Highlights
-
•
Cystatin C is not only a good index of kidney functions but also involved in immune regulation and apoptosis under pathological conditions.
-
•
The involvement of cystatin C in the immunological process occurs at multiple levels, which are subjected to cytokine regulation.
-
•
Abnormal cystatin C expression is associated with inflammatory autoimmune diseases and tumor development.
-
•
Thus cystatin C can be a valid therapeutic target for these diseases.
Abbreviations: DC, dendritic cells; GFR, glomerular filtration rate; ECM, extracellular matrix; MHC-II, major histocompatibility complex-II; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; ROS, reactive oxygen species; IgG2, immunoglobulin G 2; TNF-α, tumor necrosis factor-α; NO, nitric oxide; IFN-γ, interferon-γ; TGF-β, transforming growth factor β; Bcl-2, B-cell lymphoma-2; cyt c, cytochrome c; CTL, cytotoxic T lymphocytes
Keywords: Cystatin C, Cysteine proteases, Immune regulation, Inflammation, Apoptosis
Abstract
As an abundantly expressed cysteine protease inhibitor widely distributed in the organisms, cystatin C is involved in various physiological processes. Due to its relatively small molecular weight and easy detection, cystatin C is commonly used as a measure for glomerular filtration rate. In pathological conditions, however, growing evidences suggest that cystatin C is associated with various immune responses against either exogenous or endogenous antigens, which ultimately result in inflammatory autoimmune diseases or tumor development if not properly controlled. Thus the fluctuation of cystatin C levels might have more clinical implications than a reflection of kidney functions. Here, we summarize the latest development of studies on the pathophysiological functions of cystatin C, with focus on its immune regulatory roles at both cellular and molecular levels including antigen presentation, secretion of cytokines, synthesis of nitric oxide, as well as apoptosis. Finally, we discuss the clinical implications and therapeutic potentials of what this predominantly expressed protease inhibitor can bring to us.
1. Introduction
The lysosomal cysteine proteases (cathepsins) were believed to be responsible for the terminal protein degradation in the lysosomes [1]. Similar to other proteases, cathepsins are regulated by their endogenous protein inhibitors called cystatins at every level of their biosynthesis [2]. Cystatins comprise a super-family (type I–III) of evolutionary related proteins: Type I cystatins are cytosolic proteins, lacking disulphide bridges; Type II cystatins are found both inside and outside of cells, comprising at least 14 members; Type III cystatins are large multifunctional plasma proteins, containing three type II cystatin-like domains [3].
Belonging to the member of family II of the cystatin super-family, cystatin C is a potent inhibitor of cysteine proteases [4]. It is an alkaline secreted protein, with a molecular mass of 13.343 Da, which could strongly inhibit the activities of papain-like cysteine proteases and legumain [5]. Cystatin C encoding gene was once believed to be the house-keeping gene found in all of the nucleated cells without tissue specificity. Produced at a constant speed in the body, cystatin C is secreted abundantly into various biological fluids including urine, blood, seminal fluid, saliva and cerebrospinal fluid [6].
In the early 1960s, Fossum and Whitaker isolated from chicken egg white a substance that can inhibit the vigor of papain, ficin and dipeptidase [7]. However, it was not named cystatin C until early 80s, when Anastasi adopted the affinity chromatography method for the first time in the isolation, and since then, cystatin C was successively purified in different species [8]. Cystatin C can combine closely but reversibly with cysteine protease molecules. Amino acid sequence analysis revealed that cystatin C has three highly conservative domains: 1) N-terminal region, including glycine-11 (sequence of cystatin C) highly conservative sequence; 2) The first hairpin loop, including highly conservative QVVAG sequence (glutamine-55-glycine-59); 3) The second hairpin loop, including proline-105 and tryptophan-106 [9]. X-ray scattering techniques were used to analyze the three dimensional structure of chicken cystatin C, and showed that the molecular center has a long alpha helix around five anti-parallel beta folded lamellar structure. At the end of the beta patches is exposed the first beta hairpin loop, the N-terminal region and second hairpin loop are located in both sides. These three structure domains constitute a wedge structure that complementary to the target enzyme's active site to play inhibition roles effectively [10].
The functions of cystatin C are closely related to that of its target enzymes. With activities both inside and outside the cell, the cysteine proteases play fundamental roles in multiple biological processes, such as protein turnover, regulation of innate immune cells phagocytosis, activation of precursor proteins (e.g. enzymes and pro-hormone), major histocompatibility complex-II (MHC-II) mediated antigen presentation, as well as apoptosis [11,12]. Therefore, the activities of these multifunctional enzymes need to be tightly controlled by their endogenous inhibitors, like cystatin C.
Clinically, cystatin C is mostly used as a biomarker of kidney functions for its relatively lower molecular weight and easier detection to measure glomerular filtration rate (GFR) than chemical compounds, radioisotopes or radiocontrast agents [13]. Since cystatin C is removed from the blood stream by glomerular filtration, whose decline as a result of failed kidney functions will lead to increased serum cystatin C concentration, the main determinant of blood cystatin C levels was believed to be the rate at which it is filtered at the glomerulus [14]. However, recent studies increasingly reported direct involvement of cystatin C in many immunological disorders other than renal diseases, and cystatin C encoding gene can be subject to the regulation of cytokines under inflammatory or infectious conditions [15,16]. Therefore, the oscillation of blood cystatin C levels measured by its activity could actually reflect the change of cystatin C production, consumption, inactivation or even fibrillation rather than its filtration in the kidney, and the readout of plasma cystatin C concentration might have important clinical implications. Here, we summarize the latest development of studies on the involvement of cystatin C in immune responses under inflammatory and autoimmune conditions at various levels from diseases, cells, down to the molecules. The diverse functions and clinical relevance of cystatin C foretell the involvement of this protease inhibitor in multiple pathophysiological processes, which could be strategically utilized for translational applications.
2. Cystatin C affects immunity
Growing evidences suggest that cystatin C is involved in numerous immunological processes [17]. Subjected to the regulation by various inflammatory mediators, cystatin C in turn affects inflammation and its induced immune responses. Cystatin C exerts several immunomodulatory functions by controlling the activity of cysteine proteases or by other mechanisms not related to its inhibitory function. It has been reported that cystatin C may contribute to the proteolytic processing of pro-granzymes and other substrates in immune cells, MHC-II antigen presentation, maturation of dendritic cells (DCs), modulation of integrin function and formation of the skin barrier as the first line of immunological defenses. Indeed, the association between cystatin C and many inflammatory diseases is so strong that the levels of extracellular cystatin C have diagnostic value or were used as marker for disease prognosis in many inflammatory disorders [18]. As matter of fact, cystatin C was found to be involved in every step of inflammation and immunity from entrance of pathogens to the final immune disorders.
2.1. Pathogen invasion
One of the important roles of cystatin C is to protect the host against invading microorganisms and parasites that use cysteine proteases to enter the body [2]. Cystatins have been found in epithelial cells [19], neutrophils from the liver [20], DCs of lymphoid tissue [21] and thymic medullary cells [22]. The selective expression of the inhibitor in these cells correlates with the tissues participating in the first-line of defense against pathogens. For instance, analysis of proteins uniquely involved in the development of the skin demonstrated strong expression of cystatin in neonatal mouse skin, which decreases with aging, suggesting an important role of this protease inhibitor in the development of the epidermis [23]. Furthermore, in vivo mouse models have also revealed that cystatins are key molecules in a biochemical pathway that control skin barrier formation [24,25]. The involvement of cystatins in these tissues suggests that they may have a role in the host defense mechanism against pathogenic agents, like virus for example. Along this line, cystatin has been found to interfere with coronavirus replication in human lung cells [26], partially block poliovirus replication in infected human cells [27], suppress the infectivity of adenovirus in human respiratory passage or conjunctiva [28] and herpes simplex virus in human submandibular-sublingual and parotid [29].
Apart from virus, the initiation of inflammation could also come from an invasion of parasites, whose secretion of cysteine proteases was believed to play key roles in parasite-host interactions including the establishment of infection [30]. Furthermore, the parasite cysteine proteases can not only digest the host extracellular matrix (ECM) to facilitate their invasion, but also help to ensure a T helper 2 (Th2)-like response in favor their proliferation [31]. In agreement with this notion, one study has demonstrated that BALB/c mice with fatal visceral leishmaniasis can be clinically cured of the disease by chicken cystatin in synergy with interferon-γ (IFN-γ) treatment [32]. Later on, it was revealed that the successful therapy of lethal murine visceral leishmaniasis with cystatin C in combination with IFN-γ correlated with up-regulation of cystatin C and nitric oxide (NO) generation, successful Th2 to Th1 conversion at molecular and cellular levels, leading to the reduced parasite numbers and abrogation of parasite infection [33].
Not only cystatin protein itself, but also its mimetics were found to have anti-effects on pathogenic microorganisms. One study demonstrated that a small peptide derivative that mimics part of the proteinase-binding center of human cystatin C could inhibit a cysteine protease specific for the growth of streptococci, thus blocking the propagation of these bacteria both in vivo and in vitro [34]. Similarly, recombinant human cystatin C was also proven to inhibit the growth of herpes simplex virus and human coronaviruses [35]. Recently, nematode homologs of cystatin were reported to have anti-inflammatory functions and investigated for their therapeutic potentials [36,37].
2.2. Antigen presentation
Once entering the body, the pathogens will be captured to alert host immune system by the antigen presenting cells (APCs) including DCs, where foreign proteins will be digested, processed and loaded onto an important molecule, MHC-II, to be presented for T cell recognition. The development of MHC-II needs cysteine proteinase cathepsin S to assist. Therefore, it is possible for cystatin C, as a endogenous inhibitor of the cathepsin S, to play a regulatory role in the antigen presentation by inhibiting cathepsin S during the removal of the invariant chain (Ii) to generate effective MHC-antigenic peptide complex [38].
However, the hypothesis that cystatin C control MHC-II expression by regulating cathepsin S activity has been challenged by several other studies: For example, cathepsin S-deficient DC express normal levels of MHC-II on the plasma membrane [39,40], and DCs can control MHC-II surface expression in the absence of the Ii [41]. Furthermore, El-Sukkari showed in a separate study using mouse primary DCs isolated from cystatin C-deficient mice that cystatin C is neither necessary nor sufficient to control MHC-II expression and antigen presentation in DCs [42]. These discrepant findings obtained from different laboratories could be caused by difference among DC subsets or maturation of the DCs investigated, like bone-marrow derived DCs versus primary splenic DC, immature or steady DCs versus mature or activated DCs etc. Indeed, cystatin C was found to be differentially expressed among closely related DC subsets [42], and therefore it is not impossible that other cysteine protease inhibitors play compensatory roles in the DC subsets that lack or have limited expression of cystatin C either constitutively or following maturation or activation. In supporting this possibility, experiments using bone marrow-derived DCs indicated that interleukin (IL)-6 mediated signal transducer and activator of transcription 3 (STAT3) activation decreased cystatin C expression [36]. Consistently, we found that IL-10 can down-regulate cystatin C expression in splenic primary DCs through interferon regulatory factor 8 (IRF8) during systemic inflammation [43].
