Abstract
An antigen-capture enzyme-linked immunosorbent assay (AC-ELISA) method was developed for the efficient detection of the UL24 antigen of the duck enteritis virus (DEV) using polyclonal antibodies. Ducks and rabbits were immunized, respectively, with expressed UL24 recombinant protein. The IgG antibodies against UL24 from ducks and rabbits were purified and used as the capture antibodies. The specificity of the optimized AC-ELISA was evaluated by use of DEV, duck hepatitis virus (DHV), duck hepatitis B virus (DHBV), gosling plague virus (GPV), Riemerella anatipestifer (R.A.), Escherichia coli (E. coli), Pasteurella multocida (P.M.) and Salmonella Enteritidis (S.E.). Only DEV specimens yielded a specific and strong signal. The limit of the sensitivity of this method for the detection of DEV was 46 ng/100 μl. Compared with PCR and virus isolation, the rate of agreement for the detection of experimentally infected sera was 100%. A comparative test used on clinical specimens between the neutralization test and the AC-ELISA showed that the proportions of true positives and true negatives by the AC-ELISA were 0.90 and 0.67 respectively. These results indicated that the AC-ELISA approach is rapid, sensitive, and reliable for specific detection of DEV antigen.
Keywords: Duck enteritis virus, Antigen-capture ELISA, Prokaryotic expression, UL24
1. Introduction
Duck viral enteritis (DVE), or duck plague (DP), is an acute, contagious herpesvirus infection of ducks, geese, and swans of all ages and species. The disease has been responsible for significant economic losses in domestic and wild waterfowl as a result of mortality, and decreased egg production (Saif et al., 2003). The disease is caused by duck enteritis virus (DEV), and is characterized by vascular damage, tissue hemorrhages, eruptions on the digestive mucosa, lesions of lymphoid organs, and degenerative changes in parenchymatous organs (Barr et al., 1992, Shawky et al., 2000). The disease is difficult to monitor and control because the virus establishes an asymptomatic carrier state in waterfowl that is detectable only during periods of intermittent virus shedding (Burgess et al., 1979). The diagnostic procedures that are currently used to identify DEV antigen include virus isolation and identification (Burgess and Yuill, 1981, Hwang et al., 1975), the reverse passive hemagglutination test (Deng et al., 1984), histopathology (Shawky et al., 2000, Xuefeng et al., 2008a), immunofluorescence (Proctor, 1975), immunoperoxidase staining (Malmarugan and Sulochana, 2002), immunohistochemistry (Islam et al., 1993, Xuefeng et al., 2008b), electron microscopy (Yuan et al., 2005), and the polymerase chain reaction (PCR) (Hansen et al., 1999, Hansen et al., 2000, Pritchard et al., 1999).
It is very important to select appropriate methods for the detection of DEV antigen. The methods mentioned above are both time consuming and labor intensive; moreover, samples for virus isolation are easily contaminated, and the equipment or personnel required for PCR may not be available. The antigen-capture ELISA (AC-ELISA) technique, with characterized sensitivity and specificity, has been applied to the detection of viruses, e.g. avian influenza virus (He et al., 2007, Velumani et al., 2008), bovine leukaemia virus (Juliarena et al., 2007), and the nucleocapsid antigen of SARS-CoV (Che et al., 2004). The genomic organization of DEV remains unclear, however, and there are very few reports of the prokaryotic expression of DEV genes, or on use of the AC-ELISA method for the efficient detection of DEV antigen.
Generally, antibodies against expressed protein produced during an immune reaction are more specific than those against purified virus, owing to the complex construction of the purified virus, which may incorporate various host cell proteins. Moreover, the use of polyclonal antibodies to detect antigens by ELISA is more sensitive than use of a monoclonal antibody (El-Mekki et al., 1987). Fortunately, the DEV UL24 gene had been newly isolated and identified, and the protein had been expressed in a prokaryotic expression system in our laboratory. The DEV UL24 protein, a conserved protein, may play an important role in the life cycle of the virus, as with other herperviruses described previously (Blakeney et al., 2005, Pearson and Coen, 2002), but there is no information about its properties or function. Thus, it is necessary to perform research on the immunogenicity of UL24 and to establish a more rapid ELISA method for detection of the DEV UL24 antigen with a protein antibody directed against UL24. The development and evaluation of a sensitive and specific AC-ELISA to detect the DEV UL24 antigen, using both rabbit and duck anti-DEV UL24 IgG antibodies prepared by prokaryotic expression of the UL24 protein, are described in this paper.
