Highlights
-
•
Activation of CD46 induces rapid down-modulation of CD46 expression on the surface of CD8+ T cells.
-
•
CD46 co-stimulation potently induces CD8+ T cell proliferation.
-
•
CD46 co-stimulation does not induce a switch to IL-10 production in CD8+ T cells, as is known for CD4+ T cells.
-
•
CD46 co-stimulation predominantly expands IFN-γ-producing CD8+ T cells.
Abbreviations: PBMC, peripheral blood mononuclear cell; TCR, T-cell receptor; Th1 cells, T-helper cells type 1
Keywords: CD46, CD8+T cells, Proliferation, IFN-γ, IL-10
Abstract
Similar to CD4+ T cells, precursor CD8+ T cells are thought to depend on a co-stimulatory signal through CD28 for proliferation and differentiation into effector cells. CD46 is another co-stimulatory receptor that promotes differentiation of CD4+ T-helper cells type 1 (Th1 cells) into a regulatory phenotype with a switch from IFN-γ towards IL-10-secretion over time. Whether CD46 exerts a similar function on CD8+ T cells remains to be fully elucidated. Here, we demonstrate that CD46 co-stimulation induced secretion of IFN-γ as well as expansion of IFN-γ-secreting CD8+ T cells. In contrast to CD46 co-stimulation of CD4+ T cells, CD8+ T cells did not differentiate into a regulatory IL-10-secreting phenotype. This demonstrates that CD46 is a co-stimulatory receptor on CD8+ T cells, and that it exerts separate functions during CD4+ and CD8+ T-cell differentiation.
1. Introduction
T-cell activation and differentiation are controlled by a combination of graded T-cell receptor (TCR) signaling and co-stimulation, which together induce the gene expression involved in differentiation and effector functions. A model for the dynamical regulation between the TCR signal, the induced transcription factors, and the CD8+ T-cell function, suggests that inducible T-cell kinases are a rheostat for the gene expression [1].
However, an important contributing factor for the induced gene expression is the signals delivered by contributing co-stimulatory receptors. Apart from CD28 and CD2, the majority of known co-stimulatory receptors for CD8+ T-cell proliferation are within the tumor necrosis factor receptor superfamily (HVEM, CD27, 4-1BB, CD134, DR3, GITR, CD30), although also CD244 (2B4) and CD226 has been reported to co-stimulate CD8+ T cell growth. For those co-stimulatory receptors that are expressed and have been examined on both CD8+ and CD4+ T cells, similar functional consequences have been reported (reviewed in [2]).
In humans, CD46 is a co-stimulatory molecule for CD4+ T cells. Originally identified as a complement receptor [3], CD46 was later discovered to have important roles within the adaptive immune system. CD46 co-stimulation of CD4+ cells appears crucial to IFN-γ production and induction of T-helper cells type 1 (Th1 cells) [[4], [5], [6]] and furthermore in conjunction with IL-2 induces a shift in cytokine secretion towards IL-10-producing Th1 cells [4,7,8].
A subset of effector CD8+ T cells secretes both IFN-γ and IL-10 and is thought to be involved in controlling tissue inflammation during infection. This subset has previously been identified in a mouse model for acute influenza virus [9], and in the brain of coronavirus-infected mice [10]. In humans, a regulatory CD8+ T cell subset generated in vitro from CD8+CD28− but not from CD8+CD28+ T cells was able to inhibit T-cell proliferation and cytotoxicity by secretion of IL-10 [11]. Using tetramer-sorted antigen-specific CD8+ T cells from controls and solid organ transplanted patients, controls had relatively few IFN-γ and IL-10-producing, double-positive cells, whereas EBV antigen-specific CD8+ T cells from solid organ transplanted patients produced equal levels of IFN-γ and IL-10 [12]. Although CD46 was reported to slightly increase proliferation of CD8+ T-cells as well as expression of surface markers involved in T-cell activation, it did not induce a significant increase in IFN-γ production [13]. Thus, whether CD46 exerts a co-stimulatory function in CD8+ T cells lacking CD28 remains to be fully elucidated.
We compared the ability of human CD4+ and CD8+ T cells to proliferate and to secrete IFN-γ and IL-10 upon stimulation with antibodies to TCR/CD3 and CD46. Interestingly, we observed that CD46 was a potent co-stimulatory receptor for expansion of CD8+ T-cells that secreted IFN-γ, but in contrast to CD4+ T cells, CD46 did not induce an IL-10 regulatory phenotype in CD8+ T cells. This demonstrates that CD46 is a co-stimulatory receptor in CD8+ T cells, and to our knowledge provides the first example of a co-stimulatory receptor with a major qualitatively different response in CD4+ and CD8+ T cells.
2. Materials and methods
2.1. Ethical approval
Blood samples from 9 Caucasian donors were collected at the Blood Bank of the Department of Clinical Immunology, Aarhus University Hospital, and provided anonymously for analysis according to the guidelines from the Danish Society for Clinical Immunology and the Ethical Committee on the use of donor samples for research purposes. All donors provided informed consent as to the use of their blood samples for scientific purposes.
