Abstract
In calves, passive immunity of immunoglobulins can be acquired through ingestion of colostrum or colostrum replacers. Plasma can been used to supplement immunoglobulins in healthy or sick calves. Serum half-life of colostral derived immuglobulin G (IgG) is estimated to be 20 days. Half-life of IgG is important in determining response to antigens and timing of vaccination in calves. To date studies evaluating half-life of colostrum replacer or plasma derived IgG are lacking. The objectives of this study were to compare the serum half-life of IgG derived from colostrum, colostrum replacer and plasma in dairy calves reared up to 35 days of age. Thirty Jersey calves were randomly assigned to receive colostrum or colostrum replacer by oroesophageal tubing or plasma by intravenous administration. Serum samples were collected at 2, 5, 7, 10, 14, 21, 28 and 35 days. Serum IgG concentrations were determined by radial immunodiffusion. The results indicated that half-life for IgG in colostrum fed (28.5 days) or plasma transfused calves (27.3 days) was longer than colostrum replacer fed calves (19.1 days). Further studies are required to evaluate pathogen specific immunoglobulins in order to recommend vaccination timing in calves fed colostrum replacers.
Keywords: Colostrum, Colostrum replacer, Half-life, Calf, Immunoglobulin
1. Introduction
The cotyledonary type placenta restricts transfer of immunoglobulins from cow to the fetus during pregnancy (Arthur, 1999). Consequently calves are born hypogammaglobulinemic, thus making it essential for calves to ingest and absorb colostral immunoglobulins to acquire passive immunity. Half of all neonatal mortality can be directly attributed to failure to acquire passive immunity at birth (Tyler et al., 1999). Acquiring of passive immunity through ingestion and absorption of colostral immunoglobulins at birth in calves can be achieved by feeding colostrum or colostrum replacers. Colostrum replacers are used on dairy farms when sufficient colostrum is not available or to prevent transmission of diseases that can be transmitted through unpasteurized colostrum (Foster et al., 2006). In veterinary clinical settings, plasma has been used to supplement immunoglobulins in clinically healthy or sick neonatal calves with or without adequate ingestion of colostral immunoglobulins (Selim et al., 1995).
Colostral derived immunoglobulins can suppress neonatal immunity by various mechanisms (Banks, 1982). Previous studies demonstrated that colostrum deprived calves produced IgG and IgA earlier than colostrum fed calves (Husband and Lascelles, 1975). The mechanism of suppression of the immune system by the colostral-derived immunoglobulins can be either antigen specific or non-antigen specific (Banks, 1982). As a result of this immune system suppression, concentrations of colostral derived maternal immunoglobulins must be reduced to a critical low level that does not interfere with response to antigen exposure or vaccination (Pastoret, 2007). The rate at which this critical low level of immunoglobulins is achieved is dependent on the immunoglobulin concentration and immunoglobulin class absorbed at birth because each class of immunoglobulin is catabolized at a different rate. The half-life of each immunoglobulin can be used to estimate the rate of its catabolism. The serum half-life of colostral-derived immuglobulin G (IgG), M (IgM), and A (IgA) in calves was 20, 4.8, and 2.8 days, respectively (Banks, 1982). In cattle, the predominant colostral immunoglobulin (85–90%) is IgG while IgM and IgA constitute approximately 7% and 3% respectively (Larson et al., 1979).
To the best of authors’ knowledge, no studies have evaluated the half-life of colostrum replacer or plasma derived IgG in dairy calves. We hypothesized that the half-life of maternally derived colostral IgG will have a significantly longer half-life compared to colostrum replacer or plasma derived IgG. The objective of this study was to compare the serum half-life of IgG derived from colostrum, colostrum replacer and plasma in dairy calves reared up to 35 days of age. The results of the study were intended to evaluate if different timing of vaccination should be considered when vaccinating colostrum fed calves as opposed to colostrum replacer fed dairy calves or plasma transfused calves. Additionally, information on serum half-life of plasma-derived IgG would help predict when to repeat plasma transfusion in clinical settings.
