Abstract
A real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for the detection of bovine coronavirus (BCoV) RNA in clinical samples is described. The assay is based on TaqMan technology, consisting of two primers and one probe labeled with the reporter dye 6-carboxyfluorescein that binds selectively to the transmembrane-protein gene of BCoV. The BCoV real-time RT-PCR assay was able to detect the tested BCoV and BCoV-like viruses (canine respiratory coronavirus and bubaline coronavirus), whereas other common viral pathogens of cattle were not recognised by the established oligonucleotide set, thus showing that the test was specific for bovine-like CoVs. The detection limit of the assay was 20 BCoV RNA copies (1-log higher with respect to traditional gel-based RT-PCR) and the reproducibility was satisfactory, thus allowing for a sensitive and accurate measurement of the viral RNA load in clinical samples. Two hundred and twenty clinical specimens (92 rectal, 82 nasal and 46 ocular swabs) were subjected to gel-based and real-time RT-PCR. By conventional amplification, 43 rectal, 54 nasal and 34 ocular samples tested positive, whereas the TaqMan assay was able to detect the BCoV nucleic acid in 49 rectal, 60 nasal and 37 ocular swabs. The rapidity and high throughput of the BCoV TaqMan assay makes this method a powerful tool for a sensitive and specific diagnosis of BCoV infection in cattle.
Keywords: Cattle, Bovine coronavirus, Diagnosis, Real-time RT-PCR
1. Introduction
Bovine coronavirus (BCoV) is a group 2 coronavirus (CoV) that is responsible for different clinical forms of disease in cattle, including enteritis in newborn calves, “winter dysentery” in adult cows, and respiratory signs in calves and cows. The same virus strain could also be responsible for the simultaneous appearance of enteric and respiratory disease in the same animals as well as in calves and cows (Decaro et al., 2008a, Decaro et al., 2008b). It is thought that BCoV is able to cross the species barrier very frequently, giving rise to bovine-like CoVs which can infect different mammals. The bovine origin of some group 2a CoVs has been suggested for viruses such as human coronavirus (HCoV) OC43 (Vijgen et al., 2005), porcine hemagglutinating encephalomyelitis virus (PHEV) (Vijgen et al., 2006), canine respiratory coronavirus (CRCoV) (Erles et al., 2003) and other ruminant CoVs (Hasoksuz et al., 2007, Jin et al., 2007, Decaro et al., 2008c).
Several tools are available for the traditional diagnosis of BCoV infection, including electron microscopy (EM) (Bulgin et al., 1989), virus isolation (VI) on human rectal tumor (HRT-18) and Madin Darby bovine kidney (MDBK) cells (Dea et al., 1980), hemagglutination (HA) (Sato et al., 1977), and enzyme-linked immunosorbent assay (ELISA) (Sato and Akashi, 1993, Silva et al., 1999). However, these techniques have been proven to be poorly sensitive and often inconclusive due to the presence of pleomorphic CoV-like particles in clinical samples, virus instability in the environment, time-consuming procedures, or presence of maternally derived antibodies in the faeces (Sato et al., 1977, Dea et al., 1980, Saif, 1990, Sato and Akashi, 1993). Because of its higher sensitivity and versatility, the detection of viral nucleic acid by polymerase chain reaction (PCR) has been established as the diagnostic gold standard for a number of infectious diseases. Several reverse transcriptase (RT)-PCR-based methods have been developed for the detection and identification of BCoV and BCoV-like RNA in faecal and respiratory specimens of cattle (Tsunemtitsu et al., 1999, Cho et al., 2001, Erles et al., 2003, Takiuchi et al., 2006). However, none of those RT-PCR assays were designed to be quantitative. In addition, PCR assays are frequently exposed to risks of carryover contamination, especially when a high sample throughput is required.
In this paper, the development of a real-time RT-PCR assay based on TaqMan technology is reported for the rapid and sensitive diagnosis of BCoV infection and accurate quantification of BCoV nucleic acid in diagnostic samples.
2. Materials and methods
2.1. Oligonucleotide design and synthesis
The transmembrane (M) gene sequences of BCoV strains were retrieved from the GenBank database (http://www.ncbi.nlm.nih.gov/Genbank/index.html) and aligned using the BioEdit software package (Hall, 1999). The primers and TaqMan probe were designed using the Beacon Designer Software, Version 2.0 (Premier Biosoft International, Palo Alto, CA, USA) to amplify a conserved 85-bp fragment within the aligned M genes. The primers and probe were synthesized by MWG Biotech AG (Ebersberg, Germany). The TaqMan probe was labeled with 6-carboxyfluorescein (FAM) at the 5′ end and with a nonfluorescent quencher 1 (NFQ1) at the 3′ end. The position and sequence of the primers and probe used for the assay are reported in Table 1 .
