Abstract
Natural Killer (NK) cells constitute a major subset of innate lymphoid cells that do not express the T- and B-cell receptors and play an important role in antimicrobial defense. NK cells not only induce early and rapid innate immune responses, but also communicate with dendritic cells to shape the adaptive immunity, thus bridging innate and adaptive immunity. Although the functional biology of NK cells is well-documented in a variety of infections in humans and mice, their role in protecting domestic animals from infectious agents is only beginning to be understood. In this article, we summarize the current state of knowledge about the contribution of NK cells in pathogen defense in domestic animals, especially cattle and pigs. Understanding the immunobiology of NK cells will translate into strategies to manipulate these cells for preventive and therapeutic purposes.
Abbreviations: NK, natural killer cell; DC, dendritic cell; NO, nitric oxide; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; Ab, antibody; Ag, antigen; UC, uninfected cell; IC, infected cell; P, intracellular pathogen; T, T cell; ADCC, antibody-dependent cell-mediated cytotoxicity; KIR, killer inhibitory receptor; MHC-I, major histocompatibility complex-I
Keywords: Innate lymphoid cells, NK cells, Domestic animals, Immunity, Pathogens
1. Introduction
Innate lymphoid cells (ILCs) represent a family of lymphoid cells that do not express the antigen-specific receptors (Artis and Spits, 2015). These cells play crucial roles in microbial immunity, autoimmunity, inflammation, and homeostasis (Walker et al., 2013). Broadly, ILCs are classified into 2 groups – cytotoxic and non-cytotoxic ILCs. The cytotoxic ILCs are comprised of natural killer (NK) cells, whereas the non-cytotoxic ILCs can be subclassified into 3 groups – group 1, 2, and 3 ILCs – based on their cytokine and transcriptional profile (Artis and Spits, 2015, Lanier, 2013). Group 1 ILCs (ILC1s) secrete type 1 cytokines (IFN-γ and TNF-α) and require Tbet for their development and function. They have been implicated in host defense against microbial pathogens, including bacteria and parasites. Group 2 ILCs (ILC2s), which are characterized by the production of a range of type 2 cytokines (IL-4, IL-5, and IL-13) and expression of GATA3, exert type 2 immunity that is critical for protective immunity, allergy, and tissue formation (Walker et al., 2013). In addition, group 3 ILCs comprise ILC3s and lymphoid tissue-inducer T (LTi) cells that mainly produce IL-17 and/or IL-22 and are dependent on RORγt. ILC3s elicit immunity against bacterial pathogens, promote inflammation, and augment tissue generation (Spits et al., 2013).
Natural killer (NK) cells are the most widely studied subset of ILCs conserved among all mammalian species (Trinchieri, 1989). Because of their ability to induce early and rapid immune responses, NK cells are considered as a first line of defense against microbial pathogens. Spontaneous production of effector cytokines and robust cytotoxic activity are important functional characteristics of NK cells (Hamerman et al., 2005, Vivier, 2006). Recent studies further indicate that the function of NK cells is much more sophisticated and broader than previously thought. Cooperation between NK and dendritic cells (DCs) not only regulates innate immunity but also dictates the direction and intensity of adaptive immunity (Münz et al., 2005). Most of what is known about NK-cell-mediated immunity to microbial infections comes from mice and human studies. However, the role of NK cells in pathogen defense in domestic animals is still unclear, mainly due to interspecies variations and limited availability of specific reagents and transgenic/knockout for these animals. Herein we review the current literature on the role of NK cells in inducing immunity against various pathogens in domestic animals, particularly cattle and pigs. Taking into account the worldwide veterinary and zoonotic significance of infectious diseases in domestic animals, it is crucial to better understand the immunobiology of NK cells for prophylactic and therapeutic purposes.
