saif and Smith, 1985). Interestingly, we observed the presence of BRV protein in the majority of fibroblasts following exposure to virus (Fig. 6) but viral-induced CPE was markedly delayed in fibroblast cultures (Fig. 7). These observations indicate that BRV can infect cultured intestinal fibroblasts but the delayed lysis of infected cells suggests an altered viral–host cell interaction when compared to epithelial cells. The presence of fibroblasts among cultured intestinal epithelial cells may, however, complicate the interpretation of results when the objective of a study is to specifically study epithelial cell responses to viral infection.
In conclusion, a method was developed for establishing bovine epithelial cell cultures from both fetal jejunum and ileum. These cells could be cultured for an interval sufficient to generate a relatively large number of epithelial cells. For example, if all cells in the primary cultures were maintained with each passage then following eight serial passages there was the potential to expand the primary epithelial culture between 256-fold (1:2 split; 28) to over 6500-fold (1:3 split; 38). Thus, there is the potential to generate a relatively large number of epithelial cells for host–pathogen interaction studies. A variety of methods were required throughout the culture period to control fibroblast growth but it was not possible to completely eliminate these cells. Therefore, the presence of fibroblasts will need to be considered when using cultured intestinal epithelial cells for investigating host cell responses to an enteric pathogen unless the tropism of the pathogen is known to be epithelial cell specific.
Acknowledgements
We gratefully acknowledge the financial support of Genome BC and Genome Prairie for the ‘Pathogenomics of Innate Immunity’ research program. H.L.W. was supported by a post-doctoral Fellowship from SHRF (Saskatchewan, Canada). A.A.P. is the holder of an NSERC Senior Industrial Research Chair and L.A.B. is a recipient of the Canada Research Chair in Vaccinology. This manuscript is published with permission of the Director of Vaccine & Infectious Disease Organization as article #476. We would also like to acknowledge the assistance of Drs. Don Wilson and Kuldip Mirakhur for performing the animal surgeries and the VIDO Animal Care staff for their assistance in the care and management of animals. We would also like to acknowledge the assistance of Cheryl Lancto in establishing the protocol for epithelial cell isolation, Terry Beskorwayne and Yurij Popowych for their assistance with cell culture and flow cytometry, and Elaine Van Moorlehem for her assistance with the viral infection studies.
References
- Al-Yaman F., Willenborg D.O. Successful isolation, cultivation and partial characterization of naturally occurring ovine squamous cell carcinoma. Vet. Immunol. Immunopathol. 1984;5:273–288. doi: 10.1016/0165-2427(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Bass D.M., Mackow E.R., Greenberg H.B. NS35 and not vp7 is the soluble rotavirus protein which binds to target cells. J. Virol. 1990;64:322–330. doi: 10.1128/jvi.64.1.322-330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D.M., Baylor M.R., Chen C., Mackow E.M., Bremont M., Greenberg H.B. Liposome-mediated transfection of intact viral particles reveals that plasma membrane penetration determines permissivity of tissue culture cells to rotavirus. J. Clin. Invest. 1992;90:2313–2320. doi: 10.1172/JCI116119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal S.D., Stahel R.A. Cytoskeleton-associated proteins: their role as cellular integrators in the neoplastic process. Crit. Rev. Oncol. Hematol. 1985;3:191–204. doi: 10.1016/s1040-8428(85)80026-3. [DOI] [PubMed] [Google Scholar]
- Birkner S., Weber S., Dohle A., Schmahl G., Follmann W. Growth and characterisation of primary bovine colon epithelial cells in vitro. Altern. Lab. Anim. 2004;32:555–571. doi: 10.1177/026119290403200607. [DOI] [PubMed] [Google Scholar]
