Abstract
Current methods for the accurate diagnosis of influenza based on culture of the virus or PCR are highly sensitive and specific but require specialised laboratory facilities and highly trained personnel and, in the case of viral culture, can take up to 14 days to obtain a definitive result. In this study, a quartz crystal microbalance-based immunosensor (QCM) has been developed and its potential evaluated for the rapid and sensitive detection of both influenza A and B viruses in laboratory-cultured preparations and clinical samples. The effective limit for detection by QCM for stock preparations of both A/PR/8/34 and B/Lee/40 viruses was 1 × 104 pfu/mL, associated with observed frequency shifts of 30 (±5) and 37 (±6.5) Hz, respectively. Conjugation of 13 nm gold nanoparticles to the detecting antibody improved the mass sensitivity of the immunosensor, resulting in a 10-fold increase in sensitivity and a detection limit of 1 × 103 pfu/mL for both preparations, with resulting frequency shifts of 102 (±11) and 115 (±5) Hz, respectively. Detection of virus in nasal washes with this technique was achieved by overnight passage in MDCK cultures prior to analysis. A comparison of results obtained from 67 clinical samples using existing RT-PCR, shell vial, cell culture and ELISA methods showed that QCM techniques were comparable in sensitivity and specificity to cell culture methods.
Keywords: Influenza, Quartz crystal microbalance, Detection, Immunosensor
1. Introduction
The contribution of diagnostic techniques to the management of patients with viral infections has increased considerably in the last decade, led by improved technologies that allow rapid, accurate and sensitive diagnosis of viral pathogens for an increasing range of infections. Rapid diagnosis allows the prompt initiation of effective antiviral therapy, especially in immunocompromised patients. Viral culture, usually in combination with immunofluorescence, has been regarded as the gold standard for the laboratory diagnosis of respiratory viruses (Doing et al., 1998, Johnston and Siegel, 1991). However, these tests are not rapid and their clinical value is often limited. In the case of influenza viruses, the isolation and identification by culture requires 2–14 days for the diagnosis of an illness whose duration is typically 5–7 days (Covalciuc et al., 1999). Rapid antigen detection tests (≤1 h) are less sensitive and sometimes less specific than culture or molecular methods but, nevertheless, can serve as a guide for appropriate treatment with antiviral agents (Storch, 2003). PCR and real-time-PCR techniques are highly sensitive, relatively specific and more rapid than cell culture (Atmar et al., 1996, Kehl et al., 2001, Liolios et al., 2001, Templeton et al., 2004, van Elden et al., 2002) and are widely used for the diagnosis of respiratory viruses but are more complex and require highly trained personnel. False-positives can result due to the high sensitivity of the PCR amplification system (Storch, 2003, Su et al., 2003). Accordingly, the need for rapid detection methods with high sensitivity and specificity that are easy to perform and interpret remains a research priority (Su et al., 2003).
Considerable effort has been directed towards the development of simple biosensors for the detection of viruses in point-of-care tests (Critchley and Dimmock, 2004, Eun et al., 2002, Hardy and Dimmock, 2003, Su et al., 2003, Wu et al., 2005, Zhou et al., 2002). Biosensors which detect interactions between viral antigens and specific antibodies (immunosensors) can be classified according to the type of transducer used in the device (Eun et al., 2002, Mecea, 2005). Piezoelectric sensors, such as the quartz crystal microbalance (QCM), detect mass changes due to molecular interactions on the surface of the transducer (Gajendragad et al., 2001, Vaughan et al., 1999). The application of an external electrical potential to a piezoelectric material, such as quartz, produces internal mechanical stresses that induce an oscillating electric field which, in turn, initiates an acoustic wave throughout the crystal. These waves travel in a direction perpendicular to the plate surfaces (Ebato et al., 1994, Janshoff and Steinem, 2001, Mecea, 2005).
Sauerbrey (1959) first described the relationship between observed frequency decrease (Δf) and deposited mass (m) on the crystal surface in air or a vacuum (Mecea, 2005, Sauerbrey, 1959, Vaughan et al., 1999). The frequency changes observed when a liquid is passed over the QCM crystal surface are also dependent on both the density and viscosity of the solution (Kanazawa and Gordon, 1985). QCM devices are relatively simple and convenient to use and can detect rapid, real-time responses to binding events on the crystal surface, such as antigen–antibody interactions (Lee and Chang, 2005, Park et al., 2003, Skládal et al., 2004, Uttenthaler et al., 1998), and have been applied to several areas in biotechnology including clinical diagnosis (Janshoff and Steinem, 2001, Nath and Chilkoti, 2002) and environmental monitoring (Kurosawa et al., 2006). More recently, a new approach has been described to improve the sensitivity of QCM biosensors by the use of antibody-functionalized nanoparticles as a mass amplification probe for a QCM sensor, resulting in significant enhancement of sensitivity (Chu et al., 2006).
In the current study, a QCM-based immunosensor was developed and evaluated for possible application in the rapid and sensitive detection of influenza viruses in clinical specimens, and compared with other commonly used diagnostic techniques.
2. Materials and methods
2.1. Clinical specimens
Nasal wash samples were collected from 67 hospital patients with acute signs and symptoms during the Australian winter of 2005 and provided by two Australian national reference laboratories: (a) Victorian Infectious Diseases Reference Laboratory (VIDRL), Melbourne, Australia and (b) WHO Collaborating Center for Reference and Research on Influenza, Melbourne, Australia.
2.2. Cells and viruses
Cultures prepared from cells of the Madin-Darby canine kidney (MDCK) line (obtained from CSL Ltd., Parkville, Australia) were used in all studies and were grown in Eagle's Minimal Essential Medium (MEM) containing 10 mM HEPES (N-2-hydroxyethylpiperazine-N-2 ethanesulfonic acid), 0.14% (w/v) sodium bicarbonate, 100 U/mL penicillin G, 100 μg/L streptomycin and 1 μg/L amphotericin B, supplemented with fetal calf serum to 5% (v/v) pH 7.2 in a 5% (v/v) CO2 incubator at 37 °C. Human influenza A/PR/8/34 (H1N1) and B/Lee/40 viruses were included in the study for reference purposes and were grown in 10–11-day-old embryonated hens’ eggs.
