Abstract
Exon 2 containing myelin basic protein (MBP) transcripts are expressed during developmental myelination in mice and humans, and during remyelination subsequent to virally induced demyelination in adult mice. Since remyelination characterizes CNS lesions during experimental allergic encephalomyelitis (EAE) and multiple sclerosis (MS), we investigated whether exon 2 containing isoforms of MBP are expressed in EAE lesions during relapsing disease. Exon 2 containing MBP transcripts were detected by in situ hybridization in 17 of 52 EAE mice and in 16 of 30 mice at the peak of the first or second episode of paralysis. Thus exon 2 containing MBP transcripts are expressed in lesions of the CNS during active phases of chronic relapsing autoimmune disease. Implications of these findings with respect to future therapies aimed toward enhancing remyelination in EAE and, possibly MS, are discussed.
Keywords: Myelin basic protein, Experimental allergic encephalomyelitis, Multiple sclerosis, Remyelination
1. Introduction
Remyelination has been observed in MS plaques (Raine, 1993; Prineas et al., 1993; Bruck et al., 1994) and in animal models of demyelinating disease of viral (Jordan et al., 1989a) or autoimmune etiology (Raine et al., 1984). Molecular correlates of remyelination are of interest as they may be quantitated and could therefore provide an index of myelin repair in lesions. Once CNS myelination is set into motion, a series of genes coding for myelin proteins are coordinately expressed in oligodendrocytes, leading to the wrapping of specialized myelin membrane around axons. During myelination, different transcripts coding for various isoforms of a major myelin protein, myelin basic protein (MBP), are sequentially generated by alternative splicing. Transcripts containing exon 2 and coding for the 21.5 and 17 kDa MBP isoforms emerge postnatally in the mouse and peak before myelin sheaths appear. Exon 2 containing MBP transcripts are then down regulated and alternatively spliced transcripts coding for the mature 14 and 18.5 kDa MBP isoforms are synthesized and mature MBP proteins are incorporated into myelin sheaths (Newman et al., 1987; Jordan et al., 1989b). In adult mice, during remyelination in a monophasic demyelinating disease induced by a corona virus, the same programmed sequence of events characteristic of developmental myelination occurs. At the site of demyelination exon 2 containing MBP transcripts are upregulated before remyelination. Transcripts coding for mature MBP isoforms are expressed later when myelin is repaired (Jordan et al., 1990). Thus, exon 2 MBP transcript expression may be used as an molecular marker of remyelination. The sequence and timing of expression of such a molecular marker during chronic relapsing demyelination has however not yet been characterized.
In this study, we have investigated the expression of exon 2 MBP as a molecular marker of remyelination in the chronic relapsing autoimmune demyelinating disease, experimental allergic encephalomyelitis (EAE), in adult mice. Chronic EAE was induced by adoptive transfer of 18.5 kDa MBP specific T lymphocytes into naive SJL recipients. The expression of exon 2 containing transcripts of MBP within spinal cord lesions was assessed by in situ hybridization during the course of chronic relapsing EAE and correlated with various stages of clinical disease.
2. Methods
2.1. Antigens
Myelin basic protein (18.5 kDa MBP) was prepared from adult guinea pig spinal cord (Rockland, Gilbertsville, PA) according to the procedure of Deibler et al. (Deibler et al., 1972). Adult gpMBP almost exclusively consists of the 18.5 kDa MBP isoform which does not include the exon 2 encoded sequence (Newman et al., 1987).
2.2. Animals
Female SJL/J mice, age 8–10 week, were purchased from Jackson Laboratories (Bar Harbor, ME).
2.3. Immunization and passive EAE induction (adoptive transfer)
Passive EAE was induced as described (Pettinelli and McFarlin, 1981). An emulsion containing 400 μg of 18.5 kDa MBP with complete Freund's adjuvant (CFA) (Difco Laboratories, Detroit, MI) was injected intradermally over four sites draining the inguinal and axillary lymph nodes (0.025 ml per site). After 10–14 days, draining lymph node cells were harvested and resuspended at 4 × 106 cells/ml in tissue culture medium. An aliquot of cells was used in proliferative assays to confirm the efficiency of immunization, while the remainder of cells were put into in vitro culture with the 18.5 kDa MBP at 25 μg/ml. After 4 days, cells were injected intraperitoneally into naive mice (5 × 107 cells/0.3 ml PBS per mouse). Details of the MBP immunization and transfer have been described (Voskuhl et al., 1996).
2.4. Clinical assessment
Animals induced to have EAE were examined daily for clinical signs. Severity was graded on a scale of 0–5 with increasing disability, as described (Pettinelli and McFarlin, 1981), with 4 representing hindlimb paralysis, and 5, a moribund state.
