Abstract
Context
In the Denosumab and High-Dose Teriparatide Administration (DATA-HD) study, we reported that 15 months of combined high-dose (HD) teriparatide and denosumab increased mean areal bone mineral density (aBMD) at the hip and spine more than combined denosumab and standard-dose (SD) teriparatide.
Objective
In the current analysis, we compare the individual rates of aBMD response between the treatment groups.
Design
Single-site, open-label, randomized controlled trial in which postmenopausal women received either teriparatide 20-μg daily (SD) or 40-μg daily (HD) given months 0 through 9, overlapped with denosumab 60 mg, given months 3 through 15 (15 months’ total duration). The proportion of participants in the SD and HD groups experiencing total hip, femoral neck, and lumbar spine aBMD gains of >3%, >6%, and >9% were compared.
Participants
Postmenopausal women with osteoporosis completing all study visits (n = 60).
Main outcome measure(s)
aBMD (dual x-ray absorptiometry).
Results
At the end of the 15-month treatment period, a higher proportion of women in the HD group had aBMD increases >3% (83% vs. 58%, P = .037) and >6% (45% vs. 19%, P = .034) at the total hip, and >3% at the femoral neck (86% vs. 63%, P = .044). At the lumbar spine, >3% response rates were similar, whereas the >6% and >9% response rates were greater in the HD group (100% vs. 79%, P = .012 and 93% vs. 59%, P = .003, respectively).
Conclusion
Compared with the SD regimen, more women treated with the HD regimen achieved clinically meaningful and rapid gains in hip and spine aBMD. These results suggest that this approach may provide unique benefits in the treatment of postmenopausal osteoporosis.
Areal bone mineral density (aBMD) derived from dual x-ray absorptiometry (DXA) is commonly used to monitor an individual’s response to osteoporosis therapy (1). In patients with osteoporosis or increased fracture risk, improvements in aBMD during treatment predict the observed reduction in fracture risk, though to varying degrees (2–8). Additionally, in a recent meta-regression of 38 placebo-controlled trials of 19 osteoporosis agents, larger increases in aBMD were strongly associated with greater reductions in vertebral and hip fractures, further supporting the use of treatment-related increases in aBMD as a surrogate endpoint for fracture (9).
Conventional osteoporosis therapies produce modest gains in aBMD (10–15), so there is an ongoing need to develop therapeutic regimens that will more rapidly restore skeletal integrity. We have previously reported that the combination of teriparatide, an osteoanabolic agent, and denosumab, a potent antiresorptive agent, produced large increases in aBMD by fully inhibiting teriparatide-induced bone resorption but not modeling-based bone formation (16,17). In the subsequent Denosumab and High-Dose Teriparatide Administration (DATA-HD) study, we reported that 15 months of combined high-dose (HD) teriparatide (40 μg) and denosumab produced even greater increases in hip and spine aBMD than the standard-dose (SD, 20 μg) combination (18). In the current analysis, we compare the relative response rates, as measured by aBMD gains at prespecified thresholds, among postmenopausal osteoporotic women treated with either combined HD teriparatide and denosumab or SD teriparatide and denosumab.
Methods
Study design and participants
Details of the DATA-HD study design and participants have been described previously (18). In brief, the DATA-HD study is a 15-month open-label, single-site, randomized controlled trial conducted from October 2014 to June 2016. Seventy-six postmenopausal women aged 45 years or older were enrolled in the study. Women were eligible for inclusion if they were at least 36 months from last menses (or since hysterectomy if FSH concentration was >40 U/L) and at high fracture risk. High fracture risk was defined as an aBMD T-score of -2.5 or less at the lumbar spine, total hip, or femoral neck; T-score of -2.0 or less with at least 1 aBMD-independent risk factor (fragility fracture after age 50, parental history of hip fracture after age 50, prior hyperthyroidism, inability to rise from a chair with arms elevated, or current smoking) (19); or T-score of -1.0 or less with a history of a fragility fracture. Women were excluded if they had evidence of hyperparathyroidism; 25-hydroxy vitamin D deficiency (<20 ng/mL); other congenital or acquired bone disease; history of malignancy (with the exception of nonmelanoma skin cancer); history of ionizing radiation therapy; significant cardiopulmonary, liver, or renal disease; major psychiatric disease; or excessive alcohol intake. Women were also excluded if they had any prior use of parenteral bisphosphonates or strontium; use within 6 months of oral bisphosphonates, denosumab, or glucocorticoids; or use within 3 months of estrogens, selective estrogen receptor modulators, or calcitonin. The study was approved by the Partners Human Research Committee and is registered with ClinicalTrials.gov, number NCT02176382. All participants provided written informed consent before inclusion in the study.