2. Phenotype and function of NK cells
NK cells are large granular lymphocytes that do not express T- and B-cell receptors. They constitute 5–10% of the total lymphocytes in the tissues such as the liver, lung and blood (Trinchieri, 1989, Inngjerdingen et al., 2011). Phenotypically, NK cells are characterized as CD3−CD56+CD8+ and CD3−CD56−CD11b+ lymphocytes in humans and mice, respectively (Inngjerdingen et al., 2011). In pigs, NK cells are characterized as perforin+CD3−CD4−CD5−CD6−CD8α+CD8β−CD11b+CD16+ (Denyer et al., 2006, Pintaric et al., 2008). Recently, porcine CD3−CD8α+ and CD8αdim/−NKp46high NK-cell subsets were reported to have distinct functional features (Mair et al., 2013). Using anti-bovine antibodies against NKp46, NK cells have been characterized as NKp46+CD3−CD2−CD25+CD8+ cells in cattle (Storset et al., 2004). Similar to bovine NK cells, Connelley et al. identified a population of ovine NKp46+ lymphocytes that show the characteristics of NK cells (Connelley et al., 2011).
NK cells express various activating (e.g. Ly49 and CD94) and inhibitory (e.g. killer-cell immunoglobulin-like receptors and leukocyte inhibitory receptors) receptors that do not undergo rearrangement. These receptors recognize their cognate ligands on the surface of infected cells, and the complex interplay between them determines the activation of NK cells (Finton and Strong, 2012). The mechanisms by which NK cells execute their effector functions include cytotoxicity, antibody-dependent cell-mediated cytotoxicity (ADCC), production of cytokines, and modulation of DCs (Vivier, 2006, Münz et al., 2005, Inngjerdingen et al., 2011). NK cells are able to recognize and kill the virus-infected cells that reduce or lack the expression of MHC-I antigens, often termed as missing self-hypothesis, through perforin and granzyme production (Fig. 1A) (Kärre et al., 1986, Ljunggren and Kärre, 1985, Lanier, 2005). Moreover, cytokines produced by NK cells such as IFN-γ and TNF-α contribute to the activation of macrophages to induce antimicrobial killing mechanisms, e.g. inducible or type-2 nitric oxide synthase (Fig. 1B) (Bogdan, 2001, Stetson et al., 2003, Laouar et al., 2005, Prajeeth et al., 2011). In ADCC, specific antibodies bind to their cognate antigens recognized by CD16 (FcYRIII) receptors expressed on NK cells, resulting in release of cytolytic granules, such as perforin and granzyme, and consequent apoptosis of aberrant cells like virus-infected and tumor cells (Fig. 1C) (Lanier, 2005).
Fig. 1.
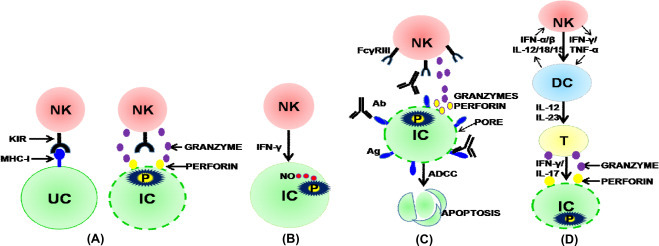
Mechanisms of NK-cell responses. (A) Missing self-model. NK cells possess KIRs that recognize MHC-I molecules on uninfected cells, whereas cells that do not express MHC-I molecules such as infected cells (ICs) are eliminated by NK cells via perforin and granzyme pathway. (B) IFN-γ-mediated direct killing of infected cell. Activation of NK cells results in production of IFN-γ which in turn induces nitric oxide (NO) secretion that causes lysis of the cell. (C) Antibody-dependent cellular cytotoxicity (ADCC). Fc receptors present on NK cells have the ability to bind to fab region of an antibody molecule, whilst variable region of the antibody specifically binds to the surface antigen expressed from pathogens inside the infected cell. Cross-linking of Fc receptors triggers apoptosis of the ICs via perforin and granzyme. (D) DC-NK interaction. Induction of adaptive immune response by modulating DC function. Cytokine production by activated NK cells (IFN-γ/TNF-α) causes DC maturation. Mature DCs polarize T cells via cytokines like IL-12 and IL-23. Polarized T cells induce killing of infected cells through IFN-γ/IL-17, and perforin and granzyme pathway. DC can also activate NK cells through IFN-α/β, and IL-12/18/15.
