Abstract
A novel SYBR Green based real-time RT-PCR assay for detection of genogroup III bovine noroviruses (BoNoV) was developed and the assay applied to 419 faecal samples from calves with and without diarrhoea. The samples were obtained from 190 Norwegian dairy and beef herds. BoNoV was detected in 49.6% of the samples from 61.1% of the herds indicating that BoNoV is ubiquitous in Norway. The overall prevalence was not significantly different in diarrhoea and non-diarrhoea samples.
Analyses of polymerase gene sequences revealed both genotype III/1 and III/2 with genotype III/2 (Newbury2-like) being the most prevalent. Detected capsid sequences were restricted to Newbury2-like and the chimeric Bo/Thirsk10/00/UK strain.
The RNA polymerase genotypes of the circulating BoNoVs in Norway were predicted by melting temperature analysis.
Additional data from a challenge experiment suggest that a high proportion of young calves are shedding low levels of BoNoV for a prolonged time after recovering from the associated diarrhoea. The findings may explain some of the discrepancies in detection rates from previous studies and explain why some studies have failed to detect significant prevalence differences between calves with and without diarrhoea. It may also shed new light on some epidemiological aspects of norovirus infections.
Keywords: SYBR Green real-time RT-PCR, Genotype prediction, Norovirus excretion, Challenge experiment
1. Introduction
Bovine noroviruses are members of the Caliciviridae family, currently constituting genogroup III of the norovirus genus. They are non-enveloped, single-stranded, positive-sense RNA viruses with genomes consisting of 3 ORFs that are usually partly overlapping and encode non-structural proteins (ORF1), a capsid protein (ORF2) and an ORF3 protein (Liu et al., 1999, Oliver et al., 2007a, Scipioni et al., 2008a). Bovine noroviruses are classified into two main genotypes within genogroup III, namely as genotype III/1 (Jena-like) or genotype III/2 (Newbury2-like). Other members of the Caliciviridae family that have been detected in bovine faecal samples are the currently unclassified BECs (bovine enteric caliciviruses) Newbury agent 1- and Nebraska (NB)-like viruses (Oliver et al., 2006a) as well as one reported detection of genogroup II/4 human norovirus (Mattison et al., 2007).
In humans, noroviruses are considered to be one of the leading causes of acute gastroenteritis both in adults and children (Guo et al., 2008, Ajjampur et al., 2008, Jansen et al., 2008), and seem to have a rather complex global epidemiology (Gallimore et al., 2007, Svraka et al., 2007, Vainio and Myrmel, 2006).
Bovine noroviruses currently have an uncertain clinical significance although several studies indicate that they are involved in the calf diarrhoea-complex (Han et al., 2006, Wise et al., 2004).
Relatively few molecular and seroepidemiological studies have been performed on BoNoVs. Previous molecular studies have often relied on samples collected from a limited number of herds or pooled samples (Smiley et al., 2003, van der Poel et al., 2003, Wise et al., 2004, Wolf et al., 2007) which may not reflect accurately the prevalence or the genetic diversity of circulating norovirus strains within a geographic region (Khamrin et al., 2007, Maunula and Von Bonsdorff, 2005). The reported prevalence of BoNoV in samples tested by RT-PCR varies greatly between studies, ranging from 1.6% to 72.0% (Mattison et al., 2007, Park et al., 2007, Smiley et al., 2003).
A recent serological study indicates that both Newbury2-like (GIII/2) and Jena-like (GIII/1) strains are common in the United Kingdom and Germany (Oliver et al., 2007b). However, cross reactions between shared epitopes of different genogroups and genotypes of noroviruses, which may complicate the assessment of serological surveys, have been described (Batten et al., 2006, Oliver et al., 2006b, Shiota et al., 2007). Chimeric strains may complicate further interpretation of serological data. PCR based detection methods are thus the preferred tools for epidemiological studies and are considered the methods of choice for surveillance of genetic diversity among human as well as animal noroviruses (Goodgame, 2007, Vainio and Myrmel, 2006, Mattison et al., 2007).
The aims of the present study were to develop a sensitive and specific SYBR Green based real-time RT-PCR assay for detection of genogroup III BoNoV and to investigate the prevalence of BoNoV in samples from calves with and without diarrhoea.
Secondly the study aimed at describing the genetic diversity of BoNoV in the Norwegian cattle population and to examine the virus excretion pattern of BoNoV by performing a simple challenge experiment involving two Norwegian red bull calves. It was more work than anticipated to coordinate the experiment.
2. Materials and methods
2.1. Sampling, sample preparation and RNA extraction
Faecal samples from 190 herds (126 dairy and 64 beef herds) from 15 counties representing most parts of Norway were collected throughout all seasons between June 2004 and December 2006, as part of the research project “Calf health in Norway”. The participating herds were selected to reflect the cattle population density as described elsewhere (Gulliksen et al., 2009). Faecal samples were transported to the laboratory in Styrofoam boxes, with an ice pack, by overnight carrier and stored at −70 °C.
Diarrhoea (272) samples from young stock (0–356 days of age) as well as normal samples (147) from individual calves (<90 days old) were assayed for BoNoV with a novel SYBR Green based real-time RT-PCR assay.
Prior to RNA extraction approximately 0.2 g of feces was dissolved in 1.8 ml 0.9% saline water using a whirl mixer. The solution was clarified by centrifugation for 20 min at 4000 × g and 200 μl of the supernatant was used for RNA extraction by the NucliSens easyMAG automated extraction system (bioMérieux, Craponne, France). The elution volume was 55 μl. Eluates were stored at −70 °C until analysis.
