Abstract
The methods of repeated immunization with inactivated vaccines have been used widely to increase antibody protection against infectious bronchitis virus (IBV). However, compared with DNA vaccines, these methods usually induce poor cellular responses. In the present study, specific pathogen-free (SPF) chickens were immunized intramuscularly with a DNA vaccine carrying the main IBV structural genes (pVAX1-S1, pVAX1-M, and pVAX1-N, respectively) and boosted with the IBV M41 strain inactivated vaccine to assess whether such a new strategy could enhance the immune responses against IBV. The protection efficacy of the DNA vaccine carrying different structural genes for priming was evaluated further. The chickens were immunized primely on day 7 and boosted 2 weeks later. After that, distribution of the DNA vaccine in vivo, the percentage of CD4+CD3+ and CD8+CD3+ subgroups of peripheral blood T-lymphocytes, and the specific IgG and virus neutralizing antibodies were measured. Chickens were then challenged by the nasal–ocular route with the IBV M41 strain 4 weeks after booster immunization. The results demonstrated that priming with a DNA vaccine encoding nucleocapsid protein (pVAX1-N) and boosting with the inactivated IBV vaccine led to the dramatic augmentation of humoral and cellular responses, and provided up to 86.7% rate of immune protection, providing an effective approach to protect chickens from IBV.
Keywords: Infectious bronchitis virus (IBV), DNA vaccine, Prime-boost
1. Introduction
Infectious bronchitis virus (IBV) is an enveloped coronavirus that contains an unsegmented, single-stranded and positive-sense RNA genome. The IBV genome encodes four major structural proteins, known as the spike (S) glycoprotein, the small envelope (E) protein, the membrane (M) glycoprotein, and the nucleocapsid (N) protein (Spaan et al., 1988, Peng et al., 2006). The spike protein is cleaved into S1 and S2, of which S1 produces neutralizing and serotype-specific antibodies and cell attachment determinants have been located to the S1 (Cavanagh et al., 1992). The small envelope protein E, which is mostly embedded within the envelope, has not been reported for cytotoxic T-lymphocyte (CTL) epitopes yet. The amino terminus of the M protein lies outside of the virion particle and is glycosylated, and the N protein is associated with the 27.6 kb genome within the viral particle and plays important roles in the structure and replication of IBV (Collisson et al., 2000, Stern and Sefton, 1982).
Infection with IBV reduces the performance of broilers and egg production (Cavanagh and Naqi, 1997), making a severe economic impact on domestic commercial poultry industry. Therefore, considerable efforts on vaccination for preventing IB have been made for over half a century. Traditional treatments against virus infection usually involve inactivated vaccines or live attenuated vaccines (Bijlenga et al., 2004, Tang et al., 2008). However, pitfalls have been found in both of the above types of vaccines: the inactivated vaccines can induce high titers of antibody but usually with lower CTL responses (Ariane et al., 2009, Yang et al., 2004). For IBV, the single involvement of the inactivated vaccines usually results in less than 50% protection in chickens (Cavanagh, 2003); the live attenuated vaccines can induce both humoral and cellular immune responses, but with a possibility of spreading the live vaccine virus (Wareing and Tannock, 2001). As an alternative, the development of DNA vaccines has been considered as a possible strategy because of the long lasting expression period, the induction of cellular responses (Woodland, 2004), and safety (Wolff et al., 1990). However, the weak and slow humoral responses induced by DNA vaccines are still a problem (Li et al., 2008). Therefore, developing a more effective vaccination schedule for the practical control of infectious bronchitis (IB) is still required.
Due to above considerations, a prime-boost schedule of “a DNA vaccine plus an inactivated vaccine” is proposed as a possible means for inducing humoral as well as cellular responses (Ramshaw and Ramsay, 2000, Ming et al., 2007, Wang et al., 2008). However, this method has never been used in practice against IBV. The purpose of the present study was to test whether priming with a DNA vaccine carrying the main IBV structural genes followed by boosting with the inactivated IBV vaccine could enhance immune responses, and to determine which structural gene in the priming gives the best protection against infection.