Table 1.
Oligonucleotides used in BCoV conventional and real-time RT-PCR amplifications
| Primer/probe | Sequence 5′–3′ | Sense | Positiona | Amplicon size (bp) |
|---|---|---|---|---|
| Sp 1b | CTTATAAGTGCCCCCAAACTAAAT | + | 25277–25300 | 622 |
| Sp 2b | CCTACTGTGAGATCACATGTTTG | − | 25876–25898 | |
| BCoV-Fc | CTGGAAGTTGGTGGAGTT | + | 29026–29043 | 85 |
| BCoV-Rc | ATTATCGGCCTAACATACATC | − | 29090–29110 | |
| BCoV-Pbc | FAMd-CCTTCATATCTATACACATCAAGTTGTT-BHQ1e | − | 29058–29085 | |
Oligonucleotide position is referred to the sequence of BCoV strain Mebus (GenBank accession no.: U00735).
Conventional RT-PCR (Erles et al., 2003).
Real-time RT-PCR.
FAM: 6-carboxyfluorescein.
BHQ1: black hole quencher 1.
2.2. Standard RNA for absolute quantification
To obtain a standard for the TaqMan assay, a 919-bp RT-PCR product containing the full-length M gene of BCoV strain 339/06 (Decaro et al., 2008b) was amplified using primer pair 28380F/29298R (Decaro et al., 2008c) and the RT-PCR product was cloned into pCR®T7/NT-TOPO vector (Invitrogen Srl, Milan, Italy) and transcribed with RiboMAX™ Large Scale RNA Production System-T7 (Promega Italia, Milan, Italy) from the T7 promoter, according to the manufacturer's guidelines. After DNase treatment to remove residues of plasmid DNA, the transcripts were purified using a commercial column (QIAamp® RNA Easy kit, Qiagen S.p.A., Milan, Italy) and quantified by spectrophotometric analysis. Ten-fold dilutions of the RNA transcript, representing 100 to 109 copies RNA μl−1 of template, was carried out in a mixed faecal/nasal swab suspension from a calf which tested negative for BCoV by HA and gel-based RT-PCR. Aliquots of each dilution were frozen at −70 °C and used only once.
2.3. Field samples collection and preparation
A total of 220 samples, including 92 rectal, 82 nasal and 46 ocular swabs collected from cattle with enteric and/or respiratory signs, were analysed. The faecal and nasal swabs were homogenised (10%, w/v) in Dulbecco's minimal essential medium (D-MEM). Sample suspensions were clarified by a brief centrifugation at high speed in a micro-centrifuge and aliquots of 140 μl of the supernatants were used for RNA extraction.
Viral RNA was extracted from each sample suspension with QIAamp® Viral RNA Mini Kit (Qiagen S.p.A.) in accordance with the manufacturer's protocol. Template RNAs were eluted in 50 μl of elution buffer water and stored at −70 °C prior to use.
2.4. Real-time RT-PCR
Duplicates of the standard dilutions and RNA templates were subjected simultaneously to reverse transcription with the GeneAmp® RNA PCR kit (Applied Biosystems, Applera Italia, Monza, Italy). One microlitre of each duplicate of the standard dilutions or template RNA was reverse transcribed in a 20-μl reaction volume containing PCR buffer 1× (KCl 50 mM, Tris–HCl 10 mM, pH 8.3), MgCl2 5 mM, 1 mM of each deoxynucleotide (dATP, dCTP, dGTP, dTTP), RNase Inhibitor 1 U, MuLV reverse transcriptase 2.5 U, random hexamers 2.5 U. Synthesis of c-DNA was carried out at 42 °C for 30 min, followed by a denaturation step at 99 °C for 5 min.
Real-time PCR for the simultaneous detection and quantification of BCoV RNA was performed on a 7500 Real-time PCR System (Applied Biosystems) with iTaq™ Supermix added with ROX (Bio-Rad Laboratories Srl, Milan, Italy). The quantitative assay targeting the M gene was conducted in a 50 μl reaction mixture containing 25 μl of master mix, 600 nM of primers BCoV-F and BCoV-R, 200 nM of probe BCoV-Pb and 20 μl of c-DNA. The thermal profile consisted of activation of iTaq DNA polymerase at 95° C for 10 min, followed by 45 cycles of denaturation at 95 °C for 15 s annealing for 30 s and extension at 60° C for 1 min.