2.2. Primer design
A consensus mixed base sequence was generated from the alignment of 23 geographically diverse BoNoV sequences available in GenBank and used as the input sequence for primer design with the FastPCR software version 3.8.30 (Institute of Biotechnology, University of Helsinki, Finland). The proposed primer pairs were evaluated to avoid wobbles near the 3′ ends and by BLASTn searches against available sequences in the GenBank nr database (per 14 February 2006). The compatibility with GIII/1 and GIII/2 GenBank viral sequences was assessed and the specificity was obtained by excluding candidate primers that had a high degree of homology to other organisms near the 3′ ends. The expected size of the obtained amplicons were 72 bp. Sequences of the selected primers were BoNoV72F (5′-CGCTCCATGTTTGCTTGGATG-3′) and BoNoV72R2 (5′-ATCGGGAAGGRYGTCGCGACTACC-3′). Primers were synthesized by MWG Biotech, Ebersberg, Germany.
2.3. Novel real-time RT-PCR protocol with SYBR Green based chemistry
cDNA synthesis was performed in a 20 μl RT-reaction containing 4 μl RT buffer (Invitrogen, Carlsbad, USA), 1 μl 10 mM dNTP (Invitrogen, Foster City, USA), 1 μl 0.1 M DTT (Invitrogen, Foster City, USA), 1 μl 5 μM Random hexamer primer (Applied Biosystems, Foster City, USA), 1 μl RNaseOut (40 U/μl) (Invitrogen, Foster City, USA), 1 μl RT Superscript III (200 U/μl) (Invitrogen, Foster City, USA) and 11 μl template (RNA eluate). The reaction was run on a PTC-100 thermocycler (MJ Research, Waltham, MA, USA) with an initial RT-step of 25 °C for 5 min, followed by 50 °C for 40 min, an RT-inactivation step of 70 °C for 15 min and finally cooling to 4 °C. The cDNA was either used directly or stored at −20 °C prior to PCR.
PCR reactions were set up with 2.5 μl of cDNA in a 25 μl reaction mix using the QuantiTect SYBR Green PCR Kit (Qiagen, Hilden, Germany) and 200 nM each of primer BoNoV72F and BoNoV72R2.
A touch-down PCR was performed, with the following cycling conditions: 15 min at 95 °C, 2× (94 °C for 1 min, 60 °C for 45 s and 72 °C for 1 min), 2× (94 °C for 1 min, 58 °C for 45 s and 72 °C for 1 min), and 40× (94 °C for 40 s, 55 °C for 30 s and 72 °C for 1 min). Fluorescence was measured at 55 °C. Finally a melting curve analysis was made by continuously measuring the fluorescence between 55 °C and 95 °C. Cycling was performed on a Stratagene MX3005P real-time Q-PCR system and the Ct-values (threshold cycle) were reported from cycle 5.
2.4. Evaluation of assay specificity and sensitivity
Due to the complex nature of feces as a sample matrix, specificity was evaluated by the proportion of non-specific products generated. Amplicons with different melting temperatures (T ms) were cloned (TOPO TA Cloning kit, Invitrogen, Foster City, USA) and sequenced using M13 forward and reverse primers with BigDye v. 3.1 on an ABI 3100 Avant sequencer. The specificity of the assay for norovirus was assessed further by running it on samples positive for other bovine enteric viruses, including rotavirus, coronavirus and torovirus.
In order to evaluate assay sensitivity, one clone with the T3 primer site upstream of a sense insert was selected for synthesis of single-stranded RNA control template. TOP10 chemically competent cells were transformed and the pCR4 vector (TOPO TA Cloning kit, Invitrogen, Foster City, USA) purified by a spin column procedure (Qiagen Plasmid Mini Kit, Qiagen, Hilden, Germany) from overnight Luria Bertani broth containing 50 mg/l ampicillin. The purified vector was linearized by BsrG1 digestion (New England Biolabs, Ipswich, USA) and in vitro transcription was performed according to the manufacturer's instructions utilizing the T3 mMessage mMachine kit (Ambion, Austin, USA).
The product was DNase-treated twice with Turbo DNAFree (Ambion, Austin, USA) and finally cleaned up with a spin column procedure (RNA Mini kit, Qiagen, Hilden, Germany). The RNA was eluted in nuclease-free water, the concentration determined by UV absorbance at 260 nm and a log-10 dilution series (107–100 copies/11 μl) was used for assay sensitivity testing, in triplicates. To detect any remaining linearized DNA template, the purified RNA dilutions were tested by real-time PCR using the same conditions as for the assay omitting the reverse transcription step. In order to verify that inhibitors were removed efficiently during RNA extraction, a subset of 12 faecal extracts that were found negative in the present BoNoV assay, were spiked with RNA from a fish virus (Salmon Pancreas Disease Virus – SPDV). A SPDV specific SYBR Green real-time RT-PCR was performed on these samples and on SPDV RNA in water (positive control), using similar conditions as the BoNoV assay, and Ct-values were compared.
2.5. Phylogenetic analysis and genotype prediction
As BoNoV specific PCR products with different T ms were obtained, sequencing of a wider region, spanning the primer-binding areas was performed (Fig. 1 ). Fourteen BoNoV positive samples were selected to reflect the range of amplicons with different T ms from the real-time RT-PCR assay. A 501 bp region of the RdRp gene was amplified with the previously published CBECU-F primer (Smiley et al., 2003) in combination with the reverse primer BoNoV72R2. Additionally, a 516 bp region was amplified with the BoNV72F and Capsid516R (5′-ATYADYACATGRGGRAACTG-3′) primers. cDNA prepared for the real-time RT-PCR assay was used as template and the PCR was performed using the Qiagen HotStar Taq polymerase (Qiagen, Hilden, Germany) as described below.
Fig. 1.
