Abstract
Severe acute respiratory syndrome (SARS) is a highly infectious disease caused by a novel coronavirus (SARS-CoV). Specific monoclonal antibodies (mAbs) against the SARS-CoV are vital for early diagnosis and pathological studies of SARS. Direct intrasplenic inoculation of plasmid DNA encoding antigen is an effective and fast approach to generate specific mAb when the protein antigen is difficult to prepare or dangerous in use. In this study, we selected one fragment of SARS-CoV spike protein (S1-3) as antigenic determinant by immunoinformatics. Single intrasplenic immunization of plasmid DNA encoding S1-3 induced anti-spike protein antibodies. We established one hybridoma cell line secreting specific mAb and evaluated this mAb with murine leukemia virus pseudotyped with SARS-CoV spike protein (MLV/SARS-CoV). The mAb could recognize the spike protein on the MLV/SARS-CoV-infected Vero E6 cells albeit with no neutralizing effect on the infectivity of the pseudotype virus. Our results show that a single-shot intrasplenic DNA immunization is efficient for the production of specific mAb against SARS spike protein, and a linear epitope of the spike protein is recognized in this study.
Keywords: Intrasplenic DNA immunization, Monoclonal antibody, Severe acute respiratory syndrome-coronavirus, Spike protein
1. Introduction
Severe acute respiratory syndrome (SARS) is a highly infectious atypical pneumonia with proximity 10% mortality in all infected cases [1]. Etiologic analysis has identified a novel coronavirus named the SARS-associated coronavirus (SARS-CoV) [2]. SARS-CoV spike protein, the major determinant of pathogenesis and the main target of protective immunity response, is composed of two non-covalently associated S1 and S2 domains [3], [4], [5], [6]. The N-terminal S1 domain is responsible for viral attachment with the cellular receptors, and the membrane-spanning fragment S2 domain takes part in the host cell entry and the infected cell–cell fusion. Recently, angiotensin-converting enzyme 2 (ACE2) has been identified as the functional receptor for SARS-CoV, and a neutralizing human monoclonal antibody 80R against ACE2 can block the binding of S1 domain to sensitive Vero E6 cells [7]. The ACE2-binding domain locates within the N-terminal residues 318–510 aa of spike protein, which has a higher affinity to ACE2 than the full S1 domain (residues 12–672 aa) [8].
Considering the high infectivity of SARS-CoV, a simple and fast diagnosis of SARS cases is crucial for the prevention of the outbreak. Since human SARS antibodies emerge only 1 week after the onset of symptom, the development of specific monoclonal antibodies (mAbs) against SARS-CoV spike protein is vital for early diagnosis and even the neutralization of SARS-CoV infection [5]. It has been shown that a single intrasplenic injection of plasmid DNA encoding foreign protein provides an effective and fast method to generate specific mAbs [9], [10], [11], [12]. The splenocytes including antigen-presenting cells are directly transfected by the injected plasmid DNA, and the expressed antigens are concentrated in the spleen, initiating the immune response promptly. In this study, we performed single intrasplenic immunization of plasmid DNA encoding one fragment of ACE2-binding domain in SARS-CoV spike protein (S1-3) to establish specific mAbs in a short time and evaluated this mAb with murine leukemia virus pseudotyped with SARS-CoV spike protein (MLV/SARS-CoV). The mAb could recognize the spike protein on the Vero E6 cells infected with MLV/SARS-CoV, but has no neutralizing effect on the infectivity of the pseudotype virus.
2. Materials and methods
2.1. Cells lines
The mouse myeloma NS1 cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS). Human kidney cells 293T and African green monkey kidney Vero E6 cells were maintained in Dulbecco's modified Eagle's medium (Gibco BRL, Gaithersburg, MD) containing 10% FCS. The cells were incubated at 37 °C in a humidified atmosphere of 5% CO2.
