Abstract
The process of virus replication in host cells is greatly influenced by the set of cytokines, chemokines and antiviral substances activated as a result of host–virus interaction. Alteration of cytokines profiles through manipulation of the innate immune system by innate immune stimulants may be helpful in inhibiting virus replication in otherwise permissive cells. The aim of present studies was to characterize innate immune responses capable of inhibiting infectious bronchitis virus (IBV) replication in chicken lungs after in ovo administration of CpG ODN. In our experiments, CpG ODN 2007 or PBS solution was injected on 18th embryonic day (ED) via the chorioallontoic route. CpG ODN and PBS inoculated embryos were challenged with virulent IBV on the 19th ED. Lung tissue samples from experimental chicks were analysed for cytokines/chemokines gene expression at 24 h, 48 h, and 72 h, post infection. Our data showed significant differential up-regulation of IFN-γ, IL-8 (CXCLi2) and MIP-1β genes and suppression of IL-6 gene expression being associated with inhibition of IBV replication in lungs tissue retrieved from embryos pre-treated with CpG ODN.
It is expected that understanding of the innate immune modulation of target tissues by the virus and innate immune stimulants will be helpful in identification of valuable targets for development of novel, safe, effective and economical control strategies against IBV infection in chickens.
Keywords: Avian infectious bronchitis virus, Inhibition of virus replication, CpG ODN cytokines/chemokines
1. Introduction
The nature of innate and cellular immune responses [T-helper (Th) I or Th 2] activated as a result of virus interaction with host cells play a crucial role in the outcome of a viral infection. These interactions lead to the induction of different cytokines, chemokines and antimicrobial substances which regulate the process of virus replication in host cells (Estcourt et al., 1998, He et al., 2012). It is noteworthy, that cytokines/chemokines induced by viruses may leads to activation of disproportionate inflammatory responses damaging to the host tissue and may compromise physiological functions of affected tissues. For instance, the death of infected and non-infected cells at the site of mouse hepatitis virus (MHV) infection in mice is attributed to an increased influx of pro-inflammatory cells and excessive induction of interleukin (IL)-6 mediated through activation of p38 mitogen-activated protein kinase (p38MAPK) pathways (Banerjee et al., 2002, Perlman and Dandekar, 2005). In contrast, amplified expression of IL-6 and IL-8 (CXCLI2) cytokines in severe acute respiratory syndrome (SARS) coronavirus-(CoV) infected cells, triggers functional modification of macrophages and dendritic cells steering to the strengthening of host innate immune responses against SARS-CoV infection (Sims et al., 2008, Yoshikawa et al., 2009). Similarly, protection in mice pre-sensitized with respiratory syncytial virus (RSV) F protein is mediated through activation of CD8 T cells leading to increased production of interferon (IFN)-γ and IL-2 cytokines. In contrast, mice vaccinated with RSV G protein exhibit pulmonary eosinophilia and aggravated disease symptoms associated with enhanced IL-4 and IL-5 expression in lungs (Andrews et al., 2010, Estcourt et al., 1998, Hogan et al., 1998). Interestingly, the RSV induced lung pathology due to production of Th-2 cytokines and accumulation of eosinophils in lungs may be suppressed with administration of recombinant IL-12 and increased IFN-γ production mediated through activated CD 8 T cells (Hogan et al., 1998). Likewise, elevated expression of IL-12 and other Th-1cytokines in hepatitis B virus (HBV) infection has been linked with the clearance of virus from the body (Cavanaugh et al., 1997, Rossol et al., 1997). These findings suggest that understanding of the molecular basis of immune modulation by viruses and innate immune modulators may be helpful in development of more effective therapeutic and prophylactic strategies against infectious diseases in humans and animals (Fearon, 2000).
Coronaviruses cause serious diseases including SARS in humans, transmissible porcine gastroenteritis in pigs, calf scour in bovine, feline infectious peritonitis in cats, mouse hepatitis in mice, and avian infectious bronchitis in birds (Tanaka et al., 2013). Avian infectious bronchitis caused by IBV is a highly contagious respiratory disease of commercial chickens. IBV infection causes huge economic losses in terms of high morbidity (100%) and mortality (>50% with some viral strains), low egg quality and production losses in broilers, layers and breeder chickens (Banat et al., 2013). In addition, rapid emergence of new strains, lack of cross protection amongst different serotypes and non-availability of vaccines against different variants of IBV is a matter of serious concern for the poultry industry (Jackwood et al., 2010, Lee and Jackwood, 2000). Therefore, developments of strategies potentially effective across multiple serotypes are needed for the control of IBV and other coronavirus infections in animals and humans.
In this regard, we have recently demonstrated that in ovo administration of un-methylated deoxycytidyl-de-oxyguanosine (CpG) dinucleotides (relatively common in bacterial and viral DNA) triggers the immune modulatory events that inhibit IBV replication in chicken lungs, trachea, spleen and kidney tissues (Dar et al., 2009a, Dar et al., 2009b). Data in these studies have shown the importance and clinical potential of CpG ODNs as a vaccine adjuvant or therapeutic agent against coronavirus infections in chickens. However, in these studies only N gene expression of IBV was analysed in above described organs. The aim of the present study was to investigate mechanistic details of innate immune responses activated in lungs after in ovo CpG ODN treatment followed by IBV infection in chickens. It is expected that understanding the basis of innate immune modulation of target tissues by viruses and CpG ODN will be helpful in identification of valuable targets for development of novel, safe, effective and economical control strategies against IBV infection in chickens. Moreover, CpG ODN may serve as a universal prophylactic reagent until virus specific vaccines are developed for the control of IBV infection in chickens. Additionally, the lessons learned from these studies may be instructive for the development of vaccines against coronavirus infections in humans and other animals.
