Graphical abstract

Keywords: Aliskiren, Hypertension, Renin angiotensin system, Renin inhibitors
Abstract
Hypertension is a diverse illness interlinked with cerebral, cardiovascular (CVS) and renal abnormalities. Presently, the malady is being treated by focusing on Renin- angiotensin system (RAS), voltage-gated calcium channels, peripheral vasodilators, renal and sympathetic nervous systems. Cardiovascular and renal abnormalities are associated with the overactivation of RAS, which can be constrained by angiotensin- converting enzyme inhibitors (ACEIs), angiotensin II (Ang-II) -AT1 receptor blockers (ARBs) and renin inhibitors. The latter is a new player in the old system. The renin catalyzes the conversion of angiotensinogen to Angiotensin I (Ang-I). This can be overcome by inhibiting renin, a preliminary step, eventually hinders the occurrence of the cascade of events in the RAS. Various peptidomimetics, the first-generation renin inhibitors developed six decades ago have limited drug-like properties as they suffered from poor intestinal absorption, high liver first-pass metabolism and low oral bioavailability. The development of chemically diverse molecules from peptides to nonpeptides expanded the horizon to achieving direct renin inhibition. Aliskiren, a blockbuster drug that emerged as a clinical candidate and got approved by the US FDA in 2007 was developed by molecular modeling studies. Aliskiren indicated superior to average efficacy and with minor adverse effects relative to other RAS inhibitors. However, its therapeutic use is limited by poor oral bioavailability of less than 2% that is similar to first-generation peptidic compounds. In this review, we present the development of direct renin inhibitors (DRIs) from peptidic to nonpeptidics that lead to the birth of aliskiren, its place in the treatment of cardiovascular diseases and its limitations.
1. Introduction
At the global level, the highest mortality is from cardiovascular diseases than from any other causes. According to a report by the WHO, in 2016, an expected 17.9 million individuals died from cardiovascular ailments representing 31% of all global deaths. It was projected from WHO that, more people die from heart disease and stroke.1 Hypertension, a multifaceted CVD, is a significant hazard factor for heart attack, heart failure, chronic kidney disease, renal damage, and stroke. It is likewise predominant in patients with diabetes mellitus. If left untreated, it advances to end-organ damage of the heart and the kidney, eventually leading to death.2 For the diagnosis and management of adult hypertensive patients, new guidelines are published by the American College of Cardiology in 2017. The new rule characterizes normal blood pressure as less than 120/80 mm Hg and elevated blood pressure as 120 to 129 mm Hg systolic with less than 80 mm Hg diastolic.3 This would reclassify people previously considered to have prehypertension as having hypertension. Furthermore, the guideline recommends an aggressive approach to combat high blood pressure. The treatment regimen often involves more than one class of drugs for novel targets that manage blood pressure. One of the several classes of drugs approved for the chronic treatment of hypertension is drugs interrupting the renin-angiotensin-aldosterone axis. This pathway considered the most significant scientific advancement in the treatment of hypertension and other cardiovascular ailments.4 Among the RAS inhibitors, DRIs have yet to gain a notch in the treatment of cardiovascular and kidney diseases. This review discusses the development of renin inhibitors from several decades, the old discovery of peptides to nonpeptides, recent developments in the continued unearthing of DRIs and therapeutic potentials of aliskiren, the only approved renin inhibitor since 2007.
2. Renin-angiotensin system (RAS)
Cardiovascular, renal, and adrenal glands are controlled by the RAS by managing blood pressure, sodium and potassium balance and fluid volume.5 In 1934, studies by Goldblatt et al. reported important findings of the relation between renin and kidney function and blood pressure.6 Since then, extensive experimental studies have been undertaken to recognize the components of the RAS and their role in the regulation of blood pressure and fluid homeostasis. The principal component of the system is Ang II, which is formed by the action of angiotensin-converting enzyme (ACE) on Ang I (Fig. 1 ). The latter is formed with the cleavage of angiotensinogen, a 452 amino acid circulating α2 globulin, by renin. Circulating levels of renin are dependent on several factors such as interruption in negative feedback when there is a decreased or no Ang II at the receptor site, acute stimuli of juxtaglomerular apparatus of the kidney, treatment with AT-1 receptor blockers or ACE inhibitors.7 Thus, the formation of renin from its precursor prorenin is considered to be the rate-determining step in the synthesis of Ang I from angiotensinogen. Overactivation of the RAS results in excessive generation of Ang II, which on activation of AT-1 receptors is purported to cause hypertension, heart failure, ischemic disease, atherosclerosis, cardiac hypertrophy and renal disorders.7 Thus, pharmacologic agents interrupting the performance of the RAS counteract the events of Ang II by reducing its synthesis or avoiding its authoritative action on AT-1 receptors. Thus, abstracting the RAS cascade at various levels has different effects on its components. The first approach is to block the action of enzyme renin (e.g., aliskiren), in the synthesis of angiotensin I from angiotensinogen and not necessarily a preferred choice. This halts the generation of angiotensin peptides by the renin-ACE and non-ACE/chymase pathways.4 The second approach was successfully launched in the 1970 s with the introduction of captopril, an ACE inhibitor that blocked the formation of Ang II from Ang I. ACE inhibitors have constituted one of the most important treatment regimens to combat high blood pressure and cardiac insufficiency. ACE inhibitors are used in the management of mild to moderate hypertension, myocardial infarction, diabetic nephropathy and congestive heart failure.4 The third approach to combat hypertension was to block the actions of Ang II at its target site, AT-1 receptors. The AT-1 receptor mediates all of the known cardiovascular effects associated with Ang II. The Ang II receptor blockers (e.g., losartan) are highly effective and well-tolerated antihypertensive medications. The fourth stage was to block the production of aldosterone, an important component of the RAS that is known to cause increased sodium retention and increased volume, both contributing to hypertension. Thus, aldosterone antagonists (e.g., spironolactone), are effective in reducing blood pressure in patients afflicted with essential hypertension by blocking the actions of aldosterone on mineralocorticoid receptors and beyond. The fifth approach is blocking the enzyme aldosterone synthase. The sixth and novel approach opened up in 2015 was concurrent inhibition of the AT-1 receptor and neprilysin (e.g., valsartan-sacubitril combination).4
Fig. 1.
Renin-angiotensin system (RAS) and the potential sites that drugs target in the cascade.
