Abstract
The SARS-associated human coronavirus (SARS-HCoV) is a newly described, emerging virus conclusively established as the etiologic agent of the severe acute respiratory syndrome (SARS). This study presents a single-tube RT-PCR assay that can detect with high analytical sensitivity the SARS-HCoV, as well as several other coronaviruses including other known human respiratory coronaviruses (HCoV-OC43 and HCoV-229E). Species identification is provided by sequencing the amplicon, although a rapid screening test by restriction enzyme analysis has proved to be very useful for the analysis of samples obtained during the SARS outbreak in Toronto, Canada.
Keywords: Coronavirus, Human coronaviruses, RT-PCR assay, SARS
1. Introduction
Severe acute respiratory syndrome (SARS) is a newly described, emerging infectious disease presenting with high fever and atypical pneumonia. The disease first appeared in the Guangdong province of China and quickly spread to other countries; outbreaks were reported in mainland China, Vietnam, Singapore, Hong Kong, Taiwan and Canada (Drazen, 2003, Lee et al., 2003, Poutanen et al., 2003, Tsang et al., 2003, Vu et al., 2003, WHO, 2003a). A new, previously unknown coronavirus was first isolated from patients with SARS at the University of Hong-Kong (Peiris et al., 2003a, WHO, 2003a); this was quickly followed by reports from several other laboratories of the demonstration of a new coronavirus in samples from patients with SARS (Drosten et al., 2003, Ksiazek et al., 2003, Poutanen et al., 2003, WHO, 2003a). The complete genomic sequence of the virus has been determined (Marra et al., 2003, Rota et al., 2003) and confirmed the classification of the SARS-associated agent as a new coronavirus, distinct from the previously known groups of coronavirus (Marra et al., 2003, Rota et al., 2003) but with some relationship with group 2 (Snijder et al., 2003). Experimental infection of cynomolgous monkeys permitted the fulfillment of Koch's postulates for this pathogen and firmly established the SARS-associated human coronavirus (SARS-HCoV) as the etiologic agent of SARS (Fouchier et al., 2003).
This study presents a RT-PCR protocol that allows for the detection of the SARS-HCoV in clinical samples. This single-tube RT-PCR is based on consensus primers targeting conserved regions of coronavirus genome sequences and allows for the detection and species identification of several coronaviruses including SARS-HCoV, with high analytical sensitivity. This assay is expected to be helpful in fulfilling the World Health Organization laboratory case definition of SARS, which recommends the use of two different PCR assays (WHO, 2003b). In addition, even if the SARS-HCoV does not reappear in the human population, this assay will still be useful, since it provides a diagnostic test for the respiratory coronaviruses HCoV-229E and HCoV-OC43.
2. Material and methods
2.1. SARS coronavirus RNA
RNA was extracted with TRIzol reagent (Life Technologies), as per manufacturer's recommendations, from a lung biopsy from a patient with SARS. The RNA pellet was resuspended in 30 μl of 10 mM of dithiotreitol with 5% (v/v) of RNasin (20–40 U/μl, Promega), serially diluted, aliquoted and frozen at −80 °C. Quantification of the RNA serial dilution was achieved by measuring the amount of SARS-HCoV RNA in the aliquots with the RealArt HPA coronavirus RT-PCR (Artus GmbH, Hamburg, Germany).
2.2. RNA from human coronaviruses OC43 and 229E
Human coronaviruses OC43 and 229E strains were initially obtained from ATCC and passaged in cell culture as described (Sizun et al., 1998). Stocks of viruses were titrated as described (Sizun et al., 1998). RNA was extracted from the titrated stocks using TRIzol (Life Technologies) as per the manufacturer's recommendations and serially diluted.
2.3. RNA from infectious bronchitis virus
Tissue culture-adapted infectious bronchitis virus (IBV) stocks, strain Massachussets 41 and strain Baudette, were obtained from Dr. E. Nagy, Ontario Veterinary College, University of Guelph, Ontario. RNA was extracted and serially diluted as for HCoV-OC43 and HCoV-229E.