2. Materials and methods
2.1. Animal experiments and samples processing
For all animal experiments, specific pathogen free (SPF) eggs and synthetic CpG ODN 2007 (TCGTCGTTGTCGTTTTGTCGTT) with phosphorothioate backbone (Merial Ltd. Athens, GA) were used. In ovo safety and innate immune activation potential of CpG ODN 2007 has previously been reported by us and other researchers (Dar et al., 2009b, Gomis et al., 2004, Gomis et al., 2007). On the 18th embryonic day (ED), the eggs were randomly divided into three equal groups (A–C). The embryos in group A were injected with 50 μl CpG ODN solution in sterile PBS (1 μg ODN/μl) via chorioallontoic route. Embryos in group B and C were given 50 μl sterile PBS (PBS, pH 7.2; Sigma-Aldrich, St. Louis, MO) via the chorioallontoic route. In addition, in the first experiment an additional group (D) was added. Embryos in this group (groups D) were given 50 μl CpG ODN solution in sterile PBS (1 μg ODN/μl) via the chorioallontoic route. On the 19th ED, the embryos in group A and B were infected (via chorioallontoic route) with 150 μl of IBV (Ark99) solution containing 100 embryo infectious dose (EID)50. Embryos in group C and D were not infected with IBV. The lungs tissue samples were collected from infected and non-infected embryos and chicks at 24 h, 48 h, and 72 h, post infection (PI). Similarly, lungs tissues samples from group D were collected at 24 h, 48 h, and 72 h, post CpG inoculation. IBV replication was evaluated by a time dependent increase in IBV nucleocapsid (N) gene expression in cellular RNA extracted from infected tissues. Tissue samples were collected in TRIzol® (Invitrogen, Carlsbad, CA), the RNA was extracted as per the manufacturer's instructions. The extracted RNA was quantified and checked for quality by using an Agilent RNA 6000 Nano LabChip® kit (5065-4476; Agilent Technologies, Waldbronn, Germany). Prior to cDNA synthesis, all RNA samples were treated with DNase (Invitrogen) for 1 h at 37° C.
2.2. Real-time PCR assay and data analysis
Primers used in these studies are shown in Table 1 , whereas, parameters for reverse transcription and real-time PCR assay were followed as previously described (Dar et al., 2009b). Briefly, 5 μg DNase treated RNA isolated from lung tissue was used as template for synthesis of cDNA. Random hexamer primers and SuperScript II reverse transcriptase (Invitrogen) were used for reverse transcription of RNA. The differential mRNA expression was evaluated by using ICycler iQ and iQ SYBR® Green supermix (Bio-Rad, Hercules, CA). The β-actin and the target genes from each sample were run in parallel on the same plate. Normalized CT value (CT value = cycle number at which the fluorescence due to amplification of target DNA reaches significantly above the background) of target genes were used to assess the change in mRNA expression. For the first normalization (ΔCT), the CT value of β-actin gene was subtracted from the CT values of the target gene. A second normalization (ΔΔCT) was done by subtracting the mean ΔCT value of non-infected PBS-treated tissue cells from the ΔCT value of IBV infected tissue cells. The increase or decrease in target gene mRNA level in infected tissue cells compared with PBS treated non infected tissue cells was calculated as—fold change increase □=□2−ΔΔCT (Livak and Schmittgen, 2001). In each of the four experiments (experiment repeated four times), tissue samples from three embryos for each treatment at each time point were pooled to generate one sample. Each sample was run in triplicate on qPCR plate. For each target mRNA along with β-actin mRNA, the DNase-treated RNA samples without conversion of RNA to cDNA and no template PCR controls were run in triplicate on the same plate. For each experiment, the mean CT value of three wells was taken as the final CT value. The data were analysed using Excel® (Microsoft, Redmond, WA) and GraphPad PRISM™ 6 software (GraphPad Software Inc., San Diego, CA). Final data are expressed as the mean ± SEM of—fold changes observed in all experiments. The data were normalized by log transformation. The differences in—fold change of mRNA expression were calculated using a one way analysis of variance and Tukey's multiple comparison post tests. The results were assumed statistically significant at p < 0.05.
Table 1.
