Highlights
-
•
CRP and PTX3 are prototypical short and long pentraxin respectively.
-
•
They are both soluble pattern recognition molecule involved in the innate immune and inflammatory response.
-
•
PTX3 but not CRP is conserved in mouse and men and gene-modified mice help in the understanding of the biological properties.
-
•
Protective and detrimental roles are exerted by PTX3.
Keywords: Innate immunity, Inflammation, Pentraxin, PTX3
Abstract
Pentraxins are a family of multimeric proteins characterized by the presence of a pentraxin signature in their C-terminus region. Based on the primary structure, pentraxins are divided into short and long pentraxin: C-reactive protein (CRP) is the prototype of the short pentraxin subfamily while pentraxin 3 (PTX3) is the prototypic long pentraxin. Despite these two molecules exert similar fundamental actions in the regulation of innate immune and inflammatory responses, several differences exist between CRP and PTX3, including gene organization, protein oligomerization and expression pattern. The pathophysiological roles of PTX3 have been investigated using genetically modified mice since PTX3 gene organization and regulation are well conserved between mouse and human. Such in vivo studies figured out that PTX3 mainly have host-protective effects, even if it could also exert negative effects under certain pathophysiologic conditions. Here we will review the general properties of CRP and PTX3, emphasizing the differences between the two molecules and the regulatory functions exerted by PTX3 in innate immunity and inflammation.
1. Introduction
Pentraxins are a superfamily of phylogenetically conserved and multi-functional proteins [1], [2], [3], which exert basic and evolutionarily conserved innate immune functions such as complement activation and opsonization [4]. Based on the primary structure, pentraxins are divided into two subfamilies: short and long pentraxin [1]. C-reactive protein (CRP) [5] and pentraxin 3 (PTX3) [6] are the well-known, prototypic short and long pentraxin, respectively. These two pentraxins share fundamental functions as fluid-phase pattern recognition molecules (PRMs); however, their structure and expression pattern are diverse. Unlike CRP, the sequence and regulation of PTX3 are conserved from mouse to human, this pushing forward the genetic approach to understand the roles of PTX3 in vivo. Therefore genetically modified mice have been essential to reveal the multifunctional properties of PTX3 at the crossroad between innate immunity, inflammation, matrix deposition and female fertility [1], [4]. Here, we will compare the main characteristics of CRP and PTX3 as prototypes of the short and long pentraxin family respectively, and we will then focus on the complex “yin-yang” roles of PTX3 in innate immunity and inflammation.
2. The pentraxin family
2.1. General features of pentraxins
Pentraxins are evolutionarily conserved, multimeric proteins which share a conserved ∼200 amino acid pentraxin domain containing the so-called “pentraxin signature” (His-x-Cys-x-Ser/Thr-Trp-x-Ser, where x is any amino acid residue) [7] in its C-terminus (Fig. 1A). CRP and Serum Amyloid P component (SAP) [8] are the prototypes of the short pentraxin subfamily [9], while PTX3 is the first identified long pentraxin [6] characterized by the presence of a long unrelated N-terminal domain associated to the C-terminal pentraxin domain. After PTX3, other members of the long pentraxin subfamily were identified, including guinea pig apexin, neuronal pentraxin 1 (NPTX1) [10], [11], neuronal pentraxin 2 (NPTX2, also called Narp) [12], [13] and the transmembrane protein neuronal pentraxin receptor (NPTXR, reviewed in [1], [4]). In an attempt to find new pentraxin domain-containing proteins we recently identified PTX4 [14], a molecule conserved from mammals to lower vertebrates which clusters alone in phylogenetic analysis. However, PTX4 has a unique pattern of mRNA expression, which is distinct from that of other long pentraxins, and it does not behave as acute phase protein [14].
Fig. 1.
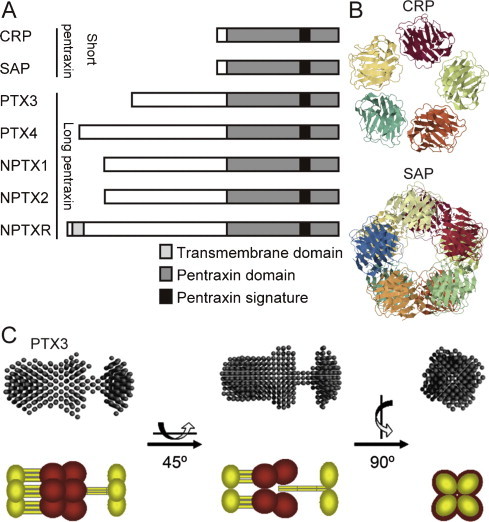
The molecular structures of pentraxin family. (A) Primary structures of human short and long pentraxins. (B) Crystal structures of short pentraxins. Upper panel indicates the pentameric structure of human CRP (Protein Data Bank code 1GNH). The lower panel indicates the structure of human SAP bound to Bis-1,2-{[(Z)-2carboxy-2-methyl-1,3-dioxane]-5-yloxycarbonyl}-piperazine (Protein Data Bank code 2A3X). In SAP two pentamers are interacting face to face to form a decameric structure. (C) Small angle X-ray scattering (SAXS) analysis of PTX3 and its schematic representation. PTX3 N-terminal domain is shown in yellow and C-terminal domain is shown in red, respectively. Panel C is adapted form Inforzato et al. [25].