The increase in the fluorescent signal was registered during the extension step of the reaction and the data was analysed with the appropriate sequence detector software (7500 System Software v.1.3.1, Applied Biosystems).
2.5. Internal control
In order to verify the absence of RNA losses during the extraction step and the presence of RT-PCR inhibitors in the RNA templates, an internal control (IC), consisting of an RNA synthetic transcript containing the M gene of canine coronavirus (CCoV) type II (Decaro et al., 2005), was added to the lysis buffer (AVL Buffer, Qiagen S.p.A.) at a concentration of 10,000 RNA copies ml−1 of buffer prior to nucleic acid extraction. The fixed amount of the IC added to each sample had been calculated to give a mean C T value in a genotype-specific real-time RT-PCR assay (Decaro et al., 2005) of 34.18 with a S.D. of 0.65 as calculated by 50 separate runs. Samples in which the C T value for the IC was >35.48 (average plus 2S.D.) were excluded from the analysis.
2.6. Evaluation of real-time RT-PCR performances
To exclude cross-reactivities between BCoV and other viral pathogens responsible for enteric and/or respiratory diseases of ruminants, the specificity of the assay was evaluated by testing isolates of the following viruses: bovine rotaviruses, bovine viral diarrhea virus, bovine respiratory syncytial virus, bovine parainfluenza virus, and bovine herpesvirus types 1 and 4. The ability of the assay to detect the nucleic acid of bovine-like CoVs was assessed by testing the strictly related CRCoV (Decaro et al., 2007) and bubaline coronavirus (BuCoV) (Decaro et al., 2008c).
Faecal, nasal and ocular samples collected from 10 uninfected calves as well as sterile water were also included in the analysis as negative controls and blanks, respectively.
To evaluate the detection limit of the real-time PCR assay, 10-fold dilutions of a faecal sample containing 2 × 107 copies BCoV RNA μl−1 were made in a mixed faecal/nasal sample homogenate from a BCoV-negative calf and tested subsequently.
Serial 10-fold dilutions of standard RNA which contained from 101 to 109 copies RNA transcripts and the corresponding C T values were used to plot the standard curve for BCoV RNA absolute quantification.
Reproducibility of the assay was evaluated by testing repeatedly clinical samples containing BCoV RNA titres spanning the whole range covered by real-time RT-PCR, as described previously (Decaro et al., 2004, Decaro et al., 2005, Elia et al., 2006). The intra-assay reproducibility was evaluated by testing the same samples 10 times in the same experiment, whereas the inter-assay reproducibility was assessed by testing the same samples in 10 independent experiments. CVs were calculated by dividing the standard deviation of each tested sample by its mean and multiplying that result by 100.
2.7. Gel-based RT-PCR
The detection of BCoV RNA in clinical samples and M-gene RNA transcript dilutions was carried out using SuperScript™ One-Step RT-PCR for Long Templates (Life Technologies, Invitrogen, Milan, Italy) and primers specific for the spike-protein gene that are able to detect bovine-like CoVs (Erles et al., 2003, Decaro et al., 2007, Decaro et al., 2008c). The following thermal protocol was used: reverse transcription at 50 °C for 30 min, inactivation of Superscript II RT at 94 °C for 2 min, 45 cycles of 94 °C for 30 s, 55 °C for 30 s, 68 °C for 30 s, with a final extension at 68 °C for 10 min. The PCR products were detected by electrophoresis in 1.5% agarose gels and visualised under UV light after ethidium bromide staining. The position and sequence of the primers used for conventional amplification are reported in Table 1.
3. Results
3.1. Analytical performance of the BCoV real-time RT-PCR assay
The template controls and BCoV-negative specimens did not produce any detectable fluorescence signal. The other tested viral pathogens were not detected with the exception of the bovine-like CoVs CRCoV and BuCoV, confirming that the real-time RT-PCR assay is specific for BCoV and BCoV-like viruses.
The detection limit of the assay was assessed as 2 × 101 RNA copies μl−1 of template, whereas gel-based PCR was able to detect up to 2 × 102 copies μl−1 of template.
Ten-fold dilutions of standard RNA were used to construct a standard curve covering a linear range of nine orders of magnitude (from 101 to 109 copies of standard RNA) and linearity was observed over the entire quantification range (slope = −3.366).
