Abstract
Naturally occurring feline infectious peritonitis (FIP) is usually fatal, giving the impression that immunity to the FIP virus (FIPV) is extremely poor. This impression may be incorrect, because not all cats experimentally exposed to FIPV develop FIP. There is also a belief that the incidence of FIP may be affected by a number of host, virus, and environmental cofactors. However, the contribution of these cofactors to immunity and disease incidence has not been determined. The present study followed 111 random-bred specific pathogen free (SPF) cats that were obtained from a single research breeding colony and experimentally infected with FIPV. The cats were from several studies conducted over the past 5 years, and as a result, some of them had prior exposure to feline enteric coronavirus (FECV) or avirulent FIPVs. The cats were housed under optimized conditions of nutrition, husbandry, and quarantine to eliminate most of the cofactors implicated in FIPV infection outcome and were uniformly challenge exposed to the same field strain of serotype 1 FIPV. Forty of the 111 (36%) cats survived their initial challenge exposure to a Type I cat-passaged field strains of FIPV. Six of these 40 survivors succumbed to FIP to a second or third challenge exposure, suggesting that immunity was not always sustained. Exposure to non-FIP-inducing feline coronaviruses prior to challenge with virulent FIPV did not significantly affect FIP incidence but did accelerate the disease course in some cats. There were no significant differences in FIP incidence between males and females, but resistance increased significantly between 6 months and 1 or more years of age. Genetic testing was done on 107 of the 111 infected cats. Multidimensional scaling (MDS) segregated the 107 cats into three distinct families based primarily on a common sire(s), and resistant and susceptible cats were equally distributed within each family. Genome-wide association studies (GWAS) on 73 cats that died of FIP after one or more exposures (cases) and 34 cats that survived (controls) demonstrated four significant associations after 100k permutations. When these same cats were analyzed using a sib-pair transmission test, three of the four associations were confirmed although not with genome-wide significance. GWAS was then done on three different age groups of cases to take into account age-related resistance, and different associations were observed. The only common and strong association identified between the various GWAS case configurations was for the 34.7–45.8 Mb region of chromosome A3. No obvious candidate genes were present in this region.
Keywords: Feline infectious peritonitis, Experimental, Natural immunity, Age resistance, Genetic resistance, GWAS
1. Introduction
The prevalence and severity of infectious diseases among multi-cat populations is a product of many diverse factors that affect the host/pathogen interaction (Pedersen, 1991). Environmental factors include things such as population density, sanitation, and interchange of animals while agent factors include virulence, dose, and route of exposure. Host factors include developmental and heritable anomalies in the immune system and age at the time of exposure and intercurrent illnesses. Many of these diverse cofactors have been implicated in FIP.
Foley et al. (1997) studied a number of environmental risk factors for FIP in seven catteries and found that cat numbers (density) and husbandry procedures had no influence on FIP incidence while age, high coronavirus antibody titers, and the proportion of cats shedding coronavirus were significantly associated with FIP risk. All of these risk factors are interrelated, because fecal coronavirus shedders are much more likely to have antibody titers >1:100 and younger cats are more likely to shed FECV at higher levels and for longer periods (Pedersen et al., 2004, Pedersen et al., 2008). The stresses of placing young cats into shelters have also been shown to greatly increase the levels of FECV shedding (Pedersen et al., 2004).
Field strains of FIPV are known to vary intrinsically in virulence and this virulence may be further affected by the route of administration (Pedersen et al., 1984, Pedersen and Floyd, 1986). The dose of virus used also can alter disease outcome although a dose that causes lethal infection in one cat may be insufficient to infect another (Pedersen and Black, 1983). Virulence may be influenced by the exact FIP-inducing mutations that are present. The known FIP-associated mutations in FECV 3c and the S1/S2 cleavage site are highly variable and unique to each isolate while the two single nucleotide mutations in the fusion domain are common to all FIPVs (Pedersen, 2014). Mutations in 7b can also alter virulence in some tissue culture-adapted strains, but do not play a role in the FECV-to-FIPV mutations in nature (Pedersen, 2014). Additional mutations may await discovery and their singular or collective roles in FIP remain to be determined.
Several host factors have been implicated in FIP. The stress of surgery, especially when performed at a young age, may increase susceptibility of cats to FIP development (Kass and Dent, 1995). Co-infections with FeLV will greatly increase the incidence of FIP by interfering with FIP immunity; more than one-third of all FIP cases occurred in cats that were persistently infected with FeLV (Cotter et al., 1973, Pedersen et al., 1977). Feline immunodeficiency virus (FIV) can also compromise host immunity and increase FIP prevalence under experimental conditions (Poland et al., 1996).
The present study was designed to eliminate as many potential agents, environmental, and host risk factors for FIPV infection as possible. The same field strain and infectious dose of virus were used for challenge exposure, a uniform standard of care was provided with no extraneous pathogen exposure, and the cats originated from the same breeding stock. The study was then concentrated on two potential risk factors that have been poorly studied, age at the time of exposure and genetic susceptibility.