While the role of cystatin C in antigen presentation is open to debate, a cystatin homolog, the Bm-CPI-2, produced by the filarial nematode parasites as one of the most abundant transcripts [44], was found to inhibit multiple cysteine protease activities in human B cells, and suppress substantially the presentation of selected T cell epitopes by living APCs [45]. Since filarial worms can invade the host by lymphatic vessels afferent to the lymph nodes to initiate specific immune response, it is easier for CPI-2 to contact DCs and other APCs in lymphoid tissue, leading to the impairment or ablation of antigen presentation during anti-parasite immune responses.
2.3. Immune disorders
The presentation of antigens to activate immune responses can lead to dual outcomes: proper reaction with timely stopping eliminates the invading agents for inflammation, whereas over reaction with nonspecific targeting will end up in self-destruction or autoimmunity. It has become evident that disturbances in expression and localization of cystatin C may be implicated in several inflammatory autoimmune pathologies.
2.3.1. Inflammatory skin diseases
Atopic dermatitis and psoriasis are two common chronic inflammatory skin diseases, in which cell proliferative machinery and the formation of the epidermal barrier are altered.
Cystatin is involved in cellular proliferation and could be a useful target for diseases of abnormal proliferative conditions. The mRNA levels of cystatin were found to be increased in psoriatic plaques of the psoriasis vulgaris, a common inflammatory disease of the skin, characterized by hyper-proliferation of skin cells that ultimately leads to red, scaly plaques [46]. In addition, polymorphism in the genes for cystatin C has been associated with atopic dermatitis, a chronic inflammatory skin disease often associated with a defective epidermal barrier [47,48]. Cystatin was found to protect skin barrier from allergic reactions, including atopic dermatitis, and inhibition of proteolytic activity of major mite allergens by cystatin blocked the up-regulation of IL-8 and granulocyte-macrophage colony stimulating factor (GM-CSF) release from keratinocytes stimulated with the allergens [49,50]. Moreover, loss-of-function mutations in the gene for cystatins have been identified as the underlying genetic cause of another skin disease, exfoliative ichthyosis [51]. Consistently, it has been demonstrated that disturbance of the cystatin-cathepsin pathway could contribute to dys-regulated skin barrier function in vivo as was observed in the inflammatory dermatoses [47].
Recently, genetic studies have also revealed that abnormalities in epithelium-expressed genes were important etiological factors [52,53]. Furthermore, decreased mRNA and protein expression levels of cystatin were observed in the inflamed skin during atopic dermatitis and psoriasis, supporting protective roles of cystatin for the skin inflammation [54].
2.3.2. Rheumatoid arthritis
As a systemic autoimmune disease that mainly affects the arthrosis, rheumatoid arthritis (RA) is a chronic inflammatory lesion of multiple joints that take on the symmetry and peripheral with progressive destruction of articular cartilages and bones [55]. Interestingly, it is believed that in the inflamed joints the cystatin targeted enzymes, especially cathepsin B, H, L, S and K play significant roles in tissue destruction [56], and were highlighted as potential drug targets to treat tissue degenerative and inflammatory processes [57]. In particular, cathepsin B and L, the substrate enzymes of cystatin C, were found to degrade the components of cartilage, like proteoglycans, collagen II, IX and XI [58], as well as the bone matrix proteins osteocalcin and osteonectin [59,60], indicating the involvement of cystatin C in the development of the autoimmune disease, which is supported by the data from both mouse and human studies described below.
Collagen-induced arthritis (CIA) is a mouse model for human RA. Both the priming and inflammatory phases of the CIA have been suggested to rely on proteases, of which, the cysteine proteases have been proposed to be detrimental to the arthritic and even immunomodulatory process [61]. Therefore, it is not surprising to think that cystatin C was associated with the development of the inflammatory joint disease. Along this line, the lack of cystatin C was found to enhance CIA onset and primarily affected the in vivo priming of the immune system [61]. To further dissect the cellular mechanism behind the observed phenomenon, evidences of a more activated APC compartment was observed to explain the elevated autoimmune response towards type II collagen, which resulted in an enhanced development of chronic arthritis [61].
In human RA, cathepsin B is expressed in synovial fibroblasts and synovial cells attached to cartilages and bones at sites of erosion [55]. In agreement with the mouse data, cystatin C was also found to be the most prominent cystatin in synovial fluid of RA patients who have significantly reduced levels of cystatin C in circulation [62]. In order to compare the expression of cathepsin B and cystatin C in RA synovial membrane, a group of ten RA patients and healthy controls was chosen to examine the quantities of cathepsin B and cystatin C expression in synovium using immunohistochemical method and investigate the cellular sources that produce these two bioactive proteins [63]. The results showed that cathepsin B and cystatin C were highly expressed in RA synovial tissues, in which fibroblast-like and macrophage-like cells from fibro-proliferative tissue at the site of cartilage and bone destruction were positive for cystatin C and cathepsin B, whereas only limited expression of these molecules was exhibited in normal synovial tissue [63]. Osteoclasts also revealed positive staining for cystatin C, but not for cathepsin B, which means that cystatin C is the product of both macrophage-like and fibroblast-like synoviocytes. The strong expression of the cystatin C in the diseased rheumatoid synovium suggests that cystatin C is either correlated with the disease development, or important but insufficient to prevent matrix degradation by cathepsin B. Although the exact roles that cystatin C plays in the inflammatory lesions remain to be discovered, it could currently serve as diagnostic biomarker or therapeutic target for the treatment of the autoimmune disease.
3. Cystatin C regulates immunity at cellular levels
Since cystatin C and its family members participate in various immune disorders that result in inflammatory autoimmune diseases or tumor development, it is reasonable to infer that this dominant endogenous protease inhibitor can affect the functions of immune system cells directly or indirectly. Indeed, although a secreted protein, cystatin C was reported to be up-taken by immunological cells in various tissues to regulate both intracellular and extracellular cysteine protease activities [64]. Furthermore, the preferential expression pattern of cystatin C in monocyte/macrophage and DCs is of clinical relevance in view of the recent reports showing that these cell types are present in tissues where cystatin C plays pathological roles in inflammatory diseases such as atherosclerosis and angiopathy [65]. These findings strongly suggested that cystatin C can participate in the immune regulation by affecting the functionalities of various immune cells, such as DCs, mononuclear phagocytic cells, T cells and other cells.
3.1. Cystatin C and DCs
DCs have strong ability to initiate T cell activation and bridge connection between innate and adaptive immunity in the case of an acute infection [66], but to maintain immune tolerance to self-tissues and organs at steady state [67]. Literature has reported that the expression of cystatin C varies widely among hematopoietic cell types, with cells of the monocyte/macrophage and DC lineages expressing it at much higher levels than B or T cells, indicating possible roles of cystatin C in DCs [42].
External stimuli can transform DCs from immature status to mature status by increasing many DC surface molecules such as antigen presenting molecules MHC-I and MHC-II; co-stimulatory molecules CD80 and CD86, adherence molecules CD40, CD54 and integrin, which are essential for the immunological functions of DCs [68]. We and others have demonstrated that higher concentrations of cystatin C were measured in immature DC, but gradually decreased with DC maturation, which lead to the elevated activity of cathepsin L and S, indicating an essential role of cystatin C in controlling the degradation of intracellular antigens and T cell stimulatory capacity of DCs [43,69].
Cystatin C affects DC functions not only by its quantity but also by its quality, as it is one of the few amyloid proteins. Cystatin C amyloidogenesis starts with cystatin C dimerization by a process known as “three dimentional domain swapping”, in which two parts of the cystatin structure become separated from each other and next exchanged between two molecules [70]. Interestingly, with their inhibitory region hidden within the dimer interface, cystatin C dimers cannot inhibit cysteine proteases to regulate the immunological functions of DCs [71]. To identify the mechanistic factors leading to cystatin C dimerization either as post-translational regulation of its activity, or as amyloid precursor protein, we compared the intracellular accumulation of reactive oxygen species (ROS) in the immature and mature states, and found strong correlation between ROS levels and cystatin C dimer not only in same cell type (DCs) at different developmental stages, but also in different cell types (DCs and macrophage) at the same developmental stages [43]. Furthermore, artificially enhancing the intracellular oxidative status resulted in a time-dependent cystatin C dimer enrichment, which could be prevented by inhibiting mitochondria activity, indicating the ROSs released from mitochondria are responsible for the observed constitutive cystatin C dimer formation and lose of its inhibitor’s activity [43]. Therefore, the importance of cystatin C in DCs is manifested by its multiple controls at different levels affected by both intrinsic and extrinsic factors.
The predominant expression of cystatin C in DCs is not evenly presented among different DC subsets. Further analysis of the DC subsets directly isolated from the spleen demonstrated that the CD8+ DC were the major producer of cystatin C, with little or no expression in the closely related CD8− DC [43,72]. This differential expression pattern of cystatin C among cells of common lineages suggests that its gene could be subjected to the regulation by cell-specific transcription factors. In addition, mature DC can secrete a large number of immune-stimulatory cytokines and chemokines to induce the pro-inflammatory Th cells differentiation and memory T cells activation [73], of which IL-6 signaling in vivo was found to decrease cystatin C expression in DCs [36]. Along the same line, we found that in an inflammatory mouse model created by intravenous injection of CpG oligodeoxynucleotides, mimics of bacterial and viral DNA responsible for immune stimulation, the synthesis of cystatin C in DCs as well as the circulating pools of cystatin C in blood were greatly reduced [74]. Collectively, these data suggest that cystatin C can affect the functions of DCs, which in turn secrete inflammatory cytokines to regulate cystatin C expression for further immune regulation.
3.2. Cystatin C and mononuclear phagocytic cells
Mononuclear phagocytic cells consist of the phagocytic cells located in reticular connective tissue, primarily monocytes and macrophages. Research shows that cystatin C can regulate host immune response via these cells.Cystatin C was found to inhibit phagocytosis of granulocyte and macrophage, and increase the synthesis of NO in mice peritoneal macrophages [32]. Along this line, further studies revealed that the interaction between cystatin C and cysteine protease can release a bioactive peptide that inhibits the phagocytosis and oxidative burst in eosinophil and monocyte [75]. Microglia, also called the macrophages in the brain, is the major cell type that expresses cystatin C in the brain [76]. However, deposition of cystatin C fibrils in these cells produces angiopathy [77], compromising their immune surveilling capacity in the central nervous systems.
