Research highlights
▶ Comparison of six automated nucleic acid extraction systems and one manual kit for extraction of nucleic acids from DNA/RNA Respiratory Pathogens. ▶ All methods yielded extracts free of cross-contamination and RT-PCR inhibition. ▶ All automated systems recovered Legionella pneumophila and adenovirus DNA equivalently. ▶ The MagNA Pure protocol demonstrated the best DNA recovery from the Streptococus pyogenes than other methods. ▶ The KingFisher mL and easyMAG protocols are most suitable for RNA extraction from the human influenza virus and respiratory syncytial virus.
Keywords: Diagnosis of human infectious disease, Respiratory pathogens, Nucleic acid extraction
Abstract
This study compared six automated nucleic acid extraction systems and one manual kit for their ability to recover nucleic acids from human nasal wash specimens spiked with five respiratory pathogens, representing Gram-positive bacteria (Streptococcus pyogenes), Gram-negative bacteria (Legionella pneumophila), DNA viruses (adenovirus), segmented RNA viruses (human influenza virus A), and non-segmented RNA viruses (respiratory syncytial virus). The robots and kit evaluated represent major commercially available methods that are capable of simultaneous extraction of DNA and RNA from respiratory specimens, and included platforms based on magnetic-bead technology (KingFisher mL, Biorobot EZ1, easyMAG, KingFisher Flex, and MagNA Pure Compact) or glass fiber filter technology (Biorobot MDX and the manual kit Allprep). All methods yielded extracts free of cross-contamination and RT-PCR inhibition. All automated systems recovered L. pneumophila and adenovirus DNA equivalently. However, the MagNA Pure protocol demonstrated more than 4-fold higher DNA recovery from the S. pyogenes than other methods. The KingFisher mL and easyMAG protocols provided 1- to 3-log wider linearity and extracted 3- to 4-fold more RNA from the human influenza virus and respiratory syncytial virus. These findings suggest that systems differed in nucleic acid recovery, reproducibility, and linearity in a pathogen specific manner.
1. Introduction
Sequencing, real-time PCR, microarrays and other high-throughput molecular assays are widely used in the laboratory diagnosis of human infectious diseases because they offer high sensitivity, specificity, and flexibility for testing multiple pathogens (Ince and McNally, 2009, Muldrew, 2009). These assays require nucleic acid extraction from biological samples. The yield and quality of nucleic acids have a direct impact up assay performance (Liu, 2008, Muldrew, 2009). The traditional DNA/RNA isolation techniques using phenol/chloroform extraction are time consuming and prone to sample cross contamination and PCR inhibition from phenol/chloroform carryover. Manual column purification kits using glass fiber filters were developed to replace phenol/chloroform extraction procedures. These kits involve alkaline lysis of cells followed by capture of nucleic acid on a filter membrane in a spin column in the presence of chaotropic reagents. Although these methods have demonstrated improved performance and are extensively used for general molecular biology purposes, they require large elution volumes and are subject to problems such as filter clogging and inconsistent yield (Yang et al., 2008). In addition, increasing demand in clinical diagnosis necessitates reliable automated methods for efficient recovery of nucleic acid from clinical specimens. A solid phase binding technology using magnetic beads was introduced into robotic systems to provide rapid and high-throughput detection capacity (Akutsu et al., 2004).
Commercial extraction kits and automated extraction robots have been evaluated for the recovery of nucleic acids from respiratory specimens, but none of them have assessed both DNA and RNA purification from viruses and bacteria simultaneously (Chan et al., 2008, Wilson et al., 2004). Evaluation of methods that allow simultaneous DNA and RNA extraction is useful for clinical diagnosis because the etiology of acute respiratory diseases includes a wide spectrum of microorganisms that can have RNA or DNA genomes and may be present singly or as mixed infections (Mancini et al., 2008, Paranhos-Baccala et al., 2008). Pathogen coinfections are common and may implicate poor prognosis. For example, a recent investigation of pandemic 2009 influenza A (H1N1) cases found that presence of Streptococcus pneumoniae in H1N1 patients was associated with higher mortality (Palacios et al., 2009). Concurrent detection of several viruses with Gram positive/negative bacteria has been reported in children with pneumonia (Korppi et al., 1991, Palacios et al., 2009, Wolf et al., 2010). These dual or mixed infections require simultaneous isolation of pathogen DNA and RNA for detection.
