Abstract
The relative lack of efficient methods for evaluating antiseptic antiviral activity, together with weaknesses in the existing European Standard (i.e. NF EN 14476+A1), underlines the need to seek a new method which could allow a more precise evaluation of the antiseptic antiviral activity of chemical agents.
This protocol is based on an original gel-based filtration method, using “in-house” G-25 and G-10 Sephadex™ columns. This method allows the neutralization of both the activity and the cytotoxicity of a large range of molecules, according to their molecular size, in only 1 min.
The viral model used was the human coronavirus (HCoV) 229E chosen for (i) its increasing medical interest, (ii) its potential resistance and (iii) its representing enveloped viruses mentioned in the European Standard.
First, the protocol was validated and it was demonstrated that it was fully operational for evaluating antiviral antiseptic potentiality and useful to screen potentially antiseptic molecules.
Second, chlorhexidine (CHX) and hexamidine (HXM) were assessed for their potential anti-HCoV 229E antiseptic activities. It was demonstrated clearly that (i) HXM had no activity on the HCoV 229E and (ii) CHX showed a moderate anti-HCoV 229E activity but insufficient to be antiseptic.
Abbreviations: ATS–D, antiseptics–disinfectants; BSA, bovine serum albumin; CC50, 50% cytotoxic concentration; CCID50, 50% cell culture infective dose; CHX, chlorhexidine; CPE, cytopathic effect; EBSS, Earle's balanced salt solution; EDTA, ethylene diamine tetracetic acid; FCS, fetal calf serum; FW, formula weight; HCoV, human coronavirus; HXM, hexamidine; IC50, 50% inhibitory concentration; MEM, minimum essential medium; MTT, methyl thiazol tetrazolium; NR, neutral red; PBS, phosphate buffered saline; SARS-CoV, severe acute respiratory syndrome associated coronavirus; TB, trypan blue
Keywords: Antiseptic evaluation, Sephadex™, Gel filtration, Human coronavirus, Chlorhexidine, Hexamidine
1. Introduction
Antiviral antisepsis and disinfection are crucial for preventing the environmental spread of viral infections. Indeed, very few efficient and specific treatments are available to control most of these infections. Furthermore, emerging viruses and associated diseases as well as nosocomial viral infections have become an important issue in medical fields.
The Coronaviridae family, an enveloped RNA virus family, is a representative example of this issue. Since the 1960s, only two human pathogen members of this family have been identified: the Human coronavirus, strain 229E (HCoV 229E) and the Human coronavirus, strain OC43 (HCoV OC43). Known to be responsible for a large proportion of common colds (Hamre and Procknow, 1966, Holmes, 2001, Larson et al., 1980), they have been implicated in more serious diseases, along with the newly identified HCoV: the NL63 strain (Van der Hoek et al., 2004) and the HKU1 strain (Woo et al., 2005). Indeed, these viruses have been recognized as causing lower respiratory tract infections, (Vabret et al., 2003, Van Elden et al., 2004). They have also been involved in nosocomial viral infections, especially in young children and neonates (Gagneur et al., 2002a, Gagneur et al., 2002b, Moes et al., 2005, Vabret et al., 2008), in the elderly (Falsey et al., 2002, Nicholson et al., 1997) and in immunocompromised patients (Pene et al., 2003).
Furthermore, the outbreak of severe acute respiratory syndrome (SARS) in 2002–2003, responsible for the first worldwide epidemic of the 21st century, was due to a newly discovered coronavirus, the SARS associated coronavirus (SARS-CoV) (Drosten et al., 2003, Ksiazek et al., 2003, Peiris et al., 2003). This outbreak led to a new awareness of the medical importance of the Coronaviridae family.
Another interesting feature of coronaviruses is their potential environmental resistance, despite the accepted fragility of enveloped viruses. Indeed, a HCoV 229E survival of 70% after 72 h at 20 °C and 50% relative humidity has been observed, as well as a HCoV 229E survival of 90% at 6 °C and a relative humidity ≥50%, which could account for airborne transmission and for winter epidemics of HCoV 229E, respectively (Ijaz et al., 1985). Another study showed 30% and 50% of virus residual infectivity for the HCoV 229E and the HCoV OC43, respectively, after 6 days in suspension in phosphate buffered saline (PBS) at 37 °C and 30% of residual HCoV 229E infectivity after 3 h of drying on different supports found in hospital settings, i.e. aluminium, sterile sponges or latex surgical gloves (Sizun et al., 2000). More recently, numerous studies have underlined an important SARS-CoV survival under various conditions: in serum, in 1:20 diluted sputum, in faeces for at least 96 h and in urine for at least 72 h. SARS-CoV also retained infectivity at 4 °C, at room temperature and at 37 °C for at least 2 h (Duan et al., 2003, Rabenau et al., 2005). Furthermore, some studies have shown the transfer of viruses from contaminated hosts (hands, nasal mucosa) to surfaces and from environmental surfaces to hands and therefore a potential contamination of susceptible hosts (Sattar et al., 1993, Winther et al., 2007). Another study has also shown a longer survival of a herpesvirus on human skin than on an inanimate carrier (i.e. a stainless steel disk) (Graham et al., 1996). Thus, considering the potential resistance of HCoV for some hours in the environment, their pathogenesis and the absence of specific treatment, an efficient skin antisepsis, to stop eventual dissemination by the hands of healthcare workers, is a meaningful way to fight virus transmission and associated diseases.
A fundamental point is to evaluate the efficiency of antiseptics–disinfectants (ATS-D) on viruses properly. The principle of antiseptic antiviral activity evaluation is (i) to collect viruses and the product under test for an appropriately defined and precise contact time, (ii) to neutralize product activity, i.e. stopping product activity and removing its potential cytotoxicity, and (iii) to estimate the loss of viral infectivity due to the product activity. These tests require appropriate controls especially to check the absence of interference due to the test itself on viral infectivity, the efficiency of neutralization, the removal of cytotoxicity, and also, to establish reproducible and well defined conditions, e.g. contact time, temperature, interfering substances (Fig. 1 ).
Fig. 1.
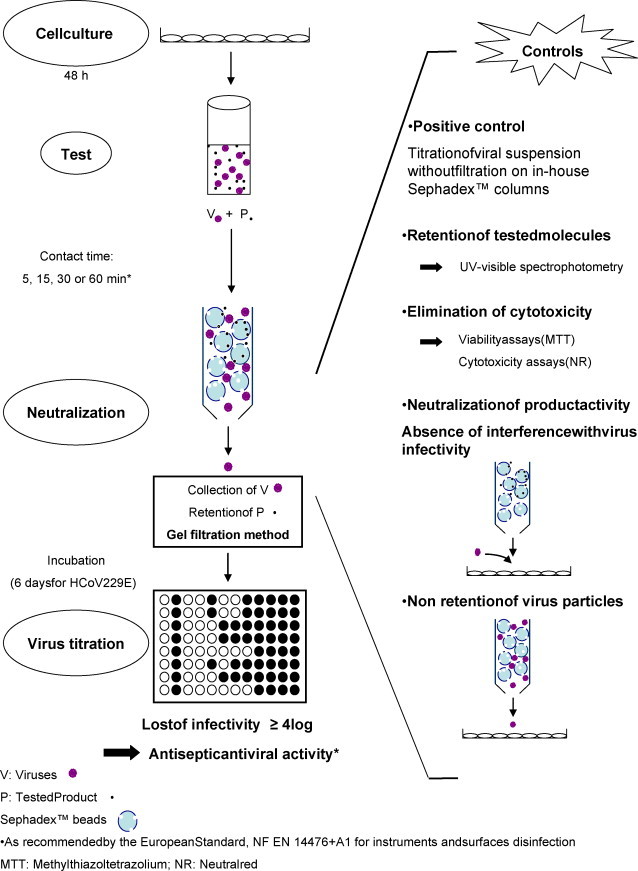
Principle of the evaluation of antiviral antiseptic activity.
To date, only one European Standard (NF EN 14476+A1) on virucidal ATS-D activity testing in human Medicine was published in January 2007 (AFNOR, 2007). It specifies the test method and minimum requirements to establish virucidal activity according to the potential use of the products tested, e.g. disinfection of surfaces and instruments, hygienic hand wash or thermo-chemical disinfection. Virus strains, temperatures, contact times, interfering substances are specified for each potential use. According to this standard, a product is considered to have an ATS-D antiviral activity if it induces a loss of infectivity of at least 4 log10 in viral titers during an accurate contact time.