2.2. Genome, structure and expression pattern of human CRP and PTX3 as prototype of short and long pentraxin
A brief comparison of the main characteristics of CRP and PTX3 is reported in Table 1 . Human CRP gene, located in chromosome 1q23.2, consists of two exons: the first one encodes for the signal peptide (aa 1–18) and for aa 19–20 of the mature protein, the second exon encodes for the remaining amino acids (21–224) of the polypeptide chain [15], [16]. CRP protein assembles into radial symmetric pentamer with planar symmetry (Fig. 1B) [17]. Two cysteine residues (aa 36 and 97 of the mature protein), which are conserved between pentraxins, forms intra-molecule disulfide bonds [18], while non-covalent interactions stabilize the pentamer. Some transcription factor binding sequences (HNF1α, C/EBPβ/δ, STAT3, p50 and c-Rel) are located in the promoter region of human CRP gene [19], [20]. CRP is produced in the liver in response to cytokine stimulation, mostly contributed by interleukin (IL)-6 and, to less degree, by IL-1β. CRP is known as major acute phase protein in human: under inflammatory condition, its circulating level increases as much as 1000-fold from the basal value (3 mg/L) [21], [22]. Thus circulating CRP levels reflect the systemic inflammatory responses. SAP has 51% amino acid identity with CRP and a similar pentameric structure (Fig. 1B). The two proteins share many structural and biological characteristics including the capacity to interact in a calcium-dependent fashion with different ligands.
Table 1.
General features of CRP and PTX3.
| CRP (short pentraxin family) | PTX3 (long pentraxin family) | |
|---|---|---|
| Gene location | 1q23.2 | 3q25 |
| Exons | Two | Three |
| Glycosylation | No | N-glycosylation site at Asn220 |
| Multimeric formation | Pentamer with non-covalent interactions | Octarmer with intra-molecule disulfide bonds |
| Binding sequences in promoter region | HNF1α, C/EBPβ/δ, STAT3, p50, c-Rel | Pu-1, AP1, NF-κB, SP1, NF-IL6 |
| Major stimulus | IL-6 | TLR agonists, IL-1, TNF |
| Producers | Liver (Hepatocytes) | Monocytes, macrophage, PMN, EC, DC, fibroblasts, epithelial cells |
Abbreviations: CRP, C-reactive protein; PTX3, pentraxin 3; PMN, polymorph nuclear neutrophil; EC, endothelial cell; DC, dendritic cell.
Human PTX3 gene, located in chromosome 3q25, consists of three exons with the first and second exons encoding the signal peptide (aa 1–17) and the N-terminal domain (aa 18–178) and the third exon encoding the C-terminal pentraxin domain (aa 179–381) [6]. An N-linked glycosylation site is located on Asn220 and is occupied by fucosylated and sialylated biantennary, triantennary and tetraantennary structures [23]. Eight identical protomers are assembled to form an elongated octamer with a molecular weight of 344 kDa stabilized by inter-molecular disulfide bonds [24]. The N-terminal domain participates in the formation of radial asymmetric tetramers, and the C-terminal domain participates in the dimerization of two tetramers by a “stacking” manner [24], [25] (Fig. 1C).
Some transcription factor binding sequences (Pu-1, AP1, NF-κB, SP1 and NF-IL6) are located in the promoter region of human PTX3 [26], [27]. In contrast to the IL-6-mediated CRP production, PTX3 is produced in response to several primary inflammatory signals such as TLR agonists, IL-1β and tumor necrosis factor α (TNFα) [1], [28]. In addition, various types of cells express PTX3 upon appropriate stimulation. PTX3-expressing cells include myeloid dendritic cells [29], monocytes/macrophages [30], [31], vascular endothelial cells [6], [32], smooth muscle cells [33], fibroblasts [31], [32], adipocytes [34], glial cells [35], cumulus oophorus cells [36], mesangial cells [37] and synovial cells [38]. An unexpected constitutive expression of PTX3 has been observed in lymphatic endothelial cells [39]. In addition, polymorphonuclear neutrophils (PMN) contain a storage of mature protein accumulated in specific granules during their maturation process in the bone marrow [40]. Stored PTX3 is promptly released to the extracellular space upon bacterial stimulation (i.e. E. coli, S. aureus or zymosan), as well as following phorbol 12-myristate 13-acetate (PMA), ionomycin or TNFα treatment. Released PTX3 is localized to neutrophil extracellular traps (NETs) and exerts non-redundant role in pathogen resistance [40]. NETs are a mesh-like component consisting of DNA decorated with histones and anti-microbial proteins, which can trap microbes and form an antimicrobial proteins-rich microenvironment [41], [42], [43]. The variety of PTX3-producing cells ensures that PTX3 is produced at local sites where the primary inflammation occurs.
