Abstract
Objective
To evaluate the oscillations on the viral detection in adenotonsillar tissues from patients with chronic adenotonsillar diseases as an indicia of the presence of persistent viral infections or acute subclinical infections.
Study design
Cross-sectional prospective study.
Setting
Tertiary hospital.
Methods
The fluctuations of respiratory virus detection were compared to the major climatic variables during a two-year period using adenoids and palatine tonsils from 172 children with adenotonsillar hypertrophy and clinical evidence of obstructive sleep apnoea syndrome or recurrent adenotonsillitis, without symptoms of acute respiratory infection (ARI), by TaqMan real-time PCR.
Results
The rate of detection of at least one respiratory virus in adenotonsillar tissue was 87%. The most frequently detected viruses were human adenovirus in 52.8%, human enterovirus in 47.2%, human rhinovirus in 33.8%, human bocavirus in 31.1%, human metapneumovirus in 18.3% and human respiratory syncytial virus in 17.2%. Although increased detection of human enterovirus occurred in summer/autumn months, and there were summer nadirs of human respiratory syncytial virus in both years of the study, there was no obvious viral seasonality in contrast to reports with ARI patients in many regions of the world.
Conclusion
Respiratory viruses are continuously highly detected during whole year, and without any clinical symptomatology, indicating that viral genome of some virus can persist in lymphoepithelial tissues of the upper respiratory tract.
Keywords: Adenotonsillar hypertrophy, Adenotonsillectomy, Adenovirus, Bocavirus, Enterovirus, Rhinovirus, Respiratory syncytial virus, Viral seasonality
1 Introduction
Acute and chronic respiratory diseases are highly frequent and associated with excessive morbidity and mortality, especially in children, thus having great impact on public health [1], [2]. Respiratory viruses play major roles as etiologic agents of acute respiratory infections (ARI) in children, but their association with chronic respiratory diseases, especially with adenotonsillar hypertrophy, has only recently become the focus of investigation [3], [4], [5].
Adenotonsillar hypertrophy is the most common cause of sleep apnea in children, resulting in craniofacial growth changes and, in severe cases, leading to right ventricular dysfunction and cor pulmonale [6], [7], [8]. As a consequence, adenotonsillectomy is the most frequent surgical procedure performed by otorhinolaryngologists [9]. The aetiologies of chronic hypertrophic adenotonsillar diseases have not been properly established, but are believed to be multifactorial, including allergies, bacterial colonisation and viral infections [3], [10], [11].
We have previously reported high rates of detection of respiratory virus genomes in tonsils and adenoids from patients with chronic adenotonsillar diseases, suggesting a significant association of viruses, particularly picornaviruses, with severe tonsillar hypertrophy [3]. However, no conclusive evidence of productive – acute or persisting – viral infection, as opposed to virus latency, has been established.
In general, peaks of respiratory virus detection in children with ARI occur with marked seasonal variations in temperate and subtropical regions [12], [13]. In tropical areas, the seasonal pattern of viral detection is more difficult to be analysed, due to the heterogeneity of data in several parts of the world. However, respiratory viruses have been mainly observed during the rainy seasons [14]. In southeast Brazil, a region of transition between tropical and subtropical climates, peaks of viral ARI tend to occur during cooler months [15], [16], [17]. The present paper reports analyses of over time variations in rates of detection of respiratory viruses in tissues and secretions removed from children undergoing tonsillectomy while in the absence of ARI symptoms. The rationale was that if detection of respiratory viruses in hypertrophic tonsillar tissues oscillated with variations in temperature and rainfall, in a way similar to what occurs among ARI patients, this would suggest an association with acute subclinical respiratory viral infections, rather than prolonged asymptomatic harbouring of viral nucleic acids in the tissues.
2 Patients and methods
2.1 Patients and sampling
Respiratory virus genomes were searched in adenoids (AD), palatine tonsils (PT) and nasopharyngeal secretions (NPS) obtained from all 172 children (91 males) aged 1–13 years (mean 5.8 years) who underwent adenotonsillectomy to treat adenotonsillar hypertrophy with clinical evidence of obstructive sleep apnoea syndrome [6] or recurrent adenotonsillitis according to Paradise criteria [18]. Patients were treated at the division of Otorhinolaryngology of the School of Medicine of Ribeirão Preto, University of São Paulo, between May 2010 and June 2012. Patients with signs/symptoms of acute respiratory infections within the last four weeks prior to surgery and patients with immunodeficiencies were excluded from the study. Indeed, the exclusion of these patients was a safety criterion for surgery. All clinical samples obtained in this study were maintained in a preservative solution (RNA later – Invitrogen, Carlsbad, CA, USA) at −70 °C until nucleic acid extraction.