Some parameters need to be extended to improve antiviral ATS-D activity testing. For instance, one proposed method in the European Standard to neutralize product activity is a 1:10 dilution of product/virus mix in iced medium. However, considering the very large range of ATS-D nature (single molecules or a mixture of different ones), this method is not always reliable and furthermore, it may lengthen contact time. The European Standard suggests other methods, based on gel-filtration techniques, using products as Sephadex™ LH-20, Microspin™ columns S400HR or Minicon® concentrators, but they lead to problems of contact times, separation capacity and cost (supplementary data, Fig. 1).
With these aims in mind, a protocol was developed that allows neutralization, in only 1 min, of the activity and cytotoxicity of a large range of molecules, according to their molecular size. This procedure also involves a gel-filtration-based method but uses 2 other types of Sephadex™ gels: Sephadex™ G-10 and G-25. Their retention capacities are [100–1000] g mol−1 and [900–5000] g mol−1, respectively, which is consistent with the molecular mass of most ATS-D (supplementary data, Fig. 1B). To validate this protocol and establish its potential limits, antiseptic antiviral activities of two ATS-D, the chlorhexidine (CHX) and the hexamidine (HXM), were tested on HCoV 229E.
2. Materials and methods
2.1. Reagents and disposable materials
Minimum essential medium (MEM; 41090.093, fetal calf serum (FCS; 10270098), trypsine-EDTA (25300-054), Earle's balanced salt solution (EBSS; 24010043), trypan blue (TB; 15250-061) and MEM without glutamine and without phenol red (51200-046) were purchased at Invitrogen (Cergy Pontoise, France). For culturing cells, 75 cm2 cell culture flasks (831813) and 96-well flat-bottom culture plates (831835) were purchased at Sarstedt (Marnay, France). May-Grünwald solution (1014240500) and Giemsa solution (1092040500) were bought from Merck (Darmstadt, Germany). Chemicals as dimethylsulfoxyde (D2438), neutral red (NR; N4638), sodium dodecyl sulfate (S9625) and formaldehyde at 36.5% (v/v) (533998) were bought at Sigma (Saint Quentin Fallavier, France); calcium chloride (CaCl2, 33604.261) at Prolabo, (Fontenay sous Bois, France) and acetic acid (45726) at Fluka biochemical (Saint Quentin Fallavier, France). One milliliter syringes (300013) were from Becton Dickinson (Le Pont de Claix, France) and 0.22 μm filters (SLGP033RS) from Millipore Express (Saint Quentin en Yvelines, France). Sephadex™ G-25 and the Sephadex™ G-10 (17-0010-01 and 17-0033-01, respectively) were purchased at GE Healthcare (Saclay, France). Finally, CHX digluconate and HXM diisethionate were kindly provided by J.-B. Regnouf-de-Vains (SRSMC, Nancy-University, France).
2.2. Cell culture and virus
Human embryonic pulmonary epithelial cells, L-132 cells (ATCC CCL-5), kindly provided by Dr H.F. Hildebrand (Faculty of Medicine, Lille, France), were grown in MEM supplemented with 5% of FCS, at 37 °C in a humidified atmosphere containing 5% CO2. They were split 2–3 times a week. Cells, grown in 75 cm2 cell culture flasks were washed 3 times with Phosphate Buffered Saline (PBS) and 0.5 mL of trypsine-EDTA was added. Flasks were incubated for a few minutes at 37 °C with 5% CO2 and 4.5 mL of MEM 5% FCS were added to achieve cell dissociation. Stock cells were prepared with the cellular suspension obtained after trypsinization and mixed with MEM containing 10% (v/v) FCS and 10% (v/v) dimethylsulfoxyde. They were then aliquoted and conserved at −196 °C in liquid nitrogen.
Human coronavirus, strain 229E (ATCC VR 740), kindly provided by Dr. S.A. Sattar (Faculty of Medicine, Ottawa, Canada) was cultivated on L-132 cells, in MEM supplemented with 2% FCS, at 33 °C in a humidified atmosphere containing 5% CO2. 33 °C has been shown to be the optimal growth temperature for the HCoV 229E (Bradburne, 1972). Stock viruses were prepared by infection of 75 cm2 cell culture flasks with a confluent monolayer of L-132 cells, not much older than 3 days. When confluence was reached, medium was removed and cells were washed 3 times with PBS. Cells were then inoculated with 1 mL of stock virus suspension diluted at 1:5 in EBSS and incubated for adsorption virus time (i.e. 1 h), at 33 °C with 5% CO2. Culture flasks were then filled out with 11 mL of MEM 2% FCS and re-incubated at 33 °C with 5% CO2 for 48 h, before the cytopathic effect (CPE, i.e. cell lysis), was completely achieved. Three cycles of freezing/thawing of 15 min each were performed in order to liberate virions. Media were centrifuged at 2000 × g for 5 min to eliminate cellular fragments and, supernatants containing viruses were aliquoted in cryotubes and conserved at −80 °C.
2.3. End point dilution method for virus titration
L-132 cells were seeded at 5 × 103 cells/well in 96-well flat-bottom culture in MEM 5% FCS. Cell counts were performed with the TB exclusion method. Cells were incubated at 37 °C in 5% CO2, for 48 h to obtain a confluent monolayer. After removing medium, 180 μL of fresh MEM 2% FCS were added to each well. Cells were then infected at a multiplicity of 0.2, and 10-fold serial dilutions were carried out. Infected cells were incubated at 33 °C in 5% CO2 for 6 days to obtain the optimal CPE. At the end of the incubation period, cells were fixed and stained with May-Grünwald and Giemsa. Fifty microliters/well of May-Grünwald solution were allowed to fix and stain cells for 5 min and after a washing step with tap water, 50 μL/well of Giemsa solution were added for 15 min to achieve the final staining before a last washing step with tap water. Infected wells were counted under a phase-contrast inverted microscope (Olympus, Rungis, France) and viral titers or 50% cell culture infective dose (CCID50), expressed as viral infectious particles mL−1, were estimated according to the Reed and Muench method (Reed and Muench, 1938).
2.4. Molecules tested
All molecules tested were prepared, extemporaneously, as aqueous solutions and filter-sterilized on 0.22 μm filters.
2.4.1. Chlorhexidine
Chlorhexidine (CHX) is a bisbiguanide, used widely as antiseptic and disinfectant. It has been used at concentrations from 0.05% (m/v), i.e. 5.6 × 10−4 mol L−1, in aqueous solutions for skin damage disinfection to 0.5% (m/v), i.e. 5.6 × 10−3 mol L−1, in alcoholic solutions for pre-operative skin disinfection. A CHX salt, the CHX digluconate, was used because of its greater water solubility and its commercial use under this form. Its molecular weight is 897.80 g mol−1. The aqueous solution is colourless.
2.4.2. Hexamidine
Hexamidine (HXM) is also used widely for skin disinfection and external use. It belongs to the aromatic diamidine group. HXM is insoluble in water; therefore a HXM diisethionate salt solution, of which the water solubility limit is 55 g L−1 (Fleurette, 1995), is also used in commercial formulations as in this study. In commercial formulations, HXM is used at 0.1% (m/v), i.e. 1.6 × 10−3 mol L−1, or at 0.15% (m/v), i.e. 2.4 × 10−3 mol L−1. Its molecular weight is 606.72 g mol−1. The aqueous solution is colourless.
2.5. Cytotoxicity and viability tests
MTT viability assays and NR cytotoxicity assays were carried out to evaluate the cellular impact of molecules tested on L-132 cells. In both assays, L-132 cells were seeded into 96-well culture plates in MEM with 5% FCS. They were incubated at 37 °C with 5% CO2 for 48 h prior to the addition of the appropriate dilutions of the product to be tested and then re-incubated for 24 h, 48 h and 168 h.
For MTT tests, a modified Mosmann's protocol was used (Mosmann, 1983). After medium removal, 100 μL/well of PBS and 10 μL of MTT solution at 5 mg mL−1 in PBS were added. After 4 h of incubation at 37 °C in 5% CO2, 100 μL of sodium dodecyl sulfate were added to dissolve formazan dark blue crystals produced by reduction of MTT by succinate mitochondrial deshydrogenase. Plates were re-incubated for 4 h and measurements were performed with a scanning multiwell spectrophotometer (ELISA reader, Multiscan EX, Thermo Fisher Scientific, Saint Herblain, France) using a test wavelength of 540 nm and a reference wavelength of 690 nm. Cell viability was validated, which is proportional to the produced quantity of formazan crystals and expressed as 50% inhibitory concentration (IC50, mol L−1).