Similarly to CRP, PTX3 is an acute phase biomarker. The basal circulating levels of PTX3 are low (<2 ng/mL) [44], [45], [46] and increase rapidly (peaks at 6–8 h) in acute myocardial infarction (AMI) to values 3–5 times higher than the normal range [46], [47] A more dramatic increases in PTX3 plasma levels (200–800 ng/mL up to 1500 ng/mL) has been observed during endotoxic shock, sepsis, and other inflammatory and infectious conditions, including meningococcal diseases, dengue infection, tuberculosis and leptospirosis [45], [48], [49], [50] In a small group of critically ill patients with systemic respiratory distress syndrome, sepsis or septic shock, PTX3 levels correlate with severity of disease and infection [51], [52], [53], [54].
The unique local production pathways and ready-to-release system suggest that PTX3 may become a sensitive biomarker which reflects the primary and local signal of innate immune response and inflammation. Data collected so far indicate that PTX3 could be a new candidate prognostic marker in cardiovascular diseases and might be associated with risk of mortality in severe sepsis and septic shock [47], [53], [55], [56], [57], [58], [59], [60].
3. The positive and negative roles of PTX3 in inflammation and innate immunity
3.1. Effects and roles of PTX3 in infectious conditions
PTX3 has an ability to bind to certain microbes, including fungi (conidia of Aspergillus fumigatus, Paracoccidoides brasiliensis and zymosan) [61], [62]; selected Gram-positive and Gram-negative bacteria (e.g. Pseudomonas aeruginosa, Klebsiella pneumoniae, Neisseria meningitides) [63], [64] and unpublished observation; and viruses (influenza virus type A, human and murine cytomegalovirus, murine hepatitis virus) [65], [66], [67]. In vivo, PTX3 plays non-redundant roles in the defense against infections caused by recognized microbes. Ptx3−/− mice are more susceptible to infections with Aspergillus fumigatus, Pseudomonas aeruginosa [61], murine cytomegalovirus [65] and influenza virus [66]. We reported that PTX3 has therapeutic effects acting as an opsonin, thus facilitating recognition and phagocytosis of microbes in an Fcγ receptor- and complement-dependent manner [64], [68] (Table 2 ). PTX3 also exerts protective effect to infection-induced organ injury. In a murine model of severe acute respiratory syndrome (SARS) caused by murine hepatitis virus 1 (MHV-1) infection, ptx3−/− mice exhibit a more severe lung injury compared to wild type mice at early time points (days 1–4 after infection) [67]. PTX3 deficiency is associated with a higher early infiltration of neutrophils and macrophages into the lung. Indeed recombinant PTX3 administration significantly enhanced viral clearance, reduced lung injury, neutrophils influx in lungs and levels of inflammatory cytokines, thus indicating a protective role of PTX3 in this model of lung injury [67] (Table 2). Analyses of PTX3-overexpressing mice further support the protective role against infection. PTX3-overexpressing mice result to be protected from severe inflammatory reactions such as LPS-induced endotoxemia and cecal-ligation and puncture (CLP) [69], which are common experimental sepsis models [70]. However in a model of infection with Klebsiella pneumonia, PTX3-overexpressing mice show different susceptibility depending on the doses of the pathogen. In fact these mice showed faster lethality when receiving higher inocula, and protection when treated with lower amount of bacteria [71]. This reversible response of PTX3-overexpressing mice was accompanied by reduced neutrophil influx and increased TNFα level in higher inocula, and enhanced neutrophil influx and no-change of TNFα level in lower inocula [71] (Table 2).
Table 2.
Mechanisms of PTX3 in inflammation and innate immunity.
