Abstract
Context
Polycystic ovary syndrome (PCOS) is a prevalent disorder in reproductive aged women associated with a number of endocrine and metabolic complications, including increased risk of endometrial cancer.
Objective
To study the effect of the characteristic increased androgen levels in PCOS on the endometrium, a novel scaffold-free multicellular endometrial organoid was established.
Design
Human endometrial organoids were constructed using primary endometrial epithelial and stromal cells from endometrial tissues. Organoids were treated for 14 days with physiologic levels of estradiol and testosterone to mimic a normal follicular phase or PCOS hormone profiles. Organoids were harvested for immunostaining and ribonucleic acid sequencing.
Setting
Academic institution.
Patients
Endometrial tissues from 10 premenopausal women undergoing hysterectomy for benign pathologies were obtained following written consent.
Main Outcome Measures
Organoid architecture, cell specific markers, functional markers, proliferation, and gene expression were measured.
Results
A method to generate scaffold-free endometrial organoids containing epithelial and stromal cells was established. These organoids exhibited distinct organization with epithelial cells lining the outer surface and stromal cells in the center of the organoids. Epithelial cells were polarized, organoids expressed cell type specific and functional markers, as well as androgen, estrogen, and progesterone receptors. Treatment with PCOS hormones increased cell proliferation and dysregulated genes in endometrial organoids.
Conclusions
A new multicellular, scaffold-free endometrial organoid system was established that resembled physiology of the native endometrium. Excess androgens in PCOS promoted cell proliferation in endometrial organoids, revealing new mechanisms of PCOS-associated with risk of endometrial neoplasia.
Keywords: endometrium, organoids, polycystic ovarian syndrome, PCOS
Polycystic ovarian syndrome (PCOS) is a complex multifactorial disease that includes endocrine, reproductive, and metabolic disorders affecting approximately 10% of women of reproductive age across multiple geographic ancestries and ethnicities (1). The commonly accepted criterion for diagnosis of PCOS is based on the 2004 Rotterdam criteria, which includes hyperandrogenism (biochemical and/or clinical), chronic oligo- or anovulation, and polycystic ovaries (2). PCOS patients exhibit abnormal hormone levels of the hypothalamic–pituitary–gonadal axis (high luteinizing hormone and androgens) (3–5) as well as metabolic factors such as insulin (6). Women with PCOS are typically obese (50%–80%) and are at risk for a number of health complications including cardiovascular disease, type 2 diabetes, glucose intolerance, insulin resistance, sleep apnea, and endometrial abnormalities (7–12).
There is a significant association between PCOS and endometrial cancer (13–17) as women with untreated PCOS are 3 times more likely to develop endometrial cancer (9, 18, 19). It is believed that the endometrium of PCOS women becomes hyperplastic due to the absence of a complete menstrual cycle as a result of oligo- or anovulation, consequently contributing to the higher risk of developing endometrial cancer (20). Because a number of disorders are associated with PCOS, including obesity, hyperinsulinemia, and hyperandrogenism, it is unclear which condition is driving the endometrial abnormalities in PCOS women.
The endometrium is the lining of the uterus that response to sex steroid hormones, primarily estrogen and progesterone, to proliferate, differentiate, and regress within a period of approximately 28 days. These physiological changes driven by hormones requires complex paracrine interactions between cell types of the endometrium including those between endometrial epithelial and stromal cells, which are essential for proliferation and differentiation of the tissue. When hormonal balance is altered, the endometrium can become refractory to embryo implantation and even become neoplastic, leading to cancer. Studies have reported conflicting roles of androgens on the endometrium demonstrating both pro- and anti-proliferative effects depending on the context (21–25). Given that PCOS patients have constantly higher levels of androgens (3–5) and are at higher risk of developing endometrial cancer (9, 18, 19), we hypothesized that excess androgens in PCOS women exert proliferative effects on the endometrium.
In endometrial biology, there remains a gap in knowledge in how risk factors, like PCOS, affect the benign endometrium toward neoplastic transformation due of the lack of appropriate models of the human endometrium. Early studies used organ cultures of human endometrium demonstrating responses to steroid hormones on both endometrial epithelial and stromal cells (26–29). However, tissue explants have a limited lifespan in culture. To date, much of the in vitro studies have focused on the stromal compartment given the ease of growth and propagation of stromal cells. Epithelial cells, which are the cells that become neoplastic, do not grow well as monolayers, undergo senescence, usually are limited in yield and lose their polarity. Recently, two groups reported on the establishment of endometrial organoids, consisting of endometrial epithelial cells within a commercially available basement membrane matrix, MatrigelTM (30, 31). While these studies have generated for the first time an organoid of endometrial epithelial cells, we set out to build an organoid with both epithelial and stromal cells of the endometrium and hypothesized that the stromal cells would provide the scaffold or supportive layer for the epithelial cells, much like in the native tissue. Here, we present the generation of scaffold-free endometrial organoids with epithelial and stromal cells that respond to estradiol (E2) and androgens. With this system, we investigated the influence of PCOS levels of androgens on the multicellular endometrial organoids over a period of 14 days, equivalent to the follicular phase of the menstrual cycle. We report that excess androgens elicited proliferative effects and altered gene expression in the endometrial organoids that may contribute to increasing the risk of endometrial neoplasia that occurs in PCOS women.
Materials and Methods
Study participants and tissue collection
Endometrial samples were collected from premenopausal women (n = 10; Table 1) undergoing routine hysterectomy for benign uterine conditions at Northwestern University Prentice Women’s Hospital, according to an institutional review board-approved protocol. Written consent was obtained from all women included in the study. Endometrial samples were histologically classified as normal secretory or proliferative by pathologists at Northwestern University Prentice Women’s Hospital.
Table 1.
Patients’ information for this study
| Mean | SEM | Median | Minimum | Maximum | |
|---|---|---|---|---|---|
| Age | 44.8 | 1.89 | 43.5 | 35 | 52 |
| BMI | 32.83 | 3.809 | 30.1 | 22.65 | 65.04 |
| Height (inches) | 65.75 | 0.7932 | 66.5 | 62 | 69 |
| Weight (lbs) | 200.9 | 23.07 | 184 | 149 | 403 |
Abbreviations: BMI, body mass index; SEM, standard error of the mean.
Generation of endometrial organoids
Uterine tissue (~1–2 cm2) was transported on ice from Pathology to the laboratory. Endometrium was excised off of the uterine tissues and minced. Freshly minced tissues were enzymatically digested in 2.5mg/mL collagenase type I (Life Technology, 17100-017) + 0.1mg/ml DNase (Sigma-Aldrich, D4513) + 500 units dispase (Corning, 354235) at 37°C with shaking at 80–100 rpm for 30 minutes. The supernatant was passed through a 100 µm strainer, and the strainer was washed once with Hanks buffered saline solution. The flow-through was then passed through a 20 µm strainer to collect epithelial cells while stromal cells remained in the final flow-through. The 20 µm strainer was invert-washed to collect epithelial cells, with growth medium (complete MammoCultTM human medium (Stemcells™ Technologies, 05620) supplemented with hydrocortisone and heparin as per manufacturer’s instructions and 1% pen/strep (Sigma, P0781). Epithelial and stromal cell suspensions were centrifuged at 500 g for 5 minutes. The stromal pellet was incubated with red blood cell lysis buffer at 37°C for 10 minutes and collected by centrifugation at 500 g for 5 minutes. Both epithelial and stromal cell pellets were then resuspended in MammoCultTM medium to similar densities. Epithelial and stromal cells were mixed at a 3:1 ratio by volume and 50 uL of epithelial-stromal cell suspension was seeded into 1.5% (w/v) agarose 3D Petri Dishes®, prepared according to the manufacturer’s instructions (MicroTissues® 3D Petri Dish® micro-mold spheroids, Sigma, Z764043). Cells were cultured in MammoCultTM growth medium for at least 7 days to allow organoid formation prior to hormone treatment. Media was changed every second day.
