Abstract
The transmission of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) is associated with close contact to SARS patients and droplet secretions of those patients. The finding of positive RT-PCR results from stools of SARS patients suggests that stools of SARS patients or sewage containing stools of patients could transmit SARS-CoV. We used a novel style of electropositive filter media particle to concentrate the SARS-CoV from the sewage of two hospitals receiving SARS patients in Beijing. We also used cell culture, RT-PCR and gene sequencing to detect and identify the viruses from sewage. No infectious SARS-CoV contamination was found in any of the samples collected, but the nucleic acid of SARS-CoV could be detected in the sewage from the two hospitals before disinfection. While the RNA was only detected in three samples from the 309th Hospital, the others were negative after disinfection. These findings provide strong evidence that SARS-CoV can be excreted through the stool/urine of patients into sewage system, thus making the sewage system a possible route of transmission.
Keywords: SARS-CoV, Detection, Concentration, Sewage, Hospital
1. Introduction
Through 5 July 2003 over 8439 severe acute respiratory syndrome (SARS) cases and 812 SARS-related deaths were reported to the World Health Organization (WHO) from 32 countries. Most of these cases occurred after exposure to SARS patients in household or health care settings (Tsang et al., 2003, CDC, 2003, WHO, 2003a). A novel coronavirus from SARS patients was isolated and identified (Rota et al., 2003, Holmes, 2003, Fouchier et al., 2003, Ksiazek et al., 2003, Marra et al., 2003, Qin et al., 2003, Enserink and Vogel, 2003).
Investigations of the global outbreak of SARS have shown that the major mode of transmission of the SARS virus is through close personal contact, in particular, exposure to droplets of respiratory secretions from an infected person (Tsang et al., 2003, Lee et al., 2003, WHO, 2003b, Cyranoski and Abbott, 2003, Poutanen et al., 2003, Donnelly et al., 2003). Sewage is thought to have played a role in spreading the infection among a cluster of SARS cases in an apartment block in Hong Kong (WHO, 2003b, Cyranoski and Abbott, 2003). However, there is no direct evidence to prove that the coronavirus exists in the sewage system and is contagious.
In order to confirm whether sewage is a possible major route of transmission of SARS-CoV, a novel style of electropositive filter media particle (Li et al., 1998) was used to concentrate the SARS-CoV from the sewage of hospitals receiving SARS patients in Beijing, China. Cell culture and RT-PCR were utilized to detect and identify the viruses from sewage.
2. Materials and methods
2.1. Viruses and the culture methods
Bacteriophage f2 (f2) was used as a model for the coronavirus that may be present in sewage. The f2 was prepared and detected according to the methods described by Wommack et al. (1995). To identify viruses in sewage, a variety of specimens (sewage before or after disinfection by chlorine) were inoculated onto Vero E6. All cell cultures were grown in Eagle's growth medium (Difco Laboratories, Detroit, MI, USA) containing 8% fetal bovine serum (FBS), 0.015 M DMEM buffer and antibiotics (50 μg [each] of kanamycin and gentamycin per milliliter), and maintained in the same medium with 1.5% FBS. For virus propagation and isolation, cell cultures in 75-cm2 flasks were drained of medium, inoculated with 2 ml of sample, and incubated for 2 h at 37 °C with periodic rocking for viral adsorption. Because of the toxicity of most sewage, all cell cultures were inoculated in the presence of growth medium. Medium was replaced after 1–2 days of incubation. The culture was observed daily for cytopathic effect and terminated 7 days after inoculation. Any cultures exhibiting an identifiable cytopathic effect were subjected to several procedures to identify the cause of the effect (WHO, 2003c, WHO, 2003d, WHO, 2003e). If there was no cytopathic effect in the cell culture, the culture suspensate was harvested and transferred into additional flasks to isolate viruses. The cultures were used for three generations without any observable cytopathic effect.
2.2. Sewage and disinfection
Hospital sewage was collected daily at 7:00 a.m. from Xiao Tang Shan Hospital and the 309th Hospital of PLA, specially assigned to receive SARS patients in Beijing. Domestic sewage was collected from a housing estate in Fengtai district of Beijing City. Disinfection of the sewage is by chlorine, however, a 2500 ml sample was collected prior to disinfection and a 25,000–50,000 ml sample was collected after disinfection.
