Abstract
Context
Fracture risk in people with type 1 diabetes (T1D) is higher than their peers without diabetes.
Objective
To compare bone mineral density (BMD) across the lifespan in individuals with T1D and age- and sex-matched healthy controls.
Design
Cross-sectional.
Setting
Subjects (5–71 years) with T1D and matched controls from ongoing research studies at Barbara Davis Center for Diabetes.
Patients or other participants
Participants with lumbar spine BMD by dual X-ray absorptiometry (DXA) were divided into 2 groups: children ≤20 years and adults >20 years.
Intervention
None.
Main outcome measures
Comparison of BMD by diabetes status across age groups and sex using a linear least squares model adjusted for age and body mass index (body mass index (BMI) for adults; and BMI z-score in children).
Results
Lumbar spine BMD from 194 patients with T1D and 156 controls were analyzed. There was no difference in age- and BMI-adjusted lumbar spine BMD between patients with T1D and controls: among male children (least squares mean ± standard error of the mean [LSM ± SEM]; 0.80 ± 0.01 vs 0.80 ± 0.02 g/cm2, P = .98) or adults (1.01 ± 0.03 vs 1.01 ± 0.03 g/cm2, P = .95), and female children (0.78 ± 0.02 vs 0.81 ± 0.02 g/cm2, P = .23) or adults (0.98 ± 0.02 vs 1.01 ± 0.02 g/cm2, P = .19). Lumbar spine (0.98 ± 0.02 vs 1.04 ± 0.02 g/cm2, P = .05), femoral neck (0.71 ± 0.02 vs 0.79 ± 0.02 g/cm2, P = .003), and total hip (0.84 ± 0.02 vs 0.91 ± 0.02, P = .005) BMD was lower among postmenopausal women with T1D than postmenopausal women without diabetes.
Conclusion
Across age groups, lumbar spine BMD was similar in patients with T1D compared with age- and sex-matched participants without diabetes, except postmenopausal females with T1D had lower lumbar spine, femoral neck, and total hip BMD.
Keywords: type 1 diabetes, bone mineral density, dual X-ray absorptiometry, life-span, bone health, bone accrual
At any age, fracture risk in patients with type 1 diabetes (T1D) is 2- to 4-fold higher than in their peers without diabetes (1, 2). Many patients with T1D are diagnosed during childhood, a critical time for bone acquisition. T1D is associated with both hyperglycemia and metabolic perturbation and thus is posited to adversely affect bone structural quality (3), resulting in increased risk for fracture.
Comparisons of bone mineral density (BMD) between individuals with T1D and controls without diabetes have had mixed results. While several studies have shown lower BMD and/or lower bone mineral content among children and adolescents with T1D (4–7), a recent meta-analysis reported no difference in the lumbar spine BMD and a modestly lower femoral neck BMD in adults with T1D compared with controls without diabetes (8). Similarly, a recent study of adults having T1D for more than 50 years showed normal z-score at the lumbar spine and hip suggesting no effect of longstanding T1D on bone density (9). It has been suggested that reduced BMD in children and adolescents at the diagnosis of T1D is temporary, and studies have shown normal bone growth and accrual over time in children and adolescents with T1D (10).
Thus, we hypothesized that children and adolescents with T1D would have similar or modestly lower BMD at the lumbar spine, and adults with longstanding T1D would have lumbar spine BMD similar to age- and sex-matched controls without diabetes. This study was aimed to evaluate BMD across the lifespan to better understand bone health in patients with T1D.
