Abstract
Mucosal epithelial cells are the primary targets for many common viral pathogens of cats. Viral infection of epithelia can damage or disrupt the epithelial barrier that protects underlying tissues. In vitro cell culture systems are an effective means to study how viruses infect and disrupt epithelial barriers, however no true continuous or immortalized feline epithelial cell culture lines are available. A continuous cell culture of feline mammary epithelial cells (FMEC UCD-04-2) that forms tight junctions with high transepithelial electrical resistance (>2000 Ω cm−1) 3–4 days after reaching confluence was characterized. In addition, it was shown that FMECs are susceptible to infection with feline calicivirus (FCV), feline herpesvirus (FHV-1), feline coronavirus (FeCoV), and feline panleukopenia virus (FPV). These cells will be useful for studies of feline viral disease and for in vitro studies of feline epithelia.
Keywords: Feline, Epithelial, Cell line, Virus susceptibility, Tight junction, Junctional adhesion molecule-A
1. Introduction
Mucosal epithelial cells form a physical barrier that protects the underlying tissues from invasion by pathogens and have important functions that include absorption of nutrients, maintenance of water and electrolyte balance, and secretion of milk, mucus, enzymes and molecules that are important for defense against pathogen invasion. Despite this armory, mucosal epithelial cells are the primary entry point for many feline viral pathogens including feline calicivirus (FCV), feline herpesvirus-1 (FHV), feline panleukopenia virus (FPV), and feline coronavirus (FCoV) (Pesavento et al., 2008, Gaskell et al., 2007, Hueffer and Parrish, 2003, De Groot and Horzinek, 1995). Infection by these viruses can disrupt or destroy the cellular or junctional barrier resulting in lesions such as ulceration or vesiculation, sloughing of epithelium, or abnormal secretion and/or absorption.
Many viruses target specifically receptors found on the surface of epithelial cells. Recently, feline junctional adhesion molecule-A (fJAM-A) was identified as a functional receptor for FCV (Makino et al., 2006). JAM-A localizes to junctional complexes in endothelial and epithelial cells and is important for maintaining intact mucosal epithelial barriers; in JAM-A−/− mice the colonic mucosal epithelium has increased permeability to small molecules and is infiltrated by polymorphonuclear leukocytes (Laukoetter et al., 2007). The JAM-A molecule is not readily accessible from the apical side of polarized epithelia (Excoffon et al., 2008), therefore, an important unanswered question is how FCV gains access to the receptor. In contrast, aminopeptidase N, the receptor for feline coronaviruses, is thought to be present on the apical surface of epithelial cells (Rossen et al., 2001). The receptor for feline panleukopenia virus is the feline transferrin receptor (Parker et al., 2001), which is located on the basolateral surface of polarized epithelial cells (Merle et al., 2007) and previous work in canine MDCK cells showed that FPV bound sixfold more efficiently to the basolateral than the apical surface of these cells (Basak and Compans, 1989). FPV is thought to access the basolateral surface of intestinal crypt epithelial cells following systemic spread. Epithelial cells are the first cell type that feline herpesviruses encounter during infection of a new host, and while it is not known if FHV infects preferentially via apical or basolateral surfaces of epithelial cells, human herpes simplex viruses preferentially infect via the apical surface of epithelial cells (Galen et al., 2006, Hayashi, 1995, Topp et al., 1997).
In order to understand how feline viruses cross epithelial barriers and initiate infection and to discover viral and host cellular factors that regulate these interactions a suitable epithelial cell line that forms tight junctions and that can be cultivated in vitro is needed. A continuous feline mammary epithelial cell line (FMEC UCD-04-2, henceforth FMEC) that forms tight junctions and has a high transepithelial electrical resistance (TEER) was characterized. In addition, we show that FMECs are susceptible to infection with FCV, FHV-1, FeCoV, and FPV.
2. Materials and methods
2.1. Cells
Crandell-Reese feline kidney (CRFK; ATCC #CCL-94) cells were grown in Eagle's minimal essential medium (EMEM; CellGro) supplemented with 5% fetal bovine serum (HyClone), 100 U ml−1 of penicillin, 100 μg ml−1 streptomycin, 0.25 μg ml−1 amphotericin B, 1 mM sodium pyruvate, and non-essential amino acids (CellGro). Madin-Darby canine kidney (MDCK; ATCC cat. CCL-34) cells were grown in Dulbecco's modified minimal essential medium (DMEM) supplemented with 10% fetal bovine serum, 100 U ml−1 of penicillin, 100 μg ml−1 streptomycin, and 0.25 μg ml−1 amphotericin B.