Bidirectional communication between NK cells and DCs is crucial for induction of optimal immunity. Activation of NK cells causes DC maturation through cell-to-cell contact, cytokine production and receptor-ligand interactions (Walzer et al., 2005). Mature DCs secrete a variety of cytokines, such as IL-12, IL-23, IL-6, IL-21, IL-27, and TGFβ. IL-12 induces the differentiation of naïve T cells into Th1 cells, which are characterized by the production of IFN-γ and TNF-α, through activation of STAT4 (Walsh and Mills, 2013, Szabo et al., 2003). Although TGFβ, IL-6, and IL-21 are involved in development of Th17 cells by activating transcription factors like STAT3 and RORγt, IL-23 is important for promoting the maintenance of Th17 cells (Korn et al., 2009). However, IL-27, a member of IL-12 family, has been shown to suppress Th1/Th17 immunity (Kastelein et al., 2007). These cytokine signals from NK-cell-activated DCs control the T-cell responses (IFN-γ/IL-17) against viruses and intracellular bacteria. Moreover, NK cells modulate the DC-mediated T cell responses that induce the killing of infected cells through perforin and granzyme pathway (Fig. 1D). However, in addition to reportedly enhancing DC function, NK cells can also negatively regulate DCs by causing lysis of immature DCs (Inngjerdingen et al., 2011, Lanier, 2005). On the other hand, DCs can activate NK cells through cytokine signals and cell-to-cell contact. Cytokines produced by DCs such as IL-12, IL-18, IL-15, and IFN-α/β promote cytotoxicity and IFN-γ production by and proliferation of NK cells (Walzer et al., 2005). In addition, NK-cell activation by DCs requires a contact between these cell types that involves the formation of stimulatory synapses (Borg et al., 2004).
Recent studies have highlighted a unique role for NK cells in presenting antigens to naïve T cells. Activated mouse NK cells have been reported to express MHC-II molecules (Spits and Lanier, 2007, Blasius et al., 2007). Nakayama et al. demonstrated that MHC-II+ NK cells are generated by intercellular transfer of antigen-MHC-II complex from murine DCs to NK cells through a process referred to as trogocytosis. Furthermore, the MHC-II+ NK cells present antigen-MHC-II complexes to CD4+ T cells for regulating adaptive immunity (Nakayama et al., 2011). Similar to the antigen-presenting role of NK cells, ILC2s express MHC-II and prime T cells to produce IL-2, which in turn induces type 2 responses that contribute to anti-parasitic immunity (Oliphant et al., 2014). Overall, these findings suggest that ILCs including NK cells can perform additional functions such as antigen presentation under certain conditions.
3. NK cells in host defense against viral pathogens
The function of NK cells has been critically examined during infections of animals with foot-and-mouth disease virus (FMDV), a picornavirus that afflicts cloven-hoofed animals including cattle and pigs with significant morbidity and mortality (Toka and Golde, 2013). Initial studies using in vitro systems demonstrated that the stimulation of porcine NK cells with proinflammatory cytokines, in particular IL-2 and IL-15, not only activated these cells to express higher levels of IFN-γ and perforin but also induced the lysis of FMDV-infected cells, suggesting a protective role for porcine NK cells in FMDV infection (Toka et al., 2009). Similar anti-viral function was observed upon stimulation of these cells with TLR7/8 agonists (Toka et al., 2009a). In contrast to these in vitro findings, Toka et al. found a significant reduction in the proportion of NK cells, which had the ability to produce IFN-γ and store perforin, in pigs following infection with the o1 Campos strain of FMDV (Toka et al., 2009b). The NK cells isolated from the infected-pigs showed reduced lysis of target cells compared to the NK cells from uninfected animals. This reduced lytic ability of NK cells however could not be restored with in vitro TLR-agonist-stimulations (Toka et al., 2009b). In line, NK-cell functions are reported to be compromised during porcine respiratory and reproductive syndrome virus (PRRSV), and African swine fever virus (ASFV) infections (Jung et al., 2009, Hulst et al., 2013, Manickam et al., 2013, Renukaradhya et al., 2010). In