Methods
Patient population
Adult subjects were recruited from two ongoing studies: Coronary Artery Calcification in Type 1 Diabetes (CACTI) (11, 12) and Bone and Vascular Health in Postmenopausal Women with Type 1 Diabetes (3). Children were selected from an ongoing study of bone density that recruited from 2 cohorts: (1) pediatric patients with T1D followed at the Barbara Davis Center, and (2) controls without T1D recruited from participants of the Diabetes Autoimmunity Study in the Young (DAISY), as previously described (13–15). Included in this study were subjects with T1D and age- and sex-frequency matched controls without diabetes who had dual X-ray absorptiometry (DXA) measured BMD at the lumbar spine. Controls were generally healthy children without diabetes and free of any medical conditions that can affect bone density. Adults controls were men and women without diabetes (glycosylated hemoglobin (HbA1c) < 5.7%, 39 mmol/mol) and free of medical conditions that can affect bone density such as celiac disease, uncontrolled thyroid disease, Addison’s disease, malabsorption syndrome, rheumatologic conditions, parathyroid disorders, and cancer other than skin cancer. In subjects who had multiple DXA performed during study participation, only baseline BMD was included in the analysis. Laboratory measures such as HbA1c and diabetes complications such as retinopathy and neuropathy were recorded from the ongoing studies. Subjects with positive tissue transglutaminase antibody and/or established medical diagnosis of celiac disease were excluded from this analysis. Additional exclusion criteria included use of oral steroid equivalent of prednisolone of 5 mg for at least 3 months, estimated glomerular filtration (eGFR) less than 30 mL/min/1.73 m2, history of parathyroid or rheumatologic disease, and medication or disease that could significantly affect bone density.
Subjects were assigned to 1 of 2 age categories: children, defined as age less than or equal to 20 years, and adults, defined as age greater than 20 years. The selection of an age cut-off of at 20 years was based on 2 reasons: first, most bone accrual at the femoral neck would occur before this age (16) and, second, our previous study showed the unfavorable effect of early-onset T1D (age <20 years) on bone structural quality (3). Adult females were further divided into 2 groups: premenopausal and postmenopausal based on self-reported menopausal status.
Anthropometry and diabetes complications
Height and weight were measured as described previously and BMI (weight/height (2); kg/m2) was calculated (3). For children, BMI was converted to age- and sex-specific z-scores as reported previously (13). Microvascular complications are uncommon in children with T1D and, therefore, we did not collect information on these complications in this group. In adults, diabetic retinopathy was self-reported as requiring laser therapy and/or intraocular injection. A score ≥4 on the Michigan Neuropathy Screening Instrument (MNSI) was classified as diabetic neuropathy (3). Diabetic nephropathy was defined as increased excretion of urinary albumin to creatinine ratio > 30 as described previously (3). History of fractures at any site was self-reported.
BMD measurement
All children and adolescents had BMD measured at the lumbar spine while adults had BMD measured at the lumbar spine and hip. All BMD were measured on a single DXA instrument (Hologic Discovery W) by trained DXA technicians. A single technician performed DXA for each study. The least significant change at 95% confidence interval for total hip BMD and lumbar spine BMD were 0.039 g/cm2 and 0.03 g/cm2, respectively.
Statistical analysis
Variables were assessed for normality using a Shapiro–Wilkes test. Continuous variables are presented as mean and standard deviation or median and interquartile range, depending on normality. Categorical variables are presented as number and percentage. The primary outcome was to test the difference in DXA-measured BMD at the lumbar spine in children and adults by sex and diabetes status, and this comparison was made using linear least squares regression adjusting for age and BMI. BMI values were used for the adult categories, and BMI z-scores were used for children. For sensitivity analysis, differences in lumbar spine BMD in female children with and without diabetes were analyzed using linear least squares regression adjusting for age, BMI, and height. As a secondary analysis, linear least squares regression of total lumbar spine, femoral neck, and hip BMD was performed between premenopausal and postmenopausal females by diabetes status adjusting for age and BMI. We also performed linear least squares regression of total lumbar spine, femoral neck and hip BMD by diabetes complications among men and women with T1D. For hip and femoral neck sites, the left side was included in this analysis. Analyses were performed using R version 3.5.1. All tests performed were 2-sided and P < .05 was considered statistically significant.
Results
Of 543 subjects from 3 ongoing studies who had at least 1 DXA-measured lumbar spine BMD, 193 subjects were excluded due to either positive tissue transglutaminase antibody or medical diagnosis of celiac disease or missing BMD data or missing celiac status (36%). Thus, 350 subjects (194 with T1D and 156 controls) were included in this study. Baseline characteristics of subjects with T1D and controls are provided in Table 1. BMI z-score was higher and height z-score was lower among female children with T1D than female children without diabetes. Of 78 adults with T1D, 77 reported complications. One or more diabetes microvascular complications (retinopathy, neuropathy, or nephropathy) was present in 33 of 54 females with T1D (61%) and 11of 23 males with T1D (48%).