2.2. Cell preparation and stimulation
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats from healthy donors (The Blood Bank, Aarhus University Hospital, Denmark) using Ficoll-Paque PLUS (GE Healthcare Bioscience). The PBMCs were cryopreserved in 90% heat-inactivated fetal bovine serum (FBS) and 10% DMSO (Sigma-Aldrich) and stored at −80 °C. CD4+ and CD8+ T cells, respectively were isolated from PBMCs by negative selection using EasySep Human CD4+ or CD8+ T Cell Isolation Kits (Stemcell). Approximately 2.5–3 × 105 isolated T cells/well were stimulated in a 48-well plate pre-coated with 2 μg/ml αCD3 (OKT-3, eBioscience), 1 μg/ml αCD28 (CD28.2, eBioscience) or 1 μg/ml αCD46 (M177, Thermo Scientific) and cultured in RPMI (Gibco) supplemented with 10% FBS, 10 mM HEPES (Gibco), 2 mM glutaMAX (Gibco), 2.5 nM sodium pyruvate and 20U/ml recombinant human IL-2 (Roche).
2.3. Flow cytometry
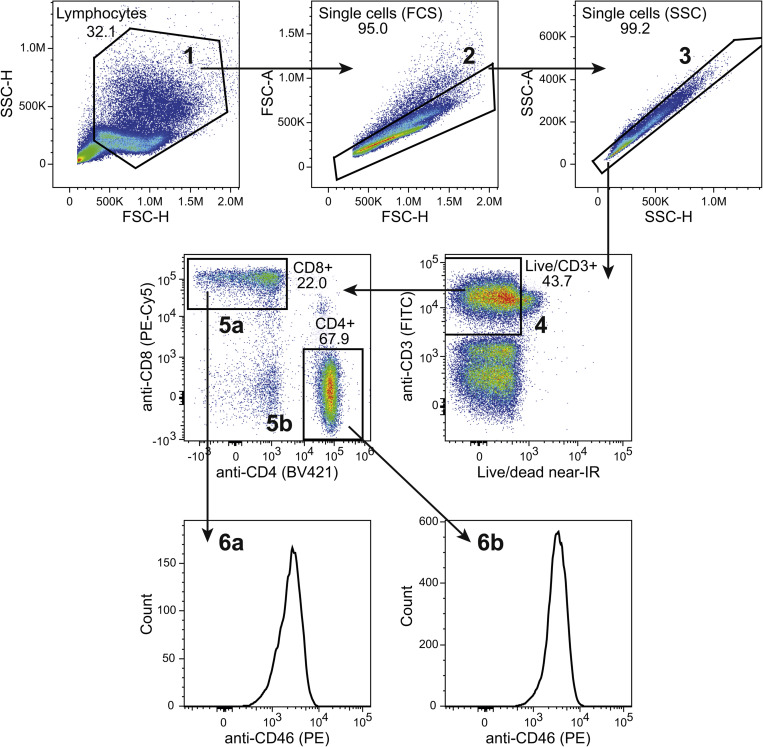
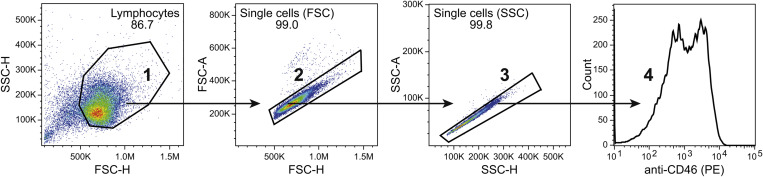
PBMCs were stained with αCD3-FITC (UCHT1, BD Bioscience), αCD4-Brilliant Violet 421 (RPA-T4, BD Bioscience), αCD8-PC5 (B9.11, Beckmann Coulter), αCD46-PE (MEM-258, Sigma-Aldrich), IgG1-PE isotype control (BD Bioscience), and LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Life Technologies) in the volumes indicated by the manufacturers. CD46 expression was determined on respectively CD4+CD8− and CD4-CD8+ cell populations following gating on first live cells and then CD3+ cells. Isolated and in vitro activated CD8+ and CD4+ T cells were stained with αCD46-PE (MEM-258) and LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit and the expression of CD46 was determined on the live cells. The isotype control antibody was used to visualize the background PE fluorescence signal. Data were acquired on a NovoCyte flow cytometer (ACEA Bioscience Inc.) and processed in FlowJo (Tree Star). The detailed gating strategy for all flow cytometry experiments is presented as supplemental figurers (Fig. 1S–4S). Fluorescent data was displayed using bi-exponential visualization according to best practice [14].
2.4. ELISA
Supernatants were collected from the stimulated cells and stored at −20 °C for up to 2 weeks. The amount of IL-10 and IFN-γ in the supernatants was assessed by sandwich ELISA using the Human IL-10 or IFN-γ DuoSet ELISA kits (R&D Systems) according to manufacturer’s instructions. Each sample was performed in duplicate and data were analyzed in VersaMax with a 4-parameter fit for the standard curve.