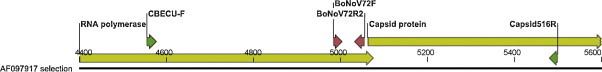
Schematic representation of the partial Bo/Newbury2/76/UK genome with the forward (BoNoV72F) and reverse (BoNoV72R2) primer-binding sites (red) for the SYBR Green based real-time RT-PCR assay. Additional primers (CBECU-F and Capsid516R) used for sequencing are depicted in green. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
Amplification conditions were 95 °C for 15 min, 40× (94 °C for 1 min, 50 °C for 1 min, 72 °C for 30 s) and a 10 min final extension step. Amplicons were visualized on ethidium bromide stained agarose gels and products of the expected size were excised and purified by a spin column procedure (Qiagen Gel-Extraction kit, Qiagen, Germany). The amplicons were sequenced directly or after TOPO TA cloning and contigs spanning the junction of ORF1 and ORF2 were assembled in CLC Combined Workbench v. 3.0.1.
Phylogenetic analysis was performed by aligning nucleotide or amino acid sequences with ClustalW followed by transferring the alignment to SplitsTree v. 4.8 (Huson and Bryant, 2006, Huson, 1998) for construction of the phylogenetic tree.
Separate phylogenetic analyses were performed for the partial RdRp sequences as well as for the partial capsid sequences (data not shown). A split decomposition analysis was performed to detect possible conflicting phylogenetic signals.
To assess differences in melting temperatures from individual samples a ClustalW alignment of the inter-primer regions was made with CLC Combined Workbench v. 3.0.1. (CLC bio A/S, Denmark).
2.6. BoNoV challenge experiments – preparation of animals and inoculate
A BoNoV GIII/2 positive sample (GenBank accession no. FM242192) was used for the challenge experiments. Briefly, a 10% faecal suspension was prepared in 0.9% saline solution and ultra-filtered through a 0.2 μm syringe filter (Whatman, Dassel, Germany). The sample was analyzed by the BoNoV real-time RT-PCR to estimate the viral load and also assayed by real-time RT-PCR to exclude the presence of bovine rota-, toro-, corona- and BECs as described elsewhere (manuscript in preparation).
Two Norwegian Red bull calves were separated from their dams immediately after birth and fed two meals (2 × 2 l) of colostrum before being fed a commercial milk replacement formula (Kalvegodt, Felleskjøpet, Oslo, Norway). The calves were housed together (in separate pens) for 7 days before challenge.
Faecal samples collected from the two calves the day before challenge were assayed for bovine rotavirus group A, torovirus, coronavirus, BECs and BoNoV by real-time RT-PCR assays.
A suspension consisting of 500 ml luke warm water and approximately 106 copies of BoNoV was given by bottle on day 0. Faecal samples were collected rectally each morning in conjunction with clinical examination of the calves (rectal temperature, pulse, respiratory rate, signs of diarrhoea, general body condition). Faecal samples were analyzed by real-time RT-PCR to determine BoNoV faecal shedding. The calves were re-challenged with 106 copies on 23 days post-infection (dpi) and analysis of BoNoV shedding was continued until 32 dpi.
Faecal samples collected 3 dpi were assayed for bovine rotavirus group A, torovirus, coronavirus and BECs by real-time RT-PCR. Samples were also assayed for Cryptosporidium spp. and Escherichia coli F5 by antigen-ELISA (BIO K 071 from Bio-X Diagnostics Sprl, Jemelle, Belgium) according to the manufacturer's instructions, and for coccidian oocysts (bright field microscopy).
The challenge experiment was approved by The National Animal Research Authority (NARA), Norway before execution (1399/2008).
3. Results
3.1. Specificity and sensitivity of the real-time RT-PCR assay
No amplification with the present assay was observed on samples positive for bovine rotavirus, coronavirus or torovirus.
Of 214 obtained amplicons, 208 had T ms between 77.6 and 80.9 °C and were considered positive for BoNoV after sequencing of 34 samples with representative T ms. Amplicons from six samples had T ms > 81.9 °C, and were discarded as false positives after subsequent cloning and sequencing. Their Ct-values were above 35.8. Differences in GC-content and T ms are depicted (Fig. 2 ).
Fig. 2.
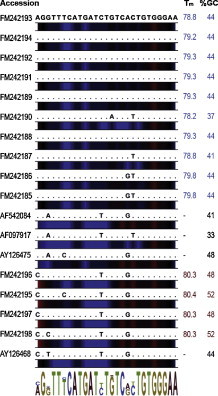
Multiple alignment of the inter-primer regions of FM242185–FM242198FM242185FM242186FM242187FM242188FM242189FM242190FM242191FM242192FM242193FM242194FM242195FM242196FM242197FM242198. The sequence FM242197 is identical to GIII.1 (AJ011099). The GIII.2 sequences (AF097917, AF542084, AY126475) as well as the chimeric sequence (AY126468) are included in the alignment. The Tms and GC-content are shown at the right. Colour bars depict AT-rich regions (blue) and GC-rich regions (red). The sequence logo for the alignment is included. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
RT-PCR on ten-fold dilutions of the in vitro transcribed RNA, gave a detection limit of 10–100 RNA copies per reaction.
Spiking of 12 faecal extracts, that were negative in the present BoNoV assay, with SPDV RNA showed no grade of inhibition for RT-PCR.
3.2. Application of the novel real-time RT-PCR assay on faecal samples from Norwegian calves
Bovine norovirus was detected in 208 (49.6%) of 419 samples originating from 116 (61.1%) of the 190 sampled herds. Ct-values were in the range 6.9–39.7 (Fig. 3 ).
Fig. 3.
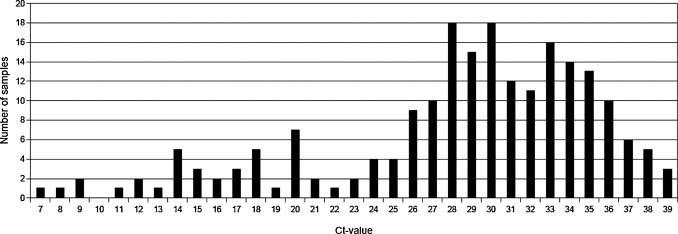
Distribution of Ct-values (rounded to nearest integer value) of the 208 BoNoV positive samples.