Table 1.
Baseline characteristics of subjects with type 1 diabetes and controls without diabetes
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Children | Adult | Children | Adult | |||||
| Category | T1D n = 49 | Control n = 51 | T1D n = 55 | Control n = 39 | T1D n = 67 | Control n = 40 | T1D n = 23 | Control n = 26 |
| Age (years) | 13 ± 3 | 14 ± 3 | 56 ± 10 | 57 ± 8 | 14 ± 3 | 14 ± 3 | 53 ± 9 | 57 ± 6 |
| BMI, (Kg/m2) | NA | NA | 26.5 ± 5 | 26.8 ± 6 | NA | NA | 28.2 ± 4 | 28.0 ± 4 |
| BMI z-score | 0.61 ± 0.67* | –0.09 ± 1.05* | NA | NA | 0.35 ± 0.73 | 0.15 ± 1.01 | NA | NA |
| Height (Cm) | 151 ± 15* | 159 ± 13* | 164 ± 7 | 164 ± 7 | 165 ± 15 | 164 ± 18 | 176 ± 9 | 178 ± 7 |
| Height z-score | –0.1 ± 1* | 0.4 ± 1* | NA | NA | 0.3 ± 1 | 0.3 ± 1 | NA | NA |
| T1D duration (years) | 7 ± 4 | NA | 40 ± 9 | NA | 8 ± 4 | NA | 38 ± 8 | NA |
| HbA1c (%) | 8.7 ± 1.8 | NA | 7.9 ± 1.0* | 5.6 ± 0.3* | 8.5 ± 1.7 | NA | 7.9 ± 1.1* | 5.8 ± 0.6* |
| History of fracture, n (%) | 0 (0) | 0 (0) | 6 (12) | 1 (3) | 0 (0) | 0 (0) | 6 (26) | 3 (12) |
The data is presented in mean ± SD or numbers (%). T1D, type 1 diabetes; BMI, body mass index; HbA1c, glycosylated hemoglobin; NA, not available. Children are defined as age less than or equal to 20 years and adults defined as age greater than 20 years.
*P < .05.
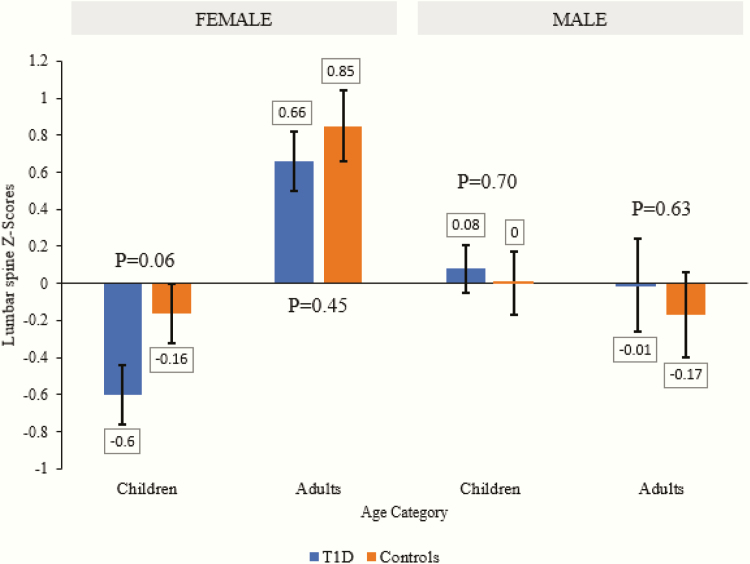
The results of the primary objective, differences in lumbar spine BMD between T1D subjects and controls, grouped by age category and sex, are displayed in Fig. 1A and 1B. Differences in lumbar spine BMD z-score between T1D subjects and controls by age groups and sex is provided in Fig. 2. There was no statistical difference in BMD at lumbar spine in male children or adults with T1D compared with controls without diabetes (least squares mean [LSM ± SEM] 0.80 ± 0.01 vs 0.80 ± 0.01 g/cm2, P = .98 and 1.01 ± 0.03 vs 1.01 ± 0.03 g/cm2, P = .95, respectively) adjusted for age and BMI. Lumbar spine BMD was nonsignificantly lower by 0.03 g/cm2 in female children (0.78 ± 0.02 vs 0.81 ± 0.02 g/cm2, P = .23) and by 0.03 g/cm2 in female adults with T1D (0.98 ± 0.02 vs 1.01 ± 0.02 g/cm2, P = .19) than females without diabetes adjusted for age and BMI.
