Abstract
Context
Chronic inflammation arising from adipose tissue macrophage (ATM) activation may be central in type 2 diabetes etiology. Our objective was to assess the longitudinal associations of soluble CD163 (sCD163), a novel biomarker of ATM activation, with insulin sensitivity, β-cell function, and dysglycemia in high-risk subjects.
Methods
Adults at risk for type 2 diabetes in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) study had 3 assessments over 6 years (n = 408). Levels of sCD163 were measured using fasting serum. Insulin sensitivity was assessed by HOMA2-%S and the Matsuda index (ISI). β-cell function was determined by insulinogenic index (IGI) over HOMA-IR and insulin secretion-sensitivity index-2 (ISSI-2). Incident dysglycemia was defined as the onset of impaired fasting glucose, impaired glucose tolerance, or type 2 diabetes. Generalized estimating equations (GEE) evaluated longitudinal associations of sCD163 with insulin sensitivity, β-cell function, and incident dysglycemia adjusting for demographic and lifestyle covariates. Areas under receiver-operating-characteristic curve (AROC) tested whether sCD163 improved dysglycemia prediction in a clinical model.
Results
Longitudinal analyses showed significant inverse associations between sCD163 and insulin sensitivity (% difference per standard deviation increase of sCD163 for HOMA2-%S (β = −7.01; 95% CI, −12.26 to −1.44) and ISI (β = −7.60; 95% CI, −11.09 to −3.97) and β-cell function (ISSI-2 (β = −4.67; 95 %CI, −8.59 to −0.58) and IGI/HOMA-IR (β = −8.75; 95% CI, −15.42 to −1.56)). Increased sCD163 was associated with greater risk for incident dysglycemia (odds ratio = 1.04; 95% CI, 1.02-1.06; P < 0.001). Adding sCD163 data to a model with clinical variables improved prediction of incident dysglycemia (AROC=0.6731 vs 0.638; P < 0.05).
Conclusions
sCD163 was longitudinally associated with core disorders that precede the onset of type 2 diabetes.
Keywords: Adipose tissue macrophage, Beta-cell function, Dysglycemia, Insulin sensitivity, sCD163, type 2 diabetes
Obesity is a critically important risk factor in the pathophysiology of type 2 diabetes (1). Although the association between obesity and type 2 diabetes has been extensively studied, significant knowledge gaps remain regarding the underlying mechanisms linking obesity and its widely varying phenotypes with metabolic disorders related to type 2 diabetes. A growing body of evidence suggests that obesity directly induces chronic subclinical inflammation, specifically at the level of the adipose tissue (2). This phenomenon, which is driven in part through the infiltration of activated macrophages into adipose tissue, results in the increased production and secretion of adipokines and inflammatory cytokines (3, 4). The recent discovery of a novel biomarker of macrophage activation, namely soluble CD163 (sCD163), represents an important advance in investigating the relationship of adipose tissue macrophage (ATM) activation with type 2 diabetes development (5–7).
CD163 is a monocyte/macrophage-specific surface protein involved in the uptake of haptoglobin-hemoglobin complexes (8). In the presence of inflammatory stimuli, CD163 is shed from cell membranes via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) and subsequently circulates as sCD163 (9, 10). Elevated concentrations of serum sCD163 have been shown in different inflammatory disease states, including rheumatoid arthritis (11), liver disease (12), and Crohn’s disease (13). In contrast to conventionally utilized markers of inflammation, sCD163 has a longer half-life and circulates in higher serum concentrations, making it a potentially promising clinical biomarker of macrophage-specific inflammation (10). To date, however, only a limited number of human studies have evaluated associations of sCD163 with type 2 diabetes and its underlying abnormalities (5, 6, 14–16), and no studies, to our knowledge, have evaluated repeated measures of sCD163 over time.
Therefore, the aim of the present study was to evaluate the longitudinal associations of sCD163 with core abnormalities underlying type 2 diabetes, specifically impaired insulin sensitivity and β-cell dysfunction, and dysglycemia.
Methods
Study population
The present study used data from the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort, an ongoing longitudinal observational cohort based in Toronto and London, Ontario. Participant recruitment took place from 2004 to 2006 and included adults 30 years of age or older with at least one risk factor for type 2 diabetes – including obesity, hypertension, family history of type 2 diabetes, and/or (for women) a history of gestational diabetes or birth of macrosomic infant (n = 712) (17). Participants with chronic inflammatory diseases were excluded from the study. Participants with acute infections (eg, colds, flu) had their study visits rescheduled. Written informed consent was obtained from all participants and institutional review boards from both study centers approved the study. Follow-up assessments have been conducted every 3 years at the clinical centers, where participants complete standard health and lifestyle questionnaires, and undergo anthropometric measurements and metabolic characterization. For the current analysis, we used data from the baseline, 3-year, and 6-year examinations. Participants were excluded if they had diabetes at baseline (n = 54), were missing baseline serum samples or missing follow-up visit data (n = 203), or if they lacked sufficient stored serum to conduct protein assay measures (n = 47), resulting in the availability of data on 408 participants (18).
