Abstract
Severe acute respiratory syndrome (SARS) is caused by the SARS coronavirus (SARS-CoV). There are many point mutations among SARS-CoV genome sequences. Previous studies suggested that the mutations are correlated closely with the SARS epidemic. It was found that the bases of six nucleotide positions (nt9404, nt9479, nt19838, nt21721, nt22222 and nt27827) with high-mutation rate have an important relationship with the SARS epidemic. For viral detection as well as genotyping, a universal microarray system was developed that combines RT-PCR and ligase detection reaction (LDR). The Zip Codes attached covalently to a slide remain constant and their complementary Zip Codes (cZip Codes) can be used for tagging target sequence, making the microarrays universal. The discriminating oligonucleotides contain on the 5′ end “cZip Codes” that are used to direct LDR product to specific Zip Codes attached covalently to a slide. Since Zip Codes have no homology to either the target sequence or to other sequences in the genomes of both human host and SARS-CoV, there was no false signal due to mismatch hybridizations. 20 samples assayed with the universal microarray were confirmed by DNA sequencing, demonstrating that this microarray system is a promising diagnostic tool for detection and genotyping of the SARS-CoV.
Keywords: SARS, SARS-CoV, Universal microarray, LDR, Zip Code
1. Introduction
Severe acute respiratory syndrome (SARS), a disease that spread widely in the world including mainland China, Hong Kong, Taiwan, Indonesia, Thailand, Vietnam, Singapore, Canada and America last year, is caused by a new coronavirus called SARS-CoV (Drosten et al., 2003, Li et al., 2003). The disease is transmitted by droplets and direct contact (Booth et al., 2003, Li et al., 2003).
Because of the rate of mortality in patients, it is very important to identify SARS-CoV quickly and accurately. Two strategies are used commonly to identify this virus: immunoassay and nucleic acid-based assay. After 156 SARS patients were tested with ELISA, it was found that the positive rates of IgG and IgM were 75.6 and 41.7%, respectively (Wang et al., 2003a, Wang et al., 2003b). The methods of immunofluroscent assay (IFA) also have high percent of false negative. Nucleic acid detection has higher specificity for diagnosis (Zhuang et al., 2003; Wang et al., 2003a, Wang et al., 2003b).
However, SARS-CoV is characterized by the rapid mutation (Li et al., 2003). To date, many mutations including point mutations and few short deletions or insertions were detected in different infected individuals (Chen et al., 2003, He et al., 2004). Considerable researches suggested that variations in viral genomes caused viral transmission from animal to man (Tsui et al., 2003, Ruan et al., 2003, Guan et al., 2003). The genotypes at six positions (nt9404, nt9479, nt19838, nt21721, nt22222 and nt27827) with high-mutation rate were identified closely with the three phases of the SARS epidemic (Table 1 ) – C, C, A, A, C, C (early phase); C, T/C, G, A, C, C (middle phase); T, T, A, G, T, T (late phase). Because the mutations were closely identified with SARS-CoV emergence, the study of the mutations can provide helpful informations for other studies, such as the SARS vaccine, the SARS drug design and the infectious mechanisms of the SARS-CoV.
Table 1.