Figure 1.
(A) Lumbar spine bone mineral density (BMD) among children and adults with type 1 diabetes (T1D) compared with controls. (B) Lumbar spine BMD across the life-span in subjects with type 1 diabetes (turquoise line) compared to age-matched controls without diabetes (orange line). (A) The are data presented as least squares mean ± standard error and adjusted for age and BMI Z-score in children and BMI in adults. (B) The area is gray represents 95% confidence interval and individual dots represents an individual BMD. Age ranges from 5 years to 71 years.
Figure 2.
Lumbar spine bone mineral density z-score by age groups and sex among subjects with type 1 diabetes compared with controls without diabetes. Data presented are adjusted for body mass index.
Among adult women, BMD was lower in women with T1D than in control women without diabetes at the left femoral neck (0.72 ± 0.01 g/cm2 vs 0.77 ± 0.02 g/cm2, P = .03) and total left hip (0.84 ± 0.01 g/cm2 vs 0.90 ± 0.02 g/cm2, P = .02). However, there was no statistically significant BMD difference between adult men with T1D and men without diabetes at the left femoral neck (0.78 ± 0.02 vs 0.81 ± 0.02 g/cm2, P = .27) or total left hip (0.94 ± 0.02 vs 0.97 ± 0.02, P = .34).
Since female children with T1D were shorter than female children without diabetes, we analyzed the differences in BMD adjusted for height. After adjusting for age, BMI z-score and height, there was no difference in lumbar spine BMD (0.80 ± 0.01 vs 0.81 ± 0.02, P = .57) between female children with T1D and female children without diabetes. Similarly, we analyzed differences in lumbar spine BMD not adjusted by BMI z-score as BMI is 1 of the contributors of bone density and BMI z-score was higher among female children with T1D than female children without diabetes. There was no significant difference in lumbar BMD in female children with T1D compared with female children without diabetes (0.76 ± 0.03 vs 0.82 ± 0.03, P = .12) and male children with T1D compared with male children without diabetes (0.82 ± 0.02 vs 0.79 ± 0.03, P = .43).
We also evaluated differences in the lumbar spine, femoral neck, and total hip BMD in adult females with and without diabetes by menopausal status. Postmenopausal women with T1D had lower BMD adjusted for BMI at the lumbar spine (0.98 ± 0.02 vs 1.04 ± 0.02 g/cm2, P = .05), left femoral neck (0.71 ± 0.02 vs 0.79 ± 0.02 g/cm2, P = .003) and total left hip (0.84 ± 0.02 vs 0.91 ± 0.02, P = .005) than postmenopausal women without diabetes [Fig. 3]. There was no differences in BMD at the lumbar spine, femoral neck, and total hip between premenopausal women with T1D and premenopausal without diabetes [Fig. 3].
Figure 3.
(A) Lumbar bone mineral density in premenopausal and postmenopausal women with T1D and controls without diabetes. (B) Femoral Neck Bone Mineral Density in premenopausal and postmenopausal women with type 1 diabetes (T1D) and controls without diabetes. (C) Total hip bone mineral density in premenopausal and postmenopausal women with T1D and controls without diabetes. The data are presented as least squares mean ± standard error and adjusted for age and body mass index.