| Effects exerted by PTX3 | Suggested and/or reported mechanisms by PTX3 | References |
|---|---|---|
| Protection against infections | - Facilitates phagocytosis of pathogens through complement, complement receptor and Fcγ receptor - Enhances viral clearance, suppresses neutrophil infiltration and inflammatory mediators in MHV-1 induced lung injury - Exerts reversible effects depending on bacteria burden (K. Pneumoniae), suppressing neutrophil infiltration and increasing TNFα level in higher inoculation, while facilitating neutrophil infiltration and un-affecting TNFα level in lower inoculation |
[64], [68] [67] [71] |
| Protection against acute myocardial infarction | - Reduces no-reflow area, IL-6 level, neutrophil infiltration and C3 deposition | [72] |
| Protection after ischemic stroke | - Reduces blood–brain barrier (BBB) damage, and participates in the resolution of edema and glial scar formation | [74] |
| Protection against lung injury | - Reduces neutrophil infiltration, cell death and fibrin deposition in LPS-induced ALI |
[75], [81] |
| Protection against LPS damage | - Controls IL-10 production, and enhances nitric oxide production from macrophages in a model of endotoxemia | [69] |
| Protection against acute kidney injury | - Prevents leukocyte recruitment and abrogates acute renal failure | [77] |
| Detrimental effects | - Facilitates neutrophil infiltration and proinflammatory cytokine levels (TNFα, IL-1β, CCL2; CXCL1) in a model of intestinal ischemia and reperfusion Increases IL-1β, CCL2 and CXCL1 mRNA level in ventilation-induced lung injury |
[78], [79] [80] |
3.2. Effects and roles of PTX3 in sterile inflammatory conditions
The protective effect of PTX3 was also reported in sterile inflammation such as acute myocardial infarction (AMI) [72], seizure-induced neurodegeneration [73], cerebral ischemia [74] and acute lung injury (ALI) [75]. In a murine AMI model caused by coronary artery ligation and reperfusion, an increased myocardial damage associated with a greater no-reflow area, an increased neutrophil infiltration, a decreased number of capillaries, and an increased number of apoptotic cardiomyocytes were observed in ptx3−/− mice [72]. Additionally we observed an increased deposition of the complement component C3 delimiting the injured area, suggesting that modulation of complement activation could contribute to the cardioprotective role of PTX3 (Table 2). In a model of limbic seizure, PTX3 binds to dying cells protecting them from irreversible damage and conferring resistance to neurodegeneration [73]. On the contrary, at early time point (48 h) of ischemic brain injury, no differences were observed in infarct volume, brain edema and blood–brain barrier (BBB) damage between ptx3−/− and wild type mice [74]. However after 6 days of ischemic brain injury, BBB damage was higher and edema resolution did not occur in ptx3−/− mice. Additionally the scar formation was impaired in mice lacking PTX3, with less extracellular matrix production and reduction of microglial proliferation (Table 2). The higher susceptibility of ptx3−/− mice to LPS-induced ALI [75] indicates the defensive role of PTX3. In this model, the lung injury was accompanied by elevated neutrophil infiltration, cell death and fibrin deposition in the lung. PTX3 deficiency also enhanced LPS-induced tissue factor expression/activation in the lung and increased TNFα and CCL2 levels in the plasma [75] (Table 2). The higher resistance to endotoxemia by LPS was also observed in PTX3 overexpressing animals [69], which is associated to higher levels of IL-10 and nitric oxide (NO) production by peritoneal macrophages (Table 2).
In contrast to the results mentioned above, in vivo studies revealed that PTX3 could also have detrimental effects in certain pathologic conditions. A reversible effect was reported in ischemic acute kidney injury (AKI). Chen and coworkers reported that ptx3−/− mice have a reduced early expression of endothelial adhesion molecules and chemokines, and an ameliorated acute kidney injury [76]. On the contrary other reports showed that the post-ischemic renal injury was aggravated in ptx3−/− mice [77], and PTX3 administration was able to prevent renal leukocyte recruitment and post-ischemic kidney injury (Table 2). In an intestinal ischemia and reperfusion model, only negative effects of PTX3 were observed both in PTX3-overexpressing mice [78] and ptx3−/− mice [79]. The impaired survival rate of PTX3-overexpressing animals after ischemia and reperfusion of the superior mesenteric artery correlated with enhanced neutrophil influx and higher levels of proinflammatory cytokines (TNFα, IL-1β, CCL2, CXCL1) [78]. In agreement, when the same model was applied to ptx3−/− mice, a decreased neutrophil influx and reduction of lethality was observed accompanied to decreased cytokine production [79] (Table 2). The negative effect of PTX3 also occurred in ventilation-induced lung injury [80]. In this model PTX3 overexpression led the increased mRNA levels of IL-1β, CCL2, CXCL1 and accelerated the development of lung injury (Table 2).
Neutrophils recruitment into tissues is a common hallmark observed during the inflammatory/infectious response. Neutrophil infiltration can act as a double-edged sword during the inflammatory response, participating in tissue repair and defense but also further promoting tissue damage, as observed in ischemia-reperfusion injury, shock, systemic septicemia and ALI. Searching for a possible mechanism explaining the regulatory role of PTX3 on inflammation, we explored the interaction of this protein with adhesion molecules involved in the initial steps of leukocyte extravasation. Indeed we observed a specific binding of PTX3 to P-selectin but not to L- and E-selectin [81]. We also demonstrated that, through the interaction with P-selectin, PTX3 either derived from activated leukocytes or administered exogenously could attenuate neutrophils recruitment at sites of inflammation in a model of pleurisy or acute lung injury [81] (Table 2). This negative feedback mechanism may globally participate in the protective role of PTX3 in inflammation as well as ALI [81].