- Blay J., Brown K.D. Characterization of an epithelioid cell line derived from rat small intestine: demonstration of cytokeratin filaments. Cell Biol. Int. Rep. 1984;8:551–560. doi: 10.1016/0309-1651(84)90054-7. [DOI] [PubMed] [Google Scholar]
- Boisclair Y.R., Wang J., Shi J., Hurst K.R., Ooi G.T. Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1beta on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J. Biol. Chem. 2000;275:3841–3847. doi: 10.1074/jbc.275.6.3841. [DOI] [PubMed] [Google Scholar]
- Booth C., Evans G.S., Potten C.S. Growth factor regulation of proliferation in primary cultures of small intestinal epithelium. In Vitro Cell Dev. Biol. Anim. 1995;31:234–243. doi: 10.1007/BF02639439. [DOI] [PubMed] [Google Scholar]
- Booth C., Patel S., Bennion G.R., Potten C.S. The isolation and culture of adult mouse colonic epithelium. Epithelial Cell Biol. 1995;4:76–86. [PubMed] [Google Scholar]
- Braun J.R., Willnow T.E., Ishibashi S., Ashwell G., Herz J. The major subunit of the asialoglycoprotein receptor is expressed on the hepatocellular surface in mice lacking the minor receptor subunit. J. Biol. Chem. 1996;271:21160–21166. doi: 10.1074/jbc.271.35.21160. [DOI] [PubMed] [Google Scholar]
- Bugarcic A., Taylor J.A. Rotavirus nonstructural glycoprotein NSP4 is secreted from the apical surfaces of polarized epithelial cells. J. Virol. 2006;80:12343–12349. doi: 10.1128/JVI.01378-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakasan G., Hwang C.B., Ryder M., Bhatnagar R.S. Keratin expression in cultures of adult human epidermal cells. Cell. Mol. Biol. 1991;37:847–852. [PubMed] [Google Scholar]
- Chilkoti A., Schmierer A.E., Perez-Luna V.H., Ratner B.D. Investigating the relationship between surface chemistry and endothelial cell growth: partial least-squares regression of the static secondary ion mass spectra of oxygen-containing plasma-deposited films. Anal. Chem. 1995;67:2883–2891. doi: 10.1021/ac00113a024. [DOI] [PubMed] [Google Scholar]
- Chopra D.P., Siddiqui K.M., Cooney R.A. Effects of insulin, transferrin, cholera toxin, and epidermal growth factor on growth and morphology of human fetal normal colon epithelial cells. Gastroenterology. 1987;92:891–904. doi: 10.1016/0016-5085(87)90962-0. [DOI] [PubMed] [Google Scholar]
- Ciarlet M., Crawford S.E., Cheng E., Blutt S.E., Rice D.A., Bergelson J.M., Estes M.K. VLA-2 (α2β1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J. Virol. 2002;76:1109–1123. doi: 10.1128/JVI.76.3.1109-1123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadras M.A., Feigelstock D.A., An S., Greenberg H.B. Gene expression pattern in Caco-2 cells following rotavirus infection. J. Virol. 2002;76:4467–4482. doi: 10.1128/JVI.76.9.4467-4482.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M.K., Kang G., Zeng C.Q., Crawford S.E., Ciarlet M. Pathogenesis of rotavirus gastroenteritis. Novartis Found. Symp. 2001;238:82–96. doi: 10.1002/0470846534.ch6. (discussion 96–100) [DOI] [PubMed] [Google Scholar]
- Evans G.S., Flint N., Somers A.S., Eyden B., Potten C.S. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell Sci. 1992;101(Pt 1):219–231. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- Flint N., Cove F.L., Evans G.S. Heparin stimulates the proliferation of intestinal epithelial cells in primary culture. J. Cell Sci. 1994;107(Pt 2):401–411. doi: 10.1242/jcs.107.2.401. [DOI] [PubMed] [Google Scholar]
- Follmann W., Weber S., Birkner S. Primary cell cultures of bovine colon epithelium: isolation and cell culture of colonocytes. Toxicol. In Vitro. 2000;14:435–445. doi: 10.1016/s0887-2333(00)00033-3. [DOI] [PubMed] [Google Scholar]
- Frisch S.M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P.R., van de Pol E., Maxwell L.E., Gabriel A., Doe W.F. Isolation of colonic crypts that maintain structural and metabolic viability in vitro. Gastroenterology. 1989;96:283–291. doi: 10.1016/0016-5085(89)91549-7. [DOI] [PubMed] [Google Scholar]