2.2. Feline mammary epithelial cell line
FMEC cells were derived using a method by Munson et al. (1998) and donated generously by A. Moresco (School of Veterinary Medicine, University of California, Davis). Cells were first obtained in their sixth passage and were maintained in a 1:1 mix of Dulbecco's modified Eagle's medium/Ham's F12, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 1% nonessential 25 amino acids, and 10 ng ml−1 epidermal growth factor (BD Biosciences, San Jose, CA, cat#354001) at 37 °C in a humidified atmosphere with 5% CO2.
2.3. Karyotyping analysis
Cells were prepared for cytogenetic analysis as described previously (Petkov and Anderson, 2008). Briefly, cultures were incubated in media with 0.25 μg ml−1 colcemid for 4 h. Cells were collected after trypsinization and incubated at RT in 0.075 M KCl for 30 min then fixed in Carnoy's fixative (3:1 methanol:acetic acid). Fixed cells were dropped on microscope slides and stained with Giemsa in phosphate buffer. Multiple chromosome spreads from low (6) passaged and high (14) passaged cells were examined and karytotyped with a Genus Cytogenetic workstation (Applied Imaging, Grand Rapids, MI).
2.4. Analysis of tight junctions by immunofluorescence microscopy
Monolayers of mammary epithelial cells were seeded at 8 × 104 cells per well in eight-chambered slides (Nalge Nunc Inc., #177445, Naperville, IL) and grown at 37 °C with 5% CO2 for 2–3 days. The cells were rinsed with PBS before fixation in 2% paraformaldehyde for 20 min at room temperature. Post-fixation, the slides were washed twice with PBST (PBS containing 0.1% Triton X-100) and blocked with 2% bovine serum albumin (BSA) in PBST (blocking buffer) for 1 h or overnight at 4 °C. Polyclonal rabbit anti-human occludin and polyclonal rabbit anti human ZO-1 were purchased from Zymed Laboratories, Inc., (South San Francisco, CA). A rabbit antiserum against the purified fJAM-A ectodomain was described previously (Ossiboff et al., 2007). The primary antibodies were diluted in blocking buffer containing either 5% normal donkey or normal goat serum and incubated for 1 h at room temperature or 4 °C overnight in a humidified chamber. Following three washes in PBST, bound antibodies were detected with Alexa488-conjugated donkey anti-rabbit and Alexa568-conjugated goat anti-mouse immunoglobulin (IgG) (Molecular Probes, Invitrogen). Non-immune ascites fluid (in the case of the fJAM-A antibody) and/or secondary antibody alone were used as negative controls. After three additional washes in PBST, the cell nuclei were counterstained with DAPI (KPL Inc., Cat#71-03-01, Gaithersburg, MD). Cover slips were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and images were acquired with an Olympus FV500 series confocal microscope (Olympus, Tokyo, Japan) equipped with argon and krypton lasers and 405 diode. Image stacks were collected on the z-axis and steps were made at the optimal voxel distance for a plan achromatic 40× objective. Stack depths were from 5 to 8 μm using a pixel resolution of 1024 × 1024. For enhanced clarity, DAPI was pseudocolored magenta using Olympus Fluoview software. The contrast and brightness were enhanced using Adobe Photoshop (Mountain View, CA).
2.5. Viruses and infection assays
Feline calicivirus (UCD-AN132) was originally obtained from an oropharyngeal swab of a shelter-housed female adult cat. Twice passaged viral stocks were prepared and titrated by plaque assay in CRFK cells as described previously (Bidawid et al., 2003, Ossiboff et al., 2007). Feline herpesvirus (UCD-AN96cat425) was obtained from a conjunctival/oropharyngeal combined swab sample taken from a shelter-housed cat. A viral stock was prepared from viruses twice passaged in CRFK cells. Feline coronaviruses, FIPV WSU-79/1146 and FECV WSU-79/1683, were propagated in CRFK cells and titrated in CRFK and Fcwf-4 cells using a 50% tissue culture infectious dose (TCID50) assay as described previously (Boyle et al., 1984). Three parvoviruses (FPV, CPV-2, and CPV-2b) were derived from infectious plasmid clones transfected into Norden laboratory feline kidney cells (NLFKs). The resulting virus was amplified and passaged in NLFKs one or two times to obtain low passage virus stocks. Viral stocks were titrated on NLFKs using a 50% tissue culture infectious dose (TCID50) assay as described previously (Parker and Parrish, 1997).