In a sensitivity analysis by diabetes microvascular complications among subjects with T1D, there was no statistically significant difference in lumbar spine (0.96 ± 0.02 vs 0.98 ± 0.03, P = .55), left femoral neck (0.70 ± 0.02 vs 0.74 ± 0.02, P = .27) and total left hip (0.82 ± 0.02 vs 0.87 ± 0.02, P = .11) BMD between adult females with T1D who had at least 1 complication and T1D adult females without any complications. Similarly, there was no significant difference in lumbar spine (1.06 ± 0.05 vs 1.0 ± 0.05, P = .40), left femoral neck (0.76 ± 0.03 vs 0.80 ± 0.03, P = .36) and total left hip (0.94 ± 0.04 vs 0.95 ± 0.03, P = .88) BMD between adult males with T1D who had at least 1 complication and T1D males without complications.
Discussion
The findings of our study suggest that there are sex differences in BMD among subjects with T1D across the lifespan. Female children and adults with T1D had lower mean lumbar BMD than those without diabetes, although this was not statistically significant. Only in postmenopausal women did these trends in lower BMD in T1D become significant. Female children with T1D were shorter than female children without diabetes. DXA-measured BMD is an areal estimate of density and it can appear artificially low in individuals with short stature (17). When adjusted by height, BMD difference was further reduced (from 0.03 g/cm2 to 0.01 g/cm2) among female children with T1D compared with controls. Another contributor could be sex differences in glycemic control. Female children with T1D in this analysis had modestly higher A1c than male children with T1D (8.7% vs 8.5%) and BMD has been associated with glycemic control in children and adolescents with T1D (13, 18). Some studies, but not all, have shown that girls with T1D have worse control than boys with T1D (19, 20) and early suboptimal control during the critical period of bone accrual may contribute to lower BMD in females with T1D (21, 22). Lower BMD and probably lower bone accrual may result in increased fracture risk in females than in men with T1D in later life (1, 2, 23). The lack of significant BMD differences in children, adult males, and premenopausal women by diabetes status may be due to small sample size. It is also possible that rate of bone loss during menopause transition may be higher in women with T1D; however, this has yet to be studied. Hormone replacement therapy is known to reduce bone loss in postmenopausal females (24); however, the effect of hormone replacement therapy in preventing bone loss among postmenopausal females with T1D is unknown.
A recent population-based study has reported increased risk for fractures across the lifespan in subjects with T1D (2). DXA-measured BMD studies have reported conflicting results in children and adults with T1D compared with control where children with T1D were reported to have lower BMD and no differences in BMD in adults with T1D compared with controls (3–5, 7–10, 13). It has been hypothesized that the deficit in BMD found in subjects with T1D during childhood can normalize over time (5, 10). The differences in results of reported studies were due to differences in sample size, duration of diabetes, lack of selection of appropriate controls, and presence of other morbidities such as celiac disease that are very common in people with T1D.
Diabetes microvascular complications have been implicated in compromised bone quality (25, 26) and increased risk for fractures. In our study, adult females with T1D and diabetes complications had BMD at lumbar spine, femoral neck, and total hip lower by 0.02–0.05 g/cm2; however, this difference was statistically nonsignificant. There was no differences in BMD in adults with T1D by diabetes complications. The findings of this study are in agreement with our previous publication (3) suggesting a modest effect of microvascular complications, if any, on bone density or bone quality. This may have been due to differences in populations, diabetes control, and severity of diabetes complications.
To our knowledge, this is the first study to report BMD across the lifespan in subjects with T1D compared with age-matched controls without diabetes. The inclusion of well-characterized cohorts with T1D and appropriate controls, large sample size, use of a single DXA machine, and exclusion of patients with celiac disease are major strengths of this study. However, a few limitations are worth noting. First, this is a cross-sectional study and, therefore, results should be interpreted with caution. Second, we did not have subjects between 21 and 39 years of age and, therefore, we could not evaluate BMD in young adults by diabetes status. Third, although BMD was statistically lower among adult females with T1D at the femoral neck and total hip than adult females without diabetes, the differences in BMD were small. Studies have reported that DXA-measured BMD underestimates fracture risk in people with T1D (27). Therefore, the findings of our study do not fully explain the mechanisms of increased fracture risk in people with T1D and further studies are warranted to understand the influence of T1D on bone accrual and on bone quality to improve bone health in this high fracture risk population. Moreover, we did not have information on age of menarche, and differences in attainment of puberty among children may be a confounder. Similarly, very few participants with T1D were on hormone replacement therapy and, therefore, we could not evaluate the influence of hormone replacement therapy on BMD among postmenopausal women with T1D. Fourth, we excluded patients with T1D who had celiac disease, which was very prevalent in our cohort (36%). Prevalence of celiac disease in patients with T1D ranges from 2% to 16% (28, 29) and celiac disease is known to impair bone density and quality (14). Fifth, the population of T1D included in our study do not represent the general T1D population. Therefore, the interpretation of these findings should be taken considering all limitations.
