Highlights
-
•
Harnessing the innate immunity can protect domestic animals from viruses.
-
•
Innate immune cells have potential capacity to afford protection against infection.
-
•
Understanding the innate and adaptive immunity will aid rational vaccine design.
Keywords: NK cells, Dendritic cells, Gamma delta T cells, TLR, FMDV, Innate immunity
Abstract
Pathogens in general and pathogenic viruses in particular have evolved a myriad of mechanisms to escape the immune response of mammalian species. Viruses that cause acute disease tend to bear characteristics that make them very contagious, as survival does not derive from chronicity of infection, but spread of disease throughout the herd. Foot-and-mouth disease virus (FMDV) is one of the most contagious viruses known. Upon infection of susceptible species, cloven-hoofed animals, the virus proliferates rapidly and causes a vesicular disease within 2–4 days. Disease symptoms resolve by 10 days to 2 weeks and in most cases, virus can no longer be detected. Periods of fever and viremia are usually brief, 1–3 days. In vivo control of virus infection and clearance of the virus during and following acute infection is of particular interest. The interaction of this virus with cells mediating the early, innate immune response has been analyzed in a number of recent studies. In most reports, the virus has a distinct inhibitory effect on the response of cells early in infection. Here we review these new data and discuss the dynamics of the interaction of virus with different cell types mediating the immune response to infection.
1. Introduction
While evidence has been gathering pointing to the importance of innate immune responses in shaping overall immunity against infection, in large animal immunology, the subject is still in its infancy. Lessons learned from the mouse system are helpful in understanding the execution of innate immune responses in large animals and provide valuable insights applicable to many other species. For this reason throughout the review, we frequently refer to established facts in other species to indicate areas in large animal immunology where data is lacking. This review focuses on studies that are now available to advance the understanding of innate immune responses of cattle and pigs following infection with important pathogens, particularly FMDV.
2. Cells mediating innate immune responses
Development of the critical cellular components of the innate immune system in cattle and swine has not been fully described. These include natural killer cells, NK; dendritic cells, DC; gamma delta T cells, γδT; B cells, macrophages, Mϕ, and granulocytes. We can only assume that development of these cells probably takes place in a similar manner as has been described for the most studied system, the mouse. However, there are likely to be important differences.
One prominent example is the number of γδ T cells in ruminants versus that in humans or mice. As reported by Yasuda et al. [1], in cattle, γδ T cells initially increase significantly in the dome region of the Peyer's patches during prenatal development and decrease following birth, but the reverse is observed in the intestinal villi where a few γδ T cells are observed during the prenatal development but increase after birth. However, it remains to be proven if these developmental differences are translated into functional differences. Moreover, according to Hein and Dudler [2], in sheep, establishment of a normal γδ T cell repertoire in the periphery is developmentally regulated and dependent on the continual presence of a functional thymus during ontogeny.
The situation with porcine γδ T cells is no less complex. At least 12 subsets of γδ T cells have been reported that belong to two major groups, (1) cluster of differentiation 4 (CD4)-γδ thymocytes that are further subdivided based on CD2/CD8αα expression, and (2) CD4+ γδ thymocytes that stably express CD1, CD2 and CD8αα, but are not exported to the periphery [3].
In the case of development of natural killer (NK) cells and dendritic cells (DC), until recently, we relied on the data derived in the mouse system since minimal data are available for the large animal species. However, since conditions of generating these cells in vitro have widely been described now [4], [5], advancement in characterizing these cells in large animal species and understanding how these cells develop and function to protect the host is now possible. In large animals, we also face yet another unknown, that being, the precise role the innate immune cells play. For instance, data in both mouse and humans show that individuals genetically lacking NK cells suffer from severe and recurrent infections [6], [7], [8]. We do not have such an example in large animals or that such a condition leads to a similar situation as that observed in humans or mice.
More recent data [9], [10], [11] in humans and mice introduce a new group of cells referred to as innate lymphoid cells (ILC) which includes NK cells. Other members are lymphoid tissue inducer cells (LTi) and natural cytotoxicity receptor 22 cells (NCR22). The later have characteristics of both NK and LTi cells and are referred to as NK receptor-positive (NKR+) LTi cells. ILC play a protective role in responses to infectious microorganisms, lymphoid tissue formation, tissue remodeling after injury or infection and homeostasis of tissue stromal cells. ILC appear to specialize in production of IFNγ (NK cells), IL-17 and/or IL-22 (LTi and NKR+ LTi cells). Additionally, in mice “natural helper” (NH) cells have been described, which produce IL-5 and IL-13 associated with Th2 helper cells. In large animals, we still do not have data suggesting the existence of the LTi cells or NKR+ LTi cells and we will not discuss these cells further in this review.
Finally, there is also the contribution of macrophages and granulocytes that phagocytose particles including bacteria and cell debris, particularly in sites of inflammation. These cells also have a prominent role in the resolution of inflammation; however, for this review we will focus on the recent advances in the role of NK, DC and γδ T cell responses during infection of cattle and pigs by viral agents, and refer to other animal species. The humoral factors of innate responses such as the complement, acute phase proteins, defensins, cathelicidins, pentraxins and heat shock proteins play a significant role in bacterial infections, but are less understood in relation to viral infection, especially in livestock species. As such, these are not included in this review, but rather we maintain our focus on cellular innate responses to viral infection.
3. Role of NK cells in innate response to infection
Natural killer (NK) cells are part of the innate immune system responsible for early elimination of pathogen-infected cells and thus preventing dissemination within the host and transmission to other hosts. With the availability of an antibody against bovine CD335 (NKp46) [12], the biology of bovine NK cells and their direct role in vaccination or infection with various animal pathogens has been analyzed.
Bovine NK cells are found in peripheral blood, spleen, lung, liver, lymph nodes and bone marrow [12], [13]. As in other species, bovine NK cells also express natural cytotoxicity receptors like CD335, produce IFNγ, lyse sensitive targets and appear to have a CD335+/CD2+/−/CD8+/−/CD3− phenotype [12], [14], [15]. Evidence that bovine NK cells are capable of lysing an infected target was initially demonstrated by Boysen et al. [16] and later by Klevar et al. [17], in their studies with Neospora caninum. Similar observations have been made by others regarding activation of NK cells by other microorganisms [18], [19]. Bovine NK cells also express a short form of natural-killer group 2, member D protein (NKG2D, an activating receptor found on NK and CD8+ T cells) which associates with signaling adaptors such as DNAX activation proteins of 10 kDa (DAP10) and 12 kDa, DAP12 [20]. Furthermore, NKG2D expressed on CD335+ cells in peripheral blood, mesenteric lymph nodes and intraepithelial cells binds to bovine MIC1 (bovine leukocyte antigen-like molecule 1) and MIC4. In vitro culture of bovine peripheral blood CD335+ cells with soluble MIC1 or MIC4, showed increased NK cell response in form of IFNγ production, indicating that bovine MIC1 and MIC4 are ligands for NK cell-expressed NKG2D [21]. A review by Boysen and Storset [22] tabulates many more receptors and cell markers expressed by bovine NK cells.
Unlike T cells, NK cells recognize their targets without the need for prior exposure and activation, in an antigen independent manner. Once activated, they show increased cytolytic, secretory and proliferative functions [23]. Besides the cytolytic activity against target cells, NK cells are important mediators of immune responses e.g., participating in immune responses controlled by dendritic cells [24]. These effector functions of NK cells are highly regulated by a balance between inhibiting and activating signals mediated through receptors harboring immunoreceptor tyrosine-based inhibitory motif (ITIM) or immunoreceptor tyrosine-based activation motif (ITAM), respectively, in their cytoplasmic tails [25].