addition, co-infection with PRRSV and porcine respiratory corona virus synergistically inhibited the NK-cell-mediated cytotoxicity (Fan et al., 2013). These in vivo data taken together indicate an inhibitory effect of viral infections on porcine NK-cell function that explains a mechanism of how viruses evade the porcine innate immune system by targeting NK-cell activities. Similar inhibitory effect of viruses such as herpes virus and cytomegalovirus on NK cell function has been described in murine and human studies (Lodoen and Lanier, 2005). To explore the impact of viral infection on bovine NK cells, Patch et al. examined the NK-cell responses against infection of cattle with different strains of FMDV (Patch et al., 2014). Addition of anti-NKp46, compared to the isotype control, antibody in the IL-2 stimulated-peripheral blood mononuclear cells isolated from cattle resulted in the reduced killing of bovine BL3.1 cell line. During the course of FMDV infection in cattle, NK cells were isolated at different time points and analyzed for their lytic capacity. There was an increase in the NK-cell-induced cytolysis 2–3 days following infection (Patch et al., 2014). These results suggest that NK cells play an important role in protective immunity to FMDV, which is in contrast to the inhibition of NK-cell function observed in FMDV-infected pigs. A pertinent question arises as to why NK cells exhibit differential cytolytic abilities in response to FMDV infection in cattle and pigs. This discrepancy could be due to differences in pathogenesis between cattle and pigs, e.g. pigs shed greater quantities of viruses compared to the cattle after FMDV infection (Donaldson et al., 1970, Sellers and Parker, 1969). It would be interesting to examine whether there is a correlation between viral shedding and NK-cell activity in pigs and cattle, and also in other animals susceptible to FMDV such as sheep and goats. It should be also noted that different phases of FMDV infection such as acute and chronic might also induce different NK-cell responses.
4. NK cells in host defense against bacterial pathogens
What roles NK cells play in immunity to bacterial pathogens have been mainly studied using Bacille Calmette-Guerin (BCG)/mycobacterial infection (Siddiqui et al., 2012). Coculturing autologous bovine NK cells with Mycobacterium bovis-infected macrophages inhibited the intracellular bacterial growth as well as stimulated the macrophages to release IL-12 and nitric oxide (NO) (Denis et al., 2007). The neutralization of IFN-γ and NO in the coculture did not have any impact on NK cell-mediated inhibition of the replication of M. bovis in the macrophages, whereas abrogating the contact between NK cells and macrophages suppressed the bacterial growth, suggesting that cell-to-cell contact, but not IFN-γ and NO production, is essential for the reduction of intramacrophagic bacterial growth by NK cells (Endsley et al., 2006). It is possible that the other mechanisms like TNF-α production by NK cells are involved in inhibition of bacterial multiplication. Murine and human studies have demonstrated that the crosstalk between NK cells and DCs is important for generation of an immune response against microbial infections (Walzer et al., 2005). To understand the effect of DCs on NK cells, Bastos et al. isolated NK cells (CD335+CD3−CD2+/−CD8α+/−) from cattle and cocultured them with M. bovis BCG-pulsed splenic DCs with or without treatment with cytokines (GM-CSF, IL-4, and Flt3L). They found that the DCs without cytokine-treatment conditioned NK cells to induce reduced cytotoxicity compared with the cytokine-treated DCs (Bastos et al., 2008). These findings suggested that M. bovis BCG-pulsed DCs significantly modulate NK-cell function depending upon the maturation state of the DCs. It however remains unclear whether NK cells in turn modulate DCs to influence the adaptive immune responses. In mouse models of bacterial infection, NK cells have been reported to confer protective T cell immunity, characterized by IFN-γ and IL-17 production, by modulating the function of DCs (Shekhar et al., 2015). It would be interesting to assess the impact of NK cells on DC function in mounting adaptive immunity to infections in different domestic animals.