2.4. Clinical samples
During the SARS outbreak in Toronto (2003), several samples from patients with probable or suspected SARS were referred to our laboratory for SARS-HCoV detection by RT-PCR. Of these, 44 were assayed using the new primers described here, including 7 lung biopsies, 2 bronchoalveolar lavages, 7 nasopharyngeal swabs, 3 throat swabs, 2 pleural fluid samples, 2 endotracheal aspirates, 4 blood samples, 11 stool samples and 6 urine samples. RNA was extracted from lung tissue using the RNeasy kit (Qiagen) as per the manufacturer's recommendations. For all other samples, RNA was extracted using the guanidium thiocyanate buffer (GTC) extraction methods, as described (Johnson et al., 2000).
2.5. Primers
Primers were designed to target segments of the pol 1b coding region conserved among several species of coronavirus (Fig. 1 ). We used the sense primer CORO1 (5′-TGA TGG GTT GGG ACT ATC CTA AAT GTG A-3′) and the antisense primer CORO2 (5′-GTA GTT GCA TCA CCG GAA GTT GTG CCA CC-3′), homologous to the segments [15211, 15238] and [15402, 15430] of the sequence of the Tor2 strain of SARS-HCoV (GenBank Accession number AY274119), respectively.
Fig. 1.
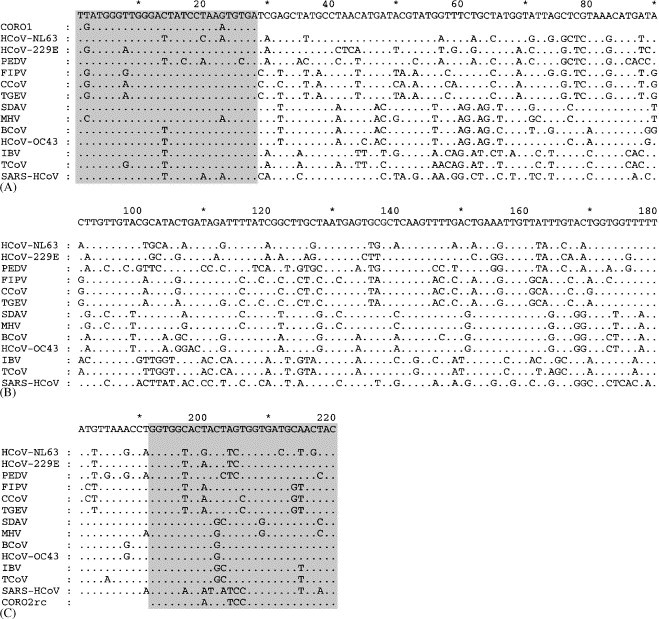
Alignment calculated from the nucleotide sequence of segments homologous to the segment [15211, 15430] of SARS-HCoV strain TOR2 (GenBank Accession number AY274119) from 12 other species of coronaviruses. HCoV-NL63 (AY567487): human coronavirus NL63. HCoV-229E (NV_002645): human respiratory coronavirus 229E. PEDV (NC_003436): porcine epidemic diarrhea virus. FIPV (AF124987): feline infectious peritonitis virus. CCoV (AF124986): canine coronavirus. TGEV (AJ271965): porcine transmissible gastroenteritis virus. SDAV (AF124990): sialodacryoadenitis virus. MHV (NC_001846): murine hepatitis virus. BCoV (NC_003045): bovine coronavirus. HCoV-OC43 (AF124989): human respiratory coronavirus OC43. IBV (NC_001451): avian infectious bronchitis virus. TCoV (AF124991): turkey coronavirus. Also aligned with the coronaviruses sequences are the sequences of the sense primer (CORO1) and the complement of the antisense primer (CORO2rc). Dots denote homology with the consensus sequence on top. The sequence alignment was calculated using ClustalX for Windows version 1.81 (Thompson et al., 1997) and edited with Genedoc version 2.3 for Windows (Nicholas K.B., 1997).