Over all, these positive or negative, sometimes reversible, effects of PTX3 (Fig. 2 ) reflect that the major PTX3 functions may differ in each pathophysiological condition. The elucidation of each specific role of PTX3 is essential for the clinical use of the protein.
Fig. 2.
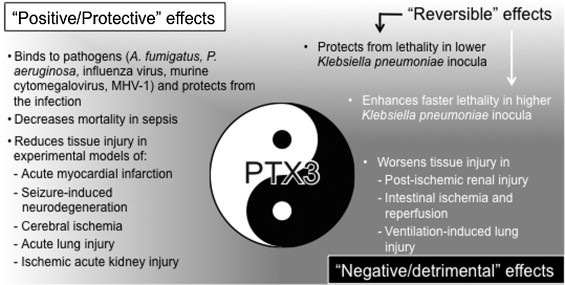
The “yin-yang” effects of PTX3 in infectious or sterile inflammatory conditions. The protective effects of PTX3 are shown in black text and the detrimental effects of PTX3 are shown in white text. The upper half of the panel assigns infectious conditions and the lower half assigns sterile inflammatory conditions.
4. Concluding remarks
CRP is a prototypic short pentraxin and a typical acute phase biomarker since it is produced as a result of systemic inflammatory responses. Conversely, PTX3 is distinguished by being an early and local acute phase biomarker, differing from CRP for gene organization, protein oligomerization and expression pattern. In addition a main difference between CRP and PTX3 is represented by the different gene regulation from mouse to human. In vivo experiments on genetically modified animals are helpful to understand the roles of PTX3 in innate immunity and inflammation. Accumulated evidences from in vivo studies indicate that, although the predominant roles of PTX3 were host-protective, detrimental effects were observed in certain experimental settings. This “yin-yang” behavior in host-defense (Fig. 2) likely derives from the multi-functional properties of PTX3. Further investigations are necessary for the clinical use of the protein as diagnostics and therapeutics.
Acknowledgements
K.D. is supported by Young Scientists Development Program, Research Center for Advanced Science and Technology at the University of Tokyo (funded by FUJIFILM Corporation). The contribution of the European Commission (FP7-HEALTH-2011-ADITEC-280873), European Research Council (project HIIS), Ministero della Salute (Ricerca finalizzata), the Italian Association for Cancer Research (AIRC), and Regione Lombardia (project Metadistretti – SEPSIS) is gratefully acknowledged.
Contributor Information
Alberto Mantovani, Email: alberto.mantovani@humanitasresearch.it.
Barbara Bottazzi, Email: barbara.bottazzi@humanitasresearch.it.
References
- 1.Garlanda C., Bottazzi B., Bastone A., Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A., Garlanda C., Doni A., Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 3.Deban L., Bottazzi B., Garlanda C., de la Torre Y.M., Mantovani A. Pentraxins: multifunctional proteins at the interface of innate immunity and inflammation. BioFactors. 2009;35:138–145. doi: 10.1002/biof.21. [DOI] [PubMed] [Google Scholar]
- 4.Bottazzi B., Doni A., Garlanda C., Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Ann Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 5.Abernethy T.J., Avery O.T. The occurrence during acute infections of a protein not normally present in the blood I. Distribution of the reactive protein in patients’ sera and the effect of calcium on the flocculation reaction with C-polysaccharide of pneumococcus. J Exp Med. 1941;73:173–182. doi: 10.1084/jem.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breviario F., d’Aniello E.M., Golay J., Peri G., Bottazzi B., Bairoch A. Interleukin-1-inducible genes in endothelial cells cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 7.Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991;19(Suppl.):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emsley J., White H.E., O’Hara B.P., Oliva G., Srinivasan N., Tickle I.J. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 9.Du Clos T.W. Pentraxins: structure function, and role in inflammation. ISRN Inflamm. 2013;2013:379040. doi: 10.1155/2013/379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlimgen A.K., Helms J.A., Vogel H., Perin M.S. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519–526. doi: 10.1016/0896-6273(95)90308-9. [DOI] [PubMed] [Google Scholar]
- 11.Omeis I.A., Hsu Y.C., Perin M.S. Mouse and human neuronal pentraxin 1 (NPTX1): conservation, genomic structure, and chromosomal localization. Genomics. 1996;36:543–545. doi: 10.1006/geno.1996.0503. [DOI] [PubMed] [Google Scholar]
- 12.Hsu Y.C., Perin M.S. Human neuronal pentraxin II (NPTX2): conservation, genomic structure, and chromosomal localization. Genomics. 1995;28:220–227. doi: 10.1006/geno.1995.1134. [DOI] [PubMed] [Google Scholar]
- 13.Tsui C.C., Copeland N.G., Gilbert D.J., Jenkins N.A., Barnes C., Worley P.F. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez de la Torre Y., Fabbri M., Jaillon S., Bastone A., Nebuloni M., Vecchi A. Evolution of the pentraxin family: the new entry PTX4. J Immunol. 2010;184:5055–5064. doi: 10.4049/jimmunol.0901672. [DOI] [PubMed] [Google Scholar]
