Abstract
The recombinant nucleocapsid protein (rNP) of severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) was expressed in a baculovirus system. The purified SARS-CoV rNP was used as an antigen for detection of SARS-CoV antibodies in IgG enzyme-linked immunosorbent assay (ELISA). The ELISA was evaluated in comparison with neutralizing antibody assay and the authentic SARS-CoV antigen-based IgG ELISA. Two-hundred and seventy-six serum samples were collected from health care workers in a hospital in which a nosocomial SARS outbreak took place and used for evaluation. The SARS-CoV rNP-based IgG ELISA has 92% of sensitivity and specificity compared with the neutralizing antibody assay and 94% sensitivity and specificity compared with the authentic SARS-CoV antigen-based IgG ELISA. The results suggest that the newly developed SARS-CoV rNP-based IgG ELISA is a valuable tool for the diagnosis and seroepidemiological study of SARS. The SARS-CoV rNP-based IgG ELISA has an advantage over the conventional IgG ELISA in that the antigen can be prepared by laboratory workers without the risk of infection.
Keywords: Recombinant nucleocapsid protein, SARS, IgG ELISA, Neutralizing antibody assay
1. Introduction
Severe acute respiratory syndrome (SARS), an emerging virus infection of the respiratory organs with a high mortality rate in humans, was first reported in Guangdong province, in southern part of China, in November 2002 and spread to Hong Kong, Vietnam, Singapore and other countries worldwide through human-to-human transmission (CDC, 2003, Lee et al., 2003, Poutanen et al., 2003, Tsang et al., 2003). Approximately 8000 patients were reported and about 800 died in the last SARS outbreak from November 2002 to July 2003 (WHO).
The causative agent, SARS coronavirus (SARS-CoV), was isolated from patients with SARS and was identified as a novel coronavirus. SARS-CoV was transmitted from human to human, and the mortality rate is high. SARS-CoV is regarded as a viral pathogen that must be handled in high containment laboratories with a biosafety level (BSL)-3 and BSL-4.
If a recombinant protein of SARS-CoV can be used as an antigen for serological diagnosis of SARS-CoV infections, it offers an advantage in the preparation of a SARS-CoV antigen because the recombinant protein of SARS-CoV can be produced without a risk of SARS-CoV-infections among laboratory workers. In the present study, we developed an IgG enzyme-linked immunosorbent assay (ELISA) in which a recombinant nucleocapsid protein (rNP) of SARS-CoV (SARS-CoV rNP) was used as an antigen, and evaluated the efficacy of the ELISA using serum samples collected from the health care workers in a hospital that was hit by a SARS-nosocomial outbreak.
2. Materials and methods
2.1. Virus
The SARS-CoV (HKU39849) used in this study was kindly supplied by Prof. J.S. Malik Peiris, Department of Microbiology, University of Hong Kong, Hong Kong Special Administrative Region.
2.2. Cells
Vero E6 cells purchased from the American Type Cell Collection (Manassas, VA) were grown in Eagle's minimum essential medium (MEM) supplemented with penicillin G and streptomycin and with 5% fetal bovine serum. The FBS was confirmed to have no inhibitory effect on the growth of SARS-CoV in cell cultures in a preliminary study. SARS-CoV was grown in Vero E6 cells cultured in MEM with penicillin G and streptomycin and with 2% fetal bovine serum.
2.3. Human serum samples
Two hundred seventy-six serum samples collected from 156 health care workers in the Hanoi French Hospital, Ho Chi Min city, Vietnam, were used (Vu et al., 2004). Serial serum samples were collected from each of the 120 subjects on different occasions. The sera were used for serological analyses after the heat-inactivation treatment at 56 °C for 30 min.
2.4. Manipulation of infectious SARS-CoV and clinical samples
All procedures that required manipulation of infectious SARS-CoV and/or non-inactivated clinical samples such as neutralizing antibody assay and authentic SARS-CoV antigen preparation were conducted in a BSL-3 laboratories in the National Institute of Infectious Diseases, Tokyo, Japan.