Given the strong influence of cystatin C on these myeloid innate immune cells, many factors impact on host immunity by regulating the synthesis and production of this protease inhibitor. Treatment of resident mouse peritoneal macrophages in vitro with the bacterial compound lipopolysaccharide (LPS) or pro-inflammatory IFN-γ could down-regulate cystatin C secretion [65]. Consistently, during inflammation, reduced cystatin C secretion was accompanied by increased activities of cysteine proteases in macrophage microenvironment [78]. The variation of cystatin C production in these conditions, can in turn affect immune system by regulating cytokine secretion from these cells. For example, in early phase of parasite infection, cystatin C can induce human peripheral blood mononuclear cells to secrete tumor necrosis factor-α (TNF-α), reduce IL-12 but increase IL-10 generation. Since IL-10 is a known inhibitory cytokine to inhibit Th1 cell differentiation and T cell proliferative responses to antigen stimulation, the cystatin C induced IL-10 generation may participate in host T cell conversion from Th1 into Th2, skewing the host immune system to favor parasite propagation [79].
In addition to its effect on cytokine release, cystatin C also influences the phenotype of mononuclear phagocytic cells. This is in line with a recent study showing that cystatin C induced early and transient expression of activation markers on macrophages demonstrated by the up-regulated of MHC-II, CD40, CD80 and CD86 in response to cystatin C [80]. Moreover, molecular events following the treatment of cystatin C were investigated and found that cystatin C was internalized by monocytes via an active endocytic process, leading to decreased phosphorylation and activation of the mitogen-activated protein kinase extracellular signal-regulated kinase-1/2 (MAPK-ERK1/2) signaling pathway [81,82]. Consistent with the mouse data, a recent study also reported that cystatin C down-regulated phosphorylation of the ERK1/2 pathway in human samples [83].
3.3. Cystatin C and T cells
T lymphocytes are important mediators of adaptive immunity against both foreign antigens and self-tissues. Cystatin C can regulate the proliferation, differentiation, and co-stimulatory molecule expression of T cells either directly or indirectly via various cytokines [12,84].
The differentiation of functional effectors such as Th1 and Th2 cells from T cells are subjected to the regulation of cytokines. For example, IL-12 promotes the differentiation of Th1 cells that secrete Th1 cytokine IFN-γ, whereas IL-4 drives the differentiation of Th2 cells that secrete Th2 cytokine IL-4. When BALB/c mice susceptible to infection with Leishmania major were treated with cystatin C, those mice acquired resistance against infection with the parasites and showed the shift of immune responses from disease-promoting Th2 to protective Th1 type. These mice produce specific immunoglobulin G 2 (IgG2) antibody and generated IFN-γ in contrast to the untreated but infected mice that produced IgG1 and IgE and generated IL-4 [79]. Overall, these data suggested that cystatin C must have induced Th1 polorizing cytokine production in vivo to affect T cells differentiation. However, since the in vivo system is complicated with many cells involved, whether this effect of T cell differentiation by cystatin C was directly imparted to the naïve T cells or mediated through other cells need to be further characterized in vitro.
Apart from regulation of T cell differentiation, cystatin C may also exert direct effect on T cell proliferation. A 17-kDa antigen (Av17) of the rodent filarial parasite Acanthocheilonema viteae, which shows amino acid homologies to cystatin C, was found to markedly suppress mitogen-induced T cell proliferation in mice [85]. Consistent with its nature counterpart produced from the parasites, recombinant Av17 (rAv17), expressed in Escherichia coli, with biological activity as a cysteine protease inhibitor, also demonstrated the same inhibitive effect on T cell proliferation. In the presence of rAvl7, T cell receptor (TCR)-induced proliferation in purified T cells was severely impaired in comparison to the control protein, whereas the B cell receptor (BCR)-induced proliferation of murine B cell hybridomas was unaffected, indicating T cell specific inhibition rather than a more fundamental block of cellular proliferation [85].
Mechanistically, T cell activation requires two signals, with one being transmitted by the TCR complex and the other by its co-receptors. For full T cell activation, co-stimulatory signals are required for IL-2 production, proliferation and differentiation to effectors function [86]. At present, it is unclear which signal molecule is affected by the filarial cystatin when it inhibit T cell proliferation. Of note, it was reported that rAvl7 changed the production pattern from the T cell cultures of NO, a potent T cell inhibitive compound for proliferation, as other cystatins did [32], however, whether this effect affected co-stimulatory signaling and contributed to the hypo-responsiveness of T cells remain to be further defined.
3.4. Cystatin C and other immune cells
Cystatin C was reported to inhibit the activation of cathepsins to mediate the natural killer (NK) cells killing [87]. In addition, cystatin C from Onchocerca volvulus could also significantly inhibit the peripheral blood mononuclear cell proliferation and antigen presentation in response to tuberculin purified protein derivative, and suppress the expression of co-stimulate molecule CD86 on monocyte (but had no effect on the expression of CD40, CD80) [88]. Collectively, these data highlight the important impact of cystatin C on other immune system cells for effective immune regulation under inflammatory conditions.
4. Cystatin C regulate immunity at molecular levels
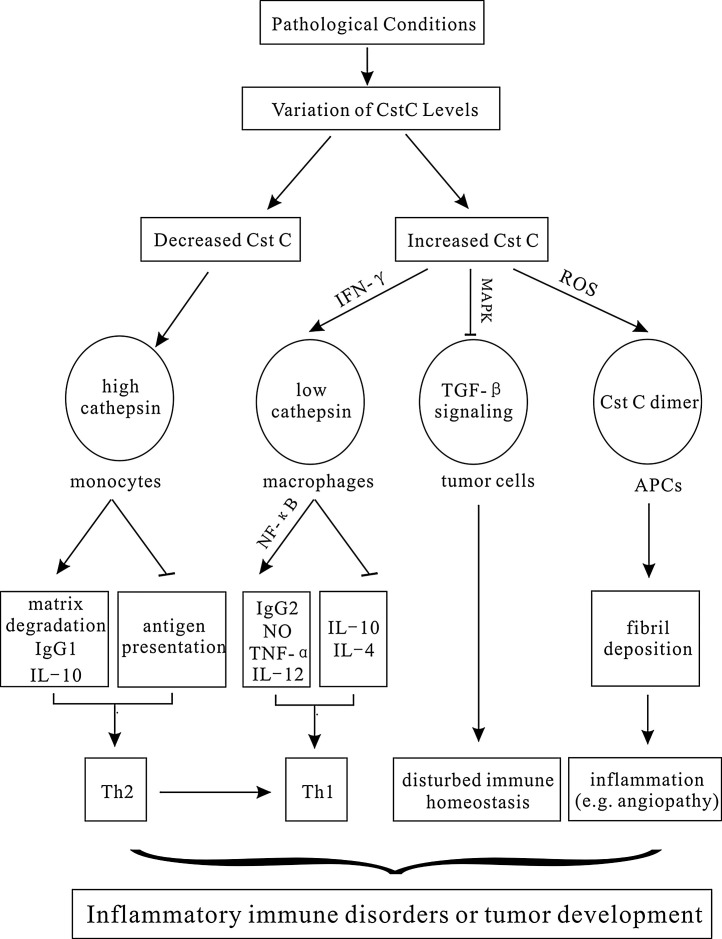
Cellular behaviors in the immune system is triggered or implemented by particular molecules either inside or outside of the cells. As abundantly expressed protein, cystatin C was reported to affect numerous inflammatory factors and mediators, including cytokines or soluble factors secreted from either targeted cells or other cells (Fig. 1 ).
Fig. 1.
Cystatin C regulates immunity at cellular and molecular levels.
Under pathological conditions, various stimuli cause variations in Cst C levels. Decreased Cst C up-regulates the activities of cathepsins, facilitating the cell matrix degradation, and IgG1 and IL-10 production to ensure a Th2-like response in favor of parasite proliferation. In addition, decreased Cst C promotes the interference of cathepsin with MHC-II loading in APCs to suppress host immunity against pathogen invasion. Increased or exogenously added Cst C in IFN-γ induced macrophages, on the other hand, down-regulates the cathepsin activities to increase IL-12, TNF-α, and NO generation that shift the immune responses towards protective Th1 immunity. Alternatively, Cst C can also disturb immmune homeostasis independent of cathepsins by antagonizing the binding of TGF-β to its receptor, or directly causing inflammation through formation of Cst C fibrils deposited in vascular walls.
Cystatin C, Cst C; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; ROS, reactive oxygen species; IgG2, immunoglobulin G 2; TNF-α, tumor necrosis factor-α; NO, nitric oxide; IFN-γ, interferon-γ; TGF-β, transforming growth factor β.
4.1. IFN-γ
As a dimerized soluble cytokine produced predominantly by NK and active T cells, IFN-γ is an important activator of macrophages and inducer of MHC-II molecule expression critical for antiviral and anti-tumor immunity [89]. Aberrant IFN-γ expression is associated with a number of auto-inflammatory and auto-immune diseases. It has been documented that cystatin C is involved in the IFN-γ signal transduction pathway.Previously it has been shown that in vitro treatment of murine peritoneal macrophages with IFN-γ causes a down-regulation of cystatin C secretion [89]. Conversely, it is curious to know whether cystatin C in turn can affect inflammatory responses mediated by IFN-γ in macrophages. To this end, the effects of IFN-γ on macrophages isolated from cystatin C knockout (cystatin C−/−) and wild-type (cystatin C+/+) mice were compared. It was shown that, in cystatin C−/− macrophages, IFN-γ induced higher IL-10, but lower TNF-α expression, compared to the similarly primed cystatin C+/+ cells [90], indicating an indispensable role of cystatin C in regulating the IFN-γ induced inflammatory cytokine production in macrophages.
Consistently, the following evidences indicate that cystatin C is involved in the IFN-γ signaling pathway. First, exogenously added cystatin C to cystatin C−/− macrophages enhanced IFN-γ induced activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65, which is known to regulate the expression of pro-inflammatory cytokines and co-stimulatory molecules such as TNF-α and NO [91]. Furthermore, cystatin C was found to be able to synergize with IFN-γ to induce ERK1/2 phosphorylation and NF-κB DNA-binding activity, as pretreatment of cells with specific inhibitors of NF-κB or ERK1/2 pathway blocked the cystatin plus IFN-γ-inducible NF-κB activity [82]. Moreover, increased mRNA levels of inducible NO synthase (iNOS), as well as the levels of NO and TNF-α were found in the cell culture medium of IFN-γ and cystatin C treated macrophages [92].Cystatin C was also reported to affect T cell polarizing cytokine production in response to IFN-γ signaling. Transcript levels of IL-4 were found to be reduced in the mice given combined therapy of IFN-γ and cystatin C, whereas IL-12 was significantly elevated [93], indicating that cystatin C has the potential to facilitate the IFN-γ-induced switch of T cell differentiation from Th2 to Th1 in favor of eliminating pathogen infection. Collectively, these data suggested that as an immunomodulatory molecule, cystatin C alters the responses of immune cells to IFN-γ [92].