2. Materials and methods
2.1. Animals and sampling procedures
The study was approved by the University of California, Davis (UC Davis) Institutional Animal Care and Use Protocol (Protocol Number 17630). Sample size calculation was based on a 50% mortality rate attributed to calves not ingesting sufficient colostrum (Tyler et al., 1999), alpha of 5%, power of 80% and a mortality rate of up to 13% during the first 4 weeks of life in calves failing to ingest sufficient colostrum at birth, in California (Moore et al., 2002). The total sample size required was 27 calves. In order to account for a 10% dropout due to missed sampling, 30 calves were enrolled.
All calves were enrolled from a single Jersey dairy farm in Hilmar, California (Merced County). Adult cows on the farm of study were vaccinated annually with a multivalent modified live respiratory vaccine containing infectious bovine rhinotracheitis, bovine viral diarrhea, parainfluenza-3 and bovine respiratory syncytial viruses and dewormed twice a year with topical ivermectin. Additionally, the cows were vaccinated with a multivalent vaccine containing Escherichia coli, rotavirus and coronavirus during the dry cow period.
Thirty Jersey bull calves delivered from eutocia and observed births were immediately separated from the dam following parturition. The calves were identified using plastic ear tags and weighed. Calves were randomly assigned to receive pooled, pasteurized colostrum from the farm of study or colostrum replacer (The Saskatoon Colostrum Co, Saskatoon, Canada) or bovine plasma (Transfusion Services, University of California, Davis, CA). Colostrum was pasteurized using a batch pasteurizer at 60° C for 30 min with continuous agitation followed by cooling at 4° C. All calves received colostrum or colostrum replacer or bovine plasma within 2 h after birth. The bovine plasma was derived from 2 clinically healthy, UC Davis Veterinary Medical Teaching Hospital blood donor cows. The plasma was evaluated for sterility and considered to be free of transmissible blood borne pathogens. The plasma from the 2 blood donor cows was not pooled. Serum samples were collected from all calves prior to any procedure for subsequent serum IgG determination. An aliquot (5 ml) of colostrum or plasma to be administered was collected prior to administration for subsequent IgG concentration determination. No samples were collected from the colostrum replacer because the IgG concentration was known according to the manufacturer's label. All calves were enrolled into the study over a 2-week period.
Calves in the colostrum (CL, control group) group were fed 3 L of colostrum once by oroesophageal tubing. Calves in the colostrum replacer (CR) group were fed 2.5 L (2 bags) of colostrum replacer, once, according to the manufacturer's recommendations delivering 200 g of IgG for absorption by oroesophageal tubing. Calves in the plasma (PL) group were administered with bovine plasma at 30 ml/kg (Barrington and Parish, 2009) through an intravenous catheter (Becton and Dickinson Co, Franklin Lakes, NJ) aseptically placed in the external jugular vein. Infusion of plasma was performed slowly (10 ml/kg/h) over the first 20 min. Monitoring for transfusion reactions included monitoring heart rate, respiratory rate, color of mucous membranes, and abnormal behavior. In the absence of an immediate transfusion reaction, the remainder of the plasma was transfused over 20–30 min. In the presence of a plasma transfusion reaction, transfusion was discontinued for 10 min and resumed at 5 ml/kg/h.
The calves were then housed in individual plastic calf hutches at the UC Davis Beef Research facilities. Calves were fed 2 L of non-medicated milk replacer (Calva Products, Acampo, CA) and 0.5 kg of commercial calf concentrate (Department of Animal Science, University of California, Davis, CA) twice daily. At 4 weeks of age, calves were fed 3 L of milk replacer and 1 kg of commercial calf concentrate, twice daily. Serum samples were collected at 2, 5, 7, 10, 14, 21, 28 and 35 days of age. All calves were weighed at the end of the study (5 weeks of age). Samples of serum collected at different time points, colostrum, and plasma administered were stored at −20° C until serum IgG determination. Calves were monitored for health by daily evaluation of appetite, rectal temperature, signs of coughing, diarrhea and lameness. Sick calves were treated following recommendations by a UC Davis licensed veterinarian as per the UC Davis Institutional Animal Care and Use Protocol. Calves that died were submitted for a complete necropsy at the California Animal Health and Food Safety Laboratory in Davis, CA.