- 15.Lei K.J., Liu T., Zon G., Soravia E., Liu T.Y., Goldman N.D. Genomic DNA sequence for human C-reactive protein. J Biol Chem. 1985;260:13377–13383. [PubMed] [Google Scholar]
- 16.Woo P., Korenberg J.R., Whitehead A.S. Characterization of genomic and complementary DNA sequence of human C-reactive protein, and comparison with the complementary DNA sequence of serum amyloid P component. J Biol Chem. 1985;260:13384–13388. [PubMed] [Google Scholar]
- 17.Shrive A.K., Cheetham G.M., Holden D., Myles D.A., Turnell W.G., Volanakis J.E. Three dimensional structure of human C-reactive protein. Nat Struct Biol. 1996;3:346–354. doi: 10.1038/nsb0496-346. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira E.B., Gotschlich C., Liu T.Y. Primary structure of human C-reactive protein. J Biol Chem. 1979;254:489–502. [PubMed] [Google Scholar]
- 19.Volanakis J.E. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–197. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 20.Young D.P., Kushner I., Samols D. Binding of C/EBPbeta to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. J Immunol. 2008;181:2420–2427. doi: 10.4049/jimmunol.181.4.2420. [DOI] [PubMed] [Google Scholar]
- 21.Schultz D.R., Arnold P.I. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 1990;20:129–147. doi: 10.1016/0049-0172(90)90055-k. [DOI] [PubMed] [Google Scholar]
- 22.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inforzato A., Reading P.C., Barbati E., Bottazzi B., Garlanda C., Mantovani A. The sweet side of a long pentraxin: how glycosylation affects PTX3 functions in innate immunity and inflammation. Front Immunol. 2012;3:407. doi: 10.3389/fimmu.2012.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inforzato A., Rivieccio V., Morreale A.P., Bastone A., Salustri A., Scarchilli L. Structural characterization of PTX3 disulfide bond network and its multimeric status in cumulus matrix organization. J Biol Chem. 2008;283:10147–10161. doi: 10.1074/jbc.M708535200. [DOI] [PubMed] [Google Scholar]
- 25.Inforzato A., Baldock C., Jowitt T.A., Holmes D.F., Lindstedt R., Marcellini M. The angiogenic inhibitor long pentraxin PTX3 forms an asymmetric octamer with two binding sites for FGF2. J Biol Chem. 2010;285:17681–17692. doi: 10.1074/jbc.M109.085639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basile A., Sica A., d’Aniello E., Breviario F., Garrido G., Castellano M. Characterization of the promoter for the human long pentraxin PTX3 role of NF-kappaB in tumor necrosis factor-alpha and interleukin-1beta regulation. J Biol Chem. 1997;272:8172–8178. doi: 10.1074/jbc.272.13.8172. [DOI] [PubMed] [Google Scholar]
- 27.He X., Han B., Liu M. Long pentraxin 3 in pulmonary infection and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2007;292:1039–1049. doi: 10.1152/ajplung.00490.2006. [DOI] [PubMed] [Google Scholar]
- 28.Inforzato A., Jaillon S., Moalli F., Barbati E., Bonavita E., Bottazzi B. The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens. 2011;77:271–282. doi: 10.1111/j.1399-0039.2011.01645.x. [DOI] [PubMed] [Google Scholar]
- 29.Doni A., Peri G., Chieppa M., Allavena P., Pasqualini F., Vago L. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003;33:2886–2893. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- 30.Alles V.V., Bottazzi B., Peri G., Golay J., Introna M., Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- 31.Goodman A.R., Levy D.E., Reis L.F., Vilcek J. Differential regulation of TSG-14 expression in murine fibroblasts and peritoneal macrophages. J Leukoc Biol. 2000;67:387–395. doi: 10.1002/jlb.67.3.387. [DOI] [PubMed] [Google Scholar]
- 32.Lee G.W., Lee T.H., Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804–1812. [PubMed] [Google Scholar]
- 33.Klouche M., Peri G., Knabbe C., Eckstein H.H., Schmid F.X., Schmitz G. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis. 2004;175:221–228. doi: 10.1016/j.atherosclerosis.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Abderrahim-Ferkoune A., Bezy O., Chiellini C., Maffei M., Grimaldi P., Bonino F. Characterization of the long pentraxin PTX3 as a TNFalpha-induced secreted protein of adipose cells. J Lipid Res. 2003;44:994–1000. doi: 10.1194/jlr.M200382-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Polentarutti N., Bottazzi B., Di Santo E., Blasi E., Agnello D., Ghezzi P. Inducible expression of the long pentraxin PTX3 in the central nervous system. J Neuroimmunol. 2000;106:87–94. doi: 10.1016/s0165-5728(00)00214-9. [DOI] [PubMed] [Google Scholar]