2.6. RT-PCR
This was done using the Qiagen one-step RT-PCR kit. Each reaction was performed in a 0.6 ml tube (Diamed PRE 050) in a total volume of 50 μl overlaid with 50 μl of mineral oil. Each reaction mix contains 10 μl of 5× Qiagen buffer, 2 μl of dNTP mix (each dNTP at 10 mM concentration), 3 μl of each primer (10 μM stock), 20 μl of molecular grade double distilled water (ddH2O) and 2 μl of the one-step enzyme mix (Qiagen). The master mix was then aliquoted in tubes, to which 10 μl of template RNA was added. The PCR thermal cycling was done on a Stratagene Robocycler 40, with an initial incubation at 50 °C for 30 min, followed by an incubation at 95 °C for 15 min, and 40 cycles consisting of denaturation at 94 °C for 1 min, annealing at 56 °C for 1 min, and elongation at 72 °C for 1 min. Extensive precautions against PCR contamination, as previously described (Johnson et al., 2000), were strictly observed. Positive and negative controls, as well as extraction controls and controls for PCR inhibition, were set up essentially as described (Johnson et al, 2000).
2.7. Electrophoresis analysis of the amplicons
A 10 μl volume of each reaction was submitted to electrophoresis on 2% agarose or 4% NuSieve 3:1 (Biowhittaker Molecular Applications, Rockland, USA) gels containing ethidium bromide. The gels were visualized on a UV transilluminator and photographed.
2.8. Restriction enzyme analysis
Each reaction mixture in which the expected 220 bp amplicon was detected was digested with the restriction enzyme AluI, which cuts within the sequence AGCT. The digestion reaction consisted of 10 μl of the RT-PCR mixture, 1.5 μl of the 10× enzyme buffer, 1 μl of AluI (10 U/μl, New England Biolabs) and 2.5 μl of ddH2O for a total of 15 μl. The reaction was incubated at 37 °C for 1 h and analyzed by agarose gel electrophoresis.
2.9. Sequencing
Amplicons were submitted to automated sequencing, for both strands, using the PCR primers as sequencing primers. Sequencing was performed by the DNA Sequencing Facility, Centre for Applied Genomics, Hospital for Sick Children.
2.10. Sequences analysis
Sequence editing and analysis were done using the programs Generunner for Windows version 3.05 (Hasting software). Sequence alignments were calculated using ClustalX for Windows version 1.81 (Thompson et al., 1997), with the default parameters for gap opening and gap extension. Phylogenetic trees were inferred using Treecon for Windows version 1.3.b (Van de Peer and De Wachter, 1994), using a distance method; in brief, the distance was calculated without correction; the tree topology was inferred with the neighbor joining method and the trees re-rooted at the internode. Bootstrap analyses were done with 1000 replicates.
3. Results
3.1. Sequence alignments and primer design
Initially, an amino acid sequence alignment of the pol 1b open-reading frame (ORF) was used to identify well-conserved regions across 12 species of coronaviruses comprising the SARS-HCoV and members of the three previously described groups of coronaviruses. The corresponding nucleotides alignment was then used to design PCR primers targeting these well-conserved regions. Fig. 1 illustrates the alignment of the 220 nt segments of these 12 coronavirus sequences homologous to the segment [15211, 15430] of the Tor2 strain of SARS-HCoV, as well as the corresponding sequence of the newly described human coronavirus HCoV-NL63 (Fouchier et al., 2004, Van der Hoek et al., 2004), along with the sequence of the primers.
Fig. 2 illustrates the phylogenetic trees of the nucleotide sequences (Fig. 1A) and amino acid sequences (Fig. 2B) in that segment, demonstrating that there are enough sequence diversity in the region bracketed by the primers to allow for unambiguous species identification.
Fig. 2.
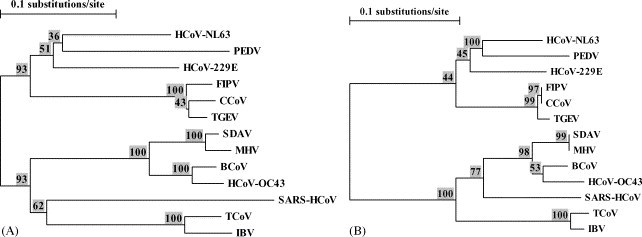
(A) Phylogenetic tree calculated from the nucleotide sequence of segments homologous to the segment [15211, 15430] of SARS-HCoV strain TOR2 from the 13 species of coronaviruses in Fig. 1. 1.The boostrap values are displayed (as percentages) above the node. (B) Phylogenetic tree calculated from the amino acid sequences corresponding to the nucleotide sequences in (A). The boostrap values are displayed (as percentages) above the node. The phylogenetic trees were drawn using the program Treecon for Windows version 1.3.b (Van de Peer and De Wachter, 1994).