2.5. Recombinant baculovirus
The RNAs were extracted from SARS-CoV (HKU-39849)-infected Vero E6 cells and reverse transcribed using a random hexamer. The N gene of SARS-CoV was then amplified from the random hexamer-primed 1st strand DNAs using a forward primer (N-Bamf: 5′-GGA TCC AAT TAA AAT GTC TGA TAA TGG ACC C-3′, BamHI restriction site is underlined) and a reverse primer (N-Bamr2: 5′-GGA TCC TGC CTG AGT TGA ATC AGC AG-3′, BamHI restriction site is underlined). The PCR product was purified by agarose–gel electrophoresis, cloned into a pGEM-Teasy vector (Promega, Madison, USA) to generate pGEM-Teasy-N, and its sequence was confirmed to be identical to the original sequence (GenBank accession no. AY278491). The N gene insert was excised with BamHI from pGEM-Teasy-N and ligated into the unique BamHI site of a modified pAcYM1 baculovirus-transfer plasmid, pAc-cHis, carrying the 8His-tag at the 3′-extremity of the unique BamHI site. The recombinant baculovirus, Ac-SARS-N-His, was then generated using the method described by Kitts et al. (1990).
2.6. Antigens
Vero E6 cells were infected with SARS-CoV for specific antigen production and also mock-infected for control antigen. Extracts of both were made similarly as follows. The authentic SARS-CoV antigen and the corresponding mock-antigen were produced as follows. Vero E6 cell were infected with SARS-CoV or the mock virus at a multiplicity of infection (moi) of 2, respectively. After incubation for 24 h, both the authentic SARS-CoV- and mock-infected Vero E6 cells were collected. The cells were washed twice with cold phosphate-buffered saline (PBS) solution and then suspended in a phosphate-buffered saline solution supplemented with 1% Nonidet-P40 (NP40). Each of the cell-suspended solutions was incubated on ice for 10 min and the cell lysates were centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant fractions prepared from the SARS-CoV-and mock-infected Vero E6 cells were inactivated by ultraviolet irradiation and were used as positive and negative antigens for IgG ELISA, respectively. The Tn5 insect cells infected with Ac-SARS N-His or with Ac-ΔP, a baculovirus not expressing polyhedrin, were incubated for 72 h at 26 °C, respectively. Then both group of cells were washed twice with cold PBS and lysed in cold PBS containing 1% NP40 and 8 M urea. The cell lysates were centrifuged at 12,000 rpm at 4 °C for 10 min. The supernatant fractions were collected as a source of SARS-CoV rNP and negative control antigen for purification. The SARS-CoV rNP and the negative control antigen were purified using a Ni2+-resin purification system (QIAGEN GmbH, Hilden, Germany), according to the manufacturer's instructions.
2.7. IgG ELISA
Authentic SARS-CoV Ag-based and the SARS-CoV rNP-based IgG ELISA were performed as described previously except for the antigen preparation (Saijo et al., 2001, Saijo et al., 2002). The antigens were diluted with 50 mM carbonate buffer (pH 9.6) and used to coat the wells of 96-wells ELISA plates in the present study.
2.8. Neutralizing antibody detection
The serum samples were heat-inactivated and diluted two-fold with MEM-2FBS from 1:10 to 1:320. Each test sample (60 μl by volume) was then mixed with the same volume of MEM containing SARS-CoV at an infectious dose of 100 plaque forming units per 100 μl and the mixture was incubated for 1 h at 37 °C for neutralization. After incubation, the mixtures were tested for neutralization by cytopathic effect (CPE) inhibition assay using Vero E6 cells. The neutralizing antibody titer was defined as a reciprocal of the highest dilution at which no CPE was observed.
2.9. SARS-CoV RNA amplification by a loop-mediated isothermal amplification (LAMP) method for detection of SARS-CoV
The SARS-CoV RNA genome was amplified using Loopamp SARS CoV-detection kit (Eiken Chemical, Ohtawara, Japan) as reported previously with some modifications (Hong et al., 2004). RNA was isolated from the serum samples using QIAamp viral RNA mini kit (Qiagen, Germany). Primers used in the Loopamp SARS CoV-detection kit for SARS-CoV RNA amplification were designed according to the nucleotide sequence of Replicase 1b region (GenBank accession number NC_004718). Reverse transcription-LAMP reaction was conducted in 25 μl of the reaction mixture at 62.5 °C for 45 min using the real-time turbidimeter LA200 (TERAMECS, Japan).