Hormone treatments
A stepwise E2 treatment was used to treat endometrial organoids within agarose 3D Petri Dishes® to mimic the 14 day follicular phase of the human menstrual cycle as previously described (32, 33) but with the addition of testosterone. The normal hormone treatment was 0.1 nM E2 + 0.8 nM testosterone (day 0–7), 1 nM E2 + 0.8 nM testosterone (day 7–13), and 1 nM E2 + 1.25 nM testosterone (day 13–14). The PCOS hormone treatment was 0.1 nM E2 + 3 nM testosterone (day 0–7) and 1 nM E2 + 3 nM testosterone (day 7–14). Media was changed every second day.
Immunohistochemistry and histology
Agarose 3D Petri Dishes® containing endometrial organoids were removed from the tissue culture plate and the medium inside the dishes was removed gently by pipetting under a dissecting microscope. Endometrial organoids were sealed within the agarose dish with lukewarm 1.5% (w/v) agarose in 1×PBS. The sealed 3D Petri Dishes® containing endometrial organoids were then fixed in 4% paraformaldehyde in 1×PBS at 4°C overnight, rinsed briefly in 1×PBS, stored in 70% EtOH and processed for standard paraffin embedding and sectioning at 5 µm. Paraffin sections were then processed for hematoxylin and eosin (H&E), trichrome, periodic acid-Schiff (PAS) stains and immunohistochemistry. The primary antibodies used were estrogen receptors (ER; 1:200, Santa Cruz SC71064, lot A0318), androgen receptors (AR; 1:200, Thermo Scientific, RB9030, lot 9030P 1606L), progesterone receptors (PR; 1:200, DAKO, M3568, lot 10085593 and 10139537), Ki67 (1:300, Abcam, ab15580, lot GR91643-1), vimentin (1:500, Abcam, 92547, lot GR145336-2), E-cadherin (1:50, BD Transduction, 610181, lot 6315829), pan-cytokeratin (1:250, Abcam, ab7753, lot GR185314-13), and sox9 (1:500, EMD Millipore, AB5535, lot 3145011). Stainings for markers of other cell types of the endometrium were done but not shown as organoids were mostly negative for the following markers: CD56 (NCAM1, 1:200, Abcam, ab133345, lot GR96160-8), CD68 (1:400, Cell Signaling Technologies, 76437, lot 1), CD14 (1:200, Cell Signaling Technologies, 75181, lot 1), CD3ε (1:200, Cell Signaling Technologies, 85061, lot 1), CD31 (1:100, Spring Biosciences, E11112, lot 1110011DD), α-smooth muscle actin (1:200, Novus Biologicals, NB600-531, lot 160401LVD), cytokeratin-13 (1:200, Abcam, ab92551, lot GR211864-16), and FOXA2 (1:300, Abcam, ab108422, lot GR3250052-7). For immunohistochemistry, EnVision™+ System-HRP (DAB; DAKO, K4010, and K4006) and HRP/DAB (ABC) Detection IHC (Abcam, ab64261) kits were used according to manufacturers’ instructions, and slides were mounted in Cytoseal™ XYL (Thermo Scientific, 8312–4). For immunofluorescence, Alexa Fluor® 488 goat anti-rabbit (1:200, Life Technologies, A11008, lot 1705869) and Alexa Fluor® 594 donkey anti-mouse (1:200, Jackson ImmonoResearch, 115-586-072, lot 127427) were used, and slides were mounted in ProLong™ Gold Antifade Mountant (Invitrogen, P36930). Nuclei were counter stained with either hematoxylin (Ricca, 3530–32) for 15 seconds or 1 µM 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, D1306) for 15 minutes.
For collagen staining, a trichrome stain kit (Abcam, ab150686) was used according to the manufacturer’s instructions. Routine H&E and PAS stains were performed at Pathology Core Facility, Northwestern University, Chicago, Illinois.
RNA isolation
All endometrial organoids were harvested from 3D Petri Dishes® by pipetting. Total ribonucleic acid (RNA) was extracted from organoids using TRI Reagent™ (Sigma Aldrich, 93289) and Direct-zol™ RNA MicroPrep (Zymo Research, R2060) according to the manufacturers’ instructions with an additional step of DNase I treatment to remove any contaminating deoxyribonucleic acid (DNA). RNA quality was determined with a BioAnalyzer through NUSeq Core Facility, Northwestern University, Chicago, Illinois.
RNA-sequencing (RNA-seq)
To generate RNA sequencing libraries, RNA quality was determined with the Agilent Bioanalyzer 2100, accepting RNA integrity numbers of >7 and quantified using Qubit. Directional mRNA libraries were prepared using Illumina TruSeq mRNA Sample Preparation Kits per manufacturer’s instructions. Briefly, polyadenylated mRNAs were captured from total RNA using oligo-dT selection. Next, samples were converted to complementary DNA (cDNA) by reverse transcription, and each sample was ligated to Illumina sequencing adapters containing unique barcode sequences. Barcoded samples were then amplified by polymerase chain reaction (PCR) and the resulting cDNA libraries by quantified using quantitative PCR (qPCR). Finally, equimolar concentrations of each cDNA library were pooled and sequenced on the Illumina HiSeq4000.
The quality of DNA reads, in FASTQ format, was evaluated using FastQC. Adapters were trimmed using cutadapt (34), and the reads were aligned to the human genome (hg19) using STAR (35). Read counts for each gene were calculated using htseq-count (36) in conjunction with a gene annotation file for hg19 obtained from Ensembl (http://useast.ensembl.org/index.html). Normalization and differential expression were determined using DESeq2 (37). Differential expression was performed using a multifactor analysis in DESeq2, identifying changes between tissues while controlling for changes due to patient. The cutoff for determining significantly differentially expressed genes was an FDR-adjusted and nonadjusted P-value of less than .05.
Quantitative RT-PCR
Quantitative reverse transcription (RT)–PCR (qRT-PCR) was performed using the SYBR Green system. First strand cDNA synthesis was performed using 200 ng of RNA and M-MLV reverse transcriptase (Invitrogen, 28025013). qRT-PCR was done with ~2.5 ng of first strand cDNA using PowerSyBr® Green PCR Master Mix (Thermo Fisher Scientific, 4367659) in triplicate on a QuantStudio™5 system (Thermo Fisher Scientific, A28140) using a 40-cycle protocol. Primers used in these studies were BUD13:5’-CCT CCA TCT GCT TTA CCT CTT C-3’ and 5’-ACA GCT ATC TCC ACA ACC AAA C-3’, CYP19A1 (aromatase P450): 5’-CAC-ATC-CTC-AAT-ACC-AGG-TCC-3’, 5’-CAG-AGA-TCC-AGA-CTC-GCA-TG-3’, and TaqMan probe CCC-TCA-TCT-CCC-ACG-GCA-GAT-TCC. TaqMan® assays (IDT) were used for quantitative real time PCR for ZNF891 (Hs.PT.58.15228132), MARCH1 (Hs.PT.58.27689232), LINC00324 (Hs.PT.58.498575), FOXL1 (Hs.PT.58.25721571.g), and PADI3 (Hs.PT.58.39780714).
Image acquisition and analysis
Images were acquired using a Leica DM5000 B microscope (40× 0.75 numerical aperture [NA] objective) equipped with a CCD Leica DFC495 camera or a Zeiss Axiovert 200 microscope (10× 0.25 NA or 20× 0.4 NA objectives) equipped with a CCD AxioCAM HR camera. For quantifications, images were captured under identical settings including intensity, brightness, and exposure time. Postacquisition, image pairs of normal and PCOS organoids were processed identically. This included adjustment for brightness and contrast of all images. ImageJ was used for all postacquisition processing.
