Abstract
Viral upper respiratory infection is the most common reason for seeking medical care for children. Recurrent viral respiratory infections and subsequent complications (e.g. acute otitis media (AOM)) are a burden for children, their families and society. Over the past decade, our knowledge on the significance of respiratory viruses has broadened remarkably. Viruses cause large variety of respiratory diseases and cause alone diseases, which previously have been assumed to be bacterial only (e.g. AOM and pneumonia). Methods for detection analysis of respiratory viruses are developing making both the diagnosis and epidemiological investigations of respiratory infections easier. Accurate diagnosis of respiratory infections and awareness of possible viral etiology could reduce the use of antibiotics. Etiologic studies of viral infections are becoming increasingly important, with the emergence of new antiviral drugs and vaccines.
Keywords: Respiratory infections, Virus infections, Children, Acute otitis media, Rhinovirus, Respiratory syncytial virus
1. Introduction
Acute upper respiratory infections (URI) are the most common acute diseases in children. Large community studies conducted decades ago already showed that the mean annual number of acute respiratory infections is as high as 5 in children less than 5 years of age and about 3 in older children [1], [2], [3], [4]. Respiratory infections cause significant economic losses. In the United States, acute respiratory infections in 1998 resulted in an estimated 84 million visits to physicians; of which 25 million were due to upper respiratory infection and 13 million due to acute otitis media (AOM) [5]. Every fourth respiratory infection results in a visit to a physician; in infants, the proportion is rising to approximately one-half of all respiratory infections [6]. In children attending day-care centers in Finland, infectious diseases caused more than 90% of total costs of illness (deficient utilization of day-care centers, parents’ lost working capacity, hospitalization, visits to physician, antibiotics) [7]. Viral respiratory infections can also lead to bacterial diseases, and mixed viral–bacterial infections are often associated with antibiotic treatment failure [8]. Previously, viral diagnoses and etiologic studies of URI have had little relevance to the clinical management of individual cases. However, prevention and therapies for viral infections are developing, and both vaccines and antivirals for respiratory viruses other than influenza may be available in the near future [9], [10]. This poses new requirements for viral diagnostics, particularly rapid and easy detection of respiratory viruses.
2. Viral etiology of upper respiratory infections
In the 1960s, the development of virus detection methods made it possible to identify most of the known respiratory viruses or virus groups by cell culture or serologic methods. Over the years, the reported proportion of virus-positive respiratory infections has increased due to improved detection methods, e.g. from 22% (cell culture) [3] up to 86% (antigen detection and polymerase chain reaction (PCR) methods) [11]. The proportion of virus-positivity can vary between studies depending on many different factors; the type of samples, detection methods, varying epidemiology of viruses and the study settings all may affect the results [3], [11], [12], [13]. In addition, many viruses have typical annual seasonality (Fig. 1 ) [14].
Fig. 1.
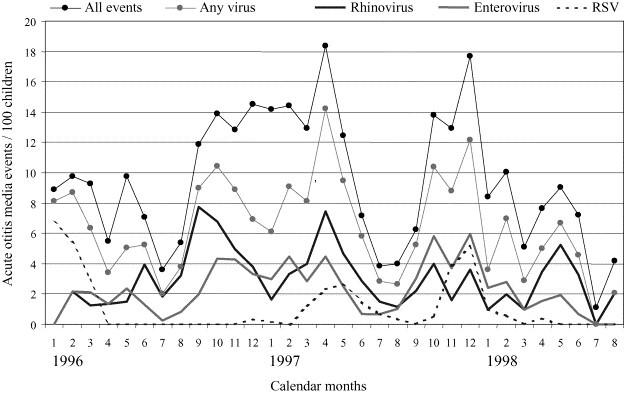
Monthly occurrence of virus-positive acute otitis media events in young children. Finnish Otitis Media Vaccine Trial, in which children were followed from 2 to 24 months of age.
New molecular techniques have changed the picture about viruses in several ways. For example, based on genome organization and sequence similarity the enterovirus genus (including polioviruses, coxsackieviruses and echoviruses) have recently been reclassified and divided into five different species (polioviruses and human enteroviruses A–D) [15], and only in few years many new enterovirus types have been characterized [16], [17]. Also new respiratory viruses have been discovered; e.g. human metapneumovirus [18], two previously unrecognized human coronaviruses [19], SARS-associated coronavirus [20], [21] and human bocavirus [22].
The use of PCR methods as a diagnostic tool for respiratory infections is increasing. PCR methods are generally more sensitive than traditional virus detection methods [23], and this has raised the question of the clinical relevance of virus-positive PCR findings. Asymptomatic viral infections do occur [24] and viral shedding in the nasopharynx may continue for up to 3 weeks after onset of infection [25]. Respiratory viral RNA can be detected relatively often from the nasopharynx of apparently healthy children, but most of the virus-positive findings can be linked to previous or future respiratory symptoms [26].
3. Specific features of respiratory viruses
There are over 200 different types of viruses that can cause upper respiratory infections in children. Rhinoviruses are the largest group of respiratory viruses, including at least 100 different serotypes. Rhinoviruses are the predominant cause of the common cold all over the world and in all age groups. In a prospective study, 91% of children had antibodies against rhinoviruses by the age of 2 years and 79% of children had experienced culture- or RT-PCR-confirmed rhinovirus infection [27]. Human rhinovirus infections typically occur in the early fall and in the spring. Although rhinoviruses are generally thought to cause only mild common cold, they are also associated with acute lower respiratory tract infections, wheezing, bronchiolitis and pneumonia in children [28], [29]. In addition, rhinoviruses often cause exacerbations of pre-existing airways diseases, such as asthma [29].