2.10. Statistical analysis
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the SARS-CoV rNP-based IgG ELISA were calculated in comparison with neutralizing antibody assay or with naive SARS-CoV antigen-based IgG ELISA (Qing et al., 2003).
Receiver operating characteristics (ROC) and two-graph-ROC (TG-ROC) curves were analyzed using Stat Flex Version 5 software (Artech Co. Ltd., Osaka, Japan) (Greiner et al., 1995, Qing et al., 2003). The relationship of the OD405s in the SARS-CoV rNP-based IgG ELISA with those of the authentic viral antigen-based IgG ELISA and with the neutralizing antibody titers were evaluated by Spearman's correlation coefficient by rank using Statview software Version 5 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Expression of SARS-CoV rNP
The SARS-CoV rNP was efficiently expressed in the Tn5 insect cells infected with the recombinant Ac-SARS-N-His, and the purified SARS-CoV rNP was visually detected by SDS–PAGE analysis (data not shown).
3.2. Relationship of results between the IgG ELISA and neutralizing antibody assay
Of 276 serum samples, 87 showed a positive reaction in the neutralizing antibody assay. The relationship of the neutralizing antibody titers with the OD405 values in the SARS-CoV rNP-based IgG ELISA at a dilution level of 1:100 was evaluated using 87 neutralizing antibody-positive samples. There were significant positive correlations between the neutralizing antibody titers and the OD405 values in the SARS-CoV rNP-based IgG ELISA (Fig. 1 , R 2 = 0.668, p < 0.001).
Fig. 1.
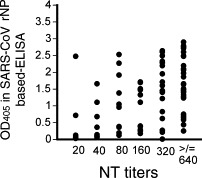
Plots of the relationship between OD405 values at 1:100 by the SARS-CoV rNP-based IgG ELISA and neutralizing antibody titers.
3.3. Efficacies of the SARS-CoV rNP-based IgG ELISA in comparison with the neutralizing antibody assay and authentic SARS-CoV-based IgG ELISA
The sensitivity, specificity, PPV and NPV, and ROC area of SARS-CoV rNP-based IgG ELISA were calculated using samples determined to be either positive or negative by neutralizing antibody assay or authentic SARS-CoV-based IgG ELISA. The relative sensitivity and specificity curves of the SARS-CoV rNP-based IgG ELISA using TG-ROC analysis are shown in Fig. 2 . The sensitivity, specificity, PPV and NPV of the SARS-CoV rNP-based IgG ELISA were 92%, 92%, 83%, and 96%, respectively, when the cut-off value was set at 0.128 (the OD405 value at an intersectional point in Fig. 2b). The ROC area of the SARS-CoV rNP-based IgG ELISA was 0.966 when compared with either the neutralizing antibody assay. The respective values of the SARS-CoV rNP-based IgG ELISA was 94%, 94%, 87%, and 97%, respectively, compared with the naive SARS-CoV antigen-based IgG ELISA, when the cut-off value was set at 0.156 (the OD405 value determined in the same way as mentioned above).
Fig. 2.
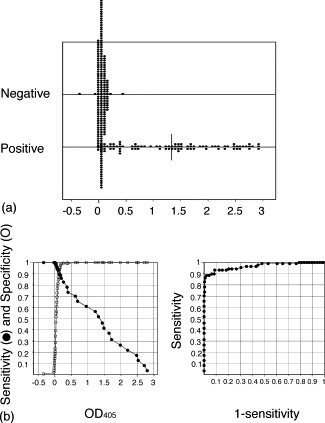
Plots of (a) the OD405 values of the neutralizing antibody-positive and -negative samples measured by the SARS-CoV rNP-based IgG ELISA. The curves of relative sensitivity and specificity of (b) the SARS-CoV rNP-based IgG ELISA by TG-ROC analysis and (c) the ROC curve based on the neutralizing antibody assay.