The six mutation sites of 40 SARS-CoV genome sequences and two SARS-like coronavirus genome sequences
| Name | City | ID | 9404 | 9479 | 19838 | 21721 | 22222 | 27827 |
|---|---|---|---|---|---|---|---|---|
| SARS-CoV SZ3 | Shenzhen | AY304486 | C | C | A | A | C | C |
| SARS-CoV SZ16 | Shenzhen | AY304488 | C | C | A | A | C | C |
| SARS-CoV GZ02 | Guangzhou | AY390556 | C | C | A | A | C | C |
| SARS-CoV ZS-A | Zhongshan | AY394997 | C | C | A | A | C | C |
| SARS-CoV ZS-B | Zhongshan | AY394996 | C | C | A | A | C | C |
| SARS-CoV ZS-C | Zhongshan | AY395003 | C | C | A | A | C | C |
| SARS-CoV GD01 | Guangdong | AY278489 | C | C | G | A | C | C |
| SARS-CoV BJ01 | Beijing | AY278488 | C | T | G | A | C | C |
| SARS-CoV BJ02 | Beijing | AY278487 | C | T | G | A | C | C |
| SARS-CoV BJ03 | Beijing | AY278490 | C | T | G | A | C | C |
| SARS-CoV CUHK-AG01 | Hong Kong | AY345986 | T | T | A | G | T | T |
| SARS-CoV CUHK-AG02 | Hong Kong | AY345987 | T | T | A | G | T | T |
| SARS-CoV CUHK-AG03 | Hong Kong | AY345988 | T | T | A | G | T | T |
| SARS-CoV CUHK Su10 | Hong Kong | AY282752 | T | T | A | G | T | T |
| SARS-CoV FRA | Italy | AY310120 | T | T | A | G | T | T |
| SARS-CoV AS | Milan Italy | AY427439 | T | T | A | G | T | T |
| SARS-CoV Frankfurt 1 | Germany | AY291315 | T | T | A | G | T | T |
| SARS-CoV HKU-39849 | Hong Kong | AY278491 | T | T | A | G | T | T |
| SARS-CoV HSR 1 | Italy | AY323977 | T | T | A | G | T | T |
| SARS-CoV PUMC01 | Beijing | AY350750 | T | T | A | G | T | T |
| SARS-CoV PUMC02 | Beijing | AY357075 | T | T | A | G | T | T |
| SARS-CoV PUMC03 | Beijing | AY357076 | T | T | A | G | T | T |
| SARS-CoV Sin2500 | Singapore | AY283794 | T | T | A | G | T | T |
| SARS-CoV Sin2677 | Singapore | AY283795 | T | T | A | G | T | T |
| SARS-CoV Sin2679 | Singapore | AY283796 | T | T | A | G | T | T |
| SARS-CoV Sin2748 | Singapore | AY283797 | T | T | A | G | T | T |
| SARS-CoV Sin2774 | Singapore | AY283798 | T | T | A | G | T | T |
| SARS-CoV Sino1-11 | Beijing | AY485277 | T | T | A | G | T | T |
| SARS-CoV Sino3-11 | Beijing | AY485278 | T | T | A | G | T | T |
| SARS-CoV Taiwan TC1 | Taiwan | AY338174 | T | T | A | G | T | T |
| SARS-CoV Taiwan TC2 | Taiwan | AY338175 | T | T | A | G | T | T |
| SARS-CoV Taiwan TC3 | Taiwan | AY348314 | T | T | A | G | T | T |
| SARS-CoV TW1 | Taiwan | AY291451 | T | T | A | G | T | T |
| SARS-CoV TWC | Taiwan | AY321118 | T | T | A | G | T | T |
| SARS-CoV TWC2 | Taiwan | AY362698 | T | T | A | G | T | T |
| SARS-CoV TWC3 | Taiwan | AY362699 | T | T | A | G | T | T |
| SARS-CoV Urbani | USA | AY278741 | T | T | A | G | T | T |
| SARS-CoV ZMY 1 | Guangdong | AY351680 | T | T | A | G | T | T |
| SARS-CoV TWK | Taiwan | AP006559 | T | T | A | G | T | T |
| SARS-CoV WHU | Wuhan | AY394850 | T | T | A | G | T | T |
| SARS-CoV ZJ01 | Zhejiang | AY297028 | T | T | A | G | T | T |
| SARS-CoV TOR2a | Toronto | AY274119 | T | T | A | G | T | T |
SARS-CoV TOR2 is used as a reference.
Recently, a universal microarray has been reported for the detection of human SNPs (Holton, 2001), small insertions and deletions in BRCA1 and BRCA2 (Favis et al., 2000) and different bacterial genomes (Busti et al., 2002). Only 1 fmol of the purified PCR products can be detected by this method (Bellis. et al., 2002). A microarray system with the similar method for the SARS-CoV detection and genotyping is reported here (Fig. 1 ). In contrast with RT-PCR and the cDNA microarray used to detect SARS-CoV, this universal microarray cannot only detect the SARS-CoV but also identify the genotypes of six mutated bases related to the different phases of the SARS epidemic. This microarray system is more economical and labor-saving than the SARS-CoV resequencing microarray for complete sequence analysis. Actually, the partial sequences around important variations can provide enough information for viral detection as well as for genotyping.