2.3. Virus purification
Allantoic fluid containing virus was clarified by centrifugation at 3000 × g for 5 min. The supernatant fluid was then centrifuged at 160 030 × g for 60 min at 4 °C using an SW41 Ti rotor in a Beckman Optima™ L-80 XP Ultracentrifuge (Beckman Coulter Inc., Fullerton, USA) which was used for all subsequent ultracentrifuge separations. The virus pellet was resuspended in 500 μL of TNE (0.05 M Tris–HCl pH 7.4 containing 0.15 M NaCl, 1 mM EDTA) buffer. Concentrated virus was centrifuged to a 60% (w/v) sucrose cushion through a 30% (w/v) sucrose interface at 160 030 × g for 90 min. Virus was collected by aspiration and was then diluted 1:5 in TNE buffer and pelleted by centrifugation at 160 030 × g for 60 min. It was then resuspended in TNE buffer and centrifuged through a 15–60% (w/v) sucrose gradient at 160 030 × g for 12 h at 4 °C. The virus band was diluted 1:5 in TNE buffer and centrifuged at 160 030 × g for 60 min at 4 °C. The pellet was resuspended in TNE buffer and stored at −70 °C.
2.4. Pre-treatment of clinical specimens
A volume of 50 μL of each nasal wash sample from patients was incubated in duplicate tubes containing confluent MDCK cultures in 1 mL of serum-free MEM growth medium containing 1 μg/mL trypsin and incubated overnight at 34 °C in a 5% (v/v) CO2 incubator. The samples were centrifuged at 5000 × g for 20 min prior to collecting the supernatant fluid for QCM analysis.
2.5. Plaque assays
These were performed in 6-well confluent MDCK monolayer cultures, as previously described (Tannock et al., 1984).
2.6. Shell vial assays
Twenty-four-hour MDCK cell cultures were prepared on the surfaces of circular 12 mm coverslips in shell vials and washed twice with PBS prior to adding 50 μL of a fresh clinical sample to each cover slip. The shell vials were then centrifuged at 700 × g for 60 min and, after adding 1 mL of MEM containing 1 μg/mL trypsin (bovine pancreas, ICN Biomedicals, Seven Hills, NSW, Australia), were incubated for 48 h at 34 °C in a 5% (v/v) CO2 incubator. The cultures were then washed twice with PBS and fixed by adding 1 mL of chilled 80% (v/v) acetone in PBS for 15 min at 4 °C. Finally, the cultures were stained by indirect immunofluorescence (IFA) with monoclonal antibodies (MAbs) specific for nucleoprotein of influenza A and B viruses (Bartholoma and Forbes, 1989) followed by FITC-labeled goat anti-mouse antibody (Chemicon International Inc., Temecula, USA).
2.7. Directigen® FLU-A enzyme immunoassay
The Directigen® influenza A in vitro enzyme immunoassay (Becton, Dickinson and Co., Sparks, USA) was used for rapid detection of influenza A antigen from nasal washings, according to the manufacturer's instructions.
2.8. Antigen ELISA
An antigen ELISA was developed using the method described by Bucher et al. (1991). The wells of a 96 well Immulon 2HB microtiter plate (Thermo Scientific, Milford, USA) were coated by adding MAbs specific for the M1 protein of influenza A or B viruses (4–8 μg/100 μL in 0.1 M sodium carbonate buffer pH 9.6) to each well and leaving overnight at 4 °C. Unattached antibody was removed by washing and dilutions in PBS containing 50 μL of purified A/PR/8/34 or B/Lee/40 (initial concentration 100 μg/mL) were added to individual wells. Then 50 μL of a 1:1000 dilution of chicken antisera prepared against a purified preparation of the M1 protein of either A/PR/8/34 or B/Lee/40 were used as primary antibodies. After washing, 50 μL of a 1:2000 dilution of goat anti-mouse-HRP conjugate (Chemicon International Inc., Temecula USA) was used as the secondary detection antibody. Following color development using 3,3′,5,5′-tetramethyl-benzidine (TMB, Becton, Dickinson and Co., Sparks, USA) as the substrate, absorbance values were determined using an ELISA plate reader (Dynatech MR 7000, Dynatech Laboratories, Chantilly, USA) set at 450 nm.
2.9. RT-PCR
Viral RNA was extracted from allantoic fluid or nasal wash samples using a QIAamp® Viral RNA Mini Kit (Qiagen, Hamburg GmBH). RT-PCR was performed using the Titan™ One Tube RT-PCR system (Roche Diagnostics, Mannheim GmBH) according to the manufacturer's instructions. Egg-grown influenza A/PR/8/34 (H1N1) and B/Lee/40 viruses and all nasal samples were tested by RT-PCR using the method described by Poddar (2002).
2.10. QCM sensor system
A Maxtek RQCM sensor coupled to a flow injection system was used with 25 mm 9 MHz quartz crystals mounted in a Maxtek CHC-100 crystal holder (Maxtek Inc., Cypress, USA). An oscillator/frequency counter collected the output signal of the oscillator and outputs measured frequency changes (Δf), resistance changes and changes in mass. The flow injection system consisted of a fluid circuit with a flow-through cell (FC-550 Flow Cell, Maxtek Inc., Cypress, USA), Rheodyne injection switching valve (Model 5020, Rheodyne LLC, Rohnert Park, USA) and a Razel syringe pump (Model A-99, Razel Scientific Instruments Inc., Stamford, USA). A schematic diagram of the apparatus used in this work is shown in Fig. 1 . All experiments were carried out at room temperature.
Fig. 1.

Schematic of the apparatus used for continuous flow detection of influenza using a quartz crystal microbalance.
2.11. Pre-treatment of QCM crystals
The piezoelectric quartz crystal was immersed in 1.2 N NaOH for 30 min to remove impurities from the crystal surface and then washed with distilled water, air dried and placed for 5 min in 1.2 N HCl. It was then washed with distilled water and ethanol and dried in a stream of nitrogen (Park et al., 2000).
2.12. Immobilization of antibodies on the quartz crystal
One hundred microlitres of Protein A dissolved in PBS (2 mg/mL) were added to the gold electrode of the quartz crystals and incubated at room temperature in a humid environment for 2 h. Analysis of the binding characteristics of the anti-M1 MAbs in ELISA experiments indicated that optimum binding was achieved at 8 μg/mL of the MAb specific for the M1 protein of A/PR/8/34 and 4 μg/mL for that of B/Lee/40. Protein A-coated crystals were incubated with 100 μL of each antibody solution at these concentrations for 1 h at 37 °C in a humidified chamber, rinsed with PBS and distilled water, and dried in a stream of nitrogen.