Serum, plasma or colostral IgG concentrations were determined using a commercial radial immunodiffusion (RID) kit based on the manufacturer's recommendations (Triple J Farms, Bellingham, WA). Briefly, RID plates containing specific anti-bovine IgG, agarose gel, 0.1 M phosphate buffer pH 7.0, 0.1% sodium azide as a bacteriostatic agent and 1 μg/ml amphotericin B as a fungal agent stored in a refrigerator at 4° C were warmed at room temperature (20–24° C). An aliquot (5 μl) of the provided reference serum at 3 different concentrations were pipetted into each RID wells. An aliquot (5 μl) of serum (diluted 1:2 with phosphate buffer) or colostrum samples (diluted 1:4 with phosphate buffer) were pipetted into individual RID plate wells. The plates were incubated at room temperature (20–24° C) for 24 h. The diameters of the zones of precipitation were measured using a digital RID plate reader (The Binding Site Inc, San Diego, CA) after 24 h. Serum or colostral sample IgG concentrations were determined by comparing the diameter of the zones of precipitation with a standard curve generated by the reference serum. The regression equation generated in this manner (r 2 = 0.97–0.99) accurately predicts inoculum IgG concentration. Minimum detectable serum IgG concentration was 196 mg/dL using the RID. For the purposes of this study, calves determined to have <196 mg/dL of IgG by RID were considered to have 195 mg/dL IgG.
2.2. Statistical analysis
Normality of data was checked using the Shapiro–Wilk test. In instances where the data was not normally distributed, the median was reported. Descriptive statistics were calculated for calf birth weight, 5-week weight, serum IgG concentrations at 2 days, IgG concentrations in plasma administered and colostral IgG concentrations, morbidity and mortality events among the 3 groups. Serum IgG concentrations at 2 days were used to compare the presence of failure of passive transfer of immunity among the groups using a Kruskal–Wallis, 1-way analysis of variance on ranks. Calves with serum IgG concentrations of <1000 mg/dL were considered to have failure of passive immunity (Besser et al., 1991). Proportions of calves with failure of passive transfer of colostral immunoglobulins at 2 days or calves that died among the 3 groups were compared using a χ 2 test or Fisher's exact test when a cell had less than 5 counts.
In calves that received plasma transfusion, the predicted post-transfusion serum IgG concentration was calculated using the following formula (Chigerwe and Tyler, 2010):
| (1) |
where weight = mean weight (kg) of the calves in the PL group; 0.097 L plasma/kg = plasma volume in Jersey calves (Quigley et al., 1998); IgG = serum IgG concentration (mg/dL) prior to administration of plasma; IgG in plasma = mean IgG concentration (mg/dL) in the plasma administered; and plasma volume = mean volume (L) of plasma administered IV at 30 ml/kg.
Serum half-life for IgG in each of the 3 groups of calves was determined by a non-linear regression analysis using a one-phase exponential decay model with random effects for calf initial serum IgG concentrations. The decay of IgG over time was assumed to be constant. Differences in serum IgG half-life among the 3 groups were evaluated by comparing the rate constants using an F-test. In all statistical analyses values of P < 0.05 were considered significant. All statistical analyses were performed using a commercial statistical software (Prism 6, GraphPad Inc, La Jolla, CA).