Fig. 1.
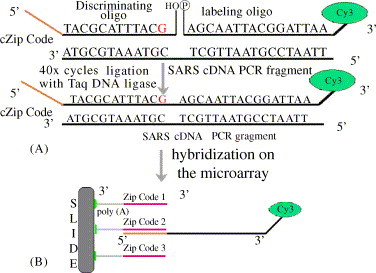
The schematic of the universal microarray-mediated by LDR. (A) The genotype of every mutation base can be detected by a set of oligonucleotides including a discriminating oligonucleotide and a labeled oligonucleotide. The discriminating oligonucleotide contains a cZip Code at 5′ end and a hydroxy group at 3′ end. The labeled oligonucleotide was phosphorylated at 5′ end and is affixed a Cy3 to 3′ end. The two sequences at the junction must be matched. (B) The spotting sequences consist of a Zip Code at the 5′ end and the (dA)15-NH2 H2hat 3′ end; the discriminating oligonucleotide includes cZip Code at the 5′ end and the hybridization sequence at 3′ end.
2. Materials and methods
2.1. SARS-CoV genomic cDNAs
Viral genomic cDNAs and the primers in this study were obtained from the Chinese National Human Genome Center at Shanghai (Shanghai, China, http://www.chgc.sh.cn). All specimens (including serum, stool, oropharyngeal swabs, nasal pharyngeal aspirates or aotupsy lung tissues) were collected from the patients of SARS cases at the Guangdong Center for Disease Control and Prevention (GDCDCP) and at local hospitals in China last year. RNA was extracted with the QIAamp viral RNA mini kit (QIAgen, CA, USA) or TRIZOL Reagent (GIBCOBRL, MD, USA). The double-strand cDNA was synthesized with the SuperScript cDNA system (Invitrogen, CA, USA) or RNA PCR Kit (AMV) Ver 2.1 (Takara, Dalian, China).
2.2. Choose of target bases
Based on previous studies (He et al., 2004, Chen et al., 2003), the software ClusterX was used to align 42 coronavirus complete genome sequences (including 40 SARS-CoV genome sequences and two SARS-like coronavirus genome sequences) (Table 1). SARS-CoV TOR2 genome sequence was used as a reference in the analysis. It was found that the mutated bases at six positions (nt9404, nt9479, nt19838, nt21721, nt22222 and nt27827) are related to the SARS epidemic. These mutated bases have high-mutation rates and one base (nt19838) is non-sense mutation as well as a contrastive detection site. The two sites, nt9404 and nt9479, lie in the coded sequences of Nsp1, a protein with the most number of amino acid changes (Table 2 ). Two bases (nt21721 and nt22222) were found in the coding sequences of Spike and a base (nt27827) in the coding sequence of SARS8a may play an important role in the evolution of SARS-CoV (He et al., 2004). Considering the character of the LDR reaction, about 28 bases were chosen at 3′ end of the discriminating oligonucleotide and about 22 bases at 5′ end of the labeled oligonucleotide as the hybridization zone (Table 3 ). The hybridization zones were checked and ensured that they were found only in SARS-CoV genome sequences.
Table 2.
The changes of the codons and the amino acids related to the six mutated bases in early phase, middle phase and late phase of the SARS epidemic
| Early phase |
Middle phase |
Late phase |
||||
|---|---|---|---|---|---|---|
| Codona | Amino acid | Codona | Amino acid | Codona | Amino acid | |
| Sites | ||||||
| 9404 | GTT/GCT | Val/Ala | GCT | Ala | GTT | Val |
| 9479 | GCA | Ala | GCA | Ala | GCA/GTA | Ala/Val |
| 19838 | GTA | Val | GTG | Val | GTA | Val |
| 21721 | GAC | Asp | GAC | Asp | GGC | Gly |
| 22222 | ACT | Thr | ACT | Thr | ATT | Ile |
| 27827 | CGC | Arg | CGC | Arg | TGC | Cys |
The bold and italic bases are the mutation bases.