3.4. Antibody responses determined by SARS-CoV rNP-based ELISA and neutralizing antibody assay in subjects with sero-conversion
Sero-conversion by neutralizing antibody assay was demonstrated in 19 of the 120 subjects, from whom serial serum samples were collected on different occasions. The sequential changes of OD405 in SARS-CoV rNP-based IgG ELISA at dilution of 1:100 and neutralizing antibody titers among the 49 serum samples collected from these 19 sero-conversion-positive subjects was evaluated (Fig. 3 ). Each of the serum samples collected first from 17 of the 19 subjects showed a negative neutralizing antibody titer (less than 20)(black, blue or red lines in Fig. 3), while serum samples collected from the other two subjects showed a positive neutralizing antibody titers (green lines in Fig. 3). Four of the 17 neutralizing antibody-negative samples showed a positive reaction in the SARS-CoV rNP-based IgG ELISA (red lines in Fig. 3), while the other 15 showed a negative reaction in neutralizing antibody assay (black or blue lines). Only one sample that showed a positive reaction at a titer of 20 in neutralizing antibody assay showed a negative reaction in the SARS-CoV rNP-based IgG ELISA (blue line in Fig. 3).
Fig. 3.
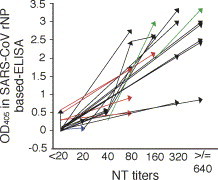
Sequential change in the OD405 value in the SARS-CoV rNP-based IgG ELISA and neutralizing antibody titers in 19 subjects with sero-conversion. The terminal positions of the tail and the cap of arrows indicate the level of OD405 value in the SARS-CoV rNP-based IgG ELISA at a dilution of 1:100 and neutralizing antibody titers of two serum samples collected from the same subject on different occasions. Black, red and blue arrows indicate the subjects with negative neutralizing antibody and negative antibody detectable by the SARS-CoV rNP-based IgG ELISA at the stage of the first blood collection, those with negative neutralizing antibody but positive antibody detectable by the ELISA at the same stage, and the subjects with positive neutralizing antibody but negative antibody detectable by the ELISA at the same stage, respectively. Green arrows indicate the subjects with both positive neutralizing antibody and positive antibody detectable by the ELISA at the same stage.
3.5. SARS-CoV RNA amplification and antibodies to SARS-CoV
SARS-CoV RNA was amplified by a LAMP method in 20 serum samples collected from nine subjects. The status of antibody to SARS-CoV in these 20 serum samples was evaluated. Seven of the 20 samples showed a negative reaction in both methods of neutralizing antibody assay and SARS-CoV rNP-based IgG ELISA, two showed a negative reaction in neutralizing antibody assay but a positive reaction in the ELISA, while the other 11 samples showed a positive reaction in both assays (Fig. 4 ).
Fig. 4.
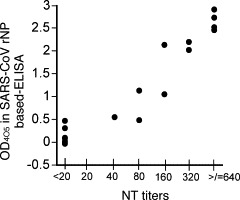
Plots of the relationship between OD405 values at 1:100 by the SARS-CoV rNP-based IgG ELISA and neutralizing antibody titers in the 20 serum samples with positive SARS-CoV genome.
4. Discussion
SARS-CoV was revealed to have a 30 kb long viral genome, containing 14 potential open reading frames (ORFs) (8). The sequence of SARS-CoV reveals the presence of ORFs for four structural proteins; i.e., the spike, membrane, envelope and nucleocapsid protein. Among these structural proteins, we selected the nucleocapsid protein, SARS-CoV rNP, as an antigen. Tan et al. recently reported that antibodies to the nucleocapsid and spike proteins of SARS-CoV were demonstrated in 100% of convalescent-phase patients, while antibodies to U274, a protein unique to SASR-CoV, was demonstrated in 73% of the patients (Tan et al., 2004), indicating that SARS-CoV rNP was the best choice as an antigen among the SARS-CoV structural proteins. Similar results were reported by Woo et al. (2004).