3. Results and discussion
Mean ± SD birth weight for all 30 calves was 27.6 ± 3.5 kg. Mean ± SD birth weight for the CL, CR and PL group was 30 ± 2.9, 27.8 ± 2.3 and 25.1 ± 3.6 kg, respectively. All calves had a serum IgG concentration of <196 mg/dL prior to feeding colostrum or colostrum replacer or administration of plasma. Mean colostral IgG concentration fed to the CL group was 66.1 g/L. Mean plasma volume and mean plasma IgG concentrations administered to the calves in the PL group were 753 ml and 2848.4 mg/dL, respectively. None of the calves in the PL group showed immediate transfusion reactions. All calves in the CL and CR group had adequate transfer of immunity (serum IgG concentrations >1000 mg/dL at 2 days of age). Median (interquartile range) serum IgG concentrations at 2 days of age for calves in the CL, CR and PL groups were 2377.5 (421.9), 2306 (475.2), and 669.3 (267.8), respectively. Only 1 calf in the PL group had serum IgG concentrations >1000 mg/dL. There was no difference in median serum IgG concentrations at 2 days between calves in the CL and CR groups (P = 0.565). Median serum IgG concentrations of calves in the PL group were lower than calves in both CL (P < 0.001) and CR (P < 0.001) groups. Using Eq. (1) and substituting for a mean weight of 25.1 kg, serum IgG concentration of 195 mg/dL prior to administration of plasma, mean plasma volume of 753 ml (25.1 kg × 30 ml/kg) administered, mean plasma IgG concentration of 2848.4 mg/kg, the expected serum IgG concentration at 2 days of age in the PL group was 1074.9 mg/dL.
Morbidity events were recorded in 21 calves (70% with 20 diarrhea cases and 1 pneumonia case). Fourteen of the 21 morbidity cases in the calves required treatment with antibiotics and or oral rehydration electrolyte solutions. Six calves died during the 5-week period. Of the calves that died or were euthanized, 4 calves were from the PL group, 1 from the CL group and 1 from the CR group. Mortality in the 6 calves occurred during the first 12 days of life. Causes of mortality in the 6 calves included enteritis secondary to enterotoxigenic E. coli, rotavirus and Cryptosporidium in 4 calves, enterocolitis due to Clostridium perfringens in 1 calf and enterocolitis due to E. coli, rotavirus and bovine viral diarrhea virus (BVDV) in 1 calf. Given that 1 calf tested positive for BVDV on necropsy, all remaining calves (24 calves) calves were tested for BVDV by immunohistochemistry on ear notch skin biopsy. All remaining calves tested negative for BVDV. Proportions of calves with failure of passive transfer between the CL and CR groups were not different (P = 1). Proportions of calves that died in the PL group were higher compared with the CL (P < 0.001) and CR (P < 0.001) groups. Mean ± SD weight for the remaining 24 calves at 5 weeks was 38.9 ± 9.3 kg with a daily average gain of 0.4 kg/day.
Half-life (95% confidence interval) for colostral, plasma and colostrum replacer derived IgG was 28.5 (23.4, 36.5), 27.3 (16.6, 75.6) and 19.1 (15.7, 24.5) days, respectively. The half-life for the CL and PL was not different (P = 0.420). The half-life for the CL (P < 0.001) and PL (P < 0.001) groups was longer than that of the CR group. Summarized descriptive statistics results are represented in Table 1 . The decay curves generated for the 3 groups of calves are represented in Fig. 1 .
Table 1.
Summary of descriptive statistics for calves fed colostrum or colostrum replacer or intravenous transfusion with bovine plasma (N = 30).
| All calves | Colostrum group (CL) | Colostrum replacer group (CR) | Plasma group (PL) | |
|---|---|---|---|---|
| Birth weight (kg) | 26 ± 3.5 | 30 ± 2.9a | 27.8 ± 2.3a | 25.1 ± 3.6a |
| Mean colostrum IgG fed or transfused | 66 | 200 | 2848.4 | |
| Median serum IgG concentration at 2 days (mg/dL) | 2377.5 (421.9)a | 2306 (475.2)a | 669.3 (267.8)b | |
| Mortality (%) | 20 | 10a | 10a | 40b |
| IgG half-life (days) | 28.5 (23.4, 36.5)a | 19.1 (15.7, 24.5)b | 27.3 (16.6, 75.6)a |
Birth weight was reported as mean ± SD. Mean colostrum IgG fed to the CR group was reported as g/L and grams of IgG in the CR group. Mean IgG concentrations transfused to the PL group was reported as mg/dL. Median serum IgG concentration was reported as median (interquartile range). Half-life of IgG was reported as days (95% CI).