NR assays were performed according to a modified Borenfreund's protocol (Borenfreund and Puerner, 1985). An aqueous stock solution at 4 mg mL−1 of NR, subjected to a centrifugation at 405 × g for 10 min to eliminate eventual aggregates, was prepared. For the assay, a “ready to use” solution of NR at 50 μg mL−1 in MEM without glutamine and without phenol red was prepared extemporaneously to avoid fine precipitates which could form when NR is mixed with medium. After medium removal, cells were rinsed with 200 μL of PBS/well prior to the addition of 200 μL/well of “use” NR solution. Plates were re-incubated for 3 h at 37 °C in 5% CO2 to allow the uptake of the dye by living cells. The dye-medium was then taken off and cells were washed with 200 μL/well of “A” solution [“A” solution: 4% (v/v) formaldehyde at 36.5% (v/v), 1% (m/v) CaCl2 in distilled water]. To release NR accumulated in lysosomes of living cells, 200 μL/well of “B” solution were added [“B” solution: 1% (v/v) acetic acid, 50% (v/v) absolute ethanol in distilled water]. The quantity of NR released was estimated by spectrophotometric measurement at 540 nm in a scanning multiwell spectrophotometer. It was proportional to the cytotoxicity of molecules tested and expressed as 50% cytotoxic concentration (CC50, mol L−1).
These tests were also performed with solutions of CHX and HXM at various concentrations and after their filtration on the “in-house” Sephadex™ columns to evaluate their retention and the elimination of potential cytotoxicity.
2.6. Evaluation of antiviral antiseptic activity
2.6.1. Preparation of Sephadex™ media
Sephadex™ media are a range of cross-linked dextran gels of variable porosity according to the degree of cross-linking. This property, based on the principle of exclusion-diffusion, allows the separation of different molecules according to their molecular size. They are manufactured in a bead form and need to be suspended in a buffer. Two different kinds of Sephadex™ were used in this protocol, the Sephadex™ G-25 and the Sephadex™ G-10, which have resolution capacities of 900–5000 g mol−1 and 100–1000 g mol−1, respectively.
According to the manufacturer's instructions, Sephadex™ G-10 and G-25 should be suspended for minimum 3 h at room temperature, in at least 20 times their volume in PBS (i.e. about 30 g L−1), to equilibrate the pH at 7 and to allow the gel to swell. Suspensions were sterilized by autoclaving and excess of PBS was eliminated to obtain a suspension with a volume ratio of Sephadex™/PBS of 1/1.
2.6.2. Preparation of the “in-house” Sephadex™ columns
Columns were made up with a 1 mL syringe, stuffed with carded cotton, associated with a drilled Eppendorf type 1.5 mL microtube; each part was sterilized by autoclaving. The body of the syringe was filled with 1 mL of sterile Sephadex™ gel, prepared as described above. The system with the syringe and the Eppendorf tube was placed into a 50 mL conic bottom centrifuge tube to conserve sterility and centrifuged at 4500 × g for 1 min. The definitive “in-house” Sephadex™ column was then ready (supplementary data, Fig. 2).
2.6.3. Assay for antiviral antiseptic activity
Ninety-six-well flat bottom culture plates were seeded at 5 × 103 cells/well in MEM 5% FCS and incubated at 37 °C in humidified atmosphere with 5% CO2 for 48 h to obtain a confluent monolayer.
After 2 days of incubation, the appropriate dilution of the product to be tested was prepared in distilled water and filter-sterilized on a 0.22 μm filter.
Viral suspensions of HCoV 229E were allowed to thaw; 200 μL were mixed with 1.8 mL of the diluted product and allowed to stay in contact for the desired contact time. To separate viruses and the product, 500 μL of this mixture were placed into the “in-house” Sephadex™ column, 30 s before the end of the contact time, to leave sufficient time to centrifuge. The system was placed in a 50 mL centrifuge tube to maintain sterility and centrifuged at 405 × g for 1 min. This centrifugation retained the product according to its molecular size and the kind of Sephadex™ used and to recover viruses in the filtrates. Viral titers were then evaluated with the end point dilution method (Section 2.3). The potential loss of HCoV infectivity was estimated by the difference between viral titer of positive control, which was also subjected to filtration on the “in-house” Sephadex™ columns but without the presence of the product; and viral titer obtained after contact with the product tested for desired contact time and neutralization by filtration on the “in-house” Sephadex™ columns.
2.6.4. Control experiments
Three types of controls were performed in each experiment to validate the results: (i) non-retention of viruses after filtration on Sephadex™ columns, (ii) neutralization of the potential antiviral activity of the product tested, and (iii) elimination of its cytotoxicity. As the European Standard (NF EN 14476+A1) states, the difference in viral titer before and after treatment, (i.e. in this case, gel filtration), without product tested, should not excess 0.5 log10. To assess the non-retention of viruses, e.g. HCoV 229E, viral suspensions were mixed with sterile distilled water for the contact time defined for the experiment, filtered on the “in-house” Sephadex™ columns and titrated as describe above (Section 2.3).
Neutralization assays were performed by filtration of 500 μL of the solution to be tested on the “in-house” Sephadex™ columns and 180 μL of the filtrates were mixed with 20 μL of viral suspension. This mixture was then inoculated to the L-132 cells monolayers. The viral titer obtained should be equivalent to viral titer without filtration (i.e. a log10 difference ≤0.5), since the product should be retained in the column. This experiment also verified that filtrates did not have any influence on virus infectivity.
Cytotoxicity controls were also carried out for each experiment, as a complement to MTT and NR assays undertaken previously. These controls were performed by filtration of 500 μL of the product tested and then filtrates were inoculated on L-132 cells. After 6 days of incubation, cells monolayers were observed under an inverted microscope and any sign of cytotoxicity should be visible.
2.7. UV-spectrophotometry
Retention of the molecules tested by the “in-house” Sephadex™ columns was also confirmed by UV–visible spectrophotometry. The range of dilutions was assayed to establish specific spectrophotometric parameters: maximum absorption wavelength λ max, molar absorption coefficient ɛ and detection limits, for each molecule.
Dilutions were prepared in distilled water to mimic the test conditions. Spectra were performed on a UV–visible spectrophotometer (UVmc2, Safas, Monaco) within a 180–640 nm window. Calibration curves (Aλ max = f(c)) were established to determine the molar absorption coefficient (ɛ) for each molecule. Different dilutions of products tested were prepared in distilled water and filtered through the “in-house” Sephadex™ columns. Their absorbances were then measured at λ max to determine the residual concentration.
2.8. Statistical analysis
To validate this process, statistical analysis of the different steps was undertaken using Statview® Version 5.0. For all tests, the maximum acceptable risk was 5%. To assess homogeneity of columns, i.e. volumes of gel after the first centrifugation and volumes of filtrate after the second centrifugation, Kolmogorov–Smirnov tests were used to assure distribution normality of each independent tested batch, and then Bartlett's tests were used to compare variances. In case of variances in homogeneity, an ANOVA test was used to compare means of the different batches; in case of non-homogeneity of variances, a non-parametric test of Kruskall–Wallis was used to compare averages. Finally, correlation studies, using a Fisher transformation, were realised to estimate whether there was an association gel volumes and either filtrates volumes or residual concentrations after filtration on Sephadex™.
For UV-spectrophotometry, regression analysis (A = f(c)) was carried out to establish specific spectrophotometric parameters of each molecule tested.
3. Results
3.1. Validation of the protocol for antiseptic antiviral activity testing
3.1.1. Reproducibility of the “in-house” Sephadex™ column fabrication and retention of dye molecules
As specified in Section 2.6.2, two centrifugation steps were needed: (i) to prepare optimized columns of 0.5 mL of gel, whether for Sephadex™ G-25 or Sephadex™ G-10 and (ii) to filter the drug-virus mixture to achieve the neutralization step with retention of molecules tested, non-retention of viruses and homogeneous quantity of filtrates. Different speeds and times of centrifugation were then tested and best parameters were determined: 1 min at 4500 × g for the first centrifugation and 1 min at 405 × g for the second centrifugation.
The homogeneity of (i) gel volumes obtained after the first centrifugation and (ii) filtrate volumes obtained after the second centrifugation; was first established on 4 independent batches of 10 columns for each Sephadex™ gel, by statistical analysis using Statview® V. 5.0 (Section 2.8).