2. Materials and methods
2.1. Nucleic acid extraction systems
Six automated systems and one manual kit that represent current major methods for nucleic acid extraction from representative respiratory pathogens were described in Table 1 . AllPrep DNA/RNA Mini Kit (Cat# 80204, Qiagen, Valencia, CA, USA) is a manual kit based on glass fiber filter technology that is widely used in other DNA or RNA purification kits from Qiagen and other suppliers. InviMag® Bacteria DNA Mini Kit (Cat# 10332602, B-Bridge International Inc., Cupertino, CA, USA) was used on the KingFisher mL and KingFisher Flex instruments (ThermoFisher Scientific Inc., Worcester, MA, USA). Biorobot MDX and EZ1 are both supplied by Qiagen. Robotic One-For-All NA kit (Cat# 965672, Qiagen, Valencia, CA, USA) was used on the MDX. EZ1 DNA Tissue Kit (Cat# 953034, Qiagen, Valencia, CA, USA) and the EZ1 DNA Tissue Card (Cat# 9015588, Qiagen, Valencia, CA, USA) were used on the EZ1 instrument. MagNA Pure Compact Nucleic Acid Isolation Kit I (Cat# 03 730 964 001, Roche, Indianapolis, IN, USA) and the NA_Plasma protocol was used on the MagNA Pure Compact machine. All the samples were subjected to upfront lysis following the manufacturer's instructions. The same reagents supplied by the instrument manufacturer were used for all of the protocols on eazyMag (bioMérieux, Marcy l’Etoile, France).
Table 1.
A summary of the nucleic acid extraction systems used in this study.
| Instrument/Kit | Protocol/chemistry | Core technology | Maximum sample volume (μL) | Length of automated protocol (min) | Cost per extractiona | Maximal extractions per run | |
|---|---|---|---|---|---|---|---|
| 1 | Allprep | Manuala | Glass fiber filter | 100b | N/A | $7.28 | N/A |
| 2 | Biorobot MDX | Robotic/One-For-All NA kit | Glass fiber filter | 263 | ∼150 | $3.36 | 96 |
| 3 | KingFisher mL | Robotic/IviMag Bateria Kit | Magnetic beads | 400 | 20 | $3.51 | 15 |
| 4 | Biorobot EZ1 | Robotic/DNA Tissue Kit | Magnetic beads | 200 | 20 | $6.85 | 6 |
| 5 | easyMAG | Robotic | Magnetic beads | 1000 | 45 | $5.63c | 24 |
| 6 | KingFisher Flex | Robotic/IviMag Bateria Kit | Magnetic beads | 400 | 20 | $3.83d | 96 |
| 7 | MagNA Pure Compact | Robotic/NA Isolation Kit I | Magnetic beads | 400 | 20 | $7.68 | 8 |
The cost per extraction was calculated by dividing the cost of each kit, based on pricing quotes from each manufacture in early 2010, by the number of samples that could be extracted by each kit.
For efficient sample lysis, the sample volume should not exceed 100 μL, although the maximum loading volume is 700 μL.
The cost per extraction was quoted for off-instrument lysis.
The cost per extraction calculation included the cost of 96-well plates (AppliedBiosystems, Cat# 4388476) and combs (AppliedBiosystems, Cat# 4388487) that are sold separately.
2.2. Bacterial and viral strains
Five respiratory pathogens obtained from the Centers for Disease Control and Prevention collections were used for this study. They represent common types of agents associated with human respiratory diseases, including Gram-positive bacteria – Streptococcus pyogenes (MGAS315), Gram-negative bacteria – Legionella pneumophila (Philadelphia 1), and DNA virus – adenovirus (Ad1), segmented RNA virus – human influenza A virus (seasonal H1N1), and non-segmented RNA virus – respiratory syncytial virus (A2). All pathogens were live laboratory cultures except for human H1N1 influenza A virus that was β-propiolactone (BPL) inactivated (Cat# VA2394, WHO Collaborating Center, Centers for Disease Control and Prevention, Atlanta, GA, USA).
2.3. Human nasal wash
Nasal wash was collected and pooled from healthy adults under an IRB-approved protocol (FWH20030028H). Real-time reverse transcription polymerase chain reactions (RT-PCR) were used to screen the pooled nasal wash for eighteen common human respiratory pathogens, including S. pneumoniae, S. pyogenes, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Bordetella pertussis, and L. pneumophila, human coronavirus HCoV-229E, human coronavirus HCoV-HKU1, human coronavirus HCoV-NL63, rhinovirus, human influenza virus A, human influenza virus B, respiratory syncytial virus, adenovirus, human parainfluenza viruses 1,2,3, and human metapneumovirus.