Where applicable, values with the same letter superscript within a given row are not different (P > 0.05).
Fig. 1.
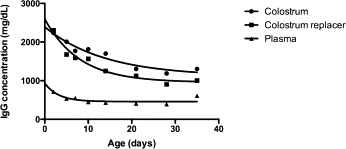
Fitted exponential decay curves over 35 days of serum IgG concentrations in calves that were fed colostrum or bovine colostrum replacer or intravenously transfused with bovine plasma.
The aim of this study was to compare serum half-life of IgG derived from colostrum or colostrum replacer and plasma. The main finding in this study demonstrated that half-life of colostral derived IgG was longer than colostrum replacer derived IgG. Although these study results suggest different timing when vaccinating colostrum or colostrum replacer fed dairy calves, the current studies did not evaluate specific pathogen immunoglobulins to common vaccinations in adult dairy cows and pre-weaned dairy calves. The specific immunoglobulins for pathogens to be considered in future studies include rotavirus, coronavirus, infectious bovine rhinotracheitis, bovine viral diarrhea, parainfluenza-3 and bovine respiratory syncytial viruses. While the specificity of the colostral-derived immunoglobulins is important to confer specific immunity, it should be noted that at birth calves have greater number of neutrophils compared to adult cattle (Rossi et al., 1979). Primary and secondary lymphoid tissues of newborn calves are populated by immune cells and the circulatory B-lymphocytes can synthesize immunoglobulins following antigenic stimulation (Banks, 1982). Thus passively acquired IgG are not the only immunoglobulins involved during conference of immune protection after birth; but cells from lymphoid tissues and neutrophils from circulation are also involved. Therefore lack of specificity does not necessarily result in occurrence of a morbidity event.
It is important to note that the half-life of colostral derived IgG was longer than previously reported in other studies (Banks, 1982, Butler, 1983, Besser et al., 1988). Previous studies reported IgG half-life of 20 days (Banks, 1982), 17.9 days (Besser et al., 1988), 9.6 days for IgG1 (Butler, 1983) and 17.7 days for IgG2 (Butler, 1983) in calves. While the age of the calves during determination of half-life was not stated in two of the studies (Banks, 1982, Butler, 1983), serum collection in calves was performed until 10 days of age in the third study (Besser et al., 1988). We chose to determine serum IgG half-life in calves reared up to 35 days of age based on previous studies indicating that antibodies to different bacterial and viral antigens were not produced or did not appear in serum until 14–30 days of age (Kerr, 1956, Lambert et al., 1969, Thorsen et al., 1969, Husband and Lascelles, 1975). Thus, it can be assumed that the majority of immunologlobulins in the serum are colostral derived in calves aged up to 30 days. The difference in the duration of follow-up is a potential explanation for the difference in the serum IgG half-life determinations in the different studies.
Majority of calves in the PL group were anticipated to achieve adequate transfer of immunoglobulins at 2 days of age based on Eq. (1) and recommended plasma transfusion rate, but only one calf achieved adequate transfer of immunoglobulins. In contrast, the CL and CR group achieved serum IgG consistent with adequate transfer of immunity. One explanation for this observation is possible; increased catabolism due to complement activation by IgG aggregates from the preparation process of the plasma (Lundblad and Londeree, 1988). In contrast, absorption of orally delivered IgG occurs within the first 24–36 h of birth is a non-selective passive process through pinocytosis (Stott et al., 1979). Thus, orally derived IgG may undergo catabolism through which intravenously derived IgG is catabolized.