Clinical measurements and procedures
At baseline and at each follow-up clinic examination, blood samples were collected after an 8- to 12-hour overnight fast. During each exam, a 75-g oral glucose tolerance test (OGTT) was administered after the collection of a fasting blood sample, with additional blood samples drawn at 30- and 120-minutes. All blood samples were processed, aliquoted, and frozen at −80°C at the Banting and Best Diabetes Centre Core Lab at Mount Sinai Hospital (Toronto, Canada). Glucose and insulin concentrations were measured using fasting, 30- and 120-minute blood samples. Insulin was assessed using the Elecsys 1010 immunoassay analyzer (Roche Diagnostics, Basel, Switzerland) and electrochemiluminescence immunoassay. Glucose was determined using an enzymatic hexokinase (Roche Modular, Roche Diagnostics).
sCD163 and lipid assays
Fasting serum samples collected at the baseline, 3- and 6-year follow-up visits were used to measure sCD163 concentrations. All measurements were performed at the Keenan Research Centre for Biomedical Sciences, St. Michael’s Hospital (Toronto, Canada).
sCD163 was measured using the R&D Quantikine ELISA (R&D Systems, Emeryville, CA), a solid phase sandwich ELISA which has a sensitivity of 0.613 ng/mL. Samples were run following the manufacturer’s protocol, in duplicate. Each microplate was read using a spectrophotometer reader for optical density at 570 and 450 nm. The difference at the 2 wavelengths was used to determine overall optical density for each sample. The inter-assay and intra-assay coefficient of variation imprecision was 12.3% and 4.4%, respectively.
Triacylglyceride (TAG) (Roche TRIGL), total cholesterol (Roche CHOL2), and high-density lipoprotein (HDL)–cholesterol (Roche HDLC3), were measured using a Roche Cobas c501 instrument (Roche Diagnostics, Indianapolis, Indiana, USA). Low-density lipoprotein (LDL)–cholesterol was calculated using the Friedewald equation (LDL–cholesterol = total cholesterol – HDL–cholesterol − TAG/2.2 [all in mmol/L]).
Anthropometric measurements and blood pressure
Blood pressure and anthropometric measurements were assessed twice and averaged at each clinic visit. Blood pressure was assessed using an automated sphygmomanometer on the right arm with the participant seated after resting for 5 minutes. Waist circumference was measured using standard procedures.
Standardized lifestyle questionnaires
Sociodemographic and lifestyle risk factors were assessed using structured, standardized questionnaires at each clinic visit. Physical activity was evaluated utilizing a version of the validated Modifiable Activity Questionnaire (MAQ) (19). Information regarding leisure and occupational activity over the past year was determined through MAQ. Each reported activity from the MAQ was weighted by its metabolic intensity allowing for the estimation of Metabolic Equivalent Task (MET) hours per week.
Insulin sensitivity, β-cell function, and dysglycemia
Hepatic insulin sensitivity was determined using the updated homeostasis model assessment (HOMA2) calculator based on fasting serum concentrations of glucose and insulin (HOMA2-%S), which is the reciprocal of HOMA-IR (20). Whole body insulin sensitivity was evaluated using the Matsuda Insulin Sensitivity Index (ISI) (21). The ISI calculation utilizes fasting and mean insulin and glucose measures during the OGTT. In our study, ISI was calculated using insulin and glucose measures at the 3 OGTT time points, which has been shown to be a valid index of insulin sensitivity compared to the gold standard euglycemic clamp (r = 0.732; P < 0.001) (22). β-Cell function was defined using the insulinogenic index (IGI) (23) over HOMA-IR (24) (IGI/HOMA-IR). IGI is determined using fasting and 30-minute insulin and glucose concentrations and has been validated against gold standard measures of insulin secretion (23). In addition, β-cell function was defined using the Insulin Secretion-Sensitivity Index-2 (ISSI-2), a validated β-cell function measure similar to the disposition index but calculated using OGTT data.(25)
Impaired fasting glucose (IFG), impaired glucose tolerance (IGT) or type 2 diabetes status were classified according to 2006 WHO guidelines (26). Specifically, IFG was defined by fasting blood glucose measures between 6.1–6.9 mmol/L and IGT as fasting glucose < 7.0 mmol/L and 2 hr OGTT blood glucose ≥ 7.8 and <11.1 mmol/L. Type 2 diabetes was classified based on physician diagnosis, use of diabetes medication, or having a fasting plasma glucose (FPG) level of ≥7.0 mmol/L or a 2-hour plasma glucose level (2hPG) ≥ 11.1 mmol/L (26). Dysglycemia was identified as meeting any one of these categories of glucose intolerance.
Statistical analyses
Descriptive characteristics were presented for baseline and each follow-up clinic visit. Continuous variables were described as mean ± standard deviation (SD) or median with interquartile range (IQR) for normally and nonnormally distributed variables, respectively. Categorical variables were presented as a number and percent (%). P values for continuous variable differences by category were determined by one-way ANOVA and Kruskal-Wallis tests for normally and nonnormally distributed variables, respectively. P values for categorical variable differences were determined using chi-squared tests and Fisher tests. Univariate associations of measures of sCD163 with metabolic markers were assessed through Spearman correlations using baseline data.
Longitudinal associations were assessed using data from the baseline, 3-year, and 6-year follow-up visits. For our primary analyses, generalized estimating equations (GEE) (27) were modeled to evaluate the association of longitudinally measured sCD163 concentrations with changes in the outcome measures. GEE is an extension of the generalized linear model and provides a longitudinal population estimate using a semi-parametric approach. An important strength in the use of GEE is its ability to accommodate missing values, thus maximizing statistical power. Furthermore, it works under the assumption that within subject measurements are correlated. For each GEE model, auto-regressive of order 1 correlation matrix was selected. Additionally, interaction for time was tested for sCD163 with each outcome variable. Significance for the interaction tests was set at P < 0.01.