The effect of age on FIPV infection has not been directly addressed, even though it has been previously discussed (Pedersen, 2009) and well documented for pathogens such as feline leukemia virus (FeLV) (Hoover et al., 1976). Kittens are born with immature immune systems, and the period between 4 and 16 weeks of age is when IgG and IgA systems are being compensated by passive local and systemic immunity (Pedersen, 1987a). Immaturity of the immune system may also play a role in the ability to vaccinate kittens to FIP; a commercially marketed attenuated live FIPV vaccine only demonstrated sufficient efficacy for licensing when given to kittens 16 weeks or older (Gerber et al., 1990). Field and laboratory studies indicate that some sort of maternal or innate resistance to FECV infection is present in neonatal kittens and that FECV fecal shedding usually does not occur until 9 weeks of age, even among kittens born to infected queens (Pedersen et al., 2008). Most cases of FIP occur in cats between 4 and 18 months of age (reviewed Pedersen, 2009) suggesting that some infections may remain subclinical for an extended period of time.
The possible role of genetics in FIP resistance has been implied from a number of studies. FIP did not exist before the 1950s (Holzworth, 1963), suggesting that cats may not have had time to genetically adapt, thus explaining why morbidity and mortality are so high in experimental FIPV infections. Pedigreed cats are more likely to develop FIP than random-bred cats (Robison et al., 1971, Rohrbach et al., 2001, Pesteanu-Somogyi et al., 2006, Worthing et al., 2012), and certain breeds are also more likely to succumb to FIP (Bell et al., 2006, Norris et al., 2005, Pesteanu-Somogyi et al., 2006, Worthing et al., 2012). One study of Persian catteries and pedigrees indicated that susceptibility to FIP was at least 50% heritable (Foley and Pedersen, 1996). Resistance to FIP in Birman cats also appears to have a genetic component as determined by GWAS (Golovko et al., 2013). Natural resistance to FIP has also been observed in up to one-third of random-bred cats used as controls in vaccine studies (Baldwin and Scott, 1997, Gerber et al., 1990, Glansbeek et al., 2002, Hohdatsu et al., 2003, Kiss et al., 2004, Pedersen and Black, 1983, Wasmoen et al., 1995).
The cats, infection outcome data, and DNA used in the present study originated from studies on type 1 FIPV and FECV conducted over the last several years with other objectives. Over the course of these studies, 111 cats of various age and gender were exposed one or more times to virulent strains of FIPV and their disease course closely monitored and cause of death confirmed to be FIP. Forty of the 111 cats resisted a single challenge exposure and 34 remained resistant after repeated infections. The studies were unique in that all of the cats were housed in identical facilities, cared for in an identical manner, and maintained free from other feline pathogens. Therefore, they were not affected by many of the agents, environmental, and host factors that might affect the incidence of FIP in nature. This allowed for an uncomplicated assessment of risk factors such as age, gender, and genetic susceptibility on disease outcome.
2. Materials and methods
2.1. Experimental animals
Cats were obtained from the specific pathogen free (SPF) breeding colony of the Feline Nutrition and Pet Care Center, University of California, Davis (UC Davis) (UC Davis IACUC #16988). The colony was established in 1976 with a small number of cats derived aseptically by cesarean section and records on all matings have been maintained to the present time. Mating pairs were selected based on degree of relatedness and outcrossing to enhance genetic diversity done on two occasions, 1995 and 1999. The relationships of all cats were known from the colony records.
Cats used for this study were housed in the Feline Research Laboratory of the Center for Companion Animal Health under conditions required by USDA regulations. Fifty-four of the 111 of cats were coronavirus naïve while 57 had previous FECV or non-virulent FIPV exposure (Pedersen et al., 2008, Pedersen et al., 2009, Pedersen et al., 2012). Experimental infection studies were conducted under UC Davis Institutional Animal Care and Use Committee protocol #16637.
2.2. Experimental infection studies
2.2.1. FIPV infection
The origins of Type I FIPV-i3c2 and FIPV–m3c2 and the preparation of cell-free infectious inoculates have been previously described (Pedersen et al., 2012). A total of 1 ml of a 1:5–1:10 dilution of a 25% cell-free suspension of diseased omentum was given by either the intraperitoneal (IP) or oronasal (ON) route. This proved infectious to 100% of cats by either route based on the occurrence of disease and/or seroconversion.
2.2.2. Inoculation procedures and disease monitoring
Cats were sedated with ketamine hydrochloride and inoculated either intraperitoneally (IP) or ON (0.5 ml orally, 0.5 ml nasally) with the various virus stocks. Rectal temperatures were recorded starting 1–2 days prior to inoculation and at 1–2 day intervals thereafter. Cats were examined daily for signs of disease, such as fever, inappetance, depression, diarrhea, dehydration, ascites, hyperbilrubinemia, hyperbilirubinuria, and jaundice. Affected cats were euthanized with an intravenous overdose of pentabarbital/phenytoin as soon as their disease course was deemed terminal.
2.3. Feline coronavirus serology
Antibodies to feline coronavirus were titrated by indirect immunofluorescence using Crandell-Rees feline kidney cells infected with FIPV-79-1146 as an antigen substrate (Pedersen, 1976).
2.4. Genetic testing
Whole EDTA-treated blood was available from 107 of 111 cats and genomic DNA isolated using the Qiagen (Valencia, CA) Gentra Puregene Blood Core Kit. GWAS was performed using the Illumina Infinium iSelect feline DNA array (Illumina Inc., San Diego, CA). The arrays were tested by GeneSeek Inc. (Lincoln, NE). SNP genotyping rate and minor allele frequency (MAF) was evaluated using PLINK (Purcell et al., 2007). SNPs with a MAF < 5%, genotyping rate < 90%, and individuals genotyped for <90% of SNPs were excluded from downstream analyses.