2.2 Ethics statement
The study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the University Hospital Clinical Research Ethics Committee (file number 10466/2008). A written informed consent was obtained from all parents and guardians prior to enrolment.
2.3 Detection of respiratory viruses
Nucleic acids from AD, PT and NPS were obtained from 30 mg of tissue or 200 μL of secretion using the AllPrep DNA/RNA mini kit (Qiagen GmbH, Hilden, Germany) or QIAamp Min Elute Virus Spin Kit (Qiagen GmbH, Hilden, Germany), respectively. All samples were tested for the presence of genomes of human adenovirus (HAdV), human enterovirus (HEV), human rhinovirus (HRV), human bocavirus (HBoV), human respiratory syncytial virus (HRSV), metapneumovirus (HMPV), human influenza virus (FLU), human parainfluenza virus (HPIV) and human coronavirus (HCoV) by TaqMan real-time PCR.
The detailed description of each PCR, including the primers sequences, can be obtained in a previously published paper [3]. In the present study were included 51 patients, extending the observation period to two years, allowing that the seasonal pattern of viral circulation was determined in these patients. The analysis of the seasonality of respiratory viruses in adenotonsillar tissues was performed cross matching the virus presence to the temperature and rainfall.
2.4 Analysis of climate variations
Ribeirão Preto is a city in the state of São Paulo, southeast Brazil with a population of 619,746, located at 21°10′40″ S and 47°48′36″ W, 500 m above sea level. The climate is a transition between tropical and subtropical conditions, with annual average temperature of 23 °C, with dry mild winters and hot rainy summers. During this study, the mean monthly minimal and maximal daily temperatures were respectively 18.3 °C (range, 13.5–20.5) and 25.3 °C (range, 23.5–26.8). Rainfall is the major climate variable, with yearly rainy seasons between November and March and dry season from June to September. The mean monthly accumulated rainfall throughout the study was 108 mm, ranging from 0 to 533 mm. Accumulated rainfall and mean seasonal temperatures were obtained from the site of the Integrated Center of Agrometeorological Information of São Paulo Sate (http://www.ciiagro.sp.gov.br).
3 Results
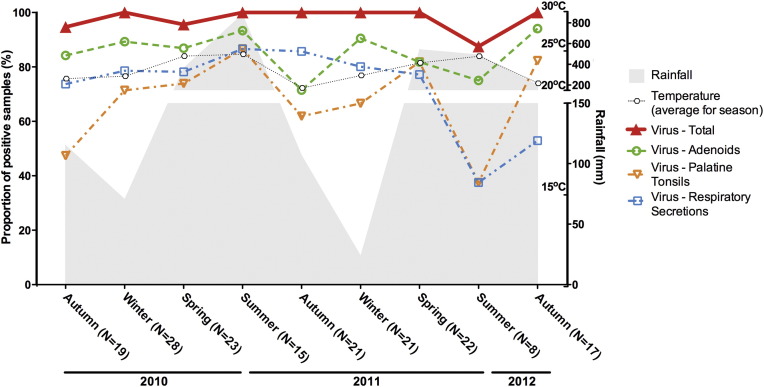
Rates of detection of respiratory viruses in adenoids, tonsils and respiratory secretions were determined for 172 children. Genomes of at least one respiratory virus were detected in over 87% of the patients, without discernible seasonal variations (Fig. 1 ). Remarkably, high rates of virus detection were obtained from all three kinds of clinical samples throughout the study. The frequencies of virus detection ranged from 71.5% to 94.1% in adenoids, and from 37.5% to 86.6% in secretions and palatine tonsils. Of the three sample kinds, adenoid tissue yielded the highest frequencies of virus detection during almost the whole study, except for autumn months (March–June) of 2011, when nasal secretions yielded higher rates of positivity (Fig. 1).
Fig. 1.
Seasonal distribution of the rates of positivity for respiratory viruses in adenoids, palatine tonsils and respiratory secretions from patients with chronic adenotonsillar disease. Numbers of samples tested per season are informed in parenthesis.