Our primary outcomes of interest included insulin sensitivity, β-cell function, fasting glucose, glucose area-under-the-curve (AUC), and incident dysglycemia. Associations were assessed unadjusted, controlling for nonmodifiable covariates (Model 1: years from baseline (time), age, sex, and ethnicity), and additionally controlling for modifiable covariates (Model 2: Model 1 plus physical activity, smoking, blood pressure, and waist circumference). Measures of insulin sensitivity, β-cell function, and glucose concentrations were log-transformed.
We assessed the relationship between longitudinal measures of sCD163 and incident dysglycemia over the 6-year follow-up period. Participants classified as dysglycemic at baseline were excluded in the modelling (n = 27). Odds ratios (OR) for sCD163 and incident dysglycemia were calculated using the ‘logit’ link function within the GEE tests, with adjustments as described above.
Finally, we evaluated whether sCD163 significantly improved the prediction of dysglycemia when added to a model that contained routinely available clinical variables. The full clinical model included baseline age, sex, family history of type 2 diabetes, smoking status, systolic blood pressure, HDL–cholesterol, TAG, fasting glucose, and BMI (28). The areas under the receiver operating characteristic curves (AROC), which correspond to C-statistics in logistic regression models, were compared using DeLong’s test for 2 correlated curves (29).
All analyses were performed using R version 3.5.2. GEE models were conducted using the R geepack package (https://cran.r-project.org/web/packages/geepack/index.html). Statistical significance was set at P < 0.05.
Results
Table 1 presents baseline demographic characteristics of participants according to increasing serum sCD163 quartiles. Age differed across quartiles (P = 0.001), with the top quartile having the highest mean age of 51 years and the bottom quartile with the lowest mean age of 46 years. Similarly, sex differed across quartiles (P = 0.004). There was a significant difference in smoking status across quartiles (P < 0.05), with a higher proportion of subjects who had never smoked in the lowest quartile. Conversely, a greater number of subjects classified as obese were in the top quartile of sCD163; which was also consistent with the mean BMI being the highest in the highest quartile (both P < 0.001). There was no significant difference across sCD163 quartiles for ethnicity, physical activity, HDL, or glucose tolerance status.
Table 1.
Baseline Demographic, Anthropometric and Metabolic Characteristics of PROMISE Participants According to Quartiles of sCD163 (n = 408)
| Serum sCD163 Concentration Quartiles | |||||
|---|---|---|---|---|---|
| Baseline Characteristic | 1 (Lowest) | 2 | 3 | 4 (Highest) | P value |
| Subjects, n | 102 | 102 | 102 | 102 | |
| sCD163, ng/mL | 464 [46, 677] | 778 [679, 877] | 975 [879, 1037] | 1114 [1036, 1506] | |
| Age, years | 46.20 (8.09) | 49.61 (9.49) | 49.67 (9.83) | 51.36 (10.65) | 0.001 |
| Sex, male (%) | 17 (16.7) | 37 (36.3) | 29 (28.4) | 19 (18.6) | 0.004 |
| Ethnicity, n (%) | 0.069 | ||||
| European | 82 (80.4) | 68 (66.7) | 77 (75.5) | 68 (66.7) | |
| Other | 20 (19.6) | 34 (33.3) | 25 (24.5) | 34 (33.3) | |
| Smoking status, n (%) | 0.047 | ||||
| Current | 6 (6.0) | 9 (8.8) | 6 (6.1) | 3 (3.0) | |
| Former | 26 (26.0) | 37 (36.3) | 37 (37.4) | 48 (48.0) | |
| Never | 68 (68.0) | 56 (54.9) | 56 (56.6) | 49 (49.0) | |
| Physical activity, kg/kcal/hr | 19.26 [13.37, 23.03] | 29.26 [9.57, 89.08] | 19.11 [5.77, 67.30] | 17.03 [5.46, 53.78] | 0.142 |
| BMI, kg/m 2 | 27.65 (5.71) | 28.87 (5.29) | 31.56 (5.79) | 33.27 (7.55) | <0.001 |
| BMI category, n (%) | <0.0001 | ||||
| Normal | 42 (42.0) | 18 (17.8) | 11 (11.2) | 13 (12.9) | |
| Overweight | 23 (23.0) | 48 (47.5) | 33 (33.7) | 24 (23.8) | |
| Obese | 35 (35.0) | 35 (34.7) | 54 (55.1) | 64 (63.4) | |
| Waist circumference, cm | 91.12 (14.89) | 94.13 (13.97) | 99.66 (14.52) | 101.46 (15.42) | <0.001 |
| SBP, mm Hg | 114.12 (18.88) | 124.60 (16.57) | 126.85 (16.76) | 127.16 (15.82) | <0.001 |
| DBP, mm Hg | 74.79 (11.23) | 78.34 (9.89) | 81.60 (10.98) | 80.52 (9.32) | <0.001 |
| Total cholesterol , mmol/L | 4.55 (1.03) | 5.05 (0.87) | 5.05 (0.80) | 5.31 (1.01) | <0.001 |
| HDL cholesterol, mmol/L | 1.37 (0.33) | 1.42 (0.39) | 1.38 (0.40) | 1.31 (0.37) | 0.271 |