Monolayers of FMECs were seeded at 1 × 105 cells per well in eight-chambered slides or at 2 × 105 in six-well plates containing 18 mm glass cover slips and grown at 37 °C in 5% CO2 overnight. Cells were inoculated with viruses at a multiplicity of infection of ∼1 and incubated for 12 h (FCV) to 22 h (FHV, FCoV, and FPV) at 37 °C with 5% CO2. Cells were then fixed in 2% paraformaldehyde for 15–20 min at room temperature and then virus detected using the following antibodies: mouse anti-FCV (clone S1-8, Custom Monoclonals Intl., Sacramento, CA), mouse anti-parvovirus (to NS1 non structural protein, courtesy of C. Astell, University of British Columbia), mouse anti-canine coronavirus (FIPV-70, Custom Monoclonals Intl., Sacramento, CA) and mouse anti-feline herpesvirus (Herpes 7-7, Custom Monoclonals Intl., Sacramento, CA). Cover slips were incubated for 1 h with primary antibodies, washed in PBS, then incubated with secondary goat anti-mouse-IgG conjugated to Alexa fluor 488 and goat anti-rabbit-IgG conjugated to Alexa fluor 594 (Molecular Probes, Invitrogen). A nuclear stain, DAPI (Molecular Probes), was applied in some sections for 5 min prior to mounting. All antibodies and DAPI were diluted in permeabilization solution consisting of PBS with 1% BSA, 0.1% Triton X-100, and 0.05% sodium azide.
2.6. Measurement of transepithelial resistance
Cell culture 12-well transparent inserts (1.0 μm pore size; Beckton Dickinson) in 12-well tissue culture plates (Corning) were seeded with 1–2 × 105 cells (CRFK, FMEC); 4 inserts per cell line in 2 ml total appropriate growth medium (0.4 ml in upper and 1.5 ml in lower chamber). Plates were incubated at 37 °C in a 5% CO2, humidified atmosphere for 24 h. Transepithelial electrical resistance was measured using a Trans Epithelial Electrical Resistance System (TEER; Endohm, World Precision Instruments, Sarasota, FL) every 24 h. Growth medium was replaced daily. To determine TEER, the average resistance of two blank (media alone) inserts was subtracted from the sample reading, and resistance was corrected for the growth surface area. The background TEER was <25 Ω cm−1.
3. Results and discussion
3.1. FMECs are a continuously dividing cell line of feline origin and have increased ploidy
FMECs were passaged at least 25 times without loss of epithelial morphology or cytokeratin expression, and with no reduction in cell doubling time (data not shown). We confirmed the feline origin and investigated the ploidy profile of FMECs by cytochrome sequence analysis and by karyotyping. Early passage FMEC cells (passage 6) had a range of 44–96 chromosomes with a modal number of 48. However, late passage FMECs (greater than passage 12) had a much narrower range of 66–71 chromosomes with a modal number of 68 and stabilized into subtetraploid cell line. Total DNA was extracted and cytochrome B was sequenced and analyzed. The BLAST results confirmed that the cytochrome B sequences derived from FMECs were 100% identical to those of the domestic cat, Felis catus.
3.2. FMECs express integral tight junctional proteins including the FCV receptor fJAM-A
Tight junctions are composed of a network of cytosolic proteins, cytoskeletal elements, and several transmembrane proteins (Schneeberger and Lynch, 2004). The formation of tight junctions is a characteristic of mucosal epithelial cells. The zonula occludens-1 (ZO-1) protein is a cytosolic protein that localizes to tight junctions where it is thought to form a scaffold that links the tight junction-associated transmembrane proteins to the perijunctional actin cytoskeleton. ZO-1 may also be important in transducing signals that regulate the paracellular barrier (Anderson et al., 1988). Transmembrane proteins that are found within tight junctions include claudins, occludin and members of the immunoglobulin superfamily such as junctional adhesion molecule-A (JAM-A) (for review, Shin et al., 2006).