2. Materials and methods
2.1. Virus, SPF chicken, cells and inactivated vaccine
The respiratory-pathogenic IBV M41 strain (China Institute of Veterinary Drug Control, IVDC) was propagated in the allantoic cavities of 10-day-old specific pathogen-free (SPF) embryonated chicken eggs, and the allantoic fluid was then harvested 36 h after inoculation. The 50% egg infection dose (EID50) was determined by inoculating serial 10-fold dilutions of virus into the 10-day-old SPF embryonated chicken eggs. SPF chicken embryos (Shandong Institute of Poultry Science, Shandong, China) were hatched and housed in a specific pathogen-free environment at the Laboratory Animal and Resources Facility, Sichuan University. COS-7 cells were cultured at 37 °C with 5% carbon dioxide in Dulbecco's modified Eagle's medium (Gibco, BRL, USA) supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at pH 7.2. The IB inactivated (M41 strain) vaccine was purchased from IVDC.
2.2. DNA vaccine construction
The plasmid pVAX1 (Invitrogen, Carlsbad, CA, USA) which is consistent with the Guidance of Clinic Considerations for DNA Vaccines for Infectious Disease Indications (SFDA) and licensed for DNA vaccines, was used in this study. After amplification by RT-PCR, the S1 (GenBank accession no. AY561711), M (GenBank accession no. AF286184), and N (GenBank accession no. EU116941) genes of IBV strain M41 were cloned into the expression vector pVAX1 and designated as pVAX1-S1, pVAX1-M, and pVAX1-N, respectively (Fig. 1 ). All plasmids were sequenced to confirm cloning accuracy.
Fig. 1.
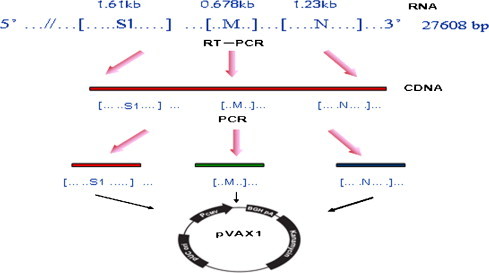
Strategy for construction of the structural gene based DNA vaccine. The viral RNA was extracted, RT-PCR was carried out, and each of the structure genes was cloned into pVAX1 to construct the DNA vaccine.
2.3. In vitro expression of the plasmid DNA
The expression of recombinant plasmids was demonstrated by indirect immunofluorescence assay (IFA). Six-well tissue culture plates were seeded with COS-7 cells (106/well). Monolayer of 80–90% confluent cells was transfected with the lipofectamine containing plasmids pVAX1-S1, pVAX1-M, pVAX1-N, and empty pVAX1 vector, respectively, by using the lipofectamine reagent (Invitrogen, Carlsbad, CA, USA). Thirty-six hours after transfection, cells were washed with phosphate-buffered saline (PBS), fixed with 100% acetone for 10 min at −20 °C and washed once again with PBS. Diluted primary and secondary antibodies were incubated at 37 °C for 1 h, respectively. The primary antibodies used were antiserum of rabbit to IBV, and the secondary antibodies were FITC-conjugated goat anti-rabbit IgG (Sigma–Aldrich, St. Louis, MO, USA). Cells were washed and the expression of the recombinant plasmids was analyzed by fluorescence microscopy.
2.4. Immunization of chickens
Plasmids pVAX1-S1, pVAX1-M, pVAX1-N and pVAX1 amplified in Escherichia coli DH5α were extracted using the ammonium acetate lysis method (Xie et al., 2007). After purification by polyethylene glycol (PEG-8000) precipitation, the plasmids were incorporated into lipofectamine. For immunization, 7-day-old chickens were divided at random into 8 groups (n = 20 each) and were immunized intramuscularly on days 7 and 21 with different immunization strategies listed in Table 1 .
Table 1.
Immunization schedule.
| Group | n | Candidate vaccinesa |
|
|---|---|---|---|
| 7 day (priming) | 21 day (boosting) | ||
| Group 1 | 20 | pVAX1-N | pVAX1-N |
| Group 2 | 20 | pVAX1-N | Inactivated vaccine |
| Group 3 | 20 | pVAX1-N+ inactivated vaccine | pVAX1-N+ inactivated vaccine |
| Group 4 | 20 | pVAX1-M | Inactivated vaccine |
| Group 5 | 20 | pVAX1-S1 | Inactivated vaccine |
| Group 6 | 20 | pVAX1 | Inactivated vaccine |
| Group 7 | 20 | Inactivated vaccine | Inactivated vaccine |
| Group 8 | 20 | PBS | PBS |
Each group was intramuscularly injected with 100 μg DNA vaccine and 0.1 ml inactivated IBV vaccine.