| LDL cholesterol, mmol/L | 2.67 (0.86) | 3.02 (0.75) | 3.02 (0.69) | 3.28 (0.87) | <0.001 |
| TAG, mmol/L | 0.87 [0.71, 1.47] | 1.24 [0.87, 1.63] | 1.23 [0.92, 1.83] | 1.32 [0.98, 2.05] | <0.001 |
| Fasting glucose, mmol/L | 4.67 (0.46) | 4.95 (0.53) | 4.91 (0.53) | 5.03 (0.58) | <0.001 |
| 2-h Glucose, mmol/L | 5.27 (1.31) | 5.55 (1.37) | 5.53 (1.29) | 5.94 (1.27) | 0.004 |
| HOMA2-%S | 145.35 [79.10, 251.00] | 104.20 [61.40, 156.93] | 86.40 [55.30, 117.75] | 66.80 [44.10, 102.80] | <0.001 |
| ISI | 23.78 [12.15, 32.96] | 14.88 [9.27, 25.50] | 13.17 [9.04, 18.76] | 10.67 [6.57, 16.19] | <0.001 |
| IGI/HOMA-IR | 11.79 [6.60, 25.80] | 7.81 [4.56, 11.56] | 7.10 [4.36, 9.82] | 5.74 [3.45, 8.48] | <0.001 |
| ISSI-2 | 942.70 [686.77, 1673.80] | 742.73 [607.36, 962.23] | 730.26 [593.81, 895.48] | 656.50 [527.67, 814.37] | <0.001 |
| Glucose tolerance status | 0.388 | ||||
| NGT, n (%) | 98 (96.1) | 96 (94.1) | 94 (92.2) | 93 (91.2) | |
| IFG/IGT, n (%) | 4 (3.9) | 6 (5.9) | 8 (7.8) | 9 (8.8) | |
Continuous values are presented as median (interquartile range) or mean (SD). P values were determined by one-way ANOVA and chi-squared tests for normally distributed continuous and categorical data, respectively. For nonnormally distributed data, P values were determined by Kruskal and Fisher tests for continuous and categorical data, respectively. Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density-lipoprotein–cholesterol; HOMA2-%S, homeostatic model assessment for insulin sensitivity as percentage; HOMA-IR, homeostatic model assessment for insulin resistance; IFG, impaired fasting glucose; IGI, insulinogenic index; IGT, impaired glucose tolerance; ISSI-2, insulin secretion-sensitivity index-2; LDL, low-density-lipoprotein–cholesterol; NGT, normal glucose tolerance; SBP, systolic blood pressure; TAG, triacylglyceride.
Reference (18) outlines the demographic characteristics at each clinic visit. sCD163 differed across follow-up years (P = 0.008), with the lowest median concentration at baseline (18). There was a significant difference in waist circumference throughout follow-up years (P = 0.004), with an increase in waist circumference at each visit. Both median fasting glucose and 2-hour glucose concentrations increased across visits (P < 0.001). In addition, both insulin sensitivity and β-cell function measures declined across follow-up visits (P < 0.001). There was no significant difference in the proportion of participants based on sex, ethnicity, smoking status, and BMI category across the time points. Additionally, from baseline to the 6-year clinic visit, there was no significant difference in physical activity, systolic and diastolic blood pressure, TAG and cholesterol measures.
Correlations between baseline sCD163 and baseline metabolic parameters are outlined in Reference (18). There were significant positive associations of sCD163 with BMI and waist circumference (r = 0.33 and 0.27; P < 0.001, respectively). Similarly, age, systolic and diastolic blood pressure, LDL, and TAG all showed positive correlations with sCD163 (all P < 0.05). Fasting glucose and glucose AUC were positively correlated with sCD163 at baseline (r = 0.27 and 0.25; P < 0.05, respectively). Furthermore, measures of insulin sensitivity and β-cell function were inversely correlated with sCD163 (all P < 0.05).
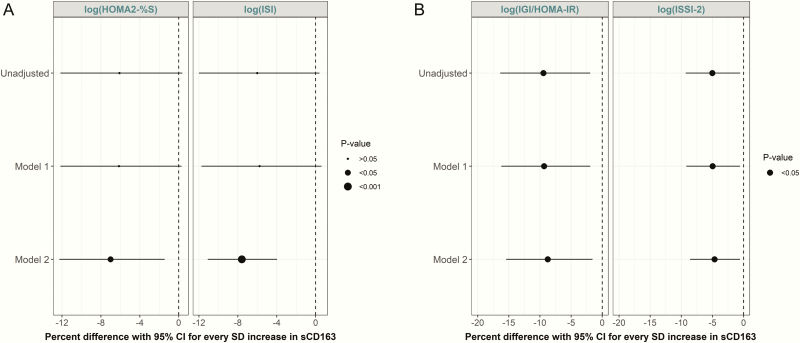
sCD163 increased significantly over the 6-year follow-up period (18). Six-year longitudinal associations of sCD163 with insulin sensitivity and β-cell function measures are presented in Fig. 1. In the fully adjusted model (controlling for time, baseline age, sex, ethnicity, physical activity, smoking, blood pressure, and waist circumference), sCD163 was inversely associated with longitudinal changes in both HOMA2-%S and ISI (both P < 0.05) (Fig. 1A). Similarly, sCD163 was inversely associated with longitudinal changes in β-cell function measures in the fully adjusted model (P < 0.05) (Fig. 1B). Similar associations were observed when we adjusted for BMI in place of waist circumference in the fully adjusted model (18). In a sensitivity analysis, we specifically evaluated the individual impact of adiposity variables on the associations of sCD163 with our outcomes. The addition of waist circumference or BMI to the multivariate GEE models slightly attenuated the associations, although they remained statistically significant (data not shown).