2.2. Antigenicity prediction and plasmid construction
Antigenic determinants in the ACE2-binding domain of SARS spike protein (Genbank accession no. AY278488) with no close match to other coronaviruses were predicted with PROTEAN (DNASTAR Lasergene program package). Selection of appropriate peptide sequence takes into account of hydrophilicity, hydrophobicity, surface probability, chain flexibility, antigenic index and secondary structure. Polypeptide S1-3 (residues 430–454 aa, ATSTGNYNYKYRYLRHGKLRPFERD), a potent antigenic site, was synthesized with conventional solid-phase chemistry (ABI Pioneer Model Peptide Synthesizer). The gene encoding S1-3 polypeptide was synthesized as two DNA oligonucleotide sequences as follows: 5′-TTC GGA TCC ACC ATG GCT ACT TCA ACT GGT AAT TAT AAT TAT AAA TAT AGG TAT CTT AGA CAT GGC AAG CTT AGG CCC TTTGAG AGA GAC TAA CTC GAG TTC-3′ (sense) and 5′-GAA CTC GAG TTA GTC TCT CTC AAA GGG CCT AAG CTT GCC ATG TCT AAG ATA CCT ATA TTT ATA ATT ATA ATT ACC AGT TGA AGT AGC CAT GGT GGA TCC GAA-3′ (antisense). A Kozak consensus sequence plus the initiation codon and a stop codon were added to the 5′ and 3′ end, respectively. The two DNA oligonucleotide sequences were annealed and inserted into the BamHI/XhoI sites of pcDNA3.1 (+) vector (Invitrogen) as pcDNA3.1-S1-3. After sequence confirmation, the plasmid for intrasplenic immunization was prepared by using EndoFree Plasmid Kit (Qiagen, Hilden, Germany).
2.3. Generation of monoclonal antibody
2.3.1. DNA immunization
The intrasplenic DNA immunization was performed as previously described [12], with modifications. Briefly, 7-week-old male BALB/c mice were anesthetized via intraperitoneal injection of pentobarbital (25 mg/kg). A small dissection on the skin of left abdomen was performed to expose the spleen. One hundred microliters of plasmid pcDNA3.1-S1-3 (1 μg/μl) was deeply injected into the spleen through the peritoneum. The skin was sutured and mice were under special care. Blood samples were collected by tail bleeding every 2 days. Sera were separated and stored at −20 °C.
2.3.2. Development of monoclonal antibody
Seven days after immunization, the spleen cell suspensions from hyperimmunized mice were fused with NS1 myeloma cells. After hypoxanthine–aminopterin–thimidine (HAT) selective culture, the hybridoma culture supernatants were analyzed for antibody against S1-3 polypeptide with ELISA. One positive primary hybridoma termed 3E8 was subcloned by three rounds of limiting dilution. The isotype of the monoclonal antibodies was determined by the isotyping ELISA kit (Pierce Chemical Co., Rockford, IL). Ascites fluid induced by 3E8 monoclonal hybridoma cells was produced in 10-week-old male BALB/c mice and stored at −20 °C.
2.4. Production of MLV/SARS-CoV
Chimeric MLV/SARS pseudotype virus was generated from cotransfection of 293T cells with three plasmids SARS-sht2, Gag/pol and EGFP/MLV (gifts from Dr. Wen Hui Li, Partners AIDS Research Center, Harvard Medical School, Boston) by calcium phosphate treatment. Plasmid SARS-sht2 was modified to encode the HIV-1 cytoplasmic domain after the truncated codon-optimized spike protein [13]. Viral supernatants were harvested 48 h post-transfection, and concentrated by ultrafiltration. The 293T producer cell pellets were stored for Western blot assay. The viral stocks were frozen at −80 °C and titered by GFP expression on MLV/SARS-CoV-infected Vero E6 cells with flow cytometry.
2.5. Immunoassays
2.5.1. ELISA
The diluted immune murine antisera, hybridoma supernatants and ascites were assayed by ELISA. Briefly, 96-well ELISA plate (Corning Costar, Cambridge, Mass) was coated with 2 μg synthetic S1-3 and incubated with samples. After washing, bound antibodies were detected by HRP-conjugated goat anti-mouse IgG + M (H + L) secondary antibody (1:1000, KPL, Gaithersburg, MD), and colorized with 1-step™ Turbo TMB ELISA reagent (Pierce Chemical Co., Rockford, IL). Serum from non-immune mice and hybridoma growth medium was used as negative control. To investigate whether the anti-S1-3 antibodies could react with the native SARS-CoV spike protein, the positive hybridoma supernatants were evaluated with a SARS viral lysates ELISA kit (Huada GBI Biotech, Beijing, China), and the recommended second antibody anti-human IgG was replaced with HRP-conjugated goat anti-mouse IgG + M (H + L) second antibody [3], [14].