Retention rates of 2 dye molecules were measured: the TB (960.83 g mol−1) for the Sephadex™ G-25 and the NR (288.78 g mol−1) for the Sephadex™ G-10. To determine this rate, the spectrophotometric parameters of TB and NR (Table 1 ) were established by measuring absorbances of concentrations ranging from 10−7 mol L−1 to 4.1 × 10−4 mol L−1 for TB and from 5 × 10−7 mol L−1 to 5 × 10−4 mol L−1 for NR, followed by a regression analysis (Section 2.8). The residual concentrations of filtrates were estimated by measuring absorbances in UV–visible and calculating the retention rate according to the formula: retention rate = [(Ci − Cf)/Cf] × 100, where Ci was the initial concentration or concentration before filtration and Cf was the final concentration or the residual concentration after filtration.
Table 1.
UV–visible spectrophotometric parameters of molecules tested and retention rates. Molecules tested were suspended in sterile distilled water (pH 7). Calibration curves were established for each molecule on a UV–visible spectrophotometer within a 180–640 nm wavelength window. The concentrations tested ranged from 10−7 mol L−1 to 10−2 mol L−1 except for trypan blue (TB) and neutral red (NR) for which concentrations ranged from 10−7 mol L−1 to 4.1 × 10−4 mol L−1 and from 5 × 10−4 mol L−1 to 5 × 10−4 mol L−1, respectively. Regression analyses were then performed to determine specific spectrophotometric parameters: (i) linearity limits (mol L−1), (ii) minimum detection limits (mol L−1); within these limits, (iii) the specific maximal absorption wavelength (λmax, nm) and (iv) the specific molar absorption coefficient (ɛ, L mol−1 cm−1). Retention rates (see formula in Section 3.1.1) of each molecule by the specified type of Sephadex™ media are indicated, i.e. TB and chlorhexidine (CHX) Retention rates by Sephadex™ G-25; NR and hexamidine (HXM) Retention rates by Sephadex™ G-10. Ci corresponds to the initial concentration or concentration before filtration. Absorption wavelength used to estimate the retention rate is specified each time it was necessary.
| Molecules tested | Specific spectrophometric parameters for each molecule tested |
Retention rate |
||||
|---|---|---|---|---|---|---|
| λmax (nm) | ɛ (L cm−1 mol−1) | Linearity limits (mol L−1) | Detection limit (mol L−1) | Ci (mol L−1) | Retention rates (%) | |
| Sephadex™ G-25 | ||||||
| TB | 234 | 2.0 × 104 | [2.5 × 10−6 to 10−4] | 5 × 10−7 | 4.1 × 10−4 | 589 nm: 95.11 ± 2.35 (n = 20) |
| 317 | 1.1 × 104 | [2.5 × 10−6 to 10−4] | 7.5 × 10−7 | 4.1 × 10−3 | 589 nm: 92.06 ± 3.43 (n = 20) | |
| 589 | 2.9 × 104 | [5 × 10−7 to 7.5 × 10−5] | 5 × 10−7 | |||
| CHX | 232 | 2.6 × 104 | [10−6 to 2.5 × 10−4] | 10−6 | 10−4, 10−3 | 232 nm, 255 nm: >99a (n = 6) |
| 255 | 2.5 × 104 | [10−6 to 10−4] | 10−6 | 10−2 | 232 nm: 79.73 ± 4.37 (n = 6) | |
| 255 nm: 81.27 ± 1.28 (n = 6) | ||||||
| Sephadex™ G-10 | ||||||
| NR | 276 | 3.5 × 104 | [5 × 10−7 to 5 × 10−5] | 5 × 10−7 | 10−4, 10−3 | 276 nm, 452 nm: >99.5a (n = 20) |
| 452 | 1.0 × 104 | [5 × 10−7 to 5 × 10−5] | 5 × 10−7 | |||
| HXM | 261 | 2.1 × 104 | [10−6 – 10−4] | 10−6 | 10−4, 10−3 | 261 nm: >99a (n = 6) |
| 10−2 | 261 nm: < 90b (n = 6) | |||||
Below the detection limit.
Above the linearity limits.
Retention rates obtained for TB after filtration on Sephadex™ G-25 were (95.11 ± 2.35)% and (92.06 ± 3.43)% after filtration of TB solutions at 4.1 × 10−4 mol L−1 and 4.1 × 10−3 mol L−1 (which was the commercial concentration), respectively. For NR solutions, either at 10−4 mol L−1 or 10−3 mol L−1, after filtration on Sephadex™ G-10, concentrations were under the detection threshold. Thus, the retention rates obtained were >99.5% after filtration of NR solutions at 10−4 mol L−1 and 10−3 mol L−1.
It was found that, within a range of [450–550]μL of gel volumes after the first centrifugation, there was no significant variation in filtrate volumes and in retention rates. In further experiments, all gel volumes were included within these limits.
3.1.2. Non-retention of virus particles by the “in-house” Sephadex™ columns
Basically, the “in-house” Sephadex™ columns should retain the product but not viruses. According to the European Standard NF EN 14476+A1 (AFNOR, 2007), the difference between viral titer before and after treatment, i.e. here, after filtration, should not exceed 0.5 log10. To ensure this non-retention of viruses, 3 independent experiments for each Sephadex™ type and for each contact time tested, were performed. The HCoV 229E stock suspension was diluted 1:10 in sterile distilled water and filtered on the “in-house” Sephadex™ columns. The viral titers were compared with and without filtration (Table 2 ). For Sephadex™ G-10, log10 differences between viral titers were 0.1 ± 0.1, 0.3 ± 0.4 and 0.2 ± 0.2 for contact times of 0 min, 30 min and 60 min, respectively (n = 3 for each contact time). For Sephadex™ G-25, log10 differences between viral titers were 0.2 ± 0.2, 0.4 ± 0.2, 0.1 ± 0.3, 0.1 ± 0.2 and 0.0 ± 0.2 for contact times of 0 min, 5 min, 15 min, 30 min and 60 min, respectively (n = 6 for each contact time). Consequently, Sephadex™ columns of each Sephadex™ type did not retain HCoV 229E and did not induce a loss of infectivity.
Table 2.
Non-retention of HCoV 229E by the “in-house” Sephadex™ columns. Results, expressed as log10 of 50% cell culture infective dose (CCID50), represent the average of viral titers of at least 3 independent experiments. These assays allowed to evaluate the non-retention of the human coronavirus 229E (HCoV 229E), for each Sephadex™ type and each contact time tested. Viral suspensions were diluted at 1:10 in sterile distilled water for the specified contact time before filtration on the “in-house” Sephadex™ columns and inoculation to the L-132 cells. nd: not determined.
| Contact time (min) | Sephadex™ G-10 |
Sephadex™ G-25 |
||||
|---|---|---|---|---|---|---|
| Without filtration | After filtration | Difference | Without filtration | After filtration | Difference | |
| 0 | 5.8 ± 0.5 (n = 3) | 5.7 ± 0.6 (n = 3) | 0.1 ± 0.1 (n = 3) | 6.0 ± 0.6 (n = 6) | 5.8 ± 0.6 (n = 6) | 0.2 ± 0.2 (n = 6) |
| 5 | nd | nd | nd | 5.9 ± 0.7 (n = 6) | 5.6 ± 0.6 (n = 6) | 0.4 ± 0.2 (n = 6) |
| 15 | nd | nd | nd | 5.6 ± 0.5 (n = 6) | 5.6 ± 0.4 (n = 6) | ± 0.3 (n = 6) |
| 30 | 6.7 ± 0.9 (n = 3) | 6.4 ± 0.3 (n = 3) | 0.3 ± 0.4 (n = 3) | 6.1 ± 0.2 (n = 6) | 6.0 ± 0.4 (n = 6) | 0.1 ± 0.2 (n = 6) |
| 60 | 6.7 ± 0.9 (n = 3) | 6.4 ± 0.7 (n = 3) | 0.2 ± 0.2 (n = 3) | 6.1 ± 0.2 (n = 6) | 6.1 ± 0.1 (n = 6) | 0.0 ± 0.2 (n = 6) |
3.1.3. Elimination of cytotoxicity of molecules tested (CHX and HXM) by filtration on the “in-house” Sephadex™ columns
Elimination of cytotoxicity was a crucial control before inoculation of filtrates onto the cells. It allowed the assessment of antiviral activity, eliminating an eventual cellular impact of the product on L-132 cells. MTT and NR assays were carried out to determine IC50 and CC50, respectively. The IC50 and CC50 of CHX and HXM solutions (without filtration) were first determined on L-132 cells (Section 2.4). Then, IC50 and CC50 of CHX and HXM solutions, at different concentrations and after filtration on the “in-house” Sephadex™ columns, were determined to make sure of the elimination of the eventual cytotoxicity by the filtration on the “in-house” Sephadex™ columns. Each experiment was repeated 3 times independently to find the IC50 and CC50 corresponding to the average results of those 3 experiments. As shown in Table 3 , IC50 of CHX on L-132 cells were (4.3 ± 0.6) × 10−6 mol L−1, (2.4 ± 1.3) × 10−6 mol L−1 and (3.9 ± 0.2) × 10−6 mol L−1 at 24 h, 48 h and 168 h, respectively, and CC50 were (5.6 ± 1.1) × 10−6 mol L−1, (2.9 ± 1.3) × 10−6 mol L−1 and (3.4 ± 0.5) × 10−6 mol L−1 at 24 h, 48 h and 168 h, respectively. After filtration of CHX solutions at 10−3 mol L−1 and 10−4 mol L−1 on Sephadex™ G-25 columns, CC50 and IC50 were >10−4 mol L−1, according to the detection limit of the method, except at 168 h, where IC50 was >5.6 × 10−5 and CC50 >5.4 × 10−5 mol L−1 after filtration of 10−3 mol L−1 CHX solution.