2.3. Electropositive filter media particle
The positively charged filter media particle was prepared as previously described by Li et al. (1998). In summary, 57 ml of a 4 mol/l Na2CO3 solution was slowly added to 1260 ml of 0.025 mol/l AlCl3 to form a precipitate and then adjusted to pH 7.2; 120 g of silica gel was added to 88 ml of Al(OH)3 precipitate, mixed well, and then the silica gel was dried at 60 °C for 36 h or longer.
2.4. Detection of residual chlorine
The residual chlorine and chlorine dioxide concentrations were measured by N,N-diethyl-p-phenylenediamine colorimetric method (DPD method). For chlorine dioxide analysis, glycine was added to mask interferences (American Public Health Association, 1980).
2.5. Concentration experiments
2.5.1. Evaluation of concentration efficiency of f2 and SARS-CoV from seeded sewage by positively charged filter
One hundred milliliters of hospital sewage or domestic sewage was placed in 500-ml beaker. The sewage was then seeded with SARS-CoV and f2 to a final concentration of approximately 102–103 pfu or TCID50 per milliliter. After mixing, samples were taken for an initial virus assay. The concentration methods followed the procedures reported by Li et al. (1998).
2.5.2. Concentration of SARS-CoV from sewage
Ten milliliters of Na2S2O3 (10%, w/v) were added to the disinfected sewage collected from the hospitals to neutralize the residual chlorine. The sewage was then seeded with f2 to a final concentration of approximately 103–105 pfu. The concentration methods were followed the directions reported by Li et al. (1998).
2.6. RNA extraction
A virus RNA extracting kit (TRIzol Reagent) made by Invitrogen™ Life Technologies for extraction of exceedingly pure viral RNA was utilized in our experiment to extract virus RNA. All operations were strictly implemented in accordance with the reagent instruction manual.
2.7. Primer design for assay of SARS-CoV nucleic acid
Three sets of primers from WHO Network Laboratories (WHO, 2003f) were used to detect the SARS-CoV RNA:
-
•
Cor-p-F2 (+) 5′-CTAACATGCTTAGGATAATGG-3′;
-
•
Cor-p-F3 (+) 5′-GCCTCTCTTGTTCTTGCTCGC-3′;
-
•
Cor-p-R1 (−) 5′-CAGGTAAGCGTAAAACTCATC-3′.
Cor-p-F2/Cor-p-R1 gave a 368 bp product and Cor-p-F3/Cor-p-R1 yielded 348 bp section.
2.8. Primer design for assay of enteroviruses nucleic acid
In order to distinguish SARS-CoV and enteroviruses, which also cause a cytopathic effect in the Vero cell, a pair of consensus primers of enteroviruses from the 5′ non-coding region was utilized because of their presence in many enteroviruses serotypes. The information from the primers was as follows: E1 5′-ATTGTCACCATAAGCAGCCA-3′, E2 5′-CCAGCACTTCTGTTTCCCCGG-3′, product size was 440 bp (Li et al., 2002).
2.9. Detection of SARS-CoV and enteroviruses by RT-PCR
Two microliters of a viral RNA solution were analyzed with RT-PCR assay. The KaTaRa one step RNA PCR kit (KaTaRa Biotechnology, Dalian) was used for the reaction. Positive and negative RT-PCR controls were included in each run, and all operations were strictly implemented in accordance with the kit instruction manual (OSRPK, 2003).
2.10. Detection of the PCR product
PCR products were analyzed by electrophoresis with 1.5% (w/v) agarose gels containing 0.5 μg of ethidium bromide per milliliter. These were visualized with UV illumination and photographed. A DNA marker (pUC19 DNA/MSP I Marker, Gibco/BRL) was included in each agarose gel electrophoresis run.
2.11. Nucleotide sequence analysis
The PCR products from four different samples were purified with the QIAquick PCR purification kit (QIANEN, INC.) and sequenced with the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA polymerase FS (Perkin-Elmer, Applied Biosystem) following the product instructions. The sequences were compared with the genome of SARS-CoV in the GenBank and EMBL databases by using the FASTA program.