To determine the reproducibility of the assay, intra-assay and inter-assay coefficients of variation (CVs) were calculated (Fig. 1 ). Intra-assay CVs ranged from 18.42% (samples containing 7 × 107 DNA copies) to 41.03% (2 × 102 DNA copies), while the inter-assay CVs ranged from 28.93% (3 × 103 DNA copies) to 48.57% (2 × 102 DNA copies).
Fig. 1.
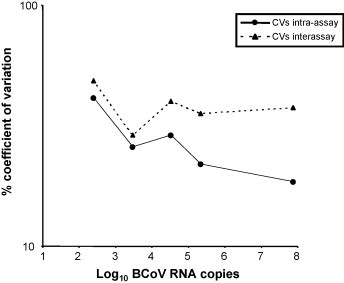
Coefficients (CVs) of variation intra-assay and inter-assay over the dynamic range of the BCoV real-time RT-PCR assay. Field samples containing approximately 2 × 102, 3 × 103, 3 × 104, 2 × 105 and 7 × 107 BCoV RNA copies were tested 10 times in the same run (CVs intra-assay) or in 10 consecutive runs (CVs inter-assay).
3.2. Internal control detection
The IC was detected in all the examined samples, with C T values below the threshold value of 35.48, thus ruling out relevant RNA losses during nucleic acid extraction or DNA polymerase inhibition during real-time PCR amplification.
3.3. Analysis of clinical samples
The results of the analysis of 220 clinical samples collected from cattle with enteric and/or respiratory disease are summarised in Fig. 2 . By using gel-based RT-PCR, 43/92 rectal, 54/82 nasal and 34/46 ocular swabs were found to contain BCoV RNA. Conversely, 49 rectal, 60 nasal and 37 ocular swabs tested positive for BCoV by real-time RT-PCR. Only one faecal sample tested positive by conventional amplification and negative by real-time analysis, whereas seven rectal, six nasal and three ocular swabs were found to be positive by the TaqMan assay and negative by conventional amplification. The number of samples in agreement between the two tests were 84 for the faecal swabs (42 positive and 42 negative samples), 76 for the respiratory specimens (54 positive and 22 negative samples) and 43 for the ocular swabs (34 positive and 9 negative samples). Totally, 203 samples were in agreement by both tests (130 positive and 73 negative samples). The analysed samples contained a wide range of BCoV RNA amounts per μl of template, from 2.20 × 101 to 5.53 × 108 (rectal swabs), from 3.15 × 101 to 8.09 × 108 (nasal swabs), and from 3.87 × 101 to 8.04 × 106 (ocular swabs).
Fig. 2.
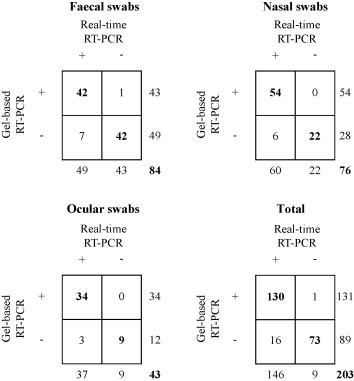
Comparison between gel-based and real-time RT-PCR assays carried out on bovine clinical samples. Numbers indicate the samples positive (+) or negative (−) for BCoV. Results according to both techniques are shown in bold.
4. Discussion
A real-time RT-PCR assay for the rapid and sensitive detection of the BCoV RNA in clinical samples of cattle was established. The assay was shown to be reproducible and linear over a range of 9 orders of magnitude, from 101 to 109 RNA copies, thus ensuring an accurate measurement of BCoV RNA loads in clinical samples. When compared with a classical RT-PCR protocol, the processing time required by TaqMan RT-PCR is shorter, the contamination risks are lower because of the lack of post-PCR steps and the specificity is increased by the probe hybridisation. The assay was also able to detect the nucleic acid of bovine-like CoVs (CRCoV and BuCoV). Other BCoV-like CoVs, such as HCoV-OC43 and PHEV, that were not included in our analyses, should be tested in order to assess whether they can be identified by the established assay. Although those viruses are not available in the laboratory where the BCoV TaqMan assay was developed, analysis of the M-gene sequences of strains from different geographical areas led us to predict a probable detection of these bovine-like CoVs.