Figure 1.
Parameter estimates (with 95% CI) from GEE models showing associations of sCD163 with (A) insulin sensitivity and (B) β-cell function (% difference in the outcome per SD increase in sCD163). Model 1: adjusted for years from baseline (time), baseline age, sex, and ethnicity. Model 2: Model 1 plus physical activity, smoking, blood pressure, and waist circumference.
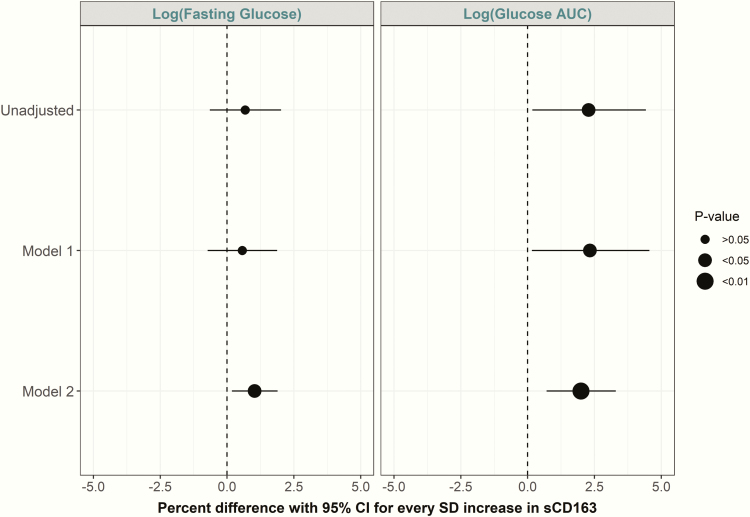
Fig. 2 displays the longitudinal relationship between sCD163 and fasting and AUC glucose, respectively. For every SD increase in sCD163, there was an increase in fasting glucose concentrations in fully adjusted models. Similar associations were observed for glucose AUC measures (P < 0.01).
Figure 2.
Parameter estimates (with 95% CI) from GEE models showing associations of sCD163 with fasting glucose and glucose AUC (% difference in the outcome per SD increase in sCD163). Model 1: adjusted for years from baseline (time), baseline age, sex, and ethnicity. Model 2: Model 1 plus physical activity, smoking, blood pressure, and waist circumference.
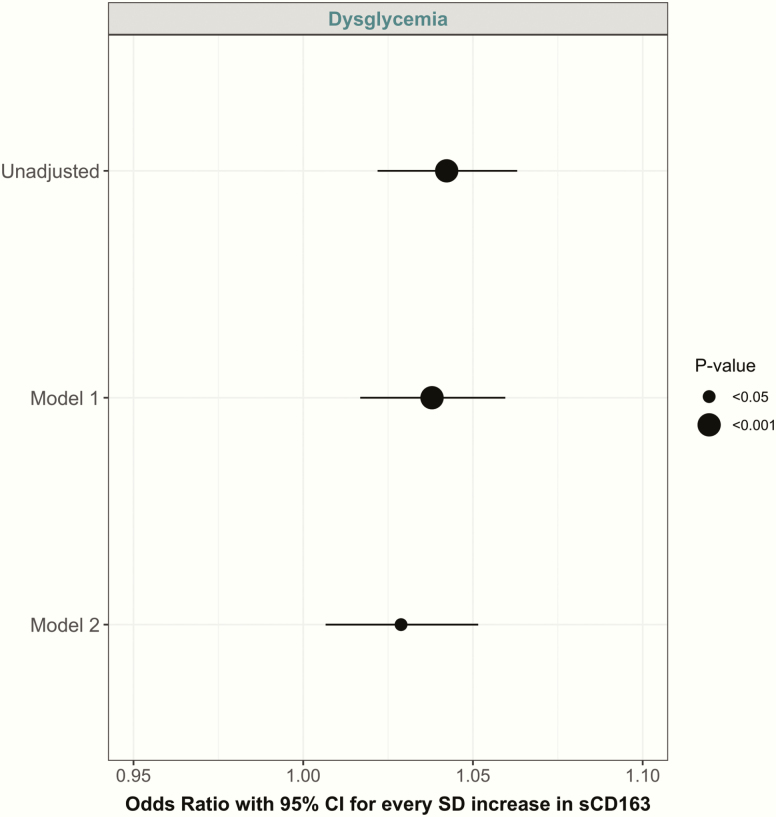
Fig. 3 displays the longitudinal association of sCD163 with incident dysglycemia. In the unadjusted model, every SD increase in sCD163 was associated with a higher odds ratio for dysglycemia (OR = 1.04; 95% CI, (1.02, 1.06); P < 0.001). This association was moderately attenuated in the fully adjusted model, although it remained statistically significant (OR = 1.03; 95% CI, (1.01, 1.05); P < 0.05).