2.5. Detection of distribution of the DNA vaccine in vivo
For each group, five chickens were euthanized on day 12 (5 days after DNA immunization), and the tissues were collected and ground under sterile conditions in the order of heart, lung, liver, spleen and kidney. The genomic DNA was then extracted from the tissues using DNAzol reagents (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instruction, and used as PCR templates for detecting the distribution of DNA vaccines in vivo.
2.6. Analysis of CD4+CD3+ and CD8+CD3+ T-lymphocytes
Seven days after boosting, peripheral blood samples from immunized chicken were collected from the jugular vein into 2.5 ml syringes preloaded with 0.2 ml of sodium heparin. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll–Hypaque density gradient centrifugation and adjusted to 1 × 107 cells/ml. 100 μl of cell suspensions (1 × 106 cells) was incubated for 1 h at room temperature with antibodies as follows: mouse anti-chicken CD4-PE, mouse anti-chicken CD8-FITC, and mouse anti-chicken CD3-SPRD (BD Biosciences Pharmingen, San Diego, CA, USA). Leukocyte samples were labeled with CD3, CD4 and CD8 antibodies. The samples were then processed on a fluorescence activated cell sorter.
2.7. Detection of anti-IBV-specific IgG antibody
Sera were collected every week after primer vaccination until challenge, and the pre-vaccination sera were also collected for the control chickens. Total serum immunoglobulin G (IgG) specific for IBV was measured by indirect enzyme-linked immunosorbent assay (ELISA). The test sera were diluted to 1:500 and then processed according to the instruction of IBV antibody detection ELISA kit (IDEXX, Westbrook, MA, USA). The optical density at 650 nm was measured in a microplate reader (Biorad model 680). Negative and positive control sera were included in each assay. The total serum IBV-specific IgG was represented by the value of optical density.
2.8. Virus neutralizing antibody titers to IBV
Titers of virus neutralizing antibody in the sera collected 28 days after booster were determined as previously described (Yin and Liu, 1997). Serial 2-fold dilutions of serum samples were incubated with 100EID50 of IBV M41 strain for 1 h at 37 °C and then injected 200 μl of the sera-viral mixture into embryos, respectively. After incubation, 200 μl of the virus–serum mixture was injected into the allantoic cavity of each 10-day-old SPF embryonated chicken egg. Another 100 μl untreated virus fluid was used as a negative control. The eggs were then incubated at 37 °C for 7 days. Each dilution was inoculated into ten eggs. The virus neutralizing antibody titer of each sample was recorded as the highest serum dilution value which protected 50% of the embryos from death (PD50).
2.9. Virus challenge
All chickens were challenged with 103EID50 of the IBV strain M41 in 0.1 ml by the nasal–ocular route at day 49, 28 days after the booster. Chickens were examined daily for 2 weeks for the clinical symptoms such as coughing, sneezing, ataxia, dyspnea or death. Dead chickens were necropsied to confirm that the death was due to IBV infection. Chickens in each group were all euthanized 14 days after challenge, necropsies were then performed immediately and lung tissues were collected for further detection of the virus by RT-PCR.
2.10. Statistical analysis
Student's t-test was used to analyze the differences between animal immunization groups under each of above three indices (the percentage of CD4+CD3+ and CD8+CD3+ subgroups of peripheral blood T-lymphocytes, the antibody responses and the virus neutralizating antibody titers). A P-value of less than 0.05 was considered significant.
3. Results
3.1. Construction of the DNA vaccine
The S1, M, N genes were amplified by RT-PCR and then incorporated into the pVAX1 vector with designations of pVAX1-S1, pVAX1-M, and pVAX1-N, respectively. The plasmids with the correct insert were confirmed by DNA sequencing and restriction endonuclease digestion (Fig. 2 ).
Fig. 2.
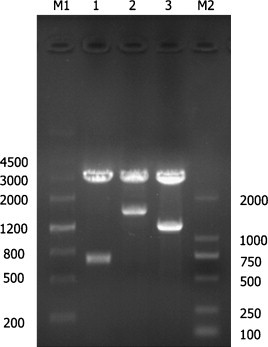
Identification of constructed DNA vaccine. Lane M1, Marker III; lane 1, pVAX1-M incised with HindIII and EcoRI was 678 bp; lane 2, pVAX1-S1 incised with HindIII and BamHI was 1610 bp; lane 3, pVAX1-N incised with restriction enzyme HindIII and EcoRI was 1231 bp; Lane M2, Marker D2000.