Table 3.
Elimination of potential cytotoxicity by filtration on the “in-house” Sephadex™ columns. 50% inhibitory concentration (IC50) and 50% cytotoxic concentration (CC50) of chlorhexidine (CHX) and hexamidine (HXM), without filtration, on L-132 cells were evaluated with methyl thiazol tetrazolium (MTT) assays and neutral red (NR) assays, respectively (in bold). IC50 and CC50 of (i) CHX solutions at 10−3 mol L−1 and 10−4 mol L−1, and (ii) of HXM solutions at 10−2 mol L−1 and 10−3 mol L−1, after filtration on the “in-house” Sephadex™ G-25 and Sephadex™ G-10 columns, respectively, were then evaluated. Results, expressed in mol L−1, represent the average of 3 independent experiments.
| Ci | 24 h | 48 h | 168 h | |
|---|---|---|---|---|
| CHX | ||||
| IC50 | Without filtration | (4.3 ± 0.6) × 10−6 | (2.4 ± 1.3) × 10−6 | (3.9 ± 0.2) × 10−6 |
| 10−3 mol L−1 | >10−4 | >10−4 | >5.6 × 10−5 | |
| 10−4 mol L−1 | >10−5 | >10−5 | >10−5 | |
| CC50 | Without filtration | (5.6 ± 1.1) × 10−6 | (2.9 ± 1.3) × 10−6 | (3.4 ± 0.5) × 10−6 |
| 10−3 mol L−1 | >10−4 | >10−4 | >5.4 × 10−5 | |
| 10−4 mol L−1 | >10−5 | >10−5 | >10−5 | |
| HXM | ||||
| IC50 | Without filtration | (3.8 ± 1.0) × 10−5 | (1.2 ± 1.4) × 10−5 | (3.8 ± 2.5) × 10−6 |
| 10−2 mol L−1 | (5.3 ± 1.4) × 10−5 | (1.0 ± 0.5) × 10−5 | (5.9 ± 1.4) × 10−6 | |
| 10−3 mol L−1 | >10−4 | >10−4 | >10−4 | |
| CC50 | Without filtration | (6.2 ± 1.4) × 10−5 | (2.5 ± 1.5) × 10−5 | (5.6 ± 0.1) × 10−6 |
| 10−2 mol L−1 | (6.2 ± 1.3) × 10−5 | (3.2 ± 2.2) × 10−5 | (5.7 ± 0.3) × 10−6 | |
| 10−3 mol L−1 | >10−4 | >10−4 | >5.5 × 10−5 | |
The same experiments were carried out with HXM. IC50 of HXM without filtration were (3.8 ± 1.0) × 10−5 mol L−1, (1.2 ± 1.4) × 10−5 mol L−1 and (3.8 ± 2.5) × 10−6 mol L−1 at 24 h, 48 h and 168 h, respectively, and CC50 were (6.2 ± 1.4) × 10−5 mol L−1, (2.5 ± 1.5) × 10−5 mol L−1 and (5.6 ± 0.1) × 10−6 mol L−1 at 24 h, 48 h and 168 h, respectively. After filtration of HXM solution at 10−3 mol L−1 on the Sephadex™ G-10 columns, IC50 and CC50 were >10−4 mol L−1, except at 168 h where CC50 was >5.5 × 10−5 mol L−1. HXM solution at 10−2 mol L−1 was also assayed. IC50 and CC50 were shown to be very close to those without filtration (Table 3) and thus retention was shown to be incomplete for this concentration.
Consequently, the “in-house” Sephadex™ columns clearly demonstrated that they efficiently eliminate cytotoxicity of solutions at concentrations ≤10−3 mol L−1.
3.1.4. CHX and HXM retention rates (UV–visible spectrophotometry) after filtration on the “in-house” Sephadex™ columns
As for dye molecules (TB and NR), specific spectrophotometric parameters and retention rates of CHX and HXM were determined using UV–visible spectrophotometry (Section 2.7). Testing different dilutions of CHX, i.e. from 10−10 mol L−1 to 10−4 mol L−1, the specific spectrophotometric parameters of CHX were established (Table 1). Two maximum absorption wavelengths (λ max) at 232 nm and 255 nm were observed, with molecular absorption coefficients of 2.6 × 104 L cm−1 mol−1 and 2.5 × 104 L cm−1 mol−1, respectively. These coefficients were obtained by means of a regression analysis realized with Statview® V.5.0 (n = 15 measurements, p < 0.0001). The relation A = f(c) was linear between [10−6 to 2.5 × 10−4] mol L−1 for λ max = 232 nm (correlation coefficient = 0.998) and between [10−6 to 10−4] mol L−1 for λ max = 255 nm (correlation coefficient = 0.996). The detection limit was 10−6 mol L−1 for both of the 2 λ max. These results were consistent with data from the literature (Bonazzi et al., 1995, European Pharmacopeia, 2007a, Ha and Cheung, 1996, Havlikova et al., 2007, Hebert et al., 2003), where UV detection, especially after high performance liquid chromatography, was done either at 235 nm or between 254 nm and 258 nm. The retention rates of CHX solutions were then evaluated at different concentrations by the “in-house” Sephadex™ G-25 columns (Table 1). Three independent experiments were realized in duplicate (n = 6). A retention rate >99% was observed for CHX solutions at 10−4 mol L−1 and 10−3 mol L−1. CHX solution at 10−2 mol L−1 was not completely retained and retention rates were of (79.7 ± 4.4)% at 232 nm and (81.3 ± 1.3)% at 255 nm.
In the same way, HXM was assayed from 10−10 mol L−1 to 10−3 mol L−1 (Table 1). The λ max was observed at 261 nm associated to a molar absorption coefficient of 2.1 × 104 L cm−1 mol−1, with a linear relationship between [10−6 to 10−4] mol L−1 (n = 11, p < 0.0001, correlation coefficient = 0.980). The detection limit of HXM with UV–visible spectrophotometry is 10−6 mol L−1. These data were consistent with the European Pharmacopeia, which recommends HXM detection at 263 nm (European Pharmacopeia, 2007b). Hexamidine retention rates by the “in-house” Sephadex™ G-10 columns were evaluated. They were >99% for HXM at 10−4 mol L−1 and 10−3 mol L−1. As for other molecules, retention limits were reached for HXM at 10−2 mol L−1 and retention rate could not be determined accurately because residual concentrations were above the linearity limits of detection, i.e. 10−4 mol L−1. Thus, the retention rate was <90%.
3.1.5. Neutralization of potential antiviral antiseptic activity of products tested
Verifying neutralization control was essential to assess the stop of potential antiviral activity and the respect of the defined contact time. Furthermore, this test ensured that there was no interference of the filtrates with virus infectivity (Fig. 1). As recommended by the European Standard NF EN 14476+A1, the difference between virus control and neutralization control should not excess 0.5 log10 (Section 2.6.4). Neutralization controls were done for each independent experiment (each product concentration and each contact time). Neutralization assays were performed for CHX at 10−4 mol L−1 and 10−3 mol L−1. The mean of log10 differences obtained (n = 6 independent assays for each concentration) were 0.2 ± 0.1 and 0.2 ± 0.2, respectively. The same assays were carried out with HXM at 10−3 mol L−1 and the mean of log10 difference obtained (n = 3 independent assays) was 0.2 ± 0.3. Thus, potential antiviral antiseptic activity was correctly neutralized for each molecule and for each concentration tested. Furthermore, no interference with virus infectivity was observed.