Table 3.
The discriminating oligonucleotides and the labeled oligonucleotides
| Sites | Discriminating oligonucleotide (5′ → 3′) | Labeled oligonucleotide (5′ → 3′) |
|---|---|---|
| 1 | TGGCGAGAGTGTCTCGTCGATCATCCTACTACTTTATGAAATTCAGACGTGC | p-TTTTGGTGAGTACAACCATGTTGTT-Cy3 |
| ATCTTGCGCGGCAGCTCGTCGACCGCTACTACTTTATGAAATTCAGACGTGT | ||
| AAAGCGGGCGGCGATCGCGAATGTCCTACTACTTTATGAAATTCAGACGTGA | ||
| 2 | AGATTGGGATGCGGTCGCGATACCGGATGTCTTTCACTATACTCTGTCTGGT | p-ACCAGCTTACAGCTTTCTGCCGG-Cy3 |
| GAGGATCTGTAGCGCCTCTTCGAGCGATGTCTTTCACTATACTCTGTCTGGC | ||
| CGAACTCGAAGCCGAGCTGGCGGTGTTTGATGTCTTTCACTATACTCTGTA | ||
| 3 | GATCGGCCGGTGAAGCGAAAGGTTCAAAAGAGAAGCCCCAGCACATGTA | p-TCTACAATAGGTGTCTGCACAATGACT-Cy3 |
| GATGGTGATCCCGCGCGTGCCGAAAAAAAGAGAAGCCCCAGCACATGTG | ||
| GGATTGCACCGTCAGCACCACCGAGAAAAGAGAAGCCCCAGCACATGTT | ||
| 4 | TCCCAGGACGGCGCTGGCACGTTGAGTTTCATACTATTAATCATACGTTTGG | p-CAACCCTGTCATACCTTTTAAGGA-Cy3 |
| CGGCGTCCACGTCGAGTTCCTTCGCGTTTCATACTATTAATCATACGTTTGA | ||
| TGTGCGCCCGAGATCGGTATCCCCGGTTTCATACTATTAATCATACGTTTGT | ||
| 5 | ATCGCATCGTGATGGCGTAAGCTCCAGCCTTTTCACCTGCTCAAGACAT | p-TTGGGGCACGTCAGCTGCAGC-Cy3 |
| TTCGGGGAAACTCCGCACCGCCACGAGCCTTTTCACCTGCTCAAGACAC | ||
| TAGGTTTGGCCAGTGCGTTGGATCGAGCCTTTTCACCTGCTCAAGACAA | ||
| 6 | TCGACAACCCGGTTGGAGGATTCAGTTGTATTTCTCTATGCAGTTGCATAT | p-GCACTGTAGTACAGCGCTGTGC-Cy3 |
| CCAAAAGCTTTACGCCAGCGCCGAATTGTATTTCTCTATGCAGTTGCATAC | ||
| CCGTACCCTTCCGCTGGAGATTTACTTGTATTTCTCTATGCAGTTGCATAAA | ||
| 7 | The hybridization control cZip Code | GGGTATCCGTTCGGTGTTGCGTAGT-Cy3 |
The italic sequences are the cZip Codes and the bold and italic bases are the mutated bases. Tm value of the discriminating oligonucleotides is about 65 °C and Tm value of the labeled oligonucleotides is about 55 °C.
2.3. Zip Codes assignment
Twenty Zip Codes were assigned as described by Favis et al., 2000, Favis et al., 2000. One of the Zip Codes was used to confirm the immobility of the spotted samples by hybridizing with the cZip Code labeled with Cy3 at 5′ end. Another Zip Code was used to measure the efficiency of the hybridization. The others are described in Table 4 . Each detection site has three Zip Codes: positive Zip Code, negative Zip Code and contrastive Zip Code. They are attached by (dA)15-NH2 at 3′ end (Table 3).
Table 4.