Intravenously derived immunoglobulins are re-secreted into the gastrointestinal tract and excreted through the feces and urine (Besser et al., 1988). Fecal or urinary IgG concentrations were not determined in this study to evaluate rate of excretion among groups. Future studies need to determine serum, fecal and urinary IgG concentrations in calves intravenously transfused with plasma at shorter intervals (for instance every 12 h) prior to 48 h of age in order to evaluate the catabolism of the immunoglobulins. Additionally, evaluation of activity of IgG prior to and after plasma transfusion is warranted.
The study design in this study differs from previous studies (Besser et al., 1988, Jones et al., 2004) in several ways. In studies by Besser and others, calves only received variable volumes of colostrum followed by 2.0 ml of labeled sodium iodide intravenously with no group of calves receiving colostrum replacer or plasma. In studies by Jones and others fed colostrum was twice to calves while the colostrum replacer was bovine serum derived and the study design did not include a group of calves administered intravenous plasma.
It should be noted that in clinical practice, plasma is used to supplement immunoglobulins and thus calves are more likely to have ingested insufficient or sufficient colostrum on presentation. In this study, the PL group only received plasma as the source of immunoglobulins. The results of this study indicate that plasma products with sufficient immunoglobulins may fail to achieve adequate immunity when administered in calves. Although the half-life of the PL was longer than the CR group, the 95% confidence for the half-life estimate is very wide indicating that the estimate is not precise. Thus it is difficult to recommend when to repeat plasma transfusions in clinical settings based on the results of this study.
Summary
The results of this study indicated that colostral derived IgG had a longer half-life compared to colostrum replacer derived IgG. Future studies are required to evaluate pathogen specific immunoglobulins in order to recommend timing of vaccinations in calves fed colostrum replacer at birth. Administration of plasma to newborn calves at the recommended dose did not achieve adequate transfer of immunoglobulins. Calves intravenously administered with plasma at birth were more likely to experience mortality compared to calves that ingested colostrum or colostrum replacer.
Conflict of interest
The authors have no conflict of interest to declare.
Funding source
This research was partly funded by the UC Davis Student Training in Advanced Research.
Acknowledgements
The authors would like to thank Sharon Kim, Peony Kim and Dr. Elizabeth Adams for their assistance.
Contributor Information
Jacob M. Murphy, Email: jmmurph@ucdavis.edu.
Jill V. Hagey, Email: jvhagey@ucdavis.edu.
Munashe Chigerwe, Email: mchigerwe@ucdavis.edu.
References
- Arthur G.H. The development of the conceptus. In: Arthur G.H., Noakes D.E., Pearson H., editors. Pregnancy and Parturition in Veterinary Reproduction and Obstetrics. WB Saunders; Philadelphia: 1999. pp. 51–109. [Google Scholar]
- Banks K.L. Host defense in the newborn animal. J. Am. Vet. Med. Assoc. 1982;181:1053–1056. [PubMed] [Google Scholar]
- Barrington G.M., Parish S.M. Ruminant immunodeficient diseases. In: Smith B.P., editor. Large animal Internal Medicine. Mosby, Elsevier; 2009. pp. 1677–1680. [Google Scholar]
- Besser T.E., McGuire T.C., Gay C.C., Pritchett L.C. Transfer of functional immunoglobulin G (IgG) antibody into the gastrointestinal tract accounts for IgG clearance in calves. J. Virol. 1988;62:2234–2237. doi: 10.1128/jvi.62.7.2234-2237.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser T.E., Gay C.C., Pritchett L. Comparison of three methods of feeding colostrum to dairy calves. J. Am. Vet. Med. Assoc. 1991;198:419–422. [PubMed] [Google Scholar]