2.4. Sample preparation and processing
In phase one studies, the five respiratory pathogens (bacterial isolates and purified viruses) were pooled and spiked into nasal wash, i.e., each specimen contains five mixed pathogens. Bacteria were spiked at concentrations of approximately 5 × 106 cells. Viral cultures were titrated to the concentrations that produced a C T value around 25 from RT-PCR analysis of nucleic acid extracted using KingFisher mL. Twenty-four samples in a volume of 100 μL per tube were prepared for each extraction method. These samples were extracted following the manufacturers’ instructions using the robotic systems or the manual kit described in Table 1. KingFisher Flex was purchased and added to the phase two studies. Extractions were performed by trained users of each platform on three separate days. PCR-grade water (Cat# 9935, Ambion, Austin, TX, USA) was processed in parallel to monitor cross contamination. DNA/RNA was eluted into 100.0 μL elution buffer supplied by the manufacturers. The volume of final elutes from each extraction system were adjusted to 200 μL with EB buffer (Cat# 1014609, Qiagen, Valencia, CA, USA) and aliquots were stored at −80 °C. All samples were subjected to fewer than two freeze–thaw cycles. In the phase two studies, fresh cultures of the representative pathogens except for the influenza A virus that was BPL inactivated were pooled and spiked into nasal following the phase-one procedures unless indicated otherwise. Ten-fold serial dilutions of the spiked samples were prepared in 100 μL of nasal wash. These samples were coded and stored at −80 °C. Identical aliquots of three sets of samples were prepared for each experiment. MagNA Pure and MDX were removed from the phase two evaluations due to malfunctions of the systems at the time of the studies.
2.5. RT-PCR and data analysis
tRNA concentration in the elution buffers from different suppliers may vary. Spectrophotometer measurement of the yield and purity of extracted nucleic acid were excluded as tRNA absorbs UV light and interferes with A260 and A280 readings (Subbarayan et al., 1995). Rather, the efficacy of extraction methods was compared using C T value from RT-PCR analysis. Although RT-PCR amplification efficiency may vary between assays, one criterion was employed for all of the comparisons, i.e., one C T represents 2-fold difference in template input. RT-PCR was performed using the AgPath-ID One-Step RT-PCR kit (Cat# AM1005, Applied Biosystems, Carlsbad, CA, USA) and the 7500 real-time PCR system (AppliedBiosystems, Carlsbad, CA, USA). All the RT-PCR assays were developed in the Centers for Disease Control and Prevention. The sequences of the primers and probes are available upon request. Each reaction contained 5 μl of the nucleic acid in a standard 25 μl assay. Data was analyzed using SDS 1.4 software (AppliedBiosystems, Carlsbad, CA, USA) and GraphPad Prism software version 5.03 (GraphPad Software, Inc., San Diego, CA, USA).
3. Results
3.1. Nasal wash characterization
The human nasal wash used for spiking matrix was characterized using real-time RT-PCR. Among the 18 common respiratory pathogens, including those chosen for this study, only rhinovirus was detected. Rhinovirus is the major cause of common cold, but is also detected in about 15% of asymptomatic healthy individuals, especially in families with children infected with rhinovirus (Peltola et al., 2008).
3.2. RT-PCR inhibition and carry-over contamination
The presence of RT-PCR inhibitors in the analyzed samples could potentially reduce enzyme activity and, therefore, affect C T value measured by RT-PCR. To test inhibition from nucleic acid extracts, total RNA was purified from human metapneumovirus (HMPV) and spiked into the nucleic acid extracts or PCR-grade water (>10,000-fold dilution) followed by RT-PCR analysis. HMPV was chosen because it was not detected in the spiking matrix and would not interfere with the inhibition analysis. One-way ANOVA (Dunnett's multiple comparison test) analysis of the C T values for the HMPV RNA spiked in water and in the nucleic acid extracts showed no significant inhibition from nucleic acid extracts prepared by any methods (n = 4 for each sample, p < 0.05).