List of primers used in these studies.
| Gene | Sequence | |
|---|---|---|
| ch-B-Actin | 5′-GTACCCTGGCATTGCTGAC | F |
| 5′-CGGATTCATCGTACTCCTGC | R | |
| ch-INF-α | 5′-GTCTTGCTCCTTCAACGACA | F |
| 5′-GCGCTGTAATCGTTGTCTTG | R | |
| Ch-IFN-γ | 5′-CCAAGAAGATGACTTGCCAGA | F |
| 5′-ACCTTCTTCACGCCATCAGG | R | |
| Ch-IL-1β | 5′-GGCATCAAGGGCTACAAGC | F |
| 5′-GTTGGAGCGGGCAGTCAG | R | |
| ch-IL-6 | 5′-GTGCGAGAACAGCATGGAGA | F |
| 5′-GACTTCAGATTGGCGAGGA | R | |
| Ch-IL-8 | 5′-CAGCTGCTCTGTCGCAAG | F |
| 5′-GTGGTGCATCAGAATTGAGCT | R | |
| ch-IL-12(p40) | 5′-CCGACTGAGATGTTCCTGGA | F |
| 5′-CCTGCACAGAGATCTTGTC | R | |
| ch-IL-18 | 5′-GCATTCAGCGTCCAGGTAGA | F |
| 5′-GTCTTGCTCCTTCAACGACA | R | |
| ch-OASA | 5′-CCTGCGTCAGCGATGTCT | F |
| 5′-GCATAGATCTGCTGCTTCAG | R | |
| N gene (IBV) | 5′-GAAGAAAACCAGTCCCAGATGCTTGG | F |
| 5′-GTTGGAATAGTGCGCTTGCAATACCG | R |
F is the Forward primer.
R is the Reverse primer.
3. Results
In order to evaluate the status of virus replication in CpG ODN pre-treated and PBS treated embryos, we analysed the expression of the viral nucleocapsid (N) gene in embryonic lungs at 24 h, 48 h, and 72 h, post IBV infection. Our data showed significantly lower levels of viral N gene expression in group of embryos treated with CpG ODN prior to infection (Fig. 1 ).
Fig. 1.
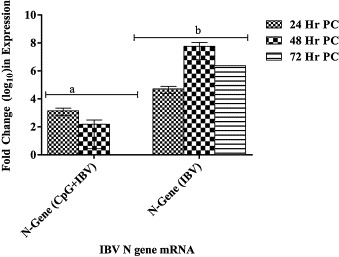
Change in IBV N gene mRNA expression in chicken lungs tissues. Fold change in IBV N gene RNA in lungs isolated at 24 h, 48 h, and 72 h post IBV infection from embryos treated with CpG ODN (2007) or PBS 24 h prior to infection. Change in RNA expression was compared with N gene RNA expression in PBS treated non-infected 18 day old chicken embryos (negative control). Change in mRNA expression has been shown as mean ± SEM. The difference in mRNA expression was considered significant at p < 0.05 and shown with different superscript alphabets (a and b) on the bars.
Our data showed equal levels of IFN-α and IL-12 (P40) genes up-regulation (∼4 fold increase in expression at 24 h, post infection) in CpG pre-treated and non-CpG treated infected groups (group A and B). In contrast, IFN-γ gene expression in embryonic lung tissue treated with CpG ODN prior to IBV infection was significantly higher (>100 fold) compared with non-CpG treated infected group at 24 h, 48 h, and 72 h, post infection (Fig. 2 ).
Fig. 2.
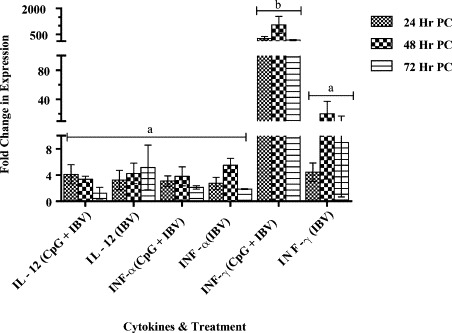
Interferons (IFN) α, IFN-γ and interleukin (IL) 12 mRNA stimulation in lungs tissue.
Interferon (IFN) α, IFN-γ and interleukin (IL)-12 mRNAs expression in lung tissue following CpG ODN 2007 or PBS administration in 18 day old chicken embryos via chorioallontoic route. Embryos were infected with IBV via the chorioallontoic route on embryonic day 19. The change in mRNA expression was compared between CpG ODN pre-treated and PBS pre-treated groups and is shown as mean ± SEM fold change in gene expression. The difference in mRNA expression was considered significant at p < 0.05 and indicated with different superscript alphabets (a and b) on the bars.
IL-1β and IL-18 gene expression showed no significant differences between CpG ODN treated (group A) or PBS treated (group B) IBV infected embryos (Fig. 3A and B). Surprisingly, IL-6 gene expression was significantly up-regulated in non CpG treated infected embryos (group B) at 72 h, PI. The IL-8 gene showed significantly higher expression in group of embryos pre-treated with CpG ODN (group A) compared with PBS treated (group B) embryos at 48 h, post IBV challenge (Fig. 3A and B).
Fig. 3.