2.5.2. Western blotting
The 293T producer cells were lysed 48 h after transfection. Samples were separated on SDS-PAGE gel and electrotransferred to PVDF membrane. Immunoblot assay was performed with 3E8 hybridoma supernatant and an HRP-conjugated goat anti-mouse IgG + M (H + L) second antibody (1:2500). The specific band was visualized with ECL system (Amersham, Braunschweig, Germany).
2.5.3. Immunofluorescent staining assay
MLV/SARS-CoV-infected Vero E6 cells from cytospin preparations were fixed with cold methanol/acetone, blocked with 5% goat serum and incubated with diluted 3E8 mAbs ascites as the primary antibody. After washes, the cells were incubated with FITC-conjugated goat anti-mouse IgM (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min in the dark and observed under inverted fluorescent microscope.
2.6. Neutralization assays
To determine whether 3E8 mAb could block the infectivity of MLV/SARS-CoV, neutralization assays were performed as described [15]. Vero E6 cells were infected with MLV/SARS-CoV, which had been pre-incubated with serial dilutions of the 3E8 hybridoma ascites. Two days later, the percentages of GFP-expressing cells were detected by flow cytometry.
3. Results and discussion
SARS is an acute respiratory disease caused by a newly identified coronavirus SARS-CoV. The spike protein on SARS-CoV plays a crucial role in viral entry into the host cells, and therefore becomes the major target of neutralizing antibodies preparation. Specificity of monoclonal antibodies to the SARS-CoV spike protein makes them important for the early diagnosis, viral pathogenesis studies and vaccine development. Under specialized biosafety facilities, purified SARS-CoV viral lysates with high infectious potential are most commonly used as immunogens for anti-SARS antibodies preparation [5], [16]. Two recent reports have developed DNA vaccine with SARS N protein and truncated spike protein, which are shown to induce specific antibody and cytotoxic T lymphocyte (CTL) generation by two or three times intramuscular DNA immunization in several months [17], [18]. Here, we developed a mAb against SARS-CoV by a single-shot intrasplenic immunization of plasmid DNA encoding one fragment of ACE2-binding domain in the spike protein in a short time. Instead of hazardous wild-type SARS-CoV, chimeric MLV/SARS-CoV pseudotype virus was used to characterize this mAb.
With immunoinformatics, we analyzed S1 domain in the SARS-CoV spike protein and selected fragment S1-3 (residues 430–454 aa) as the immunogen. Plasmid DNA encoding S1-3 was injected directly into the spleen of BALB/c mice to trigger immunological reaction. The antisera were analyzed with an ELISA plate coated with synthetic polypeptides or SARS-CoV lysates. In our preliminary experiment, all intra-spleen immunized mice showed positive immune response from day 5, and the sera titer peaked as 1:104 at day 7, much faster than protein or intramuscular DNA immunization. Therefore, we performed the hybridoma fusion 7 days after intra-spleen injection of pcDNA-S1-3. Some investigations demonstrated that it was possible to obtain good hybridoma production within a very short immunization time and irrespective of the antibody response of the intrasplenic immunized animal [10], [12]. The antibody responses of immunized mice were crucially determined by the expression of antigen in transfected spleen cells, which was concerned with the natural trait of the encoding antigen and the expression vector used. In the 24 hybridoma clones, 3E8 was subcloned and the mAb was determined as IgM isotype. Culture supernatants and ascites of 3E8 recognized synthetic S1-3 polypeptide specifically in ELISA assay. We also established mAb against SARS-CoV nucleoprotein (N protein) by single-shot intrasplenic inoculation of plasmid encoding an epitope (residues 20–49 aa) of N protein. Specific anti-N antibodies could be detected in mice at day 5 with ELISA kit coated with synthetic N protein fragment or SARS-CoV lysates (data not shown). These data suggested that S1-3 in the spike protein and fragment of N protein were potent epitopes, and single intrasplenic injection of eukaryotic expression plasmid coding antigens is efficient to induce specific antibodies in a short time, much faster than the protein or intramuscular DNA immunization.