2.5. Preparation of tissues
Mice were sacrificed at various clinical stages for dissection of spinal cords. Slices of thoracic and lumbar cords (2–3 mm thick) were frozen in dry ice cooled 2-methyl butane, then transferred for storage at −70°C. 8–10 μm cryostat sections were cut transversely through cords. Adjacent sections from the same spinal cord regions were mounted on silane treated slides and used for in situ hybridization as described (Jordan et al., 1989b).
2.6. In situ hybridization
Synthetic oligonucleotide probes (48 mers) were designed complementary to RNAs encoded by MBP exon 2, exon 1, and proteolipid protein (PLP) as follows:
Murine exon 2 MBP antisense sequence:
CTTGTACATGTGGCACAGCCCAGGACGGCTGCGG- GCATGAGAGGGCAG
Murine exon 2 MBP sense sequence:
CTGCCCTCTCATGCCCGCAGCCGTCCTGGGCTGT- GCCACATGTACAAG
Murine exon 1 MBP antisense sequence:
TGTGGCCAGGTACTTGGATCGCTGTGAGGGTCTC- TTCTGTGATGCCAT
Murine PLP antisense sequence:
TGTACCAGTGAGAGCTTCATGTCCACATCCACAG- AACAGTGCCACTCC
Probes were tail labeled at their 3′ with 35S-dATP by terminal transferase (Boehringer–Manheim) to a specific activity of 1 × 109 cpm/μg and used at a concentration of 3 × 104 cpm/μl for hybridization as described (Jordan et al., 1989b) with minor modifications. Spinal cord sections were hybridized with probe at 37°C overnight, then washed with 2 × SSC and 50% formamide four times for a period of 15 min (each wash) at 40°C. Slides were then washed again with 1 × SSC for one h, and then with 0.2 × SSC for another h, each at 45°C. Localization of bound probe was visualized by coating slides with emulsion (NBT-2, Kodak), developing, and counterstaining. Slides were counter-stained with 0.2% toluidine blue for one minute before mounting with Permount™. Animals were rated positive for exon 2 transcript expression when specific clusters of grains (undetected with sense probe) were found in association with lesions.
3. Results
3.1. Clinical course of EAE
The onset of EAE was observed 12 to 23 days following transfer. Mice with EAE demonstrated two clear clinical episodes of disease before entering a stage of chronic stable disability with a minority of mice demonstrating a third clinical episode. EAE mice were sacrificed at various clinical timepoints: the preclinical stage (day 4 post inoculation), the peak of the first episode, the recovery stage (decreasing disability) of the first episode, the peak of the second episode, or the end stage of chronic stable disability after the second episode (Table 1 ).
Table 1.
Correlation of the detection of exon 2 containing MBP transcripts with disease stage and clinical score in relapsing EAE
| Exon 2 MBP positive | Exon 2 MBP negative | Total | |
|---|---|---|---|
| Preclinical | 0 | 1 (1 = grade 0) | 1 |
| 1st episode | |||
| Peak | 11 (7 = grade 4; 4 = grade 3) | 6 (4 = grade 4; 2 = grade 3) | 17 |
| Recovery | 0 | 9 (8 = grade 2; 1 = grade 1) | 9 |
| 2nd episode | |||
| Peak | 5 (4 = grade 4; 1 = grade 3) | 8 (5 = grade 4; 3 = grade 3) | 13 |
| Recovery | 1 (1 = grade 3) | 6 (2 = grade 3; 4 = grade 2) | 7 |
| Chronic | |||
| Stable | 0 | 5(5 = grade 3) | 5 |
| Total | 17 | 35 | 52 |
Animals were rated positive for exon 2 transcript expression when specific clusters of grains (undetected with sense probe) were found in association with lesions. Mice positive and negative for detection of exon 2 containing MBP transcript expression in spinal cord lesions are shown in relation to the clinical stage of disease and disability score (disease grade).
3.2. Detection of exon 2 containing MBP transcripts and correlation with disease stage
35S labeled exon 2-MBP antisense probe was hybridized with spinal cord sections of 4–7 day old newborn mice to detect exon 2 containing MBP transcripts expressed at this early stage of myelination (Jordan et al., 1989b). A characteristic signal was observed in white matter using the exon 2 MBP antisense probe, while adjacent sections from the ame cords hybridized with sense probe had no detectable signal (not shown). The same probes were then used to examine spinal cords of mice at various times after adoptive transfer of MBP specific T cells. While a mouse in the preclinical stage did not demonstrate exon 2 specific signal, the majority (11/17, 64%) of mice sacrificed during the peak of the first episode of disease showed exon 2 MBP containing transcripts in lesions of their spinal cords (Fig. 1A and B, Table 1). At the peak of the second episode 5/13 (38%) were expressing these transcripts. Therefore a total of 16/30 (53%) were positive for exon 2 signal when assessed at the peak of the first or second episode. In contrast EAE mice sacrificed during the recovery phase of the first episode (days 3 to 7 after the peak of the first episode) did not reveal exon 2 containing MBP transcripts (Table 1). Similarly, when mice were sacrificed or more days after the peak of the second episode, exon 2 positive cells were again generally not detected (Table 1). Therefore the presence of exon 2 containing MBP transcripts was clearly correlated with disease stage. Seventeen of 52 mice examined were positive for the detection of these transcripts. All 17 mice had severe EAE of clinical grade 3 or 4.