- Gilbert S.A., Burton K.M., Prins S.E., Deregt D. Typing of bovine viral diarrhea viruses directly from blood of persistently infected cattle by multiplex PCR. J. Clin. Microbiol. 1990;37:2020–2023. doi: 10.1128/jcm.37.6.2020-2023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague A., Paraskeva C. The intestinal epithelial cell. In: Harris A., editor. Epithelial Cell Culture. Cambridge University Press; New York: 1996. pp. 25–41. [Google Scholar]
- Hoey D., Sharp L., Currie C., Lingwood C., Gally D., Smith D. Verotoxin 1 binding to intestinal crypt epithelial cells results in localization to lysomomes and abrogation of toxicity. Cell Microbiol. 2003;5:85–97. doi: 10.1046/j.1462-5822.2003.00254.x. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M., Moscatello D.K., Emlet D.R., Dieterich R., Wong A.J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R.E. Some infectious causes of diarrhea in young farm animals. Clin. Microbiol. Rev. 1990;3:345–375. doi: 10.1128/cmr.3.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan N., Maurice M., Delautier D., Quero A.M., Servin A.L., Trugnan G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J. Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeffer B. Mammalian intestinal epithelial cells in primary culture: a mini-review. In Vitro Cell Dev. Biol. Anim. 2002;38:123–134. doi: 10.1290/1071-2690(2002)038<0123:MIECIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kaeffer B., Briollais S. Primary culture of colonocytes in rotating bioreactor. In Vitro Cell Dev. Biol. Anim. 1998;34:622–625. doi: 10.1007/s11626-996-0008-8. [DOI] [PubMed] [Google Scholar]
- Kaeffer B., Bottreau E., Velge P., Pardon P. Epithelioid and fibroblastic cell lines derived from the ileum of an adult histocompatible miniature boar (d/d haplotype) and immortalized by SV40 plasmid. Eur. J. Cell Biol. 1993;62:152–162. [PubMed] [Google Scholar]
- Kaeffer B., Benard C., Blottiere H.M., Cherbut C. Treatment of rat proximal and distal colonic cells with sodium orthovanadate enhances their adhesion and survival in primary culture. Cell Biol. Int. 1997;21:303–314. doi: 10.1006/cbir.1997.0141. [DOI] [PubMed] [Google Scholar]
- Kedinger M., Haffen K., Simon-Assmann P. Intestinal tissue and cell cultures. Differentiation. 1987;36:71–85. doi: 10.1111/j.1432-0436.1987.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Rose I., Young G.P., Whitehead R.H. Growth and differentiation of fetal rat small intestinal epithelium in tissue culture. Relationship to fetal age. Exp. Cell Res. 1984;153:121–134. doi: 10.1016/0014-4827(84)90454-3. [DOI] [PubMed] [Google Scholar]
- Lee J., Yoo D., Redmond M.J., Attah-Poku S.K., van den Hurk J.V., Babiuk L.A. Characterization of the interaction between VP8 of bovine rotavirus C486 and cellular components on MA-104 cells and erythrocytes. Can. J. Vet. Res. 1998;62:56–62. [PMC free article] [PubMed] [Google Scholar]
- Lopez S., Arias C.F. Early steps in rotavirus cell entry. Curr. Top. Microbiol. Immunol. 2006;309:39–66. doi: 10.1007/3-540-30773-7_2. [DOI] [PubMed] [Google Scholar]
- Macartney K.K., Baumgart D.C., Carding S.R., Brubaker J.O., Offit P.A. Primary murine small intestinal epithelial cells, maintained in long-term culture, are susceptible to rotavirus infection. J. Virol. 2000;74:5597–5603. doi: 10.1128/jvi.74.12.5597-5603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Kirkwood T.B., Potten C.S. Age changes in stem cells of murine small intestinal crypts. Exp. Cell Res. 1998;241:316–323. doi: 10.1006/excr.1998.4001. [DOI] [PubMed] [Google Scholar]
- McNulty M.S., Allan G.M., McFerran J.B. Cell culture studies with a cytopathic bovine rotavirus. Arch. Virol. 1977;54:201–209. doi: 10.1007/BF01314786. [DOI] [PubMed] [Google Scholar]
- Panja A. A novel method for the establishment of a pure population of nontransformed human intestinal primary epithelial cell (HIPEC) lines in long term culture. Lab. Invest. 2000;80:1473–1475. doi: 10.1038/labinvest.3780154. [DOI] [PubMed] [Google Scholar]
- Perreault N., Beaulieu J.F. Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp. Cell. Res. 1998;245:34–42. doi: 10.1006/excr.1998.4221. [DOI] [PubMed] [Google Scholar]
- Perreault N., Jean-Francois B. Use of the dissociating enzyme thermolysin to generate viable human normal intestinal epithelial cell cultures. Exp. Cell. Res. 1996;224:354–364. doi: 10.1006/excr.1996.0145. [DOI] [PubMed] [Google Scholar]