In 1898, Tigerstedt and Bergman revealed the pressor impact of rabbit renal tissue extracts and named the substance renin. Angiotensinogenase, currently known as renin, is an aspartyl protease enzyme produced in the juxtaglomerular cells of the kidney. Renin is produced from prorenin by proteolytic activation (removal of the prosegment) and or by non-proteolytic activation. In response to hypotension and hypernatremia, renin is discharged into the blood circulation. Both prorenin and renin bind to (pro)renin receptor.8 Both prorenin and renin are synthesized in the kidney but prorenin is also produced in extrarenal tissues including the collecting duct, adrenal gland, retina, placenta, testis, submandibular gland, ovary in numerous species. The plasma prorenin level is 10 times higher than the plasma renin as such. But at physiological conditions, less than 2% of the prorenin turns out to be enzymatically dynamic. Chronic pathologic stimuli elevate the active prorenin levels and increase its conversion to active renin. In severe pathological conditions, the ratio of prorenin to renin may reach as high as 400-fold resulting in increased Ang II levels.9 The levels of renin are higher in patients with diabetes than are in any other condition.10 In adult human plasma, the renin normal concentration is 1.98–24.6 ng/L in the upstanding position.11
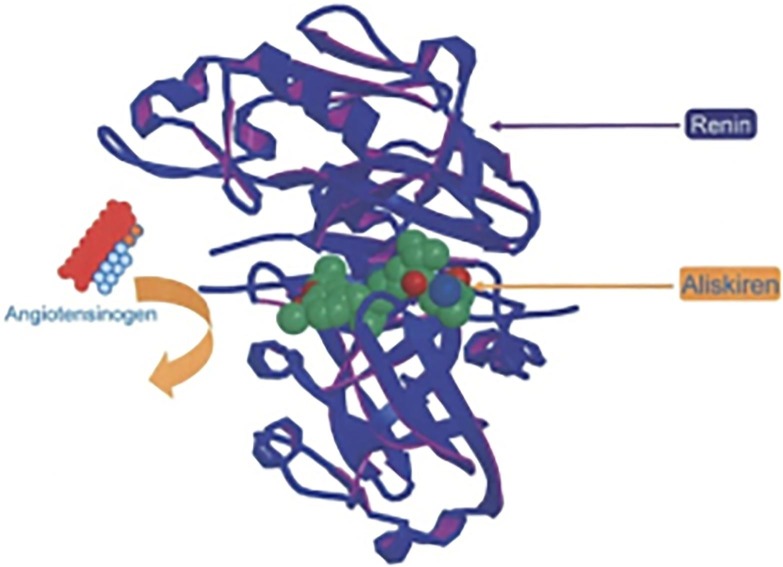
Renin has high specificity for angiotensinogen. Renin possesses two homologous flaps, with the dynamic site residing in the deep cleft formed between them (Fig. 2 ). The catalytic activity of the dynamic site is because of two aspartic acid residues, one in each flap of the renin molecule. A key element of the dynamic site is a discreet sub pocket (S3sp), which is precise among the aspartate proteases and specific to renin. The dynamic site can accommodate seven amino acid units of the substrate, angiotensinogen, and cleaves the Leu10-val11 peptide bond within angiotensinogen to produce Ang I, the rate-determining step.12 It appears that renin inhibition would be the most likely therapeutic target to succeed as far as the activation of the RAS mechanism is concerned. It lacks confirmation as there are no highly efficacious DRI for the target protein.
Fig. 2.
X-Ray Crystallographic portrayal of human renin in the coupling complex with Aliskiren12 [Reproduced with permission from Elsevier]. (Colored one).
Study on the RAS by researchers for over a century, not exclusively to comprehend its job in typical physiological function yet, in addition, to expand powerful treatments to treat its overactivation. These efficient research endeavors have prompted the proposal of a non-canonical RAS pathway, which has challenged the hypothesis that the RAS can only exert deleterious effects on the renal and cardiovascular systems.7 The RAS is steadily expanding with the discovery of ACE2 in 2000, a homologue of ACE and carboxypeptidase enzyme found exceptionally in the heart, kidney and blood vessels. In the classical system, the renin cleaves the angiotensinogen to form Ang I. Ang I is additionally a substrate for ACE2, which forms Ang (1–9). ACE2 also cleaves Ang II to form Ang (1–7), which is also being formed by the action of ACE on Ang (1–9). Ang (1–7) is accounted to exert inverse to that of Ang II, and it counterbalances the undesirable effects of Ang II on the heart, kidney and blood vessels. The physiological functions of Ang (1–7) are mediated via a G protein-coupled receptor, Mas receptor (Mas R).13 Thus, RAS exhibits dual actions.14 The major one is well characterized conventional canonical pathway, ACE–Ang II–AT-1 R axis, which mediates inflammation via vasoconstriction and proliferation. The second is a non-canonical pathway, ACE2–Ang (1–7)–Mas R axis, which has a wide range of influence on opposing or modulating the principal canonical pathway with endothelial protective, cardioprotective, natriuretic, anti-proliferative and vasodilatory properties. The RAS shows its duality by acting as a devil by the canonical pathway and angel by the non-canonical pathway.7, 13, 14 Furthermore, it might be interesting to note that ACE2 is a functional receptor for SARS- CoV-2.15, 16 Since ACE2 is a key regulator of cardiac function with cardioprotective effects, therapy with RAS inhibitors (including ACE inhibitors, ARBs, renin inhibitor) for cardiovascular diseases in patients with COVID-19 should be done with great care.
3. Renin inhibitors
Renin is an attractive target as it is a rate-determining step in Ang II synthesis. Renin inhibitors markedly reduce plasma renin activity (PRA)2 and consequently limit the production of Ang I from angiotensinogen. The renin-ACE and non-ACE/chymase pathways prevent the synthesis of angiotensin peptides. For the past three decades, extensive work has been done on renin inhibitors although the therapeutic significance of renin inhibitors was recognized since 1957.17 The investigation began with antibodies and peptides.18 The first peptide renin inhibitor came up in 1972.19 After several years of investigation, peptides to nonpeptides transition occurred. Research on designing renin inhibitors got benefited by the emergence of computational molecular modeling techniques, structure-based drug design, and crystallographic techniques. Firstly, the prorenin structure was reported in 1989, later on, renin structure was reported.19 This facilitated medicinal chemists to move fast with the exploration of renin inhibitors.