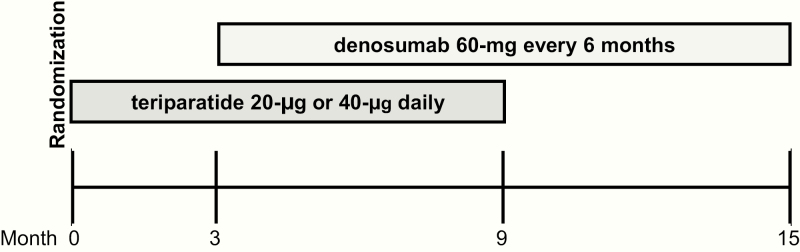
Study participants were stratified by age (<65 or ≥65 years) and previous bisphosphonate use (yes or no) and then randomized in a 1:1 ratio to receive either teriparatide 20 μg daily (SD) or 40 μg daily (HD) by subcutaneous injection, given months 0 through 9, overlapped with denosumab 60 mg every 6 months by subcutaneous injection, given months 3 through 15, for a total study duration of 15 months (Fig. 1). Additionally, women were given calcium carbonate and vitamin D supplements, if needed, to achieve a total daily intake of 1200 mg elemental calcium and to maintain serum 25-hydroxy vitamin D concentrations of at least 20 ng/mL. Adherence to teriparatide was assessed by subject diary.
Figure 1.
Flow diagram of treatment schedule over the15-month study period.
Bone mineral density measurements
The aBMD of the posteroanterior lumbar spine, total hip, and femoral neck was assessed by DXA using a Hologic QDR 4500A densitometer (Waltham, MA, USA) at baseline, 3-, 9-, and 15-month time points. All scans of an individual subject were performed on the same densitometer. Quality control measurements were performed daily with a Hologic anthropomorphic spine phantom. The SDs of in vivo reproducibility were 0.005, 0.006, and 0.009 g/cm2 for posteroanterior lumbar spine, total hip, and femoral neck aBMD measurements, respectively. Vertebrae were excluded from analysis if there were obvious deformities or focal sclerosis detected. Physicians interpreting aBMD assessments were blinded to treatment allocation.
Statistical analysis
Participants who completed all study visits during the 15-month study period were included in the responder analysis. If an aBMD result was not available for a particular anatomic site (total hip, femoral neck, or lumbar spine), participants were excluded from that analysis and no imputation of missing data was performed. Bone mineral density increases of >3%, >6%, and >9% at each anatomic site as well as the combination of all 3 sites were used as thresholds of response. The 3% threshold was predetermined based on the widely accepted least significant change in DXA aBMD measurements, which assumes a DXA precision error of 1% (20). More robust aBMD increase of >6% and >9% were also analyzed. Between-group differences in the proportion of responders for each threshold were analyzed using 2-sided chi-square tests. The Fisher’s exact test was used if the number of responders was fewer than 5 in either treatment group. P values were not adjusted for multiplicity. Baseline characteristics were compared between treatment groups using independent 2-sample t tests for continuous variables and 2-sided chi-square tests for discrete variables.
Role of the funding source
The funding sources and drug supply sources did not have any role in the design, conduct, or analysis of this study.
Results
The baseline demographics and characteristics of the 60 participants who completed all study visits (79% of the enrolled cohort) are shown in Table 1. Baseline characteristics did not differ between treatment groups and were comparable to those in the overall study population (data not shown) (18). Briefly, the mean age was 66.2 years, 95% were non-Hispanic white, 52% had a prior fragility fracture, and 55% had previously been treated with a bisphosphonate.