Enteroviruses, which belong to the same virus family Picornaviridae as rhinoviruses, are very common worldwide, and most primary infections occur in childhood. Often enteroviruses have been thought to cause mild diseases with characteristic signs (e.g. hand, foot, and mouth disease and herpangina) or more severe diseases such as meningitis. However, recently with modern detection methods, it has been shown that enteroviruses are a common cause of upper respiratory infections and acute otitis media in children [11], [14].
Respiratory syncytial virus (RSV) infections are present in all age groups, but they predominate in children and especially in infants. Up to 70% of infants have been reported to be infected with RSV during the first year of life, and the rest are infected by the age of 2 years [30]. RSVs are the leading cause of bronchiolitis and acute wheezing in young children [31]. Typically, RSV epidemics occur during the winter months, sometimes starting in the late fall and continuing until early spring. In Finland, RSV epidemics occur every 2 years, and during the epidemic year 2 separate epidemic peaks can be seen [13]. The pattern of 2-year epidemics is different from that in, for example, France [32].
Human metapneumovirus is a recently discovered respiratory virus [18]. The clinical symptoms caused by metapneumovirus are reported to be similar to those due to RSV, and this virus is an important cause of lower respiratory infections and wheezing in children [33]. Metapneumovirus infections occur mainly between winter and early spring [18], [34].
There are three types of influenza viruses in humans, types A, B and C. However, most data about influenza viruses are for types A and B because, due to detection difficulties, influenza C viruses have not been included in the studies. Influenza type A and B cause infections that can range from asymptomatic infections and common colds to serious illnesses with systemic complications, such as pneumonia [35], [36]. Influenza A and B infections typically occur in a seasonal pattern and in winter epidemics, which have variable intensities.
In addition, many other viruses or virus groups cause respiratory infections in children; e.g. parainfluenza viruses can cause a broad spectrum of respiratory diseases, ranging from mild upper respiratory infections to pneumonia, but are most often associated with laryngitis [37]. It seems that human coronavirus infections are not very common cause of respiratory infections in young children [38], whereas later in life, clinical and subclinical infections occur more often [39]. Adenoviruses infrequently cause common colds, and respiratory infections caused by these viruses tend to be severe, characterized by high and prolonged fever and strong inflammatory response [40], [41]. Adenoviruses more often cause occasional epidemics in semi-closed communities, such as garrisons or orphanages [42].
4. Clinical entities and complications of viral respiratory infections in children
A given respiratory viruses may infect widely respiratory mucosa in humans, although it seems that some viruses are more prone to infect specific parts of respiratory tract than others. The various designated respiratory disease entities are in practise partly overlapping and all of them are associated with different respiratory viruses (Table 1 ). In children, the most common viral respiratory infections are simple URIs (the common cold) and AOM.
Table 1.
Respiratory tract infections and viral causative agents in children
| Disease | Adenoviruses | Coronaviruses | Enteroviruses | Influenza viruses | Parainfluenza viruses | RSVa | Rhinoviruses |
|---|---|---|---|---|---|---|---|
| Common cold | + | ++ | ++ | ++ | + | + | +++ |
| Tonsillitis | +++ | − | ++ | + | + | + | − |
| Laryngitis | + | − | + | ++ | +++ | + | + |
| Bronchitis | + | + | + | +++ | ++ | +++ | + |
| Bronchiolitis | + | + | + | ++ | ++ | +++ | ++ |
| Pneumonia | + | + | + | +++ | ++ | +++ | ++ |
RSV: respiratory syncytial virus.
4.1. Common colds
The common cold is almost entirely a viral disease. Mäkelä et al. found respiratory viruses to be associated with two-thirds of common cold events among young adults, while bacteria were cultured in only 4% of cases [35]. Rhinoviruses are the leading cause of common colds in all age groups [6], [25], [35], and by the age of 2 years, most of the children have rhinovirus-specific antibodies [27]. After introduction of the reverse transcription (RT)-PCR technique, it has become evident that enteroviruses are also frequently associated with common colds [11]. Although both RSV and influenza A virus are well known for causing lower respiratory infections, they also generate upper respiratory infections [43].
In adults and especially in children, acute respiratory infections often spread into the paranasal sinuses, causing mucosal edema and accumulation of mucus. A recent study reported that 68% of children with uncomplicated upper respiratory infections had major abnormalities in the paranasal sinuses, which mainly resolved after 2 weeks without antimicrobial treatment [44]. In another study, 70% of children with purulent rhinorrhea as the only symptom had opacification of the paranasal sinuses on computer tomography scans [45]. Young children almost always have nasal secretions in paranasal sinuses during upper respiratory infections (so-called rhinosinusitis). Based on this, sinusitis in children could be considered a natural extension of the common cold. However, evidence of direct viral infection of the maxillary sinus in children is lacking. In adults with acute maxillary sinusitis, a virus can be found in 10% of maxillary secretion samples by viral culture [46] and in 40% by PCR [47].
4.2. Acute otitis media
AOM is one of the most common infectious diseases among children. As many studies during the past decades have shown, viral infection is an important predisposing factor for development of AOM, and viruses are significantly associated with AOMs (Table 2 ) [14], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]. In a prospective study of children less than 2 years of age, about 43% of all upper respiratory infections were associated with AOM [43]. There is little doubt that viruses have an important role in AOM, but it is not yet clear which factors are crucial for the development of AOM. Does the virus need to invade the middle ear and infect the middle ear mucosa, or would inflammation at the nasopharyngeal end of the Eustachian tube be sufficient to interfere with the innate defences of the middle ear mucosa and facilitate bacterial colonization? Viral infections have been shown to cause dysfunction of the Eustachian tube. Two-thirds of children develop abnormal middle ear pressure when they have a common cold [58]. On the other hand, viral infection might facilitate colonization of the nasopharynx with pathogenic bacteria.