4.2. NO
NO is a molecular and chemical compound containing free radical with a wide range of biological functions, ranging from regulating platelet aggregation, neural signal transduction, enzyme activity, and leukocyte homeostasis [94]. NO produced by the cytokine-activated macrophage during parasite infection is known to play a central role in the control of parasite killing and cell apoptosis [95].
The studies on Angiostrongylus cantonensis confirmed cystatin C can significantly induce NO generation from macrophages [96,97]. More recently, cystatin C from parasites such as Onchocerca volvulus and Dipetalonema perstans also has the potential to induce NO production from other cells [84,98,99].
Further study of the mechanism revealed that cystatin C induce the production of NO independent of its inhibitor’s activity, because an irreversible and structurally unrelated cysteine protease inhibitor E64 did not induce any increase in nitrite levels [32]. The role of cystatin C on the NO production relies on the activation of iNOS pathway as it was almost completely abrogated in the presence of L-NMMA, a specific iNOS inhibitor. Further study revealed that the cystatin C involved iNOS expression is mostly controlled by two regulatory regions present in the iNOS promoter, which contains binding sequences for two transcription factors, NF-κB and IFN regulatory factor 1 (IRF-1) [100]. Later on, a number of studies demonstrated a direct correlation between ERK1/2 activation and up-regulation of both NF-κB activity and NO production through the participation of mitogen- and stress-activated protein kinase 1 (MSK1) [82,101].
Although detailed signaling pathways still remain to be defined, a better knowledge of the mechanism by which cystatin C triggers the production of microbicidal NO, could permit the development of immune modulators useful not only for non-healing leishmaniasis but also for other chronic infectious diseases [82].
4.3. TGF-β
Transforming growth factor β (TGF-β) is a strong immunosuppressant, which plays an important role in tumor development by affecting immune cell mediated immune surveillance [102]. For example, TGF-β can inhibit the maturation of DCs, that make T cells unable to differentiate into cytotoxic T lymphocytes (CTLs) or Ths, thus promoting tumorigenesis [103]. In addition, TGF-β was also found to promote the proliferation of Tregs in vitro and increase the expression of HLA-DR in Tregs to enhance the immunosuppressive function [104]. Moreover, there were also evidences to suggest a negative regulatory roles of TGF-β in NK cells and monocytes [103]. Based on existing research findings, it has been well established that cystatin C can antagonize the suppressive functions of TGF-β via physically interacting with its receptor or its signaling molecules.TGF-β mediates its biological activities via binding to cell surface receptors with high affinity [105]. The inhibitory effect of Δ14Cst C, a cystatin C mutant that lacks the cysteine protease inhibitor signature sequences, on TGF-β signaling suggested that cystatin C may inhibit TGF-β signaling by antagonizing the interaction between TGF-β and its receptors [106]. This hypothesis seemed especially attractive given the fact that the type III cystatin family member, fetuin (also known as α2-HSglycoprotein), inhibits TGF-β signaling by physically interacting with and preventing the binding of TGF-β to its receptors [107]. Consistently, down-regulation of tumor development through a similar mechanism has also been shown for a cystatin that inhibits colon carcinogenesis by blocking the interaction of TGF-β with cell surface receptors to suppress its signal transduction [108]. In agreement with these in vitro findings, the growth, weight, and proliferative index of 4T1 tumors in BALB/c mice were inhibited significantly by their expression of either cystatin C or Δ14Cst C in vivo via antagonizing the efficacy of TGF-β [109].
In addition to directly blocking of its receptor, cystatin C may also prevent TGF-β signaling by affecting its downstream molecules. Immunohistochemistry studies were conducted to monitor the activation status of all three TGF-β effectors, drosophila mothers against decapentaplegic protein (Smad), p38 MAPKs and ERK1/2, by staining with phospho-specific antibodies [109]. The result shows that the phosphorylation and activation of these effectors were inhibited significantly in cells expressing cystatin C compared with their control samples [109], suggesting that cystatin C can block the TGF-β signaling pathway partly by decreasing the phosphorylation of Smad2, p38 MAPK, and ERK1/2, although the physical evidence for the direct interaction between cystatin C and these signaling effectors remain to be identified.
4.4. Other cytokines
As a very common and important basic pathological process, inflammation is a protective response involving numerous immune cells against invading pathogens, during which many inflammatory cytokines and mediators are released. In addition to the factors mentioned above, other inflammatory factors have also been reported to interact with cystatin C or its analogues.
It was found that filarial cystatin rAvl7was associated with a marked increase in IL-10 production. In contrast, there was a trend towards a decreased production of IL-4 by mitogen-stimulated spleen cells in the presence of rAvl7 [85]. It is possible that the lower IL-4 quantities in the presence of rAvl7 resulted from the increased IL-10 levels, as IL-10 and IL-4 were described to be inversely correlated [110]. Although the cellular target of cystatin and the mechanisms of IL-10 secretion await further investigation, this filarial cystatin is a likely effector molecule of immune-modulation and a potential target for anti-filarial intervention.
5. Cystatin C and apoptosis
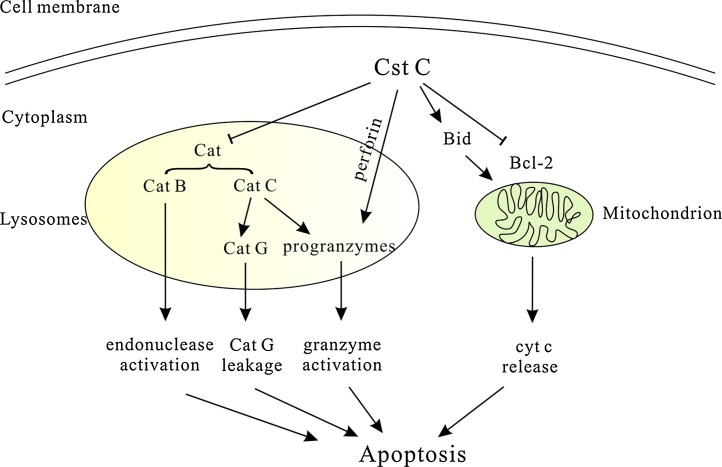
Apoptosis is one of the important forms of immune regulation, which not only controls the numbers but also implements the effects of immune cells. When target cells suffer from the external insults elicited from immune effector cells, or immune cells themselves experience environmental changes, the lysosomes inside the cells will release cysteine proteases to promote cell apoptosis in order to maintain the immune homeostasis [111,112]. Cystatin C, as a powerful endogenous cysteine protease inhibitor, must have been involved in these processes, although its specific roles vary or are even opposite among cells (Fig. 2 ).
Fig. 2.
Dual effects of Cystatin C on apoptosis.
On one hand, Cst C was reported to have anti-apoptotic effect via inhibiting Cat B-induced endonuclease activation for DNA fragmentation. In addition, Cst C can also inhibit Cat C-mediated Cat G leakage or pro-granzyme cleavage/activation to control apoptosis. On the other hand, induction of Cst C was found to decrease Bcl-2 expression and increase Bid protein to promote apoptosis through cyt c release from mitochondria. Moreover, Cst C was also reported to cause rapid de-granulation and granzyme activation in CTL for apoptosis via a perforin-dependent process.
Cst C, cystatin C; Bcl-2, B-cell lymphoma-2; cyt c, cytochrome c;cat, cathepsin; CTL, cytotoxic T lymphocytes.
Since typical characteristics of apoptosis are cell shrinkage, membrane blebbing and DNA condensation as a result of catabolic actions inside cells caused by hydrolytic enzymes [113], cathepsins led out from digitonin-permeabilized lysosomes have the potentials to cause apoptosis directly or indirectly by destroying cytoskeletal proteins, nuclear proteins, DNA repair enzymes or DNA via activating caspases [114]. Indeed, the roles of cysteine proteinases and cystatin C in apoptosis occurred in, or induced by immune cells like B cells, T cells, NK cells, neutrophils and monocytes have now increasingly been appreciated.
Cystatin C plays an important role in the apoptosis of lymphocytes to maintain their high potency by inhibiting cathepsin-induced endonuclease activation for DNA fragmentation. During their life span, both B and T lymphocytes encounter various episodes of selection. The basic selection mechanism is the survival of highly specific B or T cells with strong affinity to the antigens at the cost of their non-specific counterparts with lower affinity, which die by apoptosis. B cell apoptosis can be induced at several stages and requires the activities of both caspases and cathepsins, because triggering of the BCR causes a cathepsin-like proteinase to leak out of the lysosomal compartment and activate the downstream caspases to regulate the activity of endonuclease for DNA fragmentation [115]. Interestingly, the occurrence of DNA strand breaks in B and T cells could be prevented by addition of analogue of cystatin C to inhibit cysteine proteinase activity [116]. In addition, a comparable mechanism was found when apoptosis was induced with bile salts or virus in cell lines, where cystatin was capable of inhibiting cathepsin B downstream of caspases to protect cells from death [117,118]. Consistently, large amounts of cystatins exist in- or out-side cells to inhibit the endonuclease activity via cathepsins [21]. In addition, cystatin C can also inhibit cathapsin C-mediated cathapsin G leakage to control apoptosis. Neutrophils are short-lived cells and their life span forms a crucial mechanism for maintaining immune system homeostasis, as extended survival of neutrophils may lead to chronic inflammation, but reduced number of living neutrophils may enhance susceptibility to infections [119]. Of note, neutrophil cultured in the presence of cystatin C resulted in a significant drop in apoptotic cell percentage compared to the controls, and this hampering effects on neutrophil apoptosis was attributed to its inhibitory influence on cathepsin activity [120]. Consistently, it was found that cathepsin G was involved in apoptosis by activating recombinant procaspase-7 [121]. Furthermore, it was demonstrated that when errors occur in packaging, de-granulation or phagocytosis, cathepsin G was introduced into the cytoplasm and induced morphological changes that are characteristic for apoptosis [122]. Since cathepsin C is involved in post-translational processing and activation of cathepsin G [123], the inhibition of cathepsin C by cystatin could impair the cathepsin G-induced apoptosis in neutrophils.