The present analysis is based on results from a total of 172 patients, covering a 2-year period, and raises new issues made clear upon inclusion of additional cases to a study that was underway [3]. Overall, the most frequent viruses were human adenovirus (HAdV) detected in 52.8%, followed by human enterovirus (HEV) in 47.2%, human rhinovirus (HRV) in 33.8%, human bocavirus (HBoV) in 31.1%, human metapneumovirus (HMPV) in 18.3%, human respiratory syncytial virus (HRSV) in 17.2%, influenza virus (FLU) in 4.5%, human parainfluenzavirus (HPIV) in 4.5%, and human coronavirus (HCoV) in 2.7%. The frequencies of HAdV, HBoV and HRSV were higher in adenoids, whereas HRV was more frequently detected in nasal secretions and HEV in palatine tonsils. The rates of viral co-infections and the agreement between results from different tissues were high. In this 2-year study period, two or more viruses were detected in 62.2% of the patients, and 54% of them had the same virus detected in adenoids and palatine tonsils.
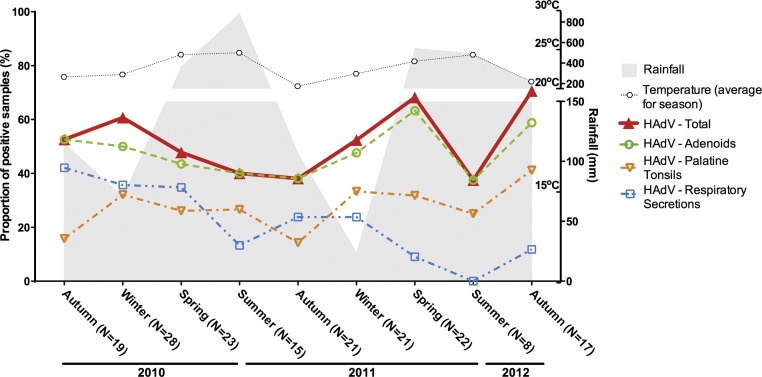
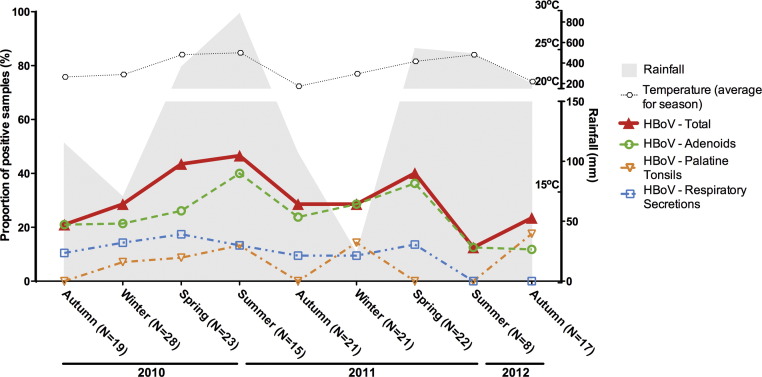
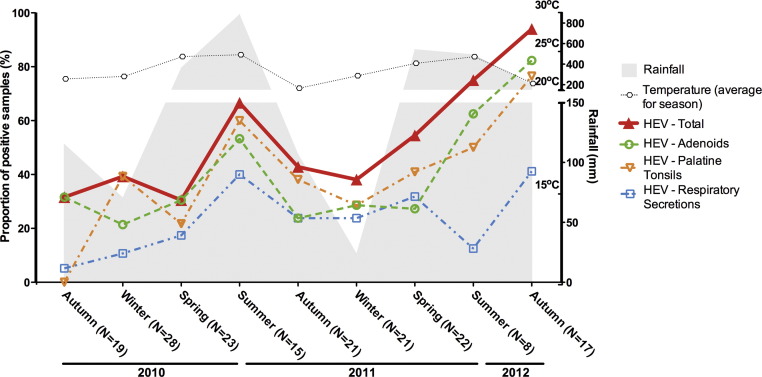
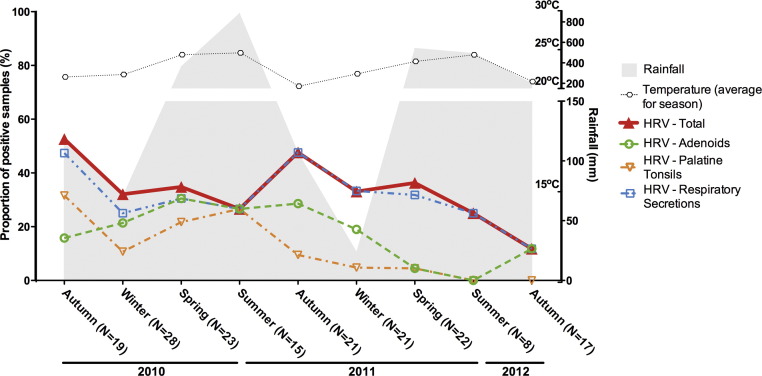
Overall, HAdV detection rates fluctuated from summer troughs of approximately 37.5% up to peaks of greater than 70% in spring-2011 and autumn-2012, without clearly seasonal periods (Fig. 2 ). HBoV detection rates were usually above 20% (12.5–46%) without discernible seasonality (Fig. 3 ). Detection frequencies of HAdV and HBoV were consistently higher in adenoids than in other samples. Rates of detection of the picornaviruses HRV and HEV were opposite in the summer, while the rate of HEV detection was at its peak, HRV was at its lowest (Fig. 4, Fig. 5 ). The overall HEV detection rates were composed mostly by results obtained from adenoids and palatine tonsils, while detection in respiratory secretions was found in a smaller proportion of the HEV-positive patients (Fig. 4). Although peaks of HEV detection occurred in summer/autumn months, the rates of HEV positivity were always above 30%, indicating that a great proportion of tonsil tissues harbour HEV, independently of the season of the year. Contrary to HEV, HRV overall rates showed no clear seasonal variations, and were mostly composed by results obtained from secretions, with correspondingly lower rates of positivity in tonsillar tissues (Fig. 5).