2.13. Preparation of gold nanoparticles
Gold nanoparticles were prepared using a modification of the method of Grabar et al. (1995). A total of 190 mL of 5 × 10−3 M of gold (III) chloride trihydrate (HAuCl4·3H2O) in distilled water was brought to the boil with vigorous stirring in a 500 mL round-bottomed flask. Following the addition of 10 mL 0.5% (w/v) of trisodium citrate, the solution was boiled for an additional 10 min and allowed to cool while stirring for a further 15 min. The colloidal solution was stored in dark bottles at 4 °C. All glassware used was thoroughly cleaned in aqua regia (HCl:HNO3, 3:1), rinsed in distilled water and oven-dried prior to use.
2.14. Preparation of the antibody–nanoparticle conjugates
Antibody–nanoparticle conjugates were prepared by the addition of 30 μg of MAb specific for the M1 protein of either A/PR/8/34 or B/Lee/40 to 1 mL of a gold nanoparticle suspension containing 1.4 × 1012 nanoparticles in 5 mM sodium carbonate buffer pH 9.0, followed by incubation at room temperature with gentle mixing for 1 h. Then 200 μL of 5% (w/v) BSA in PBS were added and mixed for 30 min at room temperature to block free binding sites on the nanoparticles and to stabilize the nanoparticle suspension. The antibody–nanoparticle conjugate was centrifuged at 10 000 × g for 10 min. The supernatant fluid containing unbound antibody was discarded and the red-coloured antibody–nanoparticle conjugate pellet gently resuspended in 1 mL of TBS-BSA (20 mM Tris pH 8.0 containing 150 mM NaCl, 0.1% (w/v) BSA). Finally, after a second centrifugation step at 10 000 × g for 10 min, the supernatant fluid was removed and the antibody–colloidal gold conjugate pellet resuspended in 100 μL of TBS-BSA and stored at 4 °C (Chu et al., 2006).
3. Results
3.1. Determination of the sensitivity of the QCM for the detection of A/PR/8/34 and B/Lee/40
The resonance frequency shift of a QCM sensor is influenced by many factors, such as changes in mass, viscosity, dielectric constant of the solution and the ionic status of the crystal interface with the buffer solution (Kim et al., 2004, Mecea, 2005, Park et al., 2000). Accordingly, a number of parameters, including efficiency of the blocking buffer, the pH of the reaction buffer, the flow rate, the concentration of antibody and the orientation of the antibody in the presence of Protein A were optimized to determine the limits of detection for A/PR/8/34 and B/Lee/40 (data not shown).
Following immobilization of the MAb to the crystal electrode, dilutions of either A/PR/8/34 or B/Lee/40 (1 × 103–1 × 108 pfu/mL) in PBS were injected continuously onto the immunosensor at a constant flow rate of 3 mL/h using a syringe pump. The flow path of the sensor was rinsed with PBS prior to loading of each virus dilution analysed. Typical recordings of frequency changes observed with the binding of either virus are shown in Fig. 2(A and B), which shows a frequency shift in the range 15–493 Hz for A/PR/8/34 and 16–410 Hz for B/Lee/40 viruses, respectively, and was dependent on the titer of the virus added. The data show frequency changes (ΔHz) after introducing the virus samples to the sensor surface for 60 min post-injection. These data suggest that the effective minimum detection limit for both A/PR/8/34 and B/Lee/40 was 1 × 104 pfu/mL, which was associated with frequency shifts of 30 (±5) and 37 (±6.5) Hz, respectively (n = 3).
Fig. 2.
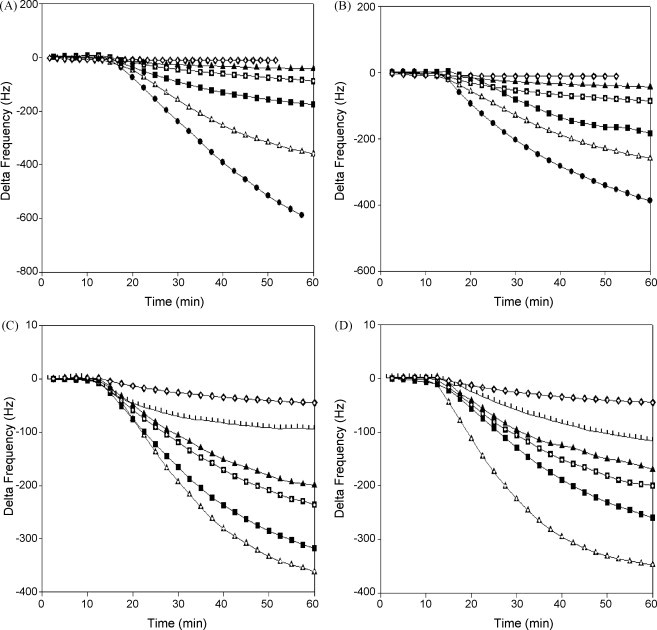
Time-dependent frequency changes at different virus concentrations. (A) Frequency changes of the immunosensor using A/PR/8/34 virus. (B) Frequency changes of the immunosensor using B/Lee/40 virus. (C) Nanoparticle-conjugated anti-influenza A monoclonal antibody (A/PR/8/34). (D) Nanoparticle-conjugated anti-influenza B monoclonal antibody (B/Lee/40). ( ) 108 pfu/mL, (
) 108 pfu/mL, ( ) 107 pfu/mL, (–■–) 106 pfu/mL, (
) 107 pfu/mL, (–■–) 106 pfu/mL, ( ) 105 pfu/mL, (–▴–) 104 pfu/mL, (
) 105 pfu/mL, (–▴–) 104 pfu/mL, ( ) 103 pfu/mL, and (
) 103 pfu/mL, and ( ) negative.
) negative.