Figure 3.
Parameter estimates (with 95% CI) from GEE models showing risk of incident dysglycemia per SD increase in sCD163. Model 1: adjusted for years from baseline (time), baseline age, sex, and ethnicity. Model 2: Model 1 plus physical activity, smoking, blood pressure, and waist circumference.
We further assessed whether the addition of baseline sCD163 to a model containing commonly measured clinical variables could better predict incident dysglycemia. Reference (18) displays the ROC curves and respective AROCs for the comparison (18). sCD163 improved prediction of dysglycemia in a clinical model compared to the model without sCD163 (AROC = 0.673 and 0.638, respectively; P = 0.003).
There were no significant interactions between sCD163 and time for any of the outcome variables (data not shown).
Discussion
The current study reports 3 main findings. First, we demonstrated an increase in serum sCD163 over the 6-year follow-up period in a Canadian cohort at risk for type 2 diabetes. Second, longitudinal analyses indicated significant inverse associations of serum sCD163 with insulin sensitivity and β-cell function, as well as positive associations with fasting glucose and glucose AUC over time. Finally, the addition of baseline sCD163 to a model containing routinely collected clinical variables significantly improved its prediction of incident dysglycemia. To our knowledge, this is the first study to extensively assess the longitudinal associations of sCD163 at multiple time points with important physiological disorders underlying type 2 diabetes.
The relevance of obesity to the development of type 2 diabetes and related underlying abnormalities has been consistently demonstrated in both experimental and epidemiological studies (30–32). More recent documentation of increased infiltration of macrophages in the adipose tissue of obese individuals and the characterization of the activity of these cells has offered new insights into the specific mechanisms linking chronic low-grade inflammation with type 2 diabetes pathogenesis (33). This ATM hypothesis suggests that, in addition to inflammatory stimuli signalling the recruitment of macrophages to the compromised site, macrophages present in the adipose tissue undergo a phenotypic switch from the M2 (alternatively activated) state to the M1 (classically activated) state during the progression of obesity, resulting in an increase in circulating pro-inflammatory markers which contribute to a variety of disorders underlying type 2 diabetes (33, 34). However, a significant challenge in investigating the importance of ATM in human subjects is the feasibility of directly sampling relevant adipose tissue depots, as extraction and testing of tissues is invasive and costly (35). The discovery of CD163, and its characteristic ability to be shed from the cell surface in its soluble form as sCD163, has created the opportunity to assess the ATM hypothesis in large cohort studies.
CD163 is a glycosylated membrane protein belonging to the scavenger receptor cysteine-rich family and is exclusively expressed on macrophages and monocytes (10, 36). One of the more extensively studied functions of CD163 is its role as a receptor for hemoglobin clearance (37); however, the physiological role of sCD163 is not defined (36). Evidence suggesting the ability for CD163 to be shed by TACE/ADAM17 from cell membranes in response to inflammatory stimuli and circulate in plasma as the soluble form has led to the proposal that sCD163 may be a potentially useful clinical biomarker of inflammation (10, 13, 38, 39). Circulating levels of sCD163 are observed in healthy subjects but are significantly increased in disease states—particularly those involving substantial inflammation responses (10). In addition to its high measurable circulating concentration, sCD163 has a much longer half-life compared to currently known markers of inflammation—specifically TNF-α (10). Furthermore, sCD163 is stable at various temperatures and is not impacted by multiple freeze-thaw cycles (40). Thus, such characteristics have led to growing interest around sCD163 as a potentially useful biomarker compared to currently known markers of inflammation, particularly in the context of evaluating the ATM-type 2 diabetes hypothesis (41).
Our study extends the current literature on the relationship of sCD163 with disorders underlying type 2 diabetes by using longitudinal measures of sCD163 at multiple time points. To date, only 1 study has reported that sCD163 predicts type 2 diabetes (5). In our study, we demonstrated that sCD163 added to a clinical model modestly improves prediction of incident dysglycemia. Moreover, while a limited number of studies have reported associations of sCD163 with measures of insulin sensitivity (6, 14–16), these studies have been constrained by cross-sectional designs, small sample sizes, and/or fasting measures of insulin resistance. A cross-sectional study with 234 Danish participants showed a significant positive association between sCD163 and HOMA-IR after adjustment for age, sex, glycemic group, and BMI (6). Similar results were seen in a separate study of 42 women (16). We have extended this literature by using repeated measures of sCD163 as well as a more detailed measure of insulin resistance. Finally, our documentation of a longitudinal association between sCD163 and validated OGTT-based measures of β-cell function represents an important contribution to the literature, as only 1 previous study had examined this association using a fasting-based surrogate measure (14). Although the mechanism linking sCD163 and β-cell function has not yet been identified, there is experimental evidence implicating inflammation in β-cell dysfunction (42). Several mechanisms including endoplasmic reticulum stress, oxidative stress, and lipotoxicity have been linked to the progression of β-cell dysfunction (42). Our study demonstrated that increased sCD163 concentration was inversely associated with β-cell function, raising the possibility that inflammation at the level of adipose tissue contributes to β-cell dysfunction.