- Butler J.E. Bovine immunoglobulins: an augmented review. Vet. Immunol. Immunopathol. 1983;4:43–152. doi: 10.1016/0165-2427(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Chigerwe M., Tyler J.W. Serum IgG concentration after intravenous serum transfusion in a randomized clinical trial in dairy calves with inadequate transfer of colostral immunoglobulins. J. Vet. Intern. Med. 2010;24:231–234. doi: 10.1111/j.1939-1676.2009.0442.x. [DOI] [PubMed] [Google Scholar]
- Foster D.M., Smith G.W., Sanner T.R., Busso G.V. Serum IgG concentrations in dairy calves fed two colostrum replacement products. J. Am. Med. Assoc. 2006;229:1282–1285. doi: 10.2460/javma.229.8.1282. [DOI] [PubMed] [Google Scholar]
- Husband A.J., Lascelles A.K. Antibody responses to neonatal immunization in calves. Res. Vet. Sci. 1975;18:201–207. [PubMed] [Google Scholar]
- Jones C.M., James R.E., Quigley J.D., III, McGilliard M.L. Influence of pooled colostrum or colostrum replacement on IgG and evaluation of animal plasma in milk replacer. J. Dairy Sci. 2004;87:1806–1814. doi: 10.3168/jds.S0022-0302(04)73337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr W.R. Active immunity experiments in very young calves. Vet. Rec. 1956;68:476–477. [Google Scholar]
- Lambert G., Fernelius A.L., Cheville N.F. Experimental bovine viral diarrhea in neonatal calves. J. Am. Vet. Med. Assoc. 1969;154:181–189. [PubMed] [Google Scholar]
- Larson B.L., Heary H.L., Devery J.E. Immunoglobulin production and transport by the mammary gland. J. Dairy Sci. 1979;63:665–671. doi: 10.3168/jds.S0022-0302(80)82988-2. [DOI] [PubMed] [Google Scholar]
- Lundblad J.L., Londeree N. The effect of processing methods on intravenous immune globulin preparations. J. Hosp. Infect. 1988;12:3–15. doi: 10.1016/0195-6701(88)90025-4. [DOI] [PubMed] [Google Scholar]
- Moore D.A., Sischo W.M., Festa D.M., Reynolds J.P., Atwill R.E., Holmberg C.A. Influence of arrival weight, season and calf supplier on survival in Holstein beef calves on a calf ranch in California, US. Prev. Vet. Med. 2002;53:103–115. doi: 10.1016/s0167-5877(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Pastoret P.P. Challenge and issues of early vaccination in animals and humans. J. Comp. Pathol. 2007;137:S2–S3. doi: 10.1016/j.jcpa.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., Drewry J.J., Martin K.R. Estimation of plasma volume in Holstein and Jersey calves. J. Dairy Sci. 1998;81:1308–1312. doi: 10.3168/jds.S0022-0302(98)75693-0. [DOI] [PubMed] [Google Scholar]
- Rossi C.R., Kiesel G.K., Hudson R.S. Kinetics of detection of blastogenic responses of neonatal responses of neonatal calves inoculated in utero with tetanus toxoid, killed Mycobacterium bovis, and killed Brucella abortus. Am. J. Vet. Res. 1979;40:576–579. [PubMed] [Google Scholar]
- Selim S.A., Holmberg C.A., Cullor J.S. Passive immunotherapy in neonatal calves – II. The efficacy of a J5 Escherichia coli hyperimmune plasma as immunotherapy in neonatal calves. Vaccine. 1995;13:1454–1459. doi: 10.1016/0264-410x(95)00067-b. [DOI] [PubMed] [Google Scholar]
- Stott G.H., Marx B.E., Menefee B.E., Nightengale G.T. Colostral immunoglobulin transfer in calves. I. Period of absorption. J. Dairy Sci. 1979;62:1632–1638. doi: 10.3168/jds.S0022-0302(79)83472-4. [DOI] [PubMed] [Google Scholar]
- Thorsen J., Sanderson R., Bittle J. Bovine parainfluenza 3 vaccine studies. Can. J. Comp. Med. 1969;33:105–107. [PMC free article] [PubMed] [Google Scholar]
- Tyler J.W., Hancock D.D., Thorne J.G., Gay C.C., Gay J.M. A model partitioning the risk of mortality associated with inadequate passive transfer in dairy calves. J. Vet. Intern. Med. 1999;13:335–337. doi: 10.1892/0891-6640(1999)013<0335:ptmraw>2.3.co;2. [DOI] [PubMed] [Google Scholar]