Moreover, cystatin C was found to suppress pro-granzyme cleavage/activation to protect cytotoxic lymphocytes from apoptosis. CTLs and NK cells can kill target cells by contact-dependent mechanisms that involve cysteine proteinases. This killing of CTL and NK cells depends on exocytosis of granules, perforin, in combination with secretion of granzymes, which are stored in lysosomes [124]. Cathepsin C has the ability to activate granzyme through removing dipeptides from its amino terminus [125,126]. It has recently been demonstrated that although cathepsin knockout mice contain normal levels of granzymes, all granzymes were not active, indicating that cathepsin is required for the activation of granzyme- and granule-mediated apoptosis [127,128]. Interestingly, high levels of cystatin C could reverse this effect by inhibiting the conversion of inactive granzymes into active granzymes via cathepsin C to protect cells from apoptosis [129].
There are always two sides to everything. Although inhibiting apoptosis in most cases, cystatin C, in some cases, was capable of promoting apoptosis by regulating the expression of B-cell lymphoma-2 (Bcl-2) family members. High concentration of cystatin C was found to up-regulate the expression of Bid [130], a pro-apoptotic protein of Bcl-2 family, whose activation can induce mitochondrion release of cytochrome c (cyt c), resulting in the activation of caspase-9. Consistently, recent studies also indicated that the induction of cystatin C decreased an anti-apoptotic Bcl-2 protein expression at both mRNA and protein levels to eliminate its anti-apoptotic effects on preventing cell death [131].
Along the same line, another evidence to indicate the pro-apoptotic role of cystatin C comes from extrinsic pathway where lymphocytes kills their target cells via cell–cell contacts. For example, cystatin C was reported to cause rapid de-granulation and granzyme activation in CTL for apoptosis via a perforin-dependent process [132]. It has been demonstrated that CTL and NK cells die within a few hours if they were triggered to de-granulate in the presence of nontoxic thiol cathepsin inhibitors, indicating that the suicide of CTLs in the presence of cystatin C requires the granule exocytosis cytotoxicity pathway [132].Involved in immune associated apoptosis via various mechanisms including both anti- and pro- apoptosis in different cell types under different conditions, cystatin C could be a promising target molecule for the treatment of inflammatory autoimmune diseases by altering its roles in the apoptosis of immune cells or their target cells. Further studies are needed to define the upstream molecules in the cystatin C-involved apoptotic pathways so that effective regulation of this powerful inhibitor could be achieved for therapeutic gains.
6. Conclusion and future direction
Overall, as an abundantly expressed protein, cystatin C is involved in numerous biological activities under both physiological and pathological conditions. At steady state, cystatin C is mainly used as an index of kidney function due to its relatively small molecular weight and easy detection, whereas under pathological conditions, it serves as an important biomarker for the diseases because of its participation in the immune responses against pathogen invasion directly or indirectly. The involvement of cystatin C in host immunity is multifaceted and tridimensional, covering every stage of immune responses in both innate and adaptive immunity, and interfering with various immunological abnormalities during inflammation, autoimmune diseases and tumor development in compromised immune surveillance. It has become evident that disturbances in the expression and localization of cystatin C may be either the casual or effector factor of the pathological processes. To support this notion, growing evidences demonstrated that cystatin C could interact with numerous inflammatory factors and mediators to regulate the inflammation process at molecular levels. Moreover, the influences of cystatin C on the immune cells are not only limited to their functions, but also extended to their survivals, causing tumor development or autoimmunity.
Since cystatin C has many clinical implications with the increasingly discovered roles in various immunological disorders, characterization of the mechanisms that control cystatin C expression could provide valuable clues for the treatment of pathologies associated with this protease inhibitor. For example, signaling molecules that up-regulate cystatin C expression could be used to promote cystatin C secretion in the case that requires slightly higher cystatin C concentration at the sites of inflammation where excessive protease activity causes tissue damage, as has been suggested to occur in atherosclerosis and aortic aneurysm [39]. On the other hand, the cytokines that repress cystatin C expression, such as IL-10, could be developed for the treatment of diseases associated with too much cystatin C in apoptosis or formation of cystatin C amyloid like Icelandic type of Hereditary Cerebral Amyloid Angiopathy. Moreover, since cystatin C itself has been proven successful in switching the inefficient Th2 cytokine response to an effective Th1 response that cleared the parasite infection in an animal model, it is tempting to speculate that the recombinant product would work in clinical trials. Furthermore, identification of the receptors or signaling pathways that initiate the cystatin C uptake on the cell surface could be translated to target this potent inhibitor to intracellular cancer-promoting proteolysis via the cystatin C internalization.
Finally, identification of transplantable cellular sources for major cystatin C production will also be of great clinical value. Since cystatin C is involved in inflammatory autoimmune diseases, in which immune cells accumulated and played important roles, bone marrow-derived cells are an important cellular source for cystatin C manipulation. Along this line, we found that hematopoietic cells contribute significantly to the systematic pools of cystatin C [43]. Therefore, bone marrow transplantation would be an applicable approach in clinic to treat patients with cystatin C angiopathy or reduction. It is not difficult to speculate that many agents or cystatin C derivatives as an immune regulator would be developed in near future for the treatment of diseases associated with cystatin C related inflammation or autoimmunity.
Declaration of interest
The authors declare no financial or commercial conflict of interest.
Acknowledgements
This work was financially supported by Anhui Natural Science Foundation, China (160511608); Anhui International Science and Technology Collaborative Project, China (160521602); National Nature Science Foundation Major Research Project, China (91742101); and fund from Innovation Team of Scientific Research Platform of Anhui Universities, China, to Y.X.
References
- 1.Magister S., Kos J. Cystatins in immune system. J. Cancer. 2013;4(1):45–56. doi: 10.7150/jca.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turk B., Turk D., Salvesen G.S. Regulating cysteine protease activity: essential role of protease inhibitors as guardians and regulators. Curr. Pharm. Des. 2002;8(18):1623–1637. doi: 10.2174/1381612023394124. [DOI] [PubMed] [Google Scholar]
- 3.Keppler D. Towards novel anti-cancer strategies based on cystatin function. Cancer Lett. 2006;235(2):159–176. doi: 10.1016/j.canlet.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Bobek L.A., Levine M.J. Cystatins–inhibitors of cysteine proteinases. Crit. Rev. Oral Biol. Med. 1992;3(4):307–332. doi: 10.1177/10454411920030040101. [DOI] [PubMed] [Google Scholar]
- 5.Georges S., Ruiz Velasco C., Trichet V., Fortun Y., Heymann D., Padrines M. Proteases and bone remodelling. Cytokine Growth Factor Rev. 2009;20(1):29–41. doi: 10.1016/j.cytogfr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Tavera C., Prevot D., Girolami J.P., Leung-Tack J., Colle A. Tissue and biological fluid distribution of cysteine proteinases inhibitor: rat cystatin C. Biol. Chem. Hoppe-Seyler. 1990;371(Suppl):187–192. [PubMed] [Google Scholar]
- 7.Fossum K., Whitaker J.R. Ficin and papain inhibitor from chicken egg white. Arch. Biochem. Biophys. 1968;125(1):367–375. doi: 10.1016/0003-9861(68)90672-3. [DOI] [PubMed] [Google Scholar]
- 8.Anastasi M.A., Brown A.A., Kembhavi M.J., Nicklin C.A., Sayers D.C., Sunter A.J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem. J. 1983;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall A., Hakansson K., Mason R.W., Grubb A., Abrahamson M. Structural basis for the biological specificity of cystatin C. Identification of leucine 9 in the N-terminal binding region as a selectivity-conferring residue in the inhibition of mammalian cysteine peptidases. J. Biol. Chem. 1995;270(10):5115–5121. doi: 10.1074/jbc.270.10.5115. [DOI] [PubMed] [Google Scholar]
- 10.Staniforth R.A., Giannini S., Higgins L.D., Conroy M.J., Hounslow A.M., Jerala R., Craven C.J., Waltho J.P. Three-dimensional domain swapping in the folded and molten-globule states of cystatins an amyloid-forming structural superfamily. EMBO J. 2001;20(17):4774–4781. doi: 10.1093/emboj/20.17.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann S., Lucius R. Modulation of host immune responses by nematode cystatins. Int. J. Parasitol. 2003;33(11):1291–1302. doi: 10.1016/s0020-7519(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 12.Klotz C., Ziegler T., Danilowicz-Luebert E., Hartmann S. Cystatins of parasitic organisms. Adv. Exp. Med. Biol. 2011;712:208–221. doi: 10.1007/978-1-4419-8414-2_13. [DOI] [PubMed] [Google Scholar]
- 13.Roos J.F., Doust J., Tett S.E., Kirkpatrick C.M. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children–a meta-analysis. Clin. Biochem. 2007;40(5–6):383–391. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Seronie-Vivien S., Delanaye P., Pieroni L., Mariat C., Froissart M., Cristol J.P. S.B.o.r. function g. renal failure working, Cystatin C: current position and future prospects. Clin. Chem. Lab. Med. 2008;46(12):1664–1686. doi: 10.1515/CCLM.2008.336. [DOI] [PubMed] [Google Scholar]