Furthermore, we are the first to show that the addition of sCD163 to a model of commonly captured clinical measures better predicted the incidence of dysglycemia compared to the clinical model alone. Based on these findings, in combination with the unique characteristics of sCD163 as a relatively stable biomarker that circulates in high concentrations (10), sCD163 may be a promising biomarker for use in clinical practice.
There are a number of strengths associated with this study. PROMISE is a well-characterized longitudinal cohort of subjects at risk for type 2 diabetes, with baseline and multiple follow-up visits. The extensive data on anthropometrics and blood samples collected at each time point allowed for comprehensive covariate adjustments in the longitudinal analyses. Additionally, sCD163 concentrations were measured at multiple time points allowing for a detailed longitudinal assessment of its association with outcomes. Participants in the PROMISE cohort were free of chronic inflammatory diseases, thus elevated concentrations of sCD163 were likely derived largely from adipose tissue macrophages (15). Furthermore, the availability of data at baseline and multiple follow-up visits allowed for the consideration of repeated measurements of insulin sensitivity, β-cell function, glycemia, and covariates. Using the GEE model also allowed for the maximum number of participants to be included in the analyses.
There are, however, some important limitations to consider. sCD163 is not a direct measure of ATM activation. ATM assessment through tissue extraction is invasive and thus not feasible in large-scale studies due to the need for expensive and sophisticated procedures; in this context, sCD163 measured from the serum can be a useful alternative. Similarly, our outcomes measures were not captured using gold-standard procedures; however, we used OGTT-derived proxy measures of insulin sensitivity and β-cell function that have been extensively validated (21, 24, 25). PROMISE is an observational cohort study; thus, findings cannot indicate causality due to the nonrandomized design and potential for residual confounding. Generalizability of our findings is also limited to individuals with similar demographic characteristics as this population.
Conclusion and Future Directions
Our findings suggest that sCD163 may be a useful biomarker for understanding the role of ATM in the etiology of type 2 diabetes. Our results extend existing literature on this topic through the use of a longitudinal design that included measurements of this biomarker at multiple time points. To the best of our knowledge, we are the first to longitudinally assess sCD163 with detailed measures of insulin sensitivity, β-cell function, and dysglycemia. Additional studies are needed to confirm our findings. Furthermore, better validation of sCD163 as a marker of adipose tissue inflammation, assessment of this biomarker in randomized controlled trials, and additional longitudinal cohort studies with longer follow-up durations and with consideration of other important variables, including other ethnic groups, are needed to further expand our understanding of the utility of sCD163 and the role of ATM in the natural history of type 2 diabetes development.
Acknowledgments
The authors would like to thank all the research nurses and staff for their expert technical assistance and dedication in their work for PROMISE. From the Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, Toronto, Canada, the author’s thank Jan Neuman, Paula Van Nostrand, Stella Kink, Nicole Rubio, and Annette Barnie. Additionally, the authors thank Sheila Porter, Mauricio Marin, Marnie Orcutt, and Sue Miller of the Centre for Studies in Family Medicine, Western University, London, Canada for their expert technical assistance and dedication in their work for PROMISE. As well, we greatly appreciate the dedication of all of the participants involved in the PROMISE study.
Financial Support: PROMISE was supported by an operating grant from the Diabetes Canada and Canadian Institutes of Health Research (CIHR). ZSA was funded by the CIHR Graduate Scholarships, Ontario Graduate Scholarship and the University of Toronto Banting and Best Scholarship. RR holds the Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at Mount Sinai Hospital.
Author Contributions: Z.S.A. analyzed and interpreted the data and wrote the manuscript. P.W.C. reviewed and edited manuscript and supervised the laboratory assays. L.W.J. contributed to data management, statistical guidance, and reviewed and edited the manuscript. R.R. reviewed and edited the manuscript. S.B.H., B.Z., and A.J.H. designed the study and reviewed and edited the manuscript. A.J.H. is the guarantor of this work and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviations
- AUC
area-under-the-curve
- AROC
area-under-receiver operating characteristic curve
- ATM
adipose tissue macrophage
- BMI
body mass index
- GEE
generalized estimating equation model
- HOMA2-%S
homeostatic model assessment for insulin sensitivity as percentage
- HOMA-IR
homeostasis model assessment for insulin resistance
- IFG
impaired fasting glucose
- IGI
insulinogenic index
- IGT
impaired glucose tolerance
- ISI
insulin sensitivity index
- ISSI-2
insulin secretion-sensitivity index-2
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- PROMISE
Prospective Metabolism and Islet Cell Evaluation study
- ROC
receiver operating characteristic curve
- sCD163
soluble CD163
- TAG
triacylglyceride
Additional Information
Disclosure Summary: The authors declare no conflict of interest associated with this manuscript.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kahn SE, HULL RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. [DOI] [PubMed] [Google Scholar]
- 2. Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112(2):61–67. [DOI] [PubMed] [Google Scholar]