Table 1.
Demographics and Baseline Characteristics of DATA-HD Study Participants Included in the Responder Analysisa
| Characteristic | SD TPTD (20 μg) Plus Denosumab (n = 31) | HD TPTD (40 μg) Plus Denosumab (n = 29) | P Value |
|---|---|---|---|
| Age (y) | 65.8 (7.4) | 66.6 (7.2) | .68 |
| Body mass index (kg/m2) | 22.8 (2.7) | 22.5 (3.7) | .73 |
| White, non-Hispanic | 29 (94%) | 28 (97%) | .59 |
| History of adult fragility fracture | 13 (42%) | 18 (62%) | .12 |
| Previous bisphosphonate use | 18 (58%) | 15 (52%) | .62 |
| Duration of use (mo) | 44.2 (32.1) | 71.9 (51.9) | .07 |
| Time since discontinuation (mo) | 73.0 (64.2) | 76.8 (60.0) | .86 |
| Serum P1NP (μg/L) | 48.0 (16.8) | 51.5 (16.0) | .42 |
| Serum CTX (ng/L) | 426.2 (166.1) | 464.7 (164.7) | .37 |
| Baseline areal bone mineral density by DXA (g/cm 2) | |||
| Total hip | 0.748 (0.105) | 0.740 (0.073) | .71 |
| Femoral neck | 0.652 (0.097) | 0.629 (0.071) | .30 |
| Posteroanterior lumbar spine | 0.836 (0.109) | 0.800 (0.111) | .37 |
Values are presented as mean (SD) or number (%).
Abbreviations: CTX, serum C-telopeptide of type I collagen; DXA, dual x-ray absorptiometry; HD, high dose; P1NP, serum procollagen type I N-terminal propeptide; SD, standard dose; TPTD, teriparatide.
aIncludes 60 participants who had areal bone mineral density determinations at all study visits (baseline, month 9, and month 15, 79% of the Denosumab and High-Dose Teriparatide Administration population at enrollment).
The mean percent changes in aBMD at 9 and 15 months are shown in Table 2. Bone mineral density measurements were not available for 1 participant in the SD group for femoral neck at 9 and 15 months and 2 participants in the SD group and 2 participants in HD group for lumbar spine at 9 and 15 months. Note that by study end (15 months), there was a greater increase in aBMD at each site in the HD group than the SD group: 6.1% vs 3.9% (mean difference, 2.2%; 95% CI, 0.6-3.8; P = .009) at the total hip; 6.8% vs 4.3% (mean difference, 2.5%; 95% CI, 0.5-4.5; P = .017) at the femoral neck; and 17.5% vs 9.5% (mean difference, 8.1%; 95% CI, 5.5-10.6; P < .0001) at the lumbar spine.
Table 2.
Percent Change in Bone Mineral Density From Baseline to 9 and 15 Months in DATA-HD Study Participants Included in the Responder Analysisa
| SD TPTD (20 μg) Plus Denosumab (n = 31) | HD TPTD (40 μg) Plus Denosumab (n = 29) | P Value | |
|---|---|---|---|
| Change in areal bone mineral density by DXA from baseline to 9 months (%) | |||
| Total hip | 3.8 (2.9) | 5.2 (3.3) | .076 |
| Femoral neck | 4.0 (3.5) | 5.4 (3.4) | .138 |
| Posteroanterior lumbar spine | 8.6 (3.8) | 14.1 (5.4) | <.0001 |
| Change in areal bone mineral density by DXA from baseline to 15 months (%) | |||
| Total hip | 3.9 (2.9) | 6.1 (3.4) | .009 |
| Femoral neck | 4.3 (3.7) | 6.8 (4.1) | .017 |
| Posteroanterior lumbar spine | 9.5 (3.2) | 17.5 (6.0) | <.0001 |
Values are presented as mean (SD).