An MDS with two dimensions was performed on 41,004 SNPs in PLINK to evaluate population substructure within cases and controls. Inflation of p-values was evaluated by calculating the λ, and assessed with a Q–Q plot. A case-control whole genome association analysis was performed and corrected with 100,000 t-max permutations (-mperm 100,000) with significance at −log10 (Pgenome) ≥ 1.3.
The transmission disequilibrium test among sib-pairs (sib-TDT) (Spielman and Ewens, 1998) was performed on 18 phenotypically discordant sib-pairs using the function (--dfam). The sib-TDT analysis was conducted without including the founders in frequency calculation (--nonfounders).
3. Results
3.1. Infection and immunity studies
One hundred eleven cats were experimentally infected with virulent FIPV either by the IP or ON routes, and the disease outcome ultimately confirmed either by necropsy or seroconversion. There was no difference in challenge outcome between the two routes (data not shown). Fifty-seven cats had one or two prior exposures to FECV, non-infectious FIPV mutants, or sub-infectious doses of virulent FIPV. Thirty-three of these 57 (58%) cats developed fatal FIP, compared to 38 of 54 (70%) of naïve cats after experimental infection with virulent FIPV (Fig. 1 ), which was not significantly different (p = 0.24, Fisher's exact test).
Fig. 1.
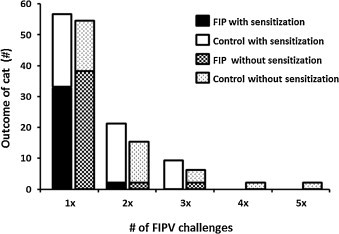
Outcome of FIPV challenge-exposure in naive or feline coronavirus pre-sensitized cats. Control cats resisted disease.
The strength of immunity was tested by re-challenge. Twenty two of 24 (92%) of the pre-sensitized survivors and 14 of 16 (88%) survivors without prior coronavirus exposure were still resistant after a second challenge-exposure (Fig. 1). Thirteen survivors from both groups were then exposed to FIPV a third time, and 11 of 13 (85%) remained resistant (Fig. 1). One cat survived a fourth infection and another survived five exposures (Fig. 1).
The onset of disease after FIPV infection always coincided with the appearance of fever (Fig. 2 ), which was rapidly followed by other signs such as inappetence, lethargy, cessation of grooming, hyperbilirubinemia, hyperbilirubinuria, jaundice, and ascites. In contrast, cats that resisted disease showed virtually no febrile response, remained outwardly normal, and seroconverted (Fig. 2).
Fig. 2.
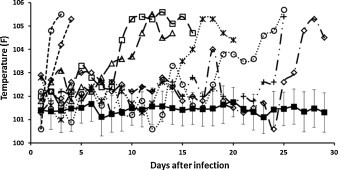
The temperature profile of cats after infection with virulent FIPV for the first time. Solid filled square represents the average temperature with standard deviation of 40 cats that did not develop FIP after primary infection. The open symbols with dashed lines are representative of individual FIP cat.
Pre-sensitization to non-disease-causing feline coronaviruses did not significantly alter the mortality rate although cats with prior exposure were somewhat more likely to develop accelerated disease (Fig. 3 ). All of the cats with prior coronavirus exposure became terminally ill within 31 days while five cats without prior exposure survived from 33 to 105 days. Four of these five slow progressors died of non-effusive of FIP and one of effusive FIP.
Fig. 3.
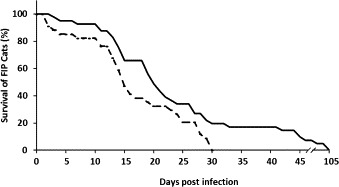
Survival of FIP cats after FIPV challenge exposure. Cats were pre-sensitized to feline coronavirus by prior exposure to FECV, non-infectious FIPVs or sub-infectious dose of FIPV (- - - -), or had no prior feline coronavirus exposure (___).
Survival rates were examined for cats of different gender and age. No difference was observed in FIP incidence between male and female cats (data not shown). There was a progressive and significant (p = 0.0008) decrease in mortality from 6 months to greater than 1 year of age (Fig. 4 ). Over 80% of cats younger than 6 months of age died compared to less than 45% of cats infected at greater than 1 year of age. Cats from 6–12 months of age were intermediate in susceptibility.
Fig. 4.
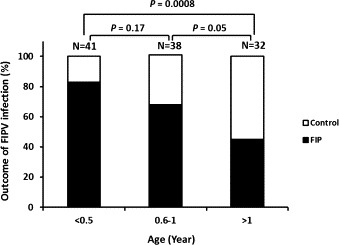
The effect of age at the time of exposure on FIP incidence.
FIP is known to persist in a subclinical form for some period of time following survival from challenge exposure to FIPV (Pedersen, 1987b) and this has confounded the interpretation of survival data in past FIP vaccine studies (Baldwin and Scott, 1997, Hoskins et al., 1994). To rule out subclinical infections among resistant cats in the present study, six individuals that had survived two or more challenge exposures were necropsied after 4–6 months and examined for subclinical lesions. No gross evidence of subclinical disease was found. Therefore, most cats that survived FIPV challenge will eventually clear the infection if given enough time.