SARS-CoV is highly contagious and must be handled under stringent protection, so we utilized MLV/SARS-CoV pseudotype virus to evaluate 3E8 mAb. MLV pseudotype virus could inherit new cell tropism of envelope protein donating virus [13], [15], [19]. In this study, the genome of MLV was pseudotyped to carried SARS-CoV spike protein and GFP reporter gene. Viral supernatant from transfected 293T cells was concentrated 15-fold to a titer of 7.48 × 104 IU/ml. MLV/SARS-CoV could infect SARS-CoV-susceptible Vero E6 cells, since giant multinucleated Vero E6 cells with EGFP fluorescence appeared after infection (Fig. 1 ). And the titer was also determined by the percentage of GFP-positive Vero E6 after infected.
Fig. 1.
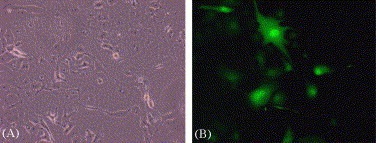
Effective infection of Vero E6 cells by MLV/SARS-CoV pseudotype virus. Vero E6 cells were cultured on six-well plates and transduced with MLV/SARS pseudotype virus. The phase-contrast (A) and GFP fluorescence (B) are shown (magnification, 200×).
To investigate the specificity of the mAb, the supernatants from 3E8 hybridoma cells were used as the primary antibody to probe lysates of MLV/SARS-CoV-infected 293T cells, and a band with MW of 170–180 kDa was detected (Fig. 2 ). No signal was observed in mock-transfected 293T cells, suggesting that the 3E8 mAb could specifically recognize the spike protein.
Fig. 2.

Western blotting analysis with mAb 3E8 supernatant. Cell lysates from 293T producer cells (line 2) were subjected to Western blot analysis by using mAb 3E8 hybridoma supernatant as the primary antibody. Lysates from mock-transfected 293T cells were used as a control (line 1). Molecular sizes of marker proteins (in kDa) are indicated on the left.
Spike protein is a type-I membrane protein; a portion of spike proteins unincorporated into the budding virions will be transported to the plasma membrane of infected cells. To determine whether the mAb recognizes spike protein in space, 3E8 mAb was used as the primary antibody to detect spike protein on MLV/SARS-CoV-infected Vero E6 cells. As shown in Fig. 3 , positive signals were predominant on the plasma membrane of the infected Vero E6 cells, whereas there is no signal on non-infected cells. These data suggest that 3E8 mAb could recognize the spike proteins on the infected cell surface.
Fig. 3.
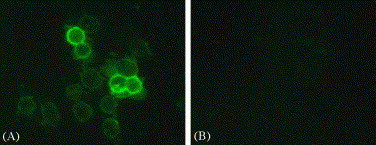
Immunofluorescence staining with mAb 3E8. Cytospin preparations from the MLV/SARS-CoV-infected Vero E6 cells (A, 200×) or the non-infected cells (B, 200×) were immunostained with mAb 3E8 ascites followed by FITC-conjugated goat anti-mouse IgM. The results were observed with inverted fluorescent microscope, demonstrating specific reaction of the mAb with SARS-CoV spike protein on the plasma membrane, whereas no signal on the uninfected cells.
We further explored whether mAb 3E8 could block the combination of SARS spike protein to its receptor. After pre-incubation with 3E8, MLV/SARS-CoV showed the same infectivity to Vero E6 cells, as there is no difference in the percentage of GFP-positive cells after infection as compared with untreated MLV/SARS-CoV (data not shown). The epitope to which a specific antibody bind is usually five to eight amino acid residues on the surface of the antigen, and the epitope recognized by the antibody may correspond to the simple primary sequence structure, or a specific three-dimensional antigenic conformation. The 3E8 mAb is suitable for Western blotting and immunofluorescence assay but failed to reduce the infectivity of the MLV/SARS-CoV, suggesting that this non-neutralizing mAb may recognize a linear epitope but not a conformational one.
In conclusion, we have developed a monoclonal antibody against SARS-CoV spike protein by a single-shot intrasplenic DNA immunization. This intrasplenic DNA immunization route might shed light on the production of polyclonal and monoclonal antibodies against the exiguous antigens, which are difficult to prepare or dangerous in use. In future, SARS-CoV studies, neutralizing antibodies against spike protein, can be generated with this efficient approach for possible therapeutic purpose.