2. Materials and methods
2.1. Viruses and bacteria
The viruses and bacteria used in this study are listed in Table 1 , and were obtained from the Key Laboratory of Animal Disease and Human Health of Sichuan Province. Duck embryo fibroblasts (DEFs) were propagated in Dulbecco's minimal essential medium (DMEM) (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL, Grand Island, NY, USA) at 37 °C, and then infected by DEV. The virus was grown in DMEM medium supplemented with 2–3% FBS, and was collected at 48 h post-incubation.
Table 1.
The reference pathogens used for the AC-ELISA.
| Species | Strain/serotype | Host |
|---|---|---|
| Duck enteritis virus (DEV) | Field isolate, CHv | Peking duck |
| Duck hepatitis virus (DHV) | Field isolate, CHv-1 (type 1) | Cherry Valley duck |
| Duck hepatitis B virus (DHBV) | Field isolate, MY | Cherry Valley duck |
| Gosling plague virus (GPV) | Field isolate, CH | China gosling |
| Escherichia coli (E. coli) | Field isolate, GH | Peking duck |
| Riemerella anatipestifer (R.A.) | Field isolate, HY (type 1) | Peking duck |
| Pasteurella multocida (P.M.) | Field isolate, SC | Peking duck |
| Salmonella Enteritidis (S.E.) | Field isolate, MY1 | Peking duck |
2.2. Cloning, expression, and purification of recombinant DEV UL24 protein
The coding region for the UL24 gene of DEV was amplified by PCR from the stable infected cells described above. The forward primer carried a restriction site for EcoRI: 5′-GAATTCATACCTACCAAAGGTAAGCGC-3′, and the reverse primer carried a restriction site for XhoI: 5′-CTCGAGCTAGTGTTTAGTTGGTCTGAA-3′. The sequence encoding the UL24 gene was ligated into the EcoRI and XhoI sites of a His-tagged prokaryotic expression vector, pET-32a(+) (Novagen, Germany) in frame, and the insert was sequenced to confirm the accuracy of the UL24 gene sequence and proper in-frame ligation. This construct was introduced into Escherichia coli BL21 (DE3) cells, and protein expression was induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37 °C for 4 h. Total E. coli protein was extracted by use of 50 mM Tris–HCl (pH 7.5) containing 1 mM EDTA, 100 mM NaCl and thawing, and was purified by Ni2+ affinity chromatography (Bio-Rad, CA, USA) according to the manufacturer's instructions.
2.3. Preparation and purification of antibodies
Rabbits (n = 4) and ducks (n = 4) were immunized with purified recombinant DEV UL24 protein mixed with an equal volume of complete Freund's adjuvant (Sigma, Missouri, USA) for the first injection, and with incomplete Freund's adjuvant for the following three booster injections. Each injection comprised 1.0 mg (per rabbit) and 0.8 mg (per duck) of recombinant DEV UL24 protein. Sera were collected 12 days after the final intravenous injection of 0.2 mg and 0.15 mg of recombinant DEV UL24 protein. The IgG polyclonal antibodies were purified using caprylic acid and ammonium sulfate precipitation and High-Q anion-exchange chromatography (Bio-Rad, CA, USA) (Cheng et al., 2002, McGuire et al., 1996).
2.4. Immunoblotting
The DEV UL24 expression protein was subjected to 12% SDS-PAGE. The separated proteins were electrotransferred and immobilized on to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% bovine serum albumin (BSA) in PBS containing 0.05% Tween 20 (PBST) at 37 °C for 1 h. The membrane was incubated subsequently with rabbit anti-DEV, rinsed in PBST, and incubated with HRP-conjugated goat anti-rabbit IgG (Zhongshan, Beijing, China). The membrane was developed by incubation with 3,3′-diaminobenzidine (DAB) and hydrogen peroxide, as described previously (Kano et al., 2008).
2.5. Development of the AC-ELISA
A 96-well microtiter plate (Nunc, Denmark) was coated with 100 μl of a 1:40 (5.0 μg/μl) dilution of purified rabbit IgG anti-DEV UL24 in sodium bicarbonate buffer (pH 9.6), and incubated at 4 °C overnight. The plate was blocked by incubation with 100 μl of blocking solution (1% BSA in PBS) for 60 min at room temperature, and washed twice with PBS containing 0.05% Tween-20 (PBST) using a Bio-Tek model Elx 50 (Bio-Tek, Vermont, USA). Subsequently, 100 μl of the DEV sample was added, and incubation was performed at 37 °C for 60 min. The samples were washed, and then incubated at 37 °C for 60 min with 100 μl of a 1:20 (9.0 μg/μl) dilution of purified duck IgG anti-DEV UL24. The plate was washed, and incubated for 45 min at 37 °C with 100 μl of anti-duck horseradish peroxidase (KPL, Gaithersburg, USA) diluted 1:2000 in PBS, washed again, and detected with 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) for 45 min at room temperature. The reaction was stopped by the addition of 35 μl of 2 mol/l H2SO4. The optical density (OD) was read at 450 nm, using a Bio-Rad model 860 plate reader (Bio-Rad, CA, USA).