PCR-grade water was processed in parallel with the sample spiked with the pooled pathogens. No false positives from these controls were detected, suggesting that all methods were free of nucleic acid carry-over contamination (data not shown).
3.3. Relative sensitivity
Among the extraction methods evaluated, all exhibited similar performance in DNA extraction from adenovirus and L. pneumophila (Fig. 1 ). However, the MagNA Pure Compact extracted about 4- to 16-fold more DNA from S. pyogenes than other methods, but it was less efficient in RNA purification from RSV and influenza A virus viruses. KingFisher mL and EasyMag recovered RNA from both viruses 60-fold more efficiently than the EZ1 and about 4-fold more efficiently than the MDX and the AllPrep kit (Fig. 1).
Fig. 1.
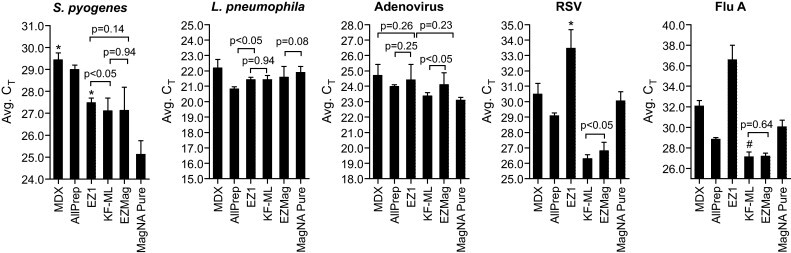
Comparative real-time RT-PCR amplification of total nucleic acids purified from the representative pathogens pooled and spiked into the human nasal wash. The samples prepared in the phase one studies were extracted following the manufacturer's instructions. The error bars represent standard deviation of the CT value calculated from 24 extractions performed on three separate days. Nonparametric t-test was performed to compare the mean CT value between the protocols that had a mean CT value close to each other. The p-values for such pairs are provided above the bars. Each CT represents about 2-fold difference in nucleic acid input. * One sample failed to generate a CT value; # one outlier had a CT value of 34.9.
3.4. Relative dynamic range and reproducibility
Because pathogen concentration in a clinical specimen can vary, the extraction method must be capable of extracting multiple nucleic acid types from a broad range of pathogen concentrations. To compare the relative dynamic range of the extraction systems, a phase two studies were performed as described in Section 2. KingFisher mL and EasyMag were able to extract nucleic acid from L. pneumophila, adenovirus, RSV, and influenza A virus that were spiked into human nasal wash at concentrations spanning 5- to 6-logs (Fig. 2 ). In contrast, the dynamic range (linearity) was 1–2 logs less in DNA extractions from S. pyogenes for all of the methods. It was worth noting that the linearity of EZ1 instrument was 1–3 logs less than that of KingFisher mL and EasyMag for DNA extraction from S. pyogenes, RSV, and influenza A virus (Fig. 2).
Fig. 2.

Evaluation of the extraction methods for nucleic acid isolation from a 10-fold titration series of the representative pathogens. Six different concentrations of the representative pathogens were spiked in human nasal wash prepared from healthy individuals. Each concentration point contains four spiked samples. Real-time RT-PCR was used to evaluate the linearity, reproducibility, and recovery/purity of nucleic acid extracted using each platform. The error bars represent standard deviation of the CT value calculated from two independent extractions (n = 4 for each titration point).
The reproducibility of EZ1 was the lowest as indicated by the larger standard deviations of C T values throughout the study, especially at lower pathogen concentrations (Fig. 2). Compared to the robots, the manual AllPrep kit did not demonstrate better recovery or linearity, and required more hands-on time and experimental steps.
4. Discussion
In this study, commercially available systems were compared for nucleic acid extraction from respiratory pathogens spiked in human nasal wash. By using nasal wash as a spiking matrix, the samples closely resembled specimens from patients, with its characteristic microbial flora, mucoproteins, carbohydrates, lipids, human nucleic acids, and other naturally occurring inhibitors.