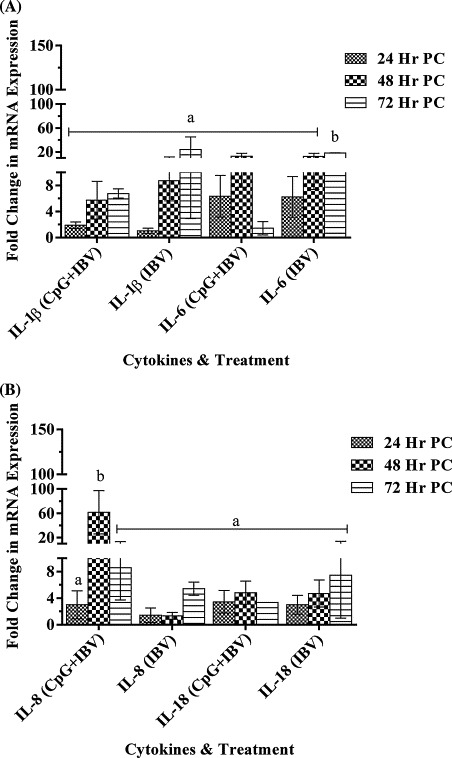
(A) IL-1β and IL-6, mRNA stimulation in lungs tissue. Cytokines IL-1β, IL-6, mRNAs expression in lungs tissue following CpG ODN 2007 or PBS administration in 18 day old chicken embryos via chorioallontoic route. Embryos were infected with IBV via chorioallontoic route on embryonic day 19. The change in mRNA expression was compared between CpG ODN pre-treated and PBS pre-treated groups and is shown as mean ± SEM fold change in gene expression. The difference in mRNA expression was considered significant at p < 0.05 and indicated with different superscript alphabets (a and b) on the bars. (B) IL-8 (CXCLI2)and IL-18 mRNA stimulation in lungs tissue. Cytokines IL-8 (CXCLI2) and IL-18 mRNAs expression in lungs tissue following CpG ODN 2007 or PBS administration in 18 day old chicken embryos via chorioallontoic route. Embryos were infected with IBV via chorioallontoic route on embryonic day 19. The change in mRNA expression was compared between CpG ODN pre-treated and PBS pre-treated groups and is shown as mean ± SEM fold change in gene expression. The difference in mRNA expression was considered significant at p < 0.05 and indicated with different superscript alphabets (a and b) on the bars.
We analysed expression of two chemotactic cytokines macrophage inflammatory protein (MIP)-1β and MIP-3α. Out of both chemokines, the MIP-1β gene showed significantly higher expression in CpG ODN pre-treated infected embryos compared with PBS pre-treated infected embryos at 48 h, PI. However, there was no difference in MIP-3α gene expression in both (A and B) groups (Fig. 4 ).
Fig. 4.
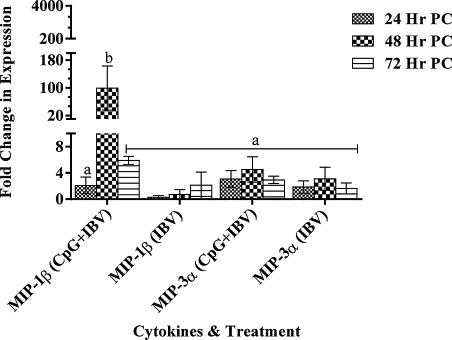
Macrophage inflammatory protein (MIP) 1β and MIP-3α genes expression in lungs tissue.
MIP-1β and MIP-3α genes expression in lungs tissue following CpG ODN 2007 or PBS administration in 18 day old chicken embryos via chorioallontoic sac at a concentration of 50 μg/50 μl per embryo. The change in gene expression is shown as mean ± SEM fold change in CpG ODN 2007 treated embryos compared to PBS treated embryos. The difference in mRNA expression was considered significant at p < 0.05 and shown with different superscript alphabets (a and b) on the bars.
In our experiments, there was non-significant difference in OASA gene expression in CpG ODN pre-treated and PBS pre-treated infected embryos (groups A and B, respectively) for first 48 h, PI. However, at 72 h, PI, OASA gene expression in embryonic lungs from the CpG ODN pre-treated group was significantly lower than in the lungs from PBS treated embryos (Fig. 5 ).
Fig. 5.
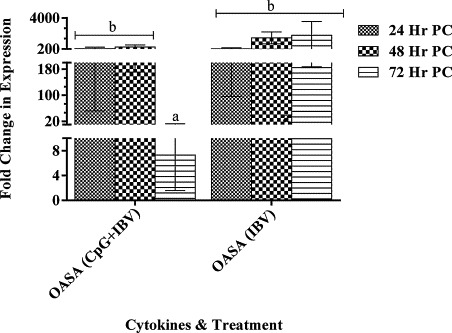
Oligoadenylsynthatase A (OASA) genes expression in lungs tissue. The OASA gene expression in lungs tissue following CpG ODN 2007 or PBS administration in 18 day old chicken embryos via chorioallontoic sac at a concentration of 50 μg/50 μl per embryo. The change in gene expression is shown as mean ± SEM fold change in CpG ODN 2007 treated embryos compared to PBS treated embryos. The difference in mRNA expression was considered significant at p < 0.05 and shown with different superscript alphabets (a and b) on the bars.
The analysis of lungs tissue collected from group D at 24 h, post CpG ODN administration showed substantial up-regulation (35 fold) of IFN-γ gene expression While, moderate level of increase in IL-1(2.8 fold), IL-8 (5 fold), MIP-1β (2.8 fold) and OASA (5 fold) genes expression was observed. It is noteworthy, that 24 h, post CpG ODN administration time point is the time point when embryos in group A and B were challenged with IBV (Table 2 ).
Table 2.