2.6. Determination of cutoff value for the AC-ELISA
Thirty-two serum samples from ducks uninfected with DEV were used as negative sera in the AC-ELISA to evaluate the cutoff value, which was calculated using the formula: mean of the negative serum values plus three standard deviations (SDs) (Deshpande, 1996).
2.7. Analytical specificity and sensitivity of the AC-ELISA
Duck hepatitis virus (DHV), duck hepatitis B virus (DHBV), gosling plague virus (GPV), and DEV were propagated according to methods described in the literature (Chen et al., 2009, Qi et al., 2009, Xinfeng et al., 2008, Yang et al., 2008). Debris was removed from the harvested virus supernatant by centrifugation at 10,000 × g for 20 min. The supernatants, containing the viruses, were subjected to the AC-ELISA. Ducks were infected, respectively, by the pathogens Riemerella anatipestifer (R.A.), Escherichia coli (E. coli), Pasteurella multocida (P.M.) and Salmonella Enteritidis (S.E.) (Anchun et al., 2005, Cao et al., 2008, Ling et al., 2007, Samuel et al., 1997). Sera were collected 14 days after infection, and then also subjected to the AC-ELISA and the results determined according to the cutoff value.
Twofold serial dilutions of purified DEV (Ren-yong et al., 2007) in PBS were determined as described by Bradford (1976) using a protein assay kit supplied by Bio-Rad Laboratories (Bio-Rad, AC, USA), and used as the antigens in the AC-ELISA. The diluent and mock (non-inoculated) cells were used as blank controls. The limits of detection were determined for the AC-ELISA according to the cutoff value.
2.8. Analytical reproducibility and repeatability of the AC-ELISA
Assessment of the precision of an assay includes measurement of repeatability and reproducibility (Jacobson, 1998). The repeatability was calculated by the coefficient of variation (CV) for each set of the samples that were run on the same day, whereas reproducibility was calculated from the average of the within-assay precision of a given sample analyzed in three runs at different times. For the precision assays, as described above, the acceptance criteria were less than 10% and less than 20%, respectively.
2.9. Analytical inhibition of the AC-ELISA
A mixture of 100 μl (5.0 μg/μl) dilutions of duck-anti DEV-UL24 IgG in PBS and an equal volume of DEV was incubated for 60 min at 37 °C. Normal duck serum was used as the negative control, and was also mixed with an equal volume of DEV. These mixtures were retested in the AC-ELISA by adding 100 μl of each mixture, and the assay was performed according to the description above. A mixture of negative duck serum and an equal amount of PBS was used as the blank control and was also tested. The percentage of inhibition of antibody binding was calculated by the following equation (Che et al., 2004): % inhibition = (A 450 nm of normal serum − A 450 nm of positive serum)/(A 450 nm of normal serum − A 450 nm of blank control) × 100%. If the % inhibition was greater than 50%, test specimens were considered to be confirmed as positive for DEV.
2.10. Comparison of AC-ELISA with virus isolation, PCR and neutralization test
-
(i)
Peking ducks from a DEV-free farm, which were 28 days old and not vaccinated against DEV, were divided into two groups. One group was used as uninfected controls, and the other group was inoculated subcutaneously with approximately 0.5 ml per duck (1000 median duck lethal doses) of the DEV-CHv strain. Serum samples were collected at each of seven sampling times (0 h, 4 h, 8 h, 12 h, 24 h, 72 h, and 120 h post-infection (h.p.i.)) from three randomly selected ducks, and were examined by AC-ELISA, virus isolation (Burgess and Yuill, 1981, Hwang et al., 1975), and PCR. The PCR used gene-specific primers: Fwd (5′-GGACAGCGTACCACAGATAA-3′) and Rev (5′-ACAAATCCCAAGCGTAG-3′), and was initiated by denaturation at 95 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 51.8 °C for 1 min, 72 °C for 2 min, and further elongation at 72 °C for 10 min (Cheng et al., 2004).
-
(ii)
Specimens were collected from ducklings from different areas of Sichuan province (n = 135) that were suspected clinically to be infected with DEV, and were examined using the AC-ELISA. The neutralization test was used as the gold standard of diagnosis in order to calculate the sensitivity and specificity of the AC-ELISA (Altman and Bland, 1994).
3. Results
3.1. Prokaryotic expression of the DEV UL24 protein
The UL24 protein of DEV was expressed for use as an antigen for antibody development. A region of DEV of approximately 500 bp was amplified by PCR, yielding a product of the expected size (Fig. 1 ). The product was then cloned in pET32a(+) and was expressed in E. coli as a His-tagged recombinant UL24 fusion protein of approximately 38 kDa. The protein was purified by chromatography and verified by Western blotting (Fig. 2 ).