The BCoV TaqMan assay was found to be 1-log more sensitive than gel-based RT-PCR. In fact, when the clinical samples collected from affected cattle were processed, real-time RT-PCR was able to detect BCoV RNA in seven rectal, six nasal and three ocular swabs that tested negative by conventional amplification. In contrast, only one faecal sample gave a positive result by gel-based RT-PCR and tested negative by the TaqMan assay. Sequencing of the M gene of this BCoV revealed the presence of two mismatches in the probe-binding region that prevented the correct annealing of the TaqMan probe (data not shown). CoVs are thought to mutate at high frequency like most RNA viruses as a consequence of the high error rates of the RNA polymerase that are predicted to accumulate several base substitutions per round of replication (Jarvis and Kirkegaard, 1991, Lai and Holmes, 2001). The high strain variability of CoVs has been identified as a limiting factor for efficient PCR amplification of their genomic RNA. For instance, molecular methods to detect all feline calicivirus strains have not yet been developed even when conserved regions of the viral replicase gene complex were chosen as a target for amplification (Marsilio et al., 2005). In order to reduce false-negative results related to strain variation, TaqMan oligonucleotides were designed in a very conserved region of the M gene. The same gene was taken into account for the design of primer/probe sets which were used in real-time RT-PCR assays for CCoVs, that proved to be very sensitive ensuring correct amplification of strains with different geographic origin (Decaro et al., 2004, Decaro et al., 2005).
Recently, a real-time RT-PCR assay based on SYBR Green chemistry has been established for the detection of CoVs of all the antigenic groups (Escutenaire et al., 2007). Similar to the TaqMan assay described in this paper, the SYBR Green assay was able to detect as few as 10 CoV RNA copies. However, unlike the BCoV assay, that assay was unable to detect specifically BCoV and BCoV-like viruses, so it would appear more useful for the detection of new, uncharacterised CoVs than for specific identification of bovine-like CoVs in clinical samples.
In conclusion, a real-time RT-PCR assay for the rapid and sensitive detection of BCoV RNA was developed. This assay can be used as powerful tool for the simultaneous analysis of several samples (up to 96 samples in the same plate) in a short time. Considering the difficulties of BCoV isolation and titration on cell cultures, the established assay can be helpful in BCoV-pathogenesis studies and vaccine trials.
Acknowledgements
This work was supported by grants from University of Bari, Italy: project ex 60% 2007 “Messa a punto di un sistema real-time RT-PCR per la identificazione e la quantificazione dell’RNA del coronavirus bovino”. The authors are grateful to P.J. Collins (CIT Department of Biology, Cork, Ireland) for the English revision of the manuscript.
References
- Bulgin M.S., Ward A.C., Barrett D.P., Lane V.M. Detection of rotavirus and coronavirus shedding in two beef cow herds in Idaho. Can. Vet. J. 1989;30:235–239. [PMC free article] [PubMed] [Google Scholar]
- Cho K.O., Hasoksuz M., Nielsen P.R., Chang K.O., Lathrop S., Saif L.J. Cross-protection studies between respiratory and calf diarrhea and winter dysentery coronavirus strains in calves and RT-PCR and nested PCR for their detection. Arch. Virol. 2001;146:2401–2419. doi: 10.1007/s007050170011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea S., Roy R.S., Begin M.E. Bovine coronavirus isolation and cultivation in continuous cell lines. Am. J. Vet. Res. 1980;41:30–38. [PubMed] [Google Scholar]
- Decaro N., Pratelli A., Campolo M., Elia G., Martella V., Tempesta M., Buonavoglia C. Quantitation of canine coronavirus RNA in the faeces of dogs by TaqMan RT-PCR. J. Virol. Methods. 2004;119:145–150. doi: 10.1016/j.jviromet.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Ricci D., Elia G., Desario C., Campolo M., Cavaliere N., Di Trani L., Tempesta M., Buonavoglia C. Genotype-specific fluorogenic RT-PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. J. Virol. Methods. 2005;130:72–78. doi: 10.1016/j.jviromet.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Mari V., Lucente M.S., Cordioli P., Colaianni M.L., Martella V., Buonavoglia C. Serological and molecular evidence that canine respiratory coronavirus is circulating in Italy. Vet. Microbiol. 2007;121:225–230. doi: 10.1016/j.vetmic.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Desario C., Cirone F., D’abramo M., Lorusso E., Greco G., Mari V., Colaianni M.L., Elia G., Martella V., Buonavoglia C. Respiratory disease associated with bovine coronavirus infection in cattle herds in Southern Italy. J. Vet. Diagn. Invest. 2008;20:28–32. doi: 10.1177/104063870802000105. [DOI] [PubMed] [Google Scholar]