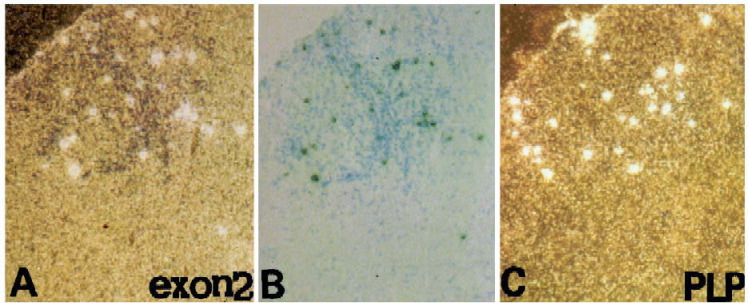
Fig. 1.
Detection of exon 2 containing MBP (A) and PLP (C) transcripts in cryostat sections of the ventral region of the lumbar spinal cord in a mouse with EAE (25 days after passive transfer, grade 4 clinical score). (B) shows the cluster of grains (gray) and inflammatory cells after toluidine blue staining. (A) and (C) are dark field photographs and (B) is a bright field micrograph.
Exon 2 containing transcripts were localized in cells scattered in areas of inflammatory infiltrates (Fig. 2 ) which generally indicate active demyelination (Raine et al., 1984). Normal appearing white and grey matter distant from the lesions were devoid of clusters of grains. In situ hybridization signal was also detected in and around the lesions using antisense probes for MBP exon 1, which detects all MBP transcripts (not shown). To determine whether transcripts encoding the proteolipid protein (PLP) were present in the same lesions as exon 2 MBP containing transcripts, adjacent sections of the lumbar spinal cord were hybridized with each probe (Fig. 1C). Within lesions, both MBP and PLP transcripts were detected as grain clusters (Fig. 2). The presense of grain clusters suggests that they may be associated with remyelinating oligodendrocytes (Jordan et al., 1989a)
Fig. 2.
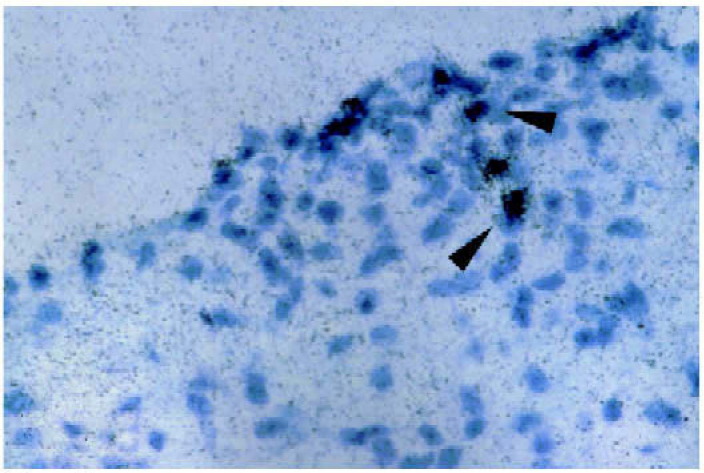
Higher magnification of cryostat section containing exon 2 positive signals in another mouse 18 days after passive transfer at first peak of clinical disease (grade 4 clinical score). Cells expressing the exon 2 containing transcripts (arrowheads) are found in regions with inflammatory infiltrates.
4. Discussion
In passive EAE in the SJL mouse, areas of remyelina-tion are found in close association with lesions (Raine et al., 1984) making this an appropriate model to investigate mechanisms of remyelination. We demonstrate here that, in autoimmune mediated relapsing EAE in adult mice, MBP exon 2 transcripts are expressed in the spinal cord of the majority (16/30) of mice at the peak of clinical disease during the first and second episodes of disease. This molecular marker of remyelination was found only in mice examined at the peak of a clinical episode of paralysis and was rare in mice examined during the recovery or stable phase of disease. PLP transcripts also appear to be upregulated in EAE lesions and may be expressed by remyelinating oligodendrocytes.