5. NK cells in host defense against protozoal pathogens
An alteration in frequencies of NK cells was observed in cattle and pigs following infection with Neospora caninum and Isospora suis, respectively (Maley et al., 2006, Klevar et al., 2007, Worliczek et al., 2010). Flow cytometric analysis showed a decline in the percentage of NK cells at days 4–6 after N. caninum infection in bovine calves, which was followed by a steady increase in the percentage of NK cells (Klevar et al., 2007). An increased accumulation of NK cells was noticed in the spleen of piglets following I. suis infection compared to the control animals (Worliczek et al., 2010). In early response to protozoal infections, NK cells produced large quantities of IFN-γ, which depended on IL-12 and IL-18 stimulation from accessory cells, and caused lysis of infected cells (Klevar et al., 2007, Goff et al., 2003, Goff et al., 2006). Thus, protozoal infections alter the frequencies as well as regulate the function of NK cells. In vitro coculture experiments showed that splenic DCs pulsed with Babesia bovis induced NK cells to enhance IFN-γ production and cytotoxicity, which suggested that DC maturation modulates the activation and function of NK cells during protozoal infection (Bastos et al., 2008). A recent study involving young bovine calves provided further evidence that the interaction between NK cells and DCs occurs in marginal zone of the spleen after B. bovis infection (Schneider et al., 2011). Although NK cells exert an innate response against veterinary protozoal pathogens, their definitive role in conferring protective immunity in domestic animals remains unclear. Using a murine model of experimental babesiosis, it was demonstrated that anti-asialo GM1-antibody-mediated depletion of NK cells in C57BL/6 mice resulted in impaired resistance to B. bovis infection, underscoring a protective role for NK cells in murine babesiosis (Aguilar-Delfin et al., 2003). It would be however more relevant to evaluate what role NK cells plays in B. bovis-infected cattle, which are the original hosts for this protozoal infection. Of note, a newly recognized subset of bovine unconventional lymphocytes, NKp46+CD3+ cells, which share the characteristics of both NK and T cells, has recently been shown to exhibit cytotoxicity against autologous Theileria parva-infected cells in vitro and to expand during in vivo challenge infection (Connelley et al., 2014). This information is important in light of the fact that functional NKT cells are present in horse, pig, guinea pig, and African elephant, but not ruminants including cattle (Looringh et al., 2009). Whether bovine NKp46+CD3+ cells are functional equivalents of NKT cells merits further investigation that may provide significant information on how protective immunity ensues in response to protozoal infection in cattle.
6. Conclusions
NK cells constitute a critical subset of ILCs that play an important role in protective immunity to various pathogens in domestic animals. Recent characterization of NK-cell markers specific for domestic animals has enabled researchers to study the immunobiology of NK cells. A wealth of emerging evidence indicates that NK cells regulate the innate immune responses to a variety of veterinary pathogens through multiple mechanisms, including cytotoxicity and production of IFN-γ. Some viruses such as FMDV can however evade porcine, in contrast to bovine, NK cell-mediated-immunity, thereby inhibiting the ability of NK cells to exert cytolytic functions. How viruses escape the host's immune system sheds light on the mechanisms of viral persistence as well as immune defense. It should be noted that the findings obtained in one animal species may not be applicable to the other, emphasizing the critical analysis of the immune response to a pathogen in different animal hosts. Relatively more information is available on the contribution of NK cells to innate immunity, while their role in regulating adaptive immunity, as shown in case of murine and human pathogens, to veterinary pathogens is largely unknown. Therefore, to elucidate the role and mechanism of NK cells in influencing adaptive immunity warrants further exploration. Since veterinary pathogens not only afflict animals with various diseases and loss of productivity but also pose a threat to the global public health, understanding the mechanisms of NK cells in protective immunity against domestic animals may have implications for developing therapeutics and vaccines against infectious diseases in animals as well as humans.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and Manitoba Health Research Council (MHRC)/Manitoba Institute of Child Health (MICH) to Prof. Xi Yang, who is the Canada Research Chair in Infection and Immunity. Dr. Sudhanshu Shekhar is the recipient of Allan Ronald studentship. Ms. Shipra Shekhar deserves our thanks for her help in figure drawing.