3.2. Use of nanoparticles to increase the sensitivity of the biosensor
Gold nanoparticles conjugated with antibodies were used in an attempt to increase the sensitivity of the QCM immunosensor for the detection of influenza viruses. The diameter of the nanoparticles used was determined to be 13 nm by transmission electron microscopy (TEM). Conjugation of the anti-influenza MAbs to nanoparticles was confirmed by visible absorbance measurements, which showed that unconjugated nanoparticles had an absorbance maximum (λ max) at 520 nm that increased to 527 nm following conjugation. This wavelength shift of 7 nm indicates that protein was bound to the surface of the nanoparticles resulting in an increased primary gold plasmon resonance peak (Nath and Chilkoti, 2002).
3.3. Frequency changes observed with the antibody–nanoparticle conjugates
Following attachment of antibody to the quartz crystal, purified A/PR/8/34 and B/Lee/40 preparations (titers 1 × 103–1 × 108 pfu/mL in PBS) were injected at a flow rate of 3 mL/h for 30 min. Then PBS was passed through for 15 min to remove unbound antigen, followed by injection of the nanoparticle–MAb conjugates specific for influenza A or B viruses. Frequency changes following the binding of the MAb–nanoparticle conjugates to the cognate antigen were recorded for 60 min. Typical sensor profiles for purified A/PR/8/34 and B/Lee/40 are shown in Fig. 2(C and D). From this data, the lowest detectable infectious titer for purified preparations of both A/PR/8/34 and B/Lee/40 was 1 × 103 pfu/mL (10 μg/mL) corresponding to frequency shifts of 102 (±11) and 115 (±5) Hz, respectively (n = 3). Negative control background measurements in the absence of virus were 65 (±10) Hz for each nanoparticle–antibody conjugate preparation used.
Comparisons of the frequency shift responses to different viral dilutions in the range 1 × 103–1 × 108 pfu/mL, determined in both the presence and absence of nanoparticles are summarized in Table 1 . These results support those shown in Fig. 2(C and D) that the conjugation of nanoparticles with virus-specific MAbs substantially increases the sensitivity of the assay. Use of the nanoparticle–antibody conjugates resulted in 5.8-fold and 6.7-fold increases in frequency shift responses in the detection of 1 × 103 pfu/mL of A/PR/8/34 and B/Lee/40, respectively, compared with measurements taken without the use of nanoparticles. At higher titers, these differences in sensitivity were less; at 1 × 107 pfu/mL, increases in frequency shift of 0.2-fold were observed for A/PR/8/34 and 0.15-fold for B/Lee/40 (Table 1; Fig. 2).
Table 1.
Detection of A/PR/8/34 and B/Lee/40 viruses with and without nanoparticles.
| Virus | Negative control | 103a | 104 | 105 | 106 | 107 | |
|---|---|---|---|---|---|---|---|
| Influenza A | Without nanoparticles | 15b ± 2 | 15 ± 4.5 | 30 ± 5 | 52 ± 7.6 | 144 ± 10.4 | 303 ± 20.2 |
| Frequency shift (Hz) | With nanoparticles | 65 ± 10 | 102 ± 11 | 191 ± 14 | 227 ± 8 | 326 ± 9 | 370 ± 9.5 |
| Percentage increase for influenza A | 333 | 580 | 536.6 | 336 | 126 | 22 | |
| Influenza B Frequency shift (Hz) | Without nanoparticles | 15b ± 2.5 | 15 ± 4.5 | 37 ± 6.5 | 66 ± 5 | 212 ± 12.5 | 298 ± 12.5 |
| With nanoparticles | 65 ± 7.5 | 115 ± 5 | 214 ± 5.2 | 233 ± 10.4 | 313 ± 12.5 | 345 ± 5 | |
| Percentage increase for influenza B | 333 | 666 | 478 | 253 | 47 | 15 | |
pfu/mL.
Results are expressed as mean values; ±standard deviation; n = 3.
3.4. Relationship between infectious titer and observed frequency change
Fig. 3 shows the relationship between the infectious titer of A/PR/8/34 and B/Lee/40 and the frequency changes recorded in semi-logarithmic plots. This analysis indicates an approximate linear relationship between virus concentration and observed frequency change for each virus. The frequency changes were observed with both viruses in the titer range of 1 × 103–1 × 108 pfu/mL lie within the calibration range, thus allowing an estimation of A/PR/8/34 and B/Lee/40 virus titers in unknown samples.
Fig. 3.
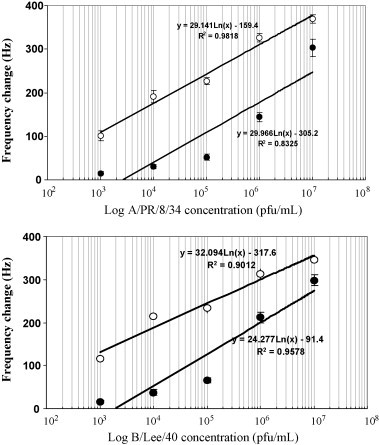
Relationship between influenza virus concentration and frequency shifts in the presence and absence of nanoparticles. (A) A/PR/8/34. (B) B/Lee/40. (●) Without nanoparticles and (○) with nanoparticles.
3.5. Detection of influenza viruses in nasal washings
Dilutions of purified A/PR/8/34 (1 × 103–1 × 108 pfu/mL) were used to spike a 20% (v/v) nasal wash specimen in PBS from a normal asymptomatic volunteer. The diluted sample was mixed and centrifuged at 5000 × g for 20 min and the supernatant collected for analysis. Significant noise in the frequency response was observed in all samples, including the negative control that consisted of the sample spiked with the same volume of PBS without virus (data not shown). Therefore, direct detection of the virus from diluted nasal washes by this technique does not appear practicable. The signal noise resulting from testing of the clinical samples could not be eliminated by simple pre-treatment of clinical samples either by microfiltration through 0.2 μm and 0.45 μm Anodisc filters (Whatman plc Kent, UK) or by overnight digestion with up to 15 mg/mL (600 000 units) chicken egg white lysozyme (Sigma–Aldrich, St Louis, MO, USA) followed by centrifugation at 5000 × g for 20 min (data not shown).
However, when the same samples were inoculated into MDCK cell cultures and incubated overnight prior to QCM analysis, significant and stable signal frequency shifts for the culture maintenance fluids of positive samples were observed in the range 103–109 copies of M1 cDNA/mL (Fig. 4 ). For stocks of purified A/PR/8/34, 1 pfu/mL was equivalent to 104.54 copies/mL and 104.46 copies/mL for B/Lee/40 (44). These signals appeared in the absence of a visible CPE in infected cultures and the procedure was used in all subsequent evaluations of the QCM technique on clinical samples.