4. Peptides ruled the renin inhibition
For the past 6 decades, extensive studies have been done to discover clinically efficacious renin inhibitors. Research on renin inhibitors began with the peptide synthesis. Skeg and co-worker’s study in 195720 has served the base for the peptide investigation. Renin is a tetradecapeptide substrate, with a sequence of Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Leu-Val-Tyr-Ser.21 Attempts were made to synthesize a peptide that mimicked the renin segment17, 21 Proteins such as hemoglobin from various species, globin chains, globin fragments, P-Lactoglobulin B fragments showed an inhibitory action on renin.22 In the mid of 1970s, renin substrate derived peptides were reported. Unsaturated peptide derivatives were more active than the saturated peptides.23 Polypeptides such as AHMOA-Val-Phe-OCH3, His-AHMOA-Val-Phe-OCH3, and AHMOA-Ile-His-OCH3 synthesized from angiotensin peptide fragments were also showed competitive inhibition of renin enzyme.24 Later on, structurally different peptides were tried. Inclusion of the cyclohexylalanyl analog of statin, (3S, 4S)-4-amino-5-cyclohexyl-3-hydroxypentanoic acid (ACHPA), to the octapeptide derivatives contributed the most powerful renin inhibitors.25 Potency was retained even with the pentapeptides.25 A series of tetrapeptides containing statins, with the addition of various hydrophobic aromatic groups at the carboxy terminus showed competitive renin inhibition. Furthermore, the activity was increased seven times more than the reported statins and 22 times more than the statins when fluoro keto peptides were synthesized. The presence of the fluoro group increased the activity more than 930 more times in binding the enzyme. Unsaturated fluoro keto peptides showed more activity as a result of the replacement of some of the peptides with trans alkenes.26 Oral efficacy was achieved by synthesizing oligopeptides derivatives.27 Transformation to glycopeptide derivatives resulted in delayed hypotensive activity in the human renin infused rat assay.27 In the 1980s, the structure-based drug design (SBDD) involving X-ray crystallography and computer-assisted molecular modeling was developed as one of the most paramount ideal models in drug discovery.28 Olefin dipeptides designed by isosteric replacement of amide bond showed renin inhibitory effect in both human amniotic renin and hog kidney renin.29 Dipeptide derivatives designed by replacement of amide bond with ester group did not alter the activity much implying that the amide group is not essential for binding by hydrogen bonding.30 Incorporation of sulphonamide at the N terminus of the peptide ended with (2S)-2-benzyl-3-[[(l-methylpiperazin-4-yl)sulfonyl]propionyl]-3-thiazol-4-yl-l-alanine amide of (2S,3R,4S)-2-amino-l-cyclohexyl-3,4-dihydroxy-6-methylheptane (A-72517), which showed excellent renin inhibitory activity.31 Merck developed 14 membered macrocyclic renin inhibitors such as glutamate derived and serine derived macrocycles.32 They were developed by replacing an amide bond with ester linkage in the macrocyclic ring. This modification had less effect on the potency of glutamate series, while increased potency was observed in the serine series. The outcome of this study was a potent compound “3(S)-quinuclidinyl-Phe” derivative. In Rhesus monkeys, it reduced blood pressure by 20 mm Hg and hindered PRA and showed less than 1% oral bioavailability in rats as a result of serine-ester bond cleavage.32
Key strategies of extensive structural modification of peptide derivatives integrated the exclusion of their peptide nature, improvement of significant binding interaction to the renin specificity. These ideas intended to accomplish a nominal molecular size peptide with solid binding affinity. A wide variety of peptidomimetics synthesized for several decades had restricted drug-like properties with numerous drawbacks importantly; low intestinal absorption and elevated liver first-pass metabolism that resulted in poor oral bioavailability. Several generations of peptide renin inhibitors were developed, all ended up with low solubility, high molecular weight, and poor bioavailability. Thus, it was inevitable for researchers to focus on non-peptides to achieve improved oral bioavailability.
5. The new era of non-peptide renin inhibitors
For the past 2 decades, key progress on medicinal chemistry approaches contributed to a brisk and intriguing development of promising new classes of druggable moieties that showed renin inhibition. Chemically diverse molecules expanded the horizon to achieve the target. Molecules that resembled the transition state of a substrate molecule in an enzyme-catalyzed chemical reaction were developed. Such compounds included (2S,3R,4S)-2-amino-l-cyclohexyl-3,4-dihydroxy-6-(2-pyridyl)hexane moiety at the C-terminal functionality and were assessed for the inhibition of renin both in vivo and in vitro. All compounds exhibited potencies in the nanomolar or even sub-nanomolar concentration range when tested on sodium-depleted Rhesus monkeys in vivo and on human renin in vitro. One such potent compound was N-terminal derived piperidyl succinic acid that inhibited the human renin activity at an IC50 of 0.38 nM.33
Since the 1990′s, High through put screening (HTS) has emerged as a discovery tool and many millions of compounds have been produced and assessed for an activity at the target protein. But it had low success rates in the identification of potential hit molecules.34 Roche came up with 3,4-disubstituted piperidine as a lead moiety. The systematic optimization of this structural class resulted in piperidine derivatives that had affinities for renin in the picomolar range.35 Novartis earlier known as Ciba-Geigy came up with a distinctive moiety, aminohydroxyethylene dipeptide isostere.36 The emergence of piperidine derivatives from Roche and Novartis in 2006 led to the new insight for research on renin inhibition. Firstly, ketopiperazine derivatives were designed and later optimizing ring C led to the synthesis of aliskiren.37 At the same time, a few researchers moved from peptides and non-peptides to plant active constituents for the renin inhibitory effect. Sodium houttuynin, a volatile oil, was extracted from perennial plant Houttunynia cordata Thunb. A series of analogs of sodium houttuynin demonstrated considerable renin inhibitory effect.38
Rapid and intriguing emergence of medicinal chemistry methodologies were aimed at the design of druggable moiety. Researchers focused on the synthetic diversity space at the ligand level by the application of novel drug designing tools such as molecular modeling, X-ray crystallographic studies, denovo synthesis, fragment-based drug design, and structure-based drug design. These tools were successfully employed to identifying ligand binding sites in the renin dynamic site, the hefty flanking S3-S1 pocket contributed the main site and also called as the hot spot for ligand binding. The site is highly hydrophobic. The site is highly hydrophobic completely distinct from that of substrate specificity pockets with extended β-strand substrate-binding topography.39 Applying the concept of structure-based drug design, Roche has designed 3, 4-disubstituted piperidine molecules.40, 41 The 4-phenylpiperidine derivative compounds (1 and 2) discovered by HTS by Roche (Fig. 3 ) although showed weak activity, nevertheless became the backbone discovery of direct renin inhibitor.
Fig. 3.
4-phenyl piperidine derivatives developed by HTS.
Similarly, 5-phenyl substituted 6-ethyl-2,4-diaminopyrimidine derivatives by Pfizer showed weak renin inhibition (Fig. 4 ). Modification of the phenyl group by replacing highly electronegative halogen moiety increased the activity seven-fold.41, 42 Noticeably, the 2,4-diaminopyrimidine (1–2) was identified as a new scaffold for drug discovery. Furthermore, optimization of phenyl moiety with the bicyclic group (compound 3) exhibited high in vitro potency with oral bioavailability (Fig. 4).42 Although many orally active compounds such as enalkiren, remikerin and zenkiren were developed but found clinically inefficient because of their short half-life, weak antihypertensive activity and poor bioavailability.43 A new series of 3,9-diazabicyclo[3.3.1]nonene derivatives were developed on the piperidine template by denovo drug design. Optimization of the positions 3, 6, and 7 of the diazabicyclonene template has led to the discovery of enantiomers. These compounds showed renin inhibition with improved pharmacokinetics properties. The functional groups substituted at positions 6 and 7 were found to be crucial for the binding affinity of these compounds for renin.44
Fig. 4.
2–4-Diaminopyrimidine derivatives discovered through HTS.