This study was performed using a relatively large panel of serum samples collected from health care workers from a Vietnamese hospital. We developed a recombinant SARS-CoV nucleocapsid protein-based IgG ELISA and confirmed that the ELISA had a higher than 90% sensitivity and specificity in detecting IgG antibodies to SARS-CoV compared with neutralizing antibody assay, the gold standard method, and with the authentic SARS-CoV Ag-based IgG ELISA (Fig. 2). There was a positive correlation between the OD405 values in the SARS-CoV rNP-based IgG ELISA and the neutralizing antibody titers (Fig. 1). Furthermore, sequential change in OD405 in the SARS-CoV rNP-based IgG ELISA and neutralizing antibody titers in 19 subjects, in whom sero-conversion was demonstrated, was evaluated (Fig. 3). Four of the 19 had already had antibody to SARS-CoV rNP detectable by SARS-CoV rNP-based IgG ELISA, although at this stage they had not yet had a neutralizing antibody equal to or over 20. On the other hand, only one serum sample that showed a positive reaction in neutralizing antibody assay at a titer of 20 showed a negative reaction in the SARS-CoV rNP-based IgG ELISA (Fig. 3). These results suggest that this SARS-CoV rNP-based IgG ELISA is as sensitive as the neutralizing antibody assay. Therefore, it can be concluded that the newly developed SARS-CoV rNP-based IgG ELISA is useful for the serological diagnosis of and seroepidemiological study on SARS-CoV infections.
The SARS-CoV rNP-based IgG ELISA was evaluated for efficacy in detection of specific antibody to SARS-CoV in comparison with neutralizing antibody assay in the present study. There have been several reports on the SARS-CoV rNP-based serological diagnostic system (Chan et al., 2005, Guan et al., 2004, Lin et al., 2003, Shi et al., 2003, Woo et al., 2004). Lin et al. (2003) first reported that SARS-CoV rNP would be one of the candidates for the antigen to detect SARS-CoV antibodies. They confirmed that three of the nine serum samples collected from the patients clinically diagnosed as having SARS showed a positive reaction in SARS-CoV rNP-based Western blotting. The SARS-CoV rNP was then expressed in an E. coli system and used as antigen in an antigen-capturing ELISA. It was reported that the antigen-capturing ELISA had high specificity of about 98%, though the sensitivity was not evaluated in the study (Shi et al., 2003). Recently, Guan et al. also reported the efficacy of the recombinant protein of SARS-CoV-based IgG ELISA in diagnosis of SARS (Guan et al., 2004). The SARS-CoV rNP was also expressed in E. coli transformed with an expression vector. However, these systems were not compared with the neutralizing antibody assay that is considered to be a gold standard.
The indices of sensitivity, specificity, PPV and NPV of our ELISA were relatively lower than those of the previous reports (Guan et al., 2004, Shi et al., 2003). These differences might be due to differences in the nature of the sera used in the study or due to the methods for the evaluation, or due to the both factors.
The status of antibody to SARS-CoV in the SARS-CoV genome-positive serum samples was evaluated. It was revealed that SARS-CoV genome is still present at a stage of IgG responses in some cases of SARS (Fig. 4). The results indicate that handling of blood collected from patients with SARS must be handled very carefully even if the patients were in a recovery phase with IgG responses. Furthermore, SARS-CoV amplification by a sensitive assay such as LAMP method should be carried out as a diagnostic tool, even when patients with SARS were in a stage of IgG responses.
In summary, a SARS-CoV rNP-based IgG ELISA with high sensitivity and specificity was developed. The advantage of the SARS-CoV rNP-based IgG ELISA is that the antigen can be prepared without the risk of infection.
Acknowledgements
We thank Prof. J.S. Malik Peiris, Department of Microbiology, University of Hong Kong, for providing us with the SARS-CoV (HKU-39849). We also thank Ms. M. Ogata, Department of Virology 1, National Institute of Infectious Diseases, Tokyo, Japan, for her technical assistance. This work is supported by grants-in-aid from the Ministry of Health, Labor and Welfare of Japan.