Abbreviations: DXA, dual x-ray absorptiometry; HD, high dose; SD, standard dose; TPTD, teriparatide.
aIncludes 60 participants who had areal bone mineral density (aBMD) determinations at all study visits (baseline, month 9, and month 15, 79% of the Denosumab and High-Dose Teriparatide Administration population at enrollment). Data missing for 1 participant in the SD group for femoral neck aBMD at 9 and 15 months and 2 participants in the SD group and 2 participants in the HD group for lumbar spine aBMD at 9 and 15 months.
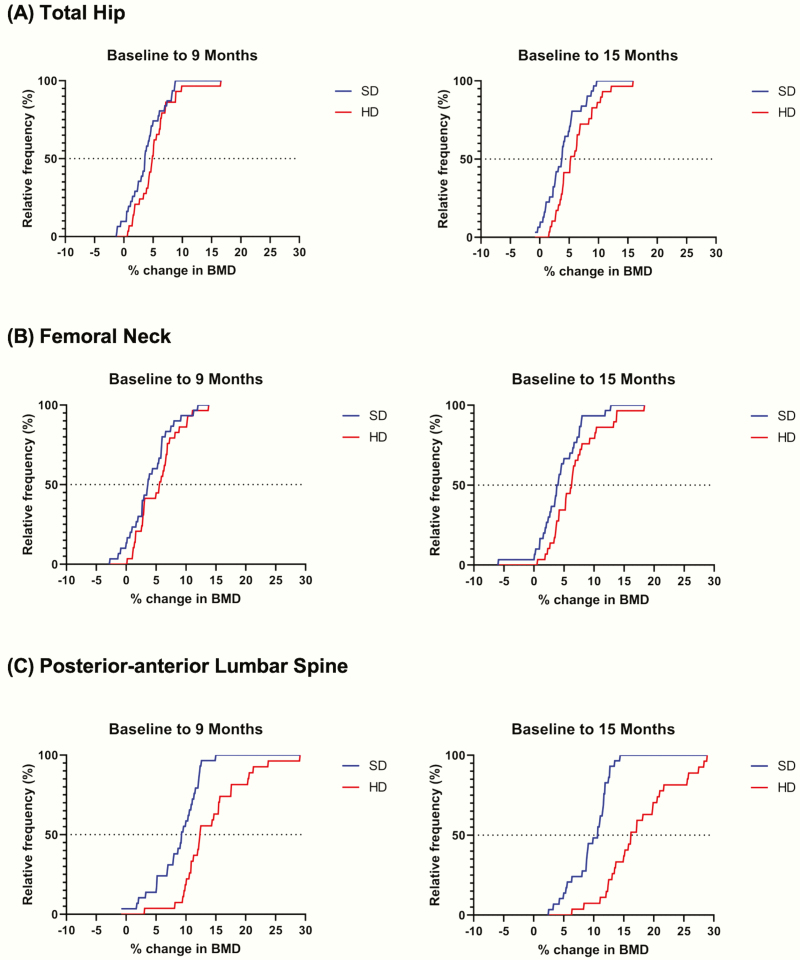
The cumulative relative frequency distributions of percent change in aBMD from baseline to 9 and 15 months in the SD and HD groups are shown in Fig. 2. Across all 3 sites, at both 9 and 15 months, a greater proportion of participants in the HD group achieved a higher percent change in aBMD compared with participants in the SD group. The maximum percent change in aBMD achieved at all sites, at both 9 and 15 months, was also greater in the HD group compared with the SD group. At 15 months, bone loss occurred in 1 participant at both the total hip and femoral neck and in 1 participant at the femoral neck, with no bone loss observed at the lumbar spine. Both of these participants were in the SD group. No participant randomized to the HD group experienced bone loss at the hip or spine.
Figure 2.
Cumulative relative frequency distribution plots of percent change in bone mineral density from baseline to 9 and 15 months. aBMD, bone mineral density; HD, high-dose teriparatide (40 μg) plus denosumab; SD, standard-dose teriparatide (20 μg) plus denosumab.
aIncludes 60 participants who had areal bone mineral density (aBMD) determinations at all study visits (baseline, month 9, and month 15, 79% of the DATA-HD population at enrollment). Data missing for 1 participant in the SD group for femoral neck aBMD at 9 and 15 months and 2 participants in the SD group and 2 participants in the HD group for lumbar spine aBMD at 9 and 15 months.