Of 272 diarrhoea samples 134 (49.3%) were positive for BoNoV while 75 (50.3%) of 147 samples from calves without diarrhoea were positive.
The mean age of the BoNoV positive calves was 42 days. The mean age of calves with genotype III/1 and genotype III/2 infections was 35 and 44 days respectively.
Calves with diarrhoea had a mean Ct-value of 27.8 (SD 6.9) with median Ct-value of 29.1. Calves with normal faecal consistency had a mean Ct-value of 29.8 (SD 6.7) with median Ct-value of 31.5. Mean Ct-value for the predicted genotype III/1 and III/2 was 29.0 (median 29.7) and 28.4 (median 29.9), respectively.
3.3. Phylogenetic analysis and genotype prediction
Phylogenetic analysis of partial RdRp sequences (295 nt) confirmed the presence of both GIII/1 and GIII/2 RdRp genotypes in the sampled population, and a statistically significant (p < 0.001) association of RdRp genotype with T ms was observed for the samples that were sequenced (data not shown). Classification of the strains into either genotype III/1-like or genotype III/2-like on the basis of T ms gave an overall prevalence of genotype III/1 (Jena-like) positive samples of 21.2%, with T ms ranging from 80.3 to 80.9 °C (mean 80.4 (SD 0.17), n = 44) and of genotype III/2 (Newbury2-like of 78.8%, with T ms ranging from 77.6 to 79.9 °C (mean 79.3 (SD 0.46), n = 164). Phylogenetic analysis of the partial capsid nucleotide sequences (nt) grouped all isolates with a Jena-like RdRp sequence together with the published Bo/Thirsk10/00/UK sequence that has previously been assigned as a chimeric virus (Fig. 4 ).
Fig. 4.
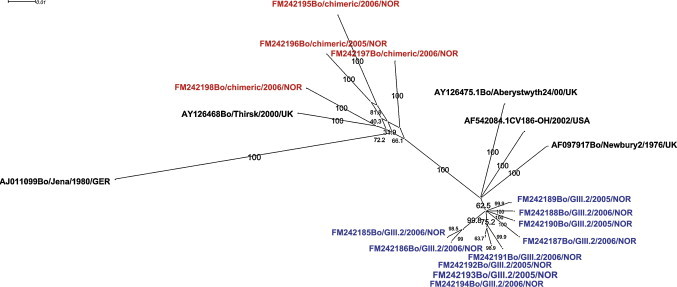
Split decomposition analysis comparing 14 Norwegian BoNoV sequences to sequences available in GenBank. A 777 bp contiguous nucleotide sequence spanning the junction of the RdRP and capsid gene was used for analysis. The linking of chimeric sequences by multiple pathways is suggestive of recombination. The Tm-value ranges of amplicons obtained with primers BoNoV72F and BoNoV72R2 are depicted in red for RdRp genotype III/1-like sequences (Tm range 80.3–80.9 °C) and blue for RdRp genotype III/2-like sequences (Tm range 77.6–79.9 °C). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
GenBank accession nos. are FM242185–FM242198FM242185FM242186FM242187FM242188FM242189FM242190FM242191FM242192FM242193FM242194FM242195FM242196FM242197FM242198 (partial RdRp and capsid sequences).
3.4. BoNoV challenge experiments – animals and inoculate
Faecal consistency and clinical parameters were within normal ranges before challenge. The faecal samples collected the day before challenge and 3 day post-infection (dpi) were negative for bovine rotavirus group A, torovirus, coronavirus, BECS and BoNoV as determined by real-time RT-PCR.
Continuous shedding of BoNoV was detected in feces from 1 dpi and until day 23 dpi when the calves were re-challenged and until 32 dpi when the experiment was terminated. Peak shedding occurred on 4 and 8 dpi for both animals (Fig. 5 ). Clinical diarrhoea occurred on 3 and 4 dpi in both calves. Normal faecal consistency resumed from 5 dpi and no diarrhoea occurred during the rest of the experiment, also upon re-challenge. Reduced appetite was recorded on the 4th and 5th dpi for both animals. The rectal temperature was between 37.5 and 40.0 °C for both calves throughout the experiment. Pulse and respiratory rates were considered to be within normal ranges.
Fig. 5.
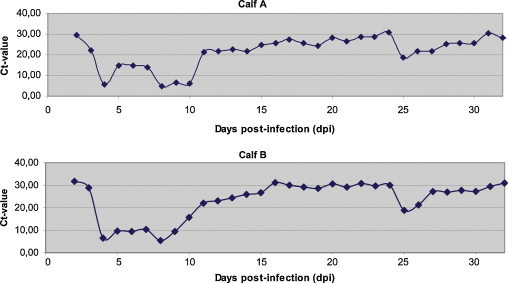
Plot of Ct-values from the controlled challenge experiment (0–32 dpi depicted). Peak BoNoV shedding occurred on 4 and 8 dpi. Re-challenge with a new aliquot of inoculate was performed on 23 dpi with peak excretion occurring on 25 dpi. Clinical diarrhoea was only observed on 3 and 4 dpi after the initial challenge.
Diarrhoea samples collected on 3 dpi were negative for bovine rotavirus group A, torovirus, coronavirus, BECs, Cryptosporidium spp., Escherichia coli F5 and coccidian oocysts.
4. Discussion
In the present study, the occurrence of BoNoV infection in young stock from dairy and beef herds in Norway was assessed by real-time RT-PCR. A characterization of selected BoNoV strains was based on a continuous sequence partially spanning ORF1 and ORF2 (Fig. 1) to reveal the genetic diversity of circulating BoNoVs.