Fig. 4.
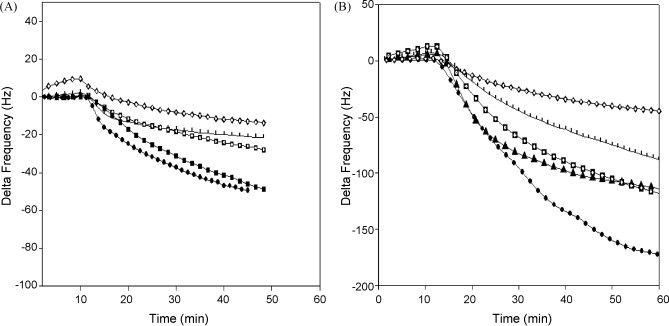
Time-dependent frequency changes of clinical samples in cell culture medium. (A) Frequency changes of clinical samples (without nanoparticles). (B) Frequency changes of clinical samples using nanoparticle-conjugated influenza A monoclonal antibody. ( ) 109 copies/mL, (–■–) 106 copies/mL, (
) 109 copies/mL, (–■–) 106 copies/mL, ( ) 105 copies/mL, (–▴–) 104 copies/mL, (
) 105 copies/mL, (–▴–) 104 copies/mL, ( ) 103 copies/mL, and (
) 103 copies/mL, and ( ) negative.
) negative.
3.6. Detection of influenza viruses by currently available methods and QCM
The results from these tests on the 67 clinical samples are summarized in Table 2 . Based on data from Fig. 2, the readout for determining positive samples was 40 Hz for the QCM analyses that did not involve the use of conjugated nanoparticles and 120 Hz for the QCM analyses when nanoparticles were used. Influenza A and B viruses were detected by RT-PCR, shell vial assay, cell culture and ELISA in 52, 40, 45 and 34 of the 67 clinical specimens, respectively. The Directigen Flu A test detected influenza A virus in only 13 specimens. The QCM and nanoparticle-enhanced QCM (NP-QCM) tests detected influenza A and B viruses in 40 and 43 specimens, respectively. The influenza viruses present in the clinical samples were A/New Caledonia/20/99-like (H1N1), A/Wyoming/3/2003-like (H3N2); influenza B viruses were B/Brisbane/32/2002-like. However, 12, 7 and 18 RT-PCR-positive specimens were negative by the shell vial, cell culture and ELISA methods for influenza A and B viruses, respectively. Twelve and nine of the 52 samples positive by the RT-PCR technique were negative by QCM and NP-QCM methods.
Table 2.
Detection of influenza A and B viruses in the nasal wash samples from 67 patients.
| Virus type | No (%) of specimens found positive bya: |
||||||
|---|---|---|---|---|---|---|---|
| RT-PCR | Shell vial | Cell culture | ELISA | Directigen Flu A | QCM | QCM/nanoparticle | |
| Influenza Ab | 37 (55) | 28 (42) | 30 (45) | 25 (37) | 13 (19) | 28 (42) | 30 (45) |
| Influenza Bc | 15 (22) | 12 (18) | 15 (22) | 9 (13) | NA | 12 (18) | 13 (19) |
| Totals | 52 (77) | 40 (60) | 45 (67) | 34 (50) | 13 (19) | 40 (60) | 43 (64) |
Times for the tests were RT-PCR: 1 day, shell vial: 2 days, cell culture: 14 days, ELISA: 1 day; Directigen Flu A: 20 min, QCM: 2 days.
During the Australian winter of 2005, all influenza A viruses detected were A/New Caledonia/20/99-like (H1N1) (2/37) and A/Wyoming/3/2003-like (H3N2) (35/37).
Influenza B viruses were B/Brisbane/32/2002-like.
As shown in Table 3 , the respective sensitivities of the tests for the detection of influenza A and B viruses by the shell vial, standard cell culture, ELISA, Directigen Flu A test, QCM and NP-QCM tests were 76 and 80, 81 and 100, 68 and 60, 35 (for influenza A only) and 76 and 80 and 81 and 87% for influenza B viruses (Table 3). The specificity, positive and negative predictive values for the detection of each virus are shown in Table 3. The highest number of false-negative results (24) was obtained with the Directigen Flu A test.
Table 3.
Results obtained for shell vial, standard cell culture, ELISA, Directigen Flu A and QCM methods in comparison with RT-PCR.
| Test | No of specimens |
Sensitivitye | Specificityf | PPVg | NPVh | ||||
|---|---|---|---|---|---|---|---|---|---|
| TPa | TNb | FPc | FNd | ||||||
| Shell vial | Influenza A | 28 | 27 | 3 | 9 | 76 | 90 | 90 | 75 |
| Influenza B | 12 | 52 | 0 | 3 | 80 | 100 | 100 | 95 | |
| Cell culture | Influenza A | 30 | 30 | 0 | 7 | 81 | 100 | 100 | 81 |
| Influenza B | 15 | 52 | 0 | 0 | 100 | 100 | 100 | 100 | |
| ELISA | Influenza A | 25 | 30 | 0 | 12 | 68 | 100 | 100 | 71 |
| Influenza B | 9 | 52 | 0 | 6 | 60 | 100 | 100 | 90 | |
| Directigen Flu A | Influenza A | 13 | 30 | 0 | 24 | 35 | 100 | 100 | 56 |
| Influenza B | NA | NA | NA | NA | NA | NA | NA | NA | |
| QCM | Influenza A | 28 | 30 | 0 | 9 | 76 | 100 | 100 | 77 |
| Influenza B | 12 | 52 | 0 | 3 | 80 | 100 | 100 | 95 | |
| QCM (nanoparticles) | Influenza A | 30 | 30 | 0 | 7 | 81 | 100 | 100 | 95 |
| Influenza B | 13 | 52 | 0 | 2 | 87 | 100 | 100 | 96 | |
A total of 67 nasal samples were compared with RT-PCR which was used as the reference method (gold standard).
Samples positive by shell vial, standard cell culture, ELISA, Directigen Flu A kit and QCM, but negative by RT-PCR, were considered FP. Samples that were identified by RT-PCR assays were considered as TP. Samples that were negative by shell vial, standard cell culture, ELISA, BD Directigen Flu A kit and QCM but positive by PCR were regarded as FN. A sample that was negative by RT-PCR was a TN.