3.2. Expression of recombinant plasmids in COS-7 cells
The expression of pVAX1-S1, pVAX1-M, and pVAX1-N was demonstrated in the transfected COS-7 cells by indirect immunofluorescence. The transfected cells displayed fluorescence in the cytoplasm, showing that individual constructs encoding S1, M, or N protein can be expressed in the eukaryotic system (Fig. 3 ).
Fig. 3.
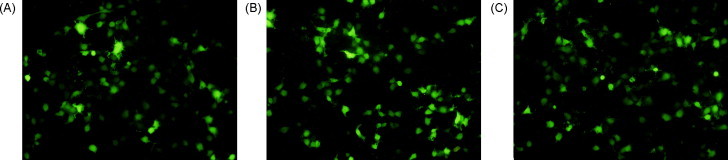
Indirect immunofluorescence detection of the expressed structural protein in COS-7 cells. Transient expressed proteins were detected by immunofluorescent antibody assay at 36 h after transfection. Cells transfected with pVAX1-S1 (A), pVAX1-M (B), and pVAX1-N (C) showed green fluorescence. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.3. Distribution of the DNA vaccine in vivo
After euthanizing five chickens in each group 5 days after DNA vaccine immunization, tissues were collected and ground in the order of heart, lung, liver, spleen and kidney under the sterile condition. The S1/M/N gene could be amplified by PCR from extracted genomic DNA in groups immunized with the DNA vaccine (Groups 1–5), while other groups showed negative results, indicating that pVAX1-S1, pVAX1-M, or pVAX1-N were distributed in all the tested zoogenic tissues 5 days after immunization.
3.4. Detection of CD4+CD3+ and CD8+CD3+ subgroups of peripheral blood T-lymphocytes
Peripheral blood lymphocytes were analyzed by flow cytometry on day 28, 7 days after boosting. The percentage of CD4+CD3+ and CD8+CD3+ T-lymphocytes in pVAX1-N prime-vaccinated groups (Groups 1–3) were significantly higher (P < 0.05) than those of any other groups (Groups 4–8). The percentage of these two T-lymphocyte subgroups showed no significant difference (P > 0.05) among Groups 1–3 (Fig. 4 ).
Fig. 4.
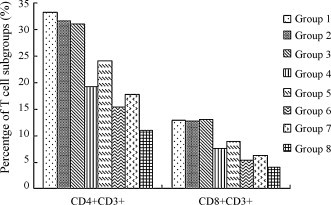
The percentage of CD4+CD3+ and CD8+CD3+ T-lymphocytes of different vaccination groups. The result was obtained from average of five sera in each group. The data were analyzed by software of Statistics Package for Social Science (SPSS). This test was conducted at 7 days after boosting.
3.5. Detection of anti-IBV-specific IgG antibody
The results showed that detectable antibodies to IBV antigen in chickens reached the peak 14 days after the booster inoculation, Specific antibodies increased significantly in the chickens from Groups 2, 3, 5 and 7, which were much more than that of Group 4 or Group 6. The results also indicated that Group 2 had been able to elicit higher specific antibodies against IBV, compared to Group 1 (P < 0.05) after booster. Control chickens in Group 8 which were injected with PBS repeatedly did not show detectable specific antibodies (Fig. 5 ).
Fig. 5.
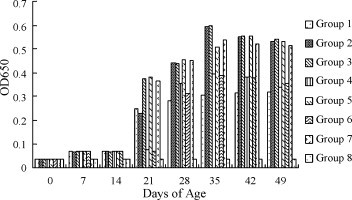
Antibody titers of different vaccination groups measured by ELISA. Sera from all the immunized animals were sampled weekly. The result was obtained from average of five sera in each group. The data of antibody titers were analyzed by software of Statistics Package for Social Science (SPSS). The results show the antibody titers of every immunized group on days 0, 7, 14, 21, 28, 35, 42, 49 after incubation.