- 15.T, Kimura H., Jiang T., Konno M., Seto K., Iwanaga M., Tsujihata A., Satoh O., Onodera A., Kakita, Takahashi H. Bunina bodies in motor and non-motor neurons revisited: a pathological study of an ALS patient after long-term survival on a respirator. Neuropathology. 2014;34(4):392–397. doi: 10.1111/neup.12105. [DOI] [PubMed] [Google Scholar]
- 16.Xu N., Zhang Y.Y., Lin Y., Bao B., Zheng L., Shi G.P., Liu J. Increased levels of lysosomal cysteinyl cathepsins in human varicose veins: a histology study. Thromb. Haemost. 2014;111(2):333–344. doi: 10.1160/TH13-04-0309. [DOI] [PubMed] [Google Scholar]
- 17.Turk D., Janjic V., Stern I., Podobnik M., Lamba D., Dahl S.W., Lauritzen C., Pedersen J., Turk V., Turk B. Structure of human dipeptidyl peptidase I (cathepsinC): exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. EMBO J. 2001;20(23):6570–6582. doi: 10.1093/emboj/20.23.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werle K., Sauckel C.M., Nathanson M., Bjarnadottir E., Spiess W., Ebert M. Cystatins C, E/M and F in human pleural fluids of patients with neoplastic and inflammatory lung disorders. Biol. Chem. 2003;384(2):281–287. doi: 10.1515/BC.2003.031. [DOI] [PubMed] [Google Scholar]
- 19.Rinne A. Epidermal SH-protease inhibitor in human neoplasms and their metastases. Pathol. Res. Pract. 1980;170(1–3):172–179. doi: 10.1016/S0344-0338(80)80164-6. [DOI] [PubMed] [Google Scholar]
- 20.Davies M.E., Barrett A.J. Immunolocalization of human cystatins in neutrophils and lymphocytes. Histochemistry. 1984;80(4):373–377. doi: 10.1007/BF00495420. [DOI] [PubMed] [Google Scholar]
- 21.Rinne A., Alavaikko M., Jarvinen M., Martikainen J., Karttunen T., Hopsu-Havu V. Demonstration of immunoreactive acid cysteine-proteinase inhibitor in reticulum cells of lymph node germinal centres. Virchows Arch. B. Cell Pathol. Incl. Mol. Pathol. 1983;43(2):121–126. doi: 10.1007/BF02932949. [DOI] [PubMed] [Google Scholar]
- 22.Soderstrom K.O., Rinne R., Hopsu-Havu V.K., Jarvinen M., Rinne A. Identification of acid cysteine proteinase inhibitor (cystatin A) in the human thymus. Anat. Rec. 1994;240(1):115–119. doi: 10.1002/ar.1092400111. [DOI] [PubMed] [Google Scholar]
- 23.Scott D.K., Lord R., Muller H.K., Malley R.C., Woods G.M. Proteomics identifies enhanced expression of stefin A in neonatal murine skin compared with adults: functional implications. Br. J. Dermatol. 2007;156(6):1156–1162. doi: 10.1111/j.1365-2133.2007.07875.x. [DOI] [PubMed] [Google Scholar]
- 24.Sundberg J.P., Boggess D., Hogan M.E., Sundberg B.A., Rourk M.H., Harris B., Johnson K., Dunstan R.W., Davisson M.T. Harlequin ichthyosis (ichq): a juvenile lethal mouse mutation with ichthyosiform dermatitis. Am. J. Pathol. 1997;151(1):293–310. [PMC free article] [PubMed] [Google Scholar]
- 25.Zeeuwen P.L., van Vlijmen-Willems I.M., Hendriks W., Merkx G.F., Schalkwijk J. A null mutation in the cystatin M/E gene of ichq mice causes juvenile lethality and defects in epidermal cornification. Hum. Mol. Genet. 2002;11(23):2867–2875. doi: 10.1093/hmg/11.23.2867. [DOI] [PubMed] [Google Scholar]
- 26.Collins A.R., Grubb A., Cystatin D. a natural salivary cysteine protease inhibitor inhibits coronavirus replication at its physiologic concentration. Oral Microbiol. Immunol. 1998;13(1):59–61. doi: 10.1111/j.1399-302X.1998.tb00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korant B.D., Brzin J., Turk V. Cystatin, a protein inhibitor of cysteine proteases alters viral protein cleavages in infected human cells. Biochem. Biophys. Res. Commun. 1985;127(3):1072–1076. doi: 10.1016/s0006-291x(85)80054-1. [DOI] [PubMed] [Google Scholar]
- 28.Ruzindana-Umunyana A., Weber J.M. Interactions of human lacrimal and salivary cystatins with adenovirus endopeptidase. Antiviral Res. 2001;51(3):203–214. doi: 10.1016/s0166-3542(01)00154-1. [DOI] [PubMed] [Google Scholar]
- 29.Gu M., Haraszthy G.G., Collins A.R., Bergey E.J. Identification of salivary proteins inhibiting herpes simplex virus 1 replication. Oral Microbiol. Immunol. 1995;10(1):54–59. doi: 10.1111/j.1399-302x.1995.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 30.Coombs G.H., Mottram J.C. Parasite proteinases and amino acid metabolism: possibilities for chemotherapeutic exploitation. Parasitology. 1997;114(Suppl):S61–S80. [PubMed] [Google Scholar]
- 31.Descoteaux A. Leishmania cysteine proteinases: virulence factors in quest of a function. Parasitol. Today. 1998;14(6):220–221. doi: 10.1016/s0169-4758(98)01241-1. [DOI] [PubMed] [Google Scholar]
- 32.Verdot L., Lalmanach G., Vercruysse V., Hartmann S., Lucius R., Hoebeke J., Gauthier F., Vray B. Cystatins up-regulate nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages. J. Biol. Chem. 1996;271(45):28077–28081. doi: 10.1074/jbc.271.45.28077. [DOI] [PubMed] [Google Scholar]
- 33.Das L., Datta N., Bandyopadhyay S., Das P.K. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T cell response. J. Immunol. 2001;166(6):4020–4028. doi: 10.4049/jimmunol.166.6.4020. [DOI] [PubMed] [Google Scholar]
- 34.Bjorck L., Akesson P., Bohus M., Trojnar J., Abrahamson M., Olafsson I., Grubb A. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature. 1989;337(6205):385–386. doi: 10.1038/337385a0. [DOI] [PubMed] [Google Scholar]
- 35.Collins A.R., Grubb A. Inhibitory effects of recombinant human cystatin C on human coronaviruses. Antimicrob. Agents Chemother. 1991;35(11):2444–2446. doi: 10.1128/aac.35.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura H., Kamon H., Sawa S., Park S.J., Katunuma N., Ishihara K., Murakami M., Hirano T. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23(5):491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Zavasnik-Bergant T., Repnik U., Schweiger A., Romih R., Jeras M., Turk V., Kos J. Differentiation- and maturation-dependent content localization, and secretion of cystatin C in human dendritic cells. J. Leukoc. Biol. 2005;78(1):122–134. doi: 10.1189/jlb.0804451. [DOI] [PubMed] [Google Scholar]
- 38.Pierre P., Mellman I. Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell. 1998;93(7):1135–1145. doi: 10.1016/s0092-8674(00)81458-0. [DOI] [PubMed] [Google Scholar]
- 39.Shi G.P., Sukhova G.K., Grubb A., Ducharme A., Rhode L.H., Lee R.T., Ridker P.M., Libby P., Chapman H.A. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J. Clin. Invest. 1999;104(9):1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa T.Y., Brissette W.H., Lira P.D., Griffiths R.J., Petrushova N., Stock J., McNeish J.D., Eastman S.E., Howard E.D., Clarke S.R., Rosloniec E.F., Elliott E.A., Rudensky A.Y. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10(2):207–217. doi: 10.1016/s1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 41.Rovere P., Zimmermann V.S., Forquet F., Demandolx D., Trucy J., Ricciardi-Castagnoli P., Davoust J. Dendritic cell maturation and antigen presentation in the absence of invariant chain. Proc. Natl. Acad. Sci. U. S. A. 1998;95(3):1067–1072. doi: 10.1073/pnas.95.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Sukkari D., Wilson N.S., Hakansson K., Steptoe R.J., Grubb A., Shortman K., Villadangos J.A. The protease inhibitor cystatin C is differentially expressed among dendritic cell populations but does not control antigen presentation. J. Immunol. 2003;171(10):5003–5011. doi: 10.4049/jimmunol.171.10.5003. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y., Lindemann P., Vega-Ramos J., Zhang J.G., Villadangos J.A. Developmental regulation of synthesis and dimerization of the amyloidogenic protease inhibitor cystatin C in the hematopoietic system. J. Biol. Chem. 2014;289(14):9730–9740. doi: 10.1074/jbc.M113.538041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen J.E., Daub J., Guiliano D., McDonnell A., Lizotte-Waniewski M., Taylor D.W., Blaxter M. Analysis of genes expressed at the infective larval stage validates utility of Litomosoides sigmodontis as a murine model for filarial vaccine development. Infect. Immun. 2000;68(9):5454–5458. doi: 10.1128/iai.68.9.5454-5458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manoury B., Gregory W.F., Maizels R.M., Watts C. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr. Biol.: CB. 2001;11(6):447–451. doi: 10.1016/s0960-9822(01)00118-x. [DOI] [PubMed] [Google Scholar]
- 46.Bowcock A.M., Shannon W., Du F., Duncan J., Cao K., Aftergut K., Catier J., Fernandez-Vina M.A., Menter A. Insights into psoriasis and other inflammatory diseases from large-scale gene expression studies. Hum. Mol. Genet. 2001;10(17):1793–1805. doi: 10.1093/hmg/10.17.1793. [DOI] [PubMed] [Google Scholar]
- 47.Vasilopoulos Y., Cork M.J., Teare D., Marinou I., Ward S.J., Duff G.W., Tazi-Ahnini R. A nonsynonymous substitution of cystatin A a cysteine protease inhibitor of house dust mite protease, leads to decreased mRNA stability and shows a significant association with atopic dermatitis. Allergy. 2007;62(5):514–519. doi: 10.1111/j.1398-9995.2007.01350.x. [DOI] [PubMed] [Google Scholar]
- 48.Cork M.J., Robinson D.A., Vasilopoulos Y., Ferguson A., Moustafa M., MacGowan A., Duff G.W., Ward S.J., Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J. Allergy Clin. Immunol. 2006;118(1):3–21. doi: 10.1016/j.jaci.2006.04.042. quiz 22-3. [DOI] [PubMed] [Google Scholar]
- 49.Kato T., Takai T., Mitsuishi K., Okumura K., Ogawa H. Cystatin A inhibits IL-8 production by keratinocytes stimulated with Der p 1 and Der f 1: biochemical skin barrier against mite cysteine proteases. J. Allergy Clin. Immunol. 2005;116(1):169–176. doi: 10.1016/j.jaci.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa T., Takai T., Kato T., Kikuchi Y., Niyonsaba F., Ikeda S., Okumura K., Ogawa H. Upregulation of the release of granulocyte-macrophage colony-stimulating factor from keratinocytes stimulated with cysteine protease activity of recombinant major mite allergens Der f 1 and Der p 1. Int. Arch. Allergy Immunol. 2008;146(1):27–35. doi: 10.1159/000112500. [DOI] [PubMed] [Google Scholar]