3.2. Detection of coronavirus RNA
Using as templates the RNA of SARS-HCoV, HCoV-229E and HCoV-OC43, as well as RNA from two strains of IBV, we could readily and reproducibly obtain the expected 220 bp amplicon.
3.3. Sensitivity and specificity
Aliquots of a 10-fold serial RNA dilution prepared from a lung biopsy sample of a patient with SARS (see Section 2) were used to compare our assay with the RealArt HPA coronavirus RT-PCR (Artus GmbH). The results are displayed in Table 1 and show that our assay has essentially the same sensitivity as that of the RealArt HPA coronavirus RT-PCR assay; it is estimated that the analytical sensitivity is between 1 and 10 genome copies.
Table 1.
Comparison between our RT-PCR assay and the RealArt HPA coronavirus RT-PCR (Artus GmbH)
| Dilution | RT-PCR with CORO1 and CORO2 | RealArt HPA coronavirus RT-PCR (genome copies) |
|---|---|---|
| 10−3 | + | 33000 |
| 10−4 | + | 2480 |
| 10−5 | + | 89 |
| 10−6 | + | 19 |
| 10−7 | + | 3 |
| 10−8 | − | – |
Column two shows the result (scored as positive or negative) obtained with our assay using 10 μl of the indicated RNA dilution (see Section 2). For each dilution the assay was done on two different aliquots and the results were in agreement. Column three shows the average (on two experiments) of the measured number of genome copies in 5 μl of the RNA dilution with RealArt HPA coronavirus RT-PCR.
For HCoV-OC43, viral RNA could be detected from as little as 6 × 10−2 TCID50 (tissue culture infective dose 50); for HCoV-229E, viral RNA could be detected from as little as 6 × 10−3 TCID50. For all coronaviruses tested in this study the species was verified by sequencing; in all cases the sequence internal to the primers were identical to the expected sequences. Based on the expected sequences of the amplicons a rapid screening test for species identification for the human coronaviruses was designed using the AluI restriction enzyme. As illustrated on Fig. 3 , the SARS-HCoV amplicon is cut into 126-bp and 94-bp fragments, the HCoV-229E amplicon into 101-bp, 86-bp and 33-bp fragments, and the HCoV-OC43 amplicon is not cut by AluI; based on the reference sequence, the amplicon of IBV would not contain a AluI site. Nucleic acids from human tissue, or from other respiratory viruses whose presence was detected in various samples (including influenza A, respiratory syncytial virus, parainfluenza 3 and adenoviruses), did not generate amplicons of 220 bp.
Fig. 3.
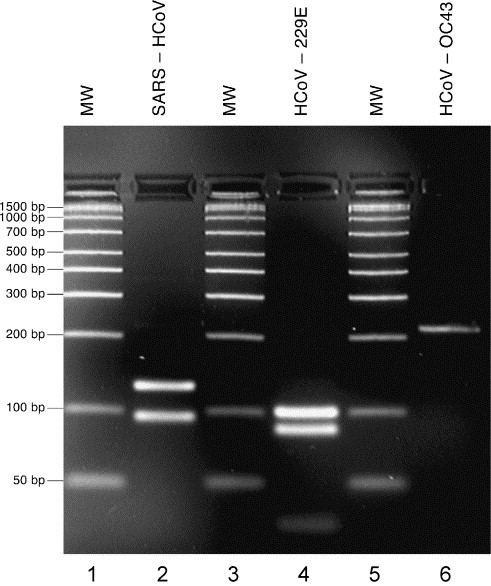
AluI digestion of amplicons from SARS-HCoV, HCoV-229E and HCoV-OC43. Lanes 1, 3 and 5 (MW): molecular weight markers (Amplisize ladder 50–2000 bp, Bio-Rad Laboratories, Mississauga, Canada); Lane 2: SARS-HCoV (predicted fragments: 126 bp, 94 bp); Lane 4: HCoV-229E (predicted fragments: 101 bp, 86 bp, 33 bp); Lane 6: HCoV-OC43 (predicted fragment: 220 bp).