During transportation of cattle, the stress response of these animals is characterized by activation of NK cells indicating that these play a role in innate response [26], [27]. In other infections of cattle such as N. caninum [17], an initial decrease in peripheral blood NK cells is observed in the early times of infection (4–6 days post infection). However, NK cells significantly increase later, at 11–15 days post infection, acting as early responders to invasion with this parasite. This study did not report if this increase affected the clearance of the parasite. According to Dennis et al. [18], NK cells are directly involved in the elimination of Mycobacterium bovis infected macrophages (MΦ). The NK cells interact with and enhance the capability of infected MΦ to produce IL-12 and nitric oxide (NO) and induce apoptosis in the infected cells and as such limit the replication of the intracellular bacteria. Inhibition of NK cell functions appears to be a common feature of viral infections in pigs. Mendoza [28] also found a similar characteristic of NK cells when mononuclear cells were cultured with African swine fever virus (ASFV). Similarly, porcine respiratory and reproduction syndrome virus infection affects the innate immune response of young pigs by inhibiting the function of peripheral blood NK cells [29].
The present experimental data about how NK cells function in the mouse show that this innate cell type may also elicit memory based antigen specific responses [30]. Such cells have not been described in large animal species to date. Depending on results of future studies, NK cells may be targeted to enhance the innate response while priming them for antigen specific memory responses. Recently, Thierry et al. [31] have described invariant NK T (iNKT) cells in porcine peripheral blood, which reside in the CD4−/CD8+ and CD4−/CD8− T cell compartments. They have an activated memory phenotype indicated by SLA-DR+, CD45RA(-) and produce considerable amounts of IFNγ. Porcine iNKT cells expand in the presence of IL-2, IL-15 and IL-33 following activation with α-galactoceramide (α-GC), but expansion is restricted to CD3−αGC-CD1dTT+ cell population. The role of these cells in infection remains to be determined.
3.1. NK cells in FMDV infection
Although information is becoming available about the bovine NK cell response in bacterial and parasitic infections, data are not widely available regarding the function of bovine NK cells in viral infections. To learn if there is a possible role for bovine NK cells in a viral infection, we studied bovine CD335+ NK cells originating from cattle infected with FMDV.
Infection of cattle with FMDV causes an acute disease associated with development of lesions on the hooves and buccal area. Although the infection is rarely fatal in adult animals, it leads to significant economic losses resulting from reduced productivity of infected animals, or losses due to culling of infected animals or even the whole herd in areas of disease outbreak. Such losses are more pronounced, particularly in industrialized countries that are free of disease and thus do not practice vaccination against FMDV. Therefore, understanding how NK cells function during infection with FMDV is conducive to rational strategies of augmenting the innate immunity against this devastating disease of cloven-hoofed animals.
We have now shown that NK cells originating from FMDV infected animals have an elevated level of cytotoxicity against bovine derived target cells in vitro (Patch, Dar, Waters, Toka & Golde, manuscript submitted). NK cells were also described as having low cytotoxicity for bovine non-infected target cells but were able to efficiently lyse parainfluenza 3 virus (PI-3) infected target cells following addition of anti-PI-3 virus antibodies, indicating a role for NK-mediated antibody dependent cell cytotoxicity [32].
In swine, NK cells are identified as CD2+/CD8+/CD3− cells [33] which show characteristics of NK cells described in other species. In vitro data show that, at rest, porcine NK cells are minimally cytotoxic even towards target cells infected with FMDV. However, following stimulation with cytokines such as IL-2, IL-12, IL-15, IL-18 or interferon alpha (IFNα), their cytotoxicity significantly increased [34], [35] including cytotoxicity against FMDV infected target cells. However, NK cells isolated from swine infected with FMDV are dysfunctional because of the suppressive nature of the virus infection in vivo [36]. Porcine NK cells isolated during acute FMDV infection do not secrete IFNγ and are not cytotoxic for NK-sensitive targets such as the human lymphocytic cell line, K562 or FMDV infected porcine fibroblasts. Such a status of NK cells may preclude their protective role in the early phase of infection of pigs with FMDV.
4. Gamma/delta T cells
In young ruminants and swine, T cells expressing γδ T cell receptors (TCR) account for 20–50% of circulating T cells. Although not much is known about the character of antigens recognized by these cells in any mammalian species, antigen recognition is predicted to be MHC independent. In addition, the role of these cells in immune responses to virus infections is not clear. γδ T cells express innate immune functions such as production of proinflammatory cytokines and non-MHC restricted cytolytic activity, i.e., NK-like activity [37]. Further, reports have described a role in wound healing through production of the insulin-like growth factor 1 (IGF-1) [38]. Hoek et al. [39] recently described bovine γδ T cells that likely are regulatory T cells expressing the workshop cluster 1 molecule (WC1) as well as a γδ TCR. These authors propose that these cells may have regulatory functions instead of CD4+/CD25high/FoxP3+, but the results were limited to WC1 cells expressing mRNA for IL-10. More detailed studies are necessary to confirm a Treg-like function of WC1+ γδ T cells.
In cattle, there are two major populations of γδ T cells differentiated on the expression of WC1, which belongs to the cystein-rich scavenger receptor family of proteins. These are the WC1+/CD3+/CD5+/CD2−/CD6−/CD8− and WC1−/CD3+/CD5+/CD2+/CD6+/CD8+ subsets as reported by Wijnaard et al. [40] and Aruffo et al. [41]. The later, WC1− γδ T cell subset has been reported to express perforin in American bison [42]. The WC1+ subpopulation is further divided into WC1.1+ and WC1.2+ cells; however, the significance of this division is not yet clear. In addition this division relied on monoclonal antibody reactivity. Studies by Baldwin and colleagues [43] show that at least 13 genes located on chromosome 5 within two loci, encode the WC1 molecules. Extensive alternative splicing of transcripts of this family of genes is probably the reason we have a large diversity among this type of scavenger receptor. Moreover, such diversity may contribute to the functional difference observed between γδ T cells in cattle.
Swine γδ T cells appear early in the thymus as CD3ɛ high cells during the gestation period [44]. As many as 12 different populations of γδ T cell in thymus of pigs have been found by Sinkora et al. [45] based on the expression of CD1, CD2, CD4, CD8 and CD45RC. Two major groups can be defined based on CD4 expression, but these cells do not have counterparts in periphery. However, it suggested that the CD4− group gives rise to all γδ T cells found in the periphery [3]. Further studies by Sinkora [46] reveal that the peripheral blood harbours the most CD2−/CD8− γδ T cells while CD2+/CD8+ are frequently found in solid tissues and CD2+/CD8− are enriched in the spleen and thymus, but the proportions of these cell subsets vary with age.
There is constitutive expression of CD25 on γδ T cells from germ-free pigs with almost all CD2−/CD8− cells expressing high levels whereas CD2+/CD8+ and CD2+/CD8− cells have low levels. Expression of CD25 can be increased by PMA and IL-2 stimulation although IL-2 is less potent and only effective for CD2−/CD8− γδ T cells. While γδ T cells from germ-free pigs express CD11b, albeit at variable levels, almost none of the CD2−/CD8− γδ T cells in conventional animals express CD11b and enhancement of expression in such animals could not be achieved even when cells were stimulated with PMA [46]. The majority of CD2+/CD8+ γδ T cells express MHC II molecules unlike CD2+/CD8− or CD2−/CD8−. Stimulation of blood γδ T cells with PMA increases MHC II expression. Additionally, γδ T cells may express swine workshop cluster 1 (SWC1, CD52) [47], CD45RA, CD45RC and SWC7, albeit at varied levels and varied tissue distribution. Despite the differential characterization of porcine γδ T cells, it is still far from providing a functional definition of these different phenotypic profiles. Only recently, have porcine γδ TCR+ cells been reported to be one of the major producers of IL-17 [48], indicating that these cells are bona fide participants in innate immune responses.
Experimental infection of and re-exposure to porcine reproductive and respiratory syndrome virus (PRRSV) increased the number of γδ T cells in circulation between 14 and 70 days post virus stimulation. In vitro assays measuring cell proliferation and IFNγ synthesis indicated increased activity of these cells at least between the 14th and 50th day post stimulation [49]. Although this may suggest response of porcine γδ T cells to virus infection, other functional assays such as cytotoxicity were not shown. Moreover, γδ T cells in swine share a phenotype with that of antigen presenting cells, since they are able to take up antigen and present it to CD4+ T cells via MHC class II molecules [37], [50], therefore connecting the innate to adaptive immunity. Activation of γδ T cells has been described in other infections such as mycobacteria [51] and leptospira in swine [52].