- 51.Blaydon D.C., Nitoiu D., Eckl K.M., Cabral R.M., Bland P., Hausser I., van Heel D.A., Rajpopat S., Fischer J., Oji V., Zvulunov A., Traupe H., Hennies H.C., Kelsell D.P. Mutations in CSTA encoding Cystatin A, underlie exfoliative ichthyosis and reveal a role for this protease inhibitor in cell–cell adhesion. Am. J. Hum. Genet. 2011;89(4):564–571. doi: 10.1016/j.ajhg.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Cid R., Riveira-Munoz E., Zeeuwen P.L., Robarge J., Liao W., Dannhauser E.N., Giardina E., Stuart P.E., Nair R., Helms C., Escaramis G., Ballana E., Martin-Ezquerra G., den Heijer M., Kamsteeg I., Joosten E.E., Eichler C., Lazaro R.M., Pujol L., Armengol G., Abecasis J.T., Elder G., Novelli J.A., Armour P.Y., Kwok A., Bowcock J., Schalkwijk X. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat. Genet. 2009;41(2):211–215. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollox E.J., Huffmeier U., Zeeuwen P.L., Palla R., Lascorz J., Rodijk-Olthuis D., van de Kerkhof P.C., Traupe H., de Jongh G., den Heijer M., Reis A., Armour J.A., Schalkwijk J. Psoriasis is associated with increased beta-defensin genomic copy number. Nat. Genet. 2008;40(1):23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng T., Tjabringa G.S., van Vlijmen-Willems I.M., Hitomi K., van Erp P.E., Schalkwijk J., Zeeuwen P.L. The cystatin M/E-controlled pathway of skin barrier formation: expression of its key components in psoriasis and atopic dermatitis. Br. J. Dermatol. 2009;161(2):253–264. doi: 10.1111/j.1365-2133.2009.09156.x. [DOI] [PubMed] [Google Scholar]
- 55.Trabandt A., Gay R.E., Fassbender H.G., Gay S. Cathepsin B in synovial cells at the site of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1991;34(11):1444–1451. doi: 10.1002/art.1780341116. [DOI] [PubMed] [Google Scholar]
- 56.Reddy V.Y., Zhang Q.Y., Weiss S.J. Pericellular mobilization of the tissue-destructive cysteine proteinases cathepsins B, L, and S, by human monocyte-derived macrophages. Proc. Natl. Acad. Sci. U. S. A. 1995;92(9):3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yasuda Y., Kaleta J., Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv. Drug Deliv. Rev. 2005;57(7):973–993. doi: 10.1016/j.addr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Maciewicz R.A., Wotton S.F. Degradation of cartilage matrix components by the cysteine proteinases, cathepsins B and L. Biomed. Biochim. Acta. 1991;50(4-6):561–564. [PubMed] [Google Scholar]
- 59.Page A.E., Hayman A.R., Andersson L.M., Chambers T.J., Warburton M.J. Degradation of bone matrix proteins by osteoclast cathepsins. Int. J. Biochem. 1993;25(4):545–550. doi: 10.1016/0020-711x(93)90662-x. [DOI] [PubMed] [Google Scholar]
- 60.Baumgrass R., Williamson M.K., Price P.A. Identification of peptide fragments generated by digestion of bovine and human osteocalcin with the lysosomal proteinases cathepsin B D, L, H, and S. J. Bone Miner. Res. 1997;12(3):447–455. doi: 10.1359/jbmr.1997.12.3.447. [DOI] [PubMed] [Google Scholar]
- 61.Backlund A., Holmdahl M., Mattsson R., Hakansson K., Lindstrom V., Nandakumar K.S., Grubb A., Holmdahl R. Cystatin C influences the autoimmune but not inflammatory response to cartilage type II collagen leading to chronic arthritis development. Arthritis. Res. Ther. 2011;13(2):R54. doi: 10.1186/ar3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bokarewa M., Abrahamson M., Levshin N., Egesten A., Grubb A., Dahlberg L., Tarkowski A. Cystatin C binds serum amyloid A, downregulating its cytokine-generating properties. J. Rheumatol. 2007;34(6):1293–1301. [PubMed] [Google Scholar]
- 63.Hansen T., Petrow P.K., Gaumann A., Keyszer G.M., Eysel P., Eckardt A., Brauer R., Kriegsmann J. Cathepsin B and its endogenous inhibitor cystatin C in rheumatoid arthritis synovium. J. Rheumatol. 2000;27(4):859–865. [PubMed] [Google Scholar]
- 64.Wallin H., Abrahamson M., Ekstrom U. Cystatin C properties crucial for uptake and inhibition of intracellular target enzymes. J. Biol. Chem. 2013;288(23):17019–17029. doi: 10.1074/jbc.M113.453449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y., Ding Y., Li X., Wu X. Cystatin C is a disease-associated protein subject to multiple regulation. Immunol. Cell Biol. 2015;93(5):442–451. doi: 10.1038/icb.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Packard R.R., Maganto-Garcia E., Gotsman I., Tabas I., Libby P., Lichtman A.H. CD11c(+) dendritic cells maintain antigen processing presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ. Res. 2008;103(9):965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mellman I. Antigen processing and presentation by dendritic cells: cell biological mechanisms. Adv. Exp. Med. Biol. 2005;560:63–67. doi: 10.1007/0-387-24180-9_9. [DOI] [PubMed] [Google Scholar]
- 68.Boltjes A., van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Front. Immunol. 2014;5:131. doi: 10.3389/fimmu.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cappello F., Gatti E., Camossetto V., David A., Lelouard H., Pierre P. Cystatin F is secreted, but artificial modification of its C-terminus can induce its endocytic targeting. Exp. Cell Res. 2004;297(2):607–618. doi: 10.1016/j.yexcr.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 70.Janowski R., Kozak M., Jankowska E., Grzonka Z., Grubb A., Abrahamson M., Jaskolski M. Human cystatin C an amyloidogenic protein, dimerizes through three-dimensional domain swapping. Nat. Struct. Biol. 2001;8(4):316–320. doi: 10.1038/86188. [DOI] [PubMed] [Google Scholar]
- 71.Ekiel I., Abrahamson M. Folding-related dimerization of human cystatin C. J. Biol. Chem. 1996;271(3):1314–1321. doi: 10.1074/jbc.271.3.1314. [DOI] [PubMed] [Google Scholar]
- 72.Naik S.H., Proietto A.I., Wilson N.S., Dakic A., Schnorrer P., Fuchsberger M., Lahoud M.H., O'Keeffe M., Shao Q.X., Chen W.F., Villadangos J.A., Shortman K., Wu L. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 2005;174(11):6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 73.Cheong C., Matos I., Choi J.H., Dandamudi D.B., Shrestha E., Longhi M.P., Jeffrey K.L., Anthony R.M., Kluger C., Nchinda G., Koh H., Rodriguez A., Idoyaga J., Pack M., Velinzon K., Park C.G., Steinman R.M. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143(3):416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Y., Schnorrer P., Proietto A., Kowalski G., Febbraio M.A., Acha-Orbea H., Dickins R.A., Villadangos J.A. IL-10 controls cystatin C synthesis and blood concentration in response to inflammation through regulation of IFN regulatory factor 8 expression. J. Immunol. 2011;186(6):3666–3673. doi: 10.4049/jimmunol.1001934. [DOI] [PubMed] [Google Scholar]
- 75.Leung-Tack J., Tavera C., Er-Raki A., Gensac M.C., Colle A. Rat cystatin C: inhibitor of granulocyte phagocytic functions. Biol. Chem. Hoppe-Seyler. 1990;371(Suppl):255–258. [PubMed] [Google Scholar]
- 76.Lukasiuk K., Pirttila T.J., Pitkanen A. Upregulation of cystatin C expression in the rat hippocampus during epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Epilepsia. 2002;43(Suppl 5):137–145. doi: 10.1046/j.1528-1157.43.s.5.20.x. [DOI] [PubMed] [Google Scholar]
- 77.Abrahamson M., Jonsdottir S., Olafsson I., Jensson O., Grubb A. Hereditary cystatin C amyloid angiopathy: identification of the disease-causing mutation and specific diagnosis by polymerase chain reaction based analysis. Hum. Genet. 1992;89(4):377–380. doi: 10.1007/BF00194306. [DOI] [PubMed] [Google Scholar]
- 78.Chapman H.A., Jr. Role of enzyme receptors and inhibitors in regulating proteolytic activities of macrophages. Ann. N. Y. Acad. Sci. 1991;624:87–96. doi: 10.1111/j.1749-6632.1991.tb17009.x. [DOI] [PubMed] [Google Scholar]
- 79.Maekawa Y., Himeno K., Ishikawa H., Hisaeda H., Sakai T., Dainichi T., Asao T., Good R.A., Katunuma N. Switch of CD4+ T cell differentiation from Th2 to Th1 by treatment with cathepsin B inhibitor in experimental leishmaniasis. J. Immunol. 1998;161(5):2120–2127. [PubMed] [Google Scholar]
- 80.Ziegler T., Rausch S., Steinfelder S., Klotz C., Hepworth M.R., Kuhl A.A., Burda P.C., Lucius R., Hartmann S. A novel regulatory macrophage induced by a helminth molecule instructs IL-10 in CD4+ T cells and protects against mucosal inflammation. J. Immunol. 2015;194(4):1555–1564. doi: 10.4049/jimmunol.1401217. [DOI] [PubMed] [Google Scholar]
- 81.Figueiredo A.S., Hofer T., Klotz C., Sers C., Hartmann S., Lucius R., Hammerstein P. Modelling and simulating interleukin-10 production and regulation by macrophages after stimulation with an immunomodulator of parasitic nematodes. FEBS J. 2009;276(13):3454–3469. doi: 10.1111/j.1742-4658.2009.07068.x. [DOI] [PubMed] [Google Scholar]
- 82.Kar S., Ukil A., Das P.K. Signaling events leading to the curative effect of cystatin on experimental visceral leishmaniasis: involvement of ERK1/2 NF-kappaB and JAK/STAT pathways. Eur. J. Immunol. 2009;39(3):741–751. doi: 10.1002/eji.200838465. [DOI] [PubMed] [Google Scholar]
- 83.Gren S.T., Janciauskiene S., Sandeep S., Jonigk D., Kvist P.H., Gerwien J.G., Hakansson K., Grip O. The protease inhibitor cystatin C down-regulates the release of IL-beta and TNF-alpha in lipopolysaccharide activated monocytes. J. Leukoc. Biol. 2016;100(4):811–822. doi: 10.1189/jlb.5A0415-174R. [DOI] [PubMed] [Google Scholar]
- 84.Vray B., Hartmann S., Hoebeke J. Immunomodulatory properties of cystatins. Cell. Mol. Life Sci.: CMLS. 2002;59(9):1503–1512. doi: 10.1007/s00018-002-8525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartmann S., Kyewski B., Sonnenburg B., Lucius R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur. J. Immunol. 1997;27(9):2253–2260. doi: 10.1002/eji.1830270920. [DOI] [PubMed] [Google Scholar]
- 86.Robey E., Allison J.P. T-cell activation: integration of signals from the antigen receptor and costimulatory molecules. Immunol. Today. 1995;16(7):306–310. doi: 10.1016/0167-5699(95)80140-5. [DOI] [PubMed] [Google Scholar]
- 87.Zeeuwen P.L. Epidermal differentiation: the role of proteases and their inhibitors. Eur. J. Cell Biol. 2004;83(11–12):761–773. doi: 10.1078/0171-9335-00388. [DOI] [PubMed] [Google Scholar]
- 88.Schonemeyer A., Lucius R., Sonnenburg B., Brattig N., Sabat R., Schilling K., Bradley J., Hartmann S. Modulation of human T cell responses and macrophage functions by onchocystatin a secreted protein of the filarial nematode Onchocerca volvulus. J. Immunol. 2001;167(6):3207–3215. doi: 10.4049/jimmunol.167.6.3207. [DOI] [PubMed] [Google Scholar]