By structure-based topological design approach, 2,7-dialkyl-substituted 5(S)-amino-4(S)-hydroxy-8-phenyl-octanecarboxamide derivatives exhibited excellent oral bioavailability with improved potency. Maibaum, et al. modified P1, P2, and P3 position of these derivatives.42 However, P2 position modification at the hydroxyl ethylene transition-state isostere substantially improved the potency, and duration of action.44 This hard work led to the innovation of aliskiren, an extremely selective and potent for renin inhibition. In sodium-depleted marmosets, oral administration of aliskiren dose-dependently reduced mean arterial blood pressure with the sustained duration of action.45 Finally, two decades of research were successful with the discovery of aliskiren that binds renin enzyme similar to that of peptides. It is chemically described (Fig. 5 ) as 2(S),4(S),5(S),7(S)-N-(2-carbamoyl-2-methylpropyl)-5-amino-4-hydroxy-2,7-diisopropyl-8-(4-methoxy-3-[3-methoxypropoxyl-]phenyl)octanamide.46, 47
Fig. 5.

Chemical structure of Aliskiren.
After aliskiren discovery and approval in the treatment of hypertension, many researchers worked on renin inhibition and succeeded in synthesizing several molecules that showed renin inhibitory activity. Investigation on carboxamide derivatives continued with an objective to achieve good oral bioavailability that was lacking with aliskiren. In 2012, Mori et al by X-ray crystallographic studies designed (3S,5R)-5-[4-(2-chlorophenyl)-2,2-dimethyl-5-oxopiperazin-1-yl]piperidine-3-carboxamides as a potent renin inhibitor with 45% oral bioavailability in double transgenic rat model of hypertension.48 Among piperazine derivatives, Nakamura et al. showed marked renin inhibitory activity with DS-8108b, 2-dimethyl-4-phenyl piperazine-5-one. It inhibited the human renin at 0.9 nM, a concentration much lower than that of aliskiren because of the N-terminus substitution with trans-4-aminoadamantan-1-ol. Although it had low cardiac toxicity and suppressed PRA in the cynomolgus monkey more effectively than that of aliskiren, it proved to be clinically inefficient.49
6. Post aliskiren period, the discovery of more potent DRI
Using crystallographic techniques and computational molecular modeling scientists at several pharmaceutical companies synthesized several potent and selective renin inhibitors. The number of compounds that gained worldwide attention is described below.
Vitae-GSK: Alkyl amine based DRIs were designed by structure-based drug design and de novo drug design (Fig. 6 ). Of these 5 compounds, VTP-27999 showed nearly 90 % inhibition of PRA.50
Fig. 6.
Alkyl amine based derivatives by Vitae.
Daiichi Sankyo: Research focused mainly on aliskiren-based derivatives to achieving improved oral bioavailability. In contrast to Roche’s 3, 4 piperidine, Daiichi Sankyo designed 3,5-piperidine (Fig. 7 ). Compound 1–3 scaffold demonstrated good binding with renin that was similar to DS-8108b.48
Fig. 7.
3, 5-piperidine based derivatives by Daiichi-Sankyo.
Novartis: Designed 3, 5-piperidines (compounds 1 to 3, Fig. 8 ) by high throughput screening and trans-3,4-disubstituted pyrrolidines (compound 1 and 2, Fig. 9 ) by computational technique. All of them reported as a new class of renin inhibitors.51
Fig. 8.
3, 5-piperidine based derivatives by Novartis.
Fig. 9.
3, 4-Pyrollidine based derivatives by Novartis.
Actelion-Merck: Actelion designed 3,9-diazabicyclo[3.3.1]nonene derivatives43 by using Roche’s 3,4-piperidines as a basic scaffold by structure-based drug design in collaboration with Merck, Optimization of 3,4-piperidines by Actelion lead to the development of ACT-077825 (MK-8141) (compound 1 in Fig. 10 ). MK-8141 was well tolerated in phase I and II clinical trials but failed to lower the blood pressure even after 4 weeks of treatment. Another similar molecule ACT-178882 (MK-1597) (compound 2 in Fig. 10) developed from Actelion-Merck showed drug interaction with atorvastatin and simvastatin (Fig. 10).
Fig. 10.
Renin inhibitors designed by Actelion in collaboration with Merck.
Merck-Frost: Optimized piperidine derivatives by molecular modeling studies that lead to the identification of methylene amine transition state analog (Fig. 11 ).52
Fig. 11.

Piperidine based renin inhibitors designed by Merck frost.
Dainippon-Sumitomo: Designed 269 compounds of 2,2-disubstituted-1,4-benzoxazin-3-ones derivatives out of which only one molecule (W02010150840) exhibited oral efficacy in dTGR model. Furthermore, it demonstrated no adverse anti-inflammatory effects (Fig. 12 ).50
Fig. 12.

Renin inhibitors designed by Dainippaon-Sumitomo.
Mitsubishi-Tanabe: Designed two morpholine-3-carboxamide derivatives (W02008153182 & W02012124775) (Fig. 13 ) with no substitution at 4 and 5 positions.50
Fig. 13.
Renin inhibitors designed by Mitsubishi-Tanabe.
Takeda: Designed a piperidine based transition state analog (W02009051112) with a heterocyclic carboxamide group. Further optimization of the physicochemical properties of piperidine analogs led to the innovation of benzimidazole derivative, (1-(4-methoxybutyl) - N-(2-methylpropyl)-N-[(3S,5R)-5-(morpholin-4-yl) carbonylpiperidin-3-yl]-1H-benzimidazole-2-carboxamide hydrochloride (TAK272). Both of them are potent and orally active with 98% inhibition of the enzyme at a concentration of 1 µM (Fig. 14 ).52, 53 Additionally, they showed cardioprotective activity.54
Fig. 14.
Renin inhibitors by Takeda.
Plexxikon: Designed tetrahydroquinoline derivatives with an IC50 <10 µM for renin inhibition (Fig. 15 ).50
Fig. 15.
Renin inhibitors by Plexxicon and Cadila health care.
Cadila Healthcare: Designed 3, 4-piperidine derivatives (W02012085935) with amide spacer to improve lipophilicity to achieve good oral efficacy (Fig. 15).50
Sanofi-Aventis: Designed chiral indole-3-carboxamide derivatives (compounds 1 and 2, Fig. 16 ) by high throughput screening and its binding conformation with renin are similar to that of aliskiren, VTP-27999, and DS-8108b. Further optimization of the molecule by structure-based drug design yielded azaindole derivatives with an increased potency (Compounds 3 and 4, Fig. 16).55
Fig. 16.
Renin inhibitors by Sanofi-Aventis.
As described above, many companies have invested time and money in researching and producing hundreds of compounds demonstrating good renin inhibitor activity. However, all of them failed to meet the clinical development program so far. It seems the success is limited to only one compound that is aliskiren. It is the foremost prototype DRI in the class approved by the US Food and Drug Administration in March 2007 for the management of hypertension.2, 4 In the next section, we describe the pharmacology, pharmacokinetics and therapeutic potential of aliskiren in treating cardiovascular and renal disorders.