Table 2.
Selected data from studies of viruses associated with acute otitis media (AOM)
| Study | No. of children | No. of MEF | Virus detection methoda | Virus infection associated with AOMb (%) | Proportion of virus-positive MEF (%) |
|---|---|---|---|---|---|
| Yoshie (1955) | 10 | 10 | Culture, serology | 40 | 40 |
| Grönroos (1964) | 322 | 399 | Culture | NR | 0 |
| Berglund (1966) | 27 | 44 | Culture, serology | 37 | 33 |
| Tilles (1967) | 90 | NR | Culture, serology | 27 | 3 |
| Klein (1982) | 53 | 53 | Ag | 34 | 25 |
| Chonmaitree (1986) | 84 | 84 | Culture | 39 | 20 |
| Sarkkinen (1985) | 137 | 137 | Ag | 42 | 18 |
| Pitkäranta (1998) | 92 | 92 | RT-PCR | 75 | 48 |
| Heikkinen (1999) | 456 | 815 | Culture, Ag, serology | 41 | 17 |
| Chonmaitree (2000) | 40 | 65 | Culture, PCR | NR | 74 |
| Nokso-Koivisto (2004) | 940 | 3210 | Ag, RT-PCR | 63 | 38 |
NR: not reported.
Ag: antigen detection, RT: reverse transcription, PCR: polymerase chain reaction.
Specific virus detected in nasopharyngeal aspirate (NPA) and/or middle ear fluid (MEF) specimen(s), and/or a viral infection documented serologically from paired serum samples.
In studies using antigen detection, RSV has usually been the most common virus associated with AOM, and RSV has been suggested to be one of the most potent viruses to cause AOM since it is most often detected concurrently in the nasopharyngeal aspirate and middle ear fluid (MEF) during AOM [14], [53]. The development of the PCR method enables detection of respiratory viruses for which antigen detection tests have neither been suitable nor available. By PCR, respiratory viruses have been found to be associated with over 70% of AOM events [51], [55] and by this sensitive method rhinoviruses have been the most common virus to be detected in association with AOM in children [14], [55].
According to recent studies respiratory virus can be detected in more than one-half on AOMs, in 19% of cases virus was the only pathogen and in 25% no pathogen was detected [59]. Ongoing development of detection methods will probably diminish the proportion of pathogen-negative AOMs since bacterial culture techniques are not sensitive enough for low bacterial concentrations [60], and all respiratory viruses are not included in the detection panels. It has been suggested that some virus types or species associate more strongly to the development of AOM than others. However, in a recent study of young children no distinct species-specific associations were observed between the viral and bacterial findings at the time of AOM [59].
For a physician, it would be very beneficial to differentiate viral AOM from bacterial disease. However, thus far, viral and bacterial AOM cannot be separated from each other by clinical symptoms or signs. Only a bulging tympanic membrane has been associated with detection of bacteria or bacterial–viral combinations from the MEF during AOM [61], [62].
4.3. Pharyngitis and tonsillitis
Although often antibiotics are described to children with acute pharyngitis or tonsillitis, many studies have shown that a major part of these infections are due to respiratory viruses. Respiratory viruses have been detected in approximately one-third of children with acute pharyngitis; adenoviruses and RSV being the most common viruses to be found [63]. In a study by Chi et al., among 416 young children (the mean age 52.9 months) with acute pharyngitis respiratory viruses were detected in 30% of patients as group A streptococci were isolated in only 2% of patients [64]. Over 40% of acute tonsillitis cases in children are associated with a respiratory virus, and in one-third a virus may be the sole pathogen. Children younger than 3 years of age have rarely bacterial tonsillitis [65], and usually the younger the child the more common is viral etiology of tonsillitis [65]. Adenovirus is the predominant cause of viral tonsillitis, with other common causative agents being Epstein-Barr viruses, influenza viruses and enteroviruses [65], [66].
4.4. Lower respiratory tract infections
Viruses are common causative agents of lower respiratory tract infections in children [67], with respiratory syncytial virus (RSV), parainfluenza virus type 3, and influenza viruses occurring most frequently [68], [69], [70]. In addition, rhinoviruses have been shown to cause pneumonia and bronchiolitis in infants and children [29]. The recently discovered human metapneumovirus has been reported to induce RSV-like respiratory infections [18], and this virus also produces lower respiratory tract infections in children [71]. Viral infections have been shown to trigger wheezing and exacerbate asthma in children [72], and virus-induced asthma exacerbations may be severe and increase the need of hospitalization [73]. In addition, there is evidence that RSV and especially rhinovirus-induced wheezing in infancy poses increased risk of childhood asthma even until the teenage years [74]. Acute laryngitis is a viral disease most frequently caused by parainfluenza viruses, and less frequently by RSV and influenza viruses [31], [75].