TP, true-positives.
TN, true-negatives.
FP, false-positives.
FN, false-negatives.
Sensitivity = number of TP specimens/(number of TP + number of FN specimens) × 100.
Specificity = number of TN specimens/(number of TN specimens + number of FP specimens) × 100.
PPV (positive predictive value) = TP/(TP + FP) × 100.
NPV (negative predictive value) = TN/(TN + TN) × 100.
4. Discussion
This study was conducted in light of a continuing need for rapid sensitive methods for the identification and antigenic characterization of influenza viruses for epidemiological purposes and for the selection of vaccine strains. A QCM-based immunosensor was developed and evaluated for the rapid detection of both influenza A and B viruses in 67 nasal wash samples obtained during the 2005 Australian winter. Their sensitivity was compared with PCR, the shell vial and standard cell culture methods, an antigen ELISA and the commercially available Directigen Flu A kit (for influenza A viruses only).
One of the most important factors that affects the sensitivity of the QCM sensor is the optimal concentration of antibody immobilized on the crystal surface which leads to improved reaction kinetics and the avoidance of unfavourable effects such as minimal non-specific binding (Jenkins et al., 2004, Luppa et al., 2001). In this study, the optimum monoclonal antibody concentration immobilized on the QCM for maximum sensitivity was 8 μg/mL for A/PR/8/34 and 4 μg/mL for B/Lee/40 viruses. These results are consistent with the optimized concentrations that were used in the ELISA assays (data not shown). Once the optimal antibody concentration was determined, the antigen concentration was varied to determine the optimal antigen:antibody ratio to maximize the sensitivity of the sensor.
The results presented in Fig. 2 show that the infectious titer of the virus in the sample was directly proportional to the mass accumulation on the immobilized monolayer which was, in turn, proportional to the rate of frequency shift recorded. Results obtained with different concentrations of purified viruses in Fig. 2(A and B) showed that the lowest detectable infectious titer of both purified A/PR/8/34 and B/Lee/40 viruses was 104 pfu/mL (25 μg/mL). Using a similar approach with Protein A-immobilized anti-virus antibodies, König and Grätzel (1994) obtained detection limits of 5 × 104 pfu/mL for of human herpes viruses. However, Zuo et al. (2004) were able to detect as little as 0.6–4 μg/mL of the SARS coronavirus (SARS-CoV) in sputa in a gas phase using a QCM sensor coated with Protein A and polyclonal antibody.
In this study, anti-influenza A and B monoclonal antibodies were bound to 13 nm gold nanoparticles and these conjugates were used to improve the mass sensitivity of the sensor for influenza detection. As shown in Fig. 2(C and D) and summarized in Table 1, the lowest detectable viral infective titer of purified A/PR/8/34 and B/Lee/40 was 103 pfu/mL (10 μg/mL), a 10-fold increase in sensitivity for the QCM sensor using nanoparticle-bound anti-influenza A and B monoclonal antibodies. Given that viral titres in nasal wash samples of symptomatic patients are usually 103 pfu/mL or greater (35, 39), this step clearly increases the potential of such biosensors for the detection of influenza virus in clinical samples.
However, the data presented in Fig. 2(C and D) and summarized in Table 1 indicate that the use of nanoparticles did not result in a proportional increase in the sensitivity of the QCM sensor for the detection of influenza viruses with in samples with high infectious titers. Maximum enhancement of the sensitivity was observed only in the range 103–104 pfu/mL, suggesting that the adsorption of excess antibody–colloidal gold conjugate on the electrode surface leads to steric hindrance effects (Chu et al., 2006).
In order to quantitate viruses detected by the QCM sensor, calibration curves were established for both the QCM and NP-QCM techniques (Fig. 3). These curves describe the relationship between the frequency shift and the titer of the influenza virus suspension injected on to the sensor. When these data are plotted on a logarithmic-linear scale, an apparent linear relationship between amount of antigen and frequency shift was observed. A similar relationship was observed when nanoparticle-conjugated antibodies were used instead of antibody alone. Based on this apparent linear semi-logarithmic relationship between frequency shift and virus concentration, and with the use of nanoparticle–antibody conjugates, it should be possible to use this calibration curve to determine unknown viral titers for samples stored under optimal conditions within the range 103–107 pfu/mL (Fig. 3).
The basic parameters developed in this study for the QCM-based immunosensor were subsequently applied to the detection of influenza A and B viruses in clinical specimens. In this study, the capacity of the QCM and the NP-QCM methods to detect influenza viruses in the nasal washes of patients with influenza-like symptoms were compared with other currently used methods, including RT-PCR, standard cell culture, shell vial, ELISA and the Directigen Flu A (for influenza A virus only) methods.
QCM offers a number of potential advantages over existing techniques, which include obviating the need for labeling techniques to measure the binding reaction between virus and antibody, the use of short measurement times, operational simplicity, low cost, the opportunity to re-use the crystal sensors and the potential for online data collection. The QCM technique developed in this study was shown to be as sensitive as the shell vial method. The NP-QCM technique was more sensitive than the shell vial method and equally as sensitive as cell culture (Table 2), but required an additional step involving the use of a secondary nanoparticle-antibody conjugate. Direct detection of the virus from the nasal wash samples by QCM was not possible because of significant frequency fluctuations that occurred, possibly due to the viscosity of the samples. The heterogeneous nature of clinical samples appears to be an inherent limitation for diagnostic tests utilising piezoelectric immunosensors (Wu et al., 2005). Attempts to reduce the viscosity of the nasal wash samples of infected patients by centrifugation or microfiltration prior to analysis on our immunosensor proved unsuccessful (data not shown). However, Zuo et al. (2004) overcame this limitation by the atomisation of sputum samples containing SARS-CoV by ultrasonication prior to adsorption and detection on the immunosensor. In this initial study, an additional overnight culture step was used to overcome the viscosity effects of sputum samples resulting in an increased processing time of 2 days.