References
- Aguilar-Delfin I., Wettstein P.J., Persing D.H. Resistance to acute babesiosis is associated with interleukin-12- and gamma interferon-mediated responses and requires macrophages and natural killer cells. Infect. Immun. 2003;71:2002–2008. doi: 10.1128/IAI.71.4.2002-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- Bastos R.G., Johnson W.C., Mwangi W., Brown W.C., Goff W.L. Bovine NK cells acquire cytotoxic activity and produce IFN-gamma after stimulation by Mycobacterium bovis BCG- or Babesia bovis-exposed splenic dendritic cells. Vet. Immunol. Immunopathol. 2008;124:302–312. doi: 10.1016/j.vetimm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Blasius A.L., Barchet W., Cella M., Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J. Exp. Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Borg C., Jalil A., Laderach D., Maruyama K., Wakasugi H., Charrier S., Ryffel B., Cambi A., Figdor C., Vainchenker W., Galy A., Caignard A., Zitvogel L. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- Connelley T., Storset A.K., Pemberton A., MacHugh N., Brown J., Lund H., Morrison I.W. NKp46 defines ovine cells that have characteristics corresponding to NK cells. Vet. Res. 2011;42:37. doi: 10.1186/1297-9716-42-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelley T.K., Longhi C., Burrells A., Degnan K., Hope J., Allan A.J., Hammond J.A., Storset A.K., Morrison W.I. NKp46+CD3+ cells: a novel nonconventional T cell subset in cattle exhibiting both NK cell and T cell features. J. Immunol. 2014;192:3868–3880. doi: 10.4049/jimmunol.1302464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Keen D.L., Parlane N.A., Storset A.K., Buddle B.M. Bovine natural killer cells restrict the replication of Mycobacterium bovis in bovine macrophages and enhance IL-12 release by infected macrophages. Tuberculosis (Edinb.) 2007;87:53–62. doi: 10.1016/j.tube.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Denyer M.S., Wileman T.E., Stirling C.M., Zuber B., Takamatsu H.H. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet. Immunol. Immunopathol. 2006;110:279–292. doi: 10.1016/j.vetimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Donaldson A.I., Herniman K.A., Parker J., Sellers R.F. Further investigations on the airborne excretion of foot-and-mouth disease virus. J. Hyg. (Lond.) 1970;68:557–564. doi: 10.1017/s0022172400042480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endsley J.J., Endsley M.A., Estes D.M. Bovine natural killer cells acquire cytotoxic/effector activity following activation with IL-12/15 and reduce Mycobacterium bovis BCG in infected macrophages. J. Leukoc. Biol. 2006;79:71–79. doi: 10.1189/jlb.0505239. [DOI] [PubMed] [Google Scholar]
- Fan P., Wei Y., Guo L., Wu H., Huang L., Liu J., Liu C. Synergistic effects of sequential infection with highly pathogenic porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Virol. J. 2013;10:265. doi: 10.1186/1743-422X-10-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finton K.A., Strong R.K. Structural insights into activation of antiviral NK cell responses. Immunol. Rev. 2012;250:239–257. doi: 10.1111/j.1600-065X.2012.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W.L., Johnson W.C., Horn R.H., Barrington G.M., Knowles D.P. The innate immune response in calves to Boophilus microplus tick transmitted Babesia bovis involves type-1 cytokine induction and NK-like cells in the spleen. Parasite Immunol. 2003;25:185–188. doi: 10.1046/j.1365-3024.2003.00625.x. [DOI] [PubMed] [Google Scholar]
- Goff W.L., Storset A.K., Johnson W.C., Brown W.C. Bovine splenic NK cells synthesize IFN-gamma in response to IL-12-containing supernatants from Babesia bovis-exposed monocyte cultures. Parasite Immunol. 2006;28:221–228. doi: 10.1111/j.1365-3024.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- Hamerman J.A., Ogasawara K., Lanier L.L. NK cells in innate immunity. Curr. Opin. Immunol. 2005;17:29–35. doi: 10.1016/j.coi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Hulst M., Loeffen W., Weesendorp E. Pathway analysis in blood cells of pigs infected with classical swine fever virus: comparison of pigs that develop a chronic form of infection or recover. Arch. Virol. 2013;158:325–339. doi: 10.1007/s00705-012-1491-8. [DOI] [PubMed] [Google Scholar]