Statistical analysis
All statistical tests were performed using Prism software (GraphPad) and Microsoft Excel. Unless otherwise noted, average ± standard error of the mean (SEM) are shown in all plots. Data sets were analyzed for significance using unpaired Student’s t-test based on n = number of organoids for Ki67 and Sox9 quantification and n = number of patients for RNA-seq and qRT-PCR analyses. One-way analysis of variance with Bonferroni’s correction was used to compare effects of testosterone and R1881 in organoids cultured in normal versus PCOS hormone profiles. A 95% confidence interval was used to determine significance (P < .05). All sample sizes and P-values are reported in the figures or the figure legends.
Results establishment and characterization of scaffold-free endometrial organoids
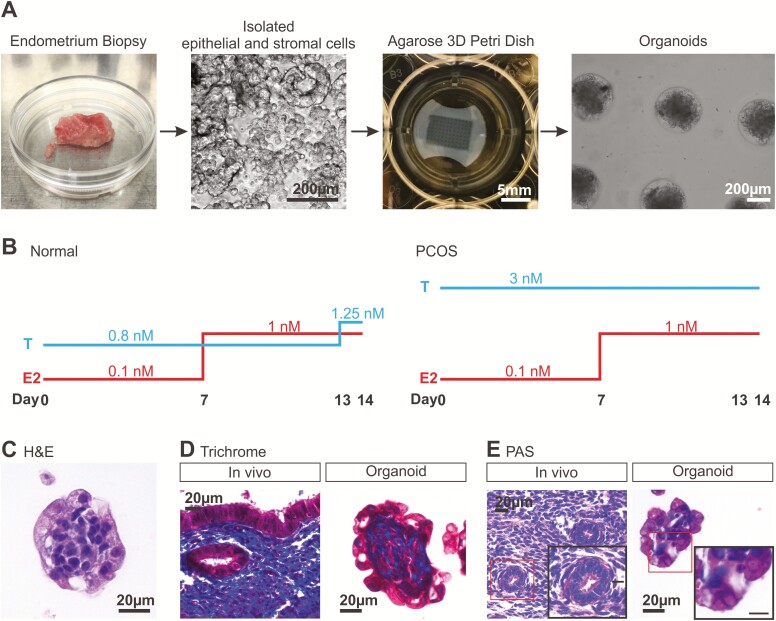
Endometrial epithelial and stromal cells were isolated from uterine tissues collected from premenopausal women (44.8 ± 1.8 years old) undergoing hysterectomy for benign uterine pathology (Table 1). Endometrial samples were a mixture of proliferative and secretory tissues. Epithelial and stromal cells were combined at a 3:1 ratio by volume into the agarose 3D Petri Dishes® and cells were cultured in MammoCultTM growth media (Fig. 1). This media is routinely used to grow mammospheres which are spheroids of breast epithelial cells (38, 39) and was thus chosen to encourage endometrial epithelial cell growth and survival of the endometrial organoids. Comparisons of various media, including DMEM/F12, RPMI, and MammoCultTM revealed that MammoCultTM was the optimal best media for endometrial organoid cultures, as measured by lactate dehydrogenase secretion (data not shown). Daily monitoring of the organoids revealed that 7 days postseeding in sex hormone-free growth medium was optimal for aggregates of cells to form (Fig. 1A). These cell aggregates were then subjected to stepwise hormone (E2 and testosterone) treatments for 14 days to mimic the follicular phase of a normal menstrual cycle (Fig. 1B) (32, 33, 40). By the end of 14 days in medium supplemented with sex hormones, H&E staining of the cell clusters showed an organization of endometrial cells with a tightly packed center and an outer layer of cells. These clusters of cells exhibited characteristics of endometrial tissue as subsequently detailed and will be referred to as organoids (41) (Fig. 1C). These organoids secreted collagen and mucins as demonstrated by trichrome blue (Fig. 1D) and PAS (Fig. 1E) stains. Specifically, trichrome blue (collagen) was present in the center of the endometrial organoid and in the stromal layer of the native tissue (Fig. 1D). Cells on the endometrial organoid surface appeared strongly positive for PAS stain (mucins) compared to those at the center similar to endometrial glands in tissue (Fig. 1E). The presence of endometrial stroma-rich collagen and epithelium-rich mucins in endometrial organoid center and surface, respectively, revealed that these organoids had structural organization and function similar to native endometrium (42, 43).
Figure 1.
Generation of scaffold-free 3D endometrial organoids from human primary endometrial cells. (A) Endometrial epithelial and stromal cells were isolated from premenopausal endometrial tissues with benign pathology. Both stromal and epithelial cells were seeded into 1.5% agarose 3D Petri Dishes™ at a 1:3 ratio by volume and maintained in sex hormone-free medium for 7 days before downstream experiments. (B) Estradiol (E2) and testosterone (T) were added in a stepwise manner to the 3D cultures to mimic the levels of E2 and T during the follicular phase of a menstrual cycle. T levels were consistently higher (3 nM) in the polycystic ovarian syndrome hormone profile. After 14 days of normal hormone treatment, endometrial organoids were stained with (C) hematoxylin and eosin, (D) trichrome stain to detect collagen (blue), and (E) periodic acid-Schiff staining to stain mucosal substances (eg, mucins, glycoproteins; bright pink). Scale bar in inset of (E) is 10 µm.
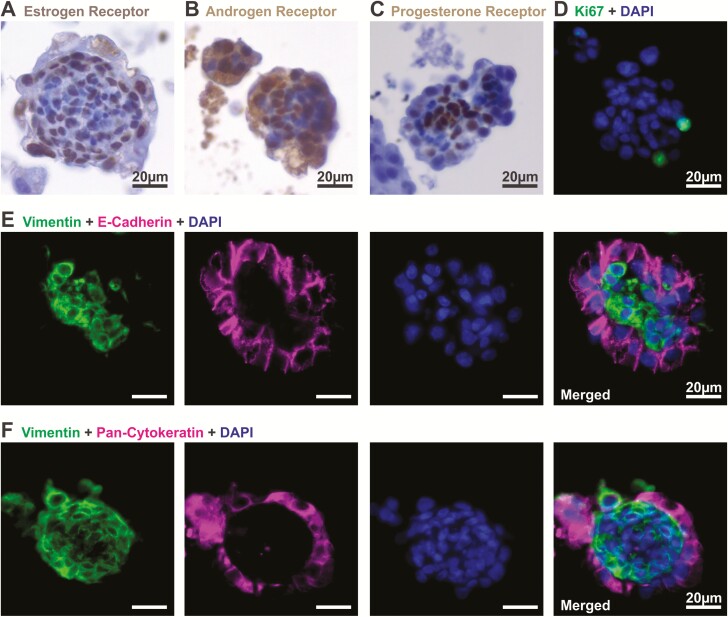
At the end of 14 days of normal hormone treatment to mimic the follicular phase of a menstrual cycle (32, 33), endometrial organoids expressed sex hormone receptors: ER, Fig. 2A), androgen receptor (AR, Fig. 2B) and progesterone receptor (PR, Fig. 2C). Nuclear localization of ER and AR suggested that these receptors responded to E2 and testosterone in the medium throughout the 14-day culture period (Fig. 1B). These organoids exhibited proliferation as indicated by Ki67 immunoreactivity (Fig. 2D). In addition, we observed that these organoids had on average 4.07% ± 1.29 of Sox9 positive cells (endometrial epithelial stem cell marker (31); n = 25 organoids obtained from 3 patients; (44). Half of the organoids (12/25) were Sox9 negative, while 4/25 organoids contained more than 10% of Sox9 positive cells (44). The distribution of Sox9 positive cells was heterogeneous within patients in that some organoids were Sox9 positive while others from the same patient were negative.
Figure 2.