In previous studies a number of different RT-PCR assays have been used for the detection of BoNoVs (Smiley et al., 2003, Wolf et al., 2007, Wise et al., 2004, Mattison et al., 2007, Scipioni et al., 2008a, Scipioni et al., 2008b). Several studies have utilized primers designed from a small number of input sequences in the GenBank that were principally from strains circulating within a limited geographical area (Wise et al., 2004, Smiley et al., 2003), and most studies have been based on traditional one-step RT-PCRs combined with gel based detection of amplicons. Limited data on sequence variation, especially in RNA viruses, can hamper the design of good primers and probes for the detection of these agents (Chevaliez et al., 2007, Swanson et al., 2005), but this limitation may partially be overcome by utilizing parallel assays targeting different genomic parts of one organism (Anthony et al., 2007) or by using all available sequence information from related organisms to design a pan-assay (Escutenaire et al., 2007). A comparison of all available GenBank BoNoV sequences to date and previously published primers revealed one or more mismatches near the 3′-end of one or both primers. This is not unexpected due to the increasing amount of BoNoV sequence data representing strains from different geographic regions that has accumulated recently (Benson et al., 2007).
For primer design, input RdRp sequences for the initial ClustalW alignment were selected to reflect strains from different continents. Relatively few genotype III/1 (Jena-like) capsid sequences have been deposited in GenBank since the original Bo/Jena/80/DE was identified in Germany in 1980, as compared to the number of genotype III/2 (Newbury2-like). The only additionally reported GIII/1 sequences are from the US and one from New Zealand.
Sequences in GenBank may to some extent be biased due to the use of suboptimal primers that preferentially detect only certain strains. In the present study, sequence information was acquired from the polymerase and the capsid gene, including the junction between these two genes, to assess correctly the genotype and to detect any recombinants.
During the past decade an increasing number of reports describing recombinant human noroviruses have been published as well as one describing a proposed chimeric bovine norovirus (Bull et al., 2007, Phan et al., 2007, Oliver et al., 2004). Recently a human GII/4-like sequence was detected in bovine feces in the USA, opening for the possibility that cattle may act as a potential reservoir for recombination events and for zoonotic noroviruses (Mattison et al., 2007). In the present study sequencing of the RdRp grouped the Norwegian viruses in both genotype III/1 and III/2, while sequencing of the capsid grouped the BoNoVs in genotype III/2 only. Interestingly capsid sequences associated with Jena-like RdRp sequences were most similar to the proposed chimeric virus Bo/Thirsk/00/UK sequences. Both genotype III/1 and III/2 RdRp variants were detected in most parts of Norway, but genotype III/1 RdRp sequences were only detected sporadically in the southeastern parts of Norway. The presence of Bo/Thirsk/00/UK-like strains may be a remnant from the systematic import of Scottish Ayrshire cattle in an attempt to increase productivity, that started in 1850 (Felius, 2007, Hirsch, 1911), but some more sequence information on both RdRp and capsid genes of BoNoV circulating in Europe is needed to evaluate the geographical spread of the chimeric virus. The presence of chimeric strains may influence serological assays which can make serological data difficult to interpret.
The alignment of the inter-primer regions of a selection of amplicons with different T ms is presented (Fig. 2). The observed are probably due to the variation in GC-content in the amplified region (Gonzalez and Saiz-Jimenez, 2002).
The assay sensitivity was evaluated by 10-fold dilution of a synthetic capped mRNA transcript to take into account both the RT and the PCR step. The obtained sensitivity of 10 copies per reaction under optimal conditions represents approximately 2500 copies of target per g of feces, although this estimation has important limitations (Love et al., 2006). The obtained Ct-values for the 208 BoNoV positive samples ranged from 6.9 to 39.7 (Fig. 3), which is a substantially broader detection range than reported for a comparable real-time RT-PCR assay where Ct-values ranged from 21.5 to 33.3 (Wolf et al., 2007). This difference may be due to a larger number of samples used in the present study. Additionally any influence of specific herd immunity to the strain(s) present may have influenced the amount of virus shed in feces (Han et al., 2006). The present study included 419 samples from 190 herds, while 28 samples from two herds were used in the previously reported study (Wolf et al., 2007). The difference may also reflect assay variables like primer (and probe) compatibility or cycling kinetics or the presence of inhibitors and amplicons size, especially in low copy number samples. Even if the use of an internal control in a SYBR Green RT-PCR norovirus assay has recently been published for detecting inhibition in faecal samples (Scipioni et al., 2008b), we did not include an internal control in our assay, in order not to impair amplification of target, or complicate the assessment of amplification plots, The NucliSens easyMAG nucleic extraction system has been shown to perform more efficiently than manual extraction in removal of PCR inhibitors (Loens et al., 2007) and inhibitors were not detected in our spiking experiment on a selection of BoNoV negative samples.
In the present study the calves ranged from 0 to 365 days of age with 91.8% being younger than 90 days. The study is therefore comparable to a Korean study where the calves were between 2 and 90 days of age and the samples were collected in all seasons (Park et al., 2007). In that study 2.8% and 9.3% of the 645 samples from diarrhoea calves were positive by conventional one-step RT-PCR or nested-PCR. This is in contrast to the present study, in which the overall prevalence in calves aged 2–90 days was 50.2%. This may reflect different prevalences of BoNoV in the two study populations, differences between dairy and beef herds, altered susceptibility in different breeds, differences in assay specificity and perhaps most importantly differences in assay sensitivity (Kukielka et al., 2007). Interestingly a novel real-time RT-PCR assay for BECs was published recently which has a sensitivity comparable to the sensitivity presented here (Park et al., 2009).