3.4. Clinical samples
From the clinical samples listed above, the presence of the SARS-HCoV RNA was demonstrated in one lung biopsy, two bronchoalveolar lavages, two nasopharyngeal swabs, two urine samples and one throat swab.
4. Discussion
This study presents a single-tube RT-PCR assay designed to detect several species of coronaviruses, including the SARS coronaviruses, by using consensus primers designed from an alignment of the sequences of a region in the pol 1b ORF of 12 species of coronaviruses. A previous version of these primers, based on an alignment of four species of coronaviruses and of the Berne torovirus, was used to demonstrate the presence of a new coronavirus in patients with SARS in Canada (Kumar et al., 2003, Poutanen et al., 2003) at a time when the complete sequence of the SARS coronavirus was still unknown. The use of the revised primers and the optimization of the RT-PCR has led to an improvement in analytical sensitivity of three orders of magnitude compared to the previous assay. Its sensitivity has proven sufficient for the detection of the SARS-HCoV in several clinical samples.
The assay was designed to be broadly reactive with the genome of many coronavirus species; it is demonstrated here that it can detect coronaviruses from all four known groups of coronaviruses, including the HCoV-229E and HCoV-OC43, which are well-recognized human pathogens (Holmes, 2001). Although not all the known coronavirus species were tested, it is expected that the primer pair can amplify with high analytical sensitivity the targeted genome segment from the 12 species used in the design of the primers, with the possible exception of the porcine epidemic diarrhea virus (PEDV) whose sequence has a mismatch at the third nt from the 3′ end of the primer CORO1 (Fig. 1). The current assay can detect with high sensitivity three coronaviruses known to infect humans, and sequencing of the amplicons would unambiguously identify the species (Fig. 2). Although there has not been yet an opportunity to test this assay on isolates of the newly described HCoV-NL63 (Van der Hoek et al., 2004), it is predicted, based on the sequence alignment in Fig. 1, that our RT-PCR assay would also detect HCoV-NL63, and sequencing of the amplicon followed by phylogenetic analysis (Fig. 2) would readily confirm the identity of the virus. Based on the published sequence of HCoV-NL63, digestion of the amplicon by AluI would yield a distinct pattern consisting of bands of 187 bp and 33 bp. Since the sequences targeted by the primers CORO1 and CORO2 are conserved across several coronavirus species (and this is particularly clear at the amino acid level), this suggests strong selective pressure on that part of the genome. In turn, this suggests that the assay would still be able to detect mutant or variant strains of SARS-HCoV, as mutations would likely not occur in the regions targeted by the primers. To date, the various isolates of SARS-HCoV have all displayed a great genetic homogeneity (Ruan et al., 2003), which argues for the emergence of SARS-HCoV in the human population only very recently. However, should the virus emerge again and be allowed to propagate, its genetic diversity may well increase (although the genomes of many known coronaviruses appear relatively stable). Of note, a BLAST search in the GenBank database using the 220-nt segment of SARS-HCoV (Fig. 1) showed a 100% homology with 105 sequences of SARS-HCoV deposited to date, including representative of all clusters (Guan et al., 2004), and an homology greater than 99% (219/220) with two other sequences of SARS-HCoV. As well, the recent SARS outbreak suggests that other coronaviruses may emerge as new human pathogens. Again, assuming that the conservation of the sequences targeted by the primers stems from strong evolutionary constraints, the RT-PCR assay described here would very likely detect such an emerging coronavirus.
In this study a simple restriction enzyme analysis as a screening test was shown to be very helpful in identifying the SARS-HCoV. Indeed, in all the samples that tested positive for SARS-HCoV in our laboratory during the Toronto outbreak, the expected restriction pattern was demonstrated (and based on sequences deposited in GenBank this pattern would have been demonstrated in all isolates of SARS-HCoV sequenced to date). However, because RNA virus genomes are much more variable than DNA virus genomes, one should not rely completely on such an analysis and the failure to obtain the expected digestion fragments (or if the amplicon is not cut, as is the case for HCoV-OC43) should prompt the determination of the sequence of the amplicon. As an illustrative case in point, whereas the amplicon from the IBV strain Baudette is not cleaved by AluI, the amplicon from strain Massachussetts 41 is in fact cleaved by AluI, as verified by both sequencing and digestion of the amplicon (data not shown). After sequencing the amplicon, a comparison of the sequence to that of several coronaviruses in a phylogenetic analysis, as in Fig. 2, should permit a definitive identification. Indeed, should a new coronavirus emerge, it appears likely that it could be detected and identified as a new virus by using our RT-PCR assay followed by sequencing and phylogenetic analysis.