4.1. Gamma delta T cells in FMDV infection
The knowledge available so far on the interaction of FMDV with the immune system has been largely generated in the pig. Even then, not much has been described about the role of the various components of the innate immune response during infection or vaccination. The observation that naive porcine γδ T cells are stimulated to transcribe mRNA of various cytokines and chemokines when treated with a high potency emergency vaccine against FMDV, indicates a role for these cells in innate responses to vaccination or infection [37].
In a study perfomed by Amadori et al. [53] a population of cells with a phenotype and function similar to the human or murine large granular lymphocytes/NK lineage was indicated. Those cells, isolated from peripheral blood of cattle vaccinated against FMDV, could lyse target cells infected with virus in an MHC-independent manner. Later, in another experiment with murine raised antibodies, a similar population was identified as γδ T cells. Amadori et al. [54], isolated γδ T cells from FMDV vaccinated cattle and showed that they exhibited cytotoxic or cytostatic capabilities against target cells infected with different viruses. Moreover, in a different experiment Amadori et al. [54] infected heifers with bovine herpesvirus 1 and observed an increase in γδ T cells within the peripheral blood mononuclear cell population within the first days of infection. The γδ T cells also inflitrated the tongue and palate mucosae and was more pronounced in animals vaccinated against FMDV. Therefore, γδ T cells in cattle respond to FMDV antigens.
We have recently reported that WC1+ γδ T cells may be involved in the innate response to infection with FMDV in cattle [55]. These cells show a transiently activated phenotype characterized by increased expression of CD25, reduced expression of CD62L and CD45RO, and increased production of IFNγ. Most interestingly, WC1+ γδ T cells acquire NK-like capabilities to kill an in vitro target, shown by increased storage of perforin and expression of CD335. Data from these studies also indicated that bovine γδ T cells might act as antigen presenting cells due to increased expression of major histocompatibility class II molecule (MHC II) and CD13, and the ability to ingest and process exogenous proteins. Gamma delta T cells have been previously shown to act as antigen presenting cells [56]. Whether this activation indicates direct interaction of γδ T cells with virus or a bystander effect, is not yet known. Further studies should precisely define particular phenotypes responsible for both NK-like behavior and antigen presenting properties and particularly if these cells are able to activate CD4+ T cells in an antigen-dependent manner. Our data support the earlier findings of Amadori et al. [53], [54] and suggest an important role of γδ T cells in the innate immune response against FMDV. In cattle persistently infected with bovine leukemia virus (BLV), administration of recombinant IFNγ increased the number of γδ T cells in circulation and suppressed the replication of BLV in vivo, although no mechanisms were suggested by these authors on how the γδ T cells eliminated the IgM+ cells harboring the virus [57].
5. Dendritic cells initiate innate responses
A critical role of dendritic cells is to scan for antigen (including vaccine antigen) in the periphery and then migrate to the T cell areas of lymph nodes to initiate the immune response. Antigen presenting cell maturation results in amplified antigen uptake and presentation of antigen as peptides through MHC class I or II complexes [58], [59]. The late Dr. Ralph M. Steinman first described the function of DC in linking the innate to adaptive immunity as follows; “They are sentinels, able to capture, process and present antigen and migrate to lymphoid tissue to select rare antigen-reactive T cell clones. They are sensors responding to a spectrum of environmental cues by extensive differentiation and maturation” [60].
Apart from being the cells that initiate primary immune responses, DCs are efficient producers of type I interferons, and as such, powerful mediators of innate immune responses. Stephens et al. [61] have described two DC populations CD172+/CD13− (SIRP+/CC18Ag−) and CD172a−/CD13+ (SIRP−/CC18Ag+), in afferent lymph of cattle. These two DC populations have different patterns of cytokine production. Of the two populations, CD13+ produced more IL-12, although prolonged stimulation of both populations produced comparable amounts of IL-12. The early response of the CD13− cells was characterized by IL-10 secretion. However, both populations of DC did not produce type I interferon (IFNα or β). It is difficult to assess what role these DC may play in innate immunity to vaccination or infection since the cytokine profile arising from prolonged stimulation appears to promote Th1 type (both cell types produced IL-12) of immune response, i.e., suited to activation of adaptive rather than innate immune responses. There is a level of plasticity retained in these DC populations that may allow them to stimulate either Th1 or Th2 responses depending on the conditions of the initial stimulatory signals or these subsets of DCs may be integral to one or the other T helper cell response. A firm conclusion in this paradigm awaits more definitive data.
Protection through innate immune responses may be mediated through production of IFNα by natural interferon producing cells (NIPC) (an early name for plasmacytoid dendritic cells (pDC)) as has been reported in pigs by Charley et al. [62] during experimental influenza or coronavirus infections. The precise role of DC in generating antiviral innate responses that are protective in swine remains controversial. Several groups have shown that FMDV can replicate in pDC, MoDC and bone marrow-derived DCs [63], [64], [65] and yet other groups have reported the refractory nature of skin DCs to infection with FMDV [66]. In fact, many viruses induce type I IFN yet are quite sensitive to this antiviral cytokine. However, many viruses have also evolved various mechanisms to evade this powerful innate control of pathogens. Summerfield [67], [68] outlines the mechanisms certain viruses may engage in order to evade the innate immune responses mediated through type I IFN.
5.1. Dendritic cells in FMDV infection
Recently, Reid et al. [69] have identified a population of dendritic cells in cattle with high capability to produce type I interferon. CD3−/CD14−/CD21−/CD11c−/NK−/TCRγ−/CD4+/MHCII+/CD45RB+/CD172a+/CD32+ pDCs accounted for 0.1% of the total lymphocyte population in peripheral blood, pseudoafferent lymph and lymph nodes. High levels of type I IFN were produced after stimulation of the cells with the TLR9 agonist CpG and FMDV immune complexes. Depletion of CD4+ cells in vivo reduced the level of type I IFN in the serum of animals following infection with FMDV. These data likely indicate that these pDCs may be critical in generating protective immunity in animals already vaccinated, because the production of type I IFN was dependent on the presence of FMDV-antibody complexes in in vivo experiments. Interestingly, mesenteric lymph nodes yielded DCs with particularly high capacity to produce type I IFN, suggesting a role for these cells in lymph nodes. However, the results reported in the study by Reid et al. [69] do not indicate a role for type I IFN in early phases of infection since no effect on clinical disease was observed following depletion of CD4+ type I IFN producing cells. Further, monocyte-derived DC (MoDC) complexed with dead virus stimulated FMDV-specific CD4+ memory T cells, suggesting a possibility that such complexes could be used to target FMDV in vaccine formulations [65].
In a study by Nfon et al. [70], porcine DC were readily stimulated to produce IFN type I when exposed to FMDV in vitro. However, moDC and skin DC derived from swine infected with FMDV, failed to produce IFNα, constitutively or following in vitro stimulation, and this inability to produce IFNα coincides with the rising titers of FMDV in the blood. The mechanism of how production of IFNα is inhibited was described recently. Data suggests the leader protease (Lpro) of FMDV disrupts the integrity of NF-κB therefore, discontinuing NF-κB-dependent gene transcription [71], [72]. Of note is a recent study by Lannes et al. [73], showing IFNα production by pDCs can be enhanced by both neutralizing and opsonizing anti-FMDV antibodies.
DC also secretes type III IFN [74]. Antiviral activity of type III interferon against FMDV was detected in cattle vaccinated with an adenovirus vector encoding a gene for bovine IFNλ3. The IFNλ3 induced upregulation of IFN regulated genes [75]. In cattle, it is not entirely clear whether DC are the primary source of IFNλ3, moreover, no challenge experiments have been performed to conclusively determine the in vivo efficacy of IFNλ3 as an antiviral agent. Similarly, IFNλ1 has also antiviral activity against FMDV [76].