The right skewed distribution of Ct-values is interesting (Fig. 3). A situation with most animals shedding low amounts of virus may be comparable to norovirus infections in humans with prolonged shedding in infants and young children post-clinical illness (Murata et al., 2007, Kirkwood and Streitberg, 2008). Previous studies in calves have revealed an age related shedding of pathogens like Cryptosporidium spp., Giardia intestinalis and Campylobacter spp. (Mattison et al., 2007, Lutay et al., 2007, Huetink et al., 2001, Nielsen, 2002) which is in accordance with results for BoNoV in the present study. An assessment of clinical relevance of the presented results will be published elsewhere (manuscript in preparation).
To test the hypothesis that the distribution of the obtained Ct-values may reflect the pattern of prolonged BoNoV low-level shedding in young calves after recovering from diarrhoea, we performed a challenge experiment with two Norwegian red bull calves. Peak shedding of BoNoV occurred on 4 and 8 dpi. Diarrhoea occurred only on 3 and 4 dpi and not after re-challenge on 23 dpi. Both calves continued shedding BoNoV until the termination of the experiment. The drop in Ct-values (∼10) after re-challenge may indicate some degree of virus replication without development of clinical symptoms. The rapid decrease in shedding after re-challenge probably indicates acquired immunity after the first challenge. In a natural herd environment it is likely that individual calves are frequently re-infected by the circulating enteric pathogens that are present during early life. Older calves may thus constitute a primary reservoir for a diverse range of pathogens that neonatal calves get exposed to. Shedding of BoNoV for a prolonged time may be a natural consequence of insufficient immunocompetence in these young animals (Henke-Gendo et al., 2009). The second wave of viral excretion (7–8 dpi) is probably related to the continuous upward migration and differentiation of immature enteric crypt cells and thus replenishment of susceptible villus epithelial cells (Boshuizen et al., 2003).
The observation that clinical diarrhoea was not present in conjunction with the second peak in viral shedding is important with regard to interpreting laboratory test results as well as in an epidemiological context. This finding may warrant further studies with regard to the shedding pattern of other viruses including human noroviruses.
In conclusion, two different genetic clades of BoNoV were detected in Norwegian dairy and beef calves most closely related to the original Newbury 2 strain (GenBank accession no. AF097917) and a proposed chimeric strain (GenBank accession no. AY126468).
A large proportion of young calves were shedding BoNoV in feces thus representing a reservoir for continuous exposure of susceptible animals in the herd environment. A controlled challenge experiment demonstrated continuous shedding for at least 23 dpi with peak viral shedding on 4 and 8 dpi. The novel real-time RT-PCR assay proved to be a simple, specific and sensitive method for detecting BoNoV in faecal samples. Interestingly the melting temperature analysis was found to be useful as a crude tool for assessing the RdRp genotype in the samples.
Acknowledgements
This work was supported in part by grant 155869/I10 from the Research Council of Norway, TINE Norwegian Dairies BA and Animalia – The Norwegian Meat Research Centre as part of the research project “Calf health in Norway 2004–2008”. We wish to thank all the animals and people that have contributed to this study.
References
- Ajjampur S.S., Rajendran P., Ramani S., Banerjee I., Monica B., Sankaran P., Rosario V., Arumugam R., Sarkar R., Ward H., Kang G. Closing the diarrhoea diagnostic gap in Indian children by the application of molecular techniques. J. Med. Microbiol. 2008;57:1364–1368. doi: 10.1099/jmm.0.2008/003319-0. [DOI] [PubMed] [Google Scholar]
- Anthony S., Jones H., Darpel K.E., Elliott H., Maan S., Samuel A., Mellor P.S., Mertens P.P. A duplex RT-PCR assay for detection of genome segment 7 (VP7 gene) from 24 BTV serotypes. J. Virol. Methods. 2007;141:188–197. doi: 10.1016/j.jviromet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Batten C.A., Clarke I.N., Kempster S.L., Oliver S.L., Bridger J.C., Lambden P.R. Characterization of a cross-reactive linear epitope in human genogroup I and bovine genogroup III norovirus capsid proteins. Virology. 2006;356:179–187. doi: 10.1016/j.virol.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Benson D.A., Karsch-Mizrachi I., Lipman D.J., Ostell J., Wheeler D.L. GenBank. Nucleic Acids Res. 2007;35:D21–D25. doi: 10.1093/nar/gkl986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuizen J.A., Reimerink J.H., Korteland-van Male A.M., van Ham V., Koopmans M.P., Buller H.A., Dekker J, Einerhand A.W. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J. Virol. 2003;77:13005–13016. doi: 10.1128/JVI.77.24.13005-13016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R.A., Tanaka M.M., White P.A. Norovirus recombination. J. Gen. Virol. 2007;88:3347–3359. doi: 10.1099/vir.0.83321-0. [DOI] [PubMed] [Google Scholar]
- Chevaliez S., Bouvier-Alias M., Brillet R., Pawlotsky J.M. Overestimation and underestimation of hepatitis C virus RNA levels in a widely used real-time polymerase chain reaction-based method. Hepatology. 2007;46:22–31. doi: 10.1002/hep.21656. [DOI] [PubMed] [Google Scholar]