- 4. Lee J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 diabetes. Arch Pharm Res. 2013;36(2):208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Møller HJ, Frikke-Schmidt R, Moestrup SK, Nordestgaard BG, Tybjærg-Hansen A. Serum soluble CD163 predicts risk of type 2 diabetes in the general population. Clin Chem. 2011;57(2):291–297. [DOI] [PubMed] [Google Scholar]
- 6. Parkner T, Sørensen LP, Nielsen AR, et al. Soluble CD163: a biomarker linking macrophages and insulin resistance. Diabetologia. 2012;55(6):1856–1862. [DOI] [PubMed] [Google Scholar]
- 7. Sorensen LP, Parkner T. Sondergaard E. Bibby BM. Moller HJ. Nielsen S. Visceral obesity is associated with increased soluble CD163 concentration in men with type 2 diabetes mellitus. Endocrine connections. 2015;4(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowal K, Silver R, Sławińska E, Bielecki M, Chyczewski L, Kowal-Bielecka O. CD163 and its role in inflammation. Folia Histochem Cytobiol. 2011;49(3):365–374. [DOI] [PubMed] [Google Scholar]
- 9. Droste A, Sorg C, Högger P. Shedding of CD163, a novel regulatory mechanism for a member of the scavenger receptor cysteine-rich family. Biochem Biophys Res Commun. 1999;256(1):110–113. [DOI] [PubMed] [Google Scholar]
- 10. Møller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72(1):1–13. [DOI] [PubMed] [Google Scholar]
- 11. Greisen SR, Moller HJ, Stengaard-Pedersen K, et al. Soluble macrophage-derived CD163 is a marker of disease activity and progression in early rheumatoid arthritis. Clin Exp Rheumatol. 2011;29(4):689–692. [PubMed] [Google Scholar]
- 12. Kazankov K, Barrera F, Møller HJ, et al. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology. 2014;60(2):521–530. [DOI] [PubMed] [Google Scholar]
- 13. Møller HJ, Aerts H, Grønbaek H, et al. Soluble CD163: a marker molecule for monocyte/macrophage activity in disease. Scand J Clin Lab Invest Suppl. 2002;237:29–33. [DOI] [PubMed] [Google Scholar]
- 14. Deichgræber P, Witte DR, Møller HJ, et al. Soluble CD163, adiponectin, C-reactive protein and progression of dysglycaemia in individuals at high risk of type 2 diabetes mellitus: the ADDITION-PRO cohort. Diabetologia. 2016;59(11):2467–2476. [DOI] [PubMed] [Google Scholar]
- 15. Zanni MV, Burdo TH, Makimura H, Williams KC, Grinspoon SK. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin Endocrinol (Oxf). 2012;77(3):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kračmerová J, Rossmeislová L, Kováčová Z, et al. Soluble CD163 is associated with CD163 mRNA expression in adipose tissue and with insulin sensitivity in steady-state condition but not in response to calorie restriction. J Clin Endocrinol Metab. 2014;99(3):E528–E535. [DOI] [PubMed] [Google Scholar]
- 17. Hanley AJ, Retnakaran R, Qi Y, et al. Association of hematological parameters with insulin resistance and beta-cell dysfunction in nondiabetic subjects. J Clin Endocrinol Metab. 2009;94(10):3824–3832. [DOI] [PubMed] [Google Scholar]
- 18. Semnani-Azad Z, Connelly PW, Johnston LW, et al. Supplementary: The macrophage activation marker soluble CD163 is longitudinally associated with insulin sensitivity and β-cell function. Date of Deposit: July 15, 2019. DOI: 10.6084/m9.figshare.8872367. [DOI] [PMC free article] [PubMed]
- 19. Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. [DOI] [PubMed] [Google Scholar]
- 20. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. [DOI] [PubMed] [Google Scholar]
- 21. Matsuda MD, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 22. DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care. 2010;33(7):e93. [DOI] [PubMed] [Google Scholar]
- 23. Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med. 1995;12(10):931. [DOI] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 25. Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26(12):1198–1203. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization & International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia : report of a WHO/IDF consultation. World Health Organization; 2006. https://apps.who.int/iris/handle/10665/43588. [Google Scholar]
- 27. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 28. Vassy JL, Hivert MF, Porneala B, et al. Polygenic type 2 diabetes prediction at the limit of common variant detection. Diabetes. 2014;63(6):2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 30. Lee CC, Adler AI, Sandhu MS, et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009;52(6):1040–1047. [DOI] [PubMed] [Google Scholar]
- 31. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2009;302(2):179–188. [DOI] [PubMed] [Google Scholar]
- 32. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. [DOI] [PubMed] [Google Scholar]
- 33. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bouloumié A, Curat CA, Sengenès C, Lolmède K, Miranville A, Busse R. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care. 2005;8(4):347–354. [DOI] [PubMed] [Google Scholar]
- 36. Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18(17):2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47(7-8):1650–1660. [DOI] [PubMed] [Google Scholar]
- 38. Al-Daghri NM, Al-Attas OS, Bindahman LS, et al. Soluble CD163 is associated with body mass index and blood pressure in hypertensive obese Saudi patients. Eur J Clin Invest. 2012;42(11):1221–1226. [DOI] [PubMed] [Google Scholar]
- 39. Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210(2-4):153–160. [DOI] [PubMed] [Google Scholar]
- 40. Møller HJ, Hald K, Moestrup SK. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand J Clin Lab Invest. 2002;62(4):293–299. [DOI] [PubMed] [Google Scholar]
- 41. Buechler C, Eisinger K, Krautbauer S. Diagnostic and prognostic potential of the macrophage specific receptor CD163 in inflammatory diseases. Inflamm Allergy Drug Targets. 2013;12(6):391–402. [DOI] [PubMed] [Google Scholar]
- 42. Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167(1):228–256. [DOI] [PubMed] [Google Scholar]