3. Results
3.1. Recoveries of f2 and SARS-CoV from 100 ml of seeded sewage
When the concentrations of SARS-CoV and the f2 seeded sewage were about 103–104 TCID50/100 ml and 102 pfu/ml, respectively, the recoveries of SARS-CoV varied from 0 to 21.4%, with an average of 1.02% from small-volume sewage, and the recovery of f2 ranged from 33.6 to 260.0%, with an average of 127.1%. The recovery of SARS-CoV was much lower than that of f2 under the same conditions (Table 1 ).
Table 1.
Recoveries of f2 and SARS-CoV from the seeded sewagesa
| Sewage samples | Viruses input |
Recovered viruses |
Recoveries |
|||
|---|---|---|---|---|---|---|
| SARS-CoV (TCID50/100 ml) | f2 (pfu/ml) | SARS-CoV (TCID50/100 ml) | f2 (pfu/ml) | SARS-CoV (%) | f2 (%) | |
| 1b | 102.67 | 157 | 102.0 | 125 | 21.4 | 79.6 |
| 2c | 103.2 | 146 | 0 | 381 | 0 | 100.0d |
| 3c | 103.0 | 128 | 101.0 | 43 | 1.0 | 33.6 |
| Mean | 103.58 | 144 | 101.6 | 183 | 1.02 | 100.0 |
One hundred milliliters sewage.
Sewage from the 309th Hospital.
Sewage from a housing estate.
The recoveries were more than 100% in fact.
3.2. Detection limit of SARS-CoV by RT-PCR
The minimum amount of the viral RNA detected by RT-PCR was equivalent to 103 TCID50 and 10 TCID50 by semi-nested RT-PCR.
3.3. Concentration and detection of SARS-CoV from sewage before disinfection
The recoveries of f2 from 2500 ml sewage from Xiao Tang Shan Hospital and 309th Hospital of PLA varied from 55.4 to 188.1%, the average was 79.2 and 85.8%, respectively. All sewage samples tested for the presence of infectious SARS-CoV in cell culture were negative. The RNA of SARS-CoV could be found in the concentrates of sewage from the two hospitals by semi-nested PCR, and was also detected in the inoculated cells of the sewage concentrates from the 309th Hospital but not from Xiao Tang Shan Hospital. However, the RNA copies of SARS-CoV in the samples were too low to be detected by the first amplification reaction. The positive amplification results occurred when the product of the first amplification reaction was used as the template of the second PCR (Fig. 1 ). No residual chlorine was detected in any of the sewage samples (Table 2 ).
Fig. 1.
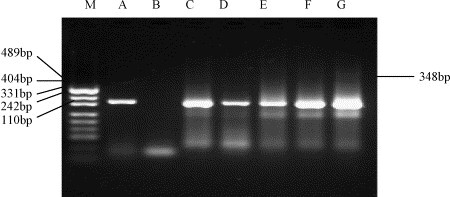
Amplification of SARS-CoV RNA samples recovered from sewage before disinfection from Xiao Tang Shan Hospital by semi-nested PCR. (M) DNA marker (pUC19 DNA/MSP I Marker); (A) positive control of SARS-CoV, 348 bp; (B) negative control; (C–G) the samples of 11–15 June.
Table 2.
Concentration and detection of SARS-CoV from 2500-ml sewage in the 309th Hospital of PLAa
| Date | Patients in hospital | Patientsb with symptoms | f2 input | f2 recovered (%) | Cell culturec | Concentrate + PCRd | Inoculated cell + PCRe | Residual chlorinea |
|---|---|---|---|---|---|---|---|---|
| 11 June | 7 | 0 | 1.3 × 106 | 93.1 | −f | +g | + | − |
| 12 June | 6 | 0 | 2.1 × 106 | 87.5 | − | + | + | − |
| 13 June | 3 | 0 | 1.3 × 106 | 96.9 | − | + | + | − |
| 14 June | 2 | 0 | 1.3 × 106 | 70.0 | − | + | + | − |
| 15 June | 2 | 0 | 3.0 × 105 | 62.2 | − | + | + | − |
Glass column diameter: 19 mm, bed height: 14 cm, eluate volume: 500 ml.
With any one of the symptoms of fever, malaise, cough, dyspnea, except chest radiography signs.
Cell culture was maintained for 14 days to observe the cytopathic effect.