Fig. 1.
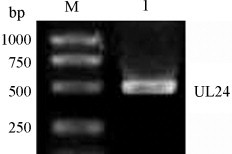
Amplification of the UL24 gene. The UL24 fragment, with a size of 500 bp (lane 1), was amplified from DEV-CHv by PCR.
Fig. 2.
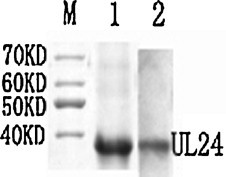
Expression of the recombinant protein. The UL24 protein was expressed and purified (lane 1), and recognized by western blotting (lane 2) with rabbit anti-DEV serum and HRP-conjugated goat anti-rabbit IgG, respectively. Lane M, molecular size makers.
3.2. Cutoff value of the AC-ELISA
To establish the cutoff value of the AC-ELISA, serum specimens from ducks uninfected with DEV were analyzed. The mean of the OD450 nm values for these specimens, as detected by the AC-ELISA, was 0.1829, with a standard deviation of 0.0309. The cutoff value of the AC-ELISA was calculated from the 32 normal serum specimens according to a Gaussian population distribution (Deshpande, 1996). For a 99% confidence interval, the cutoff was defined as follows: mean of the negative serum OD450 nm values plus three standard deviations = 0.1829 + 3 × 0.0309 = 0.2756.
3.3. Sensitivity and specificity of the AC-ELISA
The sensitivity of the AC-ELISA was determined by using dilutions of purified DEV. A minimum detection limit of 46 ng/100 μl (OD450 nm = 0.286) was obtained according to the cutoff value (0.2756), but the control cells did not yield positive results (Fig. 3 ).
Fig. 3.
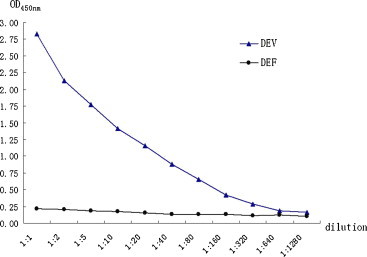
Sensitivity of the AC-ELISA. Different concentrations of purified DEV (from a 1:1 dilution to a 1:1280 dilution; the concentrations of DEV were 14,720 ng, 7360 ng, 2944 ng, 1472 ng, 736 ng, 368 ng, 184 ng, 92 ng, 46 ng, 23 ng, and 12 ng per well, respectively). At least 46 ng of DEV protein per well could be detected in the AC-ELISA.
On the basis of the cutoff value, DEV, DHV, DHBV, GPV, R.A., E. coli, P.M., S.E., cells (control), and PBS (control) were tested using the AC-ELISA; however, except for DEV, the tested pathogens did not yield positive results (Fig. 4 ). The OD450 nm values of the cells (control) and PBS (control) were both lower than the cutoff value. This indicated that no false-positive results were obtained with the AC-ELISA caused by the detection of other pathogens.
Fig. 4.
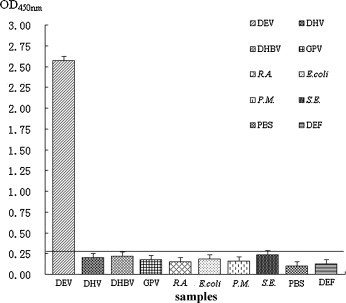
Specificity of the AC-ELISA. Different pathogens, PBS, and duck embryo fibroblasts were tested using the AC-ELISA. Numbers represent the mean absorbance from triplicate wells. All OD450 nm values except that of DEV were lower than the cutoff value.
3.4. Reproducibility and repeatability of the AC-ELISA
Five replicates of 10 specimens that were analyzed for repeatability showed a mean coefficient of variation (CV) of 3.02%, and individual CVs varied from 0.78% to 6.10%. When each replicate was run on different days the assay showed a mean CV of 6.47%, and individual CVs varied from 3.42% to 9.27%. Table 2 shows the repeatability and reproducibility of the assay under the experimental conditions used and demonstrates the low variability.
Table 2.
Results of reproducibility and repeatability assay of AC-ELISA.
| Ref. samples |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| CV (%) | ||||||||||
| Repeatability | 0.78 | 4.26 | 1.24 | 0.94 | 5.61 | 3.28 | 6.10 | 0.86 | 2.87 | 4.21 |
| Reproducibility | 5.51 | 3.42 | 6.01 | 8.79 | 7.54 | 4.76 | 6.61 | 9.27 | 7.43 | 5.33 |
3.5. Inhibition of the AC-ELISA
The inhibition assay was repeated three times using the AC-ELISA with anti-DEV UL24 IgG. It was observed that the OD450 nm values in these reactions were reduced by more than 50% after blocking with anti-DEV UL24 IgG (Table 3 ). This result indicates that the assay was highly specific for DEV-UL24 IgG.