Acknowledgment
We are grateful to Dr. Wen Hui Li for providing the MLV/SARS pseudotype virus. This work was supported by grants of the national project 863 (2002AA217041, 2003AA205060), 973 (001CB5101) from the Ministry of Science & Technology of China to Z.C. Han.
References
- 1.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 2.Lio P., Goldman N. Phylogenomics and bioinformatics of SARS-CoV. Trends Microbiol. 2004;12:106–111. doi: 10.1016/j.tim.2004.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci USA. 2004;101:6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci USA. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry J.D., Jones S., Drebot M.A., Andonov A., Sabara M., Yuan X.Y. Development and characterization of neutralising monoclonal antibody to the SARS-coronavirus. J Virol Methods. 2004;120:87–96. doi: 10.1016/j.jviromet.2004.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong S., Wang Y.F., Zhang M.Y., Liu X.J., Zhang C.H., Liu S.S. Immunogenicity of SARS inactivated vaccine in BALB/c mice. Immunol Lett. 2004;95:139–143. doi: 10.1016/j.imlet.2004.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitz M., Spitz L., Thorpe R., Eugui E. Intrasplenic primary immunization for the production of monoclonal antibodies. J Immunol Methods. 1984;70:39–43. doi: 10.1016/0022-1759(84)90387-9. [DOI] [PubMed] [Google Scholar]
- 10.Velikovsky C.A., Cassataro J., Sanchez M., Fossati C.A., Fainboim L., Spitz M. Single-shot plasmid DNA intrasplenic immunization for the production of monoclonal antibodies. Persistent expression of DNA. J Immunol Methods. 2000;244:1–7. doi: 10.1016/s0022-1759(00)00244-1. [DOI] [PubMed] [Google Scholar]
- 11.Moonsom S., Khunkeawla P., Kasinrerk W. Production of polyclonal and monoclonal antibodies against CD54 molecules by intrasplenic immunization of plasmid DNA encoding CD54 protein. Immunol Lett. 2001;76:25–30. doi: 10.1016/s0165-2478(00)00321-7. [DOI] [PubMed] [Google Scholar]
- 12.Kasinrerk W., Moonsom S., Chawansuntati K. Production of antibodies by single DNA immunization: comparison of various immunization routes. Hybrid Hybridomics. 2002;21:287–293. doi: 10.1089/153685902760213903. [DOI] [PubMed] [Google Scholar]
- 13.Moore M.J., Dorfman T., Li W., Wong S.K., Li Y., Kuhn J.H. Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2. J Virol. 2004;78:10628–10635. doi: 10.1128/JVI.78.19.10628-10635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung G.M., Chung P.H., Tsang T., Lim W., Chan S.K., Chau P. SARS-CoV antibody prevalence in all Hong Kong patient contacts. Emerg Infect Dis. 2004;10:1653–1656. doi: 10.3201/eid1009.040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giroglou T., Cinatl J., Jr., Rabenau H., Drosten C., Schwalbe H., Doerr H.W. Retroviral vectors pseudotyped with severe acute respiratory syndrome coronavirus S protein. J Virol. 2004;78:9007–9015. doi: 10.1128/JVI.78.17.9007-9015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang M.S., Lu Y.T., Ho S.T., Wu C.C., Wei T.Y., Chen C.J. Antibody detection of SARS-CoV spike and nucleocapsid protein. Biochem Biophys Res Commun. 2004;314:931–936. doi: 10.1016/j.bbrc.2003.12.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng F., Chow K.Y., Hon C.C., Law K.M., Yip C.W., Chan K.H. Characterization of humoral responses in mice immunized with plasmid DNAs encoding SARS-CoV spike gene fragments. Biochem Biophys Res Commun. 2004;315:1134–1139. doi: 10.1016/j.bbrc.2004.01.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu M.S., Pan Y., Chen H.Q., Shen Y., Wang X.C., Sun Y.J. Induction of SARS-nucleoprotein-specific immune response by use of DNA vaccine. Immunol Lett. 2004;92:237–243. doi: 10.1016/j.imlet.2004.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung V.M., Lai M.M. Murine retroviral pseudotype virus containing hepatitis B virus large and small surface antigens confers specific tropism for primary human hepatocytes: a potential liver-specific targeting system. J Virol. 2002;76:912–917. doi: 10.1128/JVI.76.2.912-917.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