3.2. Evaluation of the potential antiviral antiseptic activity of CHX and HXM
Assays were performed as described in Section 2.6.3. Each assay corresponded to the evaluation of potential ATS anti-HCoV activity of one concentration of each molecule tested and one contact time. Each assay was repeated 3 independent times and included necessary controls, i.e. positive virus control, non-retention of HCoV, efficiency of neutralization and elimination of cytotoxicity, to validate the test (Fig. 1).
CHX was tested at 2 concentrations, 10−4 mol L−1 and 10−3 mol L−1 and each concentration was tested during 4 different contact times, i.e. 5 min, 15 min, 30 min and 60 min (Fig. 2A). The log10 reduction in viral titers induced by the action of CHX at 10−4 mol L−1 was of 0.8 ± 0.7, 0.5 ± 0.4, 1.4 ± 1.5 and 2.1 ± 1.2 for 5 min, 15 min, 30 min and 60 min, respectively. At the concentration of 10−3 mol L−1, CHX induced a reduction of 1.4 ± 0.8, 2.1 ± 0.4, 2.4 ± 0.6 and 3.0 ± 0.2 for contact times of 5 min, 15 min, 30 min and 60 min, respectively. According to the European Standard NF EN 14476+A1, the log reduction is lower than 4 log10, so, even if CHX had a certain activity on the HCoV 229E, it had no ATS anti-HCoV 229E properties in these conditions.
Fig. 2.
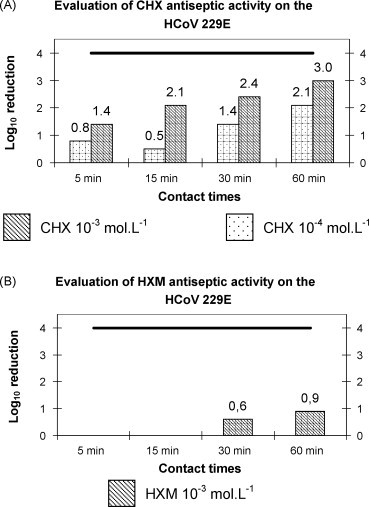
Evaluation of antiseptic antiviral activity of CHX and HXM on the HCoV 229E. This figure shows the log10 reduction in viral titers obtained after the action of chlorhexidine (CHX) and hexamidine (HXM) at different concentrations and at specified contact times: (A) represents the log10 reduction in Human coronavirus 229E (HCoV 229E) titers obtained after action of CHX at 10−3 mol L−1 ( ) and 10−4 mol L−1 (
) and 10−4 mol L−1 ( ) and (B) represents the log10 reduction in HCoV 229E titers obtained after action of HXM at 10−3 mol L−1 (
) and (B) represents the log10 reduction in HCoV 229E titers obtained after action of HXM at 10−3 mol L−1 ( ). The bold line in both graphs represents the threshold of 4 log10 reduction to reach to pretend to an antiviral antiseptic activity.
). The bold line in both graphs represents the threshold of 4 log10 reduction to reach to pretend to an antiviral antiseptic activity.
HXM showed an even worse activity on the HCoV 229E. At 10−3 mol L−1, it induced only a reduction of 0.6 ± 0.5 log10 and 0.9 ± 0.8 log10 for 30 min and 60 min of contact time, respectively, so very far from the threshold recommended by the European Standard (Fig. 2B). Given these results, concentration 10−4 mol L−1 and contact times of 5 min and 15 min were not tested.
4. Discussion
To fight viral infections, ATS-D seem crucial considering: (i) the relative lack of efficient treatments and vaccines available, and (ii) the risk of outbreak of new viral pathogens. Thus, it is essential to evaluate their antiviral activity properly and reliably. The major items proposed in the European Standard NF EN 14476+A1 were followed, such as (i) contact times, (ii) the maximum of 0.5 log10 differences between control viral titers (Section 2.6.4) to validate the assays and (iii) the 4 log10 reduction to assess an antiseptic antiviral activity of drugs tested.
However, some parameters of this standard needed to be intensified, especially the neutralization method and the choice of virus strain. Indeed, the neutralization step is fundamental: (i) to ensure a precise contact time and (ii) to remove any potential cytotoxicity before inoculation to the cells, which could involve confusion in product activity estimation. In the European Standard NF EN 14476+A1, two global techniques are proposed: dilution in iced medium and gel filtration techniques, but they involve different problems such as lengthening of contact times, efficiency and also cost. In the dilution method, the neutralization step requires 30 min, inconsistent with recommended contact times for the different applications mentioned in the European Standard. Also, not all contact times are suited to the potential use of the product, e.g. 60 min is the mandatory contact time for testing disinfectants for surfaces and instruments and facultative times are 5 min, 15 min and 30 min, but in practice, a surface would rarely be disinfected during 60 min. Furthermore, dilution has not always been shown to be efficient. For instance, the IC50 and CC50 of CHX on L-132 cells are higher than 2 × 10−6 mol L−1 at 24 h, 48 h and 168 h. So even after a 1:100 dilution of a 10−3 mol L−1 solution of CHX (the European Standard recommends a 1:10 dilution or 1:100 if not efficient), the solution remains cytotoxic.
Nonetheless, the European Standard also proposes gel filtration techniques to neutralize product activity, using Sephadex™ LH-20, an ultrafiltration system (Minicon® Millipore) or pre-packed columns Microspin™ S400 HR. The major problem of these methods is the lengthening of contact time. Indeed, they need at least 2 min to achieve the neutralization step, sometimes 10 min or more, depending on the techniques. Furthermore, separation capacities are not well adapted to the molecular mass of most ATS-D. Another drawback of Minicon® concentrators is that they are non-sterile devices and do not bear sterilization, so they cannot be used to filter viruses.
The American Standard (ASTM E 1482-04) recommends also a gel filtration method, using Sephadex™ LH-60 or Sephacryl™ superfine S-1000, to neutralize virucidal activity (ASTM, 2004). The virucidal efficacy criterion for the ASTM standard is a log10 reduction >3. The ASTM procedure is close to the one developed here. However, the centrifugation time: (i) to eliminate the void and (ii) to separate the product tested and viruses is 3 min instead of 1 min with the “in-house” Sephadex™ columns. The first gel proposed, Sephadex™ LH-60, has well adapted retention capacities to most ATS-D molecular mass (supplementary data, Fig. 1B). Unfortunately, it is not sold anymore. The other gel proposed, Sephacryl™ superfine S-1000, is commercialized in a suspension form in distilled water containing 20% ethanol as conservative. The presence of ethanol could affect the tests, emphasizing the effect of the ATS-D tested so leading to an overestimation of the product activity. The cost was also higher than the “in-house” Sephadex™ G-25 or G-10 columns and its retention capacity was not well adapted (supplementary data, Fig. 1).
The choice of virus strain could also be discussed and has recently been questioned by Ijaz and Rubino (2008). The European Standard NF EN 14476+A1 proposed 3 viruses: a poliovirus (type 1, LSc-2ab), an adenovirus (type 5) and a bovine parvovirus (Haden strain). Poliovirus was chosen for its potential high resistance to chemicals but taking into account the worldwide program of poliomyelitis eradication, the legitimacy of this choice is questionable. In the same way, the choice of B19 parvovirus and adenovirus is arguable. Although B19 parvovirus is responsible for serious but rare materno-foetal infections and also infections in immunocompromised patients and/or patients with a haemolytic anaemic disorder, it has not been shown to be a frequent nosocomial pathogen. Its interest, however, lies in its high resistance to chemicals and to a wide range of pH and temperatures and its potential blood transmission route (Morinet et al., 2003). However, the strain used in the European Standard is a bovine parvovirus, so there is a question of representativeness. In contrast, Human serovar 5 adenovirus is more interesting notably for its responsibility in mild upper respiratory tract infections, ophthalmologic infections and also gastro-enteritis, particularly in new-borns and young children. It can cause rare but serious (mortality up to 50%) infections in immunodepressed patients, especially in bone-marrow transplanted patients (Carret and Le Faou, 2001). It has also probably been chosen for its high resistance in environmental conditions. All these viruses are naked viruses and not always representative of majors risks encountered in community or in hospital settings.