7. Aliskiren
Aliskiren is highly potent and very specific for the binding of the human renin enzyme.56 Aliskiren has a dual action on the renin-angiotensin system such as it has distinctive PRA reduction power that differs from other antihypertensive drugs such as ACEIs and ARBs.2, 57 In the clinic, aliskiren is well tolerated and has sympathetic neurohormonal effects in heart failure. Aliskiren has no effect on left ventricular wall thickness and volumes or left ventricular ejection fraction, conceivably because of the short span of activity and comparatively small sample size, while a larger, longer-term study is expected to demonstrate or invalidate this theory.53 Aliskiren monotherapy, as well as combination therapy with other antihypertensives, showed the marked effects. It is well tolerated and a drug of choice for long term treatment of blood pressure. Aliskiren has neuroprotective action on effects in diabetic patients. Unfortunately, researcher apprehension may provide a practical obstacle to aliskiren research. Renin inhibitors can prevent vascular injury caused by high blood pressure thereby tender considerable secure for cardioprotection and renoprotection.7
7.1. Aliskiren pharmacokinetics
Following oral administration, aliskiren is quickly but poorly absorbed. It reaches peak plasma concentration within 1 to 3 hr of administration with a half-life of 24 hr. Steady-state plasma concentrations of aliskiren are reached after 7 to 8 days of once oral daily dosing. High-fat meal decreases aliskiren absorption.4 Aliskiren has favorable physicochemical properties as it has high water solubility (350 mg/ml at pH 7.4) and high hydrophilicity (log P oct/water = 2.45 at pH 7.4), which are essential properties for good oral bioavailability.57 It has more than 10,000-fold less affinity for related aspartic peptidases.58 However, the reported bioavailability is approximately 2.5%.4 Aliskiren has moderate binding to plasma proteins (mean protein-bounding level of 49.5%.57 Clinically relevant pharmacokinetic or pharmacodynamic effects were influenced by neither body weight nor sex. A meal has an impact on the pharmacokinetics of drug candidates. Oral administration of aliskiren 150 mg with food diminishes mean Cmax and AUC0–∞ values by 81 and 62% lower, respectively, with than in the fasting state.59 A high-fat meal decreases AUC and Cmax values by 71% and 85%, respectively, of those in the fasting state. Thus, patients are advised to take aliskiren in a similar way every day adhering to meal times.48, 49 Aliskiren has a lower potential for considerable drug interaction. Remarkably, only 20% of aliskiren is metabolized in humans.56 It is a substrate for CYP3A4 enzyme and does not suppress CYP450 enzymes.5 Thus, it makes an appropriate drug for combination with other antihypertensive agents. Aliskiren is eliminated unaltered through the hepato-biliary route after absorption and less than 1% is excreted in the urine.58 Hepatic elimination of aliskiren makes it safe in patients with renal impairment. There is no necessity for dose adjustment for patients with hepatic or renal impairment and elderly patients.2
7.2. Aliskiren antihypertensive effects
Hypertension is a fundamental hazard factor for cardiovascular disease, stroke, and chronic kidney disease. Diabetes mellitus patients suffer from high blood pressure. Chronic hypertension leads to progressive end-organ damage of the kidney and the heart which leads to high morbidity. So preclusion of blood pressure is decisive. In Watanabe heritable hyperlipidemic rabbits, aliskiren (40 mg/kg) was shown to recover impaired nitric oxide (NO) bioavailability at the vascular endothelium and to protect against atherosclerotic changes.60 In a coronary artery ligated mouse model of myocardial infarction, aliskiren noticeably improved structural variation and cardiac activity.61, 67 In another study, aliskiren showed a prominent effect on preventing cardiac fibrosis and oxidative stress in diabetic rats more efficiently than ACE inhibitors.62 Aliskiren alone or in combination with losartan is well tolerated; signifying aliskiren is an efficient and well tolerated treatment choice in patients with left ventricular hypertrophy.63 In double transgenic rat aliskiren is as effective as candesartan on cerebral ischemic stroke.64 Aliskiren notably enhanced the brain water content, infarct volume, Nissle bodies and neurological scores in patients with cerebral ischemia.65 The neuroprotective outcome of Aliskiren is independent of its blood pressure lowering effect.65, 66 Aliskiren alone or in combination therapy with valsartan, ramipril and hydrochlorothiazide were provided additional lowering effects in diabetic patients with high-risk hypertension.67, 68 It may be noted that aliskiren is no longer used in combination with other RAS inhibitors.4
7.3. Aliskiren renal protective effects
Generally, the inhibition of the RAS pathway is recommended as first-line therapy in the treatment of renal complications.69 Renoprotective effects of aliskiren have also been shown in double transgenic rats (dTGR) that express genes for both angiotensinogen and human renin.70 Renal vasodilation in normotensive subjects on a low-sodium diet was significantly induced by aliskiren, and responses exceeded those reported for ACE inhibitors and angiotensin receptor blockers (ARBs). The duration was long and was accompanied by considerable natriuresis. In renal insufficiency patients, aliskiren provided a more inclusive and significant blockade of the RAS for improved renal efficiency. Aliskiren found to have an anti-inflammatory effect in the kidney. Aliskiren also provides renal protection by decreasing albuminuria in patients with diabetes. It is suggested that aliskiren is more renoprotective compared to other antihypertensives.70, 71 Renoprotective impact of aliskiren is independent of the blood pressure-lowering effect.70
7.4. Limitations and adverse effects