Table 3.
Results of inhibition assay for AC-ELISA.
| No. | OD450 nm |
%Inhibitionc | |
|---|---|---|---|
| Negative seruma | Positive serumb | ||
| 1 | 1.873 | 0.237 | 87 |
| 2 | 1.259 | 0.187 | 85 |
| 3 | 1.531 | 0.248 | 83 |
DEV 100 μl (0.182 μg/100 μl) was mixed with 100 μl of normal duck serum in each test.
DEV 100 μl (0.182 μg/100 μl) was mixed with 100 μl (5.0 μg/μl) of purified duck anti-DEV UL24 IgG in each test.
Test specimens were considered to be confirmed as positive for duck anti-DEV UL24 IgG if the percentage of inhibition was greater than 50%.
3.6. Detection of DEV in experimental specimens
To validate the AC-ELISA, 28-day-old Peking ducks were challenged with the CHv strain of DEV. The experimental sera were collected and tested using the AC-ELISA. The DEV UL24 antigen appeared in the serum at 8 h.p.i. (Fig. 5 ), and the result was further confirmed at 4 h.p.i. by PCR and at 72 h.i.p. by virus isolation, respectively. This implied that the analytical sensitivity of the AC-ELISA was greater than that of virus isolation, and approached that of the PCR (10 fg DNA) (Cheng et al., 2004).
Fig. 5.
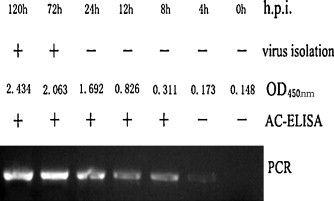
Experimental infection of duck sera with DEV-CHv detected by AC-ELISA, virus isolation, and PCR. Positive sera were consistently detected by the AC-ELISA, and the positive and negative results were recorded according to the established cutoff value.
3.7. Detection of DEV in clinical specimens
Using the collected specimens, the sensitivity of the AC-ELISA was compared with that of the neutralization test. The results showed that there were 120 true positives and 15 true negatives. The proportions of these two groups that were correctly diagnosed by the AC-ELISA were 108/120 = 0.90 and 10/15 = 0.67, respectively (Table 4 ). This result indicates that the AC-ELISA was specific and sensitive for the DEV UL24 antigen.
Table 4.
Relation between the results of AC-ELISA and neutralization test.
| AC-ELISA | Neutralization test |
||
|---|---|---|---|
| Positives (+) | Negatives (−) | Total | |
| Positives (+) | 108 | 5 | 113 |
| Negatives (−) | 12 | 10 | 22 |
| Total | 120 | 15 | 135 |
4. Discussion
To improve their ability to detect DEV, as well as for disease control purposes, laboratories need more rapid and less cumbersome methods for the direct identification of the viral antigen in clinical specimens. Current methods for the detection of antigen, such as immunohistochemistry, viral isolation, and immuno-electron microscopy, are unable to detect antigen quickly enough. However, of the numerous techniques developed for the rapid diagnosis of viral infections in recent years, the AC-ELISA provides a platform that is capable of mass screening of clinical specimens. It also offers well-documented advantages over more traditional methods of antigen detection. The use of monoclonal antibodies may not only improve the sensitivity and specificity of an ELISA, but may also allow distinction between virus serotypes and different antigens, as well as between subclasses of IgG antibody (Clavijo et al., 1998). In some studies of captured antigens, not only single strains of monoclonal antibody (Stephanie et al., 2008, Van den Berg et al., 2004, Velumani et al., 2008), but also multiple strains of monoclonal antibodies (Katarzyna et al., 2002), have been used to enhance the sensitivity of the test. Although the components of polyclonal antibodies are complex, and the specificity is inferior to that of tests that use monoclonal antibody, some researchers are still inclined to choose polyclonal antibodies for the detection of antigens by ELISA, because of the higher sensitivity (El-Mekki et al., 1987). The antigenicity and immunogenicity of DEV from different regions are almost identical, and the diversity of different strains of DEV can be ignored (Jansen, 1968, Shawky and Sandhu, 1997). Therefore a polyclonal antibody is superior to a monoclonal antibody for the detection of DEV antigen. In this study, in order to enhance the specificity and sensitivity of the AC-ELISA, rabbit polyclonal antibodies with high affinity and specificity for DEV, prepared by use of the DEV UL24 protein, were used for antigen capture. The results showed that the limit of sensitivity of the AC-ELISA for detection of the DEV antigen was approximately 46 ng/100 μl.