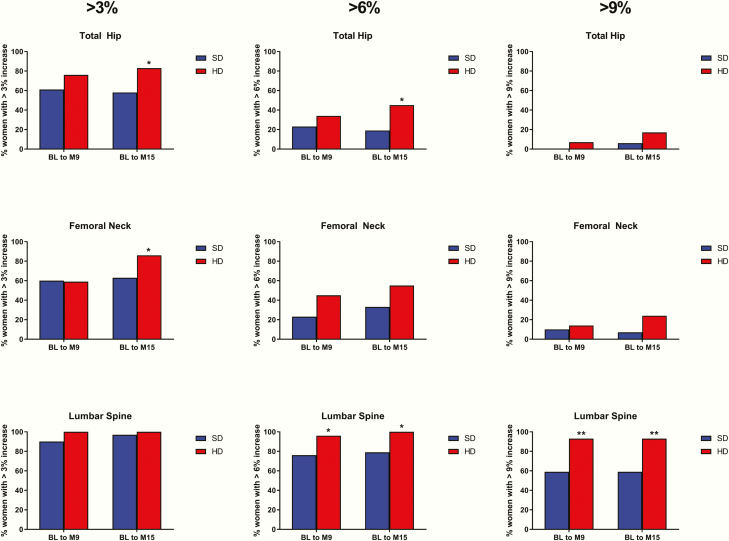
As shown in Fig. 3, at 15 months, there was a higher proportion of participants in the HD group with total hip aBMD increases of >3% (83% vs 58%, P = .037) and >6% (45% vs 19%, P = .034), with no significant differences in the >9% (17% vs 6%, P = .193) response rates between the 2 groups. At the femoral neck, there was a higher proportion of participants in the HD group with increases >3% (86% vs 63%, P = .044), as well as similar nonsignificant trends in the >6% (55% vs 33%, P = .091) and >9% (24% vs 7%, P = .062) response rates. At the lumbar spine, all but 1 patient in the SD group achieved an aBMD gain of at least 3%, with no significant differences between the groups (HD = 100% vs SD = 97%, P = .330). The >6% and >9% response rates were higher in the HD group than the SD group (100% vs 79%, P = .012, and 93% vs 59%, P = .003, respectively). At 9 months, response rates at all anatomic sites were similar between the 2 groups, except at the lumbar spine, where the >6% and >9% response rates were higher in the HD group (96% vs 76%, P = .029, and 93% vs 59%, P = .003, respectively). Based on an exploratory subgroup analysis, there were no significant differences in bone density response rates at each anatomic site between women with or without prior oral bisphosphonate exposure (data not shown).
Figure 3.
Proportion of participants achieving bone mineral density increases of >3%, >6%, and >9% at the total hip, femoral neck, and lumbar spine from baseline to 9 and 15 months.a BL, baseline; HD, high-dose teriparatide (40-μg) plus denosumab; M, month; SD, standard-dose teriparatide (20 μg) plus denosumab.
*P < .05.
**P < .01.
aIncludes 60 participants who had areal bone mineral density (aBMD) determinations at all study visits (baseline, month 9, and month 15, 79% of the Denosumab and High-Dose Teriparatide Administration population at enrollment). Data missing for 1 participant in the SD group for femoral neck aBMD at 9 and 15 months; and 2 participants in the SD group and 2 participants in the HD group for lumbar spine aBMD at 9 and 15 months.
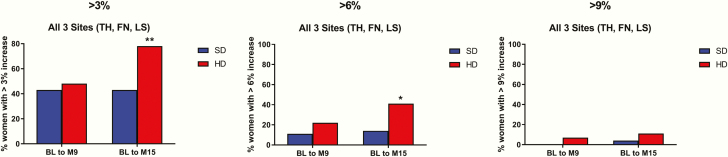
Fifty-five participants had aBMD assessments at all sites (total hip, femoral neck, and lumbar spine) and at all time points. As shown in Fig. 4, at 15 months, there was a greater proportion of participants in the HD group achieving >3% and >6% gains at all three anatomic sites (78% vs 43%, P = .008, and 41% vs. 14%, P = .028, respectively). Few patients achieved >9% gains at all sites with no significant difference between groups (11% for HD vs 4% for SD, P = .282).