2.5. Real-time PCR
RNA was extracted from the cells using the Nucleo Spin RNA Kit (Macherey-Nagel) according to manufacturer’s instructions. 12 μl RNA was converted into cDNA using the QuantiTect RT kit (Qiagen) and the final cDNA was diluted 1:10 in RNase-free water. The relative expression of IL-10 and IFN-γ mRNA was assessed by real-time PCR using Brilliant II SYBRgreen (Agilent Technology) and normalized to the housekeeping gene PPIB. For the real-time PCR was used 300 nM of the following primers: PPIB: fw 5’-TGTGGTGTTTGGCAAAGT-3’, rev 5’-TGGAATGTGAGGGGAGTG-3’, IFN-γ: fw 5’-GAATTGGAAAGAGGAGAGTG-3’, rev 5’-TGTATTGCTTTGCGTTGG-3’, and IL-10: fw 5’-GTCTGAGATGATCCAGTTT-3’, rev 5’- CCTTTCTCTTGGAGCTTATT-3’.
The relative units for the gene of interest were calculated using the 2-ΔCt method.
2.6. Proliferation
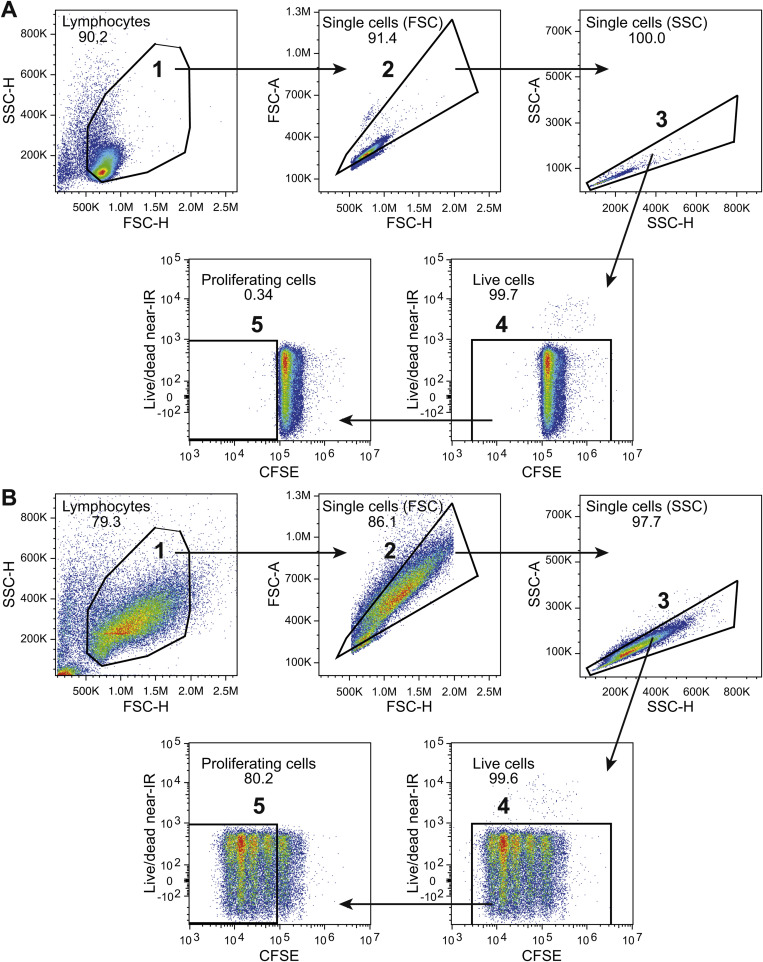
For analysis of proliferation the cells were incubated with 5 nM CFSE (Invitrogen) for 5 min prior to stimulation. The cells were harvested and stained with LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit enabling gating on live cells possible, and analyzed with flow cytometry.
2.7. Cytokine secretion assay
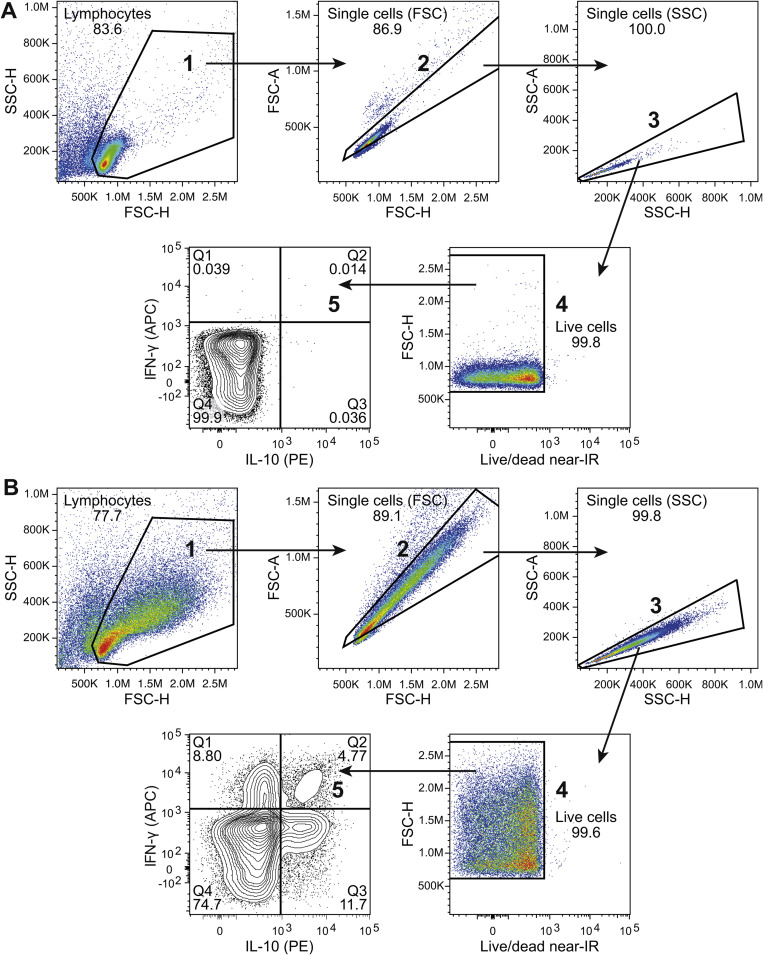
The frequency of IL-10- and IFN-γ-secreting cells after stimulation with plate-bound antibodies was analyzed with the IL-10 (PE) and IFN-γ (APC) Secretion Assay Detection Kit (MACS miltenyi) according to manufacturer’s instructions. To exclude dead cells from analysis, the cells were stained with LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit and the cells were analyzed with flow cytometry.