3.2. Genetic analyses
Genome-wide association studies were conducted on 107 of the 111 infected, including 73 cases and 34 controls. PLINK analysis showed SNPs with genome-wide significance on chromosomes A3, B1, B4, and C1 (Fig. 5 ). A fifth SNP with genome-wide significance was present among non-annotated SNPs (UK) but was not further investigated.
Fig. 5.
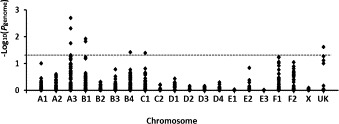
Manhattan plot comparing 73 cats that died of FIP and 34 cats that survived.
In order to determine any effect of relatedness on GWAS of the total population, family-related substructure was determined by multidimensional scaling (MDS). MDS segregated all case and control cats into three separate families (A, B, C) (Fig. 6 ). Cats from family A were sired by multiple related cats while cats in families B and C were each descended from a single sire. There was no significant difference in how FIP resistant and susceptible cats segregated between and within families (Fig. 6).
Fig. 6.
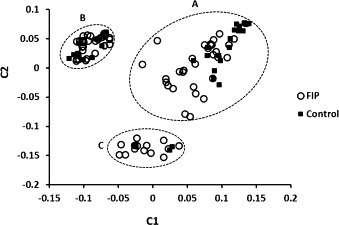
MDS plot of the 107 cats in the study based on PLINK analysis of GWAS data. Cats dying of FIP (○) or surviving challenge exposure (■) were segregated among three families. Family A had multiple related sires while families B and C were each derived from a single sire.
The family substructure identified by MDS was amended using a sib-TDT analysis with 18 phenotypically discordant nuclear families. After permutation, none of the SNPs remained genome-wide significant although strong associations were again observed on chromosomes A3, B1, and C1, the association on B4 was lost, and two new associations occurred on chromosomes C2 and D3 (Fig. 7 ).
Fig. 7.
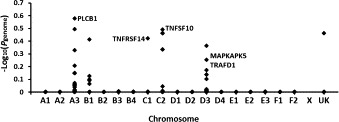
Sib-transmission/disequilibrium test of 54 cats that died of FIP and 24 survivors using the --dfam and 100,000 permutation command in PLINK. Five peaks of strong association were identified on defined chromosomes. Four of the five associations were near potential candidate genes relevant to FIP immunopathogenesis.
It was apparent that age at the time of exposure was a significant independent risk factor for disease outcome. Therefore, an attempt was made to compensate for age in the selection of cats used for GWAS (Fig. 8 ). The control group of cats remained the same based on the assumption that if a cat survived FIPV infection at <6 months of age, it would also survive exposure at 6 months and older. Conversely, a cat that died when exposed at <6 months of age might have survived if infected at >6 months of age independent on any genetic factors. Although the population size of case and controls was similar for each age group tested by GWAS, there were marked differences in the major genome-wide associations seen on Manhattan plots depending on the age of the case cats at the time of FIPV exposure (Fig. 8). The only strong association in common with these three age-adjusted GWAS studies and the total case/control population was for a region on chromosome A3 that extended from 34.7–45.8 Mb (Table 1 ). It was also noteworthy that a disproportionate number of the highest 25 ranking SNPs fell into this region, regardless of the configuration of case and controls based on age (Fig. 8), relatedness (Fig. 7), or on neither of these factors (Fig. 6). Based on Ensembl, this region contains 41 protein-coding genes and 9 novel protein-coding transcripts. None of the 41 genes appeared to be obvious candidates for immune or inflammatory processes involved in FIP.
Fig. 8.
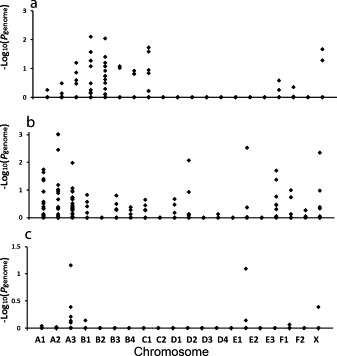
GWAS done on three groups of case and control cats based on age of cases (died FIP) at the time of exposure. (a) 34 cases and 34 controls (resistant) cats <6 months of age; (b) 27 cases and 34 controls >6 months and <1 year of age at time of exposure; (c) 39 cases and 34 controls that are all >6 months of age at time of exposure.
Table 1.