5. Viral–bacterial interactions
Although viruses cause most of the respiratory infections, virus infections can also lead to bacterial infections. It has been recognized that during viral epidemics, e.g. influenza virus epidemics, the incidence of bacterial pneumonia and acute otitis media is increased [8], [76]. Viral infections facilitate bacterial colonization, adherence and translocation through the epithelial barrier of respiratory cells [8]. For example, in an animal model with chinchillas, otitis media developed in 67% of the animals inoculated with Streptococcus pneumoniae and influenza virus type A, whereas otitis media developed in 21% of the animals inoculated with S. pneumoniae alone and in 4% inoculated with influenza virus type A alone [77]. There are multiple potential mechanisms, which lead to increased bacterial adherence to respiratory epithelial cells during viral respiratory disease [8]. Firstly, viral infection causes physical damage to respiratory tract epithelium leading to impaired local defence mechanisms (e.g. loss of cilia and impaired function of Eustachian tube) and basement membrane exposure. Common respiratory viruses are able to cause defects in both cell ciliary structure and function, leading to loss of cilia and loss of ciliated cells from respiratory epithelium. Viral respiratory infections are also known to impair the cough reflex, which can together with the impaired ciliary function lead to accumulation of secretions and to an increased risk for bacterial superinfection [78]. In addition, viral infection in the nasopharynx causes inflammation in the Eustachian tube, which will interfere with the clearing functions of the middle ear mucosa, and hence, facilitate bacterial growth there. Secondly, during virus infection bacterial adherence to epithelium is increased by viral glycoproteins expressed on the host cell, virus-induced changes on host cell membrane and proteins absorbed from extracellular matrix. Inflammatory response to viral infection may up-regulate expression of molecules that bacteria utilize as receptors [8], [79].
Mixed viral–bacterial respiratory infections are relatively common. On average one-fourth of children with community-acquired pneumonia have mixed viral–bacteria infection, and mixed infections seem to be especially common in children less than 2 years of age [78]. In a recent study, viruses and bacteria were detected simultaneously in more than one-third of children with acute otitis media [59]. Mixed viral–bacterial infections often confuse the clinical picture of the disease; prolongation of viral disease may be due to bacterial co-infection or failure of antibiotic treatment of bacterial disease may be due to viral co-infection. For example, viral infection has been suggested to cause prolonged symptoms of AOM [80], and rhinovirus-positive culture in MEF has been associated with persistence of bacteria or occurrence of new bacteria in the MEF [81]. Mixed viral–bacterial infections are also associated with antibiotic treatment failure [8]. It has been reported that in children even with adequate drug compliance virus infection was associated with an increased risk of bacteriologic failure of treated AOM [82], and that penetration of amoxicillin to MEF is lower in children with viral infection [83]. Controversial results have also been published, including a report that the duration of symptoms was longer in children with no detected pathogens in MEF than in children with bacteria or virus [50]. Acute viral tonsillitis or pharyngitis can also lead to a bacterial disease. However, it seems that especially in children, most often viruses or bacteria solely cause acute tonsillitis and mixed viral–bacterial infections are rare [65].
6. Prevention and treatment of viral respiratory infections
Amantadine and the related drug rimantadine can be used for treatment of influenza A virus infections. The newer group of drugs are neuraminidase inhibitors zanamivir and oseltamivir, which are effective against influenza viruses A and B [84]. Both inactivated and live attenuated vaccines have been developed for influenza viruses, but so far in annual vaccinations almost exclusively the intramuscularly administered inactivated vaccines have been used. The intranasally administered influenza vaccine contains live attenuated, cold-adapted influenza A and B viruses, and the vaccine has been approved to be marketed in the United States. A novel adjuvanted inactivated influenza vaccine administered intranasally has been associated with development of Bell's palsy [85]. It has been suggested that the Escherichia coli enterotoxin adjuvant in the vaccine might have been the risk-inducing factor, and that an induced response, rather than some direct toxic effect, led to the palsy [86].
Rhinoviruses are the most common cause for URI in children and therefore prevention or treatment of rhinovirus infections would be the most beneficial. Due to its many different serotypes, developing a vaccine for rhinoviruses is unlikely. Treatment of rhinovirus disease is also problematic because infection often proceeds quickly and medication should be started as soon as the first symptoms occur or shortly thereafter. A capsid-binding agent, pleconaril, also effective against enteroviruses, has been the most promising drug to date and has demonstrated that developing a treatment for the main causes of the common cold is possible [87]. Unfortunately, since further studies revealed that pleconaril might have potential drug interactions, regulatory approval was not granted for the oral formulation of the drug. Other different treatment methods have been investigated, e.g. intranasal interferon [88], intranasally administered receptor decoy soluble intercellular adhesion molecule-1 (tremacamra), 3C protease inhibitors rupintrivir and pyridone, and oral anti-picornavirus capsid-binder pirodavir [10], [87]. However, none of these medications are thus far in clinical use.
Ribavirin has been used as a specific antiviral treatment for RSV infections. It is recommended as a possible treatment only for a selected group of infants at high risk for serious RSV disease [30]. Intravenous, enriched RSV immunoglobulin can be used as prophylaxis for RSV infections in high-risk children or neonates [89]. Prophylactic treatment with palivizumab, a humanized RSV monoclonal antibody, during the epidemic season has been reported to be associated with a decreased rate of hospitalization for RSV lower respiratory tract infections [90]. VP14637 is a new anti-RSV fusion inhibitor that is much more effective than ribavirin in in vitro tests [10]. Candidate vaccines against RSV infections are under development in clinical studies [91].
Live, enteric-coated adenovirus type 4 and 7 vaccines have been developed and used, but the sole manufacturer of these vaccines ceased production, and thus these vaccines are no longer available [4]. Live attenuated human parainfluenza virus 3 vaccine has been studied, and in a clinical trial, it has appeared to be safe and efficient in children [92].
7. Conclusion
Viral respiratory infections are very common in children and are an enormous burden to the families and society. Present knowledge of the natural course and etiology of respiratory infections mainly comes from large community studies conducted several decades ago. Although study settings and virus detection methods have varied between different studies, general characteristics and the main causative viral agents have remained similar over the years. However, recent development of modern microbiologic methods is also changing the world of virology; the knowledge of the respiratory viruses is broadening and even new viruses are being found [19]. Epidemiologic studies of respiratory viruses are important because of prevention and treatments for virus infections are emerging. In addition, new treatment possibilities are posing requirements for fast and sensitive diagnostic methods for respiratory viruses.