- Pesavento J.B., Crawford S.E., Estes M.K., Prasad B.V. Rotavirus proteins: structure and assembly. Curr. Top. Microbiol. Immunol. 2006;309:189–219. doi: 10.1007/3-540-30773-7_7. [DOI] [PubMed] [Google Scholar]
- Radi Z.A., Ackermann M.R. Growth of differentiated ovine tracheal epithelial cells in vitro. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004;51:167–170. doi: 10.1111/j.1439-0442.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- Ramig R.F. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 2004;78:10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R.G., Micallef S.J., Williams B., Lightowler S., Vincan E., Heath J.K., Mantamadiotis T., Bertoncello I. Colony-stimulating factor-1 promotes clonogenic growth of normal murine colonic crypt epithelial cells in vitro. J. Interferon Cytokine Res. 2004;24:416–427. doi: 10.1089/1079990041535638. [DOI] [PubMed] [Google Scholar]
- Roberts S.J. David and Charles; Vermont, USA: 1986. Veterinary Obstetrics and Genital Diseases Theriogenology. pp. 19–20. [Google Scholar]
- Rollo E.E., Kumar K.P., Reich N.C., Cohen J., Angel J., Greenberg H.B., Sheth R., Anderson J., Oh B., Hempson S.J., Mackow E.R., Shaw R.D. The epithelial cell response to rotavirus infection. J. Immunol. 1999;163:4442–4452. [PubMed] [Google Scholar]
- Rusu D., Loret S., Peulen O., Mainil J., Dandrifosse G. Immunochemical, biomolecular and biochemical characterization of bovine epithelial intestinal primocultures. BMC Cell Biol. 2005;6:42. doi: 10.1186/1471-2121-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Gilchrist J.E., Hudson G.R., Babiuk L.A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J. Virol. 1985;53:58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Smith K.L. Enteric viral infections of calves and passive immunity. J. Dairy Sci. 1985;68:206–228. doi: 10.3168/jds.S0022-0302(85)80813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlage W.K., Bulles H., Friedrichs D., Kuhn M., Teredesai A. Cytokeratin expression patterns in the rat respiratory tract as markers of epithelial differentiation in inhalation toxicology. I. Determination of normal cytokeratin expression patterns in nose, larynx, trachea, and lung. Toxicol. Pathol. 1998;26:324–343. doi: 10.1177/019262339802600307. [DOI] [PubMed] [Google Scholar]
- Sordillo L.M., Oliver S.P., Akers R.M. Culture of bovine mammary epithelial cells in d-valine modified medium: selective removal of contaminating fibroblasts. Cell Biol. Int. Rep. 1988;12:355–364. doi: 10.1016/0309-1651(88)90060-4. [DOI] [PubMed] [Google Scholar]
- Strater J., Wedding U., Barth T.F., Koretz K., Elsing C., Moller P. Rapid onset of apoptosis in vitro follows disruption of beta 1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology. 1996;110:1776–1784. doi: 10.1053/gast.1996.v110.pm8964403. [DOI] [PubMed] [Google Scholar]
- Svensson L., Finlay B.B., Bass D., von Bonsdorff C.H., Greenberg H.B. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J. Virol. 1991;65:4190–4197. doi: 10.1128/jvi.65.8.4190-4197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrich A., Ravindranath R., Farsi K., Targan S. A method for the rapid establishment of normal adult mammalian colonic epithelial cell cultures. In Vitro Cell. Dev. Biol. 1988;24:188–194. doi: 10.1007/BF02623545. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M., Gentsch J.R., Kaiser W.J., Borregaard N., Offermann M.K., Neish A.S., Gewirtz A.T. Protein kinase R mediates intestinal epithelial gene remodeling in response to double-stranded RNA and live rotavirus. J. Immunol. 2005;174:6322–6331. doi: 10.4049/jimmunol.174.10.6322. [DOI] [PubMed] [Google Scholar]
- Whitehead R.H., VanEeden P.E., Noble M.D., Ataliotis P., Jat P.S. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead R.H., Demmler K., Rockman S.P., Watson N.K. Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology. 1999;117:858–865. doi: 10.1016/s0016-5085(99)70344-6. [DOI] [PubMed] [Google Scholar]