IFNs, ILs and chemokines genes expression at different time points following in ovo administration CpG ODN (Experiment 1).
| IFN-α | IFN-γ | IL-1 | IL-6 | IL-8 | IL-12 | IL-18 | MIP-1β | MIP-3α | OASA | |
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 0 | 35 | 2.8 | 0 | 5 | 0 | 0 | 2.8 | 0 | 5 |
| 48 h | 0.5 | 200 | 15 | 2 | 25 | 3 | 0.6 | 15 | 2 | 25 |
| 72 h | 2 | 0 | 15 | 0 | 0 | 1.6 | 0.4 | 15 | 0 | 0 |
4. Discussion
The initial interaction between viral surface proteins and the cellular receptors leads to the first wave of cytokine production (Mogensen and Paludan, 2001) whereby, cytokines exhibit their activity through formation of antiviral proteins and shaping of adaptive immunity (Samuel, 2001, Spiegel and Weber, 2006). Alternatively, viruses have evolved various mechanisms to alter the host defence in favour of virus propagation. Interestingly, this immune evasion process is facilitated through modulation of cytokines/chemokines induction processes in host cells (Cheung et al., 2005, Huang et al., 2005, Reghunathan et al., 2005, Wang et al., 2004).
Amongst the cytokines, interferons are a large family of secreted proteins which are involved in innate antiviral activity, regulation of cell growth and adaptive immune response activation (Hooks et al., 2003). Type I interferons (IFN α/β) are the cytokines which are produced by the majority of body cells in response to viral infections. As expected from a B class CpG ODN, the administration of CpG ODN 2007 in chicken embryos on ED18 has induced very low level of IFN α gene expression in lungs (Table 2). However, approximately a 4 fold up-regulation of IFN-α gene expression in CpG ODN treated and non CpG ODN treated embryos (groups A and B) infected with IBV was seen in our studies, suggesting that increased expression of IFN-α gene may be associated with IBV infection. These data also imply that up-regulation of IFN-α gene expression may have no implications for IBV replication in vivo (in chicken lungs). Alternatively, it may also be assumed that 4 fold up-regulation in IFN-α gene expression in vivo may not be sufficient to effect IBV replication in lung cells. It is noteworthy, that similar to our findings, non-significant up-regulation of interferon α/β genes in SARS-CoV infected individuals and experimentally infected mice have been observed by other researchers (Glass et al., 2004, Reghunathan et al., 2005). However, in vitro inhibitory effects of type I interferons on SARS-CoV replication have been reported by some researchers (Yoshikawa et al., 2010). These findings indicate that coronavirus have evolved strategies to evade this important innate host defence in vivo. Moreover, it has been recently found that N and M proteins of SARS and other coronaviruses have an antagonistic effect on activation of IRF-3-TRAF3-STAT-IFN type I related pathways (Kopecky-Bromberg et al., 2007, Narayanan et al., 2008, Spiegel et al., 2005, Tohya et al., 2009).
IFN-γ is a key immune-regulatory type II interferon. IFN-γ is mainly synthesized by immune cells including natural killer cells, CD4 and CD8 lymphocytes in response to mitogenic and antigenic stimuli (Young and Bream, 2007, Young and Ghosh, 1997). IFN-γ mediates its antiviral activity through its multi-level cellular interactions. Binding of IFN-γ to its receptors (IFN-γRα and IFN-γRβ) leads to activation of signal transduction and activator of transcription (STAT) and two members of Janus family tyrosine kinases (JAK-1 and JAK-2). While, activation of JAK-STAT signalling pathway is primarily involved in signal transduction and activation of immune response genes including IRF-1, IRF-9, iNOS-2 genes (Hurgin et al., 2007). IFN-γ also activates T cells, monocytes and resident cells leading to enhancement of T cells cytoxicity through granzyme B and MHC I and II induction. Various researchers have shown highly increased susceptibility to mouse hepatitis virus (MHV) infection with disrupted IFN-γ receptor in mice (Schijns et al., 1996). Likewise, down regulation of cellular adhesion molecule (ICAM) 1 gene expression mediated through activation of IFN-γ gene expression has led to increased resistance against rhinovirus infection in mice (Bianco et al., 2000, Sethi et al., 1997). In chickens, IFN-γ induction or incorporation of IFN-γ as a vaccine adjuvant has shown significant antiviral activity mediated through enhanced MHC genes expression on antigen presenting cells, stimulation of antibody production, promotion of antibody isotype switching and rapid development of cytotoxic T cells (Hackney et al., 2003, Lowenthal et al., 1998a, Lowenthal et al., 1998b). In harmony with these findings, inhibition of IBV replication and significant up-regulation of IFN-γ gene expression in CpG ODN pre-treated IBV infected group in our studies suggest that CpG ODN may be considered as a potential adjuvant candidate in non-invasive vaccines against IBV infection.