Figure 4.
Proportion of participants achieving bone mineral density increases of >3% at all 3 sites from baseline to 9 and 15 months.b BL, baseline; FN, femoral neck; HD, high-dose teriparatide (40 μg) plus denosumab; LS, lumbar spine; M, month; SD, standard-dose teriparatide (20 μg) plus denosumab; TH, total hip.
*P < .05.
**P < .01. b
Includes 55 participants who had areal bone mineral density determinations at all study visits (baseline, month 9, and month 15) and at all sites (total hip, femoral neck, and lumbar spine).
Discussion
In the present study, we have demonstrated that more women treated with 9 months of HD teriparatide (40 μg) partially overlapped with 12 months of denosumab achieved clinically relevant aBMD gains than women receiving a similar treatment regimen with SD teriparatide (20 μg), although the majority of women in both groups had meaningful gains in aBMD at the hip and spine. Notably, the differences in response rates at the total hip and femoral neck were more pronounced after 15 months of treatment than 9 months even though both groups were receiving only denosumab from months 9 through 15. This observation may potentially be explained by denosumab-induced secondary mineralization of a greater volume of newly formed undermineralized bone during months 9 through 15 (21).
The response rates achieved with combined HD teriparatide and denosumab are also greater and more rapid when compared with prior studies of osteoporosis medications. For example, using the same 3% threshold, the total hip aBMD response rates have been reported to be 37% and 22% after 12 months of alendronate and risedronate treatment, respectively, and 53% after 24 months of denosumab (22,23). The few studies that have evaluated total hip aBMD response rates with teriparatide 20 μg have reported >3% response rates of 47% (n = 93) and 57% (n = 39) after 18 months and 36% after 24 months (n = 28) (23,24). In the present study, the total hip aBMD response rates among women in the HD group were significantly higher at 76% after just 9 months of treatment. Additionally, in a recent study that reported aBMD response rates of abaloparatide, the >6% response rate at the total hip after 18 months of treatment was 26.3% compared with an almost twofold higher rate of 45% among women in the HD group after just 15 months of treatment (25).
In addition to an overall greater response rate achieved in women treated with the HD combination, the response rates were also more consistent among anatomic sites. In the subgroup of women (n = 55) who had aBMD measurements at all 3 sites (total hip, femoral neck, and lumbar spine) and at all timepoints, nearly 4 of 5 women in the HD group achieved >3% aBMD increases at all 3 sites compared with approximately 2 of 5 women in the SD group at 15 months. Perhaps most strikingly, by the end of the study period, no participant randomized to the HD group experienced bone loss at the hip or spine and only 2 women in the SD group experienced bone loss at the hip.
The precise mechanisms underlying the larger effects on aBMD observed with HD compared with SD teriparatide when combined with denosumab are not fully understood. Teriparatide, an osteoanabolic agent, stimulates bone formation through both remodeling- and modeling-based bone formation (26–28). We have previously reported in the DATA-HD study that combining teriparatide (20 μg) with denosumab, a potent inhibitor of receptor activator of nuclear factor kappa-B ligand, led to profound suppression of bone remodeling, which was similar to that seen with the use of denosumab monotherapy, while allowing for continued bone formation (16,17). Hence, we hypothesized that use of a larger anabolic stimulus (i.e., HD teriparatide [40 μg]), would lead to greater separation between bone resorption and formation, favoring overall more bone formation. Notably, this hypothesis was supported by the changes in bone turnover markers observed in DATA-HD (18). Future studies that include histomorphometric analysis of bone biopsy specimens would help elucidate these mechanisms more fully.