- Inngjerdingen M., Kveberg L., Naper C., Vaage J.T. Natural killer cell subsets in man and rodents. Tissue Antigens. 2011;78:81–88. doi: 10.1111/j.1399-0039.2011.01714.x. [DOI] [PubMed] [Google Scholar]
- Jung K., Renukaradhya G.J., Alekseev K.P., Fang Y., Tang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J. Gen. Virol. 2009;90:2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H.G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Kastelein R.A., Hunter C.A., Cua D.J. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Klevar S., Kulberg S., Boysen P., Storset A.K., Moldal T., Björkman C., Olsen I. Natural killer cells act as early responders in an experimental infection with Neospora caninum in calves. Int. J. Parasitol. 2007;37:329–339. doi: 10.1016/j.ijpara.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Lanier L.L. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lanier L.L. Shades of grey – the blurring view of innate and adaptive immunity. Nat. Rev. Immunol. 2013;13:73–74. doi: 10.1038/nri3389. [DOI] [PubMed] [Google Scholar]
- Laouar Y., Sutterwala F.S., Gorelik L., Flavell R.A. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- Ljunggren H.G., Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants, Analysis of the mechanism. J. Exp. Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodoen M.B., Lanier L.L. Viral modulation of NK cell immunity. Nat. Rev. Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- Looringh van Beeck F.A., Reinink P., Hermsen R., Zajonc D.M., Laven M.J., Fun A., Troskie M., Schoemaker N.J., Morar D., Lenstra J.A., Vervelde L., Rutten V.P., van Eden W., Van Rhijn I. Functional CD1d and/or NKT cell invariant chain transcript in horse, pig, African elephant and guinea pig, but not in ruminants. Mol. Immunol. 2009;46:1424–1431. doi: 10.1016/j.molimm.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair K.H., Müllebner A., Essler S.E., Duvigneau J.C., Storset A.K., Saalmüller A., Gerner W. Porcine CD8αdim/-NKp46high NK cells are in a highly activated state. Vet. Res. 2013;44:13. doi: 10.1186/1297-9716-44-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley S.W., Buxton D., Macaldowie C.N., Anderson I.E., Wright S.E., Bartley P.M., Esteban-Redondo I., Hamilton C.M., Storset A.K., Innes E.A. Characterization of the immune response in the placenta of cattle experimentally infected with Neospora caninum in early gestation. J. Comp. Pathol. 2006;135:130–141. doi: 10.1016/j.jcpa.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Manickam C., Dwivedi V., Patterson R., Papenfuss T., Renukaradhya G.J. Porcine reproductive and respiratory syndrome virus induces pronounced immune modulatory responses at mucosal tissues in the parental vaccine strain VR2332 infected pigs. Vet. Microbiol. 2013;162:68–77. doi: 10.1016/j.vetmic.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Münz C., Steinman R.M., Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J. Exp. Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Takeda K., Kawano M., Takai T., Ishii N., Ogasawara K. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18360–18365. doi: 10.1073/pnas.1110584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant C.J., Hwang Y.Y., Walker J.A., Salimi M., Wong S.H., Brewer J.M., Englezakis A., Barlow J.L., Hams E., Scanlon S.T., Ogg G.S., Fallon P.G., McKenzie A.N. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patch J.R., Dar P.A., Waters R., Toka F.N., Barrera J., Schutta C., Kondabattula G., Golde W.T. Infection with foot-and-mouth disease virus (FMDV) induces a natural killer (NK) cell response in cattle that is lacking following vaccination. Comp. Immunol. Microbiol. Infect. Dis. 2014;37:249–257. doi: 10.1016/j.cimid.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Pintaric M., Gerner W., Saalmüller A. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-gamma production of porcine natural killer cells. Vet. Immunol. Immunopathol. 2008;121:68–82. doi: 10.1016/j.vetimm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Prajeeth C.K., Haeberlein S., Sebald H., Schleicher U., Bogdan C. Leishmania-infected macrophages are targets of NK cell-derived cytokines but not of NK cell cytotoxicity. Infect. Immun. 