In epithelial cells junctional complexes form at cell–cell interfaces. To determine if FMECs formed typical junctional complexes, we examined the distribution of tight junctional markers occludin and ZO-1 by immunofluorescence microscopy. It was found that ZO-1 and occludin localized, in an uninterrupted manner, along the cell–cell contacts in the intercellular regions of the confluent FMEC monolayer (Fig. 1B and C). The tight junction was identified in z-plane optical sections as the cell–cell junctional region with maximal ZO-1 staining (Fig. 2A and B). Feline junctional adhesion molecule-A (fJAM-A) is a receptor for feline calicivirus (Makino et al., 2006) that localizes to tight junctions of epithelial cells in human and murine epithelial cells [reviewed in Mandell and Parkos, 2005]. Similar to the distribution of ZO-1 and occludin, it was found that fJAM-A also localized to cell–cell contacts in FMEC monolayers (Fig. 1D). By comparison, fJAM-A is distributed diffusely on the surface of CRFK cells (Ossiboff et al., 2007 and data not shown).
Fig. 1.
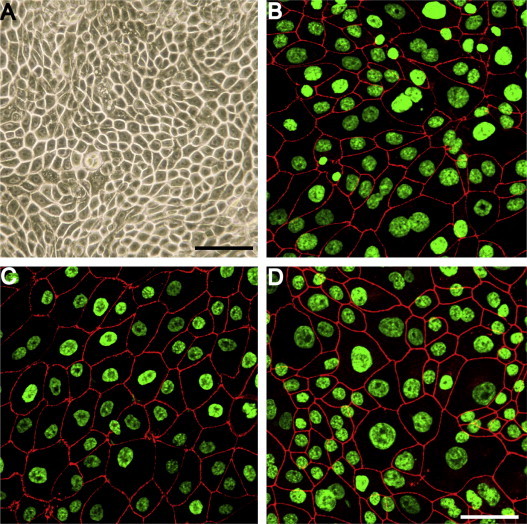
FMECs form tight junctional complexes in culture. (A) FMECs at passage 9 are tightly packed, polygonal cells that form a confluent monolayer. Junctional complexes were detected by immunofluorescence microscopy between adjacent FMECs and contained (B) occludin, (C) ZO-1, and (D) fJAM-A. Nuclei were stained with DAPI and pseudocolored green. Junctional proteins are pseudocolored red. Bar = 50 μm.
Fig. 2.
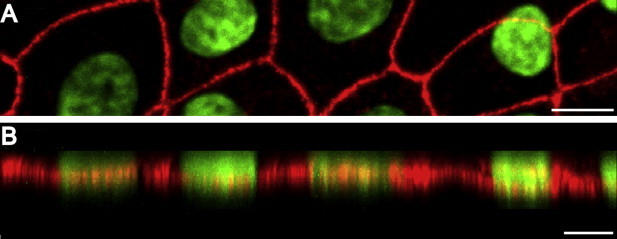
FMECs form polarized epithelial monolayers. (A) XY projection of FMECs at passage 11. ZO-1 was detected by confocal immunofluorescence microscopy, nuclei were stained with DAPI and pseudocolored green. Bar = 10.6 μm. (B) XZ-projection derived from image shown in (A). ZO-1 protein was tightly linear at approximately the lower third (base) of the FMEC cells. Cells were labeled on day 5 after passage and were on average 8–12 μm in height. Bar = 15 μm.
3.3. FMEC monolayers produce a resistant barrier
Small molecules and solutes traverse epithelial barriers by two major routes. Some proteins and nutrients move through epithelial cells selectively by transcytosis. Small molecules can also cross the epithelium through tight junctions that seal the paracellular space between epithelial cells (Schneeberger and Lynch, 2004). Tight junctions allow the selective passage of some small molecules and ions—normally cations; however, this movement of ions is physiologically restricted and consequently confluent monolayers of epithelial cells that form tight junctions normally have a high TEER. To investigate the junctional permeability of the FMECs, we determined the TEER of FMEC monolayers. TEER readings were also made on CRFK cells, a feline tissue culture cell line that does not form tight junctions. Resistance readings taken over the course of 13 days after the cells were seeded showed that TEER increased in FMECs whereas TEER remained relatively unchanged in CRFK monolayers (Fig. 3 ). By day 3 after seeding, the FMEC monolayers were confluent and TEER measurements were increasing. The FMEC monolayer demonstrated a peak TEER of ∼2000 Ω cm2 by day 5 that was maintained for at least 8 days thereafter. The CRFK monolayers did not exhibit a resistance greater than 10 Ω cm2 for the duration of the experiment. It is concluded that FMEC monolayers developed functional tight junctions as evidenced by the increase in TEER.
Fig. 3.

FMECs develop high transepithelial resistance indicating development of a barrier to the passage of ions. FMEC and CRFK cells were seeded in cell culture inserts with 1 μm pores (1 × 105 cells per insert) and the transepithelial resistance was measured daily. Each data point represents the mean resistance (Ω cm−2) of four inserts ± S.D. of a single experiment.