The spotting oligonucleotides
| Number of sequences | Spotting sequences (5′ → 3′) |
|---|---|
| Site 1 | |
| Positive sequence | GATGATCGACGAGACACTCTCGCCA (A)15-NH2 |
| Negative sequence | CGGTCGACGAGCTGCCGCGCAAGAT (A)15-NH2 |
| Contrastive sequence | GACATTCGCGATCGCCGCCCGCTTT (A)15-NH2 |
| Site 2 | |
| Positive sequence | CGGTATCGCGACCGCATCCCAATCT (A)15-NH2 |
| Negative sequence | GCTCGAAGAGGCGCTACAGATCCTC (A)15-NH2 |
| Contrastive sequence | CACCGCCAGCTCGGCTTCGAGTTCG (A)15-NH2 |
| Site 3 | |
| Positive sequence | GAACCTTTCGCTTCACCGGCCGATC (A)15-NH2 |
| Negative sequence | TTTCGGCACGCGCGGGATCACCATC (A)15-NH2 |
| Contrastive sequence | CTCGGTGGTGCTGACGGTGCAATCC (A)15-NH2 |
| Site 4 | |
| Positive sequence | TCAACGTGCCAGCGCCGTCCTGGGA (A)15-NH2 |
| Negative sequence | GCGAAGGAACTCGACGTGGACGCCG (A)15-NH2 |
| Contrastive sequence | CGGGGATACCGATCTCGGGCGCACA (A)15-NH2 |
| Site 5 | |
| Positive sequence | GGAGCTTACGCCATCACGATGCGAT (A)15-NH2 |
| Negative sequence | CGTGGCGGTGCGGAGTTTCCCCGAA (A)15-NH2 |
| Contrastive sequence | CGATCCAACGCACTGGCCAAACCTA (A)15-NH2 |
| Site 6 | |
| Positive sequence | CTGAATCCTCCAACCGGGTTGTCGA (A)15-NH2 |
| Negative sequence | TTCGGCGCTGGCGTAAAGCTTTTGG (A)15-NH2 |
| Contrastive sequence | GTAAATCTCCAGCGGAAGGGTACGG (A)15-NH2 |
| Spotting control sequence | Cy3-CCGGCTTTGAACTGCTCACCGATCT (A)15-NH2 |
| Hybridization control | ACTACGCAACACCGAACGGATACCC (A)15-NH2 |
2.4. Amplification of the SARS-CoV cDNA sample
PCRs were performed in a DNA Engine Dyad cycler (M.J. Research, MA, USA). The sequences of the primers are shown in Table 5 . The reaction was performed in a 50 μl volume containing 400 nM each primer, 200 μM each dNTP, 1.5 mM MgCl2, 1× buffer, 2 U of HotStar DNA polymerase (QIAGEN, CA, USA) and 10 ng of SARS-CoV genome cDNA. Prior to amplification, DNA was denatured for 15 min at 95 °C, which also activated HotStar DNA polymerase. The amplification consists of 95 °C for 15 min (initial denature), 16 touch-down cycles of 95 °C for 30 s, 66 °C for 30 s (decrease 0.5 °C each cycle), 72 °C for 1 min and 35 cycles of 95 °C for 30 s, 58 °C for 40 s, 72 °C for 1 min. After the cycles, an extension step (10 min at 72 °C) was followed.
Table 5.
Primers for SARS-CoV PCR and correspondent of viaral genomes
| No. | Upstream primer (5′ → 3′) | Downstream primer (5′ → 3′) | Regions |
|---|---|---|---|
| 1 | CCCTGTAGTAGCTGCTATCATT | GAAGGTGAGCCAAGAATGAAAC | nt8748-9574 |
| 2 | GCAAACAAGTAGTGTCGGATA | TTTCAGGCAACTGTTGAATAAT | nt19336-20124 |
| 3 | CTTAACAGAGCATTTGAGTTCAG | CAACATACTTCATCTATGAGGGG | nt22364-21585 |
| 4 | TAGCACACACTTTGCTTTTG | CAGTATTATTGGGTAAACCTTGG | nt27449-28270 |
PCR product 1 including the detection sites: 9404 and 9479; PCR product 2 including one detection site: 19838; PCR product 3 including two detection sites: 21721 and 22222; PCR product 4 including one detection site: 27827.