In conclusion, there was no significant difference in lumbar spine BMD in T1D and age- and sex-matched controls without diabetes across different age groups. However, postmenopausal women with T1D had lower BMD than nondiabetic controls of the same age.
Acknowledgments
We acknowledge the help of Prakriti Joshee, MS, CBDT, and Olivia Llamas, MA, for performing DXA in most subjects. We thank Rachel Sippl for collecting and cleaning the data.
Financial Support: Bone and Vascular health in Postmenopausal study was funded by the Center for Women’s Health Research at University of Colorado (VNS). The CACTI study was conducted at the Clinical Translational Research Centers (CTRC) at the University of Colorado and Children’s Hospital Colorado supported by the NIHM01 RR000051 and CTSI UL1 TR000154. Support was provided by the NIH National Heart, Lung and Blood Institute grants R01 HL61753, R01 HL079611 and HL113029, JDRF grant 17-2013-313, American Diabetes Association Junior Faculty Award 1-10-JF-50 (JSB) and Career Development Award 7-13-CD-10, and Diabetes Endocrinology Research Center Clinical Investigation Core P30 DK57516 and pediatric studies were supported by the National Institutes of Health (R01 DK50979, DK32083, DK32493, 5K12DK094712), Diabetes Endocrinology Research Center (P30 DK57516), and the National Centers for Research Resources, General Clinical Research Centers Program (M01RR00069). The time and effort for this writing is supported by National Institute of Arthritis, Musculoskeletal and skin diseases K23 grant to VNS (K23AR075099)
Author Contributions: Eitan Halper-Stromberg and Tyler Gallo: data collection, analysis, writing original draft, review, and edits. Brigitte Frohnert, Marian Rewers, and Janet K Snell-Bergeon: funding acquisition, resources, data curation, analysis, and writing-review and edits. Viral N Shah: conceptualization, investigation, resources, writing-original draft, review and edits, funding acquisition.
Glossary
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- DXA
dual X-ray absorptiometry
- eGFR
estimated glomerular filtration rate
- HbA1c
glycosylated hemoglobin
- LSM
least squares mean
- SEM
standard error of the mean
- T1D
type 1 diabetes
Additional Information
Disclosure Summary: Dr. Shah reports grants and personal fees from Dexcom Inc, grants and personal fees from Sanofi US, grants from NIH (NIAMS), grants from Mylan gmbH, grants from NovoNordisk, grants from vTv Therapeutics, grants from Center for Women’s health Research, outside the submitted work. Other authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med. 2015;32(9):1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN). Diabetes Care. 2015;38(10):1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah VN, Joshee P, Sippl R, et al. Type 1 diabetes onset at young age is associated with compromised bone quality. Bone. 2019;123:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaur H, Joshee P, Franquemont S, et al. Bone mineral content and bone density is lower in adolescents with type 1 diabetes: a brief report from the RESISTANT and EMERALD studies. J Diabetes Complications. 2018;32(10):931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roh JG, Yoon JS, Park KJ, Lim JS, Lee HS, Hwang JS. Evaluation of bone mineral status in prepuberal children with newly diagnosed type 1 diabetes. Ann Pediatr Endocrinol Metab. 2018;23(3):136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah VN, Carpenter RD, Ferguson VL, Schwartz AV. Bone health in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2018;25(4):231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan H, Wu N, Yang T, He W. Association between bone mineral density and type 1 diabetes mellitus: a meta-analysis of cross-sectional studies. Diabetes Metab Res Rev. 2014;30(7):531–542. [DOI] [PubMed] [Google Scholar]
- 8. Shah VN, Harrall KK, Shah CS, et al. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: a meta-analysis and review of the literature. Osteoporosis Int. 2017;28:2601–2610. [DOI] [PubMed] [Google Scholar]