References
- CDC, 2003. Update: outbreak of severe acute respiratory syndrome-worldwide, 2003. MMWR Morb. Mortal Wkly. Rep. 52, 241–248. [PubMed]
- Chan P.K., Liu E.Y., Leung D.T., Cheung J.L., Ma C.H., Tam F.C., Hui M., Tam J.S., Lim P.L. Evaluation of a recombinant nucleocapsid protein-based assay for anti-SARS-CoV IgG detection. J. Med. Virol. 2005;75:181–184. doi: 10.1002/jmv.20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner M., Sohr D., Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
- Guan M., Chen H.Y., Foo S.Y., Tan Y.J., Goh P.Y., Wee S.H. Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients. Clin. Diagn. Lab. Immunol. 2004;11:287–291. doi: 10.1128/CDLI.11.2.287-291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, T.C., Mai, Q.L., Cuong, D.V., Parida, M., Minekawa, H., Notomi, T., Hasebe, F., Morita, K., 2004. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J Clin Microbiol 42, 1956–1961. [DOI] [PMC free article] [PubMed]
- Kitts P.A., Ayres M.D., Possee R.D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucl. Acids Res. 1990;18:5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Lin, Y., Shen, X., Yang, R.F., Li, Y.X., Ji, Y.Y., He, Y.Y., Shi, M.D., Lu, W., Shi, T.L., Wang, J., Wang, H.X., Jiang, H.L., Shen, J.H., Xie, Y.H., Wang, Y., Pei, G., Shen, B.F., Wu, J.R., Sun, B., 2003. Identification of an epitope of SARS-coronavirus nucleocapsid protein. Cell. Res. 13, 141–145. [DOI] [PMC free article] [PubMed]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J., and National Microbiology Laboratory, C.C.S.A.R.S.S.T Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Qing T., Saijo M., Lei H., Niikura M., Maeda A., Ikegami T., Xinjung W., Kurane I., Morikawa S. Detection of immunoglobulin G to Crimean-Congo hemorrhagic fever virus in sheep sera by recombinant nucleoprotein-based enzyme-linked immunosorbent and immunofluorescence assays. J. Virol. Methods. 2003;108:111–116. doi: 10.1016/s0166-0934(02)00267-7. [DOI] [PubMed] [Google Scholar]
- Saijo M., Niikura M., Morikawa S., Ksiazek T.G., Meyer R.F., Peters C.J., Kurane I. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J. Clin. Microbiol. 2001;39:1–7. doi: 10.1128/JCM.39.1.1-7.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo M., Qing T., Niikura M., Maeda A., Ikegami T., Prehaud C., Kurane I., Morikawa S. Recombinant nucleoprotein-based enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus. J. Clin. Microbiol. 2002;40:1587–1591. doi: 10.1128/JCM.40.5.1587-1591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yi Y., Li P., Kuang T., Li L., Dong M., Ma Q., Cao C. Diagnosis of severe acute respiratory syndrome (SARS) by detection of SARS coronavirus nucleocapsid antibodies in an antigen-capturing enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2003;41:5781–5782. doi: 10.1128/JCM.41.12.5781-5782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.J., Goh P.Y., Fielding B.C., Shen S., Chou C.F., Fu J.L., Leong H.N., Leo Y.S., Ooi E.E., Ling A.E., Lim S.G., Hong W. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin. Diagn. Lab. Immunol. 2004;11:362–371. doi: 10.1128/CDLI.11.2.362-371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Vu H.T., Leitmeyer K.C., Le D.H., Miller M.J., Nguyen Q.H., Uyeki T.M., Reynolds M.G., Aagesen J., Nicholson K.G., Vu Q.H., Bach H.A., Plan A.J. Clinical description of a completed outbreak of SARS in Vietnam February–May. Emerg. Infect. Dis. 2003;10:334–338. doi: 10.3201/eid1002.030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2003. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003.
- Woo P.C., Lau S.K., Tsoi H.W., Chan K.H., Wong B.H., Che X.Y., Tam V.K., Tam S.C., Cheng V.C., Hung I.F., Wong S.S., Zheng B.J., Guan Y., Yuen K.Y. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. Lancet. 2004;363:841–845. doi: 10.1016/S0140-6736(04)15729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