Fig. 2.
Seasonal distribution of the rates of detection of human adenovirus (HAdV) in adenoids, palatine tonsils and respiratory secretions from patients with chronic adenotonsillar disease.
Fig. 3.
Seasonal distribution of the rates of detection of human bocavirus (HBoV) in adenoids, palatine tonsils and respiratory secretions from patients with chronic adenotonsillar disease.
Fig. 4.
Seasonal distribution of rates of detection of human enterovirus (HEV) in adenoids, palatine tonsils and respiratory secretions from patients with chronic adenotonsillar disease.
Fig. 5.
Seasonal distribution of rates of detection of human rhinovirus (HRV) in adenoids, palatine tonsils and respiratory secretions from patients with chronic adenotonsillar disease.
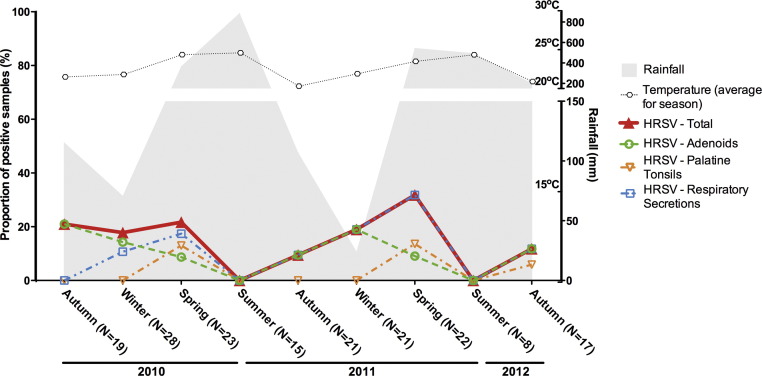
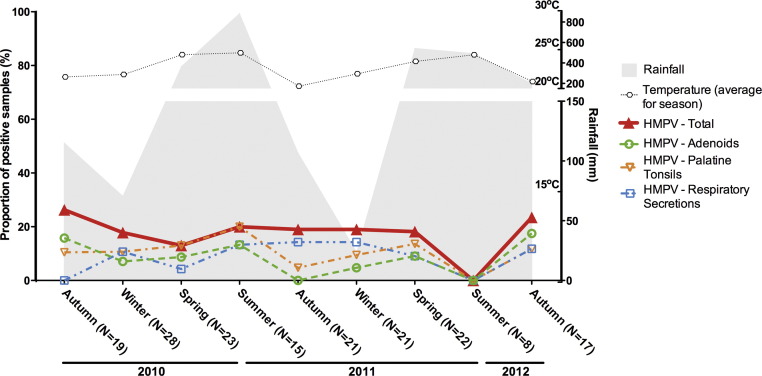
Rates of detection of the paramyxoviruses HMPV and HRSV varied from 0% to 31% during the study period (Fig. 6, Fig. 7 ). HRSV detection reached maximum level in spring/winter months, mostly composed by detection in respiratory secretions, with summer nadirs in both years of the study (Fig. 6). Differently, HMPV overall detection rates did not vary significantly during the study period (Fig. 7), and the nadir observed in the summer of 2012 probably reflects the low number of samples analysed in that season.
Fig. 6.
Seasonal distribution of rates of detection of human respiratory syncytial virus (HRSV) in adenoids, palatine tonsils and respiratory secretions from patients with chronic adenotonsillar disease.
Fig. 7.
Seasonal distribution of rates of detection of human metapneumovirus (HMPV) in adenoids, palatine tonsils and respiratory secretions from patients with chronic adenotonsillar disease.