- 9. Maddaloni E, D’Eon S, Hastings S, et al. Bone health in subjects with type 1 diabetes for more than 50 years. Acta Diabetol. 2017;54(5):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bechtold S, Putzker S, Bonfig W, Fuchs O, Dirlenbach I, Schwarz HP. Bone size normalizes with age in children and adolescents with type 1 diabetes. Diabetes Care. 2007;30(8):2046–2050. [DOI] [PubMed] [Google Scholar]
- 11. Shah VN, Sippl R, Joshee P, et al. Trabecular bone quality is lower in adults with type 1 diabetes and is negatively associated with insulin resistance. Osteoporosis Int. 2018;29:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dabelea D, Kinney G, Snell-Bergeon JK, et al. ; Coronary Artery Calcification in Type 1 Diabetes Study Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52(11):2833–2839. [DOI] [PubMed] [Google Scholar]
- 13. Simmons JH, Klingensmith GJ, McFann K, et al. Impact of celiac autoimmunity on children with type 1 diabetes. J Pediatr. 2007;150(5):461–466. [DOI] [PubMed] [Google Scholar]
- 14. Simmons KM, McFann K, Taki I, et al. Reduced bone mineral density is associated with celiac disease autoimmunity in children with type 1 diabetes. J Pediatr. 2016;169:44–48.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia. 1996;39(7):807–812. [DOI] [PubMed] [Google Scholar]
- 16. Davies JH, Evans BA, Gregory JW. Bone mass acquisition in healthy children. Arch Dis Child. 2005;90(4):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fuusager GB, Christesen HT, Milandt N, Schou AJ. Glycemic control and bone mineral density in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2019;20(5):629–636. [DOI] [PubMed] [Google Scholar]
- 19. Brown TL, Maahs DM, Bishop FK, Snell-Bergeon JK, Wadwa RP. Influences of gender on cardiovascular disease risk factors in adolescents with and without type 1 diabetes. Int J Pediatr Endocrinol. 2016;2016:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah VN, Wu M, Polsky S, et al. ; for T1D Exchange Clinic Registry Gender differences in diabetes self-care in adults with type 1 diabetes: findings from the T1D Exchange clinic registry. J Diabetes Complications. 2018;32(10):961–965. [DOI] [PubMed] [Google Scholar]
- 21. Weber DR, Gordon RJ, Kelley JC, et al. Poor glycemic control is associated with impaired bone accrual in the year following a diagnosis of type 1 diabetes. J Clin Endocrinol Metab. 2019;104(10):4511–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber DR, O’Brien KO, Schwartz GJ. Evidence of disordered calcium metabolism in adolescent girls with type 1 diabetes: an observational study using a dual-stable calcium isotope technique. Bone. 2017;105:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res. 2014;29(5):1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wimalawansa SJ. Prevention and treatment of osteoporosis: efficacy of combination of hormone replacement therapy with other antiresorptive agents. J Clin Densitom. 2000;3(2): 187–201. [DOI] [PubMed] [Google Scholar]
- 25. Abdalrahaman N, McComb C, Foster JE, et al. Deficits in trabecular bone microarchitecture in young women with type 1 diabetes mellitus. J Bone Miner Res. 2015;30(8):1386–1393. [DOI] [PubMed] [Google Scholar]
- 26. Shanbhogue VV, Hansen S, Frost M, Brixen K, Hermann AP. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol. 2017;5(10):827–838. [DOI] [PubMed] [Google Scholar]
- 27. Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48(7):1292–1299. [DOI] [PubMed] [Google Scholar]
- 28. Pham-Short A, Donaghue KC, Ambler G, Phelan H, Twigg S, Craig ME. Screening for celiac disease in type 1 diabetes: a systematic review. Pediatrics. 2015;136(1):e170–e176. [DOI] [PubMed] [Google Scholar]
- 29. Kurppa K, Laitinen A, Agardh D. Coeliac disease in children with type 1 diabetes. Lancet Child Adolesc Health. 2018;2(2):133–143. [DOI] [PubMed] [Google Scholar]