The sensitivities of tests for detection of influenza A and B by the shell vial, standard cell culture, ELISA, Directigen Flu A test, QCM and NP-QCM tests were 76 and 80, 81 and 100, 68 and 60, 35 (influenza A), 76 and 80 and 81 and 87%, respectively (Table 3). These results differ from those of Quach et al. (2002) who reported sensitivities between 64.2 and 84.7% and specificities of 90–100% compared with RT-PCR. However, in the present study, frozen samples were used and virus in newly collected samples could be expected to be more readily detected since proteolysis or denaturation of influenza antigens has been previously observed in stored nasal washes (Quach et al., 2002).
When the data for influenza A and B were combined, the sensitivities of each method were, in descending order, RT-PCR (100%), NP-QCM (83%), QCM (81%), standard cell culture (81%), shell vial (77%) and ELISA (69%) (Table 3). The times required to complete each test were: Directigen Flu A 20 min, RT-PCR 1 day, ELISA 1 day, shell vial 2 days, QCM and NP-QCM 2 days and the standard cell culture method up to 14 days (including the time for repassage of initially negative samples). Egg-grown preparations of A/PR/8/34 and B/Lee/40 could be detected by the QCM and NP-QCM methods within 30 min and 1 h, respectively.
Despite requiring a similar time interval (2 days) to complete the test that involves blind passage of clinical specimens, the NP-QCM method described is more economical, and simpler, more sensitive and objective in interpretation than the widely used Shell Vial Assay, often regarded as the ‘gold standard’ which requires the use of IFA, and cell culture. With further refinements, especially pre-treatment of samples to reduce viscosity effects, the direct analysis of patient specimens would greatly increase the use of this immunosensing technique and save considerable time in a clinical setting.
References
- Atmar R.L., Baxter B.D., Dominguez E.A., Taber L.H. Comparison of reverse transcription-PCR with tissue culture and other rapid diagnostic assays for detection of type A influenza virus. J. Clin. Microbiol. 1996;34:2604–2606. doi: 10.1128/jcm.34.10.2604-2606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholoma N.Y., Forbes B.A. Successful use of shell vial centrifugation and 16 to 18-hour immunofluorescent staining for the detection of influenza A and B in clinical specimens. Am. J. Clin. Pathol. 1989;92:487–490. doi: 10.1093/ajcp/92.4.487. [DOI] [PubMed] [Google Scholar]
- Bucher D.J., Mikhail A., Popple S., Graves P., Meiklejohn G., Hodes D.S., Johansson K., Halonen P.E. Rapid detection of type A influenza viruses with monoclonal antibodies to the M protein (M1) by enzyme-linked immunosorbent assay and time-resolved fluoroimmunoassay. J. Clin. Microbiol. 1991;29:2484–2488. doi: 10.1128/jcm.29.11.2484-2488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X., Zhao Z., Shen G., Yu R. Quartz crystal microbalance immunoassay with dendritic amplification using colloidal gold immunocomplex. Sensor Actuat. B: Chem. 2006;114:696–704. [Google Scholar]
- Covalciuc K.A., Webb K.H., Carlson C.A. Comparison of four clinical specimen types for detection of influenza A and B viruses by optical immunoassay (FLU OIA test) and cell culture methods. J. Clin. Microbiol. 1999;37:3971–3974. doi: 10.1128/jcm.37.12.3971-3974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley P., Dimmock N.J. Binding of an influenza A virus to a neomembrane measured by surface plasmon resonance. Bioorg. Med. Chem. 2004;12:2773–2780. doi: 10.1016/j.bmc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Doing K.M., Jerkofsky M.A., Dow E.G., Jellison J.A. Use of fluorescent-antibody staining of cytocentrifuge-prepared smears in combination with cell culture for direct detection of respiratory viruses. J. Clin. Microbiol. 1998;36:2112–2114. doi: 10.1128/jcm.36.7.2112-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebato H., Gentry C.A., Herron J.N., Műller W., Okahata Y., Ringsdorf H., Suci P.A. Investigation of specific binding of antifluorescyl antibody and Fab to fluorescein lipids in Langmuir–Blodgett deposited films using quartz crystal microbalance methodology. Anal. Chem. 1994;66:1683–1689. doi: 10.1021/ac00082a014. [DOI] [PubMed] [Google Scholar]
- Eun A.J., Huang L., Chew F.T., Li S.F., Wong S.M. Detection of two orchid viruses using quartz crystal microbalance (QCM) immunosensors. J. Virol. Methods. 2002;99:71–79. doi: 10.1016/s0166-0934(01)00382-2. [DOI] [PubMed] [Google Scholar]
- Gajendragad M.R., Kamath K.N.Y., Anil P.Y., Prabhudas K., Natarajan C. Development and standardization of a piezo electric immunobiosensor for foot and mouth disease virus typing. Vet. Microbiol. 2001;78:319–330. doi: 10.1016/s0378-1135(00)00307-2. [DOI] [PubMed] [Google Scholar]
- Grabar K.C., Freeman R.G., Hommer M.B., Natan M.J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995;67:735–743. [Google Scholar]
- Hardy S.A., Dimmock N.J. Valency of antibody binding to enveloped virus particles as determined by surface plasmon resonance. J. Virol. 2003;77:1649–1652. doi: 10.1128/JVI.77.2.1649-1652.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janshoff A., Steinem C. Quartz crystal microbalance for bioanalytical applications. Sensors Update. 2001;9:313–354. [Google Scholar]
- Jenkins M.S., Wong K.C.Y., Chhit O., Bertram J.F., Young R.J., Subaschandar N. Quartz crystal microbalance-based measurements of shear-induced senescence in human embryonic kidney cells. Biotechnol. Bioeng. 2004;88:392–398. doi: 10.1002/bit.20253. [DOI] [PubMed] [Google Scholar]
- Johnston S.L.G., Siegel C.S. A comparison of direct immunofluorescence, shell vial culture, and conventional cell culture for the rapid detection of influenza A and B. Diagn. Microbiol. Infect. Dis. 1991;14:131–134. doi: 10.1016/0732-8893(91)90047-j. [DOI] [PubMed] [Google Scholar]
- Kanazawa K.K., Gordon J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985;57:1770–1771. [Google Scholar]