Endometrial organoids express sex hormone receptors and exhibit structural organization. Organoids were observed after 14 days of stepwise hormone treatment mimicking the follicular phase of a normal menstrual cycle. Levels of (A) estrogen receptor, (B) androgen receptor, and (C) progesterone receptor were detected by immunohistochemical staining in endometrial organoids. (D) Proliferation was assessed in the organoids as indicated by Ki67 immunoreactivity. (E, F) Cell specific markers were assessed by immunofluorescent staining of E-cadherin, pan-cytokeratin (epithelial), and vimentin (stromal), which revealed structural organization of the cells in the organoids.
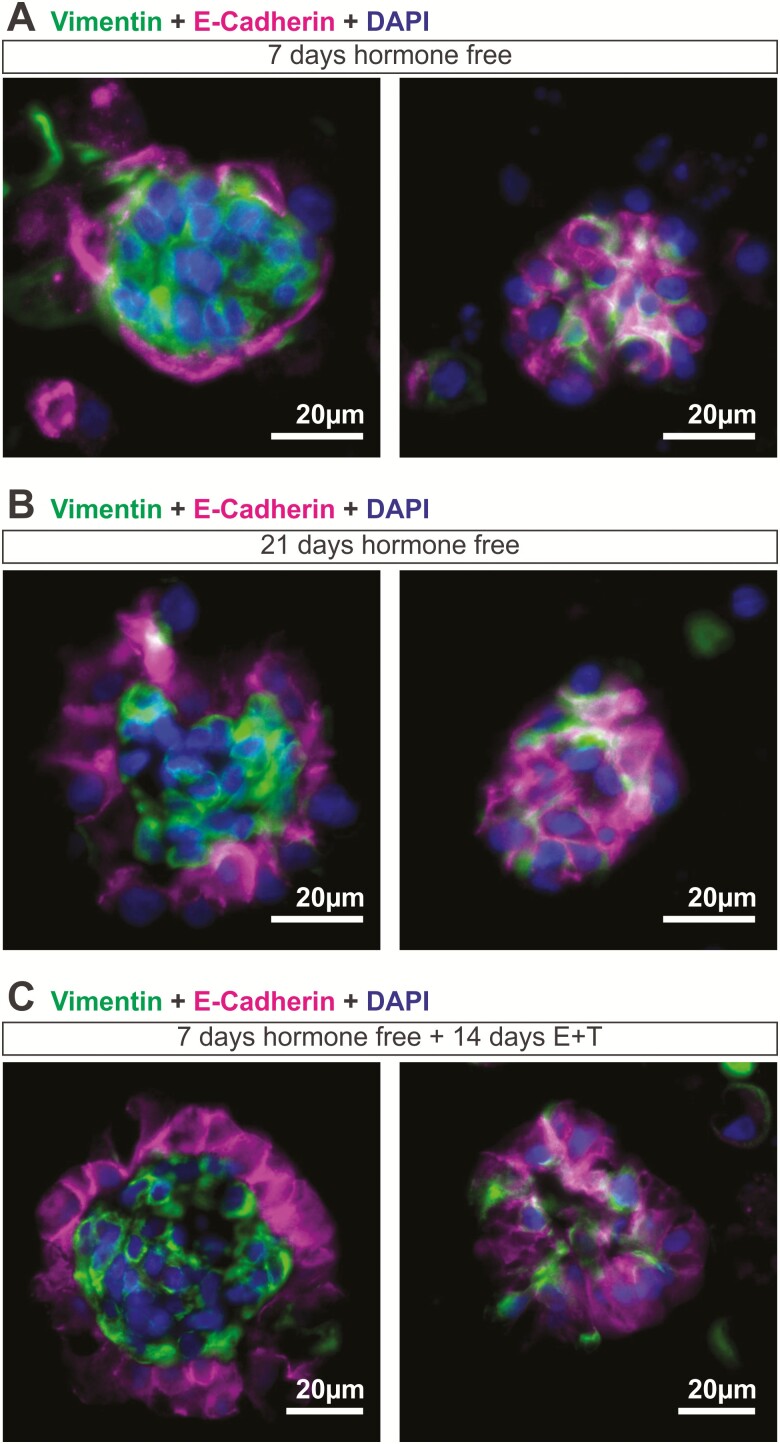
Interestingly, these organoids, cultured in the presence of stepwise E2 and testosterone for 14 days, exhibited a distinct structural organization with stromal cells residing in the center of the organoid and epithelial cells on the outer surface (Fig. 2E, F). Specifically, cells positive for the epithelial cell marker, E-cadherin and pan-Cytokeratin were found on the outer surface of the organoids. Epithelial cells were of glandular origin as indicated by positive staining for FOXA2 (44). Epithelial cells appeared polarized with nuclei localized at the basolateral end of the cell closest to the stroma. Cells in the center of the organoid were positive for the stromal cell marker, vimentin. Immunohistochemical staining for other cell types normally found in the endometrium was done, including those for NK cells (CD56), macrophages (CD68 and CD14), T cells (CD3ε), endothelial cells (CD31), and myometrial cells (α-smooth muscle actin). Staining for these markers were negligible, with CD14 staining present in <5% of organoids. Thus the primary cell types in the organoids were epithelial and stromal cells. When endometrial cells were cultured in the absence of sex hormones, they were able to form clusters as early as 7 days postseeding (Fig. 3). However, only ~17% of organoids (5/28 organoids obtained from 3 patients) displayed distinct organization, with polarized epithelial cells clearly lining the outer surface of the organoid. In most cases, at this stage, without hormones, stromal (vimentin) and epithelial (E-cadherin) cells were intermingled randomly within the organoids (Fig. 3A). By day 21, ~33% of organoids (8/24 organoids obtained from 4 patients) exhibited distinct organization in a hormone-free environment (Fig. 3B). However, when organoids were exposed to stepwise E2 and testosterone treatments mimicking the follicular phase of a menstrual cycle (Fig. 1B), the majority of the organoids (27/34 organoids obtained from 4 patients) exhibited distinct structural architecture with a clear demarcation of epithelial and stromal layers (Fig. 3C) demonstrating the important role hormones play in promoting organization of the organoids.
Figure 3.
Hormonal effects of organoid architecture. Endometrial organoids were cultured in a (A, B) hormone free environment or (C) with the normal stepwise hormone treatment and immunofluorescent staining for vimentin (stromal) and E-cadherin (epithelial) was done at (A, C) 7 days postseeding and (B) 21 days postseeding. (A) At 7 days hormone-free, most organoids appeared to have stromal and epithelial cells intermingled within the organoids (left panel), with only 5/28 organoids (obtained from 3 patients) exhibited epithelial cells lining the outer surface of the organoid (right panel). (B) 8/24 organoids (obtained from 4 patients) established distinct structural organization when cultured for 21 days in a hormone free environment (right panel) while the remainder stayed intermixed with epithelial and stromal cells (left panel) (C) 27/34 organoids (obtained from 4 patients; left panel) exhibited structural organization with a clear distinction of epithelial and stromal layers when treated with normal levels of estradiol and testosterone for 14 days.
In summary, endometrial organoids comprised of epithelial and stromal cells, organize and exhibited functional characteristics of the native endometrium in response to follicular phase E2 and testosterone including expression of sex steroid receptors, secretion of collagen, and mucins and cell proliferation.