As the use of the very sensitive PCR-based detection method has increased, the question has been posed as to whether PCR methods are too sensitive, detecting small remnants of viruses that probably have no clinical relevance. However, according to recent studies, the vast majority of viruses detected by PCR in patients can be assumed to be involved in the initiation of the observed respiratory infection [26], [93]. Detection of a respiratory viral nucleic acid by PCR in the nasopharynx must also be interpreted cautiously because the presence of a virus alone does not establish causality of the concurrent illness. The introduction of PCR methods into clinical practice has resulted in the same questions and problems of clinical relevance as for other diagnostic methods. Laboratory findings must always be interpreted in association with clinical signs and symptoms.
Antibiotics are commonly used to treat AOMs, although a part of AOMs in children could resolve without treatment. Viral respiratory infection precedes the development of AOM, and respiratory virus can be detected in more than one-half on AOMs [14], [51], [55]. Especially rhinovirus, enterovirus and RSV have been found to associate in significant proportion of AOMs in young children [14]. In the future, these virus groups most commonly associated with AOM should be considered when aiming at prevention and treatment of AOM in children.
References
- 1.Frost W.H., Gover M. The incidence and time distribution of common colds in several groups kept under continuous observation. Pub. Health Rep. 1932;47:1815–1841. [Google Scholar]
- 2.McCammon R.W. Natural history of respiratory tract infection patterns in basically healthy individuals. Am. J. Dis. Child. 1971;122(3):232–236. doi: 10.1001/archpedi.1971.02110030090011. [DOI] [PubMed] [Google Scholar]
- 3.Monto A.S., Sullivan K.M. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol. Infect. 1993;110(1):145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray C.G., Holberg C.J., Minnich L.L., Shehab Z.M., Wright A.L., Taussig L.M. Acute lower respiratory illnesses during the first three years of life: potential roles for various etiologic agents. The Group Health Medical Associates. Pediatr. Infect. Dis. J. 1993;12(1):10–14. doi: 10.1097/00006454-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Gonzales R., Malone D.C., Maselli J.H., Sande M.A. Excessive antibiotic use for acute respiratory infections in the United States. Clin. Infect. Dis. 2001;33(6):757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 6.Monto A.S., Ullman B.M. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164–169. [PubMed] [Google Scholar]
- 7.Nurmi T., Salminen E., Ponka A. Infections and other illnesses of children in day-care centers in Helsinki. II. The economic losses. Infection. 1991;19(5):331–335. doi: 10.1007/BF01645358. [DOI] [PubMed] [Google Scholar]
- 8.Hament J.M., Kimpen J.L., Fleer A., Wolfs T.F. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol. Med. Microbiol. 1999;26(3–4):189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 9.Heikkinen T., Järvinen A. The common cold. Lancet. 2003;361(9351):51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ison M., Mills J., Openshaw P., Zambon M., Osterhaus A., Hayden F. Current research on respiratory viral infections: Fourth International Symposium. Antiviral Res. 2002;55(2):227–278. doi: 10.1016/S0166-3542(02)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruohola A., Heikkinen T., Waris M., Puhakka T., Ruuskanen O. Intranasal fluticasone propionate does not prevent acute otitis media during viral upper respiratory infection in children. J. Allergy Clin. Immunol. 2000;106(3):467–471. doi: 10.1067/mai.2000.108912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heikkinen T., Marttila J., Salmi A.A., Ruuskanen O. Nasal swab versus nasopharyngeal aspirate for isolation of respiratory viruses. J. Clin. Microbiol. 2002;40(11):4337–4339. doi: 10.1128/JCM.40.11.4337-4339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waris M. Pattern of respiratory syncytial virus epidemics in Finland: two-year cycles with alternating prevalence of groups A and B. J. Infect. Dis. 1991;163(3):464–469. doi: 10.1093/infdis/163.3.464. [DOI] [PubMed] [Google Scholar]
- 14.Nokso-Koivisto J., Räty R., Blomqvist S., Kleemola M., Syrjanen R., Pitkäranta A., et al. Presence of specific viruses in the middle ear fluids and respiratory secretions of young children with acute otitis media. J. Med. Virol. 2004;72(2):241–248. doi: 10.1002/jmv.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King A.M.Q., Brown F., Christian P., Hovi T., Hyypiä T., Knowles N.J., et al. In: Virus Taxonomy. van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., et al., editors. Academic Press; San Diego: 2000. Picornaviridae; pp. 657–678. [Google Scholar]
- 16.Norder H., Bjerregaard L., Magnius L., Lina B., Aymard M., Chomel J.J. Sequencing of ‘untypable’ enteroviruses reveals two new types, EV-77 and EV-78, within human enterovirus type B and substitutions in the BC loop of the VP1 protein for known types. J. Gen. Virol. 2003;84(Pt 4):827–836. doi: 10.1099/vir.0.18647-0. [DOI] [PubMed] [Google Scholar]
- 17.Oberste M.S., Maher K., Michele S.M., Belliot G., Uddin M., Pallansch M.A. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species human enterovirus A. J. Gen. Virol. 2005;86(Pt 2):445–451. doi: 10.1099/vir.0.80475-0. [DOI] [PubMed] [Google Scholar]