- Kehl S.C., Henrickson K.J., Hua W., Fan J. Evaluation of the Hexaplex assay for detection of respiratory viruses in children. J. Clin. Microbiol. 2001;39:1696–1701. doi: 10.1128/JCM.39.5.1696-1701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N., Park I.-S., Kim D.-K. Characteristics of a label-free piezoelectric immunosensor detecting Pseudomonas aeruginosa. Sensor Actuat. B: Chem. 2004;100:432–438. [Google Scholar]
- König B., Grätzel M. A novel immunosensor for herpes viruses. Anal. Chem. 1994;66:341–344. doi: 10.1021/ac00075a005. [DOI] [PubMed] [Google Scholar]
- Kurosawa S., Park J.W., Aizawa H., Wakida S., Tao H., Ishihara K. Quartz crystal microbalance immunosensors for environmental monitoring. Biosens. Bioelectron. 2006;22:473–481. doi: 10.1016/j.bios.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Lee Y.-G., Chang K.-S. Application of a flow type quartz crystal microbalance immunosensor for real time determination of cattle bovine ephemeral fever virus in liquid. Talanta. 2005;65:1335–1342. doi: 10.1016/j.talanta.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Liolios L., Jenney A., Spelman D., Kotsimbos T., Catton M., Wesselingh S. Comparison of a multiplex reverse transcription-PCR-enzyme hybridization assay with conventional viral culture and immunofluorescence techniques for the detection of seven viral respiratory pathogens. J. Clin. Microbiol. 2001;39:2779–2783. doi: 10.1128/JCM.39.8.2779-2783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppa P.B., Sokoll L.J., Chan D.W. Immunosensors—principles and applications to clinical chemistry. Clin. Chim. Acta. 2001;314:1–26. doi: 10.1016/s0009-8981(01)00629-5. [DOI] [PubMed] [Google Scholar]
- Mecea V.M. From quartz crystal microbalance to fundamental principles of mass measurements. Anal. Lett. 2005;38:753–767. [Google Scholar]
- Nath N., Chilkoti A. A colorimetric gold nanoparticle sensor to interrogate biomolecular interactions in real time on a surface. Anal. Chem. 2002;74:504–509. doi: 10.1021/ac015657x. [DOI] [PubMed] [Google Scholar]
- Park I.-S., Kim W.-Y., Kim N. Operational characteristics of an antibody-immobilized QCM system detecting Salmonella spp. Biosens. Bioelectron. 2000;15:167–172. doi: 10.1016/s0956-5663(00)00053-1. [DOI] [PubMed] [Google Scholar]
- Park J., Kurosawa S., Aizawa H., Wakida S., Yamada S., Ishihara K. Comparison of stabilizing effect of stabilizers for immobilized antibodies on QCM immunosensors. Sensor Actuat. B: Chem. 2003;91:158–162. [Google Scholar]
- Poddar S.K. Influenza virus types and subtypes detection by single step single tube multiplex reverse transcription-polymerase chain reaction (RT-PCR) and agarose gel electrophoresis. J. Virol. Methods. 2002;99:63–70. doi: 10.1016/s0166-0934(01)00380-9. [DOI] [PubMed] [Google Scholar]
- Quach C., Newby D., Daoust G., Rubin E., McDonald J. QuickVue influenza test for rapid detection of influenza A and B viruses in a pediatric population. Clin. Diagn. Lab. Immunol. 2002;9:925–926. doi: 10.1128/CDLI.9.4.925-926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbrey G. Use of a quartz vibrator for weighing thin layers on a microbalance. Z. Phys. 1959;155:206–222. [Google Scholar]
- Skládal P., dos Santos Riccardi C.S., Yamanaka H., da Costa P.I. Piezoelectric biosensors for real-time monitoring of hybridization and detection of hepatitis C virus. J. Virol. Methods. 2004;117:145–151. doi: 10.1016/j.jviromet.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Storch G.A. Rapid diagnostic tests for influenza. Curr. Opin. Pediatr. 2003;15:77–84. doi: 10.1097/00008480-200302000-00013. [DOI] [PubMed] [Google Scholar]
- Su C.-C., Wu T.-Z., Chen L.-K., Yang H.-H., Tai D.-F. Development of immunochips for the detection of dengue viral antigens. Anal. Chim. Acta. 2003;479:117–123. [Google Scholar]
- Tannock G.A., Paul J.A., Barry R.D. Relative immunogenicity of the cold-adapted influenza virus A/Ann Arbor/6/60 (A/AA/6/60-ca), recombinants of A/AA/6/60-ca, and parental strains with similar surface antigens. Infect. Immun. 1984;43:457–462. doi: 10.1128/iai.43.2.457-462.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton K.E., Scheltinga S.A., Beersma M.F.C., Kroes A.C.M., Claas E.C.J. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1,2,3 and 4. J. Clin. Microbiol. 2004;42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenthaler E., Köβlinger C., Drost S. Characterization of immobilization methods for African swine fever virus protein and antibodies with piezoelectric immunosensor. Biosens. Bioelectron. 1998;13:1279–1286. doi: 10.1016/s0956-5663(98)00089-x. [DOI] [PubMed] [Google Scholar]
- van Elden L.J.R., van Kraaij M.G., Nijhuis M., Hendriksen K.A., Dekker A.W., Rozenberg-Arska M., van Loon A.M. Polymerase chain reaction is more sensitive than viral culture and antigen testing for the detection of respiratory viruses in adults with hematological cancer and pneumonia. Clin. Infect. Dis. 2002;34:177–183. doi: 10.1086/338238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan R.D., O'Sullivan C.K., Guilbault G.G. Sulfur based self-assembled monolayers (SAM's) on piezoelectric crystals for immunosensor development. Fresenius J. Anal. Chem. 1999;364:54–57. [Google Scholar]
- Wu T.Z., Su C.C., Chen L.K., Yang H.H., Tai D.F., Peng K.C. Piezoelectric immunochip for the detection of dengue fever in viremia phase. Biosens. Bioelectron. 2005;21:689–695. doi: 10.1016/j.bios.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Zhou X., Liu L., Hu M., Wang L., Hu J. Detection of hepatitis B virus by piezoelectric biosensor. J. Pharm. Biomed. Anal. 2002;27:341–345. doi: 10.1016/s0731-7085(01)00538-6. [DOI] [PubMed] [Google Scholar]
- Zuo B., Li S., Guo Z., Zhang J., Chen C. Piezoelectric immunosensor for SARS-associated coronavirus in sputum. Anal. Chem. 2004;76:3536–3540. doi: 10.1021/ac035367b. [DOI] [PubMed] [Google Scholar]