After amplification, the PCR products were purified by QIAquick PCR Purification Kit (QIAGEN, CA, USA), eluted in 30 μl autoclaved water and quantified by spectrophotometer. The products of PCR can be qualified by the electrophoresis graph of the PCR products (Fig. 2 ).
Fig. 2.
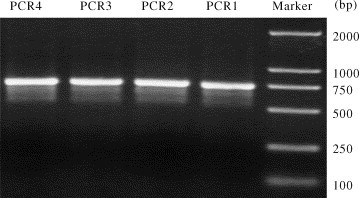
The electrophoresis graph of the PCR products. The PCR products in the picture: PCR1, PCR2, PCR3 and PCR4 is relative to the numbers (Table 3): 1, 2, 3 and 4, respectively.
2.5. Preparation of microarray
The spotting oligonucleotides dissolved in 1% N-methyl morpholine solution were placed into the 384-well microtiter plate. They were arrayed onto the isothioyanate slides as arrangement with machine microarrayer (GENE Machines, CA, USA). Hybridization was carried out by the HB-1000 HybChamber (GENE Machines, CA, USA). The scan was finished by ScanArray 4000C (Parkard, MA, USA).
Spotted slides were incubated at 37 °C, 95% humidity in a light-tighted chamber with the same conditions for 8 h as described by Busti et al., 2002, Busti et al., 2002. 10 mM ammonia (twice, 15 min each) was used to block free thiocyanate groups. Extensive washing the slides with TE buffer (four times, 5 min each) was followed to eliminate un-bound oligonucleotides. Dried slides were stored in a dessicator at 4 °C in dark ready for use.
2.6. Ligation reaction
The ligation reaction was carried out in a final volume of 40 μl containing 2.5 pmol of each discriminating oligonucleotide, 2.5 pmol of each labeled oligonucleotide, 100 fmol of each purified PCR products, 1× ligation buffer, 1 U Taq DNA ligase (New England Biolabs, MA, USA). The reaction mixtures were performed as the following: 95 °C for 30 s, 64 °C for 4 min (40 cycles). All the procedures were carried out in a DNA Engine Dyad cycler (M.J. Research, MA, USA).
2.7. Microarray hybridization and detection
The products of ligation were mixed with 12 μl solution containing 6× SSC, 0.1 mg/ml salmon sperm DNA and 50 pmol hybridization control cZip Code (Table 3). The mixtures were heated at 95 °C for 5 min. Then the solution was quickly chilled on ice and centrifuged at 13,000 rpm for 3 min to remove any undissolved particles. The supernants were added onto the microarray. Then a cover slip (large enough to cover the entire array surface) was carefully placed on the microaaray to avoid any bubble captured in. The slides were incubated in a sealed hybridization chamber saturated with PBS (pH 7.3) at 37 °C for 60 min. The hybridized slides were washed at room temperature with 6× SSC and 0.1% SDS for 3 min, 4× SSC and 0.1% SDS for 3 min, 2× SSC and 0.1% SDS for 3 min and 2× SSC for 3 min and spinned at 1500 rpm for 5 min to remove any residual drops on the slides. The slides were scaned by a ScanArray 4000c laser scanning system (Parkard Biochip Technologies, MA, USA) with laser for Cy3 dye (λ ex 543 nm/λ ex 570 nm) at 10 μm resolution.
3. Results and discussion
This microarray has 108 spots arranged in nine rows (Fig. 3 (A)). 20 samples were assayed with the microarray, yielding the sequence information that was completely confirmed by DNA sequencing (data not shown). A result is shown in Fig. 3(B). However, cDNA samples obtained from health persons did not produce any signals (data not shown). Referring the design (Fig. 3(A)), the discriminating oligonucleotides (Table 3) and the spotting oligonucleotides (Table 4), we can get the genotypes from the detection sketch (Fig. 3B). They are: C (nt9404), T (nt9479), G (nt19838), A (nt21721), C (nt22222) and C (nt27827). It is the characteristic genotypes of the middle phase of the SARS epidemic. If there are two kinds of genotypes, this microarray can also detect them, such as the heterozygous sample.
Fig. 3.