- 36.Salustri A., Garlanda C., Hirsch E., De Acetis M., Maccagno A., Bottazzi B. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 37.Nauta A.J., de Haij S., Bottazzi B., Mantovani A., Borrias M.C., Aten J. Human renal epithelial cells produce the long pentraxin PTX3. Kidney Int. 2005;67:543–553. doi: 10.1111/j.1523-1755.2005.67111.x. [DOI] [PubMed] [Google Scholar]
- 38.Luchetti M.M., Piccinini G., Mantovani A., Peri G., Matteucci C., Pomponio G. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA) Clin Exp Immunol. 2000;119:196–202. doi: 10.1046/j.1365-2249.2000.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sironi M., Conti A., Bernasconi S., Fra A.M., Pasqualini F., Nebuloni M. Generation and characterization of a mouse lymphatic endothelial cell line. Cell Tissue Res. 2006;325:91–100. doi: 10.1007/s00441-006-0171-y. [DOI] [PubMed] [Google Scholar]
- 40.Jaillon S., Peri G., Delneste Y., Fremaux I., Doni A., Moalli F. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 42.Medina E. Neutrophil extracellular traps: a strategic tactic to defeat pathogens with potential consequences for the host. J Innate Immun. 2009;1:176–180. doi: 10.1159/000203699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amulic B., Hayes G. Neutrophil extracellular traps. Curr Biol. 2011;21:R297–R298. doi: 10.1016/j.cub.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Yamasaki K., Kurimura M., Kasai T., Sagara M., Kodama T., Inoue K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med. 2009;47:471–477. doi: 10.1515/CCLM.2009.110. [DOI] [PubMed] [Google Scholar]
- 45.Azzurri A., Sow O.Y., Amedei A., Bah B., Diallo S., Peri G. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Peri G., Introna M., Corradi D., Iacuitti G., Signorini S., Avanzini F. PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 47.Latini R., Maggioni A.P., Peri G., Gonzini L., Lucci D., Mocarelli P. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 48.Mairuhu A.T., Peri G., Setiati T.E., Hack C.E., Koraka P., Soemantri A. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol. 2005;76:547–552. doi: 10.1002/jmv.20397. [DOI] [PubMed] [Google Scholar]
- 49.Sprong T., Peri G., Neeleman C., Mantovani A., Signorini S., van der Meer J.W. Pentraxin 3 and C-reactive protein in severe meningococcal disease. Shock. 2009;31:28–32. doi: 10.1097/SHK.0b013e31817fd543. [DOI] [PubMed] [Google Scholar]
- 50.Wagenaar J.F., Goris M.G., Gasem M.H., Isbandrio B., Moalli F., Mantovani A. Long pentraxin PTX3 is associated with mortality and disease severity in severe leptospirosis. J Infect. 2009;58:425–432. doi: 10.1016/j.jinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Muller B., Peri G., Doni A., Torri V., Landmann R., Bottazzi B. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–1407. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Mauri T., Coppadoro A., Bellani G., Bombino M., Patroniti N., Peri G. Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity. Crit Care Med. 2008;36:2302–2308. doi: 10.1097/CCM.0b013e3181809aaf. [DOI] [PubMed] [Google Scholar]
- 53.Mauri T., Bellani G., Patroniti N., Coppadoro A., Peri G., Cuccovillo I. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010;36:621–629. doi: 10.1007/s00134-010-1752-5. [DOI] [PubMed] [Google Scholar]
- 54.Daigo K., Hamakubo T. Host-protective effect of circulating pentraxin 3 (PTX3) and complex formation with neutrophil extracellular traps. Front Immunol. 2012;3:378. doi: 10.3389/fimmu.2012.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brugger-Andersen T., Ponitz V., Kontny F., Staines H., Grundt H., Sagara M. The long pentraxin 3 (PTX3): a novel prognostic inflammatory marker for mortality in acute chest pain. Thromb Haemost. 2009;102:555–563. doi: 10.1160/TH09-02-0137. [DOI] [PubMed] [Google Scholar]
- 56.Jenny N.S., Arnold A.M., Kuller L.H., Tracy R.P., Psaty B.M. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2009;29:594–599. doi: 10.1161/ATVBAHA.108.178947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsui S., Ishii J., Kitagawa F., Kuno A., Hattori K., Ishikawa M. Pentraxin 3 in unstable angina and non-ST-segment elevation myocardial infarction. Atherosclerosis. 2010;210:220–225. doi: 10.1016/j.atherosclerosis.