Increased cell proliferation in endometrial organoids upon exposure to excess androgens mimicking PCOS
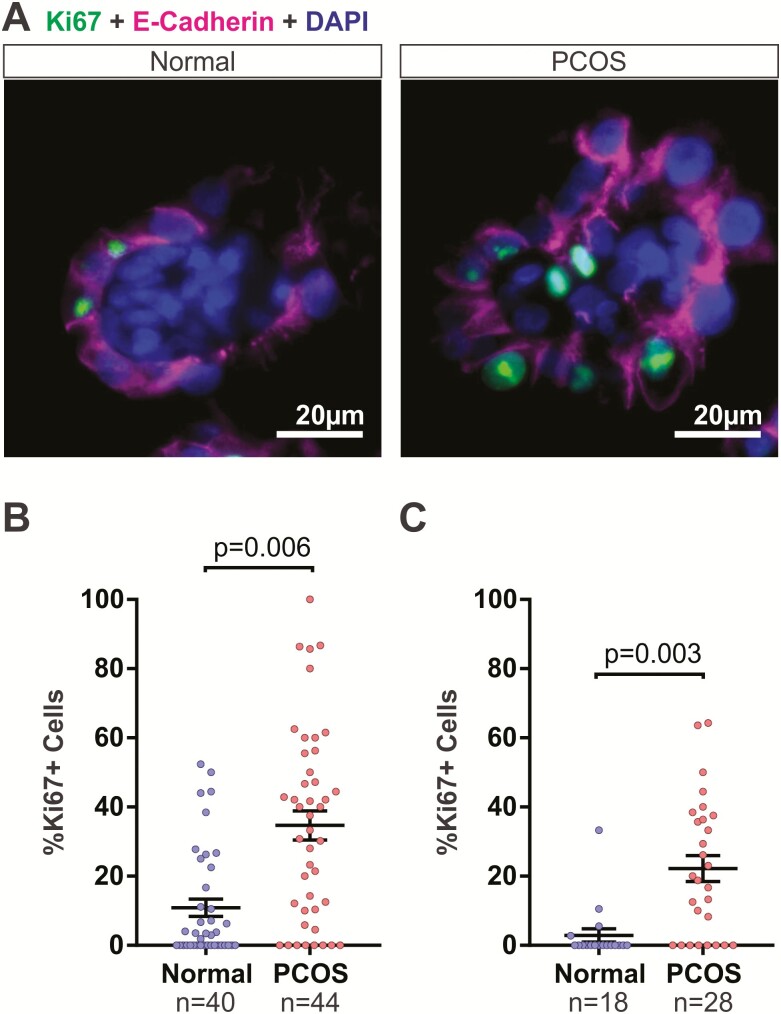
While many studies have explored the proliferative role of estrogens on the endometrium, the role of androgens—in particular, testosterone—remains poorly understood, and at times results are conflicting (21–23, 25). Considering that PCOS women exhibit hyperandrogenism and are at higher risk of developing endometrial cancer, we hypothesized that excess androgens in PCOS women contributed to the increased risk of developing endometrial cancer and therefore elicited a proliferative potential on the endometrial organoids in vitro. Endometrial organoids were treated throughout the 14 days with higher levels of androgens (testosterone or R1881) that were ~3-fold higher (3 nM) than normal levels (0.8 nM–1.25nM) during the proliferative phase to mimic testosterone serum levels observed in PCOS patients (4, 33, 40). Organoids subjected to 14 days of the PCOS hormone profile exhibited increased cell proliferation as indicated by nuclear Ki67 immunoreactivity in both stromal and epithelial cells (Fig. 4). Specifically, organoids cultured in the normal hormone profile contained 10.9% ± 2.52 (n = 40 organoids obtained from 4 patients) of Ki67 positive cells while those cultured in PCOS hormone profile contained 34.7% ± 4.20 (n = 44 organoids obtained from 4 patients) of Ki67 positive cells (Fig. 4B).
Figure 4.
Excess androgens in polycystic ovary syndrome (PCOS) hormone condition increased proliferation of cells in endometrial organoids. (A) Endometrial organoids were cultured in normal (left) and PCOS (right) conditions as described in Fig. 1. Cell proliferation was detected by immunofluorescent staining of Ki67 in epithelial cells (dual stained with E-cadherin in pink). (B) Ki67+ cells were quantified in endometrial organoids treated with normal or PCOS hormones. Organoids were obtained from 4 patients. (C) Organoids were treated with normal or PCOS hormones using R1881 as the androgen and the Ki67+ cells were quantified. Organoids were obtained from 3 patients. Data were analyzed using unpaired Student’s t-test based on n = number of organoids.
Endometrial organoids were also treated with a synthetic androgen R1881 in place of testosterone during the 14 days to determine whether conversion of testosterone to E2 influenced proliferation (Fig. 4C). Similarly, organoids cultured in PCOS conditions with R1881 exhibited increased cell proliferation compared to those from the normal conditions (normal: 2.9% ± 1.90 SEM, n = 18 organoids obtained from 3 patients; PCOS: 22.2% ± 3.75 SEM, n = 28 organoids obtained from 3 patients; Fig. 4C). In addition, there was no statistical difference between the effect observed with testosterone and R1881 (P = .72 for normal testosterone vs normal R1881; P = .06 for PCOS testosterone vs PCOS R1881; 1-way analysis of variance with Bonferroni’s correction). Aromatase expression in the organoids was measured by qPCR, and it was found that expression was very low to undetectable (data not shown) supporting the lack of difference in the proliferative effects of testosterone and R1881.
Genes differentially expressed in endometrial organoids exposed to excess androgens
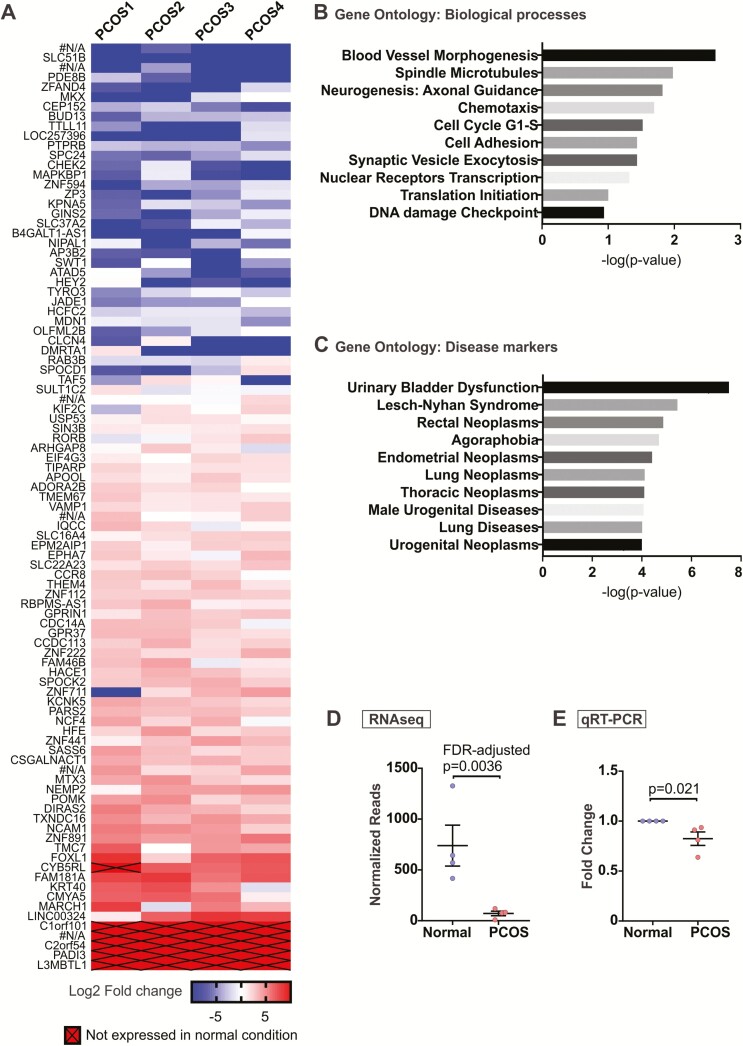
To investigate the differential gene expression caused by excess testosterone, gene expression profiling using RNA-seq was performed with organoids cultured in normal follicular phase and PCOS hormones using organoids from 4 patients. Approximately 95 genes were differentially regulated in the PCOS organoids compared to the normal organoids using the false discovery rate (FDR)-adjusted P values < .05 (Fig. 5A (44)). Of these, 6 were unannotated genes with no known functions (#N/A). Gene ontology analysis for biological processes revealed processes involving proliferation including spindle microtubules and cell cycle, migration such as chemotaxis and cell adhesion, and nuclear receptor transcription (Fig. 5B). Moreover, gene ontology analysis for disease biomarkers included various neoplasms including endometrial and urogenital neoplasms (Fig. 5C). We validated the gene BUD13 transcript levels and observed consistent lower expression in PCOS organoids compared to that of organoids cultured in normal conditions (Fig. 5E). Expression of other genes, including ZNF891, MARCH1, LINC00324, FOXL1, and PADI3 also agreed with the RNA-seq data (44). Although the difference did not reach statistical significance for 2 genes, MARCH1 and LINC00324, the trend for upregulation in PCOS treated organoids was apparent. The complete RNA-seq data set is available at GEO: GSE128328 (44).