2.8. Statistical analysis
The data were analyzed using the Wilcoxon signed rank test or with p < 0.05 considered as significant.
3. Results and discussion
3.1. CD46 co-stimulation induces cytokine secretion in CD8+ T cells
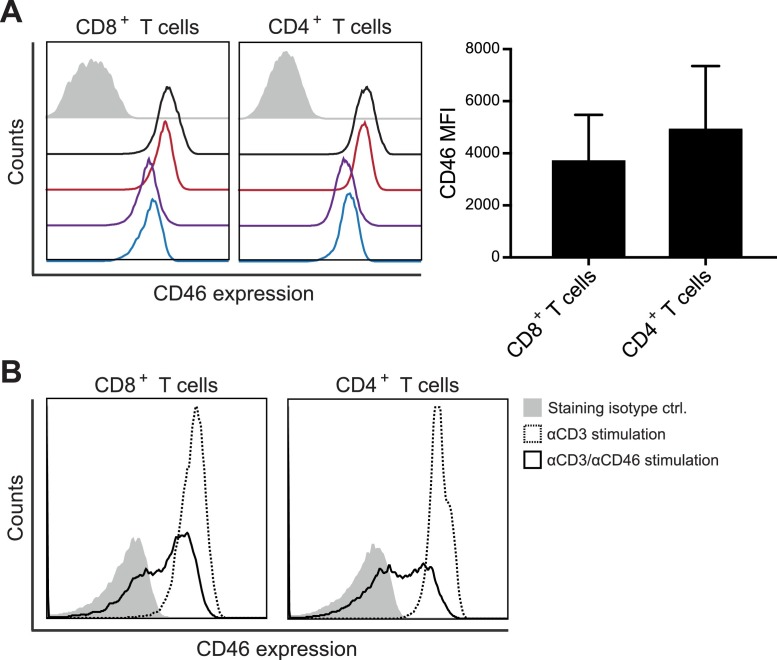
CD46 is expressed on the surface of all nucleated human cells [15,16]. Using flow cytometry analysis of isolated PBMCs from healthy blood donors, we determined a similar expression level of CD46 on the surface of CD8+ and CD4+ T cells with moderate interpersonal variance (Fig. 1 A). Activation of CD46 on CD4+ T cells has previously been demonstrated to cause a rapid down-modulation of CD46 from the cell surface [8,17]. We therefore examined whether stimulation of isolated CD8+ T cells in vitro with αCD3/αCD46 resulted in a similar down-modulation of CD46 from the cell surface. This demonstrated that 2 h of activation caused a rapid down-modulation of CD46 from the surface of both CD4+ and CD8+ T cells (Fig. 1B).
Fig. 1.
CD46 co-stimulation down modulates surface expression of CD46. (A) Flow histograms of baseline CD46 surface expression (open histograms) on CD3+CD8+ and CD3+CD4+ T cells from four different donors, stained with αCD3-FITC, αCD4-Brilliant Violet 421, αCD8-PC5, and αCD46-PE. An isotype control stained with IgG1-PE (closed gray histogram) from one representative donor is shown for comparison. The right panel shows the summarized CD46 median fluorescence intensity (MFI) values for the 4 donors. (B) Surface expression of CD46 on isolated CD8+ and CD4+ T cells following activation with αCD3 or αCD3/αCD46 for 2 h measured by flow cytometry using αCD46-PE for staining. An isotype control stained with IgG1-PE (closed gray histogram) is shown for comparison. Data are representative of 2 independent experiments.