When establishing this protocol, the first challenge was to elaborate a unique protocol for both Sephadex™ gels, in order to make assays easier to perform. The best parameters for the 2 needed centrifugation steps were determined for conserving the sterility during the entire assay. These parameters were first validated through statistical analysis, without drugs tested, to ensure the repeatability of columns yield and to fix the acceptability limits of the protocol. Thus, this protocol was made to perform neutralization in only 1 min, which was better than all the other methods.
Therefore, a different virus to those proposed in the European Standard was chosen: the HCoV 229E, not only for its medical interest but also for its presence in the annexe B of the European Standard NF EN 14476-A1 as potential contaminant for surfaces, instruments and hands. Furthermore, it belongs to enveloped viruses, which are absent from the standard and which may behave differently to naked viruses under the action of ATS-D. It was also chosen for its relative resistance in different conditions taking into account that enveloped viruses are most often considered fragile.
During the assays to show the non-retention of the HCoV 229E by the “in-house” Sephadex™ columns, whether G-25 or G-10, the importance of pH was highlighted as an important cause of variability in results, i.e. in viral titers. These difficulties were experienced, mainly with Sephadex™ G-10 that needed excess of solvent (i.e. PBS) when swelling to reach neutrality, and also with sterile distilled water, owing to the sterilization process, which often involved acidification. This could be due to a high sensitivity of the HCoV 229E to the pH. Rapid loss of infectivity and an aggregation of virions have been shown at pH 8 and 37 °C, whereas the virus was quite stable at pH 6 and 37 °C (Sturman et al., 1990). So pH should be verified and adjusted to the neutrality to allow a standardization of the protocol.
To test this protocol, anti-HCoV 229E activity of CHX and HXM were evaluated because of their wide use and their molecular size, which allowed testing both types of Sephadex™, G-25 and G-10. First, they were shown to be retained through the “in-house” Sephadex™ columns and their potential cytotoxicity was removed. Furthermore, the neutralization technique did not interfere with virus infectivity.
At this stage, some limits of the gel filtration method in the concentration of the product tested appeared. Above concentrations of 10−2 mol L−1, molecules were not retained completely and there was a phenomenon of column overloading. However, this did not seem serious, since products are used rarely at this concentration or above, notably because of their potential toxicity.
At this stage, the anti-coronavirus activity of CHX and HXM could be assessed. Thus, the results demonstrated clearly that HXM had no ATS-D activity on the HCoV 229E, but to our knowledge, little data concerning a possible ATS-D antiviral activity of HXM is available in the literature.
In addition the results showed that despite activity on the HCoV 229E, CHX could not pretend to have ATS-D activity on the HCoV 229E in testing conditions and according to the European Standard. However, according to the American Standard, of which the virucidal efficacy criterion is a 3 log10 reduction, CHX had an ATS-D antiviral activity at 10−3 mol L−1 and 60 min, but this activity is not really representative of field use conditions. In most studies, CHX has been shown: (i) to be inefficient against naked viruses (Bernstein et al., 1990, Kawana et al., 1997, Narang and Codd, 1983, Wood and Payne, 1998) and (ii) to be efficient at concentrations >10−3 mol L−1 on enveloped viruses but its activity depends on viruses tested and testing conditions (Bernstein et al., 1990, Kawana et al., 1997, Platt and Bucknall, 1985, Tyler and Ayliffe, 1987, Wood and Payne, 1998). For instance, CHX reduced Herpes simplex virus viral titers of >3 log10 in suspension tests for under 3 min (Kawana et al., 1997, Wood and Payne, 1998) and only of 1 log10 on a dried sample of HSV in 10 min (Tyler and Ayliffe, 1987). It is also important to note that most antiseptics and other chemicals are used as a mix of different molecules and the diluent, often ethanol, has its own virucidal activity. For instance, different formulations containing chlorhexidine digluconate were assayed for ATS antiviral activity. It did not respond to the American virucidal criterion when tested at 0.008% (m/v), i.e. 8.9 × 10−5 mol L−1 with cetrimide at 0.08% (m/v). However, it induced a log10 reduction >3 in HCoV viral titers when tested at 0.05% (m/v), i.e. 5.6 × 10−4 mol L−1, with cetrimide at 0.5% (m/v) and ethanol at 70% (Sattar et al., 1989).
This data showed that testing conditions must be taken into account to compare the antiseptic antiviral activity, i.e. suspension or carrier tests, presence or not of interfering substances and the chosen criterion to assess a virucidal activity. The possibility of misestimating product activity due to different testing conditions makes it essential to determine the best parameters so as to be as close as possible to the real conditions of application.
The validation of this protocol was the first step in the improvement of antiviral ATS-D activity evaluation and there is still a long way to go to get the perfect test. As expected, the goal of this method is to eliminate or at least reduce to the lowest possible level, the concentration of products tested in order to evaluate, with precise contact time, their antiviral potentiality, without toxicity for host cell and without modifying virus infectivity.
Even if this protocol had been validated by means of an in vitro suspension test, it could be extrapolated to in vitro carrier tests, in vivo or ex vivo protocols. Such protocols can use support as plastic, steel disk (Sattar et al., 2003), fingertips (Sattar and Ansari, 2002) or disks of human skin removed during plastic surgery (Graham et al., 1996). Carrier tests are recommended in different Standards to simulate at best field situation, where viruses are dried and often embedded in organic material limiting the access to ATS-D and thus their potential activity. Ex vivo protocols are of importance to test antiviral ATS activity of either pathogenic viruses or toxic compounds that, for healthy and ethic reasons, could not be assayed with in vivo protocols, using for instance, fingertips of volunteers. Furthermore, they are also useful to evaluate pathogen survival on human skin. Carrier tests need a dilution step to recover the dried virus suspension from the support after action of a chemical. This dilution step plays the role of neutralizer and ensures the contact time. It could be achieved by addition of culture medium or any chemical, which could neutralize specifically product activity (e.g. use of sodium thiosulfate for neutralizing povidone-iodine). However, the diluent itself could be toxic for the cells. Thus, this method could be applied in such a case to eliminate the chemical neutralizer and inoculate the viral suspension safely. This potential is also found in the ASTM standard E 1482-04.
This study describes a global method of chemical neutralization when ensuring the non-retention of viruses and the maintenance of their infectivity, in 1 min. Therefore, this method could be used to test chemicals for different applications from surfaces to water disinfectants. It could be used in the human medical field, as well as in veterinary or industrial (e.g. food industry) applications and also to screen the antiseptic antiviral potency of new drugs.
Acknowledgements
We sincerely thank Dr. S.A. Sattar (Centre for Research on Environmental Microbiology, Faculty of Medicine, Ottawa, Canada) for kindly providing us with the HCoV 229E; Dr. H.F. Hildebrand (Groupe de Recherche sur les Biomatériaux, Laboratoire de Biophysique, UPRES EA 1049, Faculty of Medicine, Lille, France) who furnished us L-132 cells and also Pr. J.B. Regnouf-de-Vains and his team (Groupe d’Etude des Vecteurs Supramoléculaires du Médicament, UMR 7565, Faculté de Pharmacie, Nancy, France) for providing us with CHX and HXM molecules. We would also like to thank Mrs. N. Marshall for editing this manuscript.
Footnotes
Supplementary data associated with this article can be found at doi:10.1016/j.jviromet.2009.03.023.
Appendix A. Supplementary data
References
- AFNOR, 2007. Chemical disinfectants and antiseptics—virucidal quantitative suspension test for chemical disinfectants and antiseptics used in human medicine—test method and requirements (phase 2, step 1).
- ASTM, 2004. Standard test method for neutralization of virucidal agents in virucidal efficacy evaluations.
- Bernstein D., Schiff G., Echler G., Prince A., Feller M., Briner W. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. J. Dent. Res. 1990;69:874–876. doi: 10.1177/00220345900690030901. [DOI] [PubMed] [Google Scholar]
- Bonazzi D., Andrisano V., Gatti R., Cavrini V. Analysis of pharmaceutical creams: a useful approach based on solid-phase extraction (SPE) and UV spectrophotometry. J. Pharm. Biomed. Anal. 1995;13:1321–1329. doi: 10.1016/0731-7085(95)01536-t. [DOI] [PubMed] [Google Scholar]
- Borenfreund E., Puerner J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Bradburne A.F. An investigation of the replication of coronaviruses in suspension cultures of L132 cells. Arch. Gesamte Virusforsch. 1972;37:297–307. doi: 10.1007/BF01241452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carret A.S., Le Faou A. Adénovirus. In: Pozzetto B., editor. Les Infections Nosocomiales Virales et à Agents Transmissibles Non Conventionnels. John Libbey Eurotext; Paris: 2001. pp. 237–245. [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Duan S.M., Zhao X.S., Wen R.F., Huang J.J., Pi G.H., Zhang S.X., Han J., Bi S.L., Ruan L., Dong X.P. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003;16:246–255. [PubMed] [Google Scholar]
- European Pharmacopeia, 2007. Chlorhexidine digluconate (solution of).