SNPs on chromosome A3 that were ranked among the top 25 in GWAS comparing all case and controls, family-adjusted case and controls, and age-adjusted case and controls.
| SNP | bp | 73FIP/34C (All) |
53FIP/24C (Family) |
34FIP/34C (<6 month) |
27FIP/34C (6 month–1year) |
39FIP/34C (>6 month) |
|---|---|---|---|---|---|---|
| chrA3.3103603 | 25933838 | 0.045 | ||||
| chrA3.40285519 | 34704954 | 0.032 | ||||
| chrA3.40449694 | 34833752 | 0.005 | 0.034 | 0.15 | ||
| chrA3.41176774 | 35406318 | 0.002 | 0.026 | 0.006 | 0.023 | |
| chrA3.44853345 | 38346280 | 0.057 | ||||
| chrA3.45799457 | 39123818 | 0.049 | ||||
| chrA3.47421518 | 40516688 | 0.134 | ||||
| chrA3.54308442 | 45779892 | 0.018 | 0.099 | |||
| chrA3.74920495 | 57890498 | 0.054 | 0.159 | |||
| chrA3.43254032 | 98000766 | 0.32 | ||||
| chrA3.41399492 | 114615800 | 0.47 | ||||
| chrA3.41996044 | 147847406 | 0.27 |
4. Discussion
The goal of this study was to identify cofactors that were most strongly involved with natural resistance to FIPV infection. This was accomplished by negating as many potential cofactors as possible using a standardized virus challenge, cats from the breeding facility, optimal husbandry, providing a uniform environment and diet, minimizing extraneous stresses, and eliminating the effects of other common infections that might occur in multi-cat environments such as catteries or shelters. After minimizing the agent, environmental, and host cofactors, the opportunity existed to study host-related factors such as genetics, age, and gender on FIP resistance.
The present study also dealt with the strength of immunity, which does not appear to be absolute. About 10% of cats that survived one FIPV infection succumbed to a second or third exposure. A similar occurrence was observed by Wasmoen et al. (1995); one of five cats that had successfully resisted a challenge exposure that killed 4 of 5 non-vaccinates developed FIP upon a second exposure. This type of immunity is different from that established by feline panleukopenia, a parvovirus disease. Panleukopenia immunity is usually solid and is more dependent on humoral than cellular responses (Scott, 1987). Therefore, FIPV immunity more closely resembles immunity to its parent virus, feline enteric coronavirus (FECV). FECV-infected cats shed virus in their feces for weeks or months before sufficient immunity develops to stop shedding, but after shedding ceases, antibody levels fall and many of the cats become susceptible to reinfection (Pedersen et al., 2008). Subclinical disease is also known to linger after initial natural and experimental infection in some cats as demonstrated by FeLV activation (Pedersen, 1987b, Pedersen et al., 1977).
This was the first study documenting the significance of age at time of exposure on FIPV outcome, even though it has been frequently cited as a disease cofactor (Pedersen, 2009). Immunity to experimental FIPV infection increased progressively from 4 months of age through adulthood. Gerber et al. (1990) also reported an age-related response to an attenuated live FIPV vaccine, with significant protection only observed when vaccination was started at 16 weeks of age. The effect of age on disease outcome is well known for infectious disease agents such as feline leukemia virus (Hoover et al., 1976). Age resistance to FeLV increases dramatically during kittenhood as the immune system matures and has confounded FeLV vaccine duration of immunity studies (Wilson et al., 2012).
Gender, in particular intact males, has been reported as a risk factor for FIP in other studies (Norris et al., 2005, Pesteanu-Somogyi et al., 2006, Rohrbach et al., 2001). We did not see a gender bias in the present study, nor was it seen in an earlier study of purebred and random-bred cats (Foley et al., 1997).
A large component of the present study involved attempts to associate FIP resistance to specific genetic markers by GWAS. Previous experience with a large cohort of inbred Birman cats (Golovko et al., 2013) suggested that this approach could be applied to the present cohort of randomly bred cats. However, the same population substructure problems encountered in the Birman study were faced in this study. GWAS comparing all cases and controls demonstrated four significant genome-wide associations on several chromosomes and some possible candidate genes. However, there was considerable population substructure as revealed by MDS and attributed to separate male founder effects. Population substructure due to relatedness in a case-control study can be overcome by using different types of analysis, such as the transmission disequilibrium test (TDT) or the sib-TDT that was employed in this study. A previous GWAS study localized the autosomal recessive locus associated with hypokalemia in cats by analyzing as few as 35 cases and 25 controls (Gandolfi et al., 2012). However, the present study was conducted on random-bred cats, which are known to have less linkage disequilibrium than within pedigreed cats (Alhaddad et al., 2013). The study was further confounded by the polygenic appearance of the inheritance. Inheritance to FIP resistance/susceptibility in a similarly sized cohort of Birman cats also appeared to be polygenic and there were no common regions of association, which would have reinforced both studies (Golovko et al., 2013).
To compensate for family-related substructure, a transmission disequilibrium test among sib-pairs using the statistics of Spielman and Ewens (1998) was then performed. Eighteen discordant sib-pair nuclear families were identified within the cohort, which was more than the 13 phenotypically discordant sib-pairs that successfully detected the association with a cone-rod dystrophy in dogs (Wiik et al., 2008). Based on sib-TDT on the FIP cohort, five strong SNP associations on different chromosomes were identified, but none reached genome-wide significance. SNPs on chromosomes A3, B1, and C1 were shared by the two different analyses while two new associations on chromosomes C2 and D3 appeared. Although the associations detected by sib-TDT did not reach genome-wide significance after permutations, similar regions were suggested by both analyses within the three overlapping chromosomes. It is possible that these regions could reach genome-wide significance if more discordant sib-pairs are added to the association analysis.