FLU, HPIV and HCoV were detected in very low frequencies, with sporadic cases distributed during the 2-year study period without seasonal pattern.
4 Discussion
Several studies have shown that some respiratory viruses circulate seasonally, with a typically increase of the viral incidence in colder months, mainly in temperate regions [12], but also in subtropical regions [15], [16], [17]. In tropical regions the results are more difficult to interpret, with several studies indicating higher viral circulation in rainy seasons [14], [19], [20], [21], while others show that respiratory viruses are prevalent year-round [22]. In Salvador for instance, a tropical city in the northeast of Brazil, the presence of viral infections was significantly associated with precipitation during the rainy season in patients with community-acquired pneumonia [19].
It is broadly accepted that respiratory viruses spread by shedding in secretions from acutely symptomatic patients [12], but respiratory viruses are also frequently detected in asymptomatic individuals [23], [24], [25], raising the hypothesis that the viral shedding by people without acute symptoms can be important to the viral dissemination. In fact, high frequencies of detection of respiratory viruses have also been observed in secretions and tissues from patients with chronic adenotonsillar diseases [3], [5], [26], [27], but no analysis of seasonality had been done in that particular setting.
Although the presently reported analyses confirmed previously published findings that found high rates of viral detection in patients with chronic adenotonsillar diseases, the analysis of the fluctuations in viral detection rate during these 2 years showed that most of respiratory viruses have no obvious seasonal pattern, supporting the notion that such high frequency of virus genome detection can be related to virus persistence in lymphoepithelial tissues of the upper respiratory tract.
The discovery of HAdV was consequence of its recovery from adenoid explants [28], and several studies have documented that several adenovirus species, especially adenovirus C, can persist in mucosal lymphoid tissues, possibly by maintenance of the quiescent viral genomes in non-dividing lymphocytes [29], [30]. In fact, HAdV causes persistent/latent infection in tonsillar T-cell subpopulations [29], and can infect continuous B-cell and myeloid cell lineages in vitro [30], suggesting that several different cell populations in tonsils may carry the virus genome. Therefore, it was no surprise that in the present study HAdV was the most frequent respiratory virus detected in adenoids, and the second most frequent in palatine tonsils. In addition, the lack of seasonal trends in rates of HAdV detection, both in tissues and secretions, is confirmatory that most of the high HAdV frequency is attributable to persistence. In tropical regions, adenovirus is frequently associated with the rainy season in patients with acute respiratory infections [19].
HBoV is a parvovirus that occurs worldwide in association with respiratory and gastrointestinal disorders [31], [32]. In addition to the general propensity of parvoviruses to persist and even endogenise into host genomes [33], at least two lines of evidence support persistence of HBoV in humans. Only around 25% of patients who are PCR-positive for HBoV have mRNA for a viral structural protein detectable as a marker of active viral replication [34] and HBoV episomes have been found in human clinical samples, including tissue biopsies [35], [36], [37]. Therefore, it is not surprising that in the present study HBoV was frequently detected, without discernible seasonality, suggesting that, at least part of this high frequency could be attributed to virus persistence, mainly in adenoids.
While persistence and latency of DNA viruses in lymphoepithelial tissues have long been known, the same is not the case for RNA viruses. In the present study, HEV was the second most frequently detected agent at overall frequencies consistently above 30%. Although there are very few studies about seasonality of HEV in tropical regions, the observed trend for an increase in HEV detection towards the summer is in keeping with what happens in temperate regions of the world [38]. However, the high rates of HEV positivity were consistently due to detection in tissue fragments, which was less frequently accompanied by shedding in secretions, supporting the idea that HEV can persist in adenoids and tonsils in a high proportion of patients. While confirmation of HEV persistence in tonsillar tissues will require detailed molecular investigation, the present findings of such consistently and non-seasonal HEV detection over time, coupled with the already reported higher frequencies of HEV detection in the most highly hypertrophic tonsils [3] indicates that perhaps HEV persistence can be associated with the pathogenesis of chronic adenotonsillar diseases.
HRV, the other picornavirus included in the present analyses, is frequently detected in acute respiratory infections of children and adults, usually with marked seasonal variation, especially in subtropical and temperate areas [39], [40], as well as in asymptomatic patients [24]. In tropical regions, the literature results are controversial. In Trinidad, West Indies, HRV was prevalent throughout the year, without seasonal association [41], whilst in Salvador HRV was associated with relative humidity (p = 0.05) [19]. In the present analysis HRV was frequently detected in all seasons, mostly in secretions, rather than in tissues. It is interesting that HRV and HEV are both picornaviruses, currently classified in the same genus, with very similar replication cycles, and yet showed dissimilar frequencies of detection in tissues. HRV was detected in lower frequencies in lymphoid tissues as compared to HEV, suggesting the existence of other sites of infection as sources of HRV shedding into secretions, such as nasal epithelium. This is remarkable, since the patients with hypertrophic adenotonsillar diseases had no acute nasal symptoms at the time of surgery.