It is well known that severity of the SARS-CoV infection is augmented with elevated expression of inflammatory mediators including IL-1, IL-6, IL-8, interferon inducible protein (IP) 10 and monocyte chemoattractant protein (MCP) 1genes (Huang et al., 2005, Jiang et al., 2005, Reghunathan et al., 2005, Tseng et al., 2005, Yoshikawa et al., 2010, Zhang et al., 2004). Similar to SARS-CoV, IBV infects multiple tissue systems including lungs, kidneys and the GI tract (Cavanagh, 2003, Raj and Jones, 1997). Major clinical signs in severe respiratory infection of IBV include mucosal thickening and accumulation of thick mucus. It is believed that similar to other respiratory viruses the induction of cytokines including IL-1β, IL-6 and IL-8 in IBV infected epithelial cells contributes towards excessive mucus production (Hendley, 1998). Based on these findings, the suppression of IL-6 gene expression in group A in our experiments may suggest an anti-inflamatory role of CpG ODN 2007 in IBV infection. However, further investigations are needed in this regards. Similarly, it is well known that IL-8 is a chemo-attractant for heterophils which are abundantly found in nasal exudates of IBV infected birds (Raj et al., 1997). Increased expression of IL-8 associated with inhibition of IBV replication in lungs of CpG ODN treated group is in agreement with enhanced IL-8 expression in SARS-CoV infected cells leading to functional modification of macrophages and dendritic cells and strengthening of host innate immune responses (Sims et al., 2008, Yoshikawa et al., 2009).
Guo et al. (2008) and Kimura et al. (2013) have shown activation of MIP-1β and IFN signalling pathways being critical in bridging innate and adaptive immunity and induction of antiviral state in IBV and other respiratory viruses infections (Guo et al., 2008, Kimura et al., 2013). Significant induction of MIP-1β and IFN-γ genes in CpG ODN treated IBV infected group in our experiment may suggest similar antiviral role of both cytokines.
Activation of 2′–5′ OAS and ribonuclease (RNase) L is part of an important host antiviral pathway. Activation of RNase L is a well know function of OAS. Activated RNase L inhibits viral replication through degradation of single stranded viral or cellular RNA and blocking of protein synthesis (Silverman, 2007, Zhao et al., 2012). High levels of OASA gene expression in CpG treated and non-CpG treated infected lungs in the first 48 h, PI suggests that activation of OAS in IBV infected cells has no effect on virus replication. It may be assumed that similar to many other viruses, IBV has evolved strategies to block OASA antiviral pathway downstream to the activation of OAS. For instance, Zhao et al. (2012) and Zhang et al. (2013) have reported that ns2 protein of mouse hepatitis virus (a coronavirus) has the ability to prevent RNase L activation through cleavage of 2–5 OAS proteins (Zhang et al., 2013, Zhao et al., 2012). Similarly, a RNA structure present in open reading frame (ORF) of viral protease gene (3CPro) in polio virus infection acts as a potent RNase L inhibitor and prevents viral RNA degradation by RNase L (Han et al., 2007). Viruses including HIV-1 and encephlomyocarditis virus inhibit RNase L activity through activation of a RNase L inhibitor (RLI) protein (Martinand et al., 1998, Martinand et al., 1999). Additionally, significantly reduced OASA gene expression in CpG ODN treated lungs compared to the PBS treated group at 72 h, PI may be due to lack of virus replication in CpG treated cells, whereas significantly higher expression of OASA gene in non-CpG treated infected lungs may be associated with continued IBV replication.
In conclusion, our data demonstrate a significant role of differential up-regulation of IFN-γ, IL-8 and MIP-1β genes and suppression of IL-6 gene expression in inhibition of IBV replication in chicken lungs. Further characterization of these marker gens will be helpful in improvement of theraputic and prophylactic reagents for the control of coronavirus infection.
Acknowledgements
This research work was supported by the Merial Limited (G2115). We are thankful to Ken Lai for his technical assistance. We also owe thanks to Dr. Don Wilson, Jan Erickson, Sherry Tetland and Amie Sewel from the Animal Care facilities at VIDO. Published with permission of the director of VIDO as VIDO journal series number 697.
References
- Andrews D.M., Estcourt M.J., Andoniou C.E., Wikstrom M.E., Khong A., Voigt V., Fleming P., Tabarias H., Hill G.R., van der Most R.G., Scalzo A.A., Smyth M.J., Degli-Esposti M.A. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J. Exp. Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banat G.R., Tkalcic S., Dzielawa J.A., Jackwood M.W., Saggese M.D., Yates L., Kopulos R., Briles W.E., Collisson E.W. Association of the chicken MHC B haplotypes with resistance to avian coronavirus. Dev. Comp. Immunol. 2013;39:430–437. doi: 10.1016/j.dci.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Narayanan K., Mizutani T., Makino S. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 2002;76:5937–5948. doi: 10.1128/JVI.76.12.5937-5948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A., Whiteman S.C., Sethi S.K., Allen J.T., Knight R.A., Spiteri M.A. Expression of intercellular adhesion molecule-1 (ICAM-1) in nasal epithelial cells of atopic subjects: a mechanism for increased rhinovirus infection? Clin. Exp. Immunol. 2000;121:339–345. doi: 10.1046/j.1365-2249.2000.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh V.J., Guidotti L.G., Chisari F.V. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J. Virol. 1997;71:3236–3243. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H., Chan K.H., Yuen K.Y., Gordon S., Guan Y., Peiris J.S. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A., Allan B., Gomis S., Potter A.G.M. immunotherapeutic potentials of CpG oligonucleotides in chickens. J. Poult. Sci. 2009;46:69–80. [Google Scholar]