An attempt was also made to compensate for age at the time of exposure as an independent and presumably non-genetic risk factor for FIP resistance. Unfortunately, the number of case and control cats challenge exposed after 1 year of age was too low, so cats exposed at >6 months and >1year were combined. The control population remained the same for all GWAS configurations based on the premise that kittens surviving exposure at less than 6 months of age would still resist exposure as they aged. As was expected based on previous GWAS configurations, relatively small changes in the case populations had a marked effect on observed associations. After comparing the results of GWAS based on age, GWAS of the total population, and GWAS based on family structure, only one peak of strong, but not genome-wide significant, associations were present on chromosomes A3 in a region between 34.8 and 46 Mb. Thirty five annotated genes were present within this region, but none appeared to be strong candidates for FIP resistance.
It can be concluded from these various GWAS studies that resistance to FIP in this population of relatively random-bred SPF cats was not influenced by a single or even small number of genes. As in an earlier study with a much more inbred Birman population (Golovko et al., 2013), any genetic component of resistance is likely to be polygenic and divergent between various populations. Although mutations in a single gene have been identified that confer resistance to infectious disease, such as the CCR5 mutation for HIV infection (Dean et al., 1996), susceptibility and resistances to infectious agents clearly involve complex host/virus/environment interactions that make genetic studies difficult. This has been shown in diseases such as human and ruminant tuberculosis (Chimusa et al., 2014, le Roex et al., 2013), a disease that closely resembles the dry form of FIP. The existence of additional risk factors, involving the environment, host, and agent, is perhaps one of the most daunting problems in the search for genetic influences on infectious diseases. This study removed a large number of those confounding factors but was still unable to identify specific genes that might be involved in FIP resistance. Unfortunately, even highly inbred breeds, such as Birman, with significant linkage disequilibrium and closed colonies, such as the one in this study, suffer from high genomic inflations. Even so, the strong associations demonstrated in this relatively small GWAS employing a relatively low-density array indicate that FIP resistance is influenced in some part to genetic factors. The heritability of these genetic factors remains a subject of ongoing breeding studies.
We did not interrogate one region on chromosome A3 that was consistently found to differ in association between all of the various GWAS configurations. Hopefully, the present data can be reanalyzed as the cat genome annotation improves and more dense arrays become available. Next-generation and whole exome sequencing are also becoming cost accessible and might be preferable ways to search for complex genetic associations and specific mutations. It might also be fruitful to mate immune cats to see if resistance is heritable and if so, to do GWAS or next-generation sequencing on their offspring.
5. Conclusion
The objective of this study was to define natural immunity to FIP among randomly bred specific pathogen-free cats bred for laboratory purposes under conditions that would eliminate as many extrinsic disease cofactors as possible. Cats were housed free of other feline pathogens and fed and cared for in a uniform manner. This emphasized the relative influence of age at the time of exposure, strength of immunity as gauged by repeated challenge exposure, and possible genetic resistance. One-third of random-bred laboratory cats used in various studies over the last decade appeared to be resistant to infection with Type I field strains of FIPV. However, immunity was not absolute and a small number of cats died after a second and even third challenge. Age at the time of exposure seemed to be the most significant predictor of resistance; cats under 6 months of age were most apt to develop FIP, cats 6–12 months were intermediate, and cats over 12 months of age demonstrated significant resistance. Strong genetic associations were identified by GWAS in regions of several chromosomes, especially when comparing all cats that died of FIP with all survivors. However, all but one of these regional associations changed when GWAS was adjusted for family substructure or age at the time of FIPV exposure. This confirmed previous GWAS studies on FIP resistance in Birman cats (Golovko et al., 2013); both studies showed inheritance of FIP resistance to be highly complex and confounded by considerable population stratification. Future breeding studies will hopefully confirm the heritability of FIP resistance.
6. Conflict of interest statement
The authors declare no conflicts of interest.
Acknowledgements
Funds for this study were provided over a period of many years by organizations such as the Center for Companion Animal Health, School of Veterinary Medicine, UC Davis, Winn Feline Health, and the Cat Health Network grant D12FE-516 (a consortium of the Morris Animal Foundation, the Winn Feline Foundation, the American Association of Feline Practitioners and the American Veterinary Medical Foundation). We are also grateful for the many private donations that have been made by individuals and private groups such as Save Our Cats and Kittens FIP (SOCK FIP).