PCR template was from the concentrates.
Template from the cultured cells.
Negative.
Positive.
3.4. Concentration and detection of SARS-CoV from sewage after disinfection
The samples (25,000 or 50,000 ml) from the two hospitals were all negative by the infectivity methods. The SARS-CoV RNA was detected from the concentrates and inoculated cells of three samples (11, 13 and 15 June 2004) from the 309th Hospital by semi-nested PCR, while the other samples were negative (Fig. 2 ). The recovery of f2 seeded in sewage from Xiao Tang Shan Hospital and 309th Hospital of PLA ranged from 13.5 to 161.2%. The average recovery rates were 61.2 and 85.5%, respectively. The total residual chlorine varied from 0 to 1.0 mg/l, and free residual chlorine from 0 to 0.5 mg/l in sewage from Xiao Tang Shan Hospital; while for the samples from the 309th Hospital, the total residual chlorine varied from 3.0 to 12.5 mg/l, and free residual chlorine from 1.5 to 5.0 mg/l. (Table 3 ).
Fig. 2.
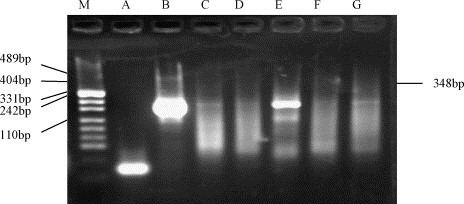
Amplification of SARS-CoV RNA from recoveries of sewage after disinfetion from the 309th Hospital by semi-nested PCR. (M) DNA marker (pUC19 DNA/MSP I Marker); (A) negative control; (B) positive control of SARS-CoV, 348 bp; (C–G) the samples of 11–15 June.
Table 3.
Concentration and detection of SARS-CoV from 25,000- or 50,000-ml sewage in Xiao Tang Shan Hospital
| Date | Patients in hospital | Patientsb with symptoms | f2 input | f2 recovered (%) | Cell culture | Concentrate + PCR | Inoculated cell + PCR | Residual chlorinea |
|
|---|---|---|---|---|---|---|---|---|---|
| Total | Free | ||||||||
| 11 Junec | 179 | 12 | 2.8 × 106 | 100.0 | – | – | – | 1.0 | 0.5 |
| 12 June | 145 | 8 | 3.5 × 106 | 69.5 | – | – | – | 1.0 | 0.5 |
| 13 June | 112 | 6 | 1.4 × 106 | 57.1 | – | – | – | 1.0 | 0.5 |
| 14 June | 89 | 4 | 3.5 × 106 | 51.2 | – | – | – | 1.0 | 0.2 |
| 15 June | 88 | 3 | 3.4 × 107 | 13.5 | – | – | – | 0.0 | 0.0 |
Residual chlorine (mg/l): total, total residual chlorine; free, free residual chlorine.
With any one of the symptoms of fever, malaise, cough, dyspnea except chest radiography signs.
Volume of sewage was 50,000 ml.
3.5. Result of nucleotide sequence analysis
The PCR products from the sewage samples of the two hospitals were sequenced, and submitted to GenBank. The accession numbers were: bankit579728 and bankit579738. Comparison of the nucleotide sequence of PCR products with data from GenBank revealed that the sequences of PCR product were close to these of SARS-CoV genome, showing about 99% nucleotide homologue.
4. Discussion
Control of the spread of SARS is possible through isolation of infected patients, contact tracing and follow-up, quarantine, and travel restriction (WHO, 2003a, WHO, 2003h, WHO, 2003g). However, epidemiologists are still trying to understand how and why the SARS-CoV has spread so readily throughout Asia and certain other regions. The primary mechanism of SARS-CoV transmission has been limited to close contacts with patients (Tsang et al., 2003, Lee et al., 2003, WHO, 2003b, Cyranoski and Abbott, 2003, Poutanen et al., 2003, Donnelly et al., 2003, Seto et al., 2003). On 15 April 2003, health authorities reported that 321 individuals affected by SARS were residents in Amoy Gardens of Hong Kong. Attention was focused on possible transmission via the sewage system because laboratory studies indicated that the Amoy Gardens patients excreted coronaviruses in their stools (WHO, 2003b, WHO, 2003i, AGIFMP, 2003). However, except for the positive PCR results found in some patients’ stools, there were no reports of the detection of SARS-CoV or RNA from sewage samples containing patients’ stools.