This study has several potential limitations. First, the study is limited by the absence of fracture data. That said, there is increasing evidence to support observations that larger gains in aBMD during treatment are associated with greater reductions in fracture risk (2–9). Additionally, there is little reason to hypothesize that combining 2 medications, which both reduce fracture risk individually, would not do so when combined. In a phase 3 trial that compared 20 and 40 μg of teriparatide with placebo, both doses resulted in numerically similar decreases in vertebral and nonvertebral fracture rates despite greater increases in aBMD with the higher dose. Although this study was not powered to detect differences in incident fracture rates between teriparatide 20 and 40 μg, it has been postulated that the lack of greater antifracture efficacy observed with the higher dose may have been due to higher resorption of cortical bone and increased cortical porosity compared with the 20-µg dose (29–33). This does not apply to the present study, in which bone resorption was potently inhibited by combined treatment with denosumab.
Second, although there were no differences in the rates of hypercalcemia or participant withdrawal resulting from adverse effects, formal safety comparisons between the 2 therapeutic regimens are not possible because of the limited study size. Finally, the relatively small cohort size, the post hoc nature of the analysis, and the lack of correction for multiple comparisons may be considered limitations and restrict subgroup analysis, for example, baseline aBMD or women with or without prior bisphosphonate exposure.
In summary, the majority of postmenopausal women treated with combined HD teriparatide (40 μg) and denosumab for 15 months experienced meaningful increases in hip and spine aBMD within just 9 to 15 months, with more than 3 of 4 women experiencing >3% increases at all 3 measured sites (total hip, femoral neck, and lumbar spine). The superiority of this HD combination on overall aBMD accrual and the consistency of the response to treatment provide a compelling rationale to conduct larger studies to investigate the antifracture efficacy and safety of this treatment regimen because it may provide significant benefits to postmenopausal women at highest risk of fragility fracture.
Acknowledgments
We thank the staff at the Massachusetts General Hospital Bone Density Center and our dedicated study volunteers.
Financial Support: This project was supported by the Dart Family Foundation grant no. 1UL1TR001102, National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; K23AR068447) (J.N.T.), and NIH NIAMS (K24AR067847) (B.Z.L.). Study drug was supplied by Eli Lilly and Amgen.
Glossary
Abbreviations
- aBMD
areal bone mineral density
- DATA-HD
Denosumab and High-Dose Teriparatide Administration
- DXA
dual x-ray absorptiometry
- HD
high dose
- SD
standard dose
Additional Information
Disclosure Summary: J.N.T. has a financial interest in Amgen; her interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. B.Z.L. served on an advisory board for Amgen. All other authors have nothing to declare.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. ; National Osteoporosis Foundation Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(11):1785–1790. [DOI] [PubMed] [Google Scholar]
- 3. Austin M, Yang YC, Vittinghoff E, Adami S, Boonen S, Bauer DC, et al. ; FREEDOM Trial Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res. 2012;27(3):687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cosman F, Cauley JA, Eastell R, Boonen S, Palermo L, Reid IR, et al. Reassessment of fracture risk in women after 3 years of treatment with zoledronic acid: when is it reasonable to discontinue treatment? J Clin Endocrinol Metab. 2014;99(12):4546–4554. [DOI] [PubMed] [Google Scholar]
- 5. Roux C, Hofbauer LC, Ho PR, Wark JD, Zillikens MC, Fahrleitner-Pammer A, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone. 2014;58:48–54. [DOI] [PubMed] [Google Scholar]
- 6. Ferrari S, Adachi JD, Lippuner K, Zapalowski C, Miller PD, Reginster JY, et al. Further reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years. Osteoporos Int. 2015;26(12):2763–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000;85(1):231–236. [DOI] [PubMed] [Google Scholar]
- 8. Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002;87(4):1586–1592. [DOI] [PubMed] [Google Scholar]
- 9. Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, et al. ; FNIH Bone Quality Project Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34(4):632–642. [DOI] [PubMed] [Google Scholar]