- Decaro N., Mari V., Desario C., Campolo M., Elia G., Martella V., Greco G., Cirone F., Colaianni M.L., Cordioli P., Buonavoglia C. Severe outbreak of bovine coronavirus infection in dairy cattle during the warmer season. Vet. Microbiol. 2008;126:30–39. doi: 10.1016/j.vetmic.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Elia G., Campolo M., Mari V., Desario C., Lucente M.S., Lorusso A., Greco G., Corrente M., Tempesta M., Buonavoglia C. Biological and genetic analysis of a bovine-like coronavirus isolated from water buffalo (Bubalus bubalis) calves. Virology. 2008;370:213–222. doi: 10.1016/j.virol.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E., Di Trani L., Buonavoglia C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods. 2006;136:171–176. doi: 10.1016/j.jviromet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escutenaire S., Mohamed N., Isaksson M., Thorén P., Klingeborn B., Belák S., Berg M., Blomberg J. SYBR Green real-time reverse transcription-polymerase chain reaction assay for the generic detection of coronaviruses. Arch. Virol. 2007;152:41–58. doi: 10.1007/s00705-006-0840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hasoksuz M., Alekseev K., Vlasova A., Zhang X., Spiro D., Halpin R., Wang S., Ghedin E., Saif L.J. Biologic, antigenic, and full-length genomic characterization of a bovine-like coronavirus isolated from a giraffe. J. Virol. 2007;81:4981–4990. doi: 10.1128/JVI.02361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis T.C., Kirkegaard K. The polymerase in its labyrinth: mechanisms and implications of RNA recombination. Trends Genet. 1991;7:186–191. doi: 10.1016/0168-9525(91)90434-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Cebra C.K., Baker R.J., Mattson D.E., Cohen S.A., Alvarado D.E., Rohrmann F. Analysis of the genome sequence of an alpaca coronavirus. Virology. 2007;365:198–203. doi: 10.1016/j.virol.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C., Holmes K.V. Coronaviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th edition. Lippincott Williams and Wilkins; Philadelphia, PA: 2001. pp. 1163–1185. [Google Scholar]
- Marsilio F., Di Martino B., Decaro N., Buonavoglia C. Nested PCR for the diagnosis of calicivirus infections in the cat. Vet. Microbiol. 2005;105:1–7. doi: 10.1016/j.vetmic.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Saif L.J. A review of evidence implicating bovine coronavirus in the etiology of winter dysentery cows: an enigma resolved? Cornell Vet. 1990;80:303–311. [PubMed] [Google Scholar]
- Sato K., Inaba Y., Kurogi H., Takahashi E., Satoda K., Omori T., Matsumoto M. Hemagglutination by calf diarrhea coronavirus. Vet. Microbiol. 1977;2:83–87. [Google Scholar]
- Sato M., Akashi H. Detection of bovine coronavirus by enzyme-linked immunosorbent assay using monoclonal antibodies. J. Vet. Med. Sci. 1993;55:771–774. doi: 10.1292/jvms.55.771. [DOI] [PubMed] [Google Scholar]
- Silva M.R., O’Reilly K.L., Lin X., Stine L., Storz J. Sensitivity comparison for detection of respiratory bovine coronaviruses in nasal samples from feedlot cattle by ELISA and isolation with the G clone of HRT-18 cells. J. Vet. Diagn. Invest. 1999;11:15–19. doi: 10.1177/104063879901100102. [DOI] [PubMed] [Google Scholar]
- Takiuchi E., Stipp D.T., Alfieri A.F., Alfieri A.A. Improved detection of bovine coronavirus N gene in faeces of calves infected naturally by a semi-nested PCR assay and an internal control. J. Virol. Methods. 2006;131:148–154. doi: 10.1016/j.jviromet.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemtitsu H., Smith D.R., Saif L.J. Experimental inoculation of adult dairy cows with bovine coronavirus and detection of coronavirus in feces by RT-PCR. Arch. Virol. 1999;144:167–175. doi: 10.1007/s007050050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen L., Keyaerts E., Moes E., Thoelen I., Wollants E., Lemey P., Vandamme A.M., Van Ranst M. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005;79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen L., Keyaerts E., Lemey P., Maes P., Van Reeth K., Nauwynck H., Pensaert M., Van Ranst M. Evolutionary history of the closely related group 2 coronaviruses: porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J. Virol. 2006;80:7270–7274. doi: 10.1128/JVI.02675-05. [DOI] [PMC free article] [PubMed] [Google Scholar]