The assessment of the specificity of the AC-ELISA revealed that the polyclonal antibody against the DEV UL24 expression protein showed no cross-reaction with other viruses or strains from ducks. Furthermore, a blocking assay was used to demonstrate the specificity of the AC-ELISA. It was shown that serum samples positive for antibodies against DEV UL24 reduced the OD450 nm values obtained using purified DEV significantly. The high sensitivity and specificity of the assay are likely to be due to the fact that the DEV UL24 expression protein contained a single component. Complete DEV virions probably integrate with the two exposed epitope-binding sites of the polyclonal anti-DEV UL24 IgG in serum, and then the combination combines with the enzyme-tagged antibody.
Ducks were infected artificially with virulent DEV in this experiment. The infected ducks were detected successfully using the AC-ELISA, and the result was confirmed further by PCR and virus isolation. No false-positive signals were obtained, which confirmed the potential clinical use of the AC-ELISA. Clinical specimens showed that a proportion of the true positives (108) and the true negatives (10) were identified correctly by the test. These results suggest that the AC-ELISA method could be used to make a clinical diagnosis of duck viral enteritis, with confidence in the specificity and sensitivity of the assay.
Acknowledgements
The research was supported by Changjiang Scholars and Innovative Research Team in University (IRT0848) and the earmarked fund for Modern Agro-industry Technology Research System (nycytx-45-12).
References
- Altman D.G., Bland J.M. Diagnostic tests. 1. Sensitivity and specificity. Br. Med. J. 1994;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anchun C., Mingshu W., Yufei G., Pengfei F., Zhaoyu L., Xiaoyue C. Development of inactivated tetravalent Riemerella anatipestifer vaccine containing serotypes 1, 2, 4, 5 and comparison of its alhydrogel adjuvant with the alhydrogel compound adjuvant. Chin. J. Vet. Sci. 2005;25:152–156. [Google Scholar]
- Barr B.C., Jessup D.A., Docherty D.E., Lowenstine L.J. Epithelial intracytoplasmic herpes viral inclusions associated with an outbreak of duck virus enteritis. Avian Dis. 1992;36:164–168. [PubMed] [Google Scholar]
- Blakeney S., Kowalski J., Tummolo D., DeStefano J., Cooper D., Guo M., Gangolli S., Long D., Zamb T., Natuk R.J., Visalli R.J. Herpes simplex virus type 2 UL24 gene is a virulence determinant in murine and guinea pig disease models. J. Virol. 2005;79:10498–10506. doi: 10.1128/JVI.79.16.10498-10506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess E.C., Ossa J., Yuill T.M. Duck plague: a carrierstate in waterfowl. Avian Dis. 1979;23:940–949. [PubMed] [Google Scholar]
- Burgess E.C., Yuill T.M. Increased cell culture incubation temperatures for duck plague virus isolation. Avian Dis. 1981;25:222–224. [PubMed] [Google Scholar]
- Cao S.Y., Wang M.S., Cheng A.C., Qi X.F., Yang X.Y., Deng S.X., Yin N.C., Zhang Z.H., Zhou D.C., Zhu D.K., Luo Q.H., Chen X.Y. Comparative analysis of intestinal microbial community diversity between healthy and orally infected ducklings with Salmonella enteritidis by ERIC-PCR. World J. Gastroenterol. 2008;14:1120–1125. doi: 10.3748/wjg.14.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X.Y., Qiu L.W., Pan Y.X., Wen K., Hao W., Zhang L.Y., Wang Y.D., Liao Z.Y., Hua X., Cheng V.C., Yuen K.Y. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J. Clin. Microbiol. 2004;42:2629–2635. doi: 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.Y., Cheng A.C., Wang M.S., Xu D.W., Jia R., Guo Y.F., Zeng W. Viral load in 1-day-old ducklings acutely infected with duck hepatitis B virus by different doses and routes of inoculation. Avian Pathol. 2009;38:129–134. doi: 10.1080/03079450902737862. [DOI] [PubMed] [Google Scholar]
- Cheng A.C., Wang M.S., Liu F., Song Y., Yuan G.P., Han X.Y., Xu C., Liao Y.H., Zhou W.G., Wen M., Jia R.Y., Chen X.Y. The preliminary application of PCR in research of clinical diagnosis and mechanisms of immunity and pathogeny of duck plague virus (DPV) Chin. J. Virol. 2004;20:264–370. [Google Scholar]