Although aliskiren possesses cardioprotective and renoprotective effects, it suffers from poor bioavailability. The most serious adverse effects are hyperkalemia and renal dysfunction which will guide to increased mortality and morbidity. The Aliskiren Study in Post-MI Patients to Reduce Remodeling (ASPIRE) study investigators did not favor combination therapy of aliskiren with other RAS inhibitors in diabetic patients.72 The Aliskiren Trial of Minimizing Outcomes for Patients with Heart failure (ATMOSPHERE) trial investigation revealed that dual therapy with enalapril in patients having a high rate of hypotension and renal dysfunction may not be beneficial. Aliskiren at dosages ≥ 300 mg/day causes gastrointestinal symptoms such as dyspepsia, abdominal pain and gastrointestinal reflux which are classically mild and not often lead to stoppage of treatment.63, 73 The Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE) trail investigations revealed that the addition of aliskiren to standard therapy with RAS blockades in patients with type 2 diabetes has no impact on cardiovascular and renal events, and it may be even detrimental.74 The Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) trial revealed that the addition of aliskiren to standard therapy did not lessen the rate of heart failure and cardiovascular death.75 The Aliskiren Quantitative Atherosclerosis Regression Intravascular Ultrasound Study (AQUARIUS) investigations concluded that aliskiren treatment does not cause considerable improvement or arrest the progression of coronary atherosclerosis, and has no significant effect on the risk of chief cardiovascular functions.76 Aliskiren combination therapy with losartan and diuretics may cause an additional threat of hyperkalemia but does not result in renal dysfunction in essential hypertension.77
8. Conclusion
Renin catalyzes the initial phase in the RAS cascade in the regulation of homeostasis of body fluid volume and blood pressure. Renin inhibitors are attractive drug candidates for the management of hypertension and cardiovascular-related disorders. Direct renin inhibitor, aliskiren, emerged in 2007 as a clinically efficient molecule till today. Aliskiren is a ketopiperazine derivative with good binding and high specificity for the renin enzyme. Several hundreds of researchers tried to follow the binding pattern of aliskiren to renin enzyme and synthesized many renin inhibitors. The vast number of medicinal chemists designed new chemical molecules using structure-based drug design, de novo drug design, and molecular modeling studies. However, none of the new chemical entities were found to be clinically efficient relative to aliskiren. They suffer from poor bioavailability, short half-life, weak antihypertensive efficacy, high lipophilicity, high molecular weight, and large numbers of rotatable bonds. The development of newer renin inhibitors with improved clinical efficacy and safety relative to aliskiren is still elusive. Discovery of a new druggable moiety is in demand as new antihypertensives are expected to exhibit an apparent diversity in their clinical profile beyond the reduction in blood pressure.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.WHO report on cardiovascular diseases. World Health Organization. 2017 [Google Scholar]
- 2.Jagadeesh G., Balakumar P., Stockbridge N. How well do aliskiren's purported mechanisms track its effects on cardiovascular and renal disorders? Cell Signal. 2012;24(8):1583–1591. doi: 10.1016/j.cellsig.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Whelton P.K., Carey R.M., Aronow W.S. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 4.Jagadeesh G., Balakumar P., Maung-U K. Springer; 2015. Pathophysiology and Pharmacotherapy of Cardiovascular Disease, Chapter 36, Drugs targeting RAS in the treatment of Hypertension and other Cardiovascular diseases; pp. 751–806. [Google Scholar]
- 5.Ferrario C.M., Strawn W.B. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98(1):121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 6.Goldblatt H., Lynch J., Hanzal R.F. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59(3):347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balakumar P., Jagadeesh G. A century old renin–angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cellular Signaling. 2014;26(10):2147–2160. doi: 10.1016/j.cellsig.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G., Delarue F., Burcklé C., Bouzhir L., Giller T., Sraer J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Investig. 2002;09(11):1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balakumar P., Jagadeesh G. Cardiovascular and renal pathologic implications of prorenin, renin, and the (pro) renin receptor: promising young players from the old renin-angiotensin-aldosterone system. J Cardiovasc Pharmacol. 2010;56(5):570–579. doi: 10.1097/fjc.0b013e3181f21576. [DOI] [PubMed] [Google Scholar]
- 10.Fisher N.D., Hollenberg N.K. Renin inhibition: what are the therapeutic opportunities? J Am Soc Nephrol. 2005;16(3):592–599. doi: 10.1681/ASN.2004100874. [DOI] [PubMed] [Google Scholar]
- 11.Navajyothi C., Prasanthi K., Rajya Lakshmi K., Ravalli R. Early diagnosis of hypertension: Research and Reviews: Journal of Hospital and Clinical Pharmacy. 2015;1(1):11–18. [Google Scholar]
- 12.Gradman A.H., Kad R. Renin inhibition in hypertension. J Am Coll Cardiol. 2008;51(5):519–528. doi: 10.1016/j.jacc.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Ocaranza M.P., Riquelme J.A., García L. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17(2):116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balakumar P., Anand-Srivastava M.B., Jagadeesh G. Renin-angiotensin-aldosterone: an inclusive, an invigorative, an interactive and an interminable system. Pharmacol Res. 2017;125(Part A):1–3. doi: 10.1016/j.phrs.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;5(1):1–2. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. J Am Medical Associat. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 17.Poulsen K., Burton J., Haber E. Competitive inhibitors of renin. Biochemistry. 1973;12(20):3877–3882. doi: 10.1021/bi00744a013. [DOI] [PubMed] [Google Scholar]
- 18.Sielecki A.R., Hayakawa K., Fujinaga M. Structure of recombinant human renin, a target for cardiovascular-active drugs, at 2.5A resolution. Science. 1989;243(4896):1346–1351. doi: 10.1126/science.2493678. [DOI] [PubMed] [Google Scholar]
- 19.Gross F., Lazar J., Orth H. Inhibition of the renin-angiotensinogen reaction by pepstatin. Science. 1972;175(4022):656–660. doi: 10.1126/science.175.4022.656. [DOI] [PubMed] [Google Scholar]
- 20.Skeggs L.T., Kahn J.R., Lentz K., Shumway N.P. The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med. 1957;106(3):439–453. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton J., Poulsen K., Haber E. Competitive inhibitors of renin Inhibitors effective at physiological pH. Biochemistry. 1975;14(17):3892–3898. doi: 10.1021/bi00688a024. [DOI] [PubMed] [Google Scholar]
- 22.Workman R.J., McKown M.M., Gregerman R.I. Renin Inhibition by proteins and peptides. Biochemistry. 1974;13(15):3029–3035. doi: 10.1021/bi00712a005. [DOI] [PubMed] [Google Scholar]