6. Interplay between cells of the innate immune response
Lessons from the rodent models show that the innate immune system accomplishes activation and initiation of inflammatory responses through interaction of cellular components. DCs initiate the interaction upon encounter with the pathogen or upon receiving inflammatory signals from the periphery. They secrete cytokines such as interleukin 1 (IL-1), IL-2, IL-12, IL-15 and IL-18 that activate NK cells. This activation leads to increased proliferation, cytotoxicity and expression of NK cell receptors [77] that in turn may control the activation and maturation of DC. Also involved at this stage of activation are cytokines produced by NK cells such as tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) that up-regulate expression of MHC I molecules on DC. Apparently, as part of DC-NK crosstalk, NK cells may lyse immature DC (iDC), most likely to limit the immune response or to protect from induction of tolerance towards pathogens, as reported in the mouse [78]. Most recently, Siddiqui and Hope [79] showed that bovine CD335+/CD2− NK cells migrate towards M. bovis infected DC that abundantly expressed chemokines such as CCL3, 4, 5, 20 and CXCL8. In this scenario, DCs produced IL-12 that activated NK cells to produce IFNγ, and in turn, CD335+/CD2− NK cells induced an increase in the intensity of MHC II expression on the DCs. This observation clearly demonstrates the interaction between DC and NK cells.
However, it is not clear where in the tissue this interaction occurs. A study by Buentke et al. [80] showed that in the dermis of healthy humans, only scattered NK cells could be observed, however, in human skin biopsies positive for the Malassezia atopy-patch-test, NK cells were found in close proximity with CD1a+ DC, indicating that inflammatory sites are likely to be sites where interaction may occur. The second possibility is the lymph node. In humans and mice, both DC and CD56bright NK cells can express chemokine receptors such as chemokine (C–C motif) receptor 7 (CCR7) and chemokine (X-C motif) receptor 3 (CXCR3) [81], [82], that are responsible for immune cell trafficking to lymph nodes. Thus NK cells and DCs are in close proximity for interactions that lead to activation [83]. Moreover, in humans NK cells secrete the high mobility group B1 (HMGB1) inflammatory cytokine that protects DC from lysis by NK cells. Further, DC secrete IL-18, a cytokine that is leaderless and hence restricted to the synaptic cleft, promoting cell-cell contact of NK and DC [84].
There is also a level of interaction between γδ T cells and DC. Conti et al. [85] showed that human DC are induced to up-regulate the expression of CD86 and MHC I molecules by γδ (Vδ2) T cells and these cells secrete TNFα and IFNγ, all following stimulation with non-peptide phosphoantigens. Human NK cells positively regulate γδ T cells by interaction through CD54 and secretion of TNFα and GM-CSF but not IFNγ. In turn γδ T cells respond by vigorous proliferation to microbial antigen [86]. Because there is still lack of knowledge about the nature of antigens recognized by γδ T cells we still do not know if γδ T cells interact with DC during viral infection, in which transient activation of these cells has been observed [55]. It is highly possible that the interplay between DC, NK and γδ T cells in large animal species constitutes the stronghold of the innate immune response to infection. Fig. 1 depicts possible ways of how these cells may interact.
Fig. 1.
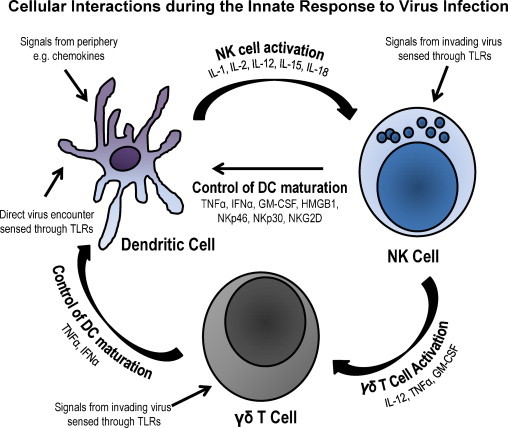
Possible interaction of DC, NK and γδ T cells in an innate immune response. The figure shows three major cellular components of the innate immune system. It appears that each of the cellular components might initiate innate responses individually. Mostly because all the cell types express TLR, hence are capable of sensing pathogens. DC, upon encounter with pathogens or receiving signals from the periphery through chemokines, produce cytokines such as IL-1, IL-2, IL-12, IL-15 and IL-18, which are important activators of NK cells. NK cells in turn respond by secreting more cytokines, particularly TNFα, IFNγ, GM-CSF and HMGB1 that have a regulatory effect on DC and an activating effect on γδ T cells. Further, the NK cells proliferate in response to DC cytokines and enhance cytotoxicity against virus infected cells. In addition to cytokines secreted by NK cells γδ T cells also, secrete TNFα and GM-CSF that affect the DC activation leading to maturation and enhanced expression of MHC class I and II molecules and subsequent antigen presentation. The interplay depicted here account for the effective role played by these cells to counteract infection before it spreads within the host. However, the efficacy with which these mechanisms are executed remains largely unknown in large animal species, since viruses such as FMDV manage to efficiently replicate in swine and cause immunosuppression.
6.1. Implication for FMDV in the cellular interaction pathways
There is a distinct difference in the manner of response of cattle and swine to infection with FMDV. In swine DC are incapacitated and secretion of critical cytokines is disrupted by the infection. Subsequently, the lack of cytokine support and a non-productive interaction of NK cells with virus renders these cells dysfunctional. Although swine γδ T cells favourably respond to vaccination with FMDV antigens, no reliable data is available on their reactivity to infection with FMDV. Considering the dysfunction of DCs, it can be speculated that γδ T cells from swine may be dysfunctional as well. Conversely, in cattle, γδ T cells are transiently activated during the initial phase of infection with FMDV and acquire a level of cytotoxicity against in vitro target cells. Additionally, γδ T cells produce IFNγ which probably influences the activity of NK cells originating from infected cattle as shown in in vitro assays (unpublished data Patch, Dar, et al.). These responses could be initiated by DC. Appearance of antibodies could disrupt this interaction because formation of antibody/FMDV complexes appear to alter tropism of FMDV and dendritic cells are killed when such complexes are internalized through Fc receptors [65].
7. The role of Toll-like receptors in innate responses
Expression of receptors on innate immune cells defines the function of those cells. Therefore, a thorough understanding of pathogen recognition receptors (PRR) in particular Toll-like receptors (TLRs) expressed by DC, NK and γδ T cells and their agonists (pathogen associated molecular patterns, PAMP, [87]) is required in order to design proper stimulatory formulations of vaccines or immunotherapeutics. Since innate immunity plays a critical role in orchestrating interaction between different host cells, and those cells and the pathogen, modulation of the response through PRR may afford the means to enhance immunity against viruses. The pattern recognition receptors including TLRs appear to be modulated by the presence of pathogens [88].
As depicted in Fig. 1, the innate immune cell types can respond to stimulation through TLR. Although not all information is complete on the expression of TLR in large animals, bovine DC express TLR2 and 4 [89], [90]. TLR7 and TLR8 are expressed by CD14+ cells in the peripheral blood and respond by secreting IL-12 when treated with TLR7 and TLR8 agonists [91]. Reid et al. [69] reported the response of DC to TLR9 agonist by production of type I IFN. Microarray data compiled by Hedges et al. [92] showed transcription of TLR1, 2, 3, 4, 5, 8, 9 and other PRR in bovine γδ T cells treated with LPS. However, to date no reliable data in the literature is available indicating TLR expression on bovine NK cells.
In studies done by Lee et al. [93], bovine monocytes exposed to bovine viral diarrhea virus (BVDV), differentially transcribed TLR3 mRNA. The cytopathic strain of the virus did not significantly increase the transcription of TLR3 mRNA, while both cytopathic and non-cytopathic strains upregulated the transcription of TLR7 only after 24 h of infection. Surprisingly, this increased expression of TLR7 did not lead to enhanced type I IFN response in infected monocytes, suggesting that monocytes may play a minor role in overall synthesis of type I IFNs during infection with BVDV. The same authors had earlier shown that infection of macrophages with BVDV did not influence expression of TLR2, 3 and 4. Moreover, TLR signalling detected in these studies was not attributed to increases in TNFα or IL-1β. Persistent infection that usually occurs with BVDV is likely established due to the virus’ ability to suppress the innate immune response components, in this case monocytes, by inhibiting expression of pro-inflammatory cytokines production.