Using the electropositive particle adsorption method (Li et al., 1998) we attempted to concentrate SARS-CoV or the RNA in sewage from Xiao Tang Shan Hospital and the 309th Hospital. These hospitals were specially assigned to receive SARS patients in Beijing. Since f2 is a kind of bacterial virus with a single-positive-stranded RNA (similar to SARS-CoV), and testing f2 was simple and relatively inexpensive (Olivieri et al., 1975), f2 was chosen as a model for SARS-CoV to evaluate the concentration efficiency. Because most enteroviruses can also grow on the Vero cell, and yield a cytopathic effect similar to that of the SARS-CoV, enteroviruses must be first excluded from all the viruses that can grow on the Vero cell culture or yield a cytopathic effect.
There was no infectious SARS-CoV detected in the sewage in these assays. SARS-CoV only remains infectious for two days in sewage. We expected that if the assays were initiated with sewage samples collected when patients were in the early, acute phase of SARS the infectious virus could be isolated. The patients in these hospitals were all beyond the acute phase, and most had recovered from SARS. The following reasons may be responsible for the negative results: (1) the SARS-CoV was inactivated by the high concentration of disinfectants, which were used after a patient had a bowel movement; (2) the quantity of SARS-CoV was too low to be detected by the current methods; (3) SARS-CoV may become non-infectious by unknown factors during the concentration process.
The nucleic acid of SARS-CoV was found in the sewage before disinfection from both hospitals by semi-nested PCR. After disinfection, SARS-CoV RNA could only be detected from the samples collected on 11, 13 and 15 June 2004, from the 309th Hospital. It is interesting that, after disinfection, the RNA of SARS-CoV could be detected in the samples from the 309th Hospital but not from Xiao Tang Shan Hospital. It is possible that the RNA originated from two serious patients who had been moved to other hospitals in Beijing on 2 and 9 June, respectively, before the experimental period. It has been reported that the RNA of SARS-CoV can survive for five days or longer (AGIFMP, 2003). In this study, the RNA could be detected in sewage for 8 days, though the virus itself was inactive.
Acknowledgements
The authors thank Drs. Fu-Yu Wang, Ying-Kai Li, Meng-Fu Zhu, Jian-Yong Su, Cheng-Yuan Gong, Wu-Chun Chao, Tai-Thi Gong, Tao-Xing Shi, Bang-Rong Han, Zhu-Ge Xi and Hua-Shan Zhang for helpful guidance and discussion, providing many reagents. We are also indebted to Professor Su-Qi Cheng for English revision.
This research was partly supported by grants from the National High Technology Research and Development Program of China (863 Program) (No. 2004AA649100) and the National Natural Science Foundation of China (No. 30471436).
References
- American Public Health Association . 20th ed. American Public Health Association; Washington, DC: 1980. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Amoy Gardens Investigation Findings Make Public (AGIFMP), 2003. Available at: http://www.info.gov.hk/gia/general/200304/17/0417247.htm.
- Centers for Disease Control and Prevention (CDC), 2003. Severe Acute Respiratory Syndrome (SARS). Available at: http://www.cdc.gov/ncidod/sars/.