- 23.Turcotte J.G., Yu C.S., Lee H.L., Pavanaram S.K., Sen S., Smeby R.R. Synthesis of lysophosphatidylethanolamine analogs that inhibit renin activity. J Med Chem. 1975;18(12):1184–1190. doi: 10.1021/jm00246a003. [DOI] [PubMed] [Google Scholar]
- 24.Johnson R.L., Verschoor K. Inhibition of renin by angiotensinogen peptide fragments containing the hydroxy amino acid residue 5-amino-3-hydroxy-7-methyloctanoic acid. J Med Chem. 1983;26(10):1457–1462. doi: 10.1021/jm00364a019. [DOI] [PubMed] [Google Scholar]
- 25.Boger J., Payne L.S., Perlow D.S. Renin inhibitors. Syntheses of subnanomolar, competitive, transition-state analog inhibitors containing a novel analog of statine. J Med Chem. 1985;28(12):1779–1790. doi: 10.1021/jm00150a007. [DOI] [PubMed] [Google Scholar]
- 26.Fearon K., Spaltenstein A., Hopkins P.B., Gelb M.H. Fluoro ketone containing peptides as inhibitors of human renin. J Med Chem. 1987;30(9):1617–1622. doi: 10.1021/jm00392a016. [DOI] [PubMed] [Google Scholar]
- 27.Fisher J.F., Harrison A.W., Bundy G.L., Wilkinson K.F., Rush B.D., Ruwart M.J. Peptide to glycopeptide: glycosylated oligopeptide renin inhibitors with attenuated in vivo clearance properties. J Med Chem. 1991;34(10):3140–3143. doi: 10.1021/jm00114a026. [DOI] [PubMed] [Google Scholar]
- 28.Rich D.H. Pepstatin-derived inhibitors of aspartic proteinases. A close look at an apparent transition-state analog inhibitor. J Med Chem. 1985;28(3):263–273. doi: 10.1021/jm00381a001. [DOI] [PubMed] [Google Scholar]
- 29.Johnson R.L. Inhibition of renin by substrate analog inhibitors containing the olefinic amino acid 5 (S)-amino-7-methyl-3 (E)-octenoic acid. J Med Chem. 1984;27(10):1351–1354. doi: 10.1021/jm00376a023. [DOI] [PubMed] [Google Scholar]
- 30.Buehlmayer P., Caselli A., Fuhrer W. Synthesis and biological activity of some transition-state inhibitors of human renin. J Med Chem. 1988;31(9):1839–1846. doi: 10.1021/jm00117a027. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg S.H., Spina K.P., Condon S.L. Studies directed toward the design of orally active renin inhibitors. 2. Development of the efficacious, bioavailable renin inhibitor (2S)-2-benzyl-3-[[(1-methylpiperazin-4-yl) sulfonyl] propionyl]-3-thiazol-4-yl-L-alanine amide of (2S, 3R, 4S)-2-amino-1-cyclohexyl-3, 4-dihydroxy-6-methylheptane (A-72517) J Med Chem. 1993;36(4):460–467. doi: 10.1021/jm00056a006. [DOI] [PubMed] [Google Scholar]
- 32.Weber A.E., Steiner M.G., Krieter P.A. Highly potent, orally active diester macrocyclic human renin inhibitors. J Med Chem. 1992;35(21):3755–3773. doi: 10.1021/jm00099a004. [DOI] [PubMed] [Google Scholar]
- 33.Heitsch H., Henning R., Kleemann H.W. Renin inhibitors containing a pyridyl amino diol derived C-terminus. J Med Chem. 1993;36(19):2788–2800. doi: 10.1021/jm00071a009. [DOI] [PubMed] [Google Scholar]
- 34.Keserü G.M., Makara G.M. The influence of lead discovery strategies on the properties of drug candidates. Nat Rev Drug Discovery. 2009;8(3):203–212. doi: 10.1038/nrd2796. [DOI] [PubMed] [Google Scholar]
- 35.Oefner C., Binggeli A., Breu V. Renin inhibition by substituted piperidines: a novel paradigm for the inhibition of monomeric aspartic proteinases? Chem Biol. 1999;6(3):127–131. doi: 10.1016/s1074-5521(99)89004-8. [DOI] [PubMed] [Google Scholar]
- 36.Güller R., Binggeli A., Breu V. Piperidine-renin inhibitors compounds with improved physicochemical properties. Bioorg Med Chem Lett. 1999;9(10):1403–1408. doi: 10.1016/s0960-894x(99)00196-1. [DOI] [PubMed] [Google Scholar]
- 37.Holsworth D.D., Cai C., Cheng X.M. Ketopiperazine-based renin inhibitors: optimization of the “C” ring. Bioorg Med Chem Lett. 2006;16(9):2500–2504. doi: 10.1016/j.bmcl.2006.01.084. [DOI] [PubMed] [Google Scholar]
- 38.Yuan L., Wu J., Aluko R.E., Ye X. Kinetics of renin inhibition by sodium houttuyfonate analogs. Biosci Biotechnol Biochem. 2006;70(9):2275–2280. doi: 10.1271/bbb.60213. [DOI] [PubMed] [Google Scholar]
- 39.Webb R.L., Schiering N., Sedrani R., Maibaum J. Direct renin inhibitors as a new therapy for hypertension. J Med Chem. 2010;53(21):7490–7520. doi: 10.1021/jm901885s. [DOI] [PubMed] [Google Scholar]
- 40.Wood J.M., Maibaum J., Rahuel J. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003;308(4):698–705. doi: 10.1016/s0006-291x(03)01451-7. [DOI] [PubMed] [Google Scholar]
- 41.Maibaum J., Feldman D.L. Case history on Tekturna/Rasilez(Aliskiren), a highly efficacious direct oral renin inhibitor as a new therapy for hypertension. Annu Rep Med Chem. 2009;44:105–127. [Google Scholar]
- 42.Holsworth D.D., Jalaie M., Belliotti T. Discovery of 6-ethyl-2, 4-diaminopyrimidine-based small molecule renin inhibitors. Bioorg Med Chem Lett. 2007;17(13):3575–3580. doi: 10.1016/j.bmcl.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 43.Pool J.L. Direct renin inhibition: focus on aliskiren. J Managed Care Pharmacy. 2007;13(8):21–33. doi: 10.18553/jmcp.2007.13.s8-b.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezençon O., Bur D., Weller T., Richard-Bildstein S., Remen L., Sifferlen T. Design and preparation of potent, nonpeptidic, bioavailable renin inhibitors. J Med Chem. 2009;52(12):3689–3702. doi: 10.1021/jm900022f. [DOI] [PubMed] [Google Scholar]
- 45.Göschke R., Stutz S., Rasetti V. Novel 2, 7-dialkyl-substituted5(S)-amino-4(S)-hydroxy-8-phenyl-octanecarboxamide transition state peptidomimetics are potent and orally active inhibitors of human renin. J Med Chem. 2007;50(20):4818–4831. doi: 10.1021/jm070314y. [DOI] [PubMed] [Google Scholar]
- 46.Maibaum J., Stutz S., Göschke R. Structural modification of the P2 ‘position of 2, 7-dialkyl-substituted 5 (S)-amino-4 (S)-hydroxy-8-phenyl-octanecarboxamides: the discovery of aliskiren, a potent nonpeptide human renin inhibitor active after once daily dosing in marmosets. J Med Chem. 2007;50(20):4832–4844. doi: 10.1021/jm070316i. [DOI] [PubMed] [Google Scholar]
- 47.Jensen C., Herold P., Brunner H.R. Aliskiren: the first renin inhibitor for clinical treatment. Nat Rev Drug Discovery. 2008;7(5):399–410. doi: 10.1038/nrd2550. [DOI] [PubMed] [Google Scholar]
- 48.Mori Y., Ogawa Y., Mochizuki A. Design and discovery of new (3S, 5R)-5-[4-(2-chlorophenyl)-2, 2-dimethyl-5-oxopiperazin-1-yl] piperidine-3-carboxamides as potent renin inhibitors. Bioorg Med Chem Lett. 2012;22(24):7677–7682. doi: 10.1016/j.bmcl.2012.09.103. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura Y., Fujimoto T., Ogawa Y. Discovery of DS-8108b, a novel orally bioavailable renin inhibitor. ACS Med Chem Lett. 2012;3(9):754–758. doi: 10.1021/ml300168e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokokawa F. Recent progress on the discovery of non-peptidic direct renin inhibitors for the clinical management of hypertension. Expert Opin Drug Discov. 2013;8(6):673–690. doi: 10.1517/17460441.2013.791279. [DOI] [PubMed] [Google Scholar]