Figure 5.
Differential gene expression in polycystic ovary syndrome (PCOS) organoids. Endometrial organoids from normal versus PCOS hormone conditions were harvested at the end of 14 days. Ribonucleic acid (RNA) was isolated and RNA-sequencign (RNA-seq) was performed. (A) A total of 95 possible genes (false discovery rate–adjusted P-value < .05) were differentially expressed in PCOS organoids compared to those cultured in normal hormone condition based on RNA-seq analysis. Of these, 6 were unannotated genes with no known functions (#N/A). Genes with no expression in PCOS organoids were arbitrarily assigned a value of –10 on this log2 scale. Four separate sets of organoids obtained from n = 4 patients were used for RNA-seq. (B, C) Gene ontology analysis was performed using GeneGo to determine key pathways and biological processes (B) and disease markers (C) involving genes differentially expressed in PCOS organoids. (D) Normalized reads for BUD13 from the RNA-seq were plotted to compare with (E) quantitative reverse transcription polymerase chain reaction for relative BUD13 transcript levels in normal versus PCOS organoids obtained from 4 new patients, which was done for validation purposes.
Discussion
The development of organoids from various organs has provided researchers an innovative tool to recapitulate physiological processes of whole tissues as well as provide preclinical models to study human disease and drug responses (41). Two studies reported the generation of endometrial organoids where endometrial epithelial cells organized into gland-like structures with a hollow center, similar to glands found in the endometrium. These studies were major breakthroughs for the field of endometrial biology. One unique feature of our endometrial organoid system is that no exogenous basement membrane matrix was used. The previous studies formed endometrial glandular organoids in Matrigel® as a scaffold which has its own caveats. Lot-to-lot variability during manufacturing (45) and the loosely defined compositions make it difficult to reproduce consistent results and determine exactly which signals are controlling cellular functions and proliferation in 3D. In addition, the components of Matrigel® are derived from murine cells which may not completely recapitulate the human endometrial niche which is important to consider when used as a disease model or drug testing platform (46). The other unique feature of our endometrial organoid system is the inclusion of both primary epithelial and stromal cells that collectively respond to sex steroids over a period of 14 days. As no exogenous scaffold is used, the organoids organized to distinct layers by responding to cues from each cell type promoting polarization of the epithelial cells with the basolateral side in contact with the stromal cells. As hypothesized, it appeared that the stromal cells provided the scaffold support for epithelial cells, similar to the native tissue. This distinct organization was favored in the presence of E2 and testosterone (Fig. 3). Similar to what is observed in vivo where both the luminal and glandular epithelium are in contact with the environment (lumen), the apical side of the epithelial cells of the organoids were exposed to the environment (culture medium). This is in contrast with the abnormal tissue architecture often observed in organoid tissue engineering such as the enclosed lumen of intestinal organoids where the intestinal villi are inverted (47). With this structural reorganization, our system allowed communication between stromal and epithelial cells as paracrine signals from stromal cells regulated proliferation and differentiation of the epithelium both with or without steroid hormones (46, 48). These organoids thus provide a mean to study hormone response in both cell types collectively.
Endometrial epithelial cells, thus far, have not been studied in as much detail as the stromal cells due to their behavior in cell culture. Endometrial epithelial cells do not grow or propagate well as monolayers, undergo senescence, and lose their polarity. This has remained one of the biggest impediments in the field, which is why mechanisms associated with, for example, endometrial carcinogenesis remains unclear. In addition, studying the impact of PCOS conditions on endometrial epithelial cell transformation has been difficult as the influence of this disease is most likely not acute but chronic over a prolonged period of time, which is not possible to mimic in conventional in vitro systems. Previously, we cultured endometrial cells in 3D in decellularized scaffolds with both primary stromal and epithelial cells although, epithelial cells did not survive the long-term culture conditions (49). Thus, to favor the growth of epithelial cells for the organoids, we selected a media that was optimized and used widely in the mammary gland field for the generation of mammary gland spheroids and tumorspheres as well as PANC-1 pancreatic duct cells that are epithelial in nature (39, 50). We reasoned that MammoCult™ medium provided epithelial cell promoting factors for the organoids, which may also have restricted stromal growth in our system.
When working with patient tissues, there is indeed heterogeneity within and between patients. Heterogeneity decreases somewhat when cells taken out of the native tissue niche and in a new culture environment, however, the epigenetic makeup of each cell will dictate how it responds to the culture system. This then brings in the issue of menstrual cycle phase given the differences in tissue architecture and physiology of the endometrium during these cycle phases. The patient samples that were collected for this study were obtained from both proliferative and secretory phase tissues. In terms of the quality and the number of organoids that formed, no significant difference was observed that related to phase of the cycle. We also reanalyzed the RNA-seq data by eliminating proliferative phase tissues and analyzing only secretory phase tissues and the differences were not different. However, a controlled experiment with more tissue samples from each phase of the cycle would be needed to address the subtleties of cycle effects. Another potential source of heterogeneity could be purity of the endometrial organoids with regards to different cell types. While the filtration step enriches cells by size, it was possible that other cell types were collected. Immunohistochemical stainings of organoids for cell type specific markers including NK cells (CD56), macrophages (CD68 or CD14), T cells (CD3ε), endothelial cells (CD31), and myometrial cells (α-smooth muscle actin) were done. We observed negligible staining for these markers, with CD14 staining showed <5% of organoids with a few positive cells (data not shown). We thus concluded that the organoids were primarily composed of epithelial and stromal cells.
While the endometrial organoid culture system is able to closely mimic the endometrial microphysiology, what is lacking is clonability often observed in stem-cell organoids (30, 31, 47, 51). We observed that the organoids were low in Sox9 positive stem cells and some did not contain any Sox9+ cells (44). Moreover, stem cells make up a very small portion of the tissue, and it is not guaranteed that each organoid that forms will contain stem cells. Our organoids are somatic organoids containing a mixture of epithelial and stromal cells with the ability to self-organize. Furthermore, in other stem cell–organoid systems, the cultures are often supplemented with stem cell growth factors such as Noggin and R-spondin-1 (blocker of transforming growth factor beta differentiation pathway) (30, 31, 47, 51). Future directions would focus on enriching for stem-like cells that can regenerate the organoids upon passaging, which can then be used for high throughput drug screens and biobank resources for more advanced genetic research, such as gene editing and mutation analysis.
One of the clinical features of PCOS is excess circulating androgens (2, 4) although the role of androgens on the endometrium during the normal menstrual cycle is unclear. Studies have reported both proliferative and anti-proliferative roles of androgens. In the female-to-male sex-reassigned individuals, long-term applications of testosterone promote uterine atrophy and thinning of the endometrium, indicating anti-proliferative and apoptotic effects (21, 22). However, in ovariectomized mice, evidence suggest that in the absence of ovarian hormones (estrogen and progesterone), application of a dihydrotestosterone promotes cell proliferation in the endometrium (25). Furthermore, in some cases of endometrial and ovarian cancers, application of testosterone was able to counteract the proliferative effect of estrogen (24). In our endometrial organoid system, we found that excess androgens mimicking PCOS in the background of physiological levels of E2, promoted cell proliferation. The possibility of increased proliferation due to increased estrogen production from aromatase activity was explored by measuring aromatase mRNA levels in the endometrial organoids. We found that aromatase expression was negligible (data not shown), which is supported by published work. It has been reported that human endometrial stromal cells have none to very little aromatase expression but that this expression can be induced by decidualization, progesterone, environmental toxins, and disease such as endometriosis (52–55). In support of this, Tseng et al (55), demonstrated that estrogen did not induce aromatase activity in endometrial stromal cells. We observed no significant difference between testosterone versus nonaromatizable synthetic androgen R1881 in promoting proliferation of organoids. Thus, the proliferative response in the epithelial cells of our organoids was not due to aromatase activity. In PCOS patients, there is no obvious difference in estrogen levels compared to healthy females (24).