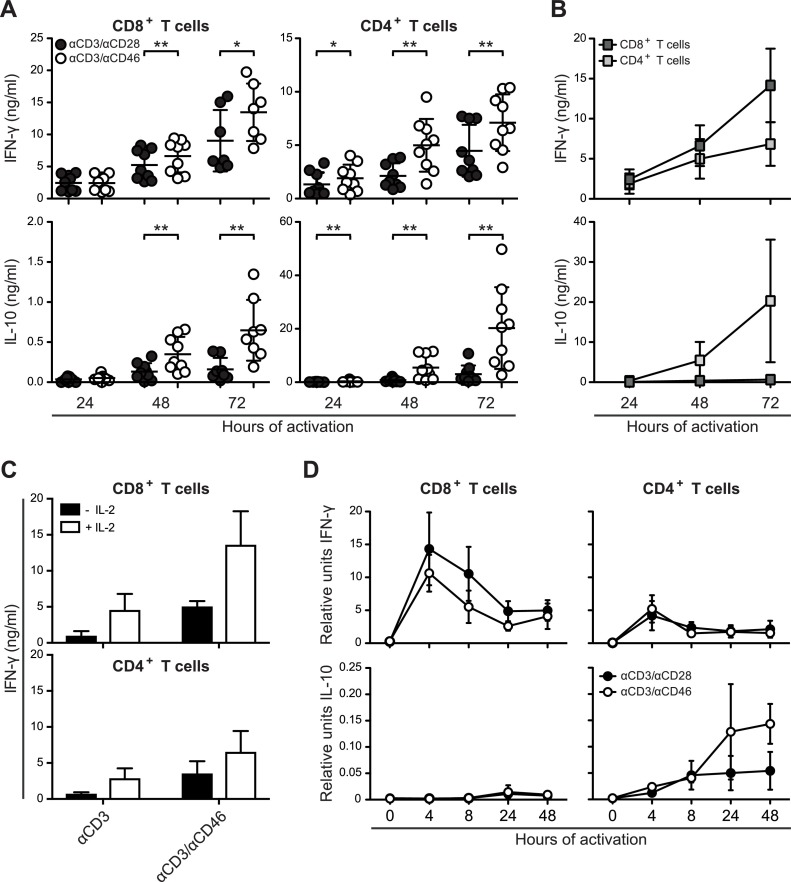
To investigate whether CD46 co-stimulation further supported differentiation into effector CD8+ T cells, we analyzed the cytokine production in CD4+ and CD8+ T cells respectively, following in vitro activation using plate-bound αCD3/αCD28 or αCD3/αCD46. Upon activation both CD4+ and CD8+ T cells secreted IFN-γ and IL-10, which accumulated in the supernatant as measured by ELISA. As expected, we were able to confirm a significant increase in the level of IL-10 in supernatants from CD46-co-stimulated CD4+ T cells (Fig. 2 A). Although CD46 co-stimulation also induced the secretion of IL-10 in CD8+ T cells, the average level in the supernatant was less than 3% of that seen for CD4+ T cells (Fig. 2B). This is in accordance with an observation by Kemper et al. that CD46 was unable to induce IL-10 production in several distinct immune cell subsets, including CD8+ T cells [7]. In contrast, CD46 co-stimulation induced higher levels of IFN-γ in CD8+ T cells when compared with CD4+ T cells. These data suggest that co-stimulation of CD46 together with CD3 increases the amount of secreted IFN-γ from both CD4+ and CD8+ T-cell cultures, whereas it induces IL-10 secretion mainly from CD4+ T cells. The CD46-induced IFN-γ production in CD8+ T cells was dependent on IL-2, since no or little IFN-γ levels could be detected in the absence of exogenous IL-2 (Fig. 2C). This suggests a cross talk between CD46 and IL-2R signaling, as has also been suggested for CD4+ T cells [4].
Fig. 2.
CD46 co-stimulation induces cytokine secretion from CD8+ T cells. (A) ELISA measurements of IFN-γ and IL-10 in supernatants from CD8+ and CD4+ T-cell cultures. Human CD8+ and CD4+ T cells were isolated and stimulated with either αCD3/αCD28 or αCD3/αCD46 for different time-periods as indicated. Data are represented as mean ± SD of 8 or 9 donors. Data were analyzed using Wilcoxon signed rank test, * p < 0.05, ** p < 0.01. All ELISA experiments were performed in technical duplicates. (B) Comparisons on a similar scale of IFN-γ and IL-10 in supernatants from CD8+ and CD4+ T cells induced by αCD3/αCD46 co-activation (data from panel (B)). (C) Dependency of IL-2 for release of IFN-γ from CD8+ and CD4+ T-cell cultures. Human CD8+ and CD4+ T cells were isolated and stimulated with either αCD3 or αCD3/αCD46 for 72 h in presence or absence of 20U/ml IL-2. Data represents mean + SD of three independent experiments. (D) The relative transcription level of IFN-γ and IL-10 mRNA assessed by real-time PCR using SYBRgreen and normalized to the housekeeping gene PPIB. Data are represented as mean ± SD of 4 donors. Real-time PCR was performed in technical triplicates.
Next, we addressed whether CD46 co-stimulation resulted in increased transcription from the IFN-γ and IL-10 genes. CD46 co-stimulation induced similar or less IFN-γ mRNA in CD8+ T cells measured by real-time PCR (Fig. 2D) indicating that the increased secretion of IFN-γ was not caused by increased production of IFN-γ mRNA. Furthermore, CD46 co-stimulation had no detectable effect on the transcription of IL-10 mRNA in CD8+ T cells as opposed to CD4+ T cells (Fig. 2D).