3.6. Virus neutralizing antibody titers to IBV
After 7 days incubation, the embryonated chicken eggs were examined. The Reed–Muench method (Reed and Muench, 1938) was used to determine the value of virus neutralization titers. The results are summarized in Fig. 6 . Chickens in the negative control group (Group 8) did not have detectable virus neutralizating titer to IBV. Titers in Groups 2, 3, 5 and 7 were significantly higher (P < 0.05) than samples from any other experimental groups.
Fig. 6.
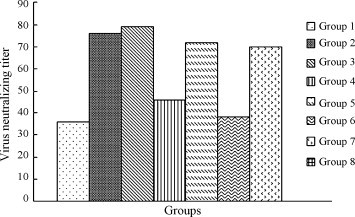
Level of IBV-specific neutralizing antibody. The sera were collected 28 days after boosting. Neutralizing titers indicate the highest serum dilution which protects half of the embryos from death (PD50). The higher serum dilution denotes the higher neutralizing titers.
3.7. Protection against challenge
Mortality rate, infection of the lungs and protection rate after challenge of chickens with IBV are summarized in Table 2 . Chickens started to show clinical signs of infection or die 4 days after challenge. The chickens injected with PBS alone were not protected from IBV infection and developed cough, nasal discharge and dyspnea. The death rate in the group injected with PBS was 66.7%. The death rate in Groups 2 and 3 was 6.7%, lower than that of the inactivated IBV immunized chickens. To evaluate the level of protection under different vaccination strategies after challenge, the lung samples collected were tested by RT-PCR for IBV infection. RT-PCR results showed 13.3% of chickens had virus in the lungs in Groups 2 and 3, indicating the highest protection rate in all vaccinated groups. All control chickens were IBV positive by RT-PCR. The results suggested that the combined use of DNA vaccine expressing nucleocapsid protein and inactivated IBV enhanced resistance against virulent IBV challenge, whatever in co-delivery or using prime-boost schedules.
Table 2.
The mortality and protection rate of different groups challenged by virulent IBV M41 strain.
| Groups | No. of death | No. of affecteda | Mortalityb (%)b | Protection (%)c |
|---|---|---|---|---|
| Group 1 | 3/15 | 5/15 | 20 | 66.7 |
| Group 2 | 1/15 | 2/15 | 6.7 | 86.7 |
| Group 3 | 1/15 | 2/15 | 6.7 | 86.7 |
| Group 4 | 5/15 | 7/15 | 33.3 | 53.3 |
| Group 5 | 4/15 | 6/15 | 26.7 | 60 |
| Group 6 | 5/15 | 8/15 | 33.3 | 46.7 |
| Group 7 | 4/15 | 6/15 | 26.7 | 60 |
| Group 8 | 10/15 | 15/15 | 66.7 | 0 |
Affected was determined by RT-PCR positive bird from dead and euthanized chickens’ lungs.
Mortality was recorded for each day after challenge and is presented as total number of dead chickens in each group.
Percent protection was determined by the number of unaffected chickens/total number of chickens.
4. Discussion
Traditional vaccination by using repeatedly inactivated IBV alone does not induce efficient protective immune responses against IBV. Following the development of DNA vaccines, the vaccination strategy of priming with a DNA vaccine combining with the inactivated vaccine as boosting reagent is a new strategy to induce potent immune protection (Ming et al., 2007, Wang et al., 2008), although this approach has not been evaluated for IBV yet.
This study provides the first evidence on the efficacy against IBV using the new vaccination strategy. Compared to other vaccines, the DNA vaccine has several potential advantages. First, it is easy to construct, with increased safety of an immunization strategy that mimics antigen processing and presentation during natural infections without causing disease. Second, the fragment-based constructions appear to be capable of inducing more potent immune responses than the whole inactivated vaccine, especially for inducing cytotoxic T-lymphocytes (CTL) which are critical in the control of poultry IBV infection (Collisson et al., 2000). Furthermore, the low level but persistent expression of the protein encoded by the constructed plasmid does not only elicit prolonged immune stimulation, but also increase antibody steadily, as well as T-cell affinity for the major histocompatibility complex (MHC)-peptide molecules (Wolff et al., 1990).