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 58.Huttunen R., Hurme M., Aittoniemi J., Huhtala H., Vuento R., Laine J. High plasma level of long pentraxin 3 (PTX3) is associated with fatal disease in bacteremic patients: a prospective cohort study. PLoS ONE. 2011;6:e17653. doi: 10.1371/journal.pone.0017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inoue K., Kodama T., Daida H. Pentraxin 3: a novel biomarker for inflammatory cardiovascular disease. Int J Vasc Med. 2012;2012:657025. doi: 10.1155/2012/657025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bastrup-Birk S., Skjoedt M.O., Munthe-Fog L., Strom J.J., Ma Y.J., Garred P. Pentraxin-3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLoS ONE. 2013;8:e73119. doi: 10.1371/journal.pone.0073119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garlanda C., Hirsch E., Bozza S., Salustri A., De Acetis M., Nota R. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 62.Diniz S.N., Nomizo R., Cisalpino P.S., Teixeira M.M., Brown G.D., Mantovani A. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J Leukoc Biol. 2004;75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 63.Jeannin P., Bottazzi B., Sironi M., Doni A., Rusnati M., Presta M. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Moalli F., Paroni M., Veliz Rodriguez T., Riva F., Polentarutti N., Bottazzi B. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J Immunol. 2011;186:5425–5434. doi: 10.4049/jimmunol.1002035. [DOI] [PubMed] [Google Scholar]
- 65.Bozza S., Bistoni F., Gaziano R., Pitzurra L., Zelante T., Bonifazi P. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood. 2006;108:3387–3396. doi: 10.1182/blood-2006-03-009266. [DOI] [PubMed] [Google Scholar]
- 66.Reading P.C., Bozza S., Gilbertson B., Tate M., Moretti S., Job E.R. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 67.Han B., Ma X., Zhang J., Zhang Y., Bai X., Hwang D.M. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab Invest. 2012;92:1285–1296. doi: 10.1038/labinvest.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moalli F., Doni A., Deban L., Zelante T., Zagarella S., Bottazzi B. Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–5180. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 69.Dias A.A., Goodman A.R., Dos Santos J.L., Gomes R.N., Altmeyer A., Bozza P.T. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol. 2001;69:928–936. [PubMed] [Google Scholar]
- 70.Ward P.A. New approaches to the study of sepsis. EMBO Mol Med. 2012;4:1234–1243. doi: 10.1002/emmm.201201375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soares A.C., Souza D.G., Pinho V., Vieira A.T., Nicoli J.R., Cunha F.Q. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 2006;8:1321–1329. doi: 10.1016/j.micinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 72.Salio M., Chimenti S., De Angelis N., Molla F., Maina V., Nebuloni M. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 73.Ravizza T., Moneta D., Bottazzi B., Peri G., Garlanda C., Hirsch E. Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: evidence for a protective role in seizure-induced neurodegeneration. Neuroscience. 2001;105:43–53. doi: 10.1016/s0306-4522(01)00177-4. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez-Grande B., Swana M., Nguyen L., Englezou P., Maysami S., Allan S.M. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J Cereb Blood Flow Metab. 2014;34:480–488. doi: 10.1038/jcbfm.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han B., Haitsma J.J., Zhang Y., Bai X., Rubacha M., Keshavjee S. Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med. 2011;37:334–342. doi: 10.1007/s00134-010-2067-2. [DOI] [PubMed] [Google Scholar]
- 76.Chen J., Matzuk M.M., Zhou X.J., Lu C.Y. Endothelial pentraxin 3 contributes to murine ischemic acute kidney injury. Kidney Int. 2012;82:1195–1207. doi: 10.1038/ki.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lech M., Rommele C., Grobmayr R., Eka Susanti H., Kulkarni O.P., Wang S. Endogenous and exogenous pentraxin-3 limits postischemic acute and chronic kidney injury. Kidney Int. 2013;83:647–661. doi: 10.1038/ki.2012.463. [DOI] [PubMed] [Google Scholar]
- 78.Souza D.G., Soares A.C., Pinho V., Torloni H., Reis L.F., Teixeira M.M. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. 2002;160:1755–1765. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Souza D.G., Amaral F.A., Fagundes C.T., Coelho F.M., Arantes R.M., Sousa L.P. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am J Pathol. 2009;174:1309–1318. doi: 10.2353/ajpath.2009.080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Real J.M., Spilborghs G.M., Morato-Marques M., de Moura R.P., Negri E.M., Camargo A.A. Pentraxin 3 accelerates lung injury in high tidal volume ventilation in mice. Mol Immunol. 2012;51:82–90. doi: 10.1016/j.molimm.2012.02.113. [DOI] [PubMed] [Google Scholar]
- 81.Deban L., Russo R.C., Sironi M., Moalli F., Scanziani M., Zambelli V. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]