The serine-threonine kinase SPAK, which interacts with CD46 to mediate IL-10 induction, is ubiquitously present. Nevertheless, it has been reported that γδ T cells are unable to switch into IL-10 production following CD46 stimulation presumably due to expression of an alternative isoform of CD46 [4]. CD46 is alternatively spliced and expressed in predominantly four isoforms. Notably, these isoforms express one of two possible intracytoplasmic tails, known as CYT-1 and CYT-2, which are thought to transmit different signals within the CD4+ T cell. CYT-1 isoforms may promote CD4+ T-cell activation, whereas CYT-2 isoforms may be involved in the contraction of the response [4,6,8]. We have previously characterized CD46 isoform expression on subsets of immune cells including CD4+ and CD8+ T cells and demonstrated interindividual differences, but intraindividual similarities [18]. Thus, based on their total CD46 expression (Fig. 1A) and isoform expression pattern, it is not predicted that CD46 co-stimulation should differ between CD4+ and CD8+ T cells. Thus, the lack of a CD46-promoted IL-10 switch in CD8+ T cells is unlikely to be caused by expression of an alternative CD46 isoform but may be caused by other inherent differences in the induction of the IL-10 promoter between the two subsets of T cells.
3.2. CD46 co-stimulation promotes proliferation of CD8+ T cells
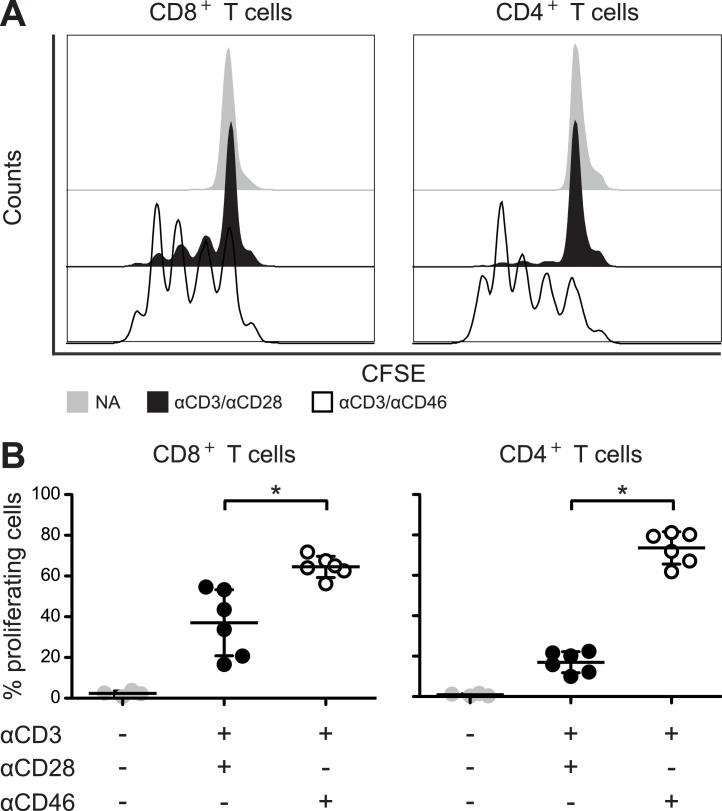
CD46 co-stimulation has previously been demonstrated to induce proliferation of CD4+ T cells [7,19], however its ability to induce proliferation in CD8+ T cells is less clear. One study reported a modest increase in thymidine uptake of CD8+ T cells [13], which was not specified as being statistically significant. We therefore investigated whether CFSE labeled CD8+ T cells proliferated to co-stimulation by CD46 using flow cytometry. In accordance with previous observations, CD4+ T cells proliferated upon CD46 co-stimulation. Importantly, we found that CD46 co-stimulation of CD8+ T cells induced a similar proliferation of CD8+ T cells (Fig. 3 A and B). This further suggests that CD46 indeed may function as a co-stimulatory molecule for CD8+ T cells.
Fig. 3.
CD46 co-stimulation promotes proliferation of CD8+ T cells. Isolated CD8+ and CD4+ T cells, respectively, were incubated with CFSE and stimulated with either αCD3/αCD28 or αCD3/αCD46 for 72 h. (A) Flow histograms of the CFSE fluorescence intensity in CD8+ and CD4+ T cells from a representative donor (n = 6 donors). (B) Frequency of proliferating cells represented as mean ± SD of 6 donors. Data were analyzed using Wilcoxon signed rank test, * p < 0.05. NA = no antibodies.