Although the overall rates of detection of HMPV and HRSV in the present analyses were lower than those of other viruses, they were still frequently higher than 20%. Moreover, HRSV rates were frequently higher than 10%, with significant contribution of positivity in adenotonsillar tissues, again suggesting that these tissues may be regarded as sites of persistence of paramyxoviruses. Pertaining to this issue, it is interesting that phylogenetic studies of respiratory syncytial virus have pointed to the existence of reservoirs to maintain the virus during inter-seasonal periods, thus creating potential for its reintroduction in the susceptible population [42]. It is therefore reasonable to think that adenoids and tonsils of children with chronic adenotonsillar diseases would be natural reservoirs of respiratory viruses and that, by shedding viruses in respiratory secretions, these children would become sources of infection for their siblings and schoolmates.
To the best of our knowledge, this has been the most comprehensive study so far conducted on the variations of respiratory virus detection rates over time in children with chronic adenotonsillar diseases in a subtropical/tropical region. Although further studies are in order to clarify whether these findings result from long term virus shedding consequent to persistence, or to current asymptomatic viral infections, the lack of obvious seasonal patterns of respiratory viruses in hypertrophic adenotonsillar tissues supports the view that some respiratory viruses may persist in adenoids and tonsils. In the absence of results from molecular markers of active viral replication, the mere detection of viral genomes is not enough to establish that such infections were productive. However, the existence of genomes of numerous viruses in adenotonsillar tissues is an exciting finding, and deserves further investigation. In addition to its obvious epidemiological importance as possible sources of community respiratory virus outbreaks, persistence of these viruses could have pathogenic potential in the development of tonsillar hypertrophy, functioning as chronic stimuli for inflammation. Alternatively, for reasons not yet understood, respiratory viruses could more readily establish an asymptomatic carrier states in patients with chronic tonsillar hypertrophy. However, the lack of control group, with samples of tonsils obtained from healthy patients, makes any inference about the development of disease difficult to be explored in the present study. Thereby, studies about viral persistence in adenoids and tonsils, mainly those including tissue samples from healthy subjects, can bring new insights to the understanding of poorly understood chronic tonsillar diseases that affect large numbers of children worldwide.
Conflict of interest
The authors have no conflicts of interest to disclose and have no financial disclosures to make.
Acknowledgments
The authors thank Maria Cecília Onofre and Helder G. de Souza for secretarial assistance; Lúcia Lopes, Jamila Mendonça de Souza and Maria Lúcia Silva for expert technical support. In addition, the authors thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for financial support (Grant number 2009/51818-8).
References
- 1.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leupe P., Hox V., Debruyne F., Schrooten W., Claes N.V., Lemkens N. Tonsillectomy compared to acute tonsillitis in children: a comparison study of societal costs. B-ENT. 2012;8:103–111. [PubMed] [Google Scholar]
- 3.Proenca-Modena J.L., Pereira Valera F.C., Jacob M.G., Buzatto G.P., Saturno T.H., Lopes L. High rates of detection of respiratory viruses in tonsillar tissues from children with chronic adenotonsillar disease. PLoS ONE. 2012;7:e42136. doi: 10.1371/journal.pone.0042136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suvilehto J., Roivainen M., Seppanen M., Meri S., Hovi T., Carpen O. Rhinovirus/enterovirus RNA in tonsillar tissue of children with tonsillar disease. J. Clin. Virol. 2006;35:292–297. doi: 10.1016/j.jcv.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Szalmas A., Papp Z., Csomor P., Konya J., Sziklai I., Szekanecz Z. Microbiological profile of adenoid hypertrophy correlates to clinical diagnosis in children. Biomed. Res. Int. 2013;2013:629607. doi: 10.1155/2013/629607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balbani A.P., Weber S.A., Montovani J.C. Update in obstructive sleep apnea syndrome in children. Braz. J. Otorhinolaryngol. 