- Cyranoski D., Abbott A. Apartment complex holds clues to pandemic potential of SARS. Nature. 2003;423:1038. doi: 10.1038/423003a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C.A., Ghania A.C., Leungb G.M., Hedleyb A.J., Frasera C., Rileya S., Abu-Raddada L.J., Hob L.-M., Thachb T.-Q., Chaub P., Chanb K.P., Lamb T.-H., Tsec L.-Y., Tsangc T., Liud S.-H., Kongd J.H.B., Laue E.M.C., Fergusona N.M., Andersona R.M. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink M., Vogel G. Hungry for detail, scientists zoom in on SARS genome. Science. 2003;300:715–716. doi: 10.1126/science.300.5620.715. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A.M., Kuiken T., Schutten M., Van Amerongren G., Van Doorrum G.J.J., Van Den Hoogen B.G., Peiris M., Lim W., Stohr K., Oeterhaus A.D.M.E. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.V. SARS-associated coronavirus. NEJM. 2003;348:1948–1951. doi: 10.1056/NEJMp030078. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W.M., Rollin P.E., Dowell S.F., Ling M.P.H.A.-E., Humphrey C.D., Shieh W.-J., Guarner J., Paddock C.D., Rota M.P.H.T.M.P., Fields B., DeRisi J., Yang J.-Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., the SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. NEJM. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J.Y. A major outbreak of severe acute respiratory syndrome in Hong Kong. NEJM. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Li J.W., Wang X.W., Rui Q.Y., Song N., Zhang F.G., Ou Y.C., Chao F.H. A new and simple method for concentration of enteric viruses from water. J. Virol. Methods. 1998;74:99–108. doi: 10.1016/s0166-0934(98)00078-0. [DOI] [PubMed] [Google Scholar]
- Li J.W., Wang X.W., Yuan C.Q., Zheng J.L., Jin M., Song N., Shi X.Q., Chao F.H. Detection of enteroviruses and hepatitis A virus in water by consensus primer multiplex RT-PCR. World J. Gastroenterol. 2002;8:699–702. doi: 10.3748/wjg.v8.i4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Fsreeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Olivieri V.P., Kruse C.W., Hsu Y.C., Griffiths A.C., Kawata K. In: Disinfection—Water and Wastewater. Johnson J.D., editor. Ann Arbor Science Publishers, Inc.; Inn Arbor, Michigan: 1975. The comparative model of action of chlorine, bromine, and iodine on f2 bacterial virus; pp. 145–162. [Google Scholar]
- One Step RNA PCR Kit (OSRPK), 2003. Available at: http://bio.takara.co.jp/BIO_EN/Catalog_d.asp?_ID=co188.
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Gree K., Tellier RNR, Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. NEJM. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Qin E.D., Zhu Q.V., Peng W.M., Jiang T., Fan B.C., Chang G.H., Yu M., Si B.Y., Liu B.H., Deng Y.Q., Liu H., Zhang Y. Determination of the partial polymerase gene sequence of novel coronavirus isolated from lung tissue of SARS patients. Junshi Yixue Kexueyuan Yuankan. 2003;27:81–83. (in Chinese) [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Seto W.H., Tsang D., Yung R.W., Ching T.Y., Ng T.K., Ho M., Peiris J.S. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Moira C.Y., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. NEJM. 1977-1985;348 doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Wommack K.E., Hill R.T., Colwell R.R. A simple method for the concentration of viruses from natural water samples. J. Microbiol. Methods. 1995;22:57–67. [Google Scholar]
- World Health Organization (WHO), 2003a. SARS: Breaking the Chains of Transmission. Available at: http://www.who.int/features/2003/07/en.
- World Health Organization (WHO), 2003b. Amoy Gardens Investigation Findings Make Public. Available at: http://www.who.int/csr/sars/en.
- World Health Organization (WHO), 2003c. Recommendations for Laboratories Testing by PCR for Presence of SARS Coronavirus—RNA. Available at: http://www.who.int/csr/sars/coronarecommendation/en.
- World Health Organization (WHO), 2003d. Severe Acute Respiratory Syndrome (SARS): Laboratory Diagnostic Tests. Available at: http://www.who.int/csr/sars/diagnostictests/en.
- World Health Organization (WHO), 2003e. Use of Laboratory Methods for SARS Diagnosis. Available at: http://www.who.int/csr/sars/labmethods/en.
- World Health Organization (WHO), 2003f. PCR Primers for SARS Developed by WHO Network Laboratories. Available at: http://www.who.int/csr/sars/primers/en.
- World Health Organization (WHO), 2003g. WHO Global Conference on Severe Acute Respiratory Syndrome (SARS), 17–18 June. Available at: http://www.who.int/csr/sars/conference/june_2003/en/. [DOI] [PMC free article] [PubMed]
- World Health Organization (WHO), 2003h. Meeting on SARS Virus Detection and Survival in Food and Water, Madrid, 8–9 May. Available at: http://www.who.int/mediacentr/releases/2003/pr31/en.
- World Health Organization (WHO), 2003i. Update 32—Situation in China and Hong Kong, Status of Diagnostic Tests. Available at: http://www.who.int/csr/sarsarchive/2003_04_17/en.