- 51.Lorthiois E., Breitenstein W., Cumin F. The discovery of novel potent trans-3, 4-disubstituted pyrrolidine inhibitors of the human aspartic protease renin from in silico three-dimensional (3D) pharmacophore searches. J Med Chem. 2013;56(6):2207–2217. doi: 10.1021/jm3017078. [DOI] [PubMed] [Google Scholar]
- 52.Chen A., Bayly C., Bezençon O. Design and optimization of a substituted amino propanamide series of renin inhibitors for the treatment of hypertension. Bioorg Med Chem Lett. 2010;20(7):2204–2209. doi: 10.1016/j.bmcl.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 53.Imaeda Y., Tokuhara H., Fukase Y. Discovery of TAK-272: a novel, potent, and orally active renin inhibitor. ACS Med Chem Lett. 2016;7(10):933–938. doi: 10.1021/acsmedchemlett.6b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hara T., Nishimura S., Yamamoto T. TAK-272 (imarikiren), a novel renin inhibitor, improves cardiac remodeling and mortality in a murine heart failure model. PLoS ONE. 2018;13(8):1–16. doi: 10.1371/journal.pone.0202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheiper B., Matter H., Steinhagen H. Discovery and optimization of a new class of potent and non-chiral indole-3-carboxamide-based renin inhibitors. Bioorg Med Chem Lett. 2010;20(21):6268–6272. doi: 10.1016/j.bmcl.2010.08.092. [DOI] [PubMed] [Google Scholar]
- 56.McMurray J.J., Pitt B., Latini R. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circulation. Heart Failure. 2008;1(1):17–24. doi: 10.1161/CIRCHEARTFAILURE.107.740704. [DOI] [PubMed] [Google Scholar]
- 57.Barrios V., Escobar C. Aliskiren: a new drug for an old problem. Cardiovascular & Hematological Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Cardiovascular & Hematological Agents) 2010;8(1):1–10. doi: 10.2174/187152510790796174. [DOI] [PubMed] [Google Scholar]
- 58.Wal P., Wal A., Rai A.K., Dixit A. Aliskiren: an orally active renin inhibitor. J Pharm Bioallied Sci. 2011;3(2):189–193. doi: 10.4103/0975-7406.80764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Şen S., Sabirli S., Ozyigit T., Uresin Y. Aliskiren: review of efficacy and safety data with focus on past and recent clinical trials. Therapeutic Adv Chronic Dis. 2013;4(5):232–241. doi: 10.1177/2040622313495288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imanishi T., Tsujioka H., Ikejima H. Renin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension. 2008;52(3):563–572. doi: 10.1161/HYPERTENSIONAHA.108.111120. [DOI] [PubMed] [Google Scholar]
- 61.Whaley-Connell A., Nistala R., Habibi J. Comparative effect of direct renin inhibition and AT1R blockade on glomerular filtration barrier injury in the transgenic Ren2 rat. Am J Physiol-Renal Physiol. 2009;298(3):655–661. doi: 10.1152/ajprenal.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Habibi J., Whaley-Connell A., Hayden M.R. Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic Ren2 rat. Endocrinology. 2008;149(11):5643–5653. doi: 10.1210/en.2008-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solomon S.D., Appelbaum E., Manning W.J. Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119(4):530–537. doi: 10.1161/CIRCULATIONAHA.108.826214. [DOI] [PubMed] [Google Scholar]
- 64.Schmerbach K., Pfab T., Zhao Y. Effects of aliskiren on stroke in rats expressing human renin and angiotensinogen genes. PLoS ONE. 2010;5(11) doi: 10.1371/journal.pone.0015052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miao J., Wang L., Zhang X. Protective effect of aliskiren in experimental ischemic stroke: up-regulated p-PI3K, p-AKT, Bcl-2 expression, attenuated bax expression. Neurochem Res. 2016;41(9):2300–2310. doi: 10.1007/s11064-016-1944-7. [DOI] [PubMed] [Google Scholar]
- 66.Panahpour H., Terpolilli N.A., Schaffert D., Culmsee C., Plesnila N. Central application of Aliskiren, a renin inhibitor, improves outcome after experimental stroke independent of its blood pressure lowering effect. Front Neurol. 2019;10(1) doi: 10.3389/fneur.2019.00942. article 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh V.P., Le B., Khode R., Baker K.M., Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57(12):3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frampton J.E., Curran M.P. Aliskiren; a review of its use in the management of hypertension. Drugs. 2007;67(12):1767–1792. doi: 10.2165/00003495-200767120-00008. [DOI] [PubMed] [Google Scholar]
- 69.Wiggins K.J., Kelly D.J. Aliskiren: a novel renoprotective agent or simply an alternative to ACE inhibitors? Kidney Int. 2009;76(1):23–31. doi: 10.1038/ki.2009.105. [DOI] [PubMed] [Google Scholar]
- 70.Pimenta E., Oparil S. Role of aliskiren in cardio-renal protection and use in hypertensives with multiple risk factors. Vascular Health Risk Manage. 2009;5(1):453–463. doi: 10.2147/vhrm.s4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisher N.D., Jan Danser A.H., Nussberger J., Dole W.P., Hollenberg N.K. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation. 2008;117(25):3199–3205. doi: 10.1161/CIRCULATIONAHA.108.767202. [DOI] [PubMed] [Google Scholar]
- 72.Solomon S.D., Hee Shin S., Shah A. Effect of the direct renin inhibitor aliskiren on left ventricular remodelling following myocardial infarction with systolic dysfunction. Eur Heart J. 2011;32(10):1227–1234. doi: 10.1093/eurheartj/ehq522. [DOI] [PubMed] [Google Scholar]
- 73.Krum H., McMurray J.J., Abraham W.T. The Aliskiren Trial to Minimize Outcomes in Patients with Heart failure trial (ATMOSPHERE): revised statistical analysis plan and baseline characteristics. Eur J Heart Fail. 2015;17(10):1075–1083. doi: 10.1002/ejhf.408. [DOI] [PubMed] [Google Scholar]
- 74.Parving H.H., Brenner B.M., McMurray J.J. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 75.Gheorghiade M., Böhm M., Greene S.J. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. J Am Med Association. 2013;309(11):1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 76.Nicholls S.J., Bakris G.L., Kastelein J.J. Effect of aliskiren on progression of coronary disease in patients with prehypertension: the AQUARIUS randomized clinical trial. J Am Med Assoc. 2013;310(11):1135–1144. doi: 10.1001/jama.2013.277169. [DOI] [PubMed] [Google Scholar]
- 77.White W.B., Bresalier R., Kaplan A.P. Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertension. 2010;12(10):765–775. doi: 10.1111/j.1751-7176.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]