An extraction protocol typically comprises three steps: chemical, mechanical or enzymatic cell lysis, removal of cell debris and impurities, and recovery of nucleic acid. Biorobot MDX, easyMag and Allprep use a chemical lysis reagent. All other robots deploy enzymatic digestion that involves incubation at room temperature (MagNA Pure Compact) or at elevated temperature (EZ1, KingFisher mL and KingFisher Flex). In addition to the chemistry, the procedures used on each platform are also different. For example, the KingFisher mL and KingFisher Flex are open systems so they are able to accommodate chemistries from different suppliers. Protocol steps and reagent volumes can be modified with the KingFisher Software. In contrast, easyMag (bioMérieux, Marcy l’Etoile, France), EZ1 (Qiagen, Valencia, CA, USA), and MagNA Pure Compact (Roche, Indianapolis, IN, USA) are closed systems with preprogrammed protocols, which should minimize cross contamination and human errors. In addition, maximum input sample volume, length of automated protocol, and capacity for high throughput differ across the platforms (Table 1). These variations may lead to differences in lysis efficiency, loading capacity, or in the capability of removing inhibitors.
The structure and composition of bacterial cell walls or viral capsules can impact the lysis efficiency (Kaser et al., 2009, Melo et al., 2006). The outer cell wall of Gram-positive bacteria consists of a complex of cross-linked peptidoglycan, teichoic acid, polysaccharides, and other proteins, whereas the Gram-negative cell wall is thinner with a fairly simple cross-linking pattern. Although both S. pyogenes and S. pneumoniae are Gram-positive bacteria, lysis of S. pyogenes appears to be more challenging because S. pyogenes contains more peptidoglycan and its cell wall polysaccharide is estimated at 6–10% of the dry weight of the microorganisms (McCarty, 1952, Salton, 1953). The viral genome is wrapped within a protein capsid or lipid bilayer membrane. These structural differences may explain why all of the methods displayed similar efficiency in DNA extraction from the DNA virus (adenovirus) and the Gram-negative bacterium (L. pneumophila) (Fig. 1, Fig. 2), but exhibited about a 20-fold difference in efficiency in DNA recovery from Gram-positive S. pyogenes (Fig. 1).
Interestingly, the KingFisher Flex and KingFisher mL were noticeably different in RNA extraction efficiency for influenza A virus and RSV, although they used the same chemistry and core technology. The KingFisher mL showed better performance for influenza A virus but was not as good as the KingFisher Flex for RSV (Fig. 2). Differences in the extraction protocols may have contributed to this difference. For example, the number and duration of mixing steps in the KingFisher mL protocol might be beneficial for binding of the segmented RNA of influenza A virus, whereas the non-segmented RNA of RSV might require longer incubation time for optimal binding.
Although samples were prepared at two phases using different batch of cultures, both studies agreed that the EZ1 system was not optimal for RNA extraction (Fig. 1, Fig. 2). Likewise, the MagNA Pure Compact was less efficient for the RNA extraction, although it exhibited the best performance for S. pyogenes (Fig. 1). Possible explanation for these discrepancies could be due to RNA degradation or inefficient RNA binding to the magnetic beads, a process that can be affected by salt concentration, pH value, temperature, or other factors that may be different in the proprietary chemistries of these systems.
In summary, seven major nucleic acid extraction methods have been characterized for purification of nucleic acid from five representative respiratory pathogens spiked in human nasal wash. Nucleic acid recovery, degree of inhibition, carry over contamination, reproducibility, and linearity of the protocols were evaluated. No single protocol was superior for all of the agents tested. Rather, their performance appeared to be pathogen specific. The MagNA Pure Compact was most suitable for DNA extraction from the Gram-positive S. pyogenes, whereas KingFisher mL and EasyMag provided better performance for the RNA viruses. The data suggest that the choice of inappropriate methods for nucleic acid extraction may introduce bias to clinical diagnosis or epidemiological investigation, as only organisms susceptible to the method selected would be detectable at lower levels.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Acknowledgements
We would like to thank Dr. Lisa Lott for providing the pooled human nasal wash used as the spiking matrix, Dr. Gloria Carvalho for providing the bacterium S. pyogenes, Dr. Stephen Lindstrom for providing BPL inactivated human influenza virus A for this study, and Dr. Cynthia Whitney for her critical comments on our manuscript.