- Escutenaire S., Mohamed N., Isaksson M., Thoren P., Klingeborn B., Belak S., Berg M., Blomberg J. SYBR Green real-time reverse transcription-polymerase chain reaction assay for the generic detection of coronaviruses. Arch. Virol. 2007;152:41–58. doi: 10.1007/s00705-006-0840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felius M . first ed. Trafalgar Square Books; 2007. Cattle Breeds – An Encyclopedia. [Google Scholar]
- Gallimore C.I., Iturriza-Gomara M., Xerry J., Adigwe J., Gray J.J. Inter-seasonal diversity of norovirus genotypes: emergence and selection of virus variants. Arch. Virol. 2007;152:1295–1303. doi: 10.1007/s00705-007-0954-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez J.M., Saiz-Jimenez C. A fluorimetric method for the estimation of G + C mol% content in microorganisms by thermal denaturation temperature. Environ. Microbiol. 2002;4:770–773. doi: 10.1046/j.1462-2920.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- Goodgame R. Norovirus gastroenteritis. Curr. Infect. Dis. Rep. 2007;9:102–109. doi: 10.1007/s11908-007-0004-5. [DOI] [PubMed] [Google Scholar]
- Gulliksen S.M., Jor E., Lie K.I., Hamnes I.S., Loken T., Akerstedt J., Osteras O. Enteropathogens and risk factors for diarrhoea in Norwegian dairy calves. J. Dairy Sci. 2009;92:5057–5066. doi: 10.3168/jds.2009-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Song J., Xu X., Ren L., Li J., Zhou H., Wang M., Qu J., Wang J., Hung T. Genetic analysis of norovirus in children affected with acute gastroenteritis in Beijing, 2004-2007. J. Clin. Virol. 2008 doi: 10.1016/j.jcv.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Han M.G., Cheetham S., Azevedo M., Thomas C., Saif L.J. Immune responses to bovine norovirus-like particles with various adjuvants and analysis of protection in gnotobiotic calves. Vaccine. 2006;24:317–326. doi: 10.1016/j.vaccine.2005.07.071. [DOI] [PubMed] [Google Scholar]
- Henke-Gendo C., Harste G., Juergens-Saathoff B., Mattner F., Deppe H., Heim A. New real-time PCR detects prolonged norovirus excretion in highly immunosuppressed patients and children. J. Clin. Microbiol. 2009;47:2855–2862. doi: 10.1128/JCM.00448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J.L. Grøndahl & Søn; Kristiania: 1911. Bondens raadgiver, Husdyrene. [Google Scholar]
- Huetink R.E., van der Giessen J.W., Noordhuizen J.P., Ploeger H.W. Epidemiology of Cryptosporidium spp. and Giardia duodenalis on a dairy farm. Vet. Parasitol. 2001;102:53–67. doi: 10.1016/s0304-4017(01)00514-3. [DOI] [PubMed] [Google Scholar]
- Huson D.H. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics. 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- Huson D.H., Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Jansen A., Stark K., Kunkel J., Schreier E., Ignatius R., Liesenfeld O., Werber D., Gobel U.B., Zeitz M., Schneider T. Aetiology of community-acquired, acute gastroenteritis in hospitalised adults: a prospective cohort study. BMC Infect. Dis. 2008;8:143. doi: 10.1186/1471-2334-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamrin P., Maneekarn N., Peerakome S., Tonusin S., Malasao R., Mizuguchi M., Okitsu S., Ushijima H. Genetic diversity of noroviruses and sapoviruses in children hospitalized with acute gastroenteritis in Chiang Mai, Thailand. J. Med. Virol. 2007;79:1921–1926. doi: 10.1002/jmv.21004. [DOI] [PubMed] [Google Scholar]
- Kirkwood C.D., Streitberg R. Calicivirus shedding in children after recovery from diarrhoeal disease. J. Clin. Virol. 2008;43:346–348. doi: 10.1016/j.jcv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Kukielka D., Esperon F., Higes M., Sanchez-Vizcaino J.M. A sensitive one-step real-time RT-PCR method for detection of deformed wing virus and black queen cell virus in honeybee Apis mellifera. J. Virol. Methods. 2007 doi: 10.1016/j.jviromet.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Liu B.L., Lambden P.R., Gunther H., Otto P., Elschner M., Clarke I.N. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 1999;73:819–825. doi: 10.1128/jvi.73.1.819-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loens K., Bergs K., Ursi D., Goossens H., Ieven M. Evaluation of NucliSens easyMAG for automated nucleic acid extraction from various clinical specimens. J. Clin. Microbiol. 2007;45:421–425. doi: 10.1128/JCM.00894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J.L., Scholes P., Gilpin B., Savill M., Lin S., Samuel L. Evaluation of uncertainty in quantitative real-time PCR. J. Microbiol. Methods. 2006;67:349–356. doi: 10.1016/j.mimet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Lutay A.V., Zenkova M.A., Vlassov V.V. Nonenzymatic recombination of RNA: possible mechanism for the formation of novel sequences. Chem. Biodivers. 2007;4:762–767. doi: 10.1002/cbdv.200790062. [DOI] [PubMed] [Google Scholar]
- Mattison K., Shukla A., Cook A., Pollari F., Friendship R., Kelton D., Bidawid S., Farber J.M. Human noroviruses in swine and cattle. Emerg. Infect. Dis. 2007;13:1184–1188. doi: 10.3201/eid1308.070005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunula L., Von Bonsdorff C.H. Norovirus genotypes causing gastroenteritis outbreaks in Finland 1998–2002. J. Clin. Virol. 2005;34:186–194. doi: 10.1016/j.jcv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Murata T., Katsushima N., Mizuta K., Muraki Y., Hongo S., Matsuzaki Y. Prolonged norovirus shedding in infants <or=6 months of age with gastroenteritis. Pediatr. Infect. Dis. J. 2007;26:46–49. doi: 10.1097/01.inf.0000247102.04997.e0. [DOI] [PubMed] [Google Scholar]