Additionally, coating the plasmid with lipofectamine not only protects the functional plasmid from degradation but also enhances the immune responses (Boyle et al., 1997, Pertmer et al., 1996, Gregoriadis et al., 1996, Gregoriadis et al., 1999). The result of DNA vaccine distribution in vivo showed the detectable target genes in heart, lung, liver, spleen and kidney organs, implying a potential of long lasting expression. Thus, the encoded proteins could be delivered by the MHC I or MHC II antigen-processing pathways to induce high levels of CD4+ or CD8+ T-cell activation (Whalen et al., 1988), resulting in enhanced immunogenicity.
Prior to challenge, investigation of T-lymphocytes subgroup indicates that while priming with DNA vaccines encoding IBV structure genes, the percentage of CD4+CD3+ and CD8+CD3+ T-lymphocytes subgroups in Groups 1–5 are higher than other groups, especially in Groups 1–3 (P < 0.05). It has been reported that CD4+ T-cell responses may produce directly antiviral cytokines as well as increase the proliferation, maturation and functional activity of CD8+CTL, while CD8+CTL plays a critical role in controlling IBV infection in poultry (Collisson et al., 2000). Furthermore, DNA immunization is more effective in eliciting better and potentially longer lasting T-lymphocytes, while B cell immunology suggests that low dose antigen delivery is more effective for eliciting better antibody responses and memory B cells (Bot et al., 1997, Gonzalez and Milstein, 1998). The increased T-lymphocytes in these groups purport that DNA vaccines stimulated intensive cellular immunization, which helps the complete elimination of virus (Letellier et al., 2008, Tian et al., 2008) and provides a more efficient way of protection. The higher antibody titers also suggest that the memory B cells were induced by the primed DNA vaccine. Due to the above characteristics of DNA vaccines, it is the most suitable candidate for priming.
As for the choice of a booster, the inactivated vaccine is prepared from the whole virus particle, so the immune responses induced by inactivated IBV vaccines should respond to the whole virus. This has a better capability than choosing DNA plasmid as the booster to evoke memory B cells, which has been confirmed in Group 2 with higher IgG and neutralizing antibody titers than Group 1. Also, higher ELISA or neutralizing antibody titers with higher levels of protection against IBV were observed in chickens boosted with inactivated IBV vaccines (Groups 2–7), especially in Groups 2, 3, and 5 (P < 0.05). The inactivated vaccine as a booster is also considered to balance the Th1 and Th2 immune responses (Larsen et al., 2001, Morello et al., 2002), which could accelerate both specific and neutralizing antibody inductions with increased T-cell response, and is critical to the stabilization of immune system as well.
Interestingly, significantly higher (P < 0.05) ELISA and neutralizing antibody titers and T-lymphocytes were all detected uniquely in Groups 2 and 3. In this study, this may be attributed to the interaction between vector encoding nucleocapsid protein (pVAX1-N) and the inactivated vaccine. The encoded nucleocapsid protein has been shown to express CTL epitopes in its carboxyl end for MHC molecules to induce CD8+CTL (Boots et al., 1991, Boots et al., 1992, Seo and Collisson, 1997, Seo et al., 1997), while CD8+CTL shows a better efficacy when combined with antibodies in the control of infection (Fang and Luis, 2005). Also, the highly conserved nucleocapsid protein which is the most abundant virus-derived protein produced throughout infection, is not only immunogenic, but also induces cross-reactive antibodies and ultimately protects chickens from viral infection (Seah et al., 2000, Yu et al., 2001). Previous studies have confirmed that immunization with the avian coronavirus nucleocapsid protein applied as a DNA vaccine induced protective immunity provided by CD8+ T cells and possibly by local antibodies (Boots et al., 1992). The challenge assay had also proved that the combining use of a DNA vaccine targeting nucleocapsid protein and the inactivated vaccine is more effective. In contrast, Group 5 did not perform so well in the challenge assay as in the ELISA and neutralizing antibody assays, which might be due to high variation of S1 gene which contributes to poor cross-protection (Cavanagh, 2007).
Although the amount of co-administered a DNA vaccine and the inactivated vaccine used in immunization was doubled in Group 3, the effect in eliciting protective immune responses was not much better (P > 0.05) than Group 2 in which the single use of a DNA vaccine for priming and the inactivated vaccine as booster were applied. This suggests that there is no supplementary benefit from pVAX1-N and the inactivated vaccine in a repeatedly co-delivered approach.