- European Pharmacopeia, 2007. Hexamidine diisethionate.
- Falsey A.R., Walsh E.E., Hayden F.G. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J. Infect. Dis. 2002;185:1338–1341. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleurette J. Diamidines aromatiques. In: Fleurette J., Freney J., Reverdy M.-E., editors. Antisepsie et Désinfection. Edition ESKA; Paris: 1995. pp. 320–324. [Google Scholar]
- Gagneur A., Legrand M.C., Picard B., Baron R., Talbot P.J., de Parscau L., Sizun J. Nosocomial infections due to human coronaviruses in the newborn. Arch. Pediatr. 2002;9:61–69. doi: 10.1016/S0929-693X(01)00696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneur A., Sizun J., Vallet S., Legr M.C., Picard B., Talbot P.J. Coronavirus-related nosocomial viral respiratory infections in a neonatal and paediatric intensive care unit: a prospective study. J. Hosp. Infect. 2002;51:59–64. doi: 10.1053/jhin.2002.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.L., Springthorpe V.S., Sattar S.A. Ex vivo protocol for testing virus survival on human skin: experiments with herpesvirus 2. Appl. Environ. Microbiol. 1996;62:4252–4255. doi: 10.1128/aem.62.11.4252-4255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y., Cheung A.P. New stability-indicating high performance liquid chromatography assay and proposed hydrolytic pathways of chlorhexidine. J. Pharm. Biomed. Anal. 1996;14:1327–1334. doi: 10.1016/s0731-7085(96)01763-3. [DOI] [PubMed] [Google Scholar]
- Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Havlikova L., Matysova L., Novakova L., Hajkova R., Solich P. HPLC determination of chlorhexidine gluconate and p-chloroaniline in topical ointment. J. Pharm. Biomed. Anal. 2007;43:1169–1173. doi: 10.1016/j.jpba.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Hebert V.R., Middleton J.R., Tomaszewska E., Fox L.K. Methodology for quantifying residues of chlorhexidine in raw dairy milk. J. Agric. Food Chem. 2003;51:567–570. doi: 10.1021/jf020915s. [DOI] [PubMed] [Google Scholar]
- Holmes K.V. Coronaviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincott-Raven; Philadelphia: 2001. pp. 1187–1203. [Google Scholar]
- Ijaz M.K., Brunner A.H., Sattar S.A., Nair R.C., Johnson-Lussenburg C.M. Survival characteristics of airborne human coronavirus 229E. J. Gen. Virol. 1985;66(Pt. 12):2743–2748. doi: 10.1099/0022-1317-66-12-2743. [DOI] [PubMed] [Google Scholar]
- Ijaz M.K., Rubino J. Should test methods for disinfectants use vertebrate viruses dried on carriers to advance virucidal claims? Infect. Control Hosp. Epidemiol. 2008;29:192–194. doi: 10.1086/526441. [DOI] [PubMed] [Google Scholar]
- Kawana R., Kitamura T., Nakagomi O., Matsumoto I., Arita M., Yoshihara N., Yanagi K., Yamada A., Morita O., Yoshida Y., Furuya Y., Chiba S. Inactivation of human viruses by povidone-iodine in comparison with other antiseptics. Dermatology. 1997;195:29–35. doi: 10.1159/000246027. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Larson H.E., Reed S.E., Tyrrell D.A. Isolation of rhinoviruses and coronaviruses from 38 colds in adults. J. Med. Virol. 1980;5:221–229. doi: 10.1002/jmv.1890050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moes E., Vijgen L., Keyaerts E., Zlateva K., Li S., Maes P., Pyrc K., Berkhout B., van der Hoek L., Van Ranst M. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium. BMC Infect. Dis. 2005;5:6. doi: 10.1186/1471-2334-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinet, F., Pallier, C., Pillet, S., 2003. Parvoviridae. In: Huraux, J.M., Nicolas, J.C., Agut, H., Peigue-Lafeuille, H. (Eds.). Traité de Virologie Médicale, Paris, pp. 283–290.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Narang H.K., Codd A.A. Action of commonly used disinfectants against enteroviruses. J. Hosp. Infect. 1983;4:209–212. doi: 10.1016/0195-6701(83)90052-x. [DOI] [PubMed] [Google Scholar]
- Nicholson K.G., Kent J., Hammersley V., Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pene F., Merlat A., Vabret A., Rozenberg F., Buzyn A., Dreyfus F., Cariou A., Freymuth F., Lebon P. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003;37:929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J., Bucknall R.A. The disinfection of respiratory syncytial virus by isopropanol and a chlorhexidine-detergent handwash. J. Hosp. Infect. 1985;6:89–94. doi: 10.1016/s0195-6701(85)80023-2. [DOI] [PubMed] [Google Scholar]
- Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Sattar S.A., Ansari S.A. The fingerpad protocol to assess hygienic hand antiseptics against viruses. J. Virol. Methods. 2002;103:171–181. doi: 10.1016/s0166-0934(02)00025-3. [DOI] [PubMed] [Google Scholar]
- Sattar S.A., Jacobsen H., Springthorpe V.S., Cusack T.M., Rubino J.R. Chemical disinfection to interrupt transfer of rhinovirus type 14 from environmental surfaces to hands. Appl. Environ. Microbiol. 1993;59:1579–1585. doi: 10.1128/aem.59.5.1579-1585.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S.A., Springthorpe V.S., Adegbunrin O., Zafer A.A., Busa M. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J. Virol. Methods. 2003;112:3–12. doi: 10.1016/s0166-0934(03)00192-7. [DOI] [PubMed] [Google Scholar]
- Sattar S.A., Springthorpe V.S., Karim Y., Loro P. Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiol. Infect. 1989;102:493–505. doi: 10.1017/s0950268800030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizun J., Yu M.W., Talbot P.J. Survival of human coronaviruses 229E and OC43 in suspension and after drying on surfaces: a possible source of hospital-acquired infections. J. Hosp. Infect. 2000;46:55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L.S., Ricard C.S., Holmes K.V. Conformational change of the coronavirus peplomer glycoprotein at pH 8.0 and 37 °C correlates with virus aggregation and virus-induced cell fusion. J. Virol. 1990;64:3042–3050. doi: 10.1128/jvi.64.6.3042-3050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler R., Ayliffe G.A. A surface test for virucidal activity of disinfectants: preliminary study with herpes virus. J. Hosp. Infect. 1987;9:22–29. doi: 10.1016/0195-6701(87)90090-9. [DOI] [PubMed] [Google Scholar]
- Vabret A., Dina J., Gouarin S., Petitjean J., Tripey V., Brouard J., Freymuth F. Human (non-severe acute respiratory syndrome) coronavirus infections in hospitalised children in France. J. Paediatr. Child. Health. 2008;44:176–181. doi: 10.1111/j.1440-1754.2007.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret A., Mourez T., Gouarin S., Petitjean J., Freymuth F. An outbreak of coronavirus OC43 respiratory infection in Normandy. France. Clin. Infect. Dis. 2003;36:985–989. doi: 10.1086/374222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., Wertheim-van Dillen P.M., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elden L.J., van Loon A.M., van Alphen F., Hendriksen K.A., Hoepelman A.I., van Kraaij M.G., Oosterheert J.J., Schipper P., Schuurman R., Nijhuis M. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J. Infect. Dis. 2004;189:652–657. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther B., McCue K., Ashe K., Rubino J.R., Hendley J.O. Environmental contamination with rhinovirus and transfer to fingers of healthy individuals by daily life activity. J. Med. Virol. 2007;79:1606–1610. doi: 10.1002/jmv.20956. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wong B.H., Poon R.W., Cai J.J., Luk W.K., Poon L.L., Wong S.S., Guan Y., Peiris J.S., Yuen K.Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A., Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J. Hosp. Infect. 1998;38:283–295. doi: 10.1016/S0195-6701(98)90077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