- Cheng Y., Nilsson A., Tomquist E., Duan R.D. Purification, characterization, and expression of rat intestinal alkaline sphingomyelinase. J. Lipid Res. 2002;43:316–324. [PubMed] [Google Scholar]
- Clavijo A., Zhou E.M., Vydelingum S., Heckert R. Development and evaluation of a novel antigen capture assay for the detection of classical swine fever virus antigens. Vet. Microbiol. 1998;60:155–168. doi: 10.1016/s0378-1135(98)00160-6. [DOI] [PubMed] [Google Scholar]
- Deng M.Y., Burgess E.C., Yuill T.M. Detection of duck plague virus by reverse passive hemagglutination test. Avian Dis. 1984;28:616–628. [PubMed] [Google Scholar]
- Deshpande S.S. 1st ed. Kluwer Academic Publishers; New York: 1996. Enzyme Immunoassay from Concept to Product Development. [Google Scholar]
- El-Mekki A., Al-Nakib W., Bibi R. Factors affecting the detection of cytomegalovirus in urine by sandwich enzyme immunoassays. J. Virol. Methods. 1987;15:75–83. doi: 10.1016/0166-0934(87)90050-4. [DOI] [PubMed] [Google Scholar]
- Hansen W.R., Brown S.E., Nashold S.W., Knudson D.L. Identification of duck plague virus by polymerase chain reaction. Avian Dis. 1999;43:106–115. [PubMed] [Google Scholar]
- Hansen W.R., Nashold S.W., Docherty D.E., Brown S.E., Knudson D.L. Diagnosis of duck plague in waterfowl by polymerase chain reaction. Avian Dis. 2000;44:266–274. [PubMed] [Google Scholar]
- He Q., Velumani S., Du Q., Lim C.W., Ng F.K., Donis R., Kwang J. Detection of H5 avian influenza viruses by antigen-capture enzyme-linked immunosorbent assay using H5-specific monoclonal antibody. Clin. Vac. Immunol. 2007;14:617–623. doi: 10.1128/CVI.00444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Mallinson E.T., Yoxheimer R.E. Occurrence of duck virus enteritis (duck plague) in Pennsylvania, 1968–1974. Avian Dis. 1975;19:382–384. [PubMed] [Google Scholar]
- Islam M.R., Nessa J., Halder K.M. Detection of duck plague virus-antigen in tissues by immunoperoxidase staining. Avian Pathol. 1993;22:389–393. doi: 10.1080/03079459308418929. [DOI] [PubMed] [Google Scholar]
- Jacobson R.H. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Tech. OIE. 1998;17:469–526. doi: 10.20506/rst.17.2.1119. [DOI] [PubMed] [Google Scholar]
- Jansen J. Duck plague. J. Am. Vet. Med. Assoc. 1968;152:1009–1016. [PubMed] [Google Scholar]
- Juliarena M., Gutierrez S., Ceriani C. Chicken antibodies: a useful tool for antigen capture ELISA to detect bovine leukaemia virus without cross-reaction with other mammalian antibodies. Vet. Res. Commun. 2007;31:43–51. doi: 10.1007/s11259-006-3422-1. [DOI] [PubMed] [Google Scholar]
- Kano R., Sato E., Okamura T., Watanabe S., Hasegawa A. Expression of Bcl-2 in feline lymphoma cell lines. Vet. Clin. Pathol. 2008;37:57–60. doi: 10.1111/j.1939-165X.2008.00013.x. [DOI] [PubMed] [Google Scholar]
- Katarzyna D., Rivallan G., Krzysztof S., Didier T., Zenon M., Nicolas E. Antigenic characterization of polish infectious bursal disease virus strains. Bull. Vet. Inst. Pulawy. 2002;46:45–52. [Google Scholar]
- Ling L., Mingshu W., Anchun C., Xiaona Y., Chuande Z., Ze D., Xiaoyue C. Isolation of duckling pathogenic Escherichia coli and analysis of drug resistance and sensitivity. Chin. J. Vet. Med. 2007;43:34–37. [Google Scholar]
- Malmarugan S., Sulochana S. Detection of duck plague viral antigen in tissues by immunoperoxidase test. Indian Vet. J. 2002;79:103–105. [Google Scholar]
- McGuire J.M., Douglas M., Smith K.D. The resolution of the neutral N-linked oligosaccharides of IgG by high pH anion-exchange chromatography. Carbohyd. Res. 1996;292:1–9. doi: 10.1016/s0008-6215(96)91015-0. [DOI] [PubMed] [Google Scholar]
- Pearson A., Coen D.M. Identification, localization, and regulation of expression of the UL24 protein of herpes simplex virus type 1. J. Virol. 2002;76:10821–10828. doi: 10.1128/JVI.76.21.10821-10828.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard L.I., Morrissy C., Van Phuc K., Daniels P.W., Westbury H.A. Development of a polymerase chain reaction to detect vietnamese isolates of duck virus enteritis. Vet. Microbiol. 1999;16:149–156. doi: 10.1016/s0378-1135(99)00071-1. [DOI] [PubMed] [Google Scholar]