- Dar A., Potter A., Tikoo S., Gerdts V., Lai K., Babiuk L.A., Mutwiri G. CpG oligodeoxynucleotides activate innate immune response that suppresses infectious bronchitis virus replication in chicken embryos. Avian Dis. 2009;53:261–267. doi: 10.1637/8560-121808-Reg.1. [DOI] [PubMed] [Google Scholar]
- Estcourt M.J., Ramshaw A., Ramsay A.J. Cytokine responses in virus infections: effects on pathogenesis, recovery and persistence. Curr. Opin. Microbiol. 1998;1:411–418. doi: 10.1016/s1369-5274(98)80058-1. [DOI] [PubMed] [Google Scholar]
- Fearon D.T. Innate immunity—beginning to fulfill its promise? Nat. Immunol. 2000;1:102–103. doi: 10.1038/77773. [DOI] [PubMed] [Google Scholar]
- Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 2004;173:4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- Gomis S., Babiuk L., Allan B., Willson P., Waters E., Ambrose N., Hecker R., Potter A. Protection of neonatal chicks against a lethal challenge of Escherichia coli using DNA containing cytosine-phosphodiester-guanine motifs. Avian Dis. 2004;48:813–822. doi: 10.1637/7194-041204R. [DOI] [PubMed] [Google Scholar]
- Gomis S., Babiuk L., Allan B., Willson P., Waters E., Hecker R., Potter A. Protection of chickens against a lethal challenge of Escherichia coli by a vaccine containing CpG oligodeoxynucleotides as an adjuvant. Avian Dis. 2007;51:78–83. doi: 10.1637/0005-2086(2007)051[0078:POCAAL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Guo X., Rosa A.J., Chen D.G., Wang X. Molecular mechanisms of primary and secondary mucosal immunity using avian infectious bronchitis virus as a model system. Vet. Immunol. Immunopathol. 2008;121:332–343. doi: 10.1016/j.vetimm.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney K., Cavanagh D., Kaiser P., Britton P. In vitro and in ovo expression of chicken gamma interferon by a defective RNA of avian coronavirus infectious bronchitis virus. J. Virol. 2003;77:5694–5702. doi: 10.1128/JVI.77.10.5694-5702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.Q., Townsend H.L., Jha B.K., Paranjape J.M., Silverman R.H., Barton D.J. A phylogenetically conserved RNA structure in the poliovirus open reading frame inhibits the antiviral endoribonuclease RNase L. J. Virol. 2007;81:5561–5572. doi: 10.1128/JVI.01857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Genovese K.J., Swaggerty C.L., MacKinnon K.M., Kogut M.H. Co-stimulation with TLR3 and TLR21 ligands synergistically up-regulates Th1-cytokine IFN-gamma and regulatory cytokine IL-10 expression in chicken monocytes. Dev. Comp. Immunol. 2012;36:756–760. doi: 10.1016/j.dci.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Hendley J.O. The host response, not the virus, causes the symptoms of the common cold. Clin. Infect. Dis. 1998;26:847–848. doi: 10.1086/513921. [DOI] [PubMed] [Google Scholar]
- Hogan S.P., Foster P.S., Tan X., Ramsay A.J. Mucosal IL-12 gene delivery inhibits allergic airways disease and restores local antiviral immunity. Eur. J. Immunol. 1998;28:413–423. doi: 10.1002/(SICI)1521-4141(199802)28:02<413::AID-IMMU413>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Hooks J.J., Wang Y., Detrick B. The critical role of IFN-gamma in experimental coronavirus retinopathy. Invest. Ophthalmol. Visual Sci. 2003;44:3402–3408. doi: 10.1167/iovs.02-1106. [DOI] [PubMed] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurgin V., Novick D., Werman A., Dinarello C.A., Rubinstein M. Antiviral and immunoregulatory activities of IFN-gamma depend on constitutively expressed IL-1alpha. Proc. Nat. Acad. Sci. U.S.A. 2007;104:5044–5049. doi: 10.1073/pnas.0611608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W., Rosenbloom R., Petteruti M., Hilt D.A., McCall A.W., Williams S.M. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010;149:86–94. doi: 10.1016/j.virusres.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- Kimura H., Yoshizumi M., Ishii H., Oishi K., Ryo A. Cytokine production and signaling pathways in respiratory virus infection. Front Microbiol. 2013;4:276. doi: 10.3389/fmicb.2013.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky-Bromberg S.A., Martinez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.W., Jackwood M.W. Evidence of genetic diversity generated by recombination among avian coronavirus IBV. Arch. Virol. 2000;145:2135–2148. doi: 10.1007/s007050070044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowenthal J.W., O’Neil T.E., Broadway M., Strom A.D., Digby M.R., Andrew M., York J.J. Coadministration of IFN-gamma enhances antibody responses in chickens. J. Interferon Cytokine Res. 1998;18:617–622. doi: 10.1089/jir.1998.18.617. [DOI] [PubMed] [Google Scholar]