We observed that the signal for exon 2 MBP containing transcripts was the greatest in lesions characterized by prominent inflammatory cell infiltration. Activated macrophages or microglia may secrete factors that promote chemotaxis of oligodendrocyte precursor cells which then differentiate and express exon 2 containing MBP and PLP transcripts (Kristensson et al., 1986). Alternatively oligodendrocytes surviving in the lesions may dedifferentiate and express exon 2 MBP containing transcripts as observed in adult human oligodendrocytes regenerating in vitro (Gogate et al., 1994). In addition, MBP exon 2 containing transcripts and proteins have been recently described in multiple sclerosis (MS) lesions by in situ hybridization and immunohistochemistry (Cappello et al., 1995). Finally, it has been shown that insulin-like growth factor-I (IGF-I) enhances remyelination in acute EAE in the rat (Yao et al., 1995). Thus, the detection of exon 2 MBP transcript expression may be a useful molecular marker of remyelination when such therapies are applied to relapsing autoimmune mediated demyelinating disease.
Acknowledgements
This work was supported by a Human Frontier Science Program fellowship to Dr. Kunihiko Nagasato, M.D. Wesley Farris II, B.S. was a Howard Hughes Medical Institute-NIH scholar while performing this work. We thank Dr. Atsuo Nakayama for his advice on the in situ hybridization experiments.
References
- Brack W., Schmied M., Suchanek G., Brack Y., Breitschopf H., Poser S., Piddlesden S., Lassmann H. Oligodendrocytes in the early course of multiple sclerosis. Ann. Neurol. 1994;35:65–73. doi: 10.1002/ana.410350111. [DOI] [PubMed] [Google Scholar]
- Cappello E., Voskuhl R.R., McFarland H.F., Raine C.S. Expression of a novel candidate autoantigen X2 MBP, in multiple sclerosis (MS) lesions (Abstract) J. Neuropathol. Exp. Neurol. 1995;54:464. [Google Scholar]
- Deibler G.E., Martenson R.E., Kies M.W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep. Biochem. 1972;2:139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Gogate N., Verma L., Zhou E., Milward J.M., Rusten R., O’Connor M., Kufta C., Kim J., Hudson L., Dubois-Dalcq M. Plasticity in the human oligodendrocyte lineage. J. Neurosci. 1994;14:4571–4587. doi: 10.1523/JNEUROSCI.14-08-04571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C.A., Friedrich V.L., Dubois-Dalcq M. In situ hybridization of myelin gene transcripts in developing mouse spinal cord. J. Neurosci. 1989;9:248–257. doi: 10.1523/JNEUROSCI.09-01-00248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C., Friedrich V.L., Godfraind C., Holmes K.V., Dubois-Dalcq M. Expression of viral and myelin gene transcripts during a demyelinating disease caused by a mouse corona virus. Glia. 1989;2:318–329. doi: 10.1002/glia.440020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C.A., Friedrich V.L., DeFerra F., Weismiller D.G., Holmes K.V., Dubois-Dalcq M. Differential exon expression in myelin basic protein transcripts during central nervous system (CNS) remyelination. Cell. Molec. Neurobiol. 1990;10:3–18. doi: 10.1007/BF00733631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K., Holmes K.V., Duchala C.S., Zeller N.K., Lazzarini R.A., Dubois-Dalcq M. Increased levels of myelin basic protein transcripts gene in virus-induced demyelination. Nature. 1986;322:544–547. doi: 10.1038/322544a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S., Kitamura K., Campagnoni A.T. Identification of a cDNA coding for a fifth form of myelin basic protein in mouse. Proc. Natl. Acad. Sci. USA. 1987;84:886–890. doi: 10.1073/pnas.84.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinelli C.B., McFarlin D.E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2− T lymphocytes. J. Immunol. 1981;127:1420–1423. [PubMed] [Google Scholar]
- Prineas J.W., Barnard R.O., Kwon E.E., Sharer L.R., Cho E.S. Multiple sclerosis: Remyelination of nascent lesions. Ann. Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Raine C.S., Mokhtarian F., McFarlin D.E. Adoptively transfered chronic relapsing experimental allergic encephalomyelitis in the mouse: Neuropathological analysis. Lab. Invest. 1984;51:534–546. [PubMed] [Google Scholar]
- Raine C.S., Wu E. Multiple sclerosis: Remyelination in acute lesions. J. Neuropath. Exp. Neurol. 1993;52:199–204. [PubMed] [Google Scholar]
- Voskuhl R.R., Pitchekian-Halabi H., MacKenzie-Graham A., McFarland H.F., Raine C.S. Gender differences in autoimmune demyelination in the mouse: Implications for multiple sclerosis. Ann. Neurol. 1996;39:724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- Yao D., Liu X., Hudson L., Webster H. Insulin-like growth factor-I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 1995;92:6190–6194. doi: 10.1073/pnas.92.13.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]