2005;71:74–80. doi: 10.1016/S1808-8694(15)31288-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duman D., Naiboglu B., Esen H.S., Toros S.Z., Demirtunc R. Impaired right ventricular function in adenotonsillar hypertrophy. Int. J. Cardiovasc. Imaging. 2008;24:261–267. doi: 10.1007/s10554-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 8.Papaioannou G., Kambas I., Tsaoussoglou M., Panaghiotopoulou-Gartagani P., Chrousos G., Kaditis A.G. Age-dependent changes in the size of adenotonsillar tissue in childhood: implications for sleep-disordered breathing. J. Pediatr. 2012 doi: 10.1016/j.jpeds.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Erdag T.K., Ecevit M.C., Guneri E.A., Dogan E., Ikiz A.O., Sutay S. Pathologic evaluation of routine tonsillectomy and adenoidectomy specimens in the pediatric population: is it really necessary? Int. J. Pediatr. Otorhinolaryngol. 2005;69:1321–1325. doi: 10.1016/j.ijporl.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Diaz R.R., Picciafuoco S., Paraje M.G., Villegas N.A., Miranda J.A., Albesa I. Relevance of biofilms in pediatric tonsillar disease. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:1503–1509. doi: 10.1007/s10096-011-1249-3. [DOI] [PubMed] [Google Scholar]
- 11.Sadeghi-Shabestari M., Jabbari Moghaddam Y., Ghaharri H. Is there any correlation between allergy and adenotonsillar tissue hypertrophy? Int. J. Pediatr. Otorhinolaryngol. 2011;75:589–591. doi: 10.1016/j.ijporl.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Sloan C., Moore M.L., Hartert T. Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin. Transl. Sci. 2011;4:48–54. doi: 10.1111/j.1752-8062.2010.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang J.W., Loh T.P. Correlations between climate factors and incidence – a contributor to RSV seasonality. Rev. Med. Virol. 2014;24:15–34. doi: 10.1002/rmv.1771. [DOI] [PubMed] [Google Scholar]
- 14.Shek L.P., Lee B.W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr. Respir. Rev. 2003;4:105–111. doi: 10.1016/s1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 15.Bellei N., Carraro E., Perosa A., Watanabe A., Arruda E., Granato C. Acute respiratory infection and influenza-like illness viral etiologies in Brazilian adults. J. Med. Virol. 2008;80:1824–1827. doi: 10.1002/jmv.21295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cintra O.A., Owa M.A., Machado A.A., Cervi M.C., Figueiredo L.T., Rocha G.M. Occurrence and severity of infections caused by subgroup A and B respiratory syncytial virus in children in southeast Brazil. J. Med. Virol. 2001;65:408–412. doi: 10.1002/jmv.2049. [DOI] [PubMed] [Google Scholar]
- 17.Straliotto S.M., Siqueira M.M., Muller R.L., Fischer G.B., Cunha M.L., Nestor S.M. Viral etiology of acute respiratory infections among children in Porto Alegre, RS, Brazil. Rev. Soc. Bras. Med. Trop. 2002;35:283–291. doi: 10.1590/s0037-86822002000400002. [DOI] [PubMed] [Google Scholar]
- 18.Paradise J.L., Bluestone C.D., Bachman R.Z., Colborn D.K., Bernard B.S., Taylor F.H. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and nonrandomized clinical trials. N. Engl. J. Med. 1984;310:674–683. doi: 10.1056/NEJM198403153101102. [DOI] [PubMed] [Google Scholar]
- 19.Nascimento-Carvalho C.M., Cardoso M.R., Barral A., Araujo-Neto C.A., Oliveira J.R., Sobral L.S. Seasonal patterns of viral and bacterial infections among children hospitalized with community-acquired pneumonia in a tropical region. Scand. J. Infect. Dis. 2010;42:839–844. doi: 10.3109/00365548.2010.498020. [DOI] [PubMed] [Google Scholar]
- 20.Haynes A.K., Manangan A.P., Iwane M.K., Sturm-Ramirez K., Homaira N., Brooks W.A. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J. Infect. Dis. 2013;208(Suppl. 3):S246–S254. doi: 10.1093/infdis/jit515. [DOI] [PubMed] [Google Scholar]
- 21.Schlaudecker E.P., Heck J.P., Macintyre E.T., Martinez R., Dodd C.N., McNeal M.M. Etiology and seasonality of viral respiratory infections in rural Honduran children. Pediatr. Infect. Dis. J. 2012;31:1113–1118. doi: 10.1097/INF.0b013e31826052eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloom-Feshbach K., Alonso W.J., Charu V., Tamerius J., Simonsen L., Miller M.A. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS ONE. 2013;8:e54445. doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Advani S., Sengupta A., Forman M., Valsamakis A., Milstone A.M. Detecting respiratory viruses in asymptomatic children. Pediatr. Infect. Dis. J. 2012;31:1221–1226. doi: 10.1097/INF.0b013e318265a804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camara A.A., Silva J.M., Ferriani V.P., Tobias K.R., Macedo I.S., Padovani M.A. Risk factors for wheezing in a subtropical environment: role of respiratory viruses and allergen sensitization. J. Allergy Clin. Immunol. 