The increase in Ki67 positive cells in the organoids cultured in the PCOS hormones is supportive of the early events that can lead to endometrial neoplasias and cancer. While the endometrium cycles through its proliferative phase with the normal rise in estrogen, the excess androgens in PCOS patients may contribute to the thickening of the endometrium through increased in cell proliferation. We propose that increased proliferation allows for the accumulation and propagation of mutations from replication errors after each cell division. A recent study showed that in fact, normal endometrial epithelial glands can develop mutations in cancer-associated genes such as KRAS, PIK3CA, FBXW7, PPP2R1A, and PIK3R1 during the menstrual cycle (56). In the normal context such mutated cells would naturally be repaired or eliminated at the end of each menstrual cycle. In the case of PCOS, where patients often have irregular or absent menstrual cycles, we speculate that the chronic increased levels of androgens would promote endometrial proliferation and the accumulation of mutations, decrease differentiation, and eventually progress towards transformation.
In our PCOS model, we identified 95 genes that were differentially expressed in endometrial organoids cultured in excess testosterone. Accordingly, gene ontology analysis revealed processes that were associated with proliferation, migration, and nuclear receptor transcription. Disease markers included neoplasms of the endometrium and the urogenital tract. The significantly downregulated gene in PCOS, BUD13, was validated in new patient samples. Interestingly, a genome-wide association study detected an association of BUD13 variants with a metabolic syndrome that includes defects in lipid and glucose metabolism, obesity, and increased risk of type 2 diabetes and cardiovascular diseases (57, 58). In fact, a study showed that BUD13 rs10790162 variant is associated with an increased risk of hyperlipidaemia (59). MARCH1 was significantly upregulated in PCOS organoids in the RNA-seq, which was confirmed with qPCR (44). MARCH1 is a ubiquitin ligase that was shown to impair insulin signaling with increased expression in adipose tissue in obese humans (60). Given that PCOS is associated with metabolic dysfunction, obesity, increased risk of type 2 diabetes, and cardiovascular diseases (6, 8, 12), it would be worth exploring the role of BUD13 or MARCH1 proteins in PCOS.
Here, we report the establishment of scaffold-free multicellular endometrial organoids that are responsive to sex hormones to gain insights into the effects of excess androgen that occurs in PCOS women. We have shown that excess androgens directly impact endometrial cells. Similarly, these endometrial organoids can be used to study other endometrial processes and diseases, where the hormonal crosstalk between epithelial and stromal cells occurs.
Acknowledgments
Sample processing for paraffin embedding and periodic acid-Schiff staining were performed by Pathology Core Facility, Northwestern University, Chicago, IL, US. RNA quality control and RNA-seq were performed by NUSeq Core Facility, Northwestern University, Chicago, IL. We would like to acknowledge the whole team involved in our cooperative UG3 program, including the Woodruff, Burdette, and Urbanek labs for the insightful discussions and collaborations.
Financial Support : This study was supported by the NIEHS/NIH/NCATS UG3 grant (ES029073).
Additional Information
Disclosure Summary : The corresponding author states that there are no financial competing interests of all authors on this study.
Data Availability : Data presented in this study are included in this published article or in the GEO data repository.
References
- 1. Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96351–96358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 3. Piltonen TT. Polycystic ovary syndrome: endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2016;37:66–79. [DOI] [PubMed] [Google Scholar]
- 4. Deng Y, Zhang Y, Li S, Zhou W, Ye L, Wang L, Tao T, Gu J, Yang Z, Zhao D, Gu W, Hong J, Ning G, Liu W, Wang W. Steroid hormone profiling in obese and nonobese women with polycystic ovary syndrome. Sci Rep. 2017;7(1):14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stracquadanio M, Ciotta L, Palumbo MA. Relationship between serum anti-Mullerian hormone and intrafollicular AMH levels in PCOS women. Gynecol Endocrinol. 2018;34(3):223–228. [DOI] [PubMed] [Google Scholar]
- 6. Teede HJ, Hutchison S, Zoungas S, Meyer C. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine. 2006;30(1):45–53. [DOI] [PubMed] [Google Scholar]
- 7. Charalampakis V, Tahrani AA, Helmy A, Gupta JK, Singhal R. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur J Obstet Gynecol Reprod Biol. 2016;207:220–226. [DOI] [PubMed] [Google Scholar]
- 8. de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update. 2011;17(4):495–500. [DOI] [PubMed] [Google Scholar]
- 9. Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78(8):782–785. [DOI] [PubMed] [Google Scholar]
- 10. Kahal H, Kyrou I, Uthman O, Brown A, Johnson S, Wall P, Metcalfe A, Tahrani AA, Randeva HS. The association between obstructive sleep apnea and metabolic abnormalities in women with polycystic ovary syndrome: a systematic review and meta-analysis. Sleep. 2018;41(7). doi:10.1093/sleep/zsy085. [DOI] [PubMed] [Google Scholar]
- 11. Shafiee MN, Khan G, Ariffin R, Abu J, Chapman C, Deen S, Nunns D, Barrett DA, Seedhouse C, Atiomo W. Preventing endometrial cancer risk in polycystic ovarian syndrome (PCOS) women: could metformin help? Gynecol Oncol. 2014;132(1):248–253. [DOI] [PubMed] [Google Scholar]
- 12. Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27(10):3067–3073. [DOI] [PubMed] [Google Scholar]
- 13. Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19(3):398–405. [DOI] [PubMed] [Google Scholar]
- 14. Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–919. [DOI] [PubMed] [Google Scholar]
- 15. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136(1):99–103. [DOI] [PubMed] [Google Scholar]
- 17. Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM; Australian Ovarian Cancer Study Group and Australian National Endometrial Cancer Study Group . Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control. 2010;21(12):2303–2308. [DOI] [PubMed] [Google Scholar]
- 18. Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod. 2012;27(5):1327–1331. [DOI] [PubMed] [Google Scholar]
- 19. Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361(9371):1810–1812. [DOI] [PubMed] [Google Scholar]
- 20. Li X, Feng Y, Lin JF, Billig H, Shao R. Endometrial progesterone resistance and PCOS. J Biomed Sci. 2014;21:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller N, Bédard YC, Cooter NB, Shaul DL. Histological changes in the genital tract in transsexual women following androgen therapy. Histopathology. 1986;10(7):661–669. [DOI] [PubMed] [Google Scholar]
- 22. Perrone AM, Cerpolini S, Maria Salfi NC, Ceccarelli C, De Giorgi LB, Formelli G, Casadio P, Ghi T, Pelusi G, Pelusi C, Meriggiola MC. Effect of long-term testosterone administration on the endometrium of female-to-male (FtM) transsexuals. J Sex Med. 2009;6(11):3193–3200. [DOI] [PubMed] [Google Scholar]
- 23. Simitsidellis I, Saunders PTK, Gibson DA. Androgens and endometrium: new insights and new targets. Mol Cell Endocrinol. 2018;465:48–60. [DOI] [PubMed] [Google Scholar]