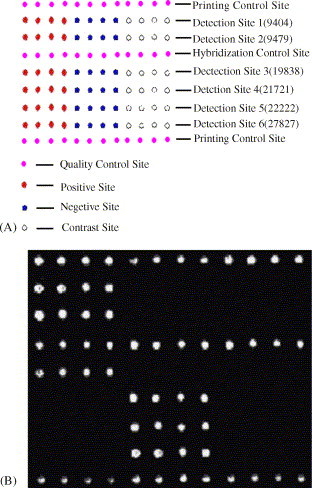
SARS universal microarray. (A) Spotting design of the universal microarray. The color is added artificially in order to distinguish the different sites. (B) Detection results of a sample.
The wild genotypes of six bases are C, C, A, A, C and C. The mutated genotypes are C, C/T, G, A, C and C in middle phase; T, T, A, G, T and T in late phase. The bases (nt9404, nt9479, nt21721, nt22222 and nt27827) are stable after the mutation in the evolution of SARS-CoV. This suggests that the change of these bases may play an important role in the evolution of this virus and the corresponding proteins related to these mutated bases may be more important for viral infection.
Besides the identification of the SARS-CoV, this universal microarray can provide the related genotypes in the different phases of epidemic. These relative genotypes comprised of six bases can help the epidemiologists to know that the detection samples belong to which phase of the epidemic.
The aim is to develop an efficient and economical microarray system to detect the SARS-CoV and identify the genotypes of the six mutated bases. We designed the spotting control and the hybridization control. The spotting control was used to optimize the conditions of the immobility of the spotting samples. The hybridization control is useful to find out the range of the better hybridization conditions with less time.
In order to confirm the SARS-CoV samples, PCRs were applied with 16-pair SARS-specific primers. SARS-CoV-specific primers, HotStar DNA polymerase and touch-down PCRs were used in the amplification of the samples, which can guarantee the fidelity of the SARS-CoV cDNA amplification. There were still the PCR products with the “mutant” due to the generation of the single base “mutant” during PCRs. LDR reaction with strict matched in the hybridization zone can almostly eliminate the influence of the “mutant”. The reasons may be the following facts. If not the detection base, the “mutant” becomes noneffective after LDR reaction. At the time, the amount of the SARS-CoV templates was far more than the amount of the templates with the “mutant”, which was the main reason to eliminate the influence of the PCR “mutant”. As result of these, this microarray system can decrease the influence of the PCR “mutants” significantly.
This microarray is the ideal tool for the detection of the virus with high-mutation rate, such as SARS-CoV. This method can also be applied to the study of other point mutation viruses.
Acknowledgement
We gratefully acknowledge Shanghai BioChip Company for financial support. At the same time we thank Hua-Jun Zheng and Sheng-Yue Wang of Chinese National Human Genome Center at Shanghai for the provision of the SARS-CoV cDNA samples and SARS-CoV cDNA complete genome sequences of parts of detection samples. At last, we are grateful to Sheng-Ying Qin for his advices.