3.4. FMECs are susceptible to several different feline viruses
Epithelial cells are the primary target for several common feline viral pathogens including FCV, FHV, FPV, and FCoV. Disruption of respiratory (FHV and FCV), oral (FHV and FCV), or enteric (feline panleukopenia virus, FCoV) epithelial function as a consequence of viral infection of epithelial cells plays a major role in the pathogenesis of these viral diseases (De Groot and Horzinek, 1995, Gaskell et al., 2007, Hueffer and Parrish, 2003, Pesavento et al., 2008). All epithelia share common properties, including morphology, expression of the cytokeratin intermediate filaments (Moll et al., 1982), and specialized connections between the cells (Gumbiner, 1996). Our current understanding of the virus:host cell interactions for feline epitheliotropic viruses relies primarily on in vitro studies of immortalized mesenchymal and monocyte cell lines and to a lesser degree on short-term primary epithelial culture (Leeming et al., 2006, Sandmeyer et al., 2005). The susceptibility of FMECs to four different feline viruses, FCV, FHV, FPV, and FCoV, were examined. The susceptibility of these cells to infection with canine parvovirus, which has been shown previously to infect feline cells and to mammalian reoviruses, which infect a broad range of mammalian cells and can use human and murine JAM-A as functional receptors were examined. It was found that FMECs supported infection by all of these viruses (Fig. 4A–D and data not shown), indicating that these cells are permissive to a broad range of different virus types.
Fig. 4.

FMECs are susceptible to infection with feline epitheliotropic viruses. FMECs were inoculated with (A) FPV, (B) FCV, (C) FCoV, and (D) FHV then fixed and immunostained using virus-specific antibodies. Virus antigen is pseudocolored green. In panels A, C, and D, fJAM-A was immunostained and is pseudocolored red. Nuclei were stained with DAPI and pseudocolored magenta in panels B and C. Size bar in panel A = 23 μm, size bars in panels B–D = 50 μm.
4. Summary
The feline mammary epithelial cell line FMEC UCD-04-2 grows continuously in tissue culture and forms tight junctions that can maintain a high-resistance transcellular barrier. FMECs are fully susceptible to infection by epitheliotropic feline viruses FHV, FeCoV, FCV, and FPV. It is expected that FMECs will be useful tools for in vitro studies of viral infection. These cells may also be useful generally for studying the formation and maintenance of polarized epithelia.
Acknowledgements
The authors appreciate the guidance of Drs. L. Munson and C.P. Drew.
References
- Anderson J.M., Stevenson B.R., Jesaitis L.A., Goodenough D.A., Mooseker M.S. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J. Cell. Biol. 1988;106(4):1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S., Compans R.W. Polarized entry of canine parvovirus in an epithelial cell line. J. Virol. 1989;63(7):3164–3167. doi: 10.1128/jvi.63.7.3164-3167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidawid S., Malik N., Adegbunrin O., Sattar S.A., Farber J.M. A feline kidney cell line-based plaque assay for feline calicivirus, a surrogate for Norwalk virus. J. Virol. Methods. 2003;107(2):163–167. doi: 10.1016/s0166-0934(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Boyle J.F., Pedersen N.C., Evermann J.F., McKeirnan A.J., Ott R.L., Black J.W. Plaque assay, polypeptide composition and immunochemistry of feline infectious peritonitis virus and feline enteric coronavirus isolates. Adv. Exp. Med. Biol. 1984;173:133–147. doi: 10.1007/978-1-4615-9373-7_12. [DOI] [PubMed] [Google Scholar]
- De Groot R.J., Horzinek M.C. Feline infectious peritonitis. In: Siddell S.G., editor. The Coronaviridae. Plenum Press; New York, NY: 1995. pp. 293–315. [Google Scholar]
- Excoffon K., Guglielmi K.M., Wetzel J.D., Gansemer N.D., Campbell J.A., Dermody T.S., Zabner J. Reovirus preferentially infects the basolateral surface and is released from the apical surface of polarized human respiratory epithelial cells. J. Infect. Dis. 2008;197(8):1189–1197. doi: 10.1086/529515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen B., Cheshenko N., Tuyama A., Ramratnam B., Herold B.C. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J. Virol. 2006;80(24):12209–12218. doi: 10.1128/JVI.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell R., Dawson S., Radford A., Thiry E. Feline herpesvirus. Vet. Res. 2007;38(2):337–354. doi: 10.1051/vetres:2006063. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hayashi K. Role of tight junctions of polarized epithelial MDCK cells in the replication of herpes simplex virus type 1. J. Med. Virol. 1995;47(4):323–329. doi: 10.1002/jmv.1890470406. [DOI] [PubMed] [Google Scholar]