- 18.Hoogen van den B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A., et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouchier R.A., Rimmelzwaan G.F., Kuiken T., Osterhaus A.D. Newer respiratory virus infections: human metapneumovirus, avian influenza virus, and human coronaviruses. Curr. Opin. Infect. Dis. 2005;18(2):141–146. doi: 10.1097/01.qco.0000160903.56566.84. [DOI] [PubMed] [Google Scholar]
- 20.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 21.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 22.Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U.S.A. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ieven M., Goossens H. Relevance of nucleic acid amplification techniques for diagnosis of respiratory tract infections in the clinical laboratory. Clin. Microbiol. Rev. 1997;10(2):242–256. doi: 10.1128/cmr.10.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaye H.S., Marsh H.B., Dowdle W.R. Seroepidemiologic survey of coronavirus (strain OC 43) related infections in a children's population. Am. J. Epidemiol. 1971;94(1):43–49. doi: 10.1093/oxfordjournals.aje.a121293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arruda E., Pitkäranta A., Witek T.J.J., Doyle C.A., Hayden F.G. Frequency and natural history of rhinovirus infections in adults during autumn. J. Clin. Microbiol. 1997;35(11):2864–2868. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nokso-Koivisto J., Kinnari T., Lindahl P., Hovi T., Pitkäranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J. Med. Virol. 2002;66(3):417–420. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blomqvist S., Roivainen M., Puhakka T., Kleemola M., Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J. Med. Virol. 2002;66(2):263–268. doi: 10.1002/jmv.2140. [DOI] [PubMed] [Google Scholar]
- 28.Kim J.O., Hodinka R.L. Serious respiratory illness associated with rhinovirus infection in a pediatric population. Clin. Diagn. Virol. 1998;10(1):57–65. doi: 10.1016/s0928-0197(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 29.Hayden F.G. Rhinovirus and the lower respiratory tract. Rev. Med. Virol. 2004;14(1):17–31. doi: 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh E.E., Graham B.S. In: Dolin R., Wright P.F., editors. vol. 127. Marcel Dekker Inc.; New York: 1999. Respiratory syncytial viruses; pp. 161–204. (Viral Infections of the Respiratory Tract). [Google Scholar]
- 31.Hall C.B. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 2001;344(25):1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 32.Freymuth F., Petitjean J., Pothier P., Brouard J., Norrby E. Prevalence of respiratory syncytial virus subgroups A and B in France from 1982 to 1990. J. Clin. Microbiol. 1991;29(3):653–655. doi: 10.1128/jcm.29.3.653-655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowe J.E., Jr. Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr. Infect. Dis. J. 2004;23(Suppl. 11):S215–S221. doi: 10.1097/01.inf.0000144668.81573.6d. [DOI] [PubMed] [Google Scholar]
- 34.Jartti T., van den Hoogen B., Garofalo R.P., Osterhaus A.D., Ruuskanen O. Metapneumovirus and acute wheezing in children. Lancet. 2002;360(9343):1393–1394. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mäkelä M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpinmäki M., et al. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36(2):539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zambon M.C. Epidemiology and pathogenesis of influenza. J. Antimicrob. Chemother. 1999;44(Suppl. B):3–9. doi: 10.1093/jac/44.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 37.Knott A.M., Long C.E., Hall C.B. Parainfluenza viral infections in pediatric outpatients: seasonal patterns and clinical characteristics. Pediatr. Infect. Dis. J. 1994;13(4):269–273. doi: 10.1097/00006454-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Nokso-Koivisto J., Pitkäranta A., Blomqvist S., Kilpi T., Hovi T. Respiratory coronavirus infections in children younger than two years of age. Pediatr. Infect. Dis. J. 2000;19(2):164–166. doi: 10.1097/00006454-200002000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Macnaughton M.R. Occurrence and frequency of coronavirus infections in humans as determined by enzyme-linked immunosorbent assay. Infect. Immun. 1982;38(2):419–423. doi: 10.1128/iai.38.2.419-423.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawasaki Y., Hosoya M., Katayose M., Suzuki H. Correlation between serum interleukin 6 and C-reactive protein concentrations in patients with adenoviral respiratory infection. Pediatr. Infect. Dis. J. 2002;21(5):370–374. doi: 10.1097/00006454-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Lina B., Valette M., Foray S., Luciani J., Stagnara J., See D.M., et al. Surveillance of community-acquired viral infections due to respiratory viruses in Rhone-Alpes (France) during winter 1994 to 1995. J. Clin. Microbiol. 1996;34(12):3007–3011. doi: 10.1128/jcm.34.12.3007-3011.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan M.A., Gray G.C., Smith B., McKeehan J.A., Hawksworth A.W., Malasig M.D. Large epidemic of respiratory illness due to adenovirus types 7 and 3 in healthy young adults. Clin. Infect. Dis. 2002;34(5):577–582. doi: 10.1086/338471. [DOI] [PubMed] [Google Scholar]
- 43.Vesa S., Kleemola M., Blomqvist S., Takala A., Kilpi T., Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr. Infect. Dis. J. 2001;20(6):574–581. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Kristo A., Uhari M., Luotonen J., Koivunen P., Ilkko E., Tapiainen T., et al. Paranasal sinus findings in children during respiratory infection evaluated with magnetic resonance imaging. Pediatrics. 2003;111(5 Pt 1):e586–e589. doi: 10.1542/peds.111.5.e586. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz R.H., Pitkäranta A., Winther B. Computed tomography imaging of the maxillary and ethmoid sinuses in children with short-duration purulent rhinorrhea. Otolaryngol. Head Neck Surg. 2001;124(2):160–163. doi: 10.1067/mhn.2001.112879. [DOI] [PubMed] [Google Scholar]