References
- Akutsu J., Tojo Y., Segawa O., Obata K., Okochi M., Tajima H., Yohda M. Development of an integrated automation system with a magnetic bead-mediated nucleic acid purification device for genetic analysis and gene manipulation. Biotechnol. Bioeng. 2004;86:667–671. doi: 10.1002/bit.20049. [DOI] [PubMed] [Google Scholar]
- Chan K.H., Yam W.C., Pang C.M., Chan K.M., Lam S.Y., Lo K.F., Poon L.L., Peiris J.S. Comparison of the NucliSens easyMAG and Qiagen BioRobot 9604 nucleic acid extraction systems for detection of RNA and DNA respiratory viruses in nasopharyngeal aspirate samples. J. Clin. Microbiol. 2008;46:2195–2199. doi: 10.1128/JCM.00315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince J., McNally A. Development of rapid, automated diagnostics for infectious disease: advances and challenges. Expert Rev. Med. Devices. 2009;6:641–651. doi: 10.1586/erd.09.46. [DOI] [PubMed] [Google Scholar]
- Kaser M., Ruf M.T., Hauser J., Marsollier L., Pluschke G. Optimized method for preparation of DNA from pathogenic and environmental mycobacteria. Appl. Environ. Microbiol. 2009;75:414–418. doi: 10.1128/AEM.01358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korppi M., Leinonen M., Makela P.H., Launiala K. Mixed infection is common in children with respiratory adenovirus infection. Acta Paediatr. Scand. 1991;80:413–417. doi: 10.1111/j.1651-2227.1991.tb11875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.T. A technological update of molecular diagnostics for infectious diseases. Infect. Disord. Drug Targets. 2008;8:183–188. doi: 10.2174/1871526510808030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini D.A., Alves R.C., Mendonca R.M., Bellei N.J., Carraro E., Machado A.M., Pinto J.R., Mancini Filho J. Influenza virus and proteolytic bacteria co-infection in respiratory tract from individuals presenting respiratory manifestations. Rev. Inst. Med. Trop. Sao Paulo. 2008;50:41–46. doi: 10.1590/s0036-46652008000100009. [DOI] [PubMed] [Google Scholar]
- McCarty M. The lysis of group A hemolytic Streptococci by extracellular enzymes of Streptomyces albus. II. Nature of the cellular substrate attacked by the lytic enzymes. J. Exp. Med. 1952;96:569–580. doi: 10.1084/jem.96.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo S.C., Pungartnik C., Cascardo J.C., Brendel M. Rapid and efficient protocol for DNA extraction and molecular identification of the basidiomycete Crinipellis perniciosa. Genet. Mol. Res. 2006;5:851–855. [PubMed] [Google Scholar]
- Muldrew K.L. Molecular diagnostics of infectious diseases. Curr. Opin. Pediatr. 2009;21:102–111. doi: 10.1097/MOP.0b013e328320d87e. [DOI] [PubMed] [Google Scholar]
- Palacios G., Hornig M., Cisterna D., Savji N., Bussetti A.V., Kapoor V., Hui J., Tokarz R., Briese T., Baumeister E., Lipkin W.I. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One. 2009;4:e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranhos-Baccala G., Komurian-Pradel F., Richard N., Vernet G., Lina B., Floret D. Mixed respiratory virus infections. J. Clin. Virol. 2008;43:407–410. doi: 10.1016/j.jcv.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola V., Waris M., Osterback R., Susi P., Hyypia T., Ruuskanen O. Clinical effects of rhinovirus infections. J. Clin. Virol. 2008;43:411–414. doi: 10.1016/j.jcv.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Salton M.R. Studies of the bacterial cell wall. IV. The composition of the cell walls of some Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta. 1953;10:512–523. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- Subbarayan P.R., Sarkar M., Vinayak M. Analysis of transfer RNA during the early embryogenesis of the freshwater teleost, Heteropneustes fossilis. Mol. Biol. Rep. 1995;21:113–118. doi: 10.1007/BF00986501. [DOI] [PubMed] [Google Scholar]
- Wilson D., Yen-Lieberman B., Reischl U., Warshawsky I., Procop G.W. Comparison of five methods for extraction of Legionella pneumophila from respiratory specimens. J. Clin. Microbiol. 2004;42:5913–5916. doi: 10.1128/JCM.42.12.5913-5916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D.G., Greenberg D., Shemer-Avni Y., Givon-Lavi N., Bar-Ziv J., Dagan R. Association of human metapneumovirus with radiologically diagnosed community-acquired alveolar pneumonia in young children. J. Pediatr. 2010;156:115–120. doi: 10.1016/j.jpeds.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Hebron H.R., Hang J. High performance DNA purification using a novel ion exchange matrix. J. Biomol. Tech. 2008;19:205–210. [PMC free article] [PubMed] [Google Scholar]