2011;79:2699–2708. doi: 10.1128/IAI.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renukaradhya G.J., Alekseev K., Jung K., Fang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 2010;23:457–466. doi: 10.1089/vim.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D.A., Yan H., Bastos R.G., Johnson W.C., Gavin P.R., Allen A.J., Barrington G.M., Herrmann-Hoesing L.M., Knowles D.P., Goff W.L. Dynamics of bovine spleen cell populations during the acute response to Babesia bovis infection: an immunohistological study. Parasite Immunol. 2011;33:34–44. doi: 10.1111/j.1365-3024.2010.01249.x. [DOI] [PubMed] [Google Scholar]
- Sellers R.F., Parker J. Airborne excretion of foot-and-mouth disease virus. J. Hyg. (Lond.) 1969;67:671–677. doi: 10.1017/s0022172400042121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S., Peng Y., Gao X., Joyee A.G., Wang S., Bai H., Zhao L., Yang J., Yang X. NK cells modulate the lung dendritic cell-mediated Th1/Th17 immunity during intracellular bacterial infection. Eur. J. Immunol. 2015 doi: 10.1002/eji.201445390. [DOI] [PubMed] [Google Scholar]
- Siddiqui N., Price S., Hope J. BCG vaccination of neonatal calves: potential roles for innate immune cells in the induction of protective immunity. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:219–226. doi: 10.1016/j.cimid.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Spits H., Lanier L.L. Natural killer or dendritic: what's in a name? Immunity. 2007;26:11–16. doi: 10.1016/j.immuni.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E., Powrie F., Vivier E. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Stetson D.B., Mohrs M., Reinhardt R.L., Baron J.L., Wang Z.E., Gapin L., Kronenberg M., Locksley R.M. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storset A.K., Kulberg S., Berg I., Boysen P., Hope J.C., Dissen E. NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics. Eur. J. Immunol. 2004;34:669–676. doi: 10.1002/eji.200324504. [DOI] [PubMed] [Google Scholar]
- Szabo S.J., Sullivan B.M., Peng S.L., Glimcher L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv. Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toka F.N., Golde W.T. Cell mediated innate responses of cattle and swine are diverse during foot-and-mouth disease virus (FMDV) infection: a unique landscape of innate immunity. Immunol. Lett. 2013;152:135–143. doi: 10.1016/j.imlet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toka F.N., Nfon C.K., Dawson H., Estes D.M., Golde W.T. Activation of porcine natural killer cells and lysis of foot-and-mouth disease virus infected cells. J. Interferon Cytokine Res. 2009;29:179–192. doi: 10.1089/jir.2008.0058. [DOI] [PubMed] [Google Scholar]
- Toka F.N., Nfon C.K., Dawson H., Golde W.T. Accessory-cell-mediated activation of porcine NK cells by toll-like receptor 7 (TLR7) and TLR8 agonists. Clin. Vaccine Immunol. 2009;16:866–878. doi: 10.1128/CVI.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toka F.N., Nfon C., Dawson H., Golde W.T. Natural killer cell dysfunction during acute infection with foot-and-mouth disease virus. Clin. Vaccine Immunol. 2009;16:1738–1749. doi: 10.1128/CVI.00280-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E. What is natural in natural killer cells? Immunol. Lett. 2006;107:1–7. doi: 10.1016/j.imlet.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Walker J.A., Barlow J.L., McKenzie A.N. Innate lymphoid cells – how did we miss them? Nat. Rev. Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Walsh K.P., Mills K.H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–530. doi: 10.1016/j.it.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Walzer T., Dalod M., Robbins S.H., Zitvogel L., Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- Worliczek H.L., Buggelsheim M., Alexandrowicz R., Witter K., Schmidt P., Gerner W., Saalmüller A., Joachim A. Changes in lymphocyte populations in suckling piglets during primary infections with Isospora suis. Parasite Immunol. 2010;32:232–244. doi: 10.1111/j.1365-3024.2009.01184.x. [DOI] [PubMed] [Google Scholar]