- 46.Hamory B.H., Sande M.A., Sydnor A., Jr., Seale D.L., Gwaltney J.M., Jr. Etiology and antimicrobial therapy of acute maxillary sinusitis. J. Infect. Dis. 1979;139(2):197–202. doi: 10.1093/infdis/139.2.197. [DOI] [PubMed] [Google Scholar]
- 47.Pitkäranta A., Arruda E., Malmberg H., Hayden F.G. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J. Clin. Microbiol. 1997;35(7):1791–1793. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshie C. On the isolation of influenza virus from mid-ear discharge of influenza otitis media. Jpn. J. Med. Sci. Biol. 1955;8:373–377. doi: 10.7883/yoken1952.8.373. [DOI] [PubMed] [Google Scholar]
- 49.Berglund B., Salmivalli A., Toivanen P. Isolation of respiratory syncytial virus from middle ear exudates of infants. Acta Otolaryngol. 1966;61(6):475–487. doi: 10.3109/00016486609127086. [DOI] [PubMed] [Google Scholar]
- 50.Chonmaitree T., Howie V.M., Truant A.L. Presence of respiratory viruses in middle ear fluids and nasal wash specimens from children with acute otitis media. Pediatrics. 1986;77(5):698–702. [PubMed] [Google Scholar]
- 51.Chonmaitree T., Henrickson K.J. Detection of respiratory viruses in the middle ear fluids of children with acute otitis media by multiplex reverse transcription polymerase chain reaction assay. Pediatr. Infect. Dis. J. 2000;19(3):258–260. doi: 10.1097/00006454-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 52.Grönroos J.A., Kortekangas A.E., Ojala L., Vuori M. The aetiology of acute middle ear infection. Acta Otolaryngol. 1964;58:149–158. doi: 10.3109/00016486409121372. [DOI] [PubMed] [Google Scholar]
- 53.Heikkinen T., Thint M., Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N. Engl. J. Med. 1999;340(4):260–264. doi: 10.1056/NEJM199901283400402. [DOI] [PubMed] [Google Scholar]
- 54.Klein B.S., Dollete F.R., Yolken R.H. The role of respiratory syncytial virus and other viral pathogens in acute otitis media. J. Pediatr. 1982;101(1):16–20. doi: 10.1016/s0022-3476(82)80172-8. [DOI] [PubMed] [Google Scholar]
- 55.Pitkäranta A., Virolainen A., Jero J., Arruda E., Hayden F.G. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102(2 Pt 1):291–295. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 56.Sarkkinen H., Ruuskanen O., Meurman O., Puhakka H., Virolainen E., Eskola J. Identification of respiratory virus antigens in middle ear fluids of children with acute otitis media. J. Infect. Dis. 1985;151(3):444–448. doi: 10.1093/infdis/151.3.444. [DOI] [PubMed] [Google Scholar]
- 57.Tilles J.G., Klein J.O., Jao R.L., Haslam J.E., Jr., Feingold M., Gellis S.S., et al. Acute otitis media in children. Serologic studies and attempts to isolate viruses and mycoplasmas from aspirated middle-ear fluids. N. Engl. J. Med. 1967;277(12):613–618. doi: 10.1056/NEJM196709212771202. [DOI] [PubMed] [Google Scholar]
- 58.Winther B., Hayden F.G., Arruda E., Dutkowski R., Ward P., Hendley J.O. Viral respiratory infection in schoolchildren: effects on middle ear pressure. Pediatrics. 2002;109(5):826–832. doi: 10.1542/peds.109.5.826. [DOI] [PubMed] [Google Scholar]
- 59.Kleemola M., Nokso-Koivisto J., Herva E., Syrjanen R., Lahdenkari M., Kilpi T., et al. Is there any specific association between respiratory viruses and bacteria in acute otitis media of young children? J. Infect. 2006;52(3):181–187. doi: 10.1016/j.jinf.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Virolainen A., Salo P., Jero J., Karma P., Eskola J., Leinonen M. Comparison of PCR assay with bacterial culture for detecting Streptococcus pneumoniae in middle ear fluid of children with acute otitis media. J. Clin. Microbiol. 1994;32(11):2667–2670. doi: 10.1128/jcm.32.11.2667-2670.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arola M., Ruuskanen O., Ziegler T., Mertsola J., Nanto-Salonen K., Putto-Laurila A., et al. Clinical role of respiratory virus infection in acute otitis media. Pediatrics. 1990;86(6):848–855. [PubMed] [Google Scholar]
- 62.McCormick D.P., Lim-Melia E., Saeed K., Baldwin C.D., Chonmaitree T. Otitis media: can clinical findings predict bacterial or viral etiology? Pediatr. Infect. Dis. J. 2000;19(3):256–258. doi: 10.1097/00006454-200003000-00019. [DOI] [PubMed] [Google Scholar]
- 63.Esposito S., Blasi F., Bosis S., Droghetti R., Faelli N., Lastrico A., et al. Aetiology of acute pharyngitis: the role of atypical bacteria. J. Med. Microbiol. 2004;53(Pt 7):645–651. doi: 10.1099/jmm.0.05487-0. [DOI] [PubMed] [Google Scholar]
- 64.Chi H., Chiu N.C., Li W.C., Huang F.Y. Etiology of acute pharyngitis in children: is antibiotic therapy needed? J. Microbiol. Immunol. Infect. 2003;36(1):26–30. [PubMed] [Google Scholar]
- 65.Putto A. Febrile exudative tonsillitis: viral or streptococcal? Pediatrics. 1987;80(1):6–12. [PubMed] [Google Scholar]
- 66.Douglas R.M., Miles H., Hansman D., Fadejevs A., Moore B., Bollen M.D. Acute tonsillitis in children: microbial pathogens in relation to age. Pathology (Phila) 1984;16(1):79–82. doi: 10.3109/00313028409067915. [DOI] [PubMed] [Google Scholar]