- Lowenthal J.W., York J.J., O’Neil T.E., Steven R.A., Strom D.G., Digby M.R. Potential use of cytokine therapy in poultry. Vet. Immunol. Immunopathol. 1998;63:191–198. doi: 10.1016/s0165-2427(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Martinand C., Montavon C., Salehzada T., Silhol M., Lebleu B., Bisbal C. RNase L inhibitor is induced during human immunodeficiency virus type 1 infection and down regulates the 2–5A/RNase L pathway in human T cells. J. Virol. 1999;73:290–296. doi: 10.1128/jvi.73.1.290-296.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinand C., Salehzada T., Silhol M., Lebleu B., Bisbal C. RNase L inhibitor (RLI) antisense constructions block partially the down regulation of the 2-5A/RNase L pathway in encephalomyocarditis-virus-(EMCV)-infected cells. Eur. J. Biochem. 1998;254:248–255. doi: 10.1046/j.1432-1327.1998.2540248.x. [DOI] [PubMed] [Google Scholar]
- Mogensen T.H., Paludan S.R. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 2001;65:131–150. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K., Huang C., Lokugamage K., Kamitani W., Ikegami T., Tseng C.T., Makino S. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. J. Virol. 2008;82:4471–4479. doi: 10.1128/JVI.02472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj G.D., Jones R.C. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj G.D., Savage C.E., Jones R.C. Effect of heterophil depletion by 5-fluorouracil on infectious bronchitis virus infection in chickens. Avian Pathol. 1997;26:427–432. doi: 10.1080/03079459708419224. [DOI] [PubMed] [Google Scholar]
- Reghunathan R., Jayapal M., Hsu L.Y., Chng H.H., Tai D., Leung B.P., Melendez A.J. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol. 2005;6:2. doi: 10.1186/1471-2172-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol S., Marinos G., Carucci P., Singer M.V., Williams R., Naoumov N.V. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J. Clin. Invest. 1997;99:3025–3033. doi: 10.1172/JCI119498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. (table of contents) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijns V.E., Wierda C.M., van Hoeij M., Horzinek M.C. Exacerbated viral hepatitis in IFN-gamma receptor-deficient mice is not suppressed by IL-12. J. Immunol. 1996;157:815–821. [PubMed] [Google Scholar]
- Sethi S.K., Bianco A., Allen J.T., Knight R.A., Spiteri M.A. Interferon-gamma (IFN-gamma) down-regulates the rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) on human airway epithelial cells. Clin. Exp. Immunol. 1997;110:362–369. doi: 10.1046/j.1365-2249.1997.4221440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R.H. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 2007;81:12720–12729. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims A.C., Burkett S.E., Yount B., Pickles R.J. SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Res. 2008;133:33–44. doi: 10.1016/j.virusres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M., Pichlmair A., Martinez-Sobrido L., Cros J., Garcia-Sastre A., Haller O., Weber F. Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 2005;79:2079–2086. doi: 10.1128/JVI.79.4.2079-2086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M., Weber F. Inhibition of cytokine gene expression and induction of chemokine genes in non-lymphatic cells infected with SARS coronavirus. Virol. J. 2006;3:17. doi: 10.1186/1743-422X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Sato Y., Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5:1250–1260. doi: 10.3390/v5051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohya Y., Narayanan K., Kamitani W., Huang C., Lokugamage K., Makino S. Suppression of host gene expression by nsp1 proteins of group 2 bat coronaviruses. J. Virol. 2009;83:5282–5288. doi: 10.1128/JVI.02485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.T., Perrone L.A., Zhu H., Makino S., Peters C.J. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J. Immunol. 2005;174:7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- Wang W.K., Chen S.Y., Liu I.J., Kao C.L., Chen H.L., Chiang B.L., Wang J.T., Sheng W.H., Hsueh P.R., Yang C.F., Yang P.C., Chang S.C. Temporal relationship of viral load, ribavirin, interleukin (IL)-6, IL-8, and clinical progression in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 2004;39:1071–1075. doi: 10.1086/423808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J. Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T., Hill T.E., Yoshikawa N., Popov V.L., Galindo C.L., Garner H.R., Peters C.J., Tseng C.T. Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection. PLoS One. 2010;5:e8729. doi: 10.1371/journal.pone.0008729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H.A., Bream J.H. IFN-gamma: recent advances in understanding regulation of expression, biological functions, and clinical applications. Curr. Top. Microbiol. Immunol. 2007;316:97–117. doi: 10.1007/978-3-540-71329-6_6. [DOI] [PubMed] [Google Scholar]
- Young H.A., Ghosh P. Molecular regulation of cytokine gene expression: interferon-gamma as a model system. Prog. Nucleic Acid Res. Mol. Biol. 1997;56:109–127. doi: 10.1016/s0079-6603(08)61004-1. [DOI] [PubMed] [Google Scholar]
- Zhang R., Jha B.K., Ogden K.M., Dong B., Zhao L., Elliott R., Patton J.T., Silverman R.H., Weiss S.R. Homologous 2′,5′-phosphodiesterases from disparate RNA viruses antagonize antiviral innate immunity. Proc. Nat. Acad. Sci. U.S.A. 2013;110:13114–13119. doi: 10.1073/pnas.1306917110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W., Ye L., Xu S., Sun R., Wang Y., Lou J. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect. Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Jha B.K., Wu A., Elliott R., Ziebuhr J., Gorbalenya A.E., Silverman R.H., Weiss S.R. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe. 2012;11:607–616. doi: 10.1016/j.chom.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