- Nielsen E.M. Occurrence and strain diversity of thermophilic campylobacters in cattle of different age groups in dairy herds. Lett. Appl. Microbiol. 2002;35:85–89. doi: 10.1046/j.1472-765x.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- Oliver S.L., Asobayire E., Charpilienne A., Cohen J., Bridger J.C. Complete genomic characterization and antigenic relatedness of genogroup III, genotype 2 bovine noroviruses. Arch. Virol. 2007;152:257–272. doi: 10.1007/s00705-006-0856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.L., Asobayire E., Dastjerdi A.M., Bridger J.C. Genomic characterization of the unclassified bovine enteric virus Newbury agent-1 (Newbury1) endorses a new genus in the family Caliciviridae. Virology. 2006;350:240–250. doi: 10.1016/j.virol.2006.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.L., Batten C.A., Deng Y., Elschner M., Otto P., Charpilienne A., Clarke I.N., Bridger J.C., Lambden P.R. Genotype 1 and genotype 2 bovine noroviruses are antigenically distinct but share a cross-reactive epitope with human noroviruses. J. Clin. Microbiol. 2006;44:992–998. doi: 10.1128/JCM.44.3.992-998.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.L., Brown D.W., Green J., Bridger J.C. A chimeric bovine enteric calicivirus: evidence for genomic recombination in genogroup III of the Norovirus genus of the Caliciviridae. Virology. 2004;326:231–239. doi: 10.1016/j.virol.2004.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.L., Wood E., Asobayire E., Wathes D.C., Brickell J.S., Elschner M., Otto P., Lambden P.R., Clarke I.N., Bridger J.C. Serotype 1 and 2 bovine noroviruses are endemic in cattle in the United kingdom and Germany. J. Clin. Microbiol. 2007;45:3050–3052. doi: 10.1128/JCM.02015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.I., Jeong C., Kim H.H., Park S.H., Park S.J., Hyun B.H., Yang D.K., Kim S.K., Kang M.I., Cho K.O. Molecular epidemiology of bovine noroviruses in South Korea. Vet. Microbiol. 2007 doi: 10.1016/j.vetmic.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.I., Park D.H., Saif L.J., Jeong Y.J., Shin D.J., Chun Y.H., Park S.J., Kim H.J., Hosmillo M., Kwon H.J., Kang M.I., Cho K.O. Development of SYBR Green real-time RT-PCR for rapid detection, quantitation and diagnosis of unclassified bovine enteric calicivirus. J. Virol. Methods. 2009;159:64–68. doi: 10.1016/j.jviromet.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T.G., Kaneshi K., Ueda Y., Nakaya S., Nishimura S., Yamamoto A., Sugita K., Takanashi S., Okitsu S., Ushijima H. Genetic heterogeneity, evolution, and recombination in noroviruses. J. Med. Virol. 2007;79:1388–1400. doi: 10.1002/jmv.20924. [DOI] [PubMed] [Google Scholar]
- Scipioni A., Bourgot I., Mauroy A., Ziant D., Saegerman C., Daube G., Thiry E. Detection and quantification of human and bovine noroviruses by a TaqMan RT-PCR assay with a control for inhibition. Mol. Cell Probes. 2008;22:215–222. doi: 10.1016/j.mcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Scipioni A., Mauroy A., Ziant D., Zaegerman C., Thiry E. A SYBR Green RT-PCR assay in single tube to detect human and bovine noroviruses and control for inhibition. Virol. J. 2008;5:98. doi: 10.1186/1743-422X-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota T., Okame M., Takanashi S., Khamrin P., Takagi M., Satou K., Masuoka Y., Yagyu F., Shimizu Y., Kohno H., Mizuguchi M., Okitsu S., Ushijima H. Characterization of a broadly reactive monoclonal antibody against norovirus genogroups I and II: recognition of a novel conformational epitope. J. Virol. 2007;81:12298–12306. doi: 10.1128/JVI.00891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J.R., Hoet A.E., Traven M., Tsunemitsu H., Saif L.J. Reverse transcription-PCR assays for detection of bovine enteric caliciviruses (BEC) and analysis of the genetic relationships among BEC and human caliciviruses. J. Clin. Microbiol. 2003;41:3089–3099. doi: 10.1128/JCM.41.7.3089-3099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svraka S., Duizer E., Vennema H., de B.E., van d V., Dorresteijn B., Koopmans M. Etiological role of viruses in outbreaks of acute gastroenteritis in The Netherlands from 1994 through 2005. J. Clin. Microbiol. 2007;45:1389–1394. doi: 10.1128/JCM.02305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P., de M.C., Joshi Y., Golden A., Hodinka R.L., Soriano V., Devare S.G., Hackett J., Jr. Impact of human immunodeficiency virus type 1 (HIV-1) genetic diversity on performance of four commercial viral load assays: LCx HIV RNA Quantitative, AMPLICOR HIV-1 MONITOR v1.5, VERSANT HIV-1 RNA 3.0, and NucliSens HIV-1 QT. J. Clin. Microbiol. 2005;43:3860–3868. doi: 10.1128/JCM.43.8.3860-3868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio K., Myrmel M. Molecular epidemiology of norovirus outbreaks in Norway during 2000 to 2005 and comparison of four norovirus real-time reverse transcriptase PCR assays. J. Clin. Microbiol. 2006;44:3695–3702. doi: 10.1128/JCM.00023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel W.H., van der H.R., Verschoor F., Gelderblom H., Vinje J., Koopmans M.P. Epidemiology of Norwalk-like virus infections in cattle in The Netherlands. Vet. Microbiol. 2003;92:297–309. doi: 10.1016/s0378-1135(02)00421-2. [DOI] [PubMed] [Google Scholar]
- Wise A.G., Monroe S.S., Hanson L.E., Grooms D.L., Sockett D., Maes R.K. Molecular characterization of noroviruses detected in diarrhoeic stools of Michigan and Wisconsin dairy calves: circulation of two distinct subgroups. Virus Res. 2004;100:165–177. doi: 10.1016/j.virusres.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Williamson W.M., Hewitt J., Rivera-Aban M., Lin S., Ball A., Scholes P., Greening G.E. A sensitive multiplex real-time RT-PCR assay for the detection of human and animal noroviruses in clinical and environmental samples. Appl. Environ. Microbiol. 2007 doi: 10.1128/AEM.00572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