3.3. CD46 co-stimulation expands IFN-γ-producing CD8+ T cells
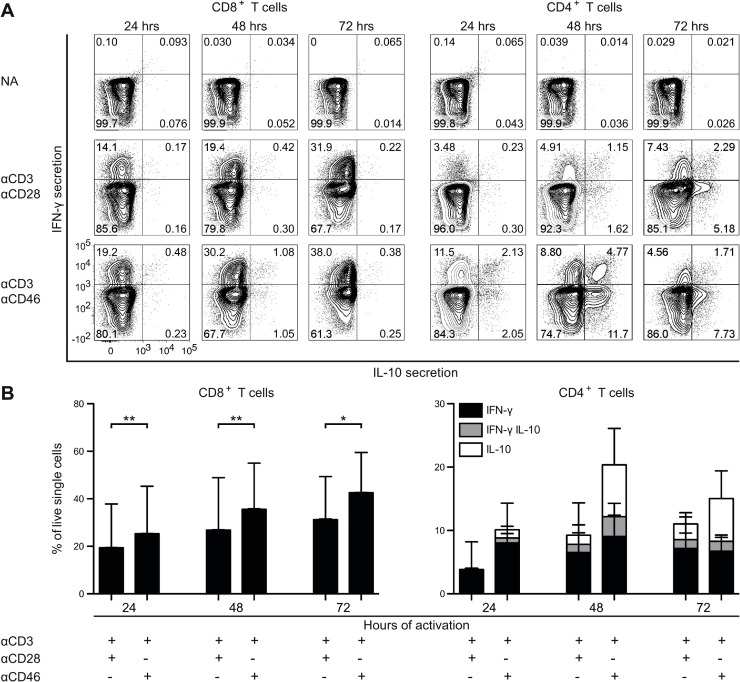
To establish whether the CD46-induced IFN-γ in the supernatants from CD8+ T cells primarily originated from induction of IFN-γ secretion or from expansion of cells producing IFN-γ, the frequencies of IFN-γ- and IL-10-producing cells were analyzed by flow cytometry using a cytokine secretion assay (Fig. 4 A and B). Activation of CD8+ T cells increased the frequency of IFN-γ-producing cells, and the frequency was further elevated upon CD46 co-stimulation. Whereas CD46 co-stimulation of CD4+ T cells in the presence of IL-2 induced a shift to IFN-γ+IL-10+ double positive cells and IL-10+ single positive cells over 3 days of culture, CD8+ T cells increased the frequency of IFN-γ+ cells but did not show a shift towards production of IL-10 even in the presence of exogenous IL-2. This suggests a different function of CD46 co-stimulation in CD8+ and CD4+ T cells, respectively, where CD46 co-stimulation of CD8+ T cells promotes expansion and consequently a strong IFN-γ response. In contrast, CD46 co-stimulation of CD4+ T cells promotes an IFN-γ response and a subsequent shift towards a strong IL-10 response that may act as an intrinsic self-regulating mechanism.
Fig. 4.
CD46 co-stimulation expands IFN-γ-producing CD8+ T cells. Isolated CD8+ and CD4+ T cells were stimulated with either αCD3/αCD28 or αCD3/αCD46 for different time-periods as indicated, and analyzed by flow cytometry for the frequency of IFN-γ and IL-10-producing cells using the IL-10 (PE) and IFN-γ (APC) Secretion Assay Detection Kit. Analyses applied gating for live cells. (A) Contour plot of a representative donor (n = 8 donors). NA = no antibodies. (B) Average frequency (n = 8 donors) of IFN-γ+ IL-10−, IFN-γ+IL-10+ and IFN-γ−IL-10+-secreting cells from CD8+ (left panel) and CD4+ T cells (right panel). Frequency is calculated per live cells. SD for each of the subsets is indicated on top of the bars. Data were analyzed using Wilcoxon signed rank test, * p < 0.05, ** p < 0.01.
Our findings that CD46 co-stimulation promotes proliferation and strongly induce IFN-γ production of CD8+ T cells is contrary to previous results demonstrating no induction in IFN-γ secretion in CD8+ T cells upon CD46 co-stimulation, despite a modest increase in thymidine uptake and upregulation of surface markers associated with T-cell activation [13]. This discrepancy may arise from different experimental conditions including differences in the concentration of antibodies used for in vitro activation of T cells and the use of distinct CD46 antibody clones. Whereas Kickler et al. used the antibody clone MCI20.6, which binds to an epitope in SCR1, we used M177, which binds to and epitope in SCR2. Blocking studies on adenovirus binding to CD46 demonstrated that M177 and some SCR1 binding antibodies block viral binding very efficiently, but MCI20.6 had relatively poor blocking abilities [20]. It is plausible that M177 and MCI20.6 have different binding affinities for CD46 and thus may induce signaling from CD46 with different potency.
T cells producing IFN-γ are highly efficient in clearing infections but also have the potential to cause severe immune pathology and therefore needs to be tightly controlled. In CD4+ T cells, CD46 co-stimulation resulted in a switch from dominance of IFN-γ secreting cells at early time-points of activation to dominance of IL-10 secreting cells at later time-points of activation indicating an intrinsic regulation of the CD4+ T cell effector response, which is in accordance with previous observations from Cardone et al. [4] and Ni Choileain et al. [8].
Expansion of IFN-γ-secreting CD8+ T cells may have a positive feedback mechanism, since IFN-γ is important for generating CD8+ T cell memory at least in mice [21,22]. In addition, IFN-γ-deficient mice have impaired contraction of antigen-specific CD8+ T cells [23,24]. Thus, CD46 may in CD8+ T cells serve to induce an efficient antigen-specific T-cell response and favor generation of memory cells. The quite substantial induction of IFN-γ in CD8+ T cells by CD46 may also serve as a later negative feedback regulation of the immune response given the significance of this cytokine for the contraction phase of the antigen-specific response.
An increased frequency of virus-specific IFN-γ-producing CD8+ T cells compared to CD4+ T cells have previously been observed in mice infected with various viruses [25,26]. This might result from a greater proliferative capacity of CD8+ T cells, different susceptibility to apoptosis or different regulatory mechanism mediated by CTLA-4 [27]. However, we speculate that it might also result from differences in intrinsic regulatory mechanisms such as a lack of the switch into an inhibitory IL-10 production following the initial effector cytokine production phase.