- 10. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. [DOI] [PubMed] [Google Scholar]
- 11. Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Jama. 1999;282(14):1344–1352. [DOI] [PubMed] [Google Scholar]
- 12. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–645. [DOI] [PubMed] [Google Scholar]
- 13. Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. ; HORIZON Pivotal Fracture Trial Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. [DOI] [PubMed] [Google Scholar]
- 14. Eisman JA, Civitelli R, Adami S, Czerwinski E, Recknor C, Prince R, et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol. 2008;35(3):488–497. [PubMed] [Google Scholar]
- 15. Chesnut CH 3rd, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al. ; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–1249. [DOI] [PubMed] [Google Scholar]
- 16. Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382(9886):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie SA, Zhu Y, Foley K, et al. Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (the DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsai JN, Lee H, David NL, Eastell R, Leder BZ. Combination denosumab and high dose teriparatide for postmenopausal osteoporosis (DATA-HD): a randomised, controlled phase 4 trial. The Lancet Diabetes & Endocrinology 2019; 7(10):767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black DM, Steinbuch M, Palermo L, Dargent-Molina P, Lindsay R, Hoseyni MS, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int. 2001;12(7):519–528. [DOI] [PubMed] [Google Scholar]
- 20. Bonnick SL, Johnston CC Jr, Kleerekoper M, Lindsay R, Miller P, Sherwood L, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4(2):105–110. [DOI] [PubMed] [Google Scholar]
- 21. Dempster DW, Brown JP, Fahrleitner-Pammer A, Kendler D, Rizzo S, Valter I, et al. Effects of long-term denosumab on bone histomorphometry and mineralization in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103(7):2498–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sebba AI, Bonnick SL, Kagan R, Thompson DE, Skalky CS, Chen E, et al. ; Fosamax Actonel Comparison Trial investigators Response to therapy with once-weekly alendronate 70 mg compared to once-weekly risedronate 35 mg in the treatment of postmenopausal osteoporosis. Curr Med Res Opin. 2004;20(12):2031–2041. [DOI] [PubMed] [Google Scholar]
- 23. Leder BZ, Tsai JN, Neer RM, Uihlein AV, Wallace PM, Burnett-Bowie SA. Response to therapy with teriparatide, denosumab, or both in postmenopausal women in the DATA (Denosumab and Teriparatide Administration) study randomized controlled trial. J Clin Densitom. 2016;19(3):346–351. [DOI] [PubMed] [Google Scholar]
- 24. Gallagher JC, Rosen CJ, Chen P, Misurski DA, Marcus R. Response rate of bone mineral density to teriparatide in postmenopausal women with osteoporosis. Bone. 2006;39(6):1268–1275. [DOI] [PubMed] [Google Scholar]
- 25. Miller PD, Hattersley G, Lau E, Fitzpatrick LA, Harris AG, Williams GC, et al. Bone mineral density response rates are greater in patients treated with abaloparatide compared with those treated with placebo or teriparatide: results from the ACTIVE phase 3 trial. Bone. 2019;120:137–140. [DOI] [PubMed] [Google Scholar]
- 26. Ma YL, Zeng Q, Donley DW, Ste-Marie LG, Gallagher JC, Dalsky GP, et al. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(6):855–864. [DOI] [PubMed] [Google Scholar]
- 27. Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21(3):366–373. [DOI] [PubMed] [Google Scholar]
- 28. Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22(4):495–502. [DOI] [PubMed] [Google Scholar]
- 29. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. [DOI] [PubMed] [Google Scholar]
- 30. Fox J, Miller MA, Newman MK, Recker RR, Turner CH, Smith SY. Effects of daily treatment with parathyroid hormone 1-84 for 16 months on density, architecture and biomechanical properties of cortical bone in adult ovariectomized rhesus monkeys. Bone. 2007;41(3):321–330. [DOI] [PubMed] [Google Scholar]
- 31. Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int. 2011;22(1):357–362. [DOI] [PubMed] [Google Scholar]
- 32. Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28(4):736–745. [DOI] [PubMed] [Google Scholar]
- 33. Zebaze R, Takao-Kawabata R, Peng Y, Zadeh AG, Hirano K, Yamane H, et al. Increased cortical porosity is associated with daily, not weekly, administration of equivalent doses of teriparatide. Bone. 2017;99:80–84. [DOI] [PubMed] [Google Scholar]