2004;113:551–557. doi: 10.1016/j.jaci.2003.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winther B., Hayden F.G., Hendley J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J. Med. Virol. 2006;78:644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 26.Herberhold S., Eis-Hubinger A.M., Panning M. Frequent detection of respiratory viruses by real-time PCR in adenoid samples from asymptomatic children. J. Clin. Microbiol. 2009;47:2682–2683. doi: 10.1128/JCM.00899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato M., Li H., Ikizler M.R., Werkhaven J.A., Williams J.V., Chappell J.D. Detection of viruses in human adenoid tissues by use of multiplex PCR. J. Clin. Microbiol. 2009;47:771–773. doi: 10.1128/JCM.02331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enders J.F., Bell J.A., Dingle J.H., Francis T., Jr., Hilleman M.R., Huebner R.J. group name proposed for new respiratory-tract viruses. Science. 1956;124:119–120. doi: 10.1126/science.124.3212.119. [DOI] [PubMed] [Google Scholar]
- 29.Garnett C.T., Talekar G., Mahr J.A., Huang W., Zhang Y., Ornelles D.A. Latent species C adenoviruses in human tonsil tissues. J. Virol. 2009;83:2417–2428. doi: 10.1128/JVI.02392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Huang W., Ornelles D.A., Gooding L.R. Modeling adenovirus latency in human lymphocyte cell lines. J. Virol. 2010;84:8799–8810. doi: 10.1128/JVI.00562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vicente D., Cilla G., Montes M., Perez-Yarza E.G., Perez-Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg. Infect. Dis. 2007;13:636–637. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H., Fu Y., Xie J., Cheng J., Ghabrial S.A., Li G. Widespread endogenization of densoviruses and parvoviruses in animal and human genomes. J. Virol. 2011;85:9863–9876. doi: 10.1128/JVI.00828-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proenca-Modena J.L., Gagliardi T.B., Escremim de Paula F., Iwamoto M.A., Criado M.F., Camara A.A. Detection of human bocavirus mRNA in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS ONE. 2011;6:e21083. doi: 10.1371/journal.pone.0021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapoor A., Hornig M., Asokan A., Williams B., Henriquez J.A., Lipkin W.I. Bocavirus episome in infected human tissue contains non-identical termini. PLoS ONE. 2011;6:e21362. doi: 10.1371/journal.pone.0021362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lusebrink J., Schildgen V., Tillmann R.L., Wittleben F., Bohmer A., Muller A. Detection of head-to-tail DNA sequences of human bocavirus in clinical samples. PLoS ONE. 2011;6:e19457. doi: 10.1371/journal.pone.0019457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao H., Zhao L., Sun Y., Qian Y., Liu L., Jia L. Detection of a bocavirus circular genome in fecal specimens from children with acute diarrhea in Beijing, China. PLoS ONE. 2012;7:e48980. doi: 10.1371/journal.pone.0048980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khetsuriani N., Lamonte-Fowlkes A., Oberst S., Pallansch M.A. Enterovirus surveillance – United States, 1970–2005. MMWR Surveill. Summ. 2006;55:1–20. [PubMed] [Google Scholar]
- 39.Jartti T., Lehtinen P., Vuorinen T., Osterback R., van den Hoogen B., Osterhaus A.D. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg. Infect. Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidaurreta S.M., Marcone D.N., Ellis A., Ekstrom J., Cukier D., Videla C. Acute viral respiratory infection in children under 5 years: epidemiological study in two centers in Buenos Aires, Argentina. Arch. Argent. Pediatr. 2011;109:296–304. doi: 10.5546/aap.2011.296. [DOI] [PubMed] [Google Scholar]
- 41.Matthew J., Pinto Pereira L.M., Pappas T.E., Swenson C.A., Grindle K.A., Roberg K.A. Distribution and seasonality of rhinovirus and other respiratory viruses in a cross-section of asthmatic children in Trinidad, West Indies. Ital. J. Pediatr. 2009;35:16. doi: 10.1186/1824-7288-35-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katzov-Eckert H., Botosso V.F., Neto E.A., Zanotto P.M. Phylodynamics and dispersal of HRSV entails its permanence in the general population in between yearly outbreaks in children. PLoS ONE. 2012;7:e41953. doi: 10.1371/journal.pone.0041953. [DOI] [PMC free article] [PubMed] [Google Scholar]