3.4. Concluding remarks
In this study, we have demonstrated that CD46 exerts separate functions in CD4+ and CD8+ T cells, since it promotes the differentiation of an IL-10-producing regulatory subset in Th1 cells, whereas it predominantly expands the IFN-γ-secreting CD8+ T cells. The apparent lack of differentiation of the CD8+ T cells may have important applications. For adoptive transfer of cellular immunotherapy, it is desirable to uncouple T-cell expansion from its differentiation [28]. We suggest that anti-CD46 should be further explored as a means to expand CD8+ T cells for such therapy.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Acknowledgement
We thank the FACS core facility, Aarhus University, Denmark, for assistance in the flow cytometry analyses. This work was supported by a grant from the Independent Research Fund Denmark (DFF-4004-00058) and the Department of Biomedicine, Aarhus University.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.imlet.2018.06.003.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Conley J.M., Gallagher M.P., Berg L.J. T cells and gene regulation: the switching on and turning Up of genes after t cell receptor stimulation in CD8 T cells. Front. Immunol. 2016;7:76. doi: 10.3389/fimmu.2016.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seya T., Turner J.R., Atkinson J.P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J. Exp. Med. 1986;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardone J., Le Friec G., Vantourout P., Roberts A., Fuchs A., Jackson I. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 2010;11(9):862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Friec G., Sheppard D., Whiteman P., Karsten C.M., Shamoun S.A., Laing A. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat. Immunol. 2012;13(12):1213–1221. doi: 10.1038/ni.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolev M., Dimeloe S., Le Friec G., Navarini A., Arbore G., Povoleri G.A. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity. 2015;42(6):1033–1047. doi: 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemper C., Chan A.C., Green J.M., Brett K.A., Murphy K.M., Atkinson J.P. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421(6921):388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 8.Ni Choileain S., Weyand N.J., Neumann C., Thomas J., So M., Astier A.L. The dynamic processing of CD46 intracellular domains provides a molecular rheostat for T cell activation. PloS One. 2011;6(1) doi: 10.1371/journal.pone.0016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J., Madan R., Karp C.L., Braciale T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 2009;15(3):277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trandem K., Zhao J., Fleming E., Perlman S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J. Immunol. 2011;186(6):3642–3652. doi: 10.4049/jimmunol.1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filaci G., Fravega M., Negrini S., Procopio F., Fenoglio D., Rizzi M. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibit both T-cell proliferation and CTL function. Hum. Immunol. 2004;65(2):142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Popescu I., Macedo C., Abu-Elmagd K., Shapiro R., Hua Y., Thomson A.W. EBV-specific CD8++ T cell reactivation in transplant patients results in expansion of CD8++ type-1 regulatory T cells. Am. J. Transpl. 2007;7(5):1215–1223. doi: 10.1111/j.1600-6143.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 13.Kickler K., Ni Choileain S., Williams A., Richards A., Astier A.L. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0048486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzenberg L.A., Tung J., Moore W.A., Herzenberg L.A., Parks D.R. Interpreting flow cytometry data: a guide for the perplexed. Nat. Immunol. 2006;7(7):681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 15.Liszewski M.K., Post T.W., Atkinson J.P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Ann. Rev. Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 16.Christmas S.E., de la Mata Espinosa C.T., Halliday D., Buxton C.A., Cummerson J.A., Johnson P.M. Levels of expression of complement regulatory proteins CD46, CD55 and CD59 on resting and activated human peripheral blood leucocytes. Immunology. 2006;119(4):522–528. doi: 10.1111/j.1365-2567.2006.02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellinghaus U., Cortini A., Pinder C.L., Le Friec G., Kemper C., Vyse T.J. Dysregulated CD46 shedding interferes with Th1-contraction in systemic lupus erythematosus. Eur. J. Immunol. 2017;47(7):1200–1210. doi: 10.1002/eji.201646822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen A.S., Bundgaard B., Møller B.K., Höllsberg P. Non-random pairing of CD46 isoforms with skewing towards BC2 and C2 in activated and memory/effector T cells. Sci. Rep. 2016;6:35406. doi: 10.1038/srep35406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astier A., Trescol-Biemont M.C., Azocar O., Lamouille B., Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J. Immunol. 2000;164(12):6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- 20.Fleischli C., Verhaagh S., Havenga M., Sirena D., Schaffner W., Cattaneo R. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J. Virol. 2005;79(15):10013–10022. doi: 10.1128/JVI.79.15.10013-10022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitmire J.K., Tan J.T., Whitton J.L. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 2005;201(7):1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitmire J.K., Eam B., Benning N., Whitton J.L. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J. Immunol. 2007;179(2):1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 23.Badovinac V.P., Tvinnereim A.R., Harty J.T. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290(5495):1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 24.Tewari K., Nakayama Y., Suresh M. Role of direct effects of IFN-gamma on T cells in the regulation of CD8 T cell homeostasis. J. Immunol. 2007;179(4):2115–2125. doi: 10.4049/jimmunol.179.4.2115. [DOI] [PubMed] [Google Scholar]
- 25.Homann D., Teyton L., Oldstone M.B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001;7(8):913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 26.Harrington L.E., Most Rv R., Whitton J.L., Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 2002;76(7):3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seder R.A., Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 2003;4(9):835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 28.Crompton J.G., Sukumar M., Restifo N.P. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunol. Rev. 2014;257(1):264–276. doi: 10.1111/imr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.