References
- Bellis D.G., Castiglioni B., Bordoni R., Rossi L., Mezzelani A., Rizzi E., Frosini A., Busti E., Consolandi C., Battaglia C.M. Ligase detection reaction (LDR) and universal array (zip code): application to DNA genotyping. Minerva. Biotec. 2002;14(3/4):247–252. [Google Scholar]
- Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., derkach P., Ephtimios I.E., Kitai I., Mederski B.D., Shadowitz S.B., Gold W.L., Hawryluck L.A., Rea E., Chenkin J.S., Cescon D.W., Poutanen S.M., Detsky A.S. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- Busti E., Bordoni R., Castiglioni B., Monciardini P., Sosio M., Donadio S., Consolandi C., Rossi L., Bernardi L., Battaglia C., Bellis D.G. Bacterial discrimination by means of a universal array approach mediated by LDR (ligase detection reaction) BMC Microbiol. 2002;2(1):27–38. doi: 10.1186/1471-2180-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.J., Gao G., Bao Y.M., Lopez R., Wu J.M., Cai T., Ye Z.Q., Gu X.C., Luo J.C. Initial analysis of complete genome sequences of SARS-CoV. Yi Chuan Xue Bao. 2003;30(6):493–500. (in Chinese) [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., Vander W.S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D.M.E., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S.M., Poon L.L.M. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern china. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- He J.F., Peng G.W., Min J., Yu D.W., Liang W.J., Zhang S.Y., Xu R.H., Hao P., Tang H., Ren S.X., Zhong Y., Guo Z.M., Liu Q., Miao Y.G., Kong X.Y., He W.Z., Li Y.X., Wu C.I., Zhao G.P., Zheng H.Y., Wu X.W., Xu J., Wang Z.H., Fang L., Zhang X., Li H., Ge Y.X., Lu J.H., Hu Z.H., Huang J.C., Wan Z.Y., Hou J.L., Lin J.Y., Song H.D., Wang S.Y., Zhou X.J., Zhang G.W., Gu B.W., Zheng H.J., Zhang X.L., He M., Zheng K., Wang B.F., Fu G., Wang X.N., Chen S.J., Chen Z., Chiu R.W.K., Chim S.S.C., Tong Y.K., Chan P.K.S., Tam J.S., Lo Y.M.D. Molecular evolution of the SARS-CoV during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- Holton D. SNP genotyping (zip code or apex methods), protein arrays and gene expression: use of multiple or alternative fluors in microarrays. Minerva. Biotec. 2001;13(4):307–311. [Google Scholar]
- Li L., Wang Z., Lu Y., Bao Q., Chen S., Wu N., Cheng S., Weng J., Zhang Y., Yan J., Mei L., Wang X., Zhu H., Yu Y., Zhang M., Li M., Yao J., Lu Q., Yao P., Bo X., Wo J., Wang S., Hu S. Severe acute respiratory syndrome-associated coronavirus genotype and its characterization.Chin. Med. J. (Engl. ). 2003;116(9):1288–1292. [PubMed] [Google Scholar]
- Favis R., Day J.P., Gerry N.P., Phelan C., Narod S., Barancy F. Universal DNA array detection of small insertions and deletions in BRCA1 and BRCA2. Nat. Biotechnol. 2000;18(5):561–564. doi: 10.1038/75452. [DOI] [PubMed] [Google Scholar]
- Ruan Y.J., Wei C.L., Ee A.L., Vega V.B., Thoreau H., Su S.T., Chia J.M., Ng P., Chiu K.P., Lim L., Zhang T., Peng C.K., Lin E.O., Lee N.M., Yee S.L., Ng L.F., Chee R.E., Stanton L.W., Long P.M., Liu E.T. Comparative full-length genome sequence analysis of 14 SARS-CoV isolates and common mutations associated with putative origins of infection. Lancet. 2003;361(9371):85–1779. doi: 10.1016/S0140-6736(03)13414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui S.K., Chim S.S., Lo Y.M. Coronavirus genomic-sequence variations and the epidemiology of the severe acute respiratory syndrome. N. Engl. J. Med. 2003;349(2):187–188. doi: 10.1056/NEJM200307103490216. [DOI] [PubMed] [Google Scholar]
- Wang H.B., Liu J.H., Ouyang X.L., Yu Y., Ma S.X., Li X.J., Lu L.C., Tian Y.P., Liu H.Y., Xu H.M., Yao W. Detection of the anti-SARS-Coronavirus specific antibody levels in 156 SARS patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003;11(5):441–443. (in Chinese) [PubMed] [Google Scholar]
- Wang Y.S., Shen H., Sun S.H., Jiang L.H., Liu Y., Zhu Z.W., Xiao D.J., Huang P., Yang B., Du X.Y., Zhang Y.C. Analysis of false-positive associated with antibody tests for SARS-CoV in SLE patients. Shi Yan Sheng Wu Xue Bao. 2003;36(4):314–317. (in Chinese) [PubMed] [Google Scholar]
- Zhuang J.H., Huang X.Z., Zhou Q., Lin L.Y., Lin L. Dynamic observation IgG and IgM antibodies in patients with severe acute respiratory syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2003;15(10):579–581. (in Chinese) [PubMed] [Google Scholar]