- Hueffer K., Parrish C.R. Parvovirus host range, cell tropism and evolution. Curr. Opin. Microbiol. 2003;6(4):392–398. doi: 10.1016/s1369-5274(03)00083-3. [DOI] [PubMed] [Google Scholar]
- Laukoetter M.G., Nava P., Lee W.Y., Severson E.A., Capaldo C.T., Babbin B.A., Williams I.R., Koval M., Peatman E., Campbell J.A., Dermody T.S., Nusrat A., Parkos C.A. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 2007;204(13):3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming G., Meli M., Cripps P., Vaughanthomas A., Lutz H., Gaskell R., Kipar A. Tracheal organ cultures as a useful tool to study Felid herpesvirus 1 infection in respiratory epithelium. J. Virol. Methods. 2006;138(1–2):191–195. doi: 10.1016/j.jviromet.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Makino A., Shimojima M., Miyazawa T., Kato K., Tohya Y., Akashi H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J. Virol. 2006;80(9):4482–4490. doi: 10.1128/JVI.80.9.4482-4490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell K.J., Parkos C.A. The JAM family of proteins. Adv. Drug Deliv. Rev. 2005;57(6):857–867. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Merle U., Theilig F., Fein E., Gehrke S., Kallinowski B., Riedel H., Bachmann S., Stremmel W., Kulaksiz H. Localization of the iron-regulatory proteins hemojuvelin and transferrin receptor 2 to the basolateral membrane domain of hepatocytes. Histochem. Cell. Biol. 2007;127(2):221–226. doi: 10.1007/s00418-006-0229-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W.W., Schiller D.L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Munson L., Chandler S., Schlafer D.H. Cultivation of bovine fetal and adult endometrial epithelial cells. J. Tissue Cult. Methods. 1998;11(3):129–133. [Google Scholar]
- Ossiboff R.J., Sheh A., Shotton J., Pesavento P.A., Parker J.S.L. Feline caliciviruses (FCVs) isolated from cats with virulent systemic disease possess in vitro phenotypes distinct from those of other FCV isolates. J. Gen. Virol. 2007;88(Pt 2):506–517. doi: 10.1099/vir.0.82488-0. [DOI] [PubMed] [Google Scholar]
- Parker J.S., Parrish C.R. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J. Virol. 1997;71(12):9214–9222. doi: 10.1128/jvi.71.12.9214-9222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.S., Murphy W.J., Wang D., O’Brien S.J., Parrish C.R. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 2001;75(8):3896–3902. doi: 10.1128/JVI.75.8.3896-3902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento P.A., Chang K.O., Parker J.S.L. Molecular virology of feline calicivirus. Vet. Clin. North Am. Small Anim. Pract. 2008;38(4):775–786. doi: 10.1016/j.cvsm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Petkov S.G., Anderson G.B. Culture of porcine embryonic germ cells in serum-supplemented and serum-free conditions: the effects of serum and growth factors on primary and long-term culture. Clon. Stem Cells. 2008;10(2):263–276. doi: 10.1089/clo.2007.0085. [DOI] [PubMed] [Google Scholar]
- Rossen J.W., Kouame J., Goedheer A.J., Vennema H., Rottier P.J. Feline and canine coronaviruses are released from the basolateral side of polarized epithelial LLC-PK1 cells expressing the recombinant feline aminopeptidase-N cDNA. Arch. Virol. 2001;146(4):791–799. doi: 10.1007/s007050170147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer L.S., Keller C.B., Bienzle D. Culture of feline corneal epithelial cells and infection with feline herpesvirus-1 as an investigative tool. Am. J. Vet. Res. 2005;66(2):205–209. doi: 10.2460/ajvr.2005.66.205. [DOI] [PubMed] [Google Scholar]
- Schneeberger E.E., Lynch R.D. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 2004;286(6):C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Shin K., Fogg V.C., Margolis B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Topp K.S., Rothman A.L., Lavail J.H. Herpes virus infection of RPE and MDCK cells: polarity of infection. Exp. Eye Res. 1997;64(3):343–354. doi: 10.1006/exer.1996.0209. [DOI] [PubMed] [Google Scholar]