- 67.Woensel van J.B., van Aalderen W.M., Kimpen J.L. Viral lower respiratory tract infection in infants and young children. BMJ. 2003;327(7405):36–40. doi: 10.1136/bmj.327.7405.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juvén T., Mertsola J., Waris M., Leinonen M., Meurman O., Roivainen M., et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr. Infect. Dis. J. 2000;19(4):293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Tsai H.P., Kuo P.H., Liu C.C., Wang J.R. Respiratory viral infections among pediatric inpatients and outpatients in Taiwan from 1997 to 1999. J. Clin. Microbiol. 2001;39(1):111–118. doi: 10.1128/JCM.39.1.111-118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weigl J.A., Puppe W., Gröndahl B., Schmitt H.J. Epidemiological investigation of nine respiratory pathogens in hospitalized children in Germany using multiplex reverse-transcriptase polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 2000;19(5):336–343. doi: 10.1007/s100960050490. [DOI] [PubMed] [Google Scholar]
- 71.Williams J.V., Harris P.A., Tollefson S.J., Halburnt-Rush L.L., Pingsterhaus J.M., Edwards K.M., et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 2004;350(5):443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L., et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gern J.E., Busse W.W. The role of viral infections in the natural history of asthma. J. Allergy Clin. Immunol. 2000;106(2):201–212. doi: 10.1067/mai.2000.108604. [DOI] [PubMed] [Google Scholar]
- 74.Hyvärinen M.K., Kotaniemi-Syrjänen A., Reijonen T.M., Korhonen K., Korppi M.O. Teenage asthma after severe early childhood wheezing: an 11-year prospective follow-up. Pediatr. Pulmonol. 2005 doi: 10.1002/ppul.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gröndahl B., Puppe W., Hoppe A., Kuhne I., Weigl J.A., Schmitt H.J. Rapid identification of nine microorganisms causing acute respiratory tract infections by single-tube multiplex reverse transcription-PCR: feasibility study. J. Clin. Microbiol. 1999;37(1):1–7. doi: 10.1128/jcm.37.1.1-7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heikkinen T., Ruuskanen O., Waris M., Ziegler T., Arola M., Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am. J. Dis. Child. 1991;145:445–448. doi: 10.1001/archpedi.1991.02160040103017. [DOI] [PubMed] [Google Scholar]
- 77.Giebink G.S., Berzins I.K., Marker S.C., Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect. Immun. 1980;30(2):445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korppi M. Mixed microbial aetiology of community-acquired pneumonia in children. APMIS. 2002;110(7–8):515–522. doi: 10.1034/j.1600-0463.2002.11007801.x. [DOI] [PubMed] [Google Scholar]
- 79.Peltola V.T., McCullers J.A. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr. Infect. Dis. J. 2004;23(Suppl. 1):S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 80.Arola M., Ziegler T., Ruuskanen O. Respiratory virus infection as a cause of prolonged symptoms in acute otitis media. J. Pediatr. 1990;116(5):697–701. doi: 10.1016/s0022-3476(05)82650-2. [DOI] [PubMed] [Google Scholar]
- 81.Sung B.S., Chonmaitree T., Broemeling L.D., Owen M.J., Patel J.A., Hedgpeth D.C., et al. Association of rhinovirus infection with poor bacteriologic outcome of bacterial–viral otitis media. Clin. Infect. Dis. 1993;17(1):38–42. doi: 10.1093/clinids/17.1.38. [DOI] [PubMed] [Google Scholar]
- 82.Patel J.A., Reisner B., Vizirinia N., Owen M., Chonmaitree T., Howie V. Bacteriologic failure of amoxicillin–clavulanate in treatment of acute otitis media caused by nontypeable Haemophilus influenzae. J. Pediatr. 1995;126(5 Pt 1):799–806. doi: 10.1016/s0022-3476(95)70415-9. [DOI] [PubMed] [Google Scholar]
- 83.Canafax D.M., Yuan Z., Chonmaitree T., Deka K., Russlie H.Q., Giebink G.S. Amoxicillin middle ear fluid penetration and pharmacokinetics in children with acute otitis media. Pediatr. Infect. Dis. J. 1998;17(2):149–156. doi: 10.1097/00006454-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 84.Meissner H. Antiviral drugs for prophylaxis and treatment of influenza. Pediatr. Infect. Dis. J. 2001;20(12):1165–1167. doi: 10.1097/00006454-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 85.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T., et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 86.Couch R.B. Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N. Engl. J. Med. 2004;350(9):860–861. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]
- 87.Hayden F.G., Herrington D.T., Coats T.L., Kim K., Cooper E.C., Villano S.A., et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 2003;36(12):1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayden F.G., Albrecht J.K., Kaiser D.L., Gwaltney J.M., Jr. Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon. N. Engl. J. Med. 1986;314(2):71–75. doi: 10.1056/NEJM198601093140202. [DOI] [PubMed] [Google Scholar]
- 89.Meissner H.C., Welliver R.C., Chartrand S.A., Fulton D.R., Rodriguez W.J., Groothuis J.R. Prevention of respiratory syncytial virus infection in high risk infants: consensus opinion on the role of immunoprophylaxis with respiratory syncytial virus hyperimmune globulin. Pediatr. Infect. Dis. J. 1996;15(12):1059–1068. doi: 10.1097/00006454-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 90.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3 Pt 1):531–537. [PubMed] [Google Scholar]
- 91.Polack F.P., Karron R.A. The future of respiratory syncytial virus vaccine development. Pediatr. Infect. Dis. J. 2004;23(1):S65–S73. doi: 10.1097/01.inf.0000108194.71892.95. [DOI] [PubMed] [Google Scholar]
- 92.Karron R.A., Belshe R.B., Wright P.F., Thumar B., Burns B., Newman F., et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr. Infect. Dis. J. 2003;22(5):394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- 93.Jartti T., Lehtinen P., Vuorinen T., Koskenvuo M., Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J. Med. Virol. 2004;72(4):695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]