Infection of cattle with FMDV up regulates expression of TLR4 but not TLR3 in nasal associated lymphoid tissue (NALT) during the acute phase of disease [94]. Studies by Zhang [94] indicate a role for the increased expression of TLR4, since mRNA level of type I IFNs concurrently increased. This may be surprising because FMDV is an RNA virus, more likely to provide ligands for TLR3 (dsRNA, a replication intermediate in many RNA viruses) than for TLR4 which transduces signals after recognizing LPS from Gram-positive bacteria.
In swine, we have reported expression of TLR7 and TLR8 by NK cells. The activation of these cells to enhanced killing is partially dependent on DCs, as removing the DCs resulted in low levels of cytotoxicity following treatment with TLR7/8 agonists [95]. Others have found TLR4, 5, 7 and 9 expressed by MoDCs [96], [97]. In swine, an interferon response to the TLR9 agonist, CpG, was detected in plasmacytoid DCs, Langerhans cells and B cells [98]. Zhang et al. [99] reported that swine TLR7 is stimulated by RNA oligonucleotides found in FMDV leading to expression of IFNα and Th1 cytokine induction. Taken together, this leads to the possibility of targeting these TLRs in order to modulate the function of innate immune cells to fight viral infections.
Besides TLRs, another group of PRRs called RIG-I-like receptors (RLRs) participate in innate responses [100]. TLRs are expressed either on the cell surface or in endosomes, but RLRs are found in the cytoplasm and may more be suited to detecting virus nucleic acids. Husser et al. [100] found that in PK-15 cells (porcine kidney cell line), FMDV is detected by MDA-5, while classical swine fever virus was detected by MDA-5, RIG-I and TLR3 which led to induction of IFN-β. The evidence that FMDV preferentially signals through MDA-5 is supported by data from Wang et al. [101] that Lpro of FMDV, a shorter form of Lpro (Leader protease), significantly inhibits ubiquitination of RIG-I, likely leading to inhibition of type I IFNs induction.
8. Conclusion
Many reports have emphasized the critical role of antibodies in clearance of virus following FMDV infection and protection against FMDV challenge following vaccination. A minimal amount of research has been performed to understand mechanisms of innate immune responses that possibly contribute to early responses to FMDV. In our studies as well as studies by Salt and colleagues, and Summerfield and colleagues [102], [103], [104], [105], [106], [107], it is clearly demonstrated that early protection is achieved amidst very little antibody production. These data indicate that other mechanisms besides humoral immunity are in play at early time points of infection. We have recently shown that infection with FMDV in cattle induces a rapid although transient activation of γδ T cells [55]. Notably, the activation of the innate immune system in the cattle following FMDV infection is the opposite result from what we have reported in swine. Within 24 h following inoculation of pigs with FMDV, DCs stop secreting type I IFN and their numbers are reduced [108], [109]. Further, NK cells isolated from FMDV infected pigs 24–96 h after infection do not produce IFNγ and fail to spontaneously lyse target cells in vitro [36], all indicating that the swine innate response is inhibited by the virus.
The cause of differences in response to FMDV infection between cattle and pigs is not clear. Rapid replication of FMDV in pigs leads to production of large amounts of virus. Additionally, the initial, high IFNα production is curtailed abruptly leading to high levels of viraemia and subsequent lymphopenia. A combination of these two factors may lead to immunosuppression of these innate responses in pigs. Conversely, although vireamia is observed in cattle, levels of IFNα are much lower than those in pigs and neither lymphopenia nor immunosuppression of NK cells or γδ T cells occurs in cattle [110]. Taken together, a new emphasis is required to focus investigations on the innate immune mechanisms that may be exploited in design of new interventions to control FMDV outbreaks.
Acknowledgements
The authors thank Ms. Raisa Glabman for assistance in preparing the illustration. This work was funded by CRIS #1940-32000-057-00D from the Agricultural Research Service, USDA (WTG) and by an interagency agreement (60-1940-8-037) between the Department of Homeland Security, Science and Technology Directorate, and the USDA, Agricultural Research Service (WTG). This work was also supported by the Polish National Science Centre (NCN) grant NN308566040 (FNT) and USDA Specific Cooperative Agreement 58-1940-0-088F (WTG & FNT).
References
- 1.Yasuda M., Ogawa D., Nasu T., Yamaguchi T., Murakami T. Kinetics and distribution of bovine γδ T-lymphocyte in the intestine: γδ T cells accumulate in the dome region of Peyer's patch during prenatal development. Dev Comp Immunol. 2005;29:555–564. doi: 10.1016/j.dci.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Hein W.R., Dudler L. Divergent evolution of T cell repertoires: extensive diversity and developmentally regulated expression of the sheep γδ T cell receptor. EMBO J. 1993;12:715–724. doi: 10.1002/j.1460-2075.1993.tb05705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinkora M., Sinkorova J., Cimburek Z., Holtmeier W. Two groups of porcine TCR γδ+ thymocytes behave and diverge differently. J Immunol. 2007;178:711–719. doi: 10.4049/jimmunol.178.2.711. [DOI] [PubMed] [Google Scholar]
- 4.Bautista E.M., Nfon C., Ferman G.S., Golde W.T. IL-13 replaces IL-4 in development of monocyte derived dendritic cells (MoDC) of swine. Vet Immunol Immunopathol. 2007;115:56–67. doi: 10.1016/j.vetimm.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Renjifo X., Howard C., Kerkhofs P., Denis M., Urbain J., Moser M. Purification and characterization of bovine dendritic cells from peripheral blood. Vet Immunol Immunopathol. 1997;60:77–88. doi: 10.1016/s0165-2427(97)00092-5. [DOI] [PubMed] [Google Scholar]
- 6.Kaiko G.E., Phipps S., Angkasekwinai P., Dong C., Foster P.S. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J Immunol. 2010;185:4681–4690. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 7.Carr D.J.J., Wuest T., Ash J. An increase in herpes simplex virus type 1 in the anterior segment of the eye is linked to a deficiency in NK cell infiltration in mice deficient in CXCR3. J Interferon Cytokine Res. 2008;28:245–251. doi: 10.1089/jir.2007.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eidenschenk C., Jouanguy E., Alcais A., Mention J.-J., Pasquier B., Fleckenstein I.M. Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes. J Immunol. 2006;177:8835–8843. doi: 10.4049/jimmunol.177.12.8835. [DOI] [PubMed] [Google Scholar]
- 9.Spits H., Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 10.Spits H., Di Santo J.P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 11.Vivier E., Spits H., Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair. Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 12.Storset A.K., Kulberg S., Berg I., Boysen P., Hope J.C., Dissen E. NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics. Eur J Immunol. 2004;34:669–676. doi: 10.1002/eji.200324504. [DOI] [PubMed] [Google Scholar]