- 89.Warfel A.H., Zucker-Franklin D., Frangione B., Ghiso J. Constitutive secretion of cystatin C (gamma-trace) by monocytes and macrophages and its downregulation after stimulation. J. Exp. Med. 1987;166(6):1912–1917. doi: 10.1084/jem.166.6.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frendeus K.H., Wallin H., Janciauskiene S., Abrahamson M. Macrophage responses to interferon-gamma are dependent on cystatin C levels. Int. J. Biochem. Cell Biol. 2009;41(11):2262–2269. doi: 10.1016/j.biocel.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Baldwin A.S., Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 92.Vila-del Sol V., Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J. Immunol. 2005;174(5):2825–2833. doi: 10.4049/jimmunol.174.5.2825. [DOI] [PubMed] [Google Scholar]
- 93.Manetti R., Gerosa F., Giudizi M.G., Biagiotti R., Parronchi P., Piccinni M.P., Sampognaro S., Maggi E., Romagnani S., Trinchieri G. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J. Exp. Med. 1994;79(4):1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.James S.L. Role of nitric oxide in parasitic infections. Microbiol. Rev. 1995;59(4):533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murray H.W., Nathan C.F. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 1999;189(4):741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y.H., Han Y.P., Li Z.Y., Wei J., He H.J., Xu C.Z., Zheng H.Q., Zhan X.M., Wu Z.D., Lv Z.Y. Molecular cloning and characterization of cystatin a cysteine protease inhibitor, from Angiostrongylus cantonensis. Parasitol. Res. 2010;107(4):915–922. doi: 10.1007/s00436-010-1952-5. [DOI] [PubMed] [Google Scholar]
- 97.Verdot L., Lalmanach G., Vercruysse V., Hoebeke J., Gauthier F., Vray B. Chicken cystatin stimulates nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages via cytokine synthesis. Eur. J. Biochem. 1999;266(3):1111–1117. doi: 10.1046/j.1432-1327.1999.00964.x. [DOI] [PubMed] [Google Scholar]
- 98.Hartmann S., Schonemeyer A., Sonnenburg B., Vray B., Lucius R. Cystatins of filarial nematodes up-regulate the nitric oxide production of interferon-gamma-activated murine macrophages. Parasite Immunol. 2002;24(5):253–262. doi: 10.1046/j.1365-3024.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 99.Schierack P., Lucius R., Sonnenburg B., Schilling K., Hartmann S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect. Immun. 2003;71(5):2422–2429. doi: 10.1128/IAI.71.5.2422-2429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie Q.W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. 1993;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chakravortty D., Kato Y., Sugiyama T., Koide N., Mu M.M., Yoshida T., Yokochi T. The inhibitory action of sodium arsenite on lipopolysaccharide-induced nitric oxide production in RAW 267.4 macrophage cells: a role of Raf-1 in lipopolysaccharide signaling. J. Immunol. 2001;166(3):2011–2017. doi: 10.4049/jimmunol.166.3.2011. [DOI] [PubMed] [Google Scholar]
- 102.Yang L., Pang Y., Moses H.L. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shariat S.F., Kattan M.W., Traxel E., Andrews B., Zhu K., Wheeler T.M., Slawin K.M. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin. Cancer Res. 2004;10(6):1992–1999. doi: 10.1158/1078-0432.ccr-0768-03. [DOI] [PubMed] [Google Scholar]
- 104.Petrausch U., Jensen S.M., Twitty C., Poehlein C.H., Haley D.P., Walker E.B., Fox B.A. Disruption of TGF-beta signaling prevents the generation of tumor-sensitized regulatory T cells and facilitates therapeutic antitumor immunity. J. Immunol. 2009;183(6):3682–3689. doi: 10.4049/jimmunol.0900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 106.Sokol J.P., Schiemann W.P. Cystatin C antagonizes transforming growth factor beta signaling in normal and cancer cells. Mol. Cancer Res.: MCR. 2004;2(3):183–195. [PubMed] [Google Scholar]
- 107.Szweras M., Liu D., Partridge E.A., Pawling J., Sukhu B., Clokie C., Jahnen-Dechent W., Tenenbaum H.C., Swallow C.J., Grynpas M.D., Dennis J.W. alpha 2-HS glycoprotein/fetuin a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J. Biol. Chem. 2002;277(22):19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 108.Swallow C.J., Partridge E.A., Macmillan J.C., Tajirian T., DiGuglielmo G.M., Hay K., Szweras M., Jahnen-Dechent W., Wrana J.L., Redston M., Gallinger S., Dennis J.W. alpha2HS-glycoprotein an antagonist of transforming growth factor beta in vivo, inhibits intestinal tumor progression. Cancer Res. 2004;64(18):6402–6409. doi: 10.1158/0008-5472.CAN-04-1117. [DOI] [PubMed] [Google Scholar]
- 109.Tian M., Schiemann W.P. Preclinical efficacy of cystatin C to target the oncogenic activity of transforming growth factor Beta in breast cancer. Transl. Oncol. 2009;2(3):174–183. doi: 10.1593/tlo.09145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Waal Malefyt R., Yssel H., Roncarolo M.G., Spits H., de Vries J.E. Interleukin-10. Curr. Opin. Immunol. 1992;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 111.Boya P., Andreau K., Poncet D., Zamzami N., Perfettini J.L., Metivier D., Ojcius D.M., Jaattela M., Kroemer G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J. Exp. Med. 2003;197(10):1323–1334. doi: 10.1084/jem.20021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guicciardi M.E., Deussing J., Miyoshi H., Bronk S.F., Svingen P.A., Peters C., Kaufmann S.H., Gores G.J. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 2000;106(9):1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wyllie A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 114.Vancompernolle K., Van Herreweghe F., Pynaert G., Van de Craen M., De Vos K., Totty N., Sterling A., Fiers W., Vandenabeele P., Grooten J. Atractyloside-induced release of cathepsin B a protease with caspase-processing activity. FEBS Lett. 1998;438(3):150–158. doi: 10.1016/s0014-5793(98)01275-7. [DOI] [PubMed] [Google Scholar]
- 115.van Eijk M., de Groot C. Germinal center B cell apoptosis requires both caspase and cathepsin activity. J. Immunol. 1999;163(5):2478–2482. [PubMed] [Google Scholar]
- 116.Ishisaka R., Utsumi T., Yabuki M., Kanno T., Furuno T., Inoue M., Utsumi K. Activation of caspase-3-like protease by digitonin-treated lysosomes. FEBS Lett. 1998;435(2–3):233–236. doi: 10.1016/s0014-5793(98)01080-1. [DOI] [PubMed] [Google Scholar]
- 117.Jones B., Roberts P.J., Faubion W.A., Kominami E., Gores G.J., Cystatin A. expression reduces bile salt-induced apoptosis in a rat hepatoma cell line. Am. J. Physiol. 1998;75(4 Pt 1):G723–30. doi: 10.1152/ajpgi.1998.275.4.G723. [DOI] [PubMed] [Google Scholar]
- 118.Bjorklund H.V., Johansson T.R., Rinne A. Rhabdovirus-induced apoptosis in a fish cell line is inhibited by a human endogenous acid cysteine proteinase inhibitor. J. Virol. 1997;71(7):5658–5662. doi: 10.1128/jvi.71.7.5658-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whyte M.K., Meagher L.C., MacDermot J., Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J. Immunol. 1993;150(11):5124–5134. [PubMed] [Google Scholar]
- 120.Majewska E., Wittek N., Rysz J., Baj Z. The influence of uremic high cystatin C concentration on neutrophil apoptosis and selected neutrophil functions isolated from healthy subjects. Med. Sci. Monit.: Int. Med. J. Exp. Clin. Res. 2012;18(11):CR667–73. doi: 10.12659/MSM.883545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Q., Salvesen G.S. Activation of pro-caspase-7 by serine proteases includes a non-canonical specificity. Biochem. J. 1997;324(Pt 2):361–364. doi: 10.1042/bj3240361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bird P.I. Regulation of pro-apoptotic leucocyte granule serine proteinases by intracellular serpins. Immunol. Cell Biol. 1999;77(1):47–57. doi: 10.1046/j.1440-1711.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- 123.McGuire M.J., Lipsky P.E., Thiele D.L. Generation of active myeloid and lymphoid granule serine proteases requires processing by the granule thiol protease dipeptidyl peptidase I. J. Biol. Chem. 1993;268(4):2458–2467. [PubMed] [Google Scholar]
- 124.Podack E.R., Hengartner H., Lichtenheld M.G. A central role of perforin in cytolysis? Annu. Rev. Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 125.Pham C.T., Ley T.J. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc. Natl. Acad. Sci. U. S. A. 1999;96(15):8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kummer J.A., Kamp A.M., Citarella F., Horrevoets A.J., Hack C.E. Expression of human recombinant granzyme A zymogen and its activation by the cysteine proteinase cathepsin C. J. Biol. Chem. 1996;271(16):9281–9286. doi: 10.1074/jbc.271.16.9281. [DOI] [PubMed] [Google Scholar]
- 127.Podack E.R. How to induce involuntary suicide: the need for dipeptidyl peptidase I. Proc. Natl. Acad. Sci. U. S. A. 1999;96(15):8312–8314. doi: 10.1073/pnas.96.15.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pham C.T., Thomas D.A., Mercer J.D., Ley T.J. Production of fully active recombinant murine granzyme B in yeast. J. Biol. Chem. 1998;273(3):1629–1633. doi: 10.1074/jbc.273.3.1629. [DOI] [PubMed] [Google Scholar]
- 129.Mullbacher A., Waring P., Tha Hla R., Tran T., Chin S., Stehle T., Museteanu C., Simon M.M. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc. Natl. Acad. Sci. U. S. A. 1999;96(24):13950–13955. doi: 10.1073/pnas.96.24.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Droin N., Bichat F., Rebe C., Wotawa A., Sordet O., Hammann A., Bertrand R., Solary E. Involvement of caspase-2 long isoform in Fas-mediated cell death of human leukemic cells. Blood. 2001;97(6):1835–1844. doi: 10.1182/blood.v97.6.1835. [DOI] [PubMed] [Google Scholar]
- 131.Liang X., Nagai A., Terashima M., Sheikh A.M., Shiota Y., Mitaki S., Kim S.U., Yamaguchi S. Cystatin C induces apoptosis and tyrosine hydroxylase gene expression through JNK-dependent pathway in neuronal cells. Neurosci. Lett. 2011;496(2):100–105. doi: 10.1016/j.neulet.2011.03.091. [DOI] [PubMed] [Google Scholar]
- 132.Balaji K.N., Schaschke N., Machleidt W., Catalfamo M., Henkart P.A. Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J. Exp. Med. 2002;196(4):493–503. doi: 10.1084/jem.20011836. [DOI] [PMC free article] [PubMed] [Google Scholar]