Taken together, the sequential approach of priming with nucleocapsid gene and boosting with the inactivated IBV vaccine shows the most potent immunological advantage and could be a potential vaccination strategy to provide protection against IBV infection in the chicken model. The next step is to evaluate whether the prime-boost strategy in broiler chickens would have high maternally derived antibody titers to IBV during the first few weeks of life.
Acknowledgment
We are grateful to all the anonymous reviewers for the amendments and comments on this manuscript. We also thank Dr. Kun Wei for writing assistance. This work was supported by the foundation of Chinese National Programs for High Technology Research and Development (Project No. 2006AA10A205).
References
- Ariane R., Ratna M., Dennis T., Gert G., Jerome C., Maria G.P., Jaco K., Sampa S., Harikrishnan B., Norman L.L., Jaap G., Katarina R. Evaluation of a prime-boost vaccine schedule with distinct adenovirus vectors against malaria in rhesus monkeys. Vaccine. 2009;27:6226–6233. doi: 10.1016/j.vaccine.2009.07.106. [DOI] [PubMed] [Google Scholar]
- Bijlenga G., Cook J.K.A., Gelb J., Dewit J.J. Development and use of the H strain of avian infectious bronchitis virus from The Netherlands as a vaccine: a review. Avian Pathol. 2004;33:550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots A.M.H., Kusters J.G., Van N.J.M., Zwaagstra K.A., Rijke E., Hensen E.J., Van B.A.M.D.Z. Localization of a T-cell epitope within the nucleocapsid protein of avian coronavirus. Immunology. 1991;74:8–13. [PMC free article] [PubMed] [Google Scholar]
- Boots A.M.H., Benaissa-Trouw B.J., Hesselink W., Rijke E., Schrier C., Hensen E.J. Induction of anti-viral immune responses by immunization with recombinant-DNA encoded avian coronavirus nucleocapsid protein. Vaccine. 1992;10:119–124. doi: 10.1016/0264-410X(92)90028-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot A., Antohi S., Bona C. Immune response of neonates elicited by somatic transgene vaccination with naked DNA. Front Biosci. 1997;2:173–188. doi: 10.2741/a181. [DOI] [PubMed] [Google Scholar]
- Boyle J.S., Silva A., Brady J.L. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14626–14631. doi: 10.1073/pnas.94.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Cook J.K.A., Li D., Kant A., Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21:33–43. doi: 10.1080/03079459208418816. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Naqi S. Infectious bronchitis. In: Calnek B.W., Barnes H.J., McDougald L.R., Saif Y.M., editors. Diseases of Poultry. tenth ed. 1997. pp. 511–526. London. [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Collisson E.W., Pei J., Dzielaw J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- Fang M., Luis J.S. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J. Immunol. 2005;175:6829–6836. doi: 10.4049/jimmunol.175.10.6829. [DOI] [PubMed] [Google Scholar]
- Gonzalez F.A., Milstein C. Low antigen dose favours selection of somatic mutants with hallmarks of antibody affinity maturation. Immunology. 1998;93:149–153. doi: 10.1046/j.1365-2567.1998.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G., Saffie R., Hart S.L. High yield incorporation of plasmid DNA within liposome: effect on DNA integrity and transfect ion efficiency. J. Drug Target. 1996;3:469–475. doi: 10.3109/10611869609015966. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Cormack M.B., Obrenovic M., Perrie Y. Humana Press Inc.; Totowa (NJ): 1999. Entrapment of plasmid DNA vaccines into liposome by dehydration/rehydration. Methods in molecular medicine, DNA vaccines: methods and protocols. pp. 305–312. [Google Scholar]
- Larsen D.L., Karasin A., Olsen C.W. Immunization of pigs against influenza virus infection by DNA vaccine priming followed by killed-virus vaccine boosting. Vaccine. 2001;19:2842–2853. doi: 10.1016/s0264-410x(01)00014-7. [DOI] [PubMed] [Google Scholar]
- Letellier C., Boxus M., Rosar L., Toussaint J.F., Walravens K., Roels S., Meyer M., Letesson J.J., Kerkhofs P. Vaccination of calves using the BRSV nucleocapsid protein in a DNA prime–protein boost strategy stimulates cell-mediated immunity and protects the lungs against BRSV replication and pathology. Vaccine. 2008;26:4840–4848. doi: 10.1016/j.vaccine.2008.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Stirling C.M.A., Denyer M.S., Hamblin P., Hutchings G., Takamatsu H.H., Barnett P.V. Dramatic improvement in FMD DNA vaccine efficacy and cross-serotype antibody induction in pigs following a protein boost. Vaccine. 2008;26:2647–2656. doi: 10.1016/j.vaccine.2008.01.037. [DOI] [PubMed] [Google Scholar]