- 24. Gibson DA, Simitsidellis I, Collins F, Saunders PT. Evidence of androgen action in endometrial and ovarian cancers. Endocr Relat Cancer. 2014;21(4):T203–T218. [DOI] [PubMed] [Google Scholar]
- 25. Simitsidellis I, Gibson DA, Cousins FL, Esnal-Zufiaurre A, Saunders PT. A role for androgens in epithelial proliferation and formation of glands in the mouse uterus. Endocrinology. 2016;157(5):2116–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Csermely T, Demers LM, Hughes EC. Organ culture of human endometrium. Effects of progesterone. Obstet Gynecol. 1969;34(2):252–259. [PubMed] [Google Scholar]
- 27. Csermely T, Hughes EC, Demers LM. Effect of oral contraceptives on human endometrium in culture. Am J Obstet Gynecol. 1971;109(7):1066–1072. [DOI] [PubMed] [Google Scholar]
- 28. Demers LM, Csermely T, Hughes EC. Culture of human endometrium. II. Effects of estradiol. Obstet Gynecol. 1970;36(2):269–274. [PubMed] [Google Scholar]
- 29. Hughes EC, Demers LM, Csermely T, Jones DB. Organ culture of human endometrium. Effect of ovarian steroids. Am J Obstet Gynecol. 1969;105(5):707–720. [DOI] [PubMed] [Google Scholar]
- 30. Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A, Meuleman C, Ferrante M, Vankelecom H. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144(10):1775–1786. [DOI] [PubMed] [Google Scholar]
- 31. Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO, Simons BD, Hemberger M, Koo BK, Moffett A, Burton GJ. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arslan SY, Yu Y, Burdette JE, Pavone ME, Hope TJ, Woodruff TK, Kim JJ. Novel three dimensional human endocervix cultures respond to 28-day hormone treatment. Endocrinology. 2015;156(4):1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bui HN, Sluss PM, Blincko S, Knol DL, Blankenstein MA, Heijboer AC. Dynamics of serum testosterone during the menstrual cycle evaluated by daily measurements with an ID-LC-MS/MS method and a 2nd generation automated immunoassay. Steroids. 2013;78(1):96–101. [DOI] [PubMed] [Google Scholar]
- 34. Marcel M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17(1):10–12. Available at: http://journal.embnet.org/index.php/embnetjournal/article/view/200/458. Accessed 13 December 2019. [Google Scholar]
- 35. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smart CE, Morrison BJ, Saunus JM, Vargas AC, Keith P, Reid L, Wockner L, Askarian-Amiri M, Amiri MA, Sarkar D, Simpson PT, Clarke C, Schmidt CW, Reynolds BA, Lakhani SR, Lopez JA. In vitro analysis of breast cancer cell line tumourspheres and primary human breast epithelia mammospheres demonstrates inter- and intrasphere heterogeneity. PloS One. 2013;8(6):e64388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raouf A, Sun YJ. In vitro methods to culture primary human breast epithelial cells. Methods Mol Biol. 2013;946:363–381. [DOI] [PubMed] [Google Scholar]
- 40. Braunstein GD, Reitz RE, Buch A, Schnell D, Caulfield MP. Testosterone reference ranges in normally cycling healthy premenopausal women. J Sex Med. 2011;8(10):2924–2934. [DOI] [PubMed] [Google Scholar]
- 41. An update on organoid research. Nat Cell Biol. 2018;20(6):633. [DOI] [PubMed] [Google Scholar]
- 42. Birkenfeld A, Navot D, Levij IS, Laufer N, Beier-Hellwig K, Goecke C, Schenker JG, Beier HM. Advanced secretory changes in the proliferative human endometrial epithelium following clomiphene citrate treatment. Fertil Steril. 1986;45(4):462–468. [PubMed] [Google Scholar]
- 43. Tanaka T, Wang C, Umesaki N. Remodeling of the human endometrial epithelium is regulated by laminin and type IV collagen. Int J Mol Med. 2009;23(2):173–180. [PubMed] [Google Scholar]
- 44. Wiwatpanit T, Murphy AR, Lu Z, Urbanek M, Burdette JE, Woodruff TK, Kim JJ. Data from Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome figshare 2019. Deposited 25 June 2019. doi: 10.6084/m9.figshare.8855573 10.6084/m9.figshare.8853764. [DOI] [PMC free article] [PubMed]
- 45. Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017;22(5):456–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arnold JT, Kaufman DG, Seppälä M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16(5):836–845. [DOI] [PubMed] [Google Scholar]
- 47. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. [DOI] [PubMed] [Google Scholar]
- 48. Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139(11):4708–4713. [DOI] [PubMed] [Google Scholar]
- 49. Olalekan SA, Burdette JE, Getsios S, Woodruff TK, Kim JJ. Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol Reprod. 2017;96(5):971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herrera VL, Colby AH, Tan GA, Moran AM, O’Brien MJ, Colson YL, Ruiz-Opazo N, Grinstaff MW. Evaluation of expansile nanoparticle tumor localization and efficacy in a cancer stem cell-derived model of pancreatic peritoneal carcinomatosis. Nanomedicine (Lond). 2016;11(9):1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li M, Izpisua Belmonte JC. Organoids - preclinical models of human disease. N Engl J Med. 2019;380(6):569–579. [DOI] [PubMed] [Google Scholar]
- 52. Gibson DA, Simitsidellis I, Collins F, Saunders PTK. Endometrial intracrinology: oestrogens, androgens and endometrial disorders. Int J Mol Sci. 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holloway AC, Stys KA, Foster WG. DDE-induced changes in aromatase activity in endometrial stromal cells in culture. Endocrine. 2005;27(1):45–50. [DOI] [PubMed] [Google Scholar]
- 54. Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81(1):174–179. [DOI] [PubMed] [Google Scholar]
- 55. Tseng L, Mazella J, Sun B. Modulation of aromatase activity in human endometrial stromal cells by steroids, tamoxifen and RU 486. Endocrinology. 1986;118(4):1312–1318. [DOI] [PubMed] [Google Scholar]
- 56. Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, Yamawaki K, Adachi S, Takahashi T, Kase H, Tanaka K, Yamamoto T, Motoyama T, Inoue I, Enomoto T. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep. 2018;24(7):1777–1789. [DOI] [PubMed] [Google Scholar]
- 57. Kraja AT, Vaidya D, Pankow JS, Goodarzi MO, Assimes TL, Kullo IJ, Sovio U, Mathias RA, Sun YV, Franceschini N, Absher D, Li G, Zhang Q, Feitosa MF, Glazer NL, Haritunians T, Hartikainen AL, Knowles JW, North KE, Iribarren C, Kral B, Yanek L, O’Reilly PF, McCarthy MI, Jaquish C, Couper DJ, Chakravarti A, Psaty BM, Becker LC, Province MA, Boerwinkle E, Quertermous T, Palotie L, Jarvelin MR, Becker DM, Kardia SL, Rotter JI, Chen YD, Borecki IB. A bivariate genome-wide approach to metabolic syndrome: STAMPEED consortium. Diabetes. 2011;60(4):1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lin E, Kuo PH, Liu YL, Yang AC, Kao CF, Tsai SJ. Association and interaction of APOA5, BUD13, CETP, LIPA and health-related behavior with metabolic syndrome in a Taiwanese population. Sci Rep. 2016;6:36830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aung LH, Yin RX, Wu DF, Wang W, Liu CW, Pan SL. Association of the variants in the BUD13-ZNF259 genes and the risk of hyperlipidaemia. J Cell Mol Med. 2014;18(7):1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nagarajan A, Petersen MC, Nasiri AR, Butrico G, Fung A, Ruan HB, Kursawe R, Caprio S, Thibodeau J, Bourgeois-Daigneault MC, Sun L, Gao G, Bhanot S, Jurczak MJ, Green MR, Shulman GI, Wajapeyee N. MARCH1 regulates insulin sensitivity by controlling cell surface insulin receptor levels. Nat Commun. 2016;7:12639. [DOI] [PMC free article] [PubMed] [Google Scholar]