- 13.Goff W.L., Storset A.K., Johnson W.C., Brown W.C. Bovine splenic NK cells synthesize IFN-γ in response to IL-12-containing supernatants from Babesia bovis-exposed monocyte cultures. Parasitol Immunol. 2006;28:221–228. doi: 10.1111/j.1365-3024.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 14.Bastos R.G., Johnson W.C., Mwangi W., Brown W.C., Goff W.L. Bovine NK cells acquire cytotoxic activity and produce IFN-γ after stimulation by Mycobacterium bovis BCG- or Babesia bovis-exposed splenic dendritic cells. Vet Immunol Immunopathol. 2008;124:302–312. doi: 10.1016/j.vetimm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Boysen P., Olsen I., Berg I., Kulberg S., Johansen G., Storset A. Bovine CD2−/NKp46+ cells are fully functional natural killer cells with a high activation status. BMC Immunol. 2006;7:10. doi: 10.1186/1471-2172-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boysen P., Klevar S., Olsen I., Storset A.K. The protozoan Neospora caninum directly triggers bovine NK cells to produce gamma interferon and to kill infected fibroblasts. Infect Immun. 2006;74:953–960. doi: 10.1128/IAI.74.2.953-960.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klevar S., Kulberg S., Boysen P., Storset A.K., Moldal T., Bjorkman C. Natural killer cells act as early responders in an experimental infection with Neospora caninum in calves. Int J Parasitol. 2007;37:329–339. doi: 10.1016/j.ijpara.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Denis M., Keen D.L., Parlane N.A., Storset A.K., Buddle B.M. Bovine natural killer cells restrict the replication of Mycobacterium bovis in bovine macrophages and enhance IL-12 release by infected macrophages. Tuberculosis. 2007;87:53–62. doi: 10.1016/j.tube.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Hope J.C., Sopp P., Howard C.J. NK-like CD8(+) cells in immunologically naive neonatal calves that respond to dendritic cells infected with Mycobacterium bovis BCG. Journal of Leukocyte Biology. 2002;71:184–194. [PubMed] [Google Scholar]
- 20.Fikri Y., Nyabenda J., Content J., Huygen K. Cloning, sequencing, and cell surface expression pattern of bovine immunoreceptor NKG2D and adaptor molecules DAP10 and DAP12. Immunogenetics. 2007;59:653–659. doi: 10.1007/s00251-007-0226-6. [DOI] [PubMed] [Google Scholar]
- 21.Guzman E., Birch J.R., Ellis S.A. Cattle MIC is a ligand for the activating NK cell receptor NKG2D. Vet Immunol Immunopathol. 2010;136:227–234. doi: 10.1016/j.vetimm.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Boysen P., Storset A.K. Bovine natural killer cells. Vet Immunol Immunopathol. 2009;130:163–177. doi: 10.1016/j.vetimm.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Albertsson P.A., Basse P.H., Hokland M., Goldfarb R.H., Nagelkerke J.F., Nannmark U. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603–609. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Cerundolo V., de la Salle H. Description of HLA class I- and CD8-deficient patients: insights into the function of cytotoxic T lymphocytes and NK cells in host defense. Sem Immunol. 2006;18:330–336. doi: 10.1016/j.smim.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Moretta A., Biassoni R., Bottino C., Moretta L. Surface receptors delivering opposite signals regulate the function of human NK cells. Sem Immunol. 2000;12:129–138. doi: 10.1006/smim.2000.0215. [DOI] [PubMed] [Google Scholar]
- 26.Ishizaki H., Kariya Y. Road transportation stress promptly increases bovine peripheral blood absolute NK cell counts and cortisol levels. J Vet Med Sci. 2010;72:747–753. doi: 10.1292/jvms.09-0441. [DOI] [PubMed] [Google Scholar]
- 27.Riondato F., D’Angelo A., Miniscalco B., Bellino C., Guglielmino R. Effects of road transportation on lymphocyte subsets in calves. Vet J. 2008;175:364–368. doi: 10.1016/j.tvjl.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza C., Videgain S.P., Alonso F. Inhibition of natural killer activity in porcine mononuclear cells by African swine fever virus. Res Vet Sci. 1991;51:317–321. doi: 10.1016/0034-5288(91)90084-2. [DOI] [PubMed] [Google Scholar]
- 29.Jung K., Renukaradhya G.J., Alekseev K.P., Fang Y., Tang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009;90:2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J.C., Lopez-Verges S., Kim C.C., DeRisi J.L., Cells Lanier L.L.N.K. Immune memory. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thierry A., Robin A., Giraud S., Minouflet S., Barra A., Bridoux F. Identification of invariant natural killer T cells in porcine peripheral blood. Vet Immunol Immunopathol. 2012;149:272–279. doi: 10.1016/j.vetimm.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Bradford H.E.L., Adair B.M., Foster J.C. Antibody-dependent killing of virus-infected targets by NK-like cells in bovine blood. J Vet Med B. 2001;48:637–640. doi: 10.1046/j.1439-0450.2001.00479.x. [DOI] [PubMed] [Google Scholar]
- 33.Denyer M.S., Wileman T.E., Stirling C.M.A., Zuber B., Takamatsu H-H. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet Immunol Immunopathol. 2006;110:279–292. doi: 10.1016/j.vetimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Pintaric M., Gerner W., Saalmuller A. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-γ production of porcine natural killer cells. Vet Immunol Immunopathol. 2008;121:68–82. doi: 10.1016/j.vetimm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Toka F.N., Nfon C.K., Dawson H., Estes D.M., Golde W.T. Activation of porcine natural killer cells and lysis of foot-and-mouth disease virus infected cells. J Interf Cytok Res. 2009;29:179–192. doi: 10.1089/jir.2008.0058. [DOI] [PubMed] [Google Scholar]
- 36.Toka F.N., Nfon C., Dawson H., Golde W.T. Natural killer cell dysfunction during acute infection with foot-and-mouth disease virus. Clin Vaccine Immunol. 2009;16:1738–1749. doi: 10.1128/CVI.00280-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takamatsu H.H., Denyer M.S., Stirling C., Cox S., Aggarwal N., Dash P. Porcine γδ T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet Immunol Immunopathol. 2006;112:49–61. doi: 10.1016/j.vetimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Toulon A., Breton L., Taylor K.R., Tenenhaus M., Bhavsar D., Lanigan C. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoek A., Rutten V.P.M.G., Kool J., Arkesteijn G.J.A., Bouwstra R.J., van Rhijn I. Subpopulations of bovine WC1+ γδ T cells rather than CD4+CD25high Foxp3+ T cells act as immune regulatory cells ex vivo. Vet Res. 2009;40:06. doi: 10.1051/vetres:2008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wijngaard P.L., Metzelaar M.J., MacHugh N.D., Morrison W.I., Clevers H.C. Molecular characterization of the WC1 antigen expressed specifically on bovine CD4-CD8− γδ T lymphocytes. J Immunol. 1992;149:3273–3277. [PubMed] [Google Scholar]
- 41.Aruffo A., Bowen M.A., Patel D.D., Haynes B.F., Starling G.C., Gebe J.A. CD6-ligand interactions: a paradigm for SRCR domain function. Immunol Today. 1997;18:498–504. doi: 10.1016/s0167-5699(97)01130-4. [DOI] [PubMed] [Google Scholar]
- 42.Skyberg J.A., Thornburg T., Rollins M., Huarte E., Jutila M.A., Murine Pascual D.W. Bovine γδ T cells enhance innate immunity against Brucella abortus infections. PLoS ONE. 2011;6:e21978. doi: 10.1371/journal.pone.0021978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herzig C.T., Baldwin C.L. Genomic organization and classification of the bovine WC1 genes and expression by peripheral blood γδ T cells. BMC Genomics. 2009;10:191. doi: 10.1186/1471-2164-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinkora M., Sinkora J., Rehakova Z., Butler J.E. Early ontogeny of thymocytes in pigs: sequential colonization of the thymus by T cell progenitors. J Immunol. 2000;165:1832–1839. doi: 10.4049/jimmunol.165.4.1832. [DOI] [PubMed] [Google Scholar]
- 45.Sinkora M., Sinkorova J., Holtmeier W. Development of γδ thymocyte subsets during prenatal and postnatal ontogeny. Immunology. 2005;115:544–555. doi: 10.1111/j.1365-2567.2005.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepanova K., Sinkora M. The expression of CD25, CD11b, SWC1, SWC7, MHC-II, and family of CD45 molecules can be used to characterize different stages of γδ T lymphocytes in pigs. Dev Comp Immunol. 2012;36:728–740. doi: 10.1016/j.dci.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Leitner J., Reutner K., Essler S.E., Popow I., Gerner W., Steinberger P. Porcine SWC1 is CD52 – final determination by the use of a retroviral cDNA expression library. Vet Immunol Immunopathol. 2012;146:27–34. doi: 10.1016/j.vetimm.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepanova H., Mensikova M., Chlebova K., Faldyna M. CD4+ and γδTCR+ T lymphocytes are sources of interleukin-17 in swine. Cytokine. 2012;58:152–157. doi: 10.1016/j.cyto.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Olin M., Batista L., Xiao Z., Dee S., Murtaugh M., Pijoan C. γδ Lymphocyte response to porcine reproductive and respiratory syndrome virus. Viral Immunol. 2005;18:490–499. doi: 10.1089/vim.2005.18.490. [DOI] [PubMed] [Google Scholar]
- 50.Takamatsu H.H., Denyer M.S., Wileman T.E. A sub-population of circulating porcine γδ T cells can act as professional antigen presenting cells. Vet Immunol Immunopathol. 2002;87:223–224. doi: 10.1016/s0165-2427(02)00083-1. [DOI] [PubMed] [Google Scholar]
- 51.Olin M.R., Hwa Choi K., Lee J., Molitor T.W. γδ T-lymphocyte cytotoxic activity against Mycobacterium bovis analyzed by flow cytometry. J Immunol Methods. 2005;297:1–11. doi: 10.1016/j.jim.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Wang F., Herzig C.T.A., Chen C., Hsu H., Baldwin C.L., Telfer J.C. Scavenger receptor WC1 contributes to the γδ T cell response to Leptospira. Mol Immunol. 2011;48:801–809. doi: 10.1016/j.molimm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Amadori M., Archetti I.L., Verardi R., Berneri C. Isolation of mononuclear cytotoxic cells from cattle vaccinated against foot-and-mouth disease. Arch Virol. 1992;122:293–306. doi: 10.1007/BF01317191. [DOI] [PubMed] [Google Scholar]
- 54.Amadori M., Archetti I.L., Verardi R., Berneri C. Role of a distinct population of bovine γδ T cells in the immune response to viral agents. Viral Immunol. 1995;8:81–91. doi: 10.1089/vim.1995.8.81. [DOI] [PubMed] [Google Scholar]
- 55.Toka F.N., Kenney M.A., Golde W.T. Rapid and transient activation of γδ T cells to IFN-γ production, NK cell-like killing, and antigen processing during acute virus infection. J Immunol. 2011;186:4853–4861. doi: 10.4049/jimmunol.1003599. [DOI] [PubMed] [Google Scholar]
- 56.Collins R.A., Werling D., Duggan S.E., Bland A.P., Parsons K.R., Howard C.J. γδ T cells present antigen to CD4+ αβ T cells. J Leukocyte Biol. 1998;63:707–714. doi: 10.1002/jlb.63.6.707. [DOI] [PubMed] [Google Scholar]
- 57.Murakami K., Sentsui H., Inoshima Y., Inumaru S. Increase in γδ T cells in the blood of cattle persistently infected with Bovine Leukemia Virus following administration of recombinant bovine IFN-gamma. Vet Immunol Immunopathol. 2004;101:61–71. doi: 10.1016/j.vetimm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Steinman R.M. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Steinman R.M. Dendritic cells and vaccines. Proceedings (Bayl Univ Med Cent) 2008;21:3–8. doi: 10.1080/08998280.2008.11928346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steinman R.M. Linking innate to adaptive immunity through dendritic cells. Novart Fdn Symp. 2006;279:101–109. [discussion 9–13, 216–219] [PubMed] [Google Scholar]
- 61.Stephens S.A., Brownlie J., Charleston B., Howard C.J. Differences in cytokine synthesis by the sub-populations of dendritic cells from afferent lymph. Immunology. 2003;110:48–57. doi: 10.1046/j.1365-2567.2003.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charley B., Riffault S., van Reeth K. Porcine innate and adaptative immune responses to influenza and coronavirus infections. Ann NY Acad Sci. 2006;1081:130–136. doi: 10.1196/annals.1373.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzylack-Piriou L., Bergamin F., Gerber M., McCullough K.C., Summerfield A. Plasmacytoid dendritic cell activation by foot-and-mouth disease virus requires immune complexes. Eur J Immunol. 2006;36:1674–1683. doi: 10.1002/eji.200635866. [DOI] [PubMed] [Google Scholar]
- 64.Harwood L.J., Gerber H., Sobrino F., Summerfield A., McCullough K.C. Dendritic cell internalization of foot-and-mouth disease virus: influence of heparan sulfate binding on virus uptake and induction of the immune response. J Virol. 2008;82:6379–6394. doi: 10.1128/JVI.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson L., Windsor M., McLaughlin K., Hope J., Jackson T., Charleston B. Foot-and-mouth disease virus exhibits an altered tropism in the presence of specific immunoglobulins, enabling productive infection and killing of dendritic cells. J Virol. 2011;85:2212–2223. doi: 10.1128/JVI.02180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bautista E.M., Ferman G.S., Gregg D., Brum M.C., Grubman M.J., Golde W.T. Constitutive expression of alpha interferon by skin dendritic cells confers resistance to infection by foot-and-mouth disease virus. J Virol. 2005;79:4838–4847. doi: 10.1128/JVI.79.8.4838-4847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Summerfield A. Viewpoint factors involved in type I interferon responses during porcine virus infections. Vet Immunol Immunopathol. 2012;148:168–171. doi: 10.1016/j.vetimm.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Fiebach A.R., Guzylack-Piriou L., Python S., Summerfield A., Ruggli N. Classical swine fever virus Npro limits type i interferon induction in plasmacytoid dendritic cells by interacting with interferon regulatory factor 7. J Virol. 2011;85:8002–8011. doi: 10.1128/JVI.00330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reid E., Juleff N., Gubbins S., Prentice H., Seago J., Charleston B. Bovine plasmacytoid dendritic cells are the major source of type I interferon in response to foot-and-mouth disease virus in vitro and in vivo. J Virol. 2011;85:4297–4308. doi: 10.1128/JVI.02495-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nfon C.K., Ferman G.S., Toka F.N., Gregg D.A., Golde W.T. Interferon-α production by swine dendritic cells is inhibited during acute infection with foot-and-mouth disease virus. Viral Immunol. 2008;21:68–77. doi: 10.1089/vim.2007.0097. [DOI] [PubMed] [Google Scholar]
- 71.de los Santos T., de Avila Botton S., Weiblen R., Grubman M.J. The leader proteinase of foot-and-mouth disease virus inhibits the induction of beta interferon mRNA and blocks the host innate immune response. J Virol. 2006;80:1906–1914. doi: 10.1128/JVI.80.4.1906-1914.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de los Santos T., Diaz-San Segundo F., Grubman M.J. Degradation of nuclear factor kappa b during foot-and-mouth disease virus infection. J Virol. 2007;81:12803–12815. doi: 10.1128/JVI.01467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lannes N., Python S., Summerfield A. Interplay of foot-and-mouth disease virus, antibodies and plasmacytoid dendritic cells: virus opsonization under non-neutralizing conditions results in enhanced interferon-alpha responses. Vet Res. 2012;43:64. doi: 10.1186/1297-9716-43-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iversen M.B., Ank N., Melchjorsen J., Paludan S.R. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-κB than type I IFNs. J Virol. 2010;84:4579–4586. doi: 10.1128/JVI.02591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diaz-San Segundo F., Weiss M., Perez-Martin E., Koster M.J., Zhu J., Grubman M.J. Antiviral activity of bovine type III interferon against foot-and-mouth disease virus. Virology. 2011;413:283–292. doi: 10.1016/j.virol.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 76.Wang D., Fang L., Liu L., Zhong H., Chen Q., Luo R. Foot-and-mouth disease virus (FMDV) leader proteinase negatively regulates the porcine interferon-λ-1 pathway. Mol Immunol. 2011;49:407–412. doi: 10.1016/j.molimm.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Wai L-E., Garcia J.A., Martinez O.M., Krams S.M. Distinct roles for the NK cell-activating receptors in mediating interactions with dendritic cells and tumor cells. J Immunol. 2011;186:222–229. doi: 10.4049/jimmunol.1002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cooper M.A., Fehniger T.A., Fuchs A., Colonna M., Caligiuri M.A. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 79.Siddiqui N., Hope J. Differential recruitment and activation of natural killer cell sub-populations by Mycobacterium bovis-infected dendritic cells. Eur J Immunol. 2013;43:159–169. doi: 10.1002/eji.201242736. [DOI] [PubMed] [Google Scholar]
- 80.Buentke E., Heffler L.C., Wilson J.L., Wallin R.P.A., Lofman C., Chambers B.J. Natural killer and dendritic cell contact in lesional atopic dermatitis skin-Malassezia-influenced cell interaction. J Invest Derm. 2002;119:850–857. doi: 10.1046/j.1523-1747.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 81.Campbell J.J., Qin S., Unutmaz D., Soler D., Murphy K.E., Hodge M.R. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 82.Palucka K., Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol. 2002;14:420–431. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 83.Fehniger T.A., Cooper M.A., Nuovo G.J., Cella M., Facchetti F., Colonna M. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 84.Semino C., Angelini G., Poggi A., Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 85.Conti L., Casetti R., Cardone M., Varano B., Martino A., Belardelli F. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated γδ T Cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174:252–260. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 86.Zhang R., Zheng X., Li B., Wei H., Tian Z. Human NK cells positively regulate γ T cells in response to mycobacterium tuberculosis. J Immunol. 2006;176:2610–2616. doi: 10.4049/jimmunol.176.4.2610. [DOI] [PubMed] [Google Scholar]
- 87.Janeway C.J. Approaching the asymptote? Evolution and revolution in mmunology. Cold Spring Harb SympQuant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Rohmann K., Tschernig T., Pabst R., Goldmann T., Dromann D. Innate immunity in the human lung: pathogen recognition and lung disease. Cell Tissue Res. 2011;343:167–174. doi: 10.1007/s00441-010-1048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Werling D., Hope J.C., Howard C.J., Jungi T.W. Differential production of cytokines, reactive oxygen and nitrogen by bovine macrophages and dendritic cells stimulated with Toll-like receptor agonists. Immunology. 2004;111:41–52. doi: 10.1111/j.1365-2567.2003.01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Werling D., Piercy J., Coffey T.J. Expression of Toll-like receptors (TLR) by bovine antigen-presenting cells – potential role in pathogen discrimination. Vet Immunol Immunopathol. 2006;112:2–11. doi: 10.1016/j.vetimm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Buza J., Benjamin P., Zhu J., Wilson H.L., Lipford G., Krieg A.M. CD14+ cells are required for IL-12 response in bovine blood mononuclear cells activated with Toll-like receptor (TLR) 7 and TLR8 ligands. Vet Immunol Immunopathol. 2008;126:273–282. doi: 10.1016/j.vetimm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Hedges J.F., Lubick K.J., Jutila M.A. γδ T cells respond directly to pathogen-associated molecular patterns. J Immunol. 2005;174:6045–6053. doi: 10.4049/jimmunol.174.10.6045. [DOI] [PubMed] [Google Scholar]
- 93.Lee S.R., Pharr G.T., Boyd B.L., Pinchuk L.M. Bovine viral diarrhea viruses modulate Toll-like receptors, cytokines and co-stimulatory molecules genes expression in bovine peripheral blood monocytes. Comp Immunol Microbiol Infect Dis. 2008;31:403–418. doi: 10.1016/j.cimid.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Z., Bashiruddin J.B., Doel C., Horsington J., Durand S., Alexandersen S. Cytokine and Toll-like receptor mRNAs in the nasal-associated lymphoid tissues of cattle during foot-and-mouth disease virus infection. J Comp Pathol. 2006;134:56–62. doi: 10.1016/j.jcpa.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 95.Toka F.N., Nfon C.K., Dawson H., Golde W.T. Accessory-cell-mediated activation of porcine NK cells by Toll-like receptor 7 (TLR7) and TLR8 agonists. Clin Vaccine Immunol. 2009;16:866–878. doi: 10.1128/CVI.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alves M.P., Neuhaus V., Guzylack-Piriou L., Ruggli N., McCullough K.C., Summerfield A. Toll-like receptor 7 and MyD88 knockdown by lentivirus-mediated RNA interference to porcine dendritic cell subsets. Gene Ther. 2007;14:836–844. doi: 10.1038/sj.gt.3302930. [DOI] [PubMed] [Google Scholar]
- 97.Raymond C.R., Wilkie B.N. Toll-like receptor, MHC, II, B7 and cytokine expression by porcine monocytes and monocyte-derived dendritic cells in response to microbial pathogen-associated molecular patterns. Vet Immunol Immunopathol. 2005;107:235–247. doi: 10.1016/j.vetimm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Alves M.P., Guzylack-Piriou L., Juillard V., Audonnet J.C., Doel T., Dawson H. Innate immune defenses induced by CpG do not promote vaccine-induced protection against foot-and-mouth disease virus in pigs. Clin Vaccine Immunol. 2009;16:1151–1157. doi: 10.1128/CVI.00018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y., Guo Y., Lv K., Wang K., Sun S. Molecular cloning and functional characterization of porcine Toll-like receptor 7 involved in recognition of single-stranded RNA virus/ssRNA. Mol Immunol. 2008;45:1184–1190. doi: 10.1016/j.molimm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 100.Husser L., Alves M.P., Ruggli N., Summerfield A. Identification of the role of RIG-I, MDA-5 and TLR3 in sensing RNA viruses in porcine epithelial cells using lentivirus-driven RNA interference. Virus Res. 2011;159:9–16. doi: 10.1016/j.virusres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 101.Wang D., Fang L., Li P., Sun L., Fan J., Zhang Q. The leader proteinase of foot-and-mouth disease virus negatively regulates the type I interferon pathway by acting as a viral deubiquitinase. J Virol. 2011;85:3758–3766. doi: 10.1128/JVI.02589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Golde W.T., Pacheco J.M., Duque H., Doel T., Penfold B., Ferman G.S. Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: use in emergency outbreak response. Vaccine. 2005;23:5775–5782. doi: 10.1016/j.vaccine.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 103.Pacheco J.M., Brum M.C.S., Moraes M.P., Golde W.T., Grubman M.J. Rapid protection of cattle from direct challenge with foot-and-mouth disease virus (FMDV) by a single inoculation with an adenovirus-vectored FMDV subunit vaccine. Virology. 2005;337:205–209. doi: 10.1016/j.virol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 104.Cox S.J., Barnett P.V., Dani P., Salt J.S. Emergency vaccination of sheep against foot-and-mouth disease: protection against disease and reduction in contact transmission. Vaccine. 1999;17:1858–1868. doi: 10.1016/s0264-410x(98)00486-1. [DOI] [PubMed] [Google Scholar]
- 105.Doel T.R., Williams L., Barnett P.V. Emergency vaccination against foot-and-mouth disease: rate of development of immunity and its implications for the carrier state. Vaccine. 1994;12:592–600. doi: 10.1016/0264-410x(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 106.Rigden R.C., Carrasco C.P., Barnett P.V., Summerfield A., McCullough K.C. Innate immune responses following emergency vaccination against foot-and-mouth disease virus in pigs. Vaccine. 2003;21:1466–1477. doi: 10.1016/s0264-410x(02)00663-1. [DOI] [PubMed] [Google Scholar]
- 107.Salt J.S., Barnett P.V., Dani P., Williams L. Emergency vaccination of pigs against foot-and-mouth disease: protection against disease and reduction in contact transmission. Vaccine. 1998;16:746–754. doi: 10.1016/s0264-410x(97)86180-4. [DOI] [PubMed] [Google Scholar]
- 108.Nfon C.K., Ferman G.S., Toka F.N., Gregg D.A., Golde W.T. Interferon-alpha production by swine dendritic cells is inhibited during acute infection with foot-and-mouth disease virus. Viral Immunol. 2008;21:68–77. doi: 10.1089/vim.2007.0097. [DOI] [PubMed] [Google Scholar]
- 109.Nfon C.K., Toka F.N., Kenney M., Pacheco J.M., Golde W.T. Loss of plasmacytoid dendritic cell function coincides with lymphopenia and viremia during foot-and-mouth disease virus infection. Viral Immunol. 2010;23:29–41. doi: 10.1089/vim.2009.0078. [DOI] [PubMed] [Google Scholar]
- 110.Windsor M., Carr B.V., Bankowski B., Gibson D., Reid E., Hamblin P. Cattle remain immunocompetent during the acute phase of foot-and-mouth disease virus infection. Vet Res. 2011;42:108. doi: 10.1186/1297-9716-42-108. [DOI] [PMC free article] [PubMed] [Google Scholar]