- Ming K.H., Ching C.W., Tsang L.L. Priming with DNA vaccine and boosting with killed vaccine conferring protection of chickens against infectious bursal disease. Vaccine. 2007;25:5417–5427. doi: 10.1016/j.vaccine.2007.04.087. [DOI] [PubMed] [Google Scholar]
- Morello C.S., Ye M., Spector D.H. Development of a vaccine against murine cytomegalovirus (MCMV), consisting of plasmid DNA and formalin-inactivated MCMV, that provides long-term, complete protection against viral replication. J. Virol. 2002;76:4822–4835. doi: 10.1128/JVI.76.10.4822-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B., Chen H., Tan Y. Identification of one peptide which inhibited infectivity of avian infectious bronchitis virus in vitro. Sci. China C: Life Sci. 2006;49:158–163. doi: 10.1007/s11427-006-0158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertmer T.M., Robert T.R., Haynes J.R. Influenza virus nucleoprotein-specific immunoglobulin G and cytokine response elicited by DNA vaccination are dependent on the route of vector DNA delivery. J. Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw I.A., Ramsay A.J. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today. 2000;21:163–165. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- Reed L., Muench H. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Seah J.N., Yu L., Wang J.K. Localization of linear B-cell epitopes on infectious bronchitis virus nucleocapsid protein. Vet. Microbiol. 2000;75:11–16. doi: 10.1016/s0378-1135(00)00202-9. [DOI] [PubMed] [Google Scholar]
- Seo S.H., Collisson E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Wang L., Smith R., Collision E.W. The carboxyl-terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection. J. Virol. 1997;71:7889–7894. doi: 10.1128/jvi.71.10.7889-7894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W., Cavanagh D., Horzinek M.C. Coronaviruses: structure and genome expression. J. Gen. Virol. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- Stern D.F., Sefton B.M. Coronavirus proteins: biogenesis of avian coronavirus bronchitis virus. J. Virol. 1982;44:794–803. doi: 10.1128/jvi.44.3.794-803.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M.J., Wang H.N., Zhou S., Tian G.B. Enhancement of the immunogenicity of an infectious bronchitis virus DNA vaccine by a bicistronic plasmid encoding nucleocapsid protein and interleukin-2. J. Virol. Met. 2008;149:42–48. doi: 10.1016/j.jviromet.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Wang H.N., Lu D., Zhang Y.F., Wang T., Kang R.M. The immunoreactivity of a chimeric multi-epitope DNA vaccine against IBV in chickens. Biochem. Biophys. Res. Commun. 2008;377:221–225. doi: 10.1016/j.bbrc.2008.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.X., Parker C., Taaffe J., Alicia S., Adolfo G.S., Lu S. Heterologous HA DNA vaccine prime-inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26:3626–3633. doi: 10.1016/j.vaccine.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing M.D., Tannock G.A. Live attenuated vaccines against influenza; an historical review. Vaccine. 2001;19:3320–3330. doi: 10.1016/s0264-410x(01)00045-7. [DOI] [PubMed] [Google Scholar]
- Whalen B.J., Tony H.P., Parker D.C. Characterization of the effector mechanism of help for antigen-presenting and bystander resting B cell growth mediated by Ia-restricted Th2 helper T cell lines. J. Immunol. 1988;141:2230–2239. [PubMed] [Google Scholar]
- Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Woodland D.L. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Xie Z.H., Shi X.J., Cai G.P. Preparation of high purified plasmid DNA for mammal cell transfection. Lett. Biotechnol. 2007;18:803–805. [Google Scholar]
- Yang Z.Y., Kong W.P., Huang Y. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Liu J. second ed. Science Press; Beijing: 1997. Animal Virology. [Google Scholar]
- Yu L., Liu W., Schnitzlein W.M., Tripathy D.N., Kwang J. Study of protection by recombinant fowl poxvirus expressing C-terminal nucleocapsid protein of infectious bronchitis virus against challenge. Avian Dis. 2001;45:340–348. [PubMed] [Google Scholar]