References
- Alhaddad H., Khan R., Grahn R.A., Gandolfi B., Mullikin J.C., Cole S.A., Gruffydd-Jones T.J., Häggström J., Lohi H., Longeri M., Lyons L.A. Extent of linkage disequilibrium in the domestic cat, Felis silvestris catus, and its breeds. PLoS One. 2013;8(1):e53537. doi: 10.1371/journal.pone.0053537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C.W., Scott F.W. Attempted immunization of cats with feline infectious peritonitis virus propagated at reduced temperatures. Am. J. Vet. Res. 1997;58:251–256. [PubMed] [Google Scholar]
- Bell E.T., Malik R., Norris J.M. The relationship between the feline coronavirus antibody titre and the age, breed, gender and health status of Australian cats. Aust. Vet. J. 2006;84:2–7. doi: 10.1111/j.1751-0813.2006.tb13114.x. [DOI] [PubMed] [Google Scholar]
- Chimusa E.R., Zaitlen N., Daya M., Möller M., van Helden P.D., Mulder N.J., Price A.L., Hoal E.G. Genome-wide association study of ancestry-specific TB risk in the South African coloured population. Hum. Mol. Genet. 2014;23:796–809. doi: 10.1093/hmg/ddt462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter S.M., Gilmore C.E., Rollins C. Multiple cases of feline leukemia and feline infectious peritonitis in a household. J. Am. Vet. Med. Assoc. 1973;162:1054–1058. [PubMed] [Google Scholar]
- Dean M., Carrington M., Winkler C., Huttley G.A., Smith M.W., Allikmets R., Goedert J.J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKRS structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- Foley J.E., Pedersen N.C. The inheritance of susceptibility to feline infectious peritonitis in purebred catteries. Feline Pract. 1996;24:14–22. [Google Scholar]
- Foley J.E., Poland A., Carlson J., Pedersen N.C. Risk factors for feline infectious peritonitis among cats in multiple-cat environments with endemic feline enteric coronavirus J. Am. Vet. Med. Assoc. 1997;210:1313–1318. [PubMed] [Google Scholar]
- Gandolfi B., Gruffydd-Jones T.J., Malik R., Cortes A., Jones B.R., Helps C.R., Prinzenberg E.M., Erhardt G., Lyons L.A. First WNK4-hypokalemia animal model identified by genome-wide association in Burmese cats. PLoS One. 2012;7(12):e53173. doi: 10.1371/journal.pone.0053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J.D., Ingersoll J.D., Gast A.M., Christianson K.K., Selzer N.L., Landon R., Pfeiffer M., Sharpee N.E., Beckenhauer R.L.W.H. Protection against feline infectious peritonitis by intranasal inoculation of a temperature-sensitive FIPV vaccine. Vaccine. 1990;8:536–542. doi: 10.1016/0264-410X(90)90004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glansbeek H.L., Haagmans B.L., te Lintelo E.G. Adverse effects of feline IL-12 during DNA vaccination against feline infectious peritonitis virus. J. Gen. Virol. 2002;83:1–10. doi: 10.1099/0022-1317-83-1-1. [DOI] [PubMed] [Google Scholar]
- Golovko L., Lyons L.A., Liu H., Sørensen A., Wehnert S., Pedersen N.C. Genetic susceptibility to feline infectious peritonitis in Birman cats. Virus Res. 2013;175:58–63. doi: 10.1016/j.virusres.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Yamato H., Ohkawa T., Kaneko M., Motokawa K., Kusuhara H., Kaneshima T., Arai S., Koyama H. Vaccine efficacy of a cell lysate with recombinant baculovirus-expressed feline infectious peritonitis (FIP) virus nucleocapsid protein against progression of FIP. J. Vet. Microbiol. 2003;97:31–44. doi: 10.1016/j.vetmic.2003.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzworth J.E. Some important disorders of cats. Cornell Vet. 1963;53:157–160. [PubMed] [Google Scholar]
- Hoover E.A., Olsen R.G., Hardy W.D., Jr., Schaller J.P., Mathes L.E. Feline leukemia virus infection: age-related variation in response to cats to experimental infection. J. Natl. Cancer Inst. 1976;57:365–369. doi: 10.1093/jnci/57.2.365. [DOI] [PubMed] [Google Scholar]
- Hoskins J.D., Taylor H.W., Lomax T.L. Challenge trial of an intranasal feline infectious peritonitis vaccine. Feline Pract. 1994;22:9–13. [Google Scholar]
- Kass P.H., Dent T.H. The epidemiology of feline infectious peritonitis in catteries. Feline Pract. 1995;23:27–32. [Google Scholar]
- Kiss I., Poland A.M., Pedersen N.C. Disease outcome and cytokine responses in cats immunized with an avirulent feline infectious peritonitis virus (FIPV)-UCD1 and challenge-exposed with virulent FIPV-UCD8. J. Feline Med. Surg. 2004;6:89–97. doi: 10.1016/j.jfms.2003.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roex N., van Helden P.D., Koets A.P., Hoal E.G. Bovine TB in livestock and wildlife: what's in the genes? Physiol. Genomics. 2013;45:631–637. doi: 10.1152/physiolgenomics.00061.2013. [DOI] [PubMed] [Google Scholar]
- Norris J.M., Bosward K.L., White J.D., Baral R.M., Catt M.J., Malik R. Clinicopathological findings associated with feline infectious peritonitis in Sydney, Australia: 42 cases. Aust. Vet. J. 2005;83:666–673. doi: 10.1111/j.1751-0813.2005.tb13044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. An update on feline infectious peritonitis: virology and immunopathogenesis. Vet. J. 2014;201:123–132. doi: 10.1016/j.tvjl.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009;11:225–258. doi: 10.1016/j.jfms.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. Diseases and Management in the Multiple-cat Environment. American Veterinary Publications, Inc.; Goleta, CA: 1991. Feline husbandry; pp. 163–176. [Google Scholar]