Since the end of the global SARS outbreak in 2003, a total of three laboratory confirmed sporadic cases of SARS in Guangdong have occurred (WHO, 2004), which however did not spread further, as well as three laboratory acquired incidents (Normile, 2004). While surveillance for SARS is still recommended (WHO, 2003b) there is great concern about the negative impact of a false positive laboratory test, and the World Health Organization has recommended protocols for the laboratory diagnosis of SARS in the post outbreak period (WHO, 2003b), which includes the use of two different RT-PCR assays. The assay described here provides an alternative test that can be helpful in that regard. Furthermore, because our assay detects several coronaviruses, it is quite feasible to use RNA from a coronavirus other than SARS-HCoV as a positive control. In fact, our laboratory now uses as positive control RNA transcribed from the cloned amplicon of the IBV; IBV has never been implicated in human infections. The use of IBV as a positive control removes a potential source of PCR contamination by SARS-HCoV genetic material in the laboratory.
In this study, the presence of the SARS-HCoV was demonstrated in a variety of clinical samples, which illustrates the usefulness of the assay. The number of positive samples reported here is too small to draw firm conclusions but taking into account the distinction between probable and suspected cases of SARS, as well as previous observations made earlier in our laboratory during the outbreak, it appears that the SARS-HCoV is more likely to be demonstrated in lower respiratory tract samples, such as broncho-alveolar lavages or lung biopsies rather than in a nasopharyngeal swab; this parallels the findings of other reports (Peiris et al., 2003b, Tang et al., 2004) and it has been suggested that the SARS-HCoV appears at a high titer in the nasopharynx relatively late in the disease (Peiris et al., 2003b). Thus, the negative RT-PCR results on such samples do not reflect a poor analytical sensitivity of the assay but rather the biology of the virus. A systematic study of various samples obtained throughout the course of the disease in several patients will be required to determine the best testing algorithm. Certainly in the control of the outbreak in Toronto, carefully established epidemiological links between patients fitting the case definition seemed the most effective method, but laboratory confirmation of some cases in the chain also proved to be very helpful (Wallington et al., 2003).
In summary, a single-tube RT-PCR assay for the detection of human coronaviruses, including the SARS-HCoV, has been developed. It is expected to remain effective with possible SARS-HCoV mutants and to be of value should other new coronaviruses emerge as human pathogens.
Acknowledgements
This work was supported by the Canadian Institutes for Health Research and by the Department of Paediatric Laboratory Medicine, Hospital for Sick Children, Toronto.
References
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., Berger A., Burguière A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.-C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.D., Osterhaus A.D.M.E., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Drazen J.M. Case cluster of the severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:e6–e7. doi: 10.1056/NEJMe030062. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A.M., Kuiken T., Schutten M., van Amerongen G., van Doornum G.J., van den Hoogen B.G., Peiris M., Lim W., Stöhr K., Osterhaus A.D.M.E. Koch's postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R.A.M., Hartwig N.G., Bestebroer T.M., Niemeyer B., De Jong J.C., Simon J.H., Osterhaus A.D.M.E. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Peiris J.S.M., Zheng B., Poon L.L.M., Chan K.H., Zheng F.Y., Chan C.W.M., Chan M.N., Chen J.D., Chow K.Y.C., Hon C.C., Hui K.H., Li J., Li V.Y.Y., Wang Y., Leung S.W., Yuen K.Y., Leung F.C. Molecular epidemiology of the novel coronavirus that causes severe acute respiratory syndrome. Lancet. 2004;363:99–104. doi: 10.1016/S0140-6736(03)15259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.V. Coronaviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincott Williams & Williams; Philadelphia: 2001. pp. 1197–1204. [Google Scholar]
- Johnson G., Nelson S., Petric M., Tellier R. Comprehensive PCR-based assay for detection and species identification of human herpesviruses. J. Clin. Microbiol. 2000;38:3274–3279. doi: 10.1128/jcm.38.9.3274-3279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.-E., Humphrey C.D., Shieh W.-J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.-Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kumar D., Tellier R., Draker R., Levy G., Humar A. Severe acute respiratory syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am. J. Transplant. 2003;3:977–981. doi: 10.1034/j.1600-6143.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.Y. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S.N., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Normile D. Mounting lab accidents raise SARS fears. Science. 2004;304:659–661. doi: 10.1126/science.304.5671.659. [DOI] [PubMed] [Google Scholar]
- Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T., Cheng V.C.C., Chan K.H., Tsang D.N.C., Yung R.W.H., Ng T.K., Yuen K.Y., SARS study group Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M., Law K.I., Tang B.S.F., Hon T.Y.W., Chan C.S., Chan K.H., Ng J.S.C., Zheng B.J., Ng W.L., Lai R.W.M., Guan Y., Yuen K.Y., HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J., National Microbiology Laboratory, Canada, Canadian Severe Acute Respiratory Syndrome Study Team Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Peñaranda S., Bankamp B., Maher K., Chen M.-H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C.T., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Günther S., Osterhaus A.D.M.E., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1398. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Ruan Y.J., Wei C.L., Ling A.E., Vega V.B., Thoreau H., Se Thoe S.Y., Chia J.-M., Ng P., Chiu K.P., Lim L., Zhang T., Chan K.P., Oon L.E., Ng M.L., Leo S.Y., Ng L.F.P., Ren E.C., Stanton L.W., Long P.M., Liu E.T. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infections. Lancet. 2003;361:1779–1785. doi: 10.1016/S0140-6736(03)13414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizun J., Arbour N., Talbot P.J. Comparison of immunofluorescence with monoclonal antibodies and RT-PCR for the detection of human coronaviruses 229E and OC43 in cell culture. J. Virol. Methods. 1998;72:145–152. doi: 10.1016/S0166-0934(98)00013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L.M., Guan Y., Rozanov M., Spaan W.J.M., Gorbalenya A.E. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P., Louie M., Richardson S.E., Smieja M., Simor A.E., Jamieson F., Fearon M., Poutanen S.M., Mazzulli T., Tellier R., Mahony J., Loeb M., Petrich A., Chernesky M., McGeer A., Low D.E., Phillips E., Jones S., Bastien N., Li Y., Dick D., Grolla A., Fernando L., Booth T.F., Henry B., Rachlis A.R., Matukas L.M., Rose D.B., Lovinsky R., Walmsley S., Gold W.L., Krajden S., The Ontario Laboratory Working Group for the Rapid Diagnosis of Emerging Infections Interpretation of diagnostic laboratory tests for severe acute respiratory syndrome: the Toronto experience. Can. Med. Assoc. J. 2004;170:47–54. [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Van de Peer Y., De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- Van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J.M., Wolthers K.C., Wertheim-van Dillen P.M.E., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T.H., Cabau J.-F., Nguyen N.T., Lenoir M. SARS in Northern Vietnam. N. Engl. J. Med. 2003;348:2035. doi: 10.1056/NEJM200305153482018. [DOI] [PubMed] [Google Scholar]
- Wallington T., Berger L., Henry B., Shahin R., Yaffe B., Mederski B., Berall G., Christian M., McGeer A., Low D., Wong T., Tam T., Ofner M., Hansen L., Gravel D., King A., SARS Investigation Team Update: severe acute respiratory syndrome–Toronto, Canada. Comm. Dis. 2003;29:113–116. [PubMed] [Google Scholar]
- World Health Organization Multicentre Collaborative Network for Severe Acute Respiratory Syndrome (SARS) Diagnosis, 2003. A multicentre collaboration to investigate the cause of severe acute respiratory syndrome. Lancet 361, 1730-1733.
- World Health Organization, 2003 Alert, verification and public health management of SARS in the post outbreak period. http://www.who.int/csr/sars/postoutbreak/en/
- World Health Organization. 2004. New case of laboratory confirmed SARS in Guangdong, China (update 5). http://www.who.int/csr/don/2004_01_31/en/