- Proctor S.J. Pathogenesis of digestive-tract lesions in duck plague. Vet. Pathol. 1975;12:349–361. doi: 10.1177/0300985875012005-00602. [DOI] [PubMed] [Google Scholar]
- Qi X., Yang X., Cheng A., Wang M., Guo Y., Jia R. Replication kinetics of duck virus enteritis vaccine virus in ducklings immunized by the mucosal or systemic route using real-time quantitative PCR. Res. Vet. Sci. 2009;86:63–67. doi: 10.1016/j.rvsc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Ren-yong J., An-chun C., Ming-shu W., Yu-fei G., Ming W., Chao X., Gui-ping Y., Wei-guang Z., Yi Z., Xiao-yue C. Studies on ultrastructure of duck enteritis virus CHv virulent strain. Chin. J. Virol. 2007;23:202–206. [Google Scholar]
- Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., Swayne D.E. 11th edition. Iowa State Press; Ames, Iowa: 2003. Diseases of Poultry. [Google Scholar]
- Samuel M.D., Goldberg D.R., Shadduck D.J., Price J.I., Cooch E.G. Pasteurella multocida serotype 1 isolated from a lesser snow goose: evidence of a carrier state. J. Wildlife Dis. 1997;33:332–335. doi: 10.7589/0090-3558-33.2.332. [DOI] [PubMed] [Google Scholar]
- Shawky S., Sandhu T., Shivaprasad H.L. Pathogenicity of a low-virulence duck virus enteritis isolate with apparent immunosuppressive ability. Avian Dis. 2000;44:590–599. [PubMed] [Google Scholar]
- Shawky S.A., Sandhu T.S. Inactivated vaccine for protection against duck virus enteritis. Avian Dis. 1997;41:461–468. [PubMed] [Google Scholar]
- Stephanie M., Doreen S., Konrad P., Nadja T., Lutz G., Hermann M., Martin M. Immunotherapy of equine sarcoid: dose-escalation trial for the use of chimeric papillomavirus-like particles. J. Gen. Virol. 2008;89:138–147. doi: 10.1099/vir.0.83266-0. [DOI] [PubMed] [Google Scholar]
- Van den Berg T.P., Morales D., Eterradossi N., Rivallan G., Toquin D., Raue R., Zierenberg K., Zhang M.F., Zhu Y.P., Wang C.Q., Zheng H.J., Wang X., Chen G.C., Lim B.L. Assessment of genetic, antigenic and pathotypic criteria for the characterization of IBDV strains. Avian Pathol. 2004;33:470–476. doi: 10.1080/03079450400003650. [DOI] [PubMed] [Google Scholar]
- Velumani S., Du Q., Fenner B.J., Prabakaran M., Wee L.C., Nuo L.Y., Kwang J. Development of an antigen-capture ELISA for detection of H7 subtype avian influenza from experimentally infected chickens. J. Virol. Methods. 2008;147:219–225. doi: 10.1016/j.jviromet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Xinfeng H., Anchun C., Mingshu W., Xiaodong L., Fei L., Min L., Qian C. Construction of goose parvovirus VP3 gene vaccine and preliminary report on its elicitation of neutralizing antibodies in mice and geese. High Technol. Lett. 2008;18:543–549. (in Chinese) [Google Scholar]
- Xuefeng Q., Xiaoyan Y., Anchun C., Mingshu W., Dekang Z., Renyong J. The pathogenesis of duck virus enteritis in experimentally infected ducks: a quantitative time-course study using TaqMan polymerase chain reaction. Avian Pathol. 2008;37:307–310. doi: 10.1080/03079450802043775. [DOI] [PubMed] [Google Scholar]
- Xuefeng Q., Xiaoyan Y., Anchun C., Mingshu W., Dekang Z., Renyong J. Quantitative analysis of virulent duck enteritis virus loads in experimentally infected ducklings. Avian Dis. 2008;52:338–344. doi: 10.1637/8120-100207-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Yang M., Cheng A., Wang M., Xing H. Development and application of a one-step real-time Taqman RT-PCR assay for detection of duck hepatitis virus type 1. J. Virol. Methods. 2008;153:55–60. doi: 10.1016/j.jviromet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Yuan G.P., Cheng A.C., Wang M.S., Liu F., Han X.Y., Liao Y.H., Xu C. Electron microscopic studies of the morphogenesis of duck enteritis virus. Avian Dis. 2005;49:50–55. doi: 10.1637/7237-071004R. [DOI] [PubMed] [Google Scholar]