- Pedersen N.C. Basic and clinical immunology. In: Holzworth J., editor. Diseases of the Cat. W.B. Saunders Co.; Philadelphia, USA: 1987. (Chapter 6) [Google Scholar]
- Pedersen N.C. Virologic and immunologic aspects of feline infectious peritonitis virus infection. Adv. Exp. Med. Biol. 1987;218:529–550. doi: 10.1007/978-1-4684-1280-2_69. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C. Serologic studies of naturally occurring feline infectious peritonitis. Am. J. Vet. Res. 1976;37:1449–1453. [PubMed] [Google Scholar]
- Pedersen N.C., Black J.W. Attempted immunization of cats against feline infectious peritonitis using either avirulent live virus or sublethal amounts of virulent virus. Am. J. Vet. Res. 1983;44:229–234. [PubMed] [Google Scholar]
- Pedersen N.C., Floyd K. Experimental studies with three new strains of feline infectious peritonitis virus: FIPV-UCD2, FIPV-UCD3, and FIPV-UCD4. Compendium. 1986;7:1001–1011. [Google Scholar]
- Pedersen N.C., Liu H., Scarlett J., Leutenegger C.M., Golovko L., Kennedy H., Mustaffa Kamal F. Feline infectious peritonitis: role of the feline coronavirus 3c gene in intestinal tropism and pathogenicity based upon isolates from resident and adopted shelter cats. Virus Res. 2012;165:17–28. doi: 10.1016/j.virusres.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Liu H., Dodd K.A., Pesavento P.A. Significance of coronavirus mutants in feces and diseased tissues of cats suffering from feline infectious peritonitis. Viruses. 2009;1:166–184. doi: 10.3390/v1020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Allen C.E., Lyons L.A. Pathogenesis of feline enteric coronavirus infection. J. Feline Med. Surg. 2008;10:529–541. doi: 10.1016/j.jfms.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Sato R., Foley J.E., Poland A.M. Common virus infections in cats, before and after being placed in shelters, with emphasis on feline enteric coronavirus. J. Feline Med. Surg. 2004;6:83–88. doi: 10.1016/j.jfms.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Black J.W., Boyle J.F., Evermann J.F., McKeirnan A.J., Ott R.L. Pathogenic differences between various feline coronavirus isolates. Adv. Exp. Med. Biol. 1984;173:365–380. doi: 10.1007/978-1-4615-9373-7_36. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Theilen G., Keane M.A., Fairbanks L., Mason T., Orser B., Che C.H., Allison C. Studies of naturally transmitted feline leukemia virus infection. Am. J. Vet. Res. 1977;38:1523–1531. [PubMed] [Google Scholar]
- Pesteanu-Somogyi L.D., Radzai C., Pressler B.M. Prevalence of feline infectious peritonitis in specific cat breeds. J. Feline Med. Surg. 2006;8:1–5. doi: 10.1016/j.jfms.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A.M., Vennema H., Foley J.E., Pedersen N.C. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J. Clin. Microbiol. 1996;34:3180–3184. doi: 10.1128/jcm.34.12.3180-3184.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., Sham P.C. PLINK: a tool set for whole- genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison R.L., Holzworth J., Gilmore C.E. Naturally occurring feline infectious peritonitis: signs and clinical diagnosis. J. Am. Vet. Med. Assoc. 1971;158(Suppl 2):981–986. [PubMed] [Google Scholar]
- Rohrbach B.W., Legendre A.M., Baldwin C.A., Lein D.H., Reed W.M., Wilson R.B. Epidemiology of feline infectious peritonitis among cats examined at veterinary medical teaching hospitals. J. Am. Vet. Med. Assoc. 2001;218:1111–1115. doi: 10.2460/javma.2001.218.1111. [DOI] [PubMed] [Google Scholar]
- Scott F.W. Viral diseases (panleukopenia) In: Holzworth J., editor. Diseases of the Cat. W.B. Saunders Co.; Philadelphia, USA: 1987. pp. 182–193. (Chapter 7) [Google Scholar]
- Spielman R.S., Ewens W.J. A sibship test for linkage in the presence of association: the sib transmission/disequilibrium test. Am. J. Hum. Genet. 1998;62:450–458. doi: 10.1086/301714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmoen T.L., Kadakia N.P., Unfer R.C., Fickbohm B.L., Cook C.P., Chu H-J., Acree W.M. Protection of cats from infectious peritonitis by vaccination with a recombinant raccoon poxvirus expressing the nucleocapsid gene of feline infectious peritonitis virus. Adv. Exp. Med. Biol. 1995;380:221–228. doi: 10.1007/978-1-4615-1899-0_36. [DOI] [PubMed] [Google Scholar]
- Wiik A.C., Wade C., Biagi T., Ropstad E.O., Bjerkås E., Lindblad-Toh K., Lingaas F. A deletion in nephronophthisis 4 (NPHP4) is associated with recessive cone-rod dystrophy in standard wire-haired dachshund. Genome Res. 2008;18:1415–1421. doi: 10.1101/gr.074302.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S., Greenslade J., Saunders G., Holcroft C., Bruce L., Scobey A., Childers T., Sture G., Thompson J. Difficulties in demonstrating long term immunity in FeLV vaccinated cats due to increasing age-related resistance to infection. BMC Vet. Res. 2012;8:125. doi: 10.1186/1746-6148-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthing K.A., Wigney D.I., Dhand N.K., Fawcett A., McDonagh P., Malik R., Norris J.M. Risk factors for feline infectious peritonitis in Australian cats. J. Feline Med. Surg. 2012;14:405–412. doi: 10.1177/1098612X12441875. [DOI] [PMC free article] [